PEMFC Semi-Empirical Model Improvement by Reconstructing Concentration Loss
Abstract
1. Introduction
2. Model Development
- (1)
- The PEMFC operates under a steady-state condition, and any transient phenomena are not included in this model.
- (2)
- The anode, cathode, and membrane are at the same temperature.
- (3)
- In the PEMFC, oxygen reduction is dominant because it is much slower than hydrogen oxidation. Therefore, the activation loss and concentration loss of the anode are ignored in the modeling.
- (4)
- The reactants are pure hydrogen and air, which are regarded as ideal gases.
- (5)
- The GDLs, catalyst layers, and membrane are treated as isotropic porous media.
- (6)
- The PEM is not electrically conductive and is impermeable to neutral reactant gases, so internal currents and fuel crossover losses are not considered.
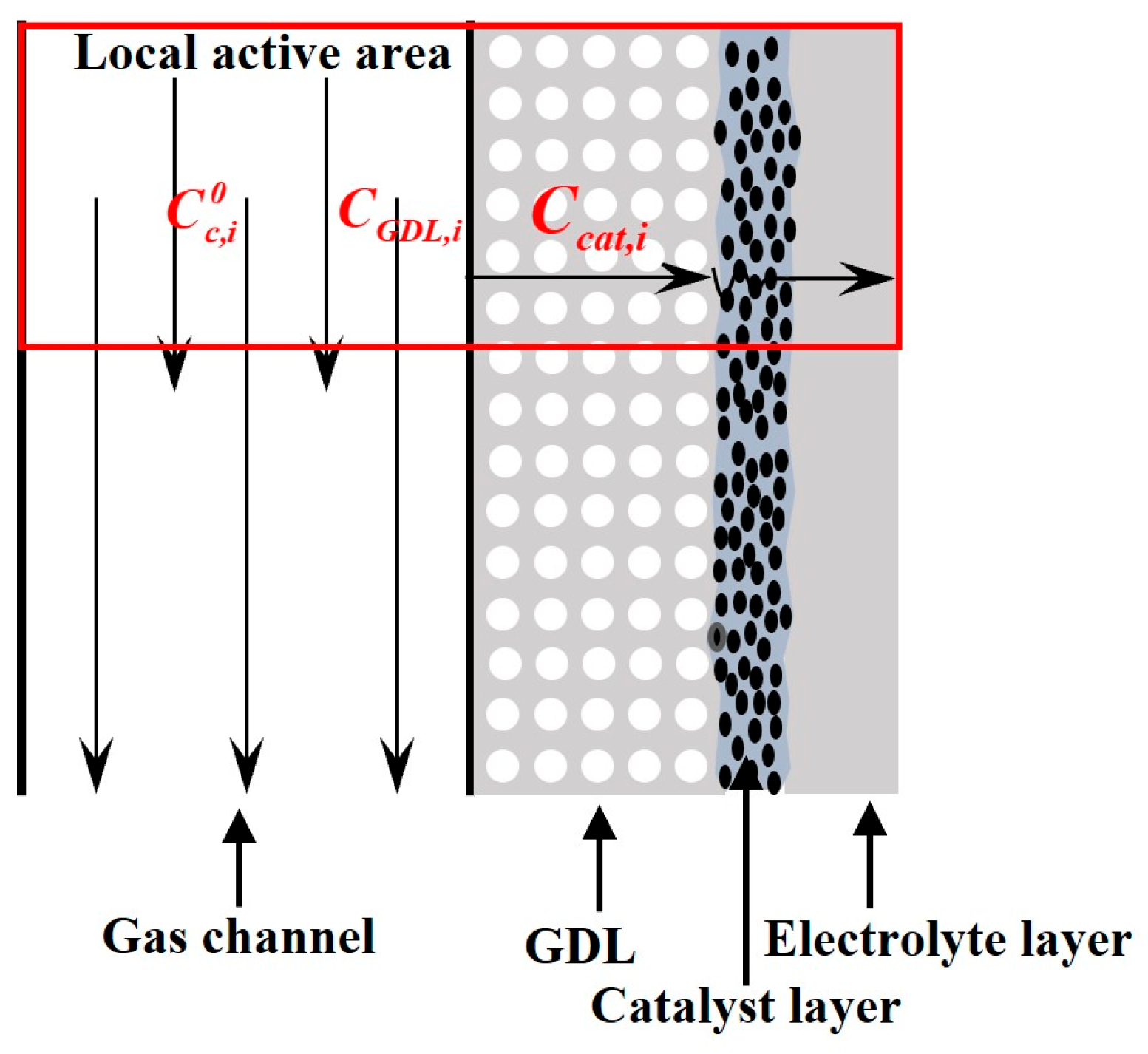
2.1. Concentration Loss
2.1.1. Gas Transport
2.1.2. Water Transport
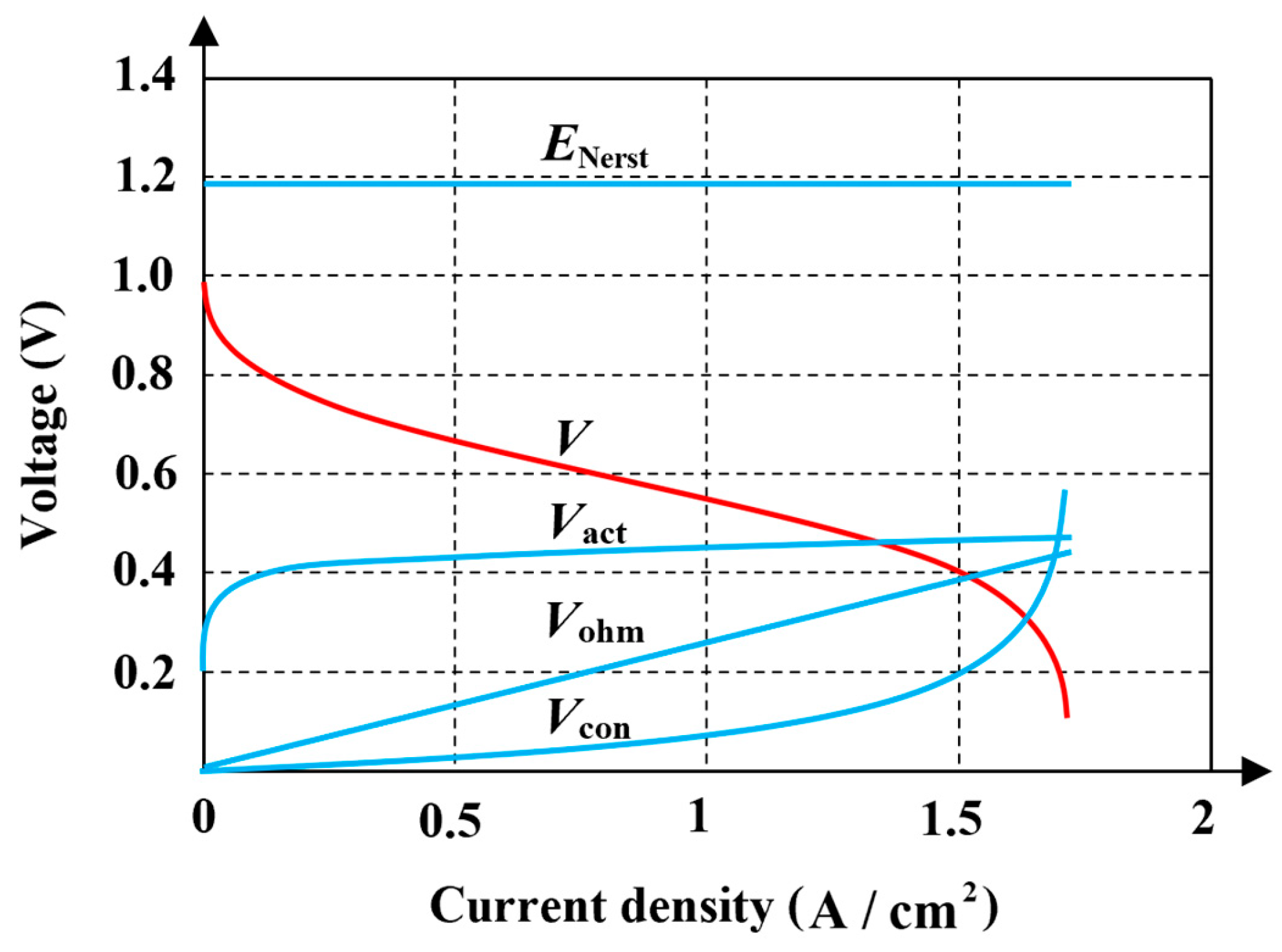
2.2. Semi-Empirical Model
2.3. Model Characteristic
3. Experimental Verification
3.1. Experimental Platform
3.2. Experimental Design and Data Collection
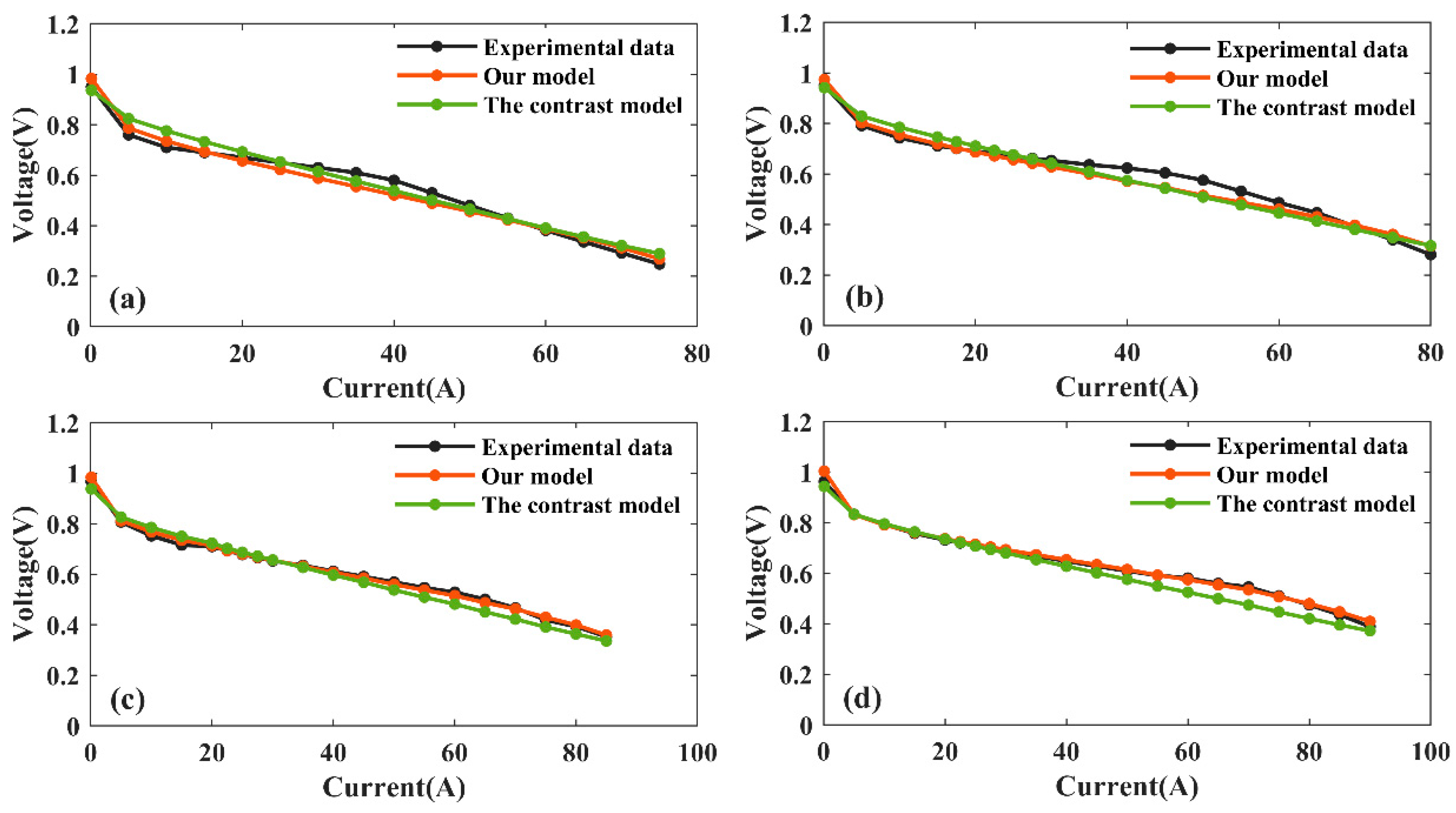
3.3. Model Verification
4. Applications of the Semi-Empirical Model
4.1. Analysis of the Effects of Operating Parameters on PEMFC Performance
