Hierarchical Core-Shell Cu@Cu-Ni-Co Alloy Electrocatalyst for Efficient Hydrogen Evolution in Alkaline Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrocatalysts’ Synthesis
2.3. Characterization Techniques
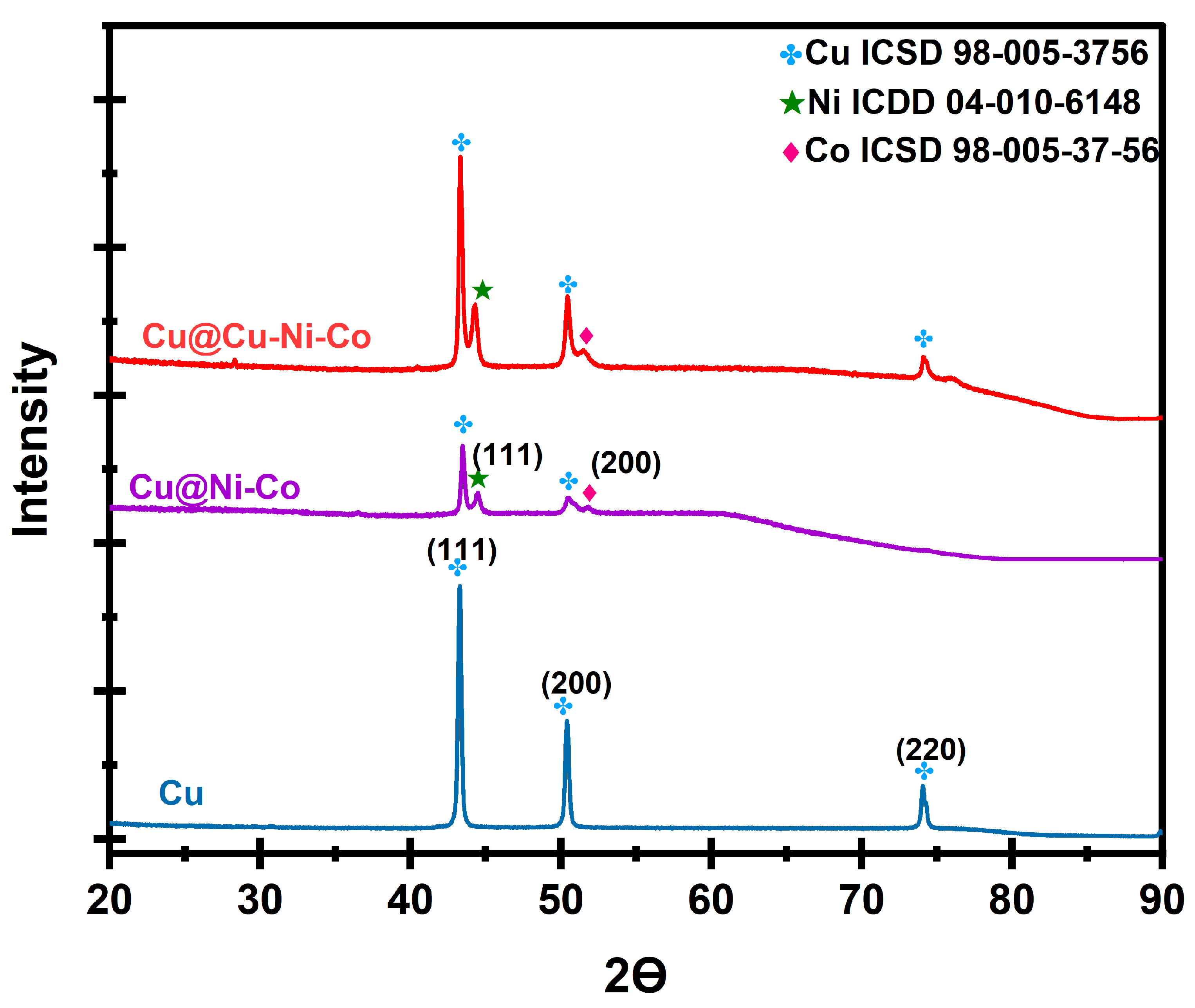
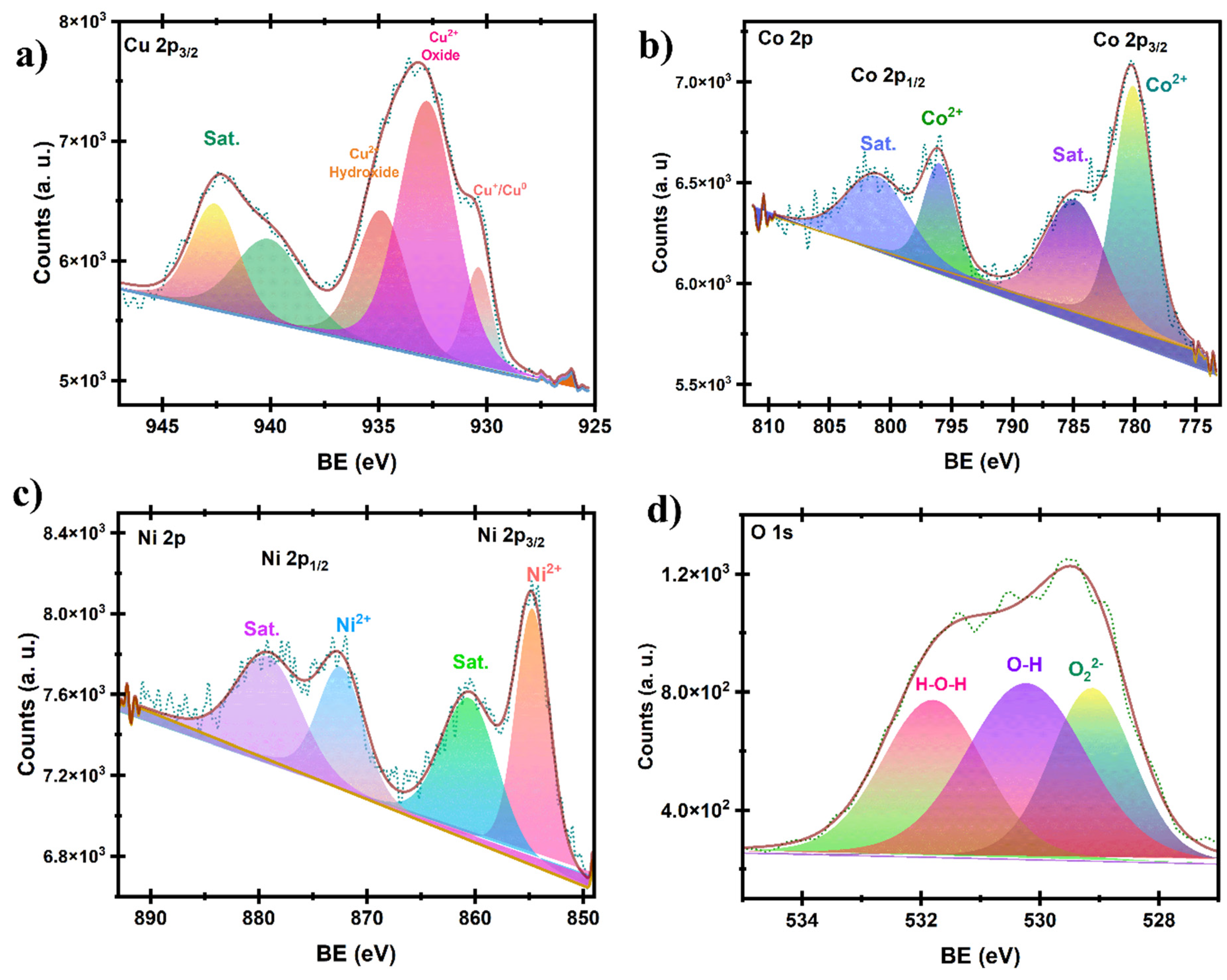
2.3.1. Structural and Chemical Analysis
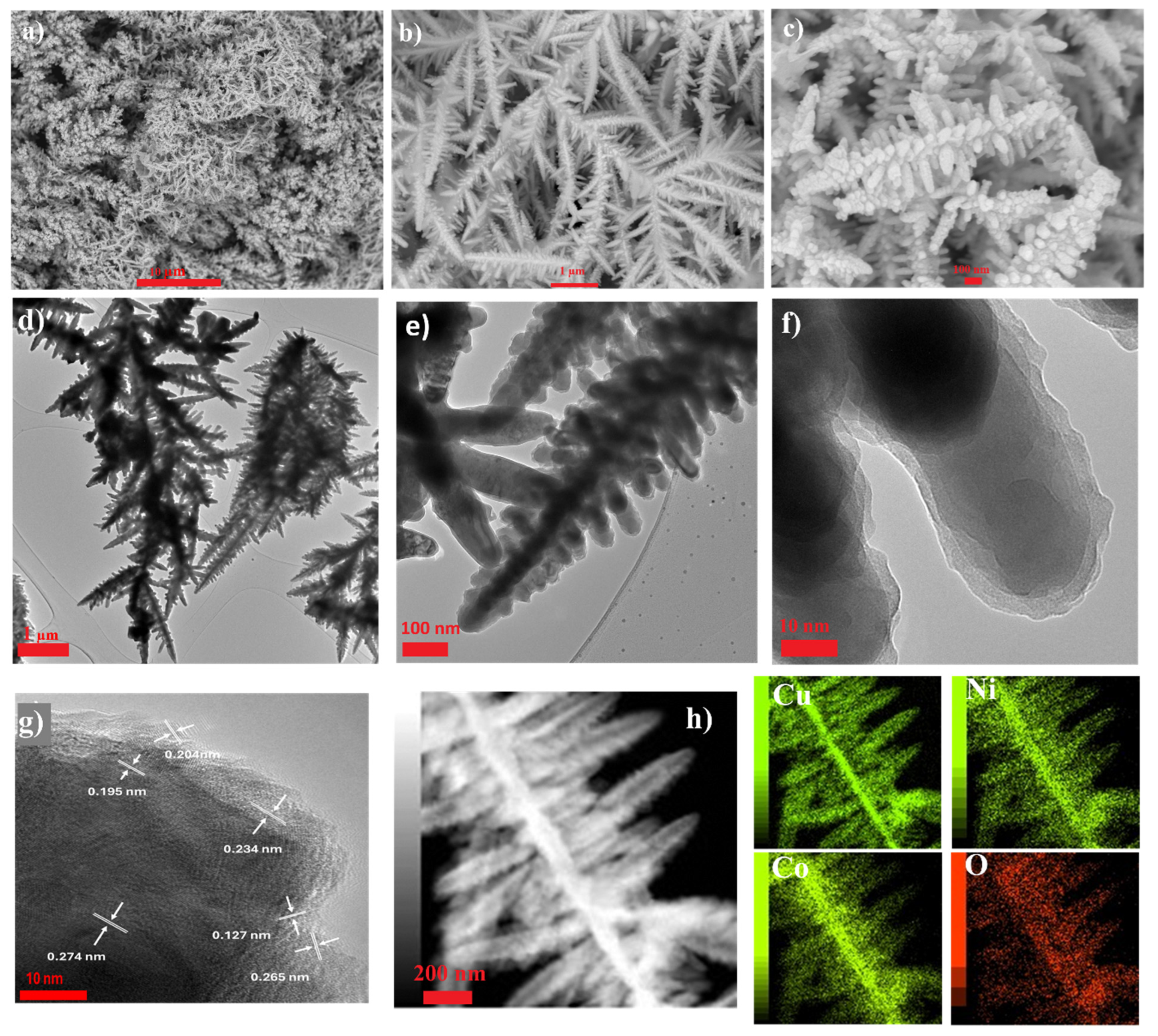
2.3.2. Morphological and Elemental Analysis
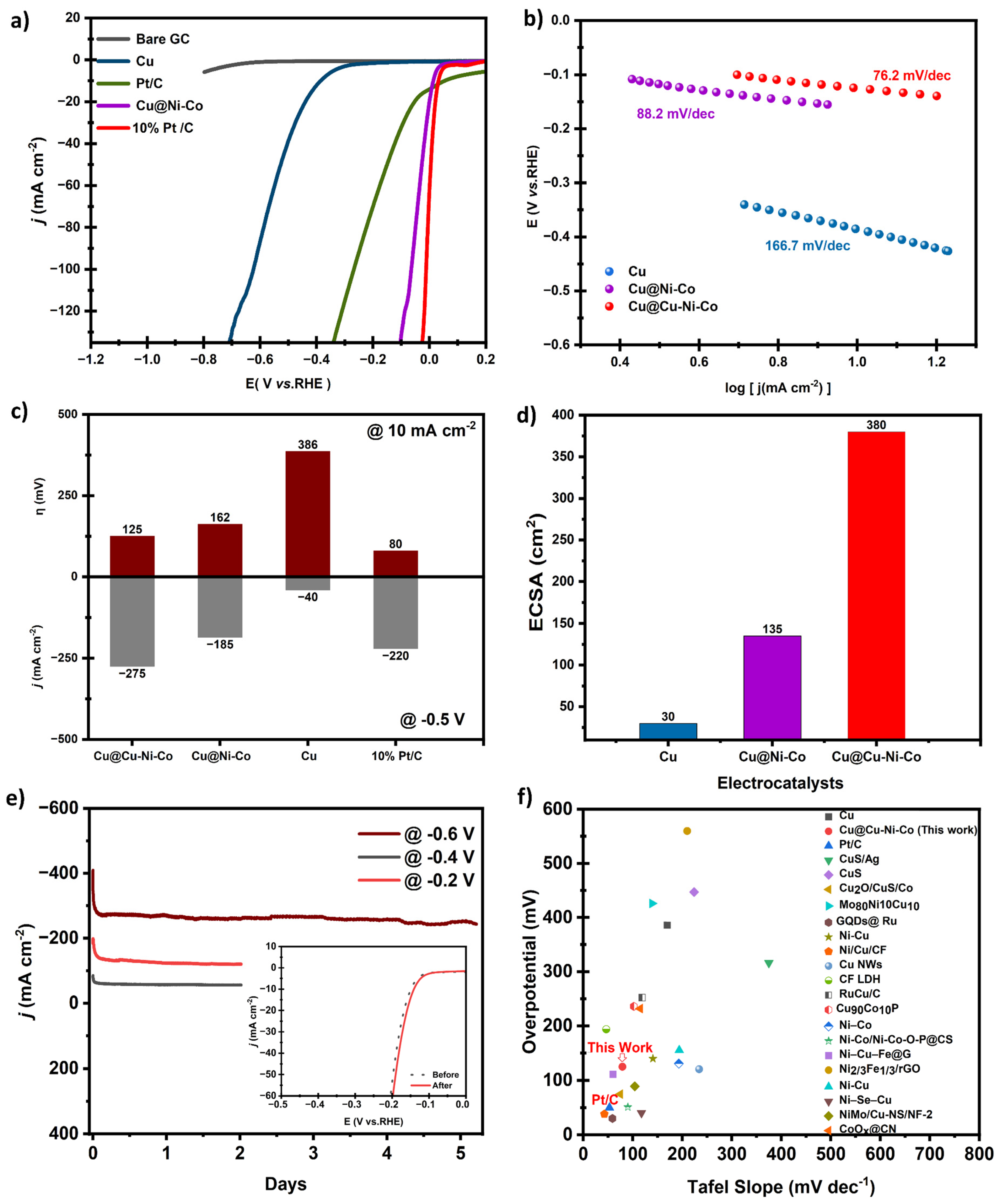
2.3.3. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IRENA. Green Hydrogen Cost Reduction: Scaling Up Electrolysers to Meet the 1.5 °C Climate Goal; IRENA: Abu Dhabi, United Arab Emirates, 2020. [Google Scholar]
- Nnabuife, S.G.; Hamzat, A.K.; Whidborne, J.; Kuang, B.; Jenkins, K.W. Integration of renewable energy sources in tandem with electrolysis: A technology review for green hydrogen production. Int. J. Hydrogen Energy 2024, 107, 218–240. [Google Scholar] [CrossRef]
- Inocêncio, C.V.M.; Holade, Y.; Morais, C.; Kokoh, K.B.; Napporn, T.W. Electrochemical hydrogen generation technology: Challenges in electrodes materials for a sustainable energy. Electrochem. Sci. Adv. 2023, 3, e2100206. [Google Scholar] [CrossRef]
- Araújo, H.F.; Gómez, J.A.; Santos, D.M.F. Proton-Exchange Membrane Electrolysis for Green Hydrogen Production: Fundamentals, Cost Breakdown, and Strategies to Minimize Platinum-Group Metal Content in Hydrogen Evolution Reaction Electrocatalysts. Catalysts 2024, 14, 845. [Google Scholar] [CrossRef]
