Usage of a Binary CO2/C3H8 (85/15 vol%) Mixture for Hydrate Production: The Role of the Memory Effect and Phase Boundary Conditions of the System
Abstract
:1. Introduction
2. Materials and Methods
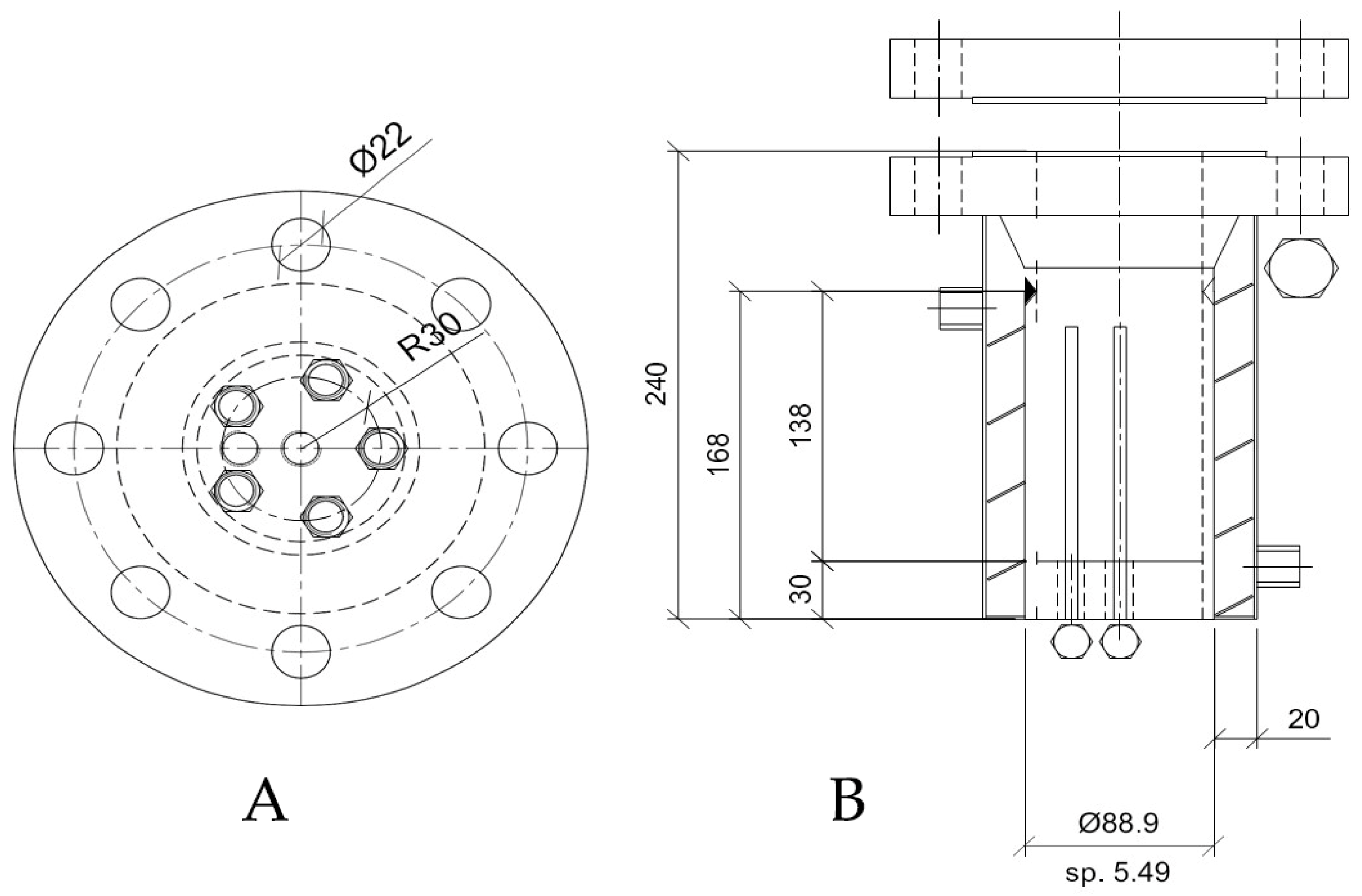
2.1. Experimental Apparatus and Materials
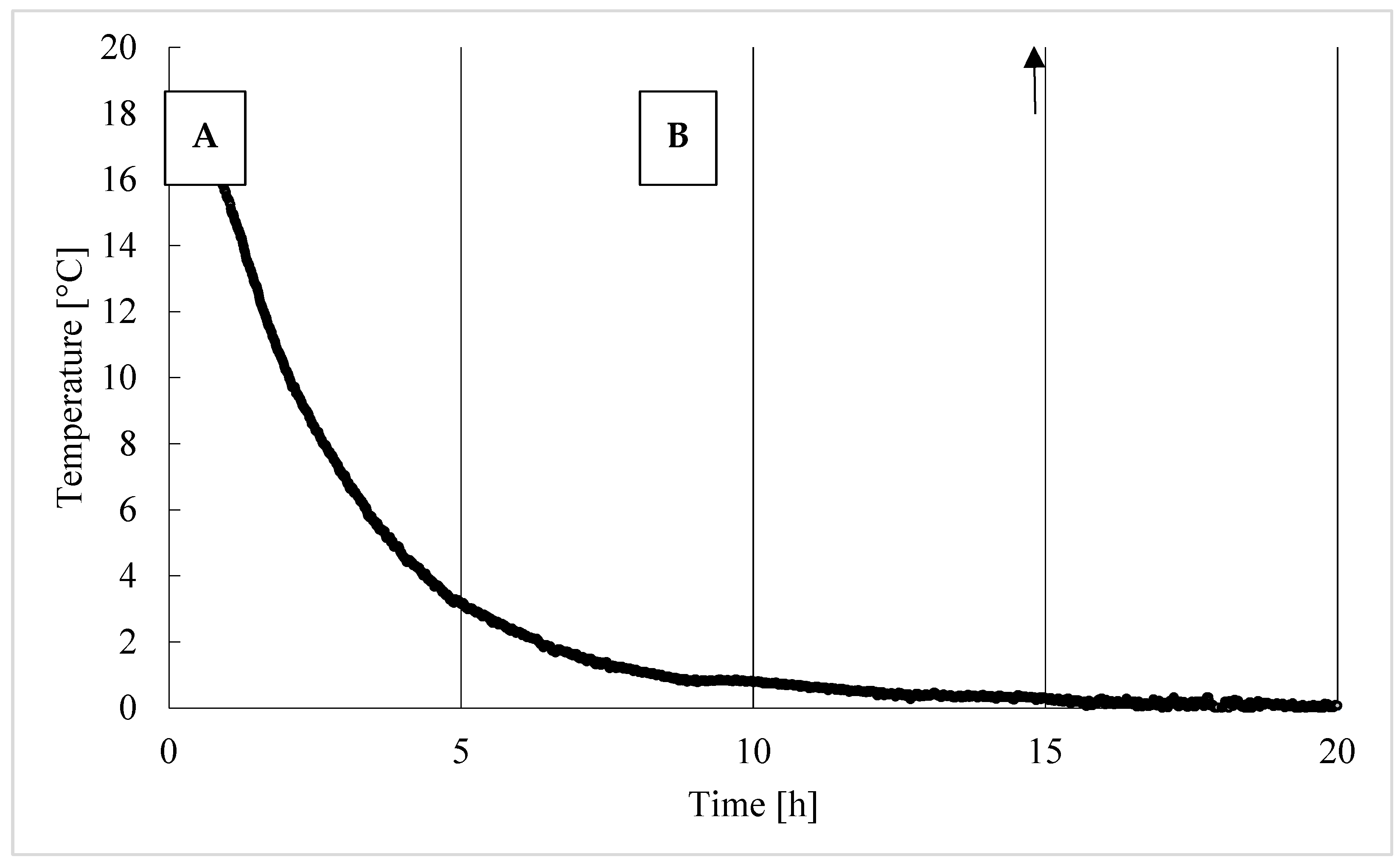
2.2. Experimental Procedure
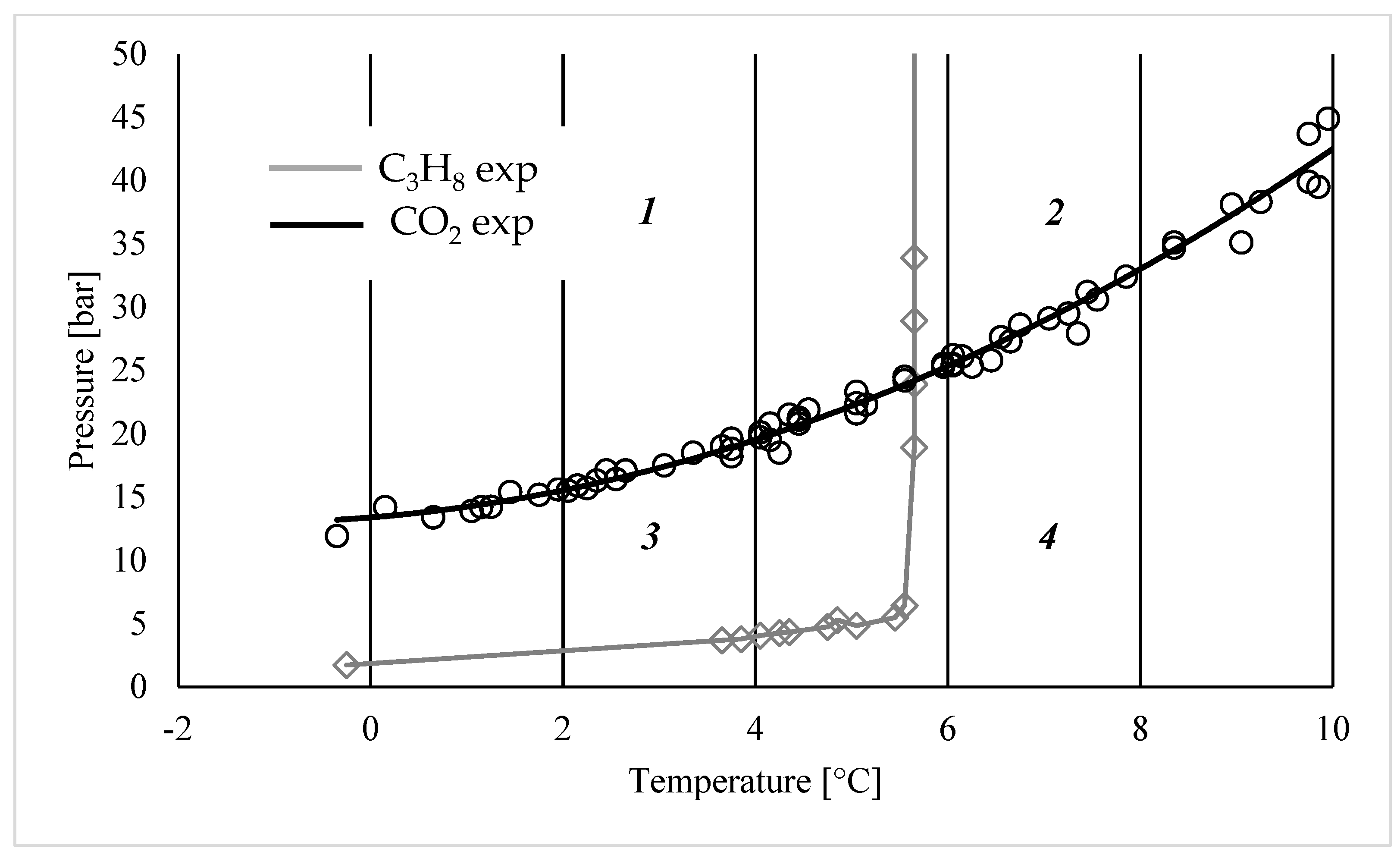
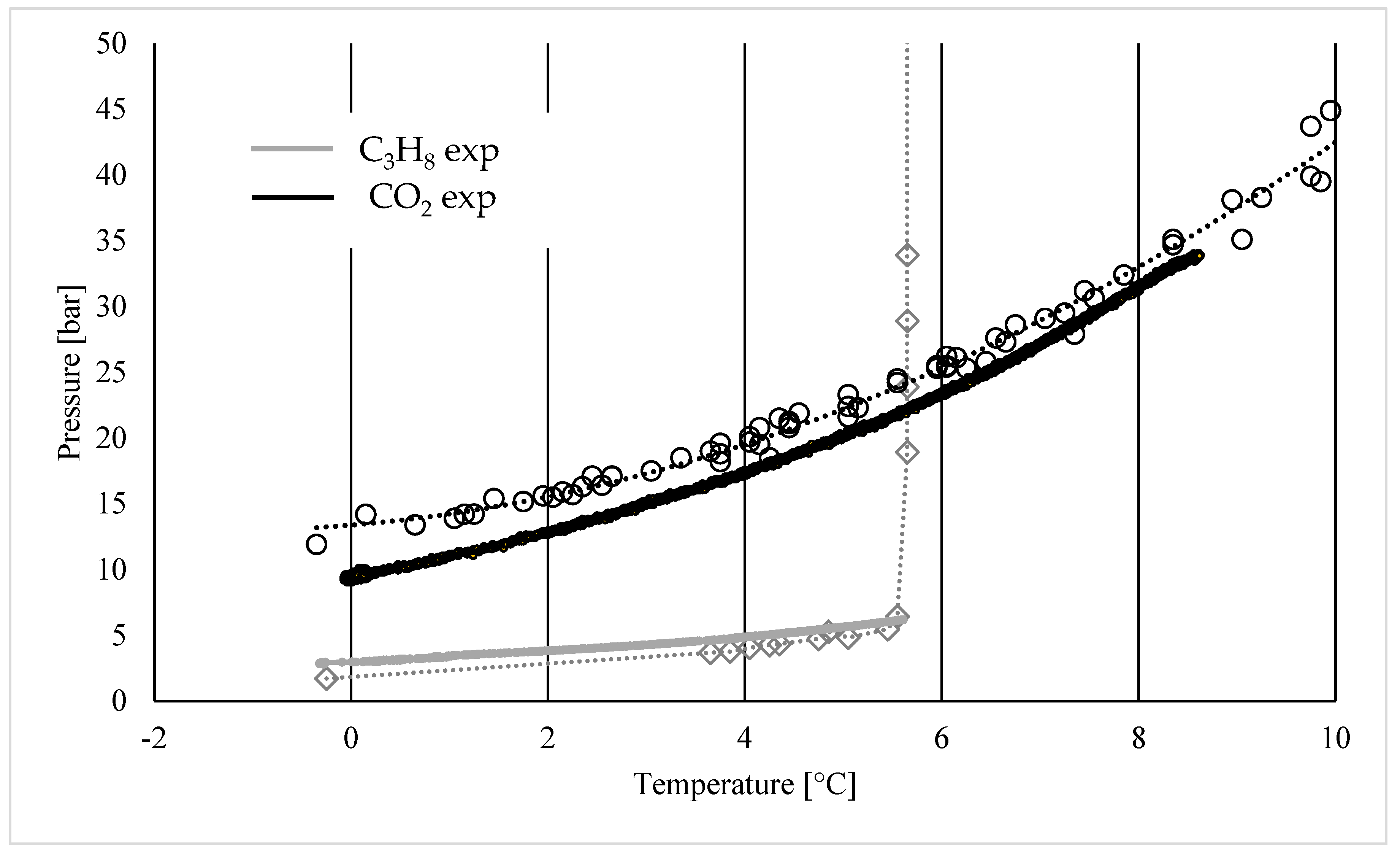
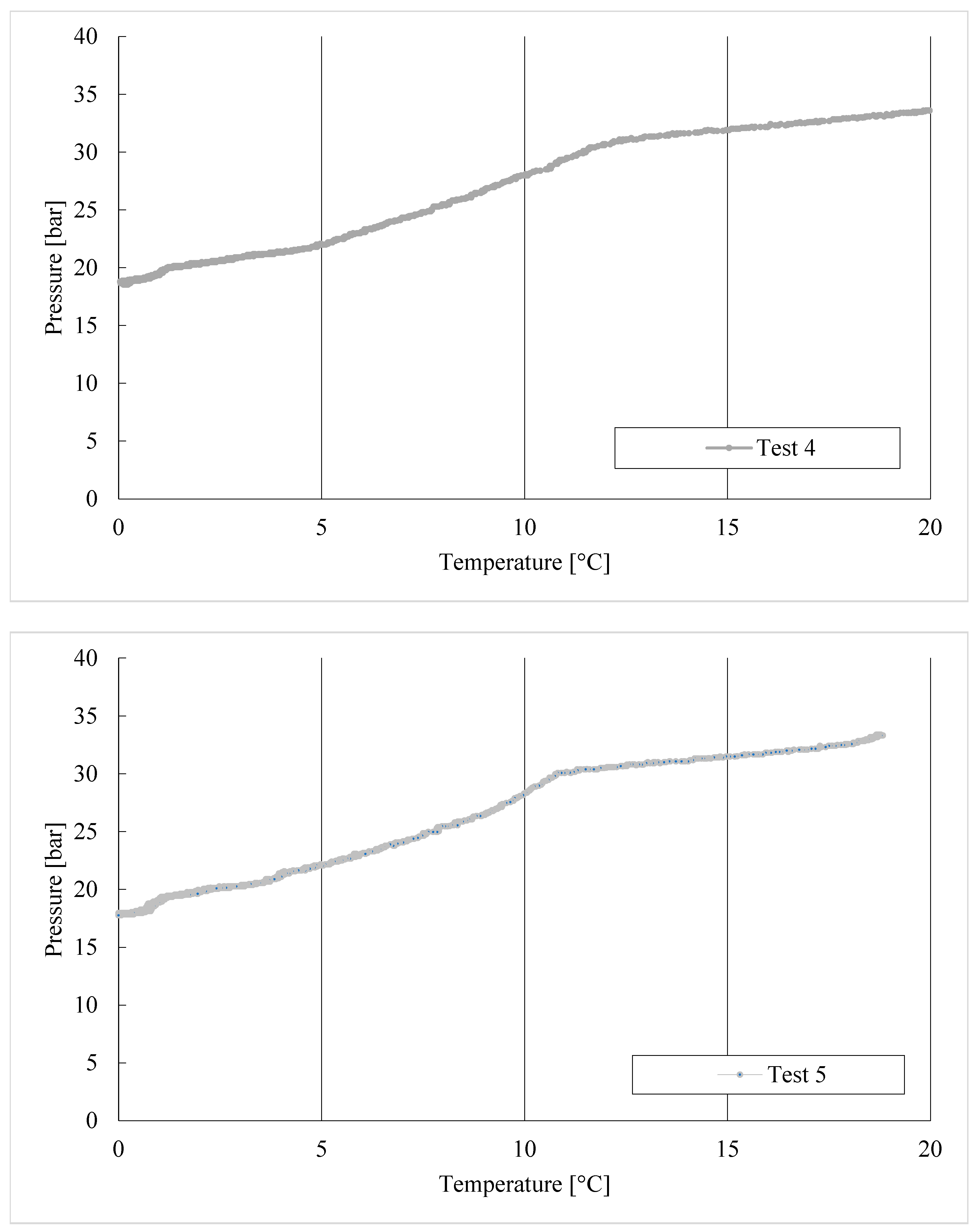
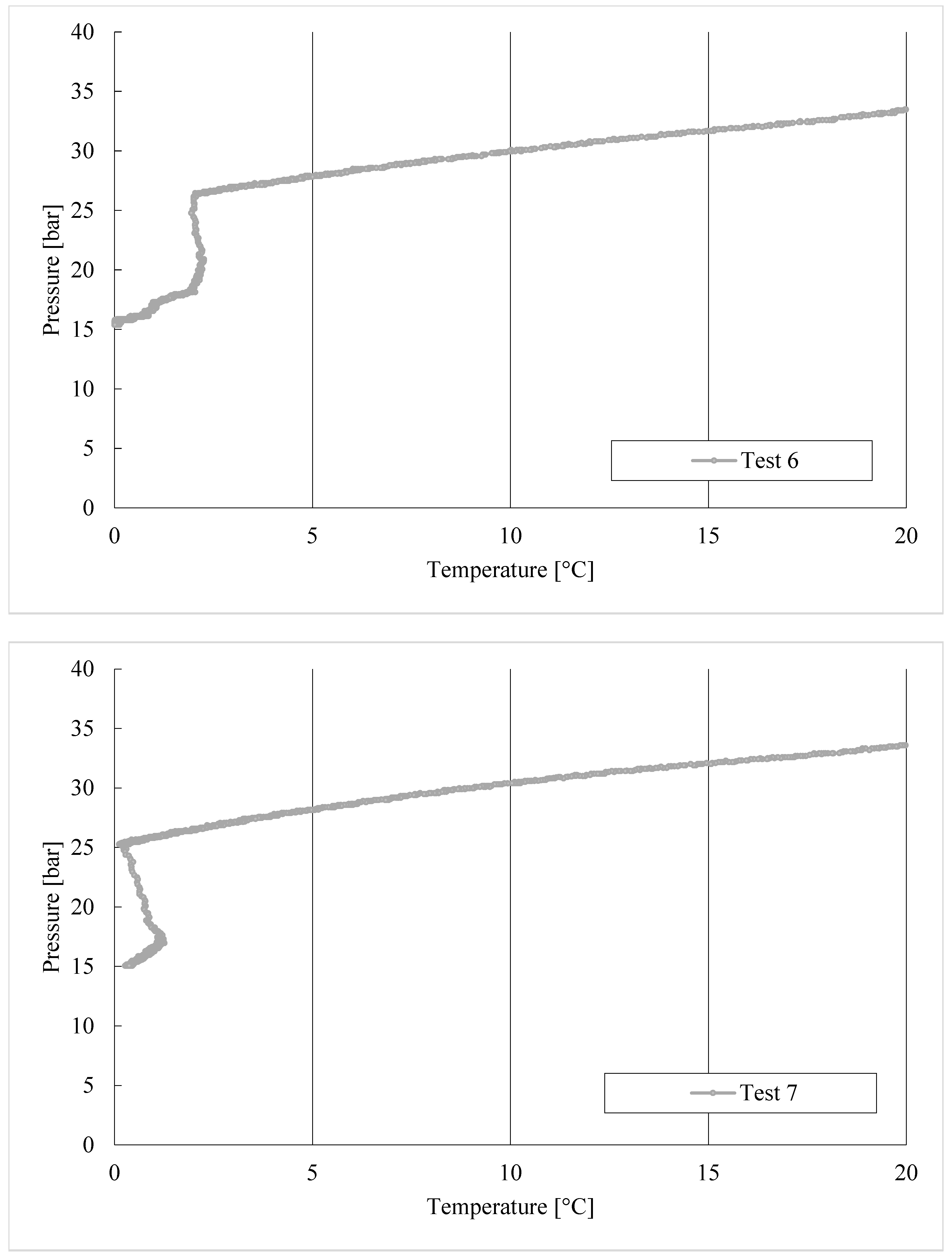
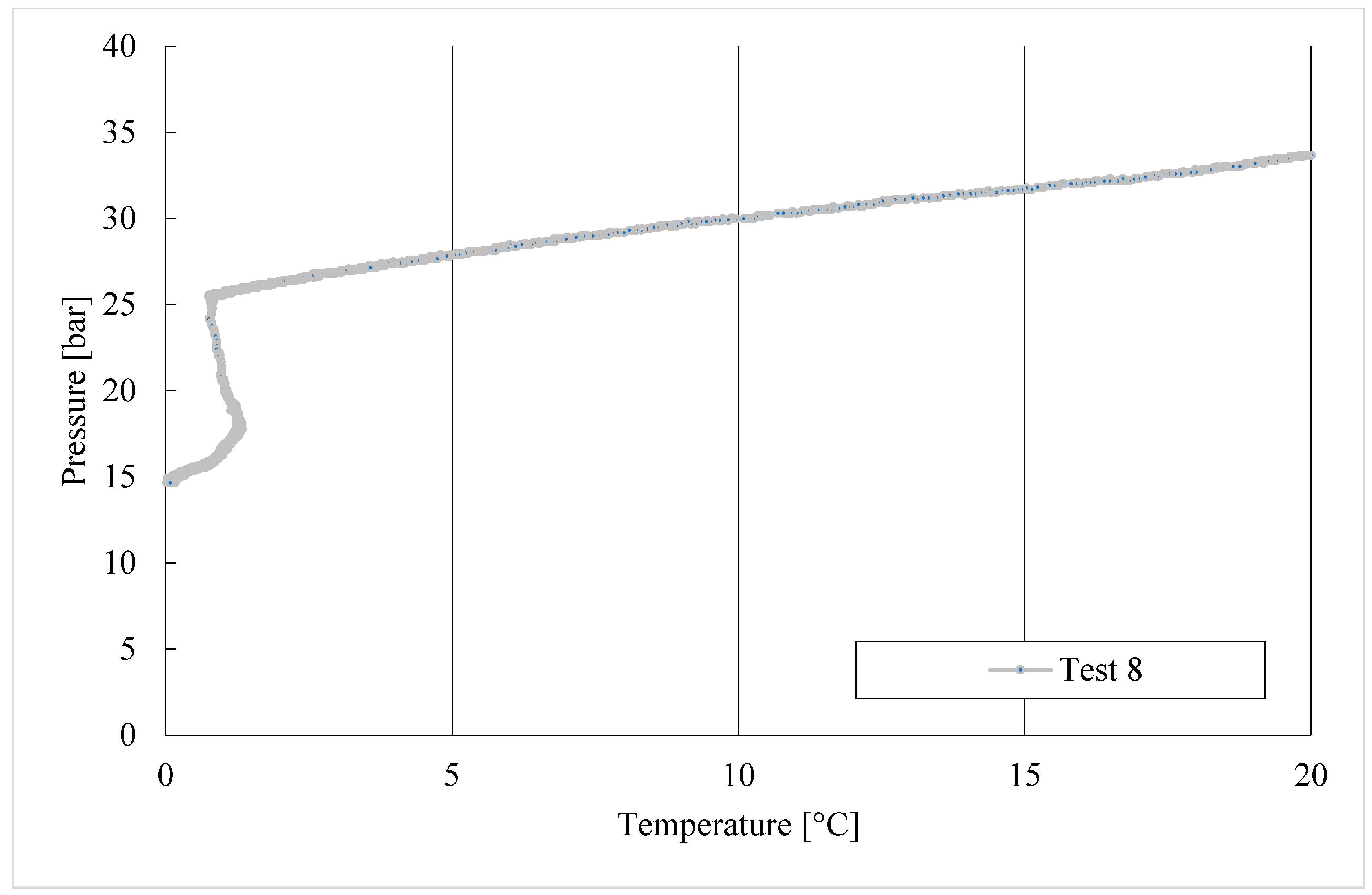
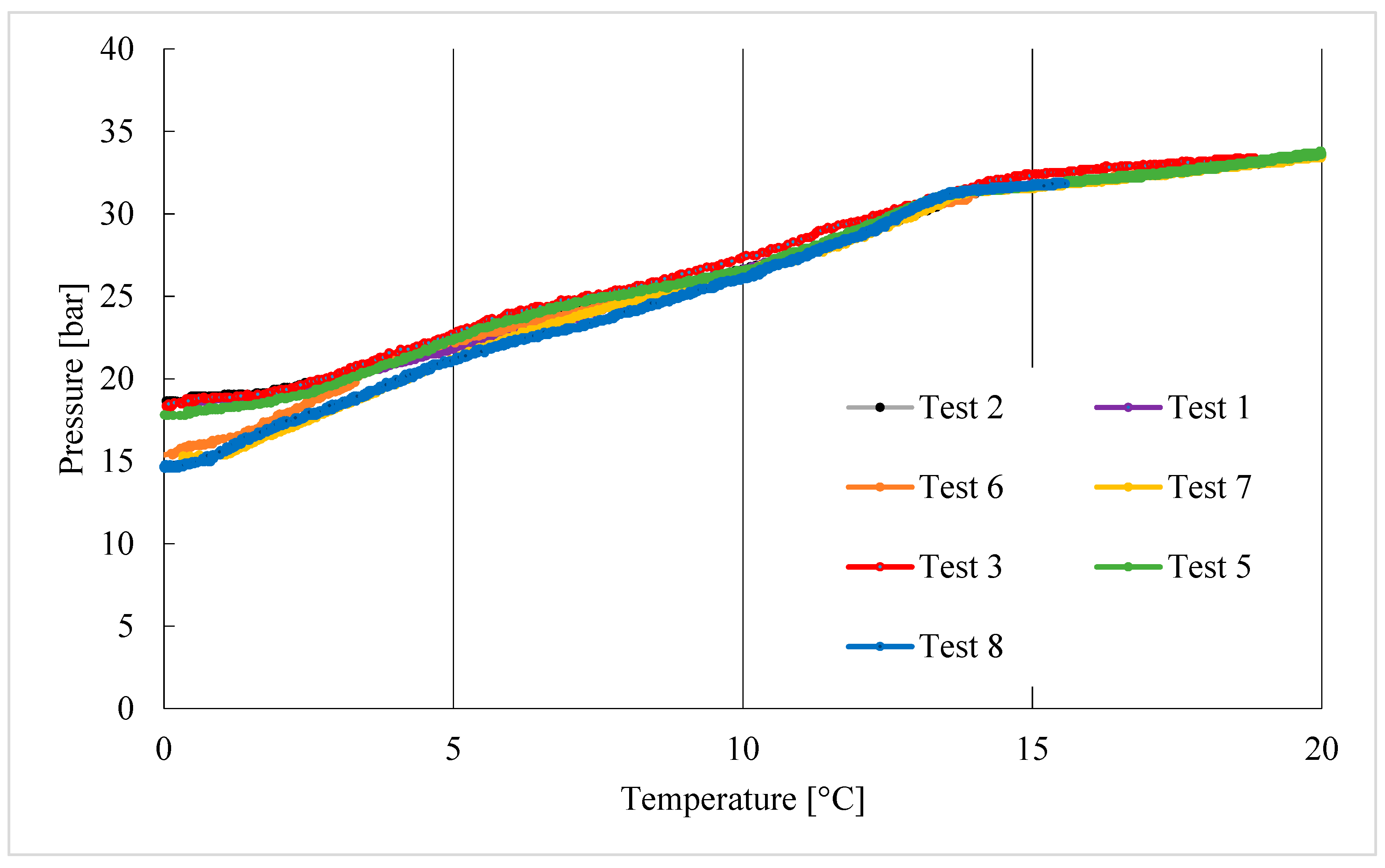
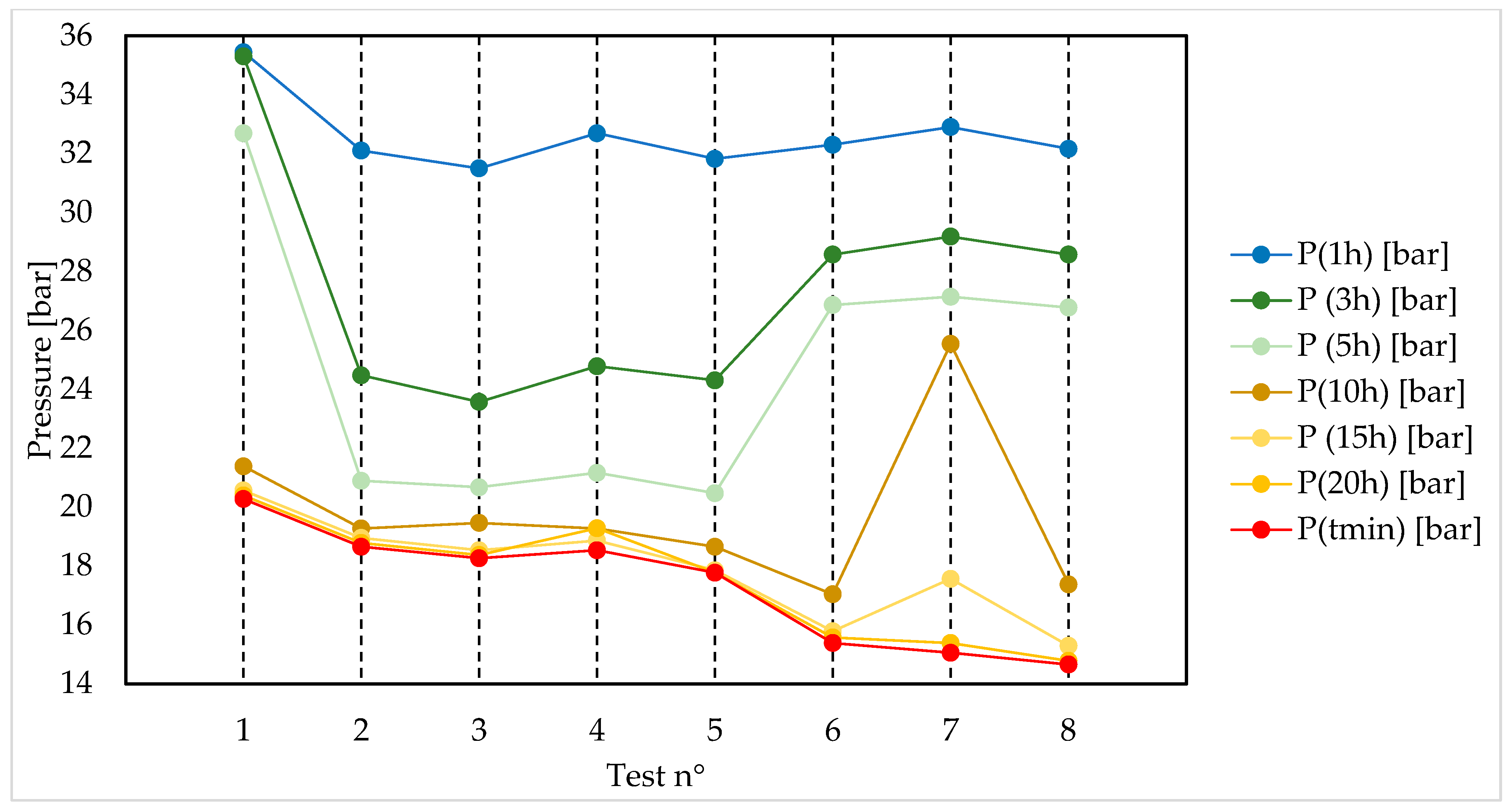
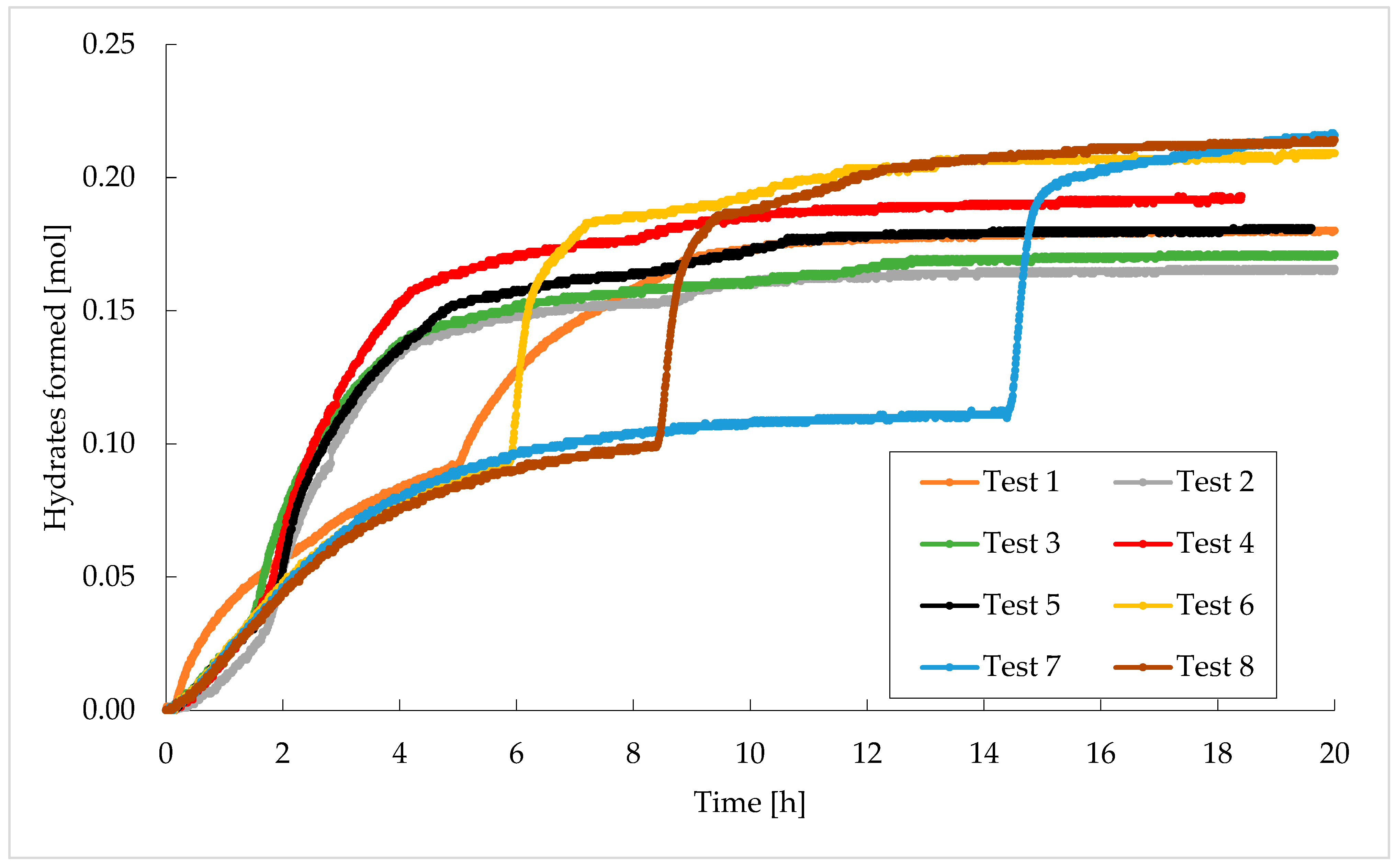
3. Results
- (1)
- Both the gaseous species can form hydrates;
- (2)
- Only carbon dioxide can form hydrates;
- (3)
- Only propane can form hydrates;
- (4)
- The conditions are not suitable for the production of hydrates.
Trend of Hydrates Formation over Time
4. Conclusions
- (i)
- As the experiments proceeded, the formation of hydrates began at gradually higher temperatures, proving that hydrates had the capability to occur at more severe thermodynamic conditions.
- (ii)
- The advantageous contribution of the memory effect is clearly visible: gradually lower minimum pressures were reached, denoting a higher production of hydrates. This can be attributed to the (partial) permanence of nucleation sites that occurred during the previous formations.
- (iii)
- Regarding the time required to reach completion of the process, the sole relevant difference was observed between Test 1 and the other experiments.
- (iv)
- Finally, it was observed that the longer the time required to detect a significant production of hydrates, the more abundant the production of hydrates.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oyama, A.; Masutani, S.M. A review of methane hydrate program in Japan. Energies 2017, 10, 1447. [Google Scholar] [CrossRef]
- Shaibu, R.; Sambo, C.; Guo, B.; Dudun, A. An assessment of methane gas production from natural gas hydrates: Challenges, technology and market outlook. Adv. Geoenergy Res. 2021, 5, 318–332. [Google Scholar] [CrossRef]