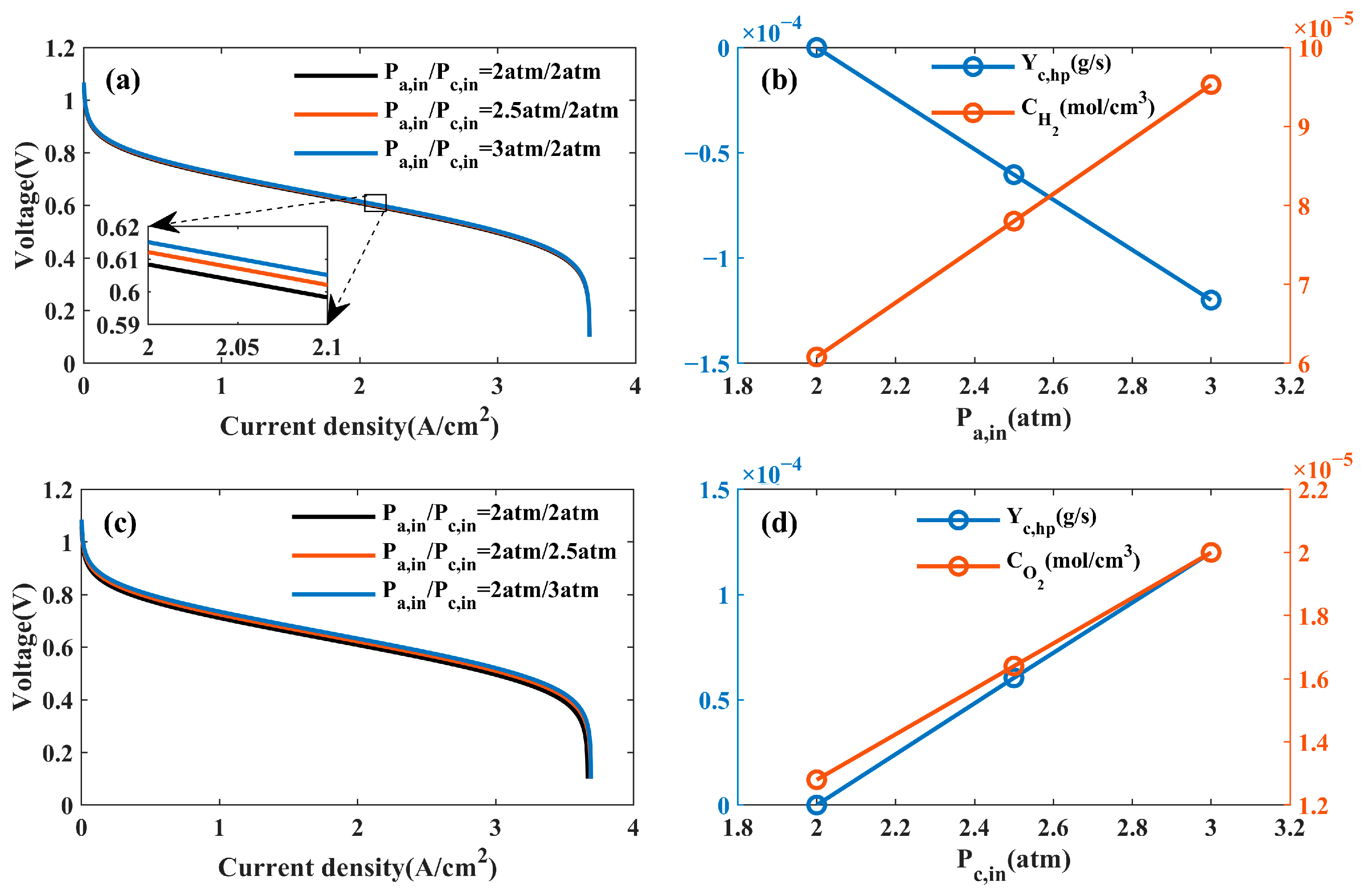
4.1.1. Effects of Pressure
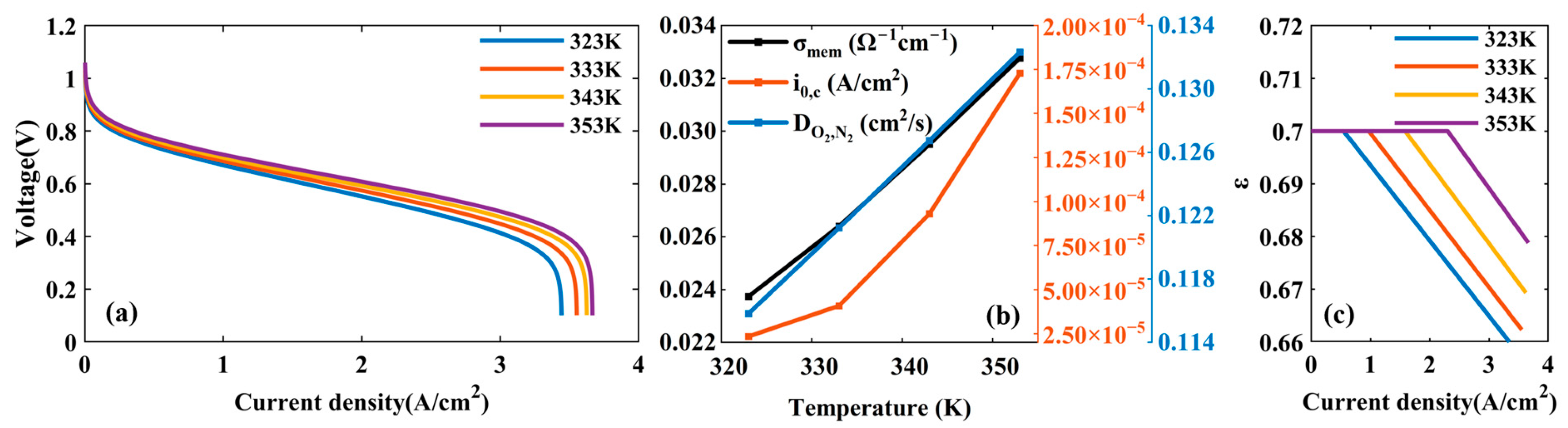
4.1.2. Effects of Temperature
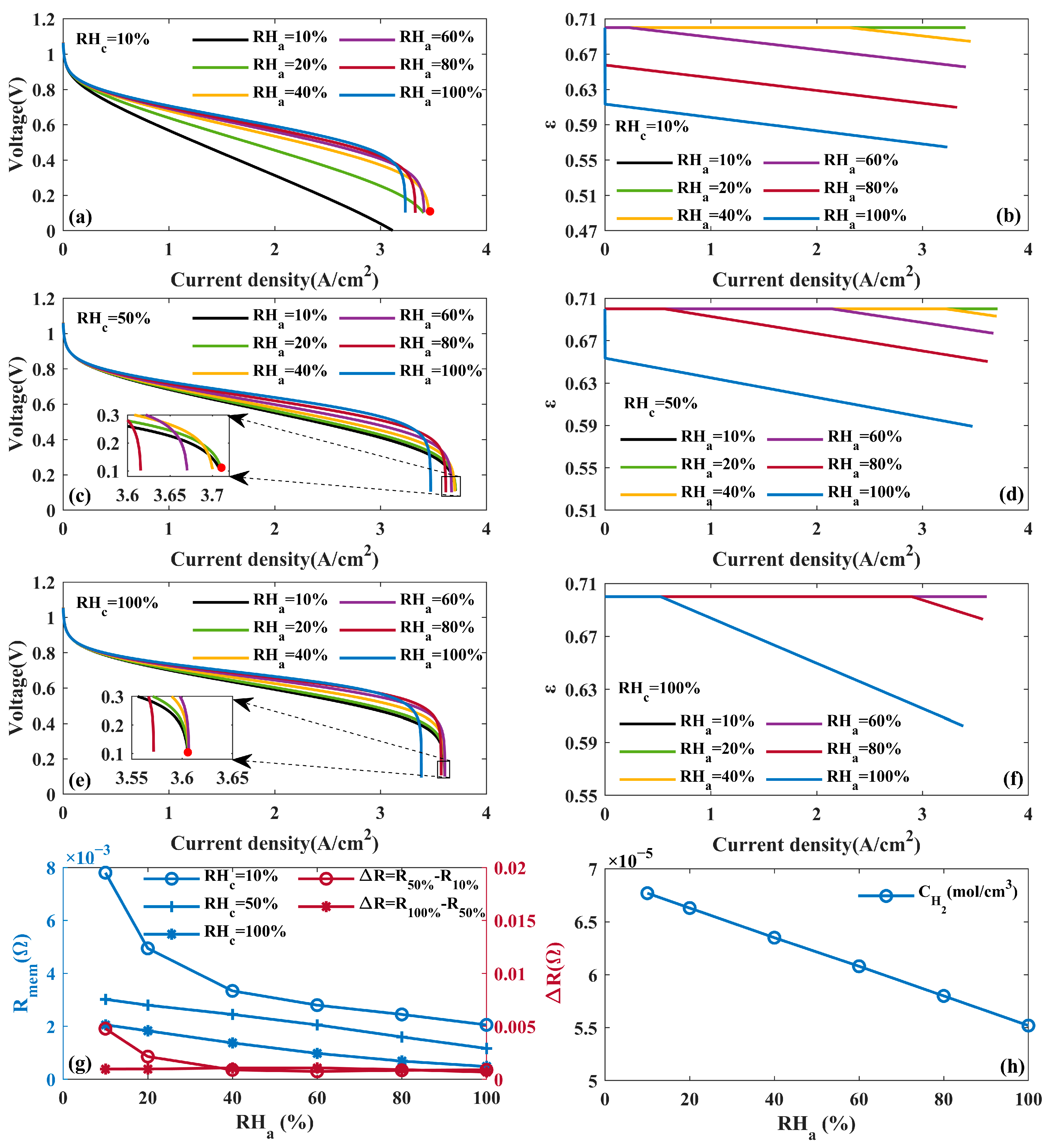
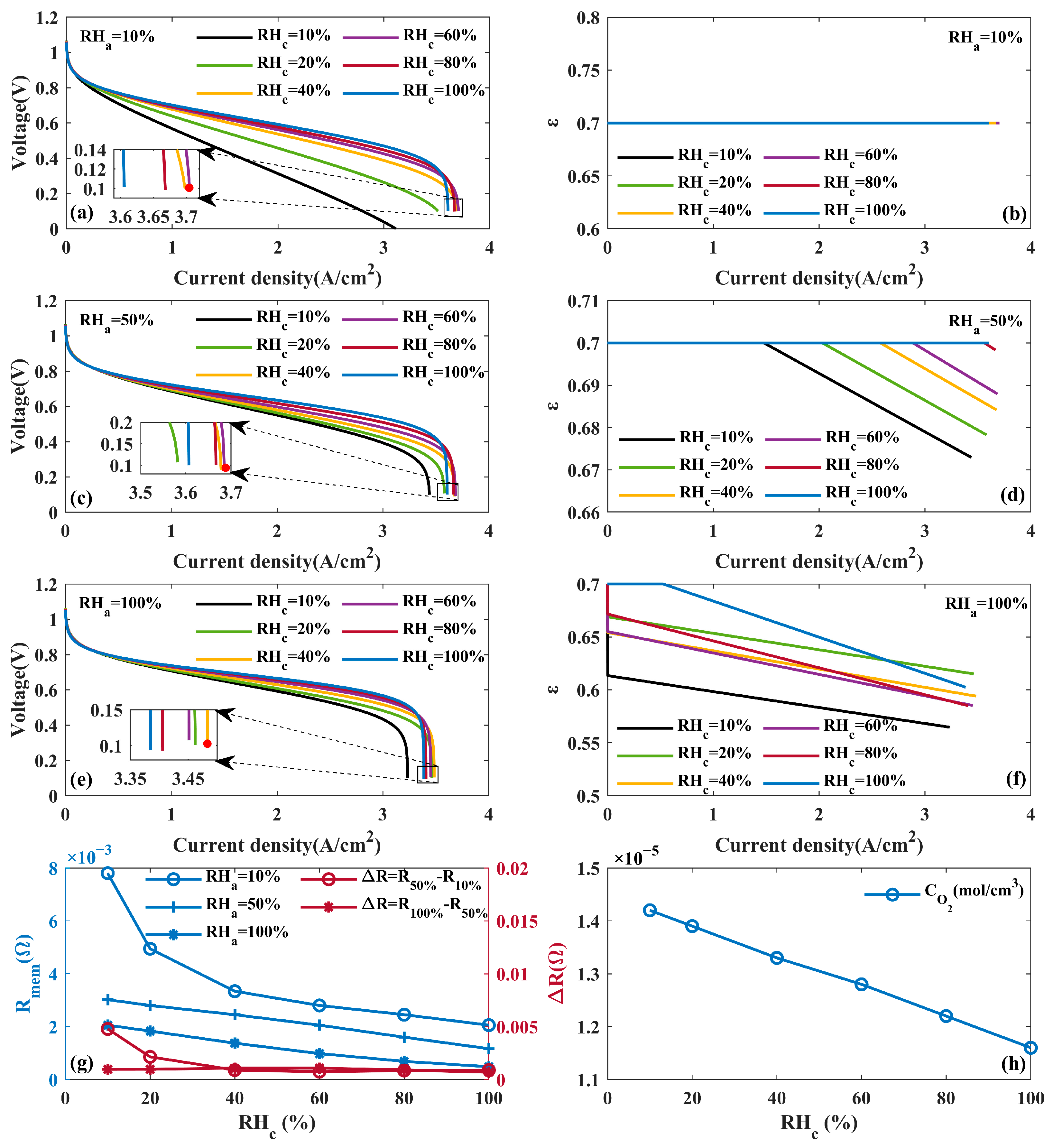
4.1.3. Effects of Humidity
4.2. Analysis of the Effects of Structural Parameters on PEMFC Performance
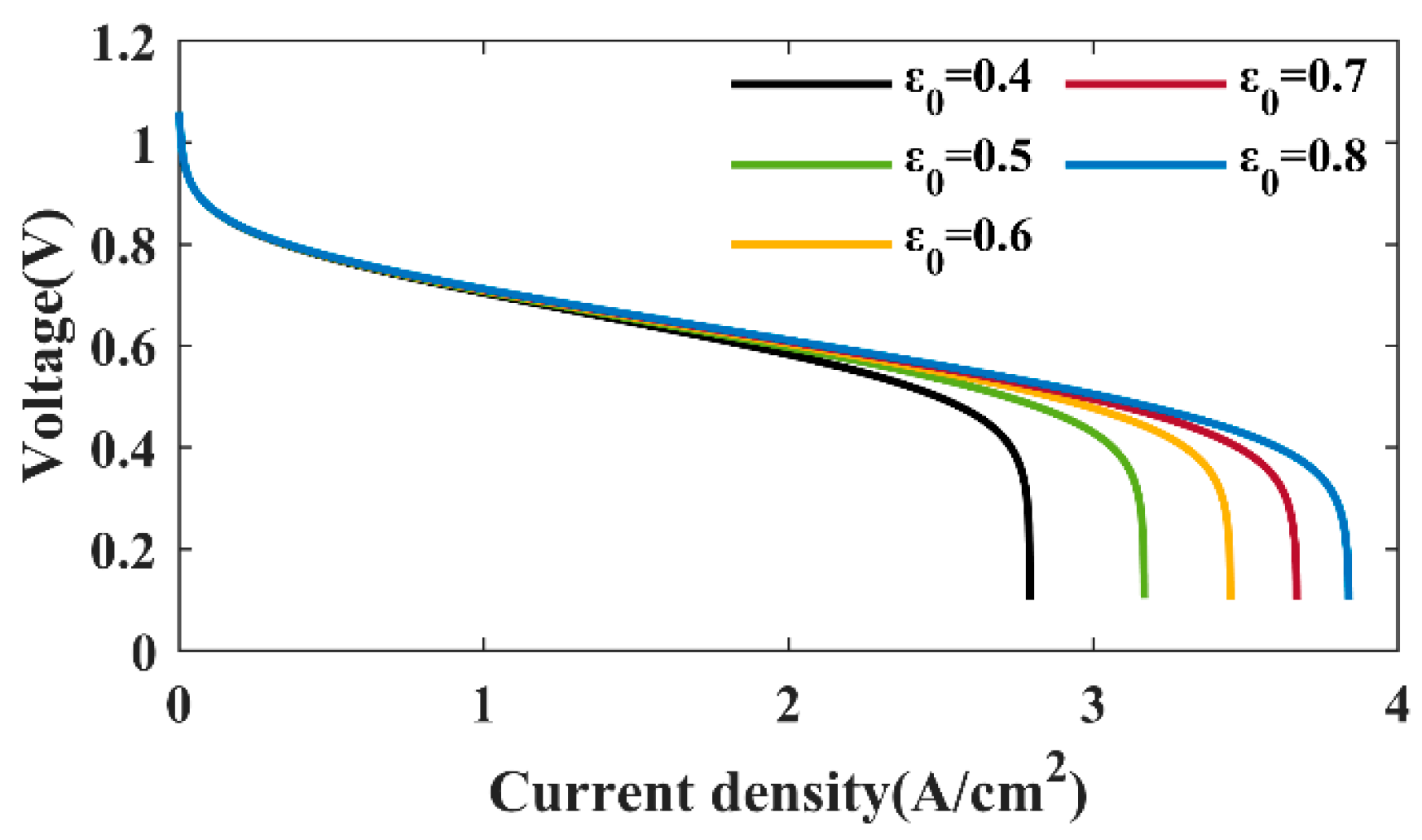
4.2.1. Effects of GDL Porosity
4.2.2. Effects of PEM Thickness
4.3. Optimization of Operating Parameters
4.4. Solution of Physical Coefficients
5. Conclusions
- (1)
- Important physical phenomena including reactant transport, water transport, and phase changes are considered in the modeling process to improve the