- Wang, H.; Huo, M.; Liang, Y.; Qin, K.; Li, Q.; Liu, W.; Xing, Z.; Chang, J. Significant Reduction of Anode Reaction Overpotential in Alkaline Water Electrolysis by Ultrathin NiFe-Layered Double Hydroxide in Ethanol-Added Electrolyte. ChemCatChem 2025, 17, e202401950. [Google Scholar] [CrossRef]
- Duan, X.; Xiao, J.; Lin, W.; Wang, S.; Wen, J. Experimental and numerical investigation of the impact of operating conditions on water electrolysis with ultrasonic. Int. J. Hydrogen Energy 2024, 49, 404–416. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Bai, K.; Liu, J.; Xiao, Z.; Fan, S. Energy consumption model of flow-through electrolyzer considering mass transfer overpotential for hydrogen production from alkaline water splitting. J. Power Sources 2024, 621, 235292. [Google Scholar] [CrossRef]
- Verma, J.; Goel, S. Cost-effective electrocatalysts for hydrogen evolution reactions (HER): Challenges and prospects. Int. J. Hydrogen Energy 2022, 47, 38964–38982. [Google Scholar] [CrossRef]
- Shtepliuk, I. 2D noble metals: Growth peculiarities and prospects for hydrogen evolution reaction catalysis. Phys. Chem. Chem. Phys. 2023, 25, 8281–8292. [Google Scholar] [CrossRef]