- Sloan, E.D. Fundamental principles and applications of natural gas hydrates. Nature 2003, 426, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Kondori, J.; Zendehboudi, S.; Hossain, M.E. A review on simulation of methane production from gas hydrate reservoirs: Molecular dynamics prospective. J. Petrol. Sci. Eng. 2017, 159, 754–772. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, G.; Liu, L.; Liu, C.; Wan, Y.; Bu, Q.; Ji, Y.; Zhang, Z.; Kong, L. Mechanical properties of gas hydrate-bearing sediments: Research progress, challenges and perspectives. Earth Sci. Rev. 2025, 262, 105058. [Google Scholar] [CrossRef]
- Wei, N.; Pei, J.; Li, H.; Zhou, S.; Zhao, J.; Kvamme, B.; Coffin, R.B.; Zhang, L.; Zhang, Y.; Xue, J. Classification of natural gas hydrate resources: Review, application and prospect. Gas Sci. Eng. 2024, 124, 205269. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Yin, S.; He, G.; Li, J.; Wu, Q. Research progress of the kinetics on natural gas hydrate replacement by CO2-containing mixed gas: A review. J. Nat. Gas Sci. Eng. 2022, 108, 104837. [Google Scholar] [CrossRef]
- Collett, T.S. Energy resource potential of natural gas hydrates. AAPG Bull. 2002, 86, 1971–1992. [Google Scholar]
- Makogon, Y.F. Hydrates of Natural Gas; PennWell: Tulsa, OK, USA, 1981. [Google Scholar]
- Li, X.S.; Xu, C.G.; Zhang, Y.; Ruan, X.K.; Li, G.; Wang, Y. Investigation into gas production from natural gas hydrate: A review. Appl. Energy 2016, 172, 286–322. [Google Scholar] [CrossRef]
- Deng, S.; Wang, H.; Wang, C.; Ma, M.; Wei, Y.; Liu, Y. Research on dynamic movement of temperature and pressure during hydrate depressurization mining. J. Energy Resour. Technol. 2022, 144, 103001. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F. Optimization of the pressure drop produced during CO2 replacement in hydrate reservoirs: Balance between gas removal and preservation of structures. J. Petrol. Sci. Eng. 2022, 217, 110936. [Google Scholar] [CrossRef]