accuracy and interpretability of the semi-empirical model. The model can be used to describe PEMFC polarization with different sizes and flow field structures, because the coefficients and , representing the flow field and GDL mass transfer capacity, are quantified in detail.
- (2)
- An orthogonal experiment was designed with temperature, humidity, anode back pressure, and cathode back pressure as experimental variables. Its higher prediction accuracy and generalization ability than the contrast model indicate that the influence of operating parameters on PEMFC performance is well-modeled in this study.
- (3)
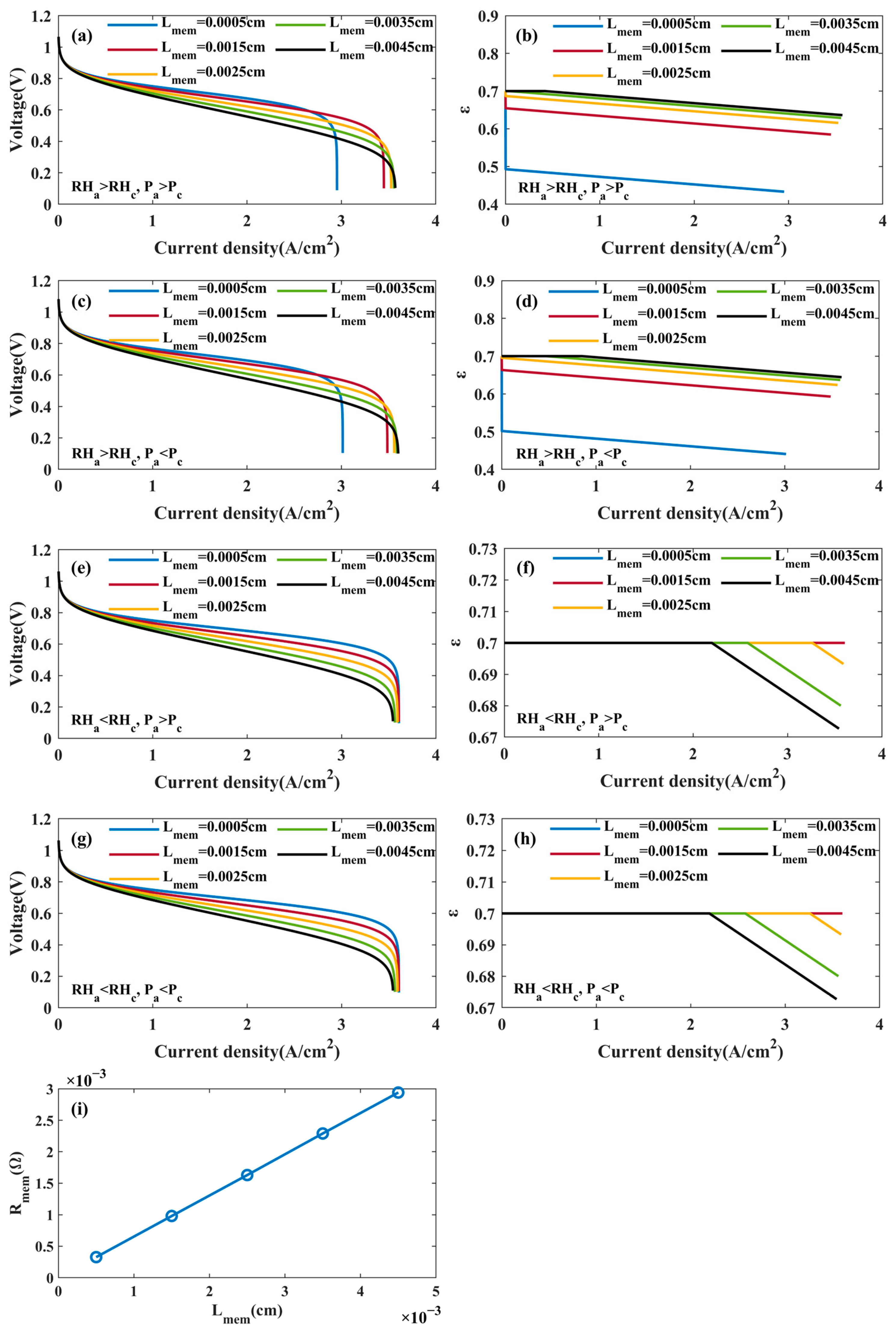
- The effects of the operating parameters and physical parameters on PEMFC performance were analyzed. It was found that a relatively high operating temperature, pressure, relative humidity, GDL porosity, and lower PEM thickness can increase PEMFC performance at a low current density. PEMFC performance will decrease if the relative humidity is too high at a high current density. Moreover, the effect of the PEM thickness on PEMFC performance is closely related to the anode and cathode humidity at a high current density. Specifically, PEMFC performance increases with an increasing PEM thickness when , and PEMFC performance decreases with an increasing PEM thickness when .