- Sultana, S.; Hossain, R.; Ahmed, K.; Jiwanti, P.K.; Wardhana, B.Y. Recent progress in development of cost effective and highly efficient pt group metal free ORR and HER electrocatalysts for next generation energy devices. J. Electrochem. Soc. 2023, 170, 100509. [Google Scholar] [CrossRef]
- Anantharaj, S.; Noda, S.; Jothi, V.R.; Yi, S.; Driess, M.; Menezes, P.W. Strategies and Perspectives to Catch the Missing Pieces in Energy-Efficient Hydrogen Evolution Reaction in Alkaline Media. Angew. Chem. Int. Ed. 2021, 60, 18981–19006. [Google Scholar] [CrossRef]
- Mahmood, J.; Li, F.; Jung, S.-M.; Okyay, M.S.; Ahmad, I.; Kim, S.-J.; Park, N.; Jeong, H.Y.; Baek, J.-B. An efficient and pH-universal ruthenium-based catalyst for the hydrogen evolution reaction. Nat. Nanotechnol. 2017, 12, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Myint, M.; Chen, J.G.; Yan, Y. Correlating the hydrogen evolution reaction activity in alkaline electrolytes with the hydrogen binding energy on monometallic surfaces. Energy Environ. Sci. 2013, 6, 1509–1512. [Google Scholar] [CrossRef]
- Conway, B.; Jerkiewicz, G. Relation of energies and coverages of underpotential and overpotential deposited H at Pt and other metals to the ‘volcano curve’ for cathodic H2 evolution kinetics. Electrochim. Acta 2000, 45, 4075–4083. [Google Scholar] [CrossRef]
- Mahmood, N.; Yao, Y.; Zhang, J.-W.; Pan, L.; Zhang, X.; Zou, J.-J. Electrocatalysts for Hydrogen Evolution in Alkaline Electrolytes: Mechanisms, Challenges, and Prospective Solutions. Adv. Sci. 2018, 5, 1700464. [Google Scholar] [CrossRef]