- Wan, Q.C.; Si, H.; Li, B.; Yin, Z.H.; Gao, Q.; Liu, S.; Han, X.; Chen, L.L. Energy recovery enhancement from gas hydrate based on the optimization of thermal stimulation modes and depressurization. Appl. Energy 2020, 278, 115612. [Google Scholar] [CrossRef]
- Pandey, J.S.; Karantonidis, C.; Karcz, A.P.; von Solms, N. Enhanced CH4-CO2 hydrate swapping in the presence of low dosage methanol. Energies 2020, 13, 5238. [Google Scholar] [CrossRef]
- Feng, J.C.; Li, G.; Li, X.S.; Li, B.; Chen, Z.Y. Evolution of hydrate dissociation by warm brine stimulation combined with depressurization in the South China Sea. Energies 2013, 6, 5402–5425. [Google Scholar] [CrossRef]
- Feng, J.C.; Li, X.S.; Li, G.; Li, B.; Chen, Z.Y.; Wang, Y. Numerical investigation of hydrate dissociation performance in the South China Sea with different horizontal well configurations. Energies 2014, 7, 4813–4834. [Google Scholar] [CrossRef]
- Li, B.; Li, G.; Li, X.S.; Chen, Z.Y.; Zhang, Y. The use of heat-assisted antigravity drainage method in the two horizontal wells in gas production from the Qillian Mountain permafrost hydrate deposits. J. Petrol. Sci. Eng. 2014, 120, 141–153. [Google Scholar] [CrossRef]
- Xu, C.G.; Li, X.S. Research progress on methane production from natural gas hydrates. RCS Adv. 2015, 5, 54672–54699. [Google Scholar] [CrossRef]
- Mohammadi, A.H.; Laurens, S.; Richon, D. Experimental study of methane hydrate phase equilibrium in the presence of polyethylene glycol-400 aqueous solution. J. Chem. Eng. Data 2009, 54, 3118–3120. [Google Scholar] [CrossRef]
- Li, Y.; Gambelli, A.M.; Rossi, F. Experimental study on the effect of SDS and micron copper particles mixture on carbon dioxide hydrates formation. Energies 2022, 15, 6540. [Google Scholar] [CrossRef]
- Mohammadi, A.H.; Richon, D. Gas hydrate phase equilibrium in the presence of ethylene glycol or methanol aqueous solution. Ind. Eng. Chem. Res. 2010, 49, 8865–8869. [Google Scholar] [CrossRef]
- Ndlovu, P.; Babaee, S.; Naidoo, P. Review on CH4-CO2 replacement for CO2 sequestration and CH4/CO2 hydrate formation in porous media. Fuel 2022, 320, 123795. [Google Scholar] [CrossRef]