- (4)
- The application of the semi-empirical model to predict PEMFC performance considering component degradation in long-term operation was discussed. The optimization of operating parameters for a PEMFC working at a high current density and the solution of important physical coefficients were also prospected.
- (5)
- The independent impact of “water–gas coupled transport” was isolated in reconstructing concentration loss. This simplification inevitably introduced limitations, such as an inability to describe PEMFC degradation and applicability verification for different flow fields. In the future, we intend to address these limitations by supplementing the degradation model and multiple flow field models on the basis of the current model framework.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jung, E.S.; Kim, H.; Kwon, S.; Oh, T.H. Fuel cell system with sodium borohydride hydrogen generator for small unmanned aerial vehicles. Int. J. Green Energy 2018, 15, 385–392. [Google Scholar] [CrossRef]
- Zhang, G.; Qu, Z.; Wang, N.; Wang, Y. Spatial distribution characteristics in segmented PEM fuel cells: Three-dimensional full-morphology simulation. Electrochim. Acta 2024, 484, 144061. [Google Scholar] [CrossRef]
- Huo, W.; Fan, L.; Xu, Y.; Benbouzid, M.; Xu, W.; Gao, F.; Li, W.; Shan, N.; Xie, B.; Huang, H. Digitally-assisted structure design of a large-size proton exchange membrane fuel cell. Energy Environ. Sci. 2025, 18, 631–644. [Google Scholar] [CrossRef]
- Wang, Y.; Diaz, D.F.R.; Chen, K.S.; Wang, Z.; Adroher, X.C. Materials, technological status, and fundamentals of PEM fuel cells–a review. Mater. Today 2020, 32, 178–203. [Google Scholar] [CrossRef]
- Wang, Y.; Seo, B.; Wang, B.; Zamel, N.; Jiao, K.; Adroher, X.C. Fundamentals, materials, and machine learning of polymer electrolyte membrane fuel cell technology. Energy AI 2020, 1, 100014. [Google Scholar] [CrossRef]
- Zine, Y.; Benmouna, A.; Becherif, M.; Hissel, D. Towards maximum efficiency of an open-cathode PEM fuel cell system: A comparative experimental demonstration. Int. J. Hydrogen Energy 2024, 86, 72–85. [Google Scholar] [CrossRef]
- Jiao, K.; Xuan, J.; Du, Q.; Bao, Z.; Xie, B.; Wang, B.; Zhao, Y.; Fan, L.; Wang, H.; Hou, Z. Designing the next generation of proton-exchange membrane fuel cells. Nature 2021, 595, 361–369. [Google Scholar] [CrossRef]
- Stoll, J.; Zhao, N.; Yuan, X.-Z.; Girard, F.; Kjeang, E.; Shi, Z. Impacts of cathode catalyst layer defects on performance and durability in PEM fuel cells. J. Power Sources 2023, 583, 233565. [Google Scholar] [CrossRef]
- Dou, S.; Hao, L.; Wang, Q.; Liu, H. Effects of agglomerate structure and operating humidity on the catalyst layer performance of PEM fuel cells. Appl. Energy 2024, 355, 122211. [Google Scholar] [CrossRef]
- Soltani, M.; Amin, H.M.; Cebe, A.; Ayata, S.; Baltruschat, H. Metal-supported perovskite as an efficient bifunctional electrocatalyst for oxygen reduction and evolution: Substrate effect. J. Electrochem. Soc. 2021, 168, 034504. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, B.; Meng, K.; Zhou, H.; Chen, W.; Zhang, N.; Deng, Q.; Yang, G.; Tu, Z. Optimal design of a cathode flow field for performance enhancement of PEM fuel cell. Appl. Energy 2023, 343, 121226. [Google Scholar] [CrossRef]
- Liu, M.; Fan, W.; Lu, G. Study on mass transfer enhancement of locally improved structures and the application in serpentine and parallel flow fields of PEM fuel cells. Int. J. Hydrogen Energy 2023, 48, 19248–19261. [Google Scholar] [CrossRef]
- Choi, J.; Park, Y.; Park, J.; Kim, C.; Heo, S.; Kim, S.-D.; Ju, H. Innovative flow field design strategies for performance optimization in polymer electrolyte membrane fuel cells. Appl. Energy 2025, 377, 124551. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; Gao, Y.; He, W.; Zhao, Y.; Wang, X. Optimal design of cathode gas diffusion layer with arrayed grooves for performance enhancement of a PEM fuel cell. Renew. Energy 2022, 199, 697–709. [Google Scholar] [CrossRef]
- Lee, F.; Ismail, M.; Zhang, K.; Ingham, D.; Aldakheel, F.; Hughes, K.; Ma, L.; El-Kharouf, A.; Pourkashanian, M. Optimisation and characterisation of graphene-based microporous layers for polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy 2024, 51, 1311–1325. [Google Scholar] [CrossRef]
- Yoon, K.R.; Lee, K.A.; Jo, S.; Yook, S.H.; Lee, K.Y.; Kim, I.D.; Kim, J.Y. Mussel-Inspired Polydopamine-Treated Reinforced Composite Membranes with Self-Supported CeOx Radical Scavengers for Highly Stable PEM Fuel Cells. Adv. Funct. Mater. 2019, 29, 1806929. [Google Scholar] [CrossRef]
- O’hayre, R.; Cha, S.-W.; Colella, W.; Prinz, F.B. Fuel Cell Fundamentals; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Abdin, Z.; Webb, C.; Gray, E.M. PEM fuel cell model and simulation in Matlab–Simulink based on physical parameters. Energy 2016, 116, 1131–1144. [Google Scholar] [CrossRef]
- Hasegawa, S.; Kimata, M.; Ikogi, Y.; Kageyama, M.; Kawase, M.; Kim, S. Modeling of fuel cell stack for high-speed computation and implementation to integrated system model. ECS Trans. 2021, 104, 3. [Google Scholar] [CrossRef]
- Jang, J.-H.; Yan, W.-M.; Shih, C.-C. Effects of the gas diffusion-layer parameters on cell performance of PEM fuel cells. J. Power Sources 2006, 161, 323–332. [Google Scholar] [CrossRef]
- Xia, L.; Ni, M.; He, Q.; Xu, Q.; Cheng, C. Optimization of gas diffusion layer in high temperature PEMFC with the focuses on thickness and porosity. Appl. Energy 2021, 300, 117357. [Google Scholar] [CrossRef]