- Zhu, W.; Song, X.; Liao, F.; Huang, H.; Shao, Q.; Feng, K.; Zhou, Y.; Ma, M.; Wu, J.; Yang, H.; et al. Stable and oxidative charged Ru enhance the acidic oxygen evolution reaction activity in two-dimensional ruthenium-iridium oxide. Nat. Commun. 2023, 14, 5365. [Google Scholar] [CrossRef]
- Wohlfahrt-Mehrens, M.; Heitbaum, J. Oxygen evolution on Ru and RuO2 electrodes studied using isotope labelling and on-line mass spectrometry. J. Electroanal. Chem. Interfacial Electrochem. 1987, 237, 251–260. [Google Scholar] [CrossRef]
- Thao, N.T.T.; Jang, J.U.; Nayak, A.K.; Han, H. Current Trends of Iridium-Based Catalysts for Oxygen Evolution Reaction in Acidic Water Electrolysis. Small Sci. 2024, 4, 2300109. [Google Scholar] [CrossRef]
- Reier, T.; Oezaslan, M.; Strasser, P. Electrocatalytic Oxygen Evolution Reaction (OER) on Ru, Ir, and Pt Catalysts: A Comparative Study of Nanoparticles and Bulk Materials. ACS Catal. 2012, 2, 1765–1772. [Google Scholar] [CrossRef]
- Deka, N.; Jones, T.E.; Falling, L.J.; Sandoval-Diaz, L.-E.; Lunkenbein, T.; Velasco-Velez, J.-J.; Chan, T.-S.; Chuang, C.-H.; Knop-Gericke, A.; Mom, R.V. On the Operando Structure of Ruthenium Oxides during the Oxygen Evolution Reaction in Acidic Media. ACS Catal. 2023, 13, 7488–7498. [Google Scholar] [CrossRef]