- Ohgaki, K.; Takano, K.; Sangawa, H.; Matsubara, T.; Nakano, S. Methane exploitation by carbon dioxide from gas hydrates–phase equilibrium for CO2–CH4 mixed hydrate systems. J. Chem. Eng. Jpn. 1996, 29, 478–483. [Google Scholar] [CrossRef]
- McConnell, D.R.; Zhang, Z.; Boswell, R. Review progress in evaluating gas hydrate drilling hazards. Mar. Pet. Geol. 2012, 34, 209–223. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, B.; Chen, Y.; Zhang, S.; Guo, W.; Cai, Y.; Tan, B.; Wang, W. Methane storage in a hydrate form as promoted by leucines for possible application to natural gas transportation and storage. Energy Technol. 2015, 3, 815–819. [Google Scholar] [CrossRef]
- Wang, T.; Sun, L.; Fan, Z.; Wei, R.; Li, Q.; Yao, H.; Dong, H.; Zhang, L.; Yang, L.; Zhao, J.; et al. Promoting CH4/CO2 replacement from hydrate with warm brine injection for synergistic energy harvest and carbon sequestration. Chem. Eng. J. 2023, 457, 141129. [Google Scholar] [CrossRef]
- Ndlovu, P.; Babaee, S.; Naidoo, P. Experimental study of CH4-CO2 replacement in gas hydrates in the presence of nitrogen and graphene nenoplatelets. J. Mol. Liq. 2023, 371, 121109. [Google Scholar] [CrossRef]
- Lee, J.; Mok, J.; Choi, W.; Seo, Y. Influence of structural transformation on guest exchange behavior in the sII hydrate-(CO2+N2) replacement for energy recovery and CO2 sequestration. Chem. Eng. J. 2023, 472, 144680. [Google Scholar] [CrossRef]
- Rossi, F.; Li, Y.; Gambelli, A.M. Thermodynamic and kinetic description of the main effects related to the memory effect during carbon dioxide hydrates formation in a confined environment. Sustainability 2021, 13, 13797. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, L.; Sun, L.; Zhou, R.; Dong, B.; Yang, L.; Li, Y.; Zhao, J.; Song, Y. Methane recovery and carbon dioxide storage from gas hydrate in fine marine sediments by using CH4/CO2 replacement. Chem. Eng. J. 2021, 425, 131562. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Presciutti, A.; Rossi, F. Review on the characteristics and advantages related to the use of flue-gas as CO2/N2 mixture for gas hydrate production. Fluid Phase Equilibr. 2021, 541, 113077. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F. Review on the usage of small-chain hydrocarbons (C2-C4) as aid gases for improving the efficiency of hydrate-based technologies. Energies 2023, 16, 3576. [Google Scholar] [CrossRef]