- Carcadea, E.; Varlam, M.; Ismail, M.; Ingham, D.B.; Marinoiu, A.; Raceanu, M.; Jianu, C.; Patularu, L.; Ion-Ebrasu, D. PEM fuel cell performance improvement through numerical optimization of the parameters of the porous layers. Int. J. Hydrogen Energy 2020, 45, 7968–7980. [Google Scholar] [CrossRef]
- Spiegel, C. PEM Fuel Cell Modeling and Simulation Using MATLAB; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Isaacson, M.; Sonin, A.A. Sherwood number and friction factor correlations for electrodialysis systems, with application to process optimization. Ind. Eng. Chem. Process Des. Dev. 1976, 15, 313–321. [Google Scholar]
- Ding, Y.; Fu, X.; Xu, L.; Li, J.; Ouyang, M.; Wu, H. Scaling analysis of diffusion–reaction process in proton exchange membrane fuel cell with the second Damköhler number. Chem. Eng. J. 2023, 465, 143011. [Google Scholar] [CrossRef]
- Wang, C.-T.; Chen, Y.-M.; Tang, R.C.O.; Garg, A.; Ong, H.-C.; Yang, Y.-C. Dominated flow parameters applied in a recirculation microbial fuel cell. Process Biochem. 2020, 99, 236–245. [Google Scholar] [CrossRef]
- Pukrushpan, J.T. Modeling and Control of Fuel Cell Systems and Fuel Processors; University of Michigan: Ann Arbor, MI, USA, 2003. [Google Scholar]
- Barbir, F. PEM Fuel Cells: Theory and Practice; Academic Press: Waltham, MA, USA, 2005. [Google Scholar] [CrossRef]
- Wu, H.-W.; Shih, G.-J.; Chen, Y.-B. Effect of operational parameters on transport and performance of a PEM fuel cell with the best protrusive gas diffusion layer arrangement. Appl. Energy 2018, 220, 47–58. [Google Scholar] [CrossRef]
- Zamel, N.; Li, X. Effective transport properties for polymer electrolyte membrane fuel cells–with a focus on the gas diffusion layer. Prog. Energy Combust. Sci. 2013, 39, 111–146. [Google Scholar] [CrossRef]
- del Real, A.J.; Arce, A.; Bordons, C. Development and experimental validation of a PEM fuel cell dynamic model. J. Power Sources 2007, 173, 310–324. [Google Scholar] [CrossRef]
- Deng, H.; Wang, D.; Xie, X.; Zhou, Y.; Yin, Y.; Du, Q.; Jiao, K. Modeling of hydrogen alkaline membrane fuel cell with interfacial effect and water management optimization. Renew. Energy 2016, 91, 166–177. [Google Scholar] [CrossRef]
- Chen, X.; Xu, J.; Liu, Q.; Chen, Y.; Wang, X.; Li, W.; Ding, Y.; Wan, Z. Active disturbance rejection control strategy applied to cathode humidity control in PEMFC system. Energy Convers. Manag. 2020, 224, 113389. [Google Scholar] [CrossRef]
- Ziogou, C.; Voutetakis, S.; Papadopoulou, S.; Georgiadis, M.C. Modeling, simulation and experimental validation of a PEM fuel cell system. Comput. Chem. Eng. 2011, 35, 1886–1900. [Google Scholar] [CrossRef]
- Ferrara, A.; Polverino, P.; Pianese, C. Analytical calculation of electrolyte water content of a Proton Exchange Membrane Fuel Cell for on-board modelling applications. J. Power Sources 2018, 390, 197–207. [Google Scholar] [CrossRef]
- Ding, Q.; Zhu, K.-Q.; Yang, C.; Chen, X.; Wan, Z.-M.; Wang, X.-D. Performance investigation of proton exchange membrane fuel cells with curved membrane electrode assemblies caused by pressure differences between cathode and anode. Int. J. Hydrogen Energy 2021, 46, 37393–37405. [Google Scholar] [CrossRef]
- Amphlett, J.C.; Baumert, R.M.; Mann, R.F.; Peppley, B.A.; Roberge, P.R.; Harris, T.J. Performance modeling of the Ballard Mark IV solid polymer electrolyte fuel cell: I. Mechanistic model development. J. Electrochem. Soc. 1995, 142, 1. [Google Scholar] [CrossRef]
- Springer, T.E.; Zawodzinski, T.; Gottesfeld, S. Polymer electrolyte fuel cell model. J. Electrochem. Soc. 1991, 138, 2334. [Google Scholar] [CrossRef]
- Nguyen, T.V.; White, R.E. A water and heat management model for proton-exchange-membrane fuel cells. J. Electrochem. Soc. 1993, 140, 2178. [Google Scholar] [CrossRef]
- Gouda, E.A.; Kotb, M.F.; El-Fergany, A.A. Jellyfish search algorithm for extracting unknown parameters of PEM fuel cell models: Steady-state performance and analysis. Energy 2021, 221, 119836. [Google Scholar] [CrossRef]
- Ashraf, H.; Elkholy, M.M.; Abdellatif, S.O.; El-Fergany, A.A. Accurate emulation of steady-state and dynamic performances of PEM fuel cells using simplified models. Sci. Rep. 2023, 13, 19532. [Google Scholar] [CrossRef]
- Chavan, S.L.; Talange, D.B. Modeling and performance evaluation of PEM fuel cell by controlling its input parameters. Energy 2017, 138, 437–445. [Google Scholar] [CrossRef]
- Ou, K.; Yuan, W.-W.; Choi, M.; Yang, S.; Kim, Y.-B. Performance increase for an open-cathode PEM fuel cell with humidity and temperature control. Int. J. Hydrogen Energy 2017, 42, 29852–29862. [Google Scholar] [CrossRef]
- Yakut, Y.B. A new control algorithm for increasing efficiency of PEM fuel cells–Based boost converter using PI controller with PSO method. Int. J. Hydrogen Energy 2024, 75, 1–11. [Google Scholar] [CrossRef]
- Yin, X.; Wang, X.; Wang, L.; Qin, B.; Liu, H.; Jia, L.; Cai, W. Cooperative control of air and fuel feeding for PEM fuel cell with ejector-driven recirculation. Appl. Therm. Eng. 2021, 199, 117590. [Google Scholar] [CrossRef]
- Newman, J.S. Electrochemical Systems, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 1991. [Google Scholar]
- Zhu, H.; Kee, R.J. A general mathematical model for analyzing the performance of fuel-cell membrane-electrode assemblies. J. Power Sources 2003, 117, 61–74. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.; Kang, T.; Sohn, Y.J.; Song, T.; Choi, K.H. Degradation modeling and operational optimization for improving the lifetime of high-temperature PEM (proton exchange membrane) fuel cells. Energy 2014, 66, 41–49. [Google Scholar] [CrossRef]