- Vijayakumar, P.; Lenus, S.; Pradeeswari, K.; Kumar, M.; Chang, J.-H.; Kandasamy, M.; Krishnamachari, M.; Dai, Z.; Al-Kahtani, A.A.; Sankar Krishnan, P. In Situ Reconstructed Layered Double Hydroxides via MOF Engineering and Ru Doping for Decoupled Acidic Water Oxidation Enhancement. Energy Fuels 2024, 38, 4504–4515. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, Y.; Ma, Z.; Zhang, Y.; Ni, W.; Younus, H.A.; Zhang, C.; Chen, Z.; Zhang, S. Supported ionic liquid phase-boosted highly active and durable electrocatalysts towards hydrogen evolution reaction in acidic electrolyte. J. Energy Chem. 2021, 54, 342–351. [Google Scholar] [CrossRef]
- Seabold, J.A.; Choi, K.-S. Effect of a Cobalt-Based Oxygen Evolution Catalyst on the Stability and the Selectivity of Photo-Oxidation Reactions of a WO3 Photoanode. Chem. Mater. 2011, 23, 1105–1112. [Google Scholar] [CrossRef]
- Mtangi, W.; Tassinari, F.; Vankayala, K.; Vargas Jentzsch, A.; Adelizzi, B.; Palmans, A.R.A.; Fontanesi, C.; Meijer, E.W.; Naaman, R. Control of Electrons’ Spin Eliminates Hydrogen Peroxide Formation During Water Splitting. J. Am. Chem. Soc. 2017, 139, 2794–2798. [Google Scholar] [CrossRef]
- Moschkowitsch, W.; Lori, O.; Elbaz, L. Recent progress and viability of PGM-free catalysts for hydrogen evolution reaction and hydrogen oxidation reaction. ACS Catal. 2022, 12, 1082–1089. [Google Scholar] [CrossRef]