- Belandria, V.; Mohammadi, A.H.; Eslamimanesh, A.; Richon, D.; Sànchez-Mora, M.F.; Galicia-Luna, L.A. Phase equilibrium measurements for semi-clathrate hydrates of the (CO2+N2+tetra-n-butylammonium bromide) aqueous solution systems: Part 2. Fluid Phase Equilibr. 2012, 322–323, 102–112. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, J.D.; Lee, H.J.; Lee, E.K.; Kim, Y. Gas hydrate formation method to capture the carbon dioxide for pre-combustion process in IGCC plant. Int. J. Hydrogen Energy 2011, 36, 1115–1121. [Google Scholar] [CrossRef]
- Sun, S.C.; Liu, C.L.; Meng, Q.G. Hydrate phase equilibrium of binary guest-mixtures containing CO2 and N2 in various systems. J. Chem. Thermodyn. 2015, 84, 1–6. [Google Scholar] [CrossRef]
- Sfaxi, I.B.A.; Durand, I.; Lugo, R.; Mohammadi, A.H.; Richon, D. Hydrate phase equilibria of CO2+N2+aqueous solution of THF, TBAB or TBAF system. Int. J. Greenh. Gas Control 2014, 26, 185–192. [Google Scholar] [CrossRef]
- Sundramoorthy, J.D.; Hammonds, P.; Lal, B.; Philips, G. Gas hydrate equilibrium measurement and observation of gas hydrate dissociation with/without a KHI. Procedia Eng. 2016, 148, 870–877. [Google Scholar] [CrossRef]
- Moojer-van den Heuvel, M.M.; Peters, C.J.; de Swaan Arons, J. Gas hydrate phase equilibria for propane in the presence of additive components. Fluid Phase Equilibr. 2002, 193, 245–249. [Google Scholar] [CrossRef]
- Buleiko, V.M.; Grigoriev, B.A.; Mendoza, J. Calorimetric investigation of hydrates of pure isobutane and iso- and normal- butane mixtures. Fluid Phase Equilibr. 2018, 462, 14–24. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F.; Gigliotti, G. Methane replacement by using CO2/C3H8 mixtures for carbon storage and enhanced methane recovery in gas hydrates. Gas Sci. Eng. 2023, 115, 205028. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F. Re-definition of the region suitable for CO2/CH4 replacement into hydrates as a function of the thermodynamic difference between CO2 hydrate formation and dissociation. Process Saf. Environ. Prot. 2023, 169, 132–141. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F. Experimental characterization of the difference in induction period between CH4 and CO2 hydrates: Motivations and possible consequences on the replacement process. J. Nat. Gas Sci. Eng. 2022, 108, 104848. [Google Scholar] [CrossRef]
- Takeya, S.; Kida, M.; Minami, H.; Sakagami, H.; Hachikubo, A.; Takahashi, N.; Shoji, H.; Soloviev, V.; Wallmann, K.; Biebow, N.; et al. Structure and thermal expansion of natural gas clathrate hydrates. Chem. Eng. Sci. 2006, 61, 2670–2674. [Google Scholar] [CrossRef]
- Aregba, A.G. Gas Hydrate—Properties, Formation and Benefits. Open J. Yangtze Gas Oil 2017, 2, 27–44. [Google Scholar] [CrossRef]
- Yu, S.H.; Zhou, S.D.; Li, X.S.; Wang, S.L. Effect of graphite nanoparticles on CO2 hydrate phase equilibrium. Fluid Phase Equilibr. 2016, 414, 23–28. [Google Scholar] [CrossRef]
- Jarrahian, A.; Nakaee, A. Hydrate-liquid-vapor equilibrium condition for N2+CO2+H2O system: Measurement and modelling. Fuel 2019, 237, 769–774. [Google Scholar] [CrossRef]
- Kyung, D.; Lee, K.; Kim, H.; Lee, W. Effect of marine environmental factors on the phase equilibrium of CO2 hydrate. Int. J. Greenh. Gas Control 2014, 20, 285–292. [Google Scholar] [CrossRef]
- Seo, Y.T.; Lee, H. Multiple-phase hydrate equilibria of the ternary carbon dioxide, methane, and water mixtures. J. Phys. Chem. B 2001, 105, 10084–10090. [Google Scholar] [CrossRef]
- Ripmeester, J.A.; Alavi, S. Some current challenges in clathrate hydrate science: Nucleation, decomposition and the memory effect. Curr. Opin. Solid State Mater. Sci. 2016, 20, 344–351. [Google Scholar] [CrossRef]
- Takeya, S.; Hori, A.; Hondoh, T.; Uchida, T. Freezing-memory effect of water on nucleation of CO2 hydrate crystals. J. Phys. Chem. B 2000, 104, 4164–4168. [Google Scholar] [CrossRef]