- Wang, B.; Wu, K.; Xi, F.; Xuan, J.; Xie, X.; Wang, X.; Jiao, K. Numerical analysis of operating conditions effects on PEMFC with anode recirculation. Energy 2019, 173, 844–856. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Srinivasan, S.; Appleby, A.J.; Martin, C.R. Electrode kinetics of oxygen reduction at carbon-supported and unsupported platinum microcrystallite/Nafion® interfaces. J. Electroanal. Chem. 1992, 339, 101–121. [Google Scholar] [CrossRef]
- Watt-Smith, M.J.; Friedrich, J.M.; Rigby, S.P.; Ralph, T.R.; Walsh, F.C. Determination of the electrochemically active surface area of Pt/C PEM fuel cell electrodes using different adsorbates. J. Phys. D Appl. Phys. 2008, 41, 174004. [Google Scholar] [CrossRef]
- Xie, Z.; Navessin, T.; Shi, K.; Chow, R.; Wang, Q.P.; Song, D.T.; Andreaus, B.; Eikerling, M.; Liu, Z.S.; Holdcroft, S. Functionally graded cathode catalyst layers for polymer electrolyte fuel cells—II. Experimental study of the effect of Nafion distribution. J. Electrochem. Soc. 2005, 152, A1171. [Google Scholar] [CrossRef]
- Maggio, G.; Recupero, V.; Pino, L. Modeling polymer electrolyte fuel cells: An innovative approach. J. Power Sources 2001, 101, 275–286. [Google Scholar] [CrossRef]
- El-Genk, M.S.; Toumier, J.M.P. Sherwood number correlation for nuclear graphite gasification at high temperature. Prog. Nucl. Energy 2013, 62, 26–36. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.; Kim, D.; Kim, G.H.; Kwon, O.; Cha, H.; Choi, H.; Yoo, H.; Park, T. Mass diffusion characteristics on performance of polymer electrolyte membrane fuel cells with serpentine channels of different width. Int. J. Heat Mass Transf. 2022, 183, 122106. [Google Scholar] [CrossRef]
- Bernardi, D.M.; Verbrugge, M.W. A Mathematical Model of the Solid-Polymer-Electrolyte Fuel Cell. J. Electrochem. Soc. 1992, 139, 2477. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Y.; Yang, F.; Xu, H. Two-dimensional multi-physics modeling of porous transport layer in polymer electrolyte membrane electrolyzer for water splitting. Int. J. Hydrogen Energy 2020, 45, 32984–32994. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, X.; Li, Y.; Fan, Z.; Chen, W.; Mao, J.; Deng, P.; Wik, T. Model optimization of a high-power commercial PEMFC system via an improved grey wolf optimization method. Fuel 2024, 357, 129589. [Google Scholar] [CrossRef]
- Majsztrik, P.W.; Satterfield, M.B.; Bocarsly, A.B.; Benziger, J.B. Water sorption, desorption and transport in Nafion membranes. J. Membr. Sci. 2007, 301, 93–106. [Google Scholar] [CrossRef]


| Gas | C | ||
|---|---|---|---|
| Air | 17.16 | 273 | 111 |
| N2 | 16.63 | 273 | 107 |
| O2 | 19.19 | 273 | 139 |
| H2 | 8.411 | 273 | 47 |
| H2O | 11.2 | 350 | 1064 |
| Substance i | Substance j | ||
|---|---|---|---|
| H2 | H2O | 298 | |
| O2 | H2O | 298 | |
| O2 | N2 | 273 | |
| N2 | H2O | 298 |
| Characteristics | Equation |
|---|---|
| Gas transport considering flow field and GDL structure | |
| Dynamic change in GDL porosity | |
| Hydraulic permeation | |
| Phase change of the water |
| Factor | Level | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| T: Temperature (K) | 323 | 333 | 343 |
| Pa,out: Anode backpressure (Kpa) | 0 | 20 | 40 |
| Pc,out: Cathode backpressure (Kpa) | 0 | 20 | 40 |
| RH: Humidity (%) | 30 | 40 | 50 |
| Test No | Levels | Test No | Levels | Test No | Levels | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | Pa,out | Pc,out | RH | T | Pa,out | Pc,out | RH | T | Pa,out | Pc,out | RH | |||
| 1 | 1 | 1 | 1 | 1 | 4 | 2 | 2 | 3 | 1 | 7 | 3 | 3 | 2 | 1 |
| 2 | 1 | 2 | 2 | 2 | 5 | 2 | 3 | 1 | 2 | 8 | 3 | 1 | 3 | 2 |
| 3 | 1 | 3 | 3 | 3 | 6 | 2 | 1 | 2 | 3 | 9 | 3 | 2 | 1 | 3 |
| Parameter | Value | Parameter | Value | Parameter | Value |
|---|---|---|---|---|---|
| N | 1 | 0.7 | 18 g/mol | ||
| A | 25 cm2 | d | 0.1 cm | 32 g/mol | |
| 0.0015 cm | 0.0515 cm | 28 g/mol | |||
| 0.025 cm | 29 | 2 g/mol | |||
| 1 g/cm3 | 21% | 28 g/mol | |||
| 1.98 g/cm3 | R | 8.314 J/mol | 1100 g/mol |
| Parameter | Value in Our Model | Value in Contrast Model | Reported Value |
|---|---|---|---|
| 0.186 | 0.336 | 0–2 [46,47] | |
| 5.3 × 10−8 | 6.23 × 10−10 | 5.38 × 10−6 [48], 1 × 10−10 [49] | |
| 42 | 9 | 2.7 [50], 111 [51], 446 [52] | |
| 0.108 | / | 0.054 [53] | |
| a | 0.0196 | / | 0.025 [54], 0.044 [24], 0.021 [24] |
| b | 0.23 | / | 0.144 [55], 0.33 [24], 0.33 [54] |
| c | 0.386 | / | −0.207 [55], 0.875 [24], 0.466 [54] |
| 6.5 × 10−21 | / | 1.8 × 10−14 [56], 5 × 10−16 [57] | |
| B | / | 0.928 | 5 × 10−4–1 [58] |
| Parameter | T | RH | ||
|---|---|---|---|---|
| Value | 353 | 60 | 60 | 60 |
| The Set of Experimental Data | Model | ||||
|---|---|---|---|---|---|
| Validation set | Our model | 56 | 0.025 | 0.019 | 0.061 |
| Contrast model | 0.032 | 0.027 | 0.067 | ||
| Testing set | Our model | 21 | 0.012 | 0.007 | 0.04 |
| Contrast model | 0.035 | 0.026 | 0.071 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Liu, X.; Xiao, G.; Zhang, Z. PEMFC Semi-Empirical Model Improvement by Reconstructing Concentration Loss. Energies 2025, 18, 1754. https://doi.org/10.3390/en18071754
Yang Q, Liu X, Xiao G, Zhang Z. PEMFC Semi-Empirical Model Improvement by Reconstructing Concentration Loss. Energies. 2025; 18(7):1754. https://doi.org/10.3390/en18071754
Chicago/Turabian StyleYang, Qinwen, Xuan Liu, Gang Xiao, and Zhen Zhang. 2025. "PEMFC Semi-Empirical Model Improvement by Reconstructing Concentration Loss" Energies 18, no. 7: 1754. https://doi.org/10.3390/en18071754
APA StyleYang, Q., Liu, X., Xiao, G., & Zhang, Z. (2025). PEMFC Semi-Empirical Model Improvement by Reconstructing Concentration Loss. Energies, 18(7), 1754. https://doi.org/10.3390/en18071754