- Lakhan, M.N.; Hanan, A.; Shar, A.H.; Ali, I.; Wang, Y.; Ahmed, M.; Aftab, U.; Sun, H.; Arandiyan, H. Transition Metals-Based Electrocatalysts for Alkaline Overall Water Splitting: Advancements, Challenges, and Perspectives. Chem. Commun. 2024, 60, 5104–5135. [Google Scholar] [CrossRef]
- Wang, J.; Yue, X.; Yang, Y.; Sirisomboonchai, S.; Wang, P.; Ma, X.; Abudula, A.; Guan, G. Earth-abundant transition-metal-based bifunctional catalysts for overall electrochemical water splitting: A review. J. Alloys Compd. 2020, 819, 153346. [Google Scholar] [CrossRef]
- Li, A.; Sun, Y.; Yao, T.; Han, H. Earth-Abundant Transition-Metal-Based Electrocatalysts for Water Electrolysis to Produce Renewable Hydrogen. Chem. Eur. J. 2018, 24, 18334–18355. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.T.; Tran, P.K.; Malhotra, D.; Nguyen, T.H.; Nguyen, T.T.; Duong, N.T.; Kim, N.H.; Lee, J.H. Current status of developed electrocatalysts for water splitting technologies: From experimental to industrial perspective. Nano Converg. 2025, 12, 9. [Google Scholar] [CrossRef]
- Liu, T.; Chen, C.; Pu, Z.; Huang, Q.; Zhang, X.; Al-Enizi, A.M.; Nafady, A.; Huang, S.; Chen, D.; Mu, S. Non-Noble-Metal-Based Electrocatalysts for Acidic Oxygen Evolution Reaction: Recent Progress, Challenges, and Perspectives. Small 2024, 20, 2405399. [Google Scholar] [CrossRef]
- Irshad, H.; Zia, M.; Al-Hajri, R.; Khattak, Z.A.K.; Al-Abri, M.; Ahmad, N.; Younus, H.A. Electrocatalysts for hydrogen and oxygen evolution reactions under neutral/near-neutral conditions: Summary and challenges. Int. J. Hydrogen Energy 2025, in press. [CrossRef]