- Li, Y.; Wu, N.; Ning, F.; Gao, D.; Hao, X.; Chen, Q.; Liu, C.; Sun, J. Hydrate-induced clogging of sand-control screen and its implication on hydrate production operation. Energy 2020, 206, 118030. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, B. Memory effect on the pressure-temperature condition and induction time of gas hydrate nucleation. J. Nat. Gas Chem. 2010, 19, 446–451. [Google Scholar] [CrossRef]
- Duchateau, C.; Glènant, P.; Pou, T.E.; Hidalgo, M.; Dicharry, C. Hydrate precursor test method for the laboratory evaluation of kinetic hydrate inhibitors. Energy Fuels 2010, 24, 616–623. [Google Scholar] [CrossRef]
- Gambelli, A.M. Variations in terms of CO2 capture and CH4 recovery during replacement process in gas hydrate reservoirs, associated to the “memory effect”. J. Clean. Prod. 2022, 360, 132154. [Google Scholar] [CrossRef]
- Kashchiev, D.; Verdoes, D.; van Rosmalen, G.M. Induction time and metastability limit in new phase formation. J. Cryst. Growth 1991, 110, 373–380. [Google Scholar] [CrossRef]
- Wang, S.R.; Yang, M.J.; Liu, W.G.; Zhao, J.F.; Song, Y.C. Investigation on the induction time of methane hydrate formation in porous media under quiescent conditions. J. Pet. Sci. Eng. 2016, 145, 565–572. [Google Scholar] [CrossRef]




| Temperature [°C] | Pressure [bar] | Tolerance Measured [bar] |
|---|---|---|
| 0.1 | 16.52 | 1.86 |
| 0.4 | 16.72 | 1.94 |
| 0.7 | 16.88 | 1.91 |
| 1.0 | 17.16 | 1.70 |
| 1.3 | 17.50 | 1.45 |
| 1.6 | 17.75 | 1.21 |
| 1.9 | 18.15 | 1.13 |
| 2.2 | 18.41 | 1.04 |
| 2.5 | 18.81 | 0.96 |
| 2.8 | 19.04 | 1.00 |
| 3.1 | 19.46 | 0.93 |
| 3.4 | 19.83 | 0.97 |
| 3.7 | 20.28 | 1.01 |
| 4.0 | 20.67 | 0.90 |
| 4.3 | 20.97 | 0.92 |
| 4.6 | 21.43 | 0.88 |
| 4.9 | 21.72 | 0.75 |
| 5.2 | 22.19 | 0.89 |
| 5.5 | 22.51 | 0.86 |
| 5.8 | 22.84 | 0.80 |
| 6.1 | 23.19 | 0.87 |
| 6.4 | 23.43 | 0.95 |
| 6.7 | 23.63 | 0.82 |
| 7.0 | 23.93 | 0.85 |
| 7.3 | 24.20 | 0.86 |
| 7.6 | 24.35 | 0.78 |
| 7.9 | 24.68 | 0.70 |
| 8.2 | 24.91 | 0.74 |
| 8.5 | 25.17 | 0.70 |
| 8.8 | 25.51 | 0.64 |
| 9.1 | 25.85 | 0.71 |
| 9.4 | 26.12 | 0.65 |
| 9.7 | 26.47 | 0.59 |
| 10.0 | 26.77 | 0.62 |
| P (Time) [bar] | P (1 h) | P (3 h) | P (5 h) | P (10 h) | P (15 h) | P (20 h) | tmin [h] | P (tmin) |
|---|---|---|---|---|---|---|---|---|
| Test 1 | 35.44 | 35.30 | 32.69 | 21.38 | 20.56 | 20.39 | 50.95 | 20.27 |
| Test 2 | 32.10 | 24.47 | 20.89 | 19.27 | 18.95 | 18.78 | 23.54 | 18.65 |
| Test 3 | 31.50 | 23.57 | 20.67 | 19.46 | 18.53 | 18.37 | 23.41 | 18.26 |
| Test 4 | 32.69 | 24.78 | 21.16 | 19.27 | 18.86 | 19.28 | 18.41 | 18.53 |
| Test 5 | 31.82 | 24.30 | 20.47 | 18.65 | 17.85 | 17.77 | 18.27 | 17.77 |
| Test 6 | 32.30 | 28.57 | 26.86 | 17.04 | 15.78 | 15.57 | 24.53 | 15.38 |
| Test 7 | 32.90 | 29.18 | 27.14 | 25.54 | 17.56 | 15.38 | 22.15 | 15.05 |
| Test 8 | 32.16 | 28.57 | 26.77 | 17.37 | 15.29 | 14.78 | 23.45 | 14.65 |
| Moles of Hydrates Formed [mol] | |
|---|---|
| Test 1 | 0.181 |
| Test 2 | 0.171 |
| Test 3 | 0.175 |
| Test 4 | 0.190 |
| Test 5 | 0.182 |
| Test 6 | 0.208 |
| Test 7 | 0.216 |
| Test 8 | 0.215 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambelli, A.M.; Rossi, F.; Gigliotti, G. Usage of a Binary CO2/C3H8 (85/15 vol%) Mixture for Hydrate Production: The Role of the Memory Effect and Phase Boundary Conditions of the System. Energies 2025, 18, 1522. https://doi.org/10.3390/en18061522
Gambelli AM, Rossi F, Gigliotti G. Usage of a Binary CO2/C3H8 (85/15 vol%) Mixture for Hydrate Production: The Role of the Memory Effect and Phase Boundary Conditions of the System. Energies. 2025; 18(6):1522. https://doi.org/10.3390/en18061522
Chicago/Turabian StyleGambelli, Alberto Maria, Federico Rossi, and Giovanni Gigliotti. 2025. "Usage of a Binary CO2/C3H8 (85/15 vol%) Mixture for Hydrate Production: The Role of the Memory Effect and Phase Boundary Conditions of the System" Energies 18, no. 6: 1522. https://doi.org/10.3390/en18061522
APA StyleGambelli, A. M., Rossi, F., & Gigliotti, G. (2025). Usage of a Binary CO2/C3H8 (85/15 vol%) Mixture for Hydrate Production: The Role of the Memory Effect and Phase Boundary Conditions of the System. Energies, 18(6), 1522. https://doi.org/10.3390/en18061522