- Younus, H.A.; Ahmad, N.; Al-Abri, M.; Al-Hajri, R.; Zhang, S. Molecular Nature of Electrodeposits in Electrochemical Water Oxidation. Adv. Energy Mater. 2023, 13, 2300896. [Google Scholar] [CrossRef]
- Younus, H.A.; Vandichel, M.; Ahmad, N.; Ahlberg, E.; Busch, M.; Verpoort, F. Engineering of a highly stable metal-organic Co-film for efficient electrocatalytic water oxidation in acidic media. Mater. Today Energy 2020, 17, 100437. [Google Scholar] [CrossRef]
- Wu, Q.; Gao, Q.; Wang, X.; Qi, Y.; Shen, L.; Tai, X.; Yang, F.; He, X.; Wang, Y.; Yao, Y. Boosting electrocatalytic performance via electronic structure regulation for acidic oxygen evolution. Iscience 2024, 27, 108738. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Li, B.; Xu, G.; Wang, L. Recent advances in interface engineering strategy for highly-efficient electrocatalytic water splitting. InfoMat 2023, 5, e12377. [Google Scholar] [CrossRef]
- You, B.; Sun, Y. Innovative strategies for electrocatalytic water splitting. Acc. Chem. Res. 2018, 51, 1571–1580. [Google Scholar] [CrossRef]
- Shit, S.; Bolar, S.; Murmu, N.C.; Kuila, T. An account of the strategies to enhance the water splitting efficiency of noble-metal-free electrocatalysts. J. Energy Chem. 2021, 59, 160–190. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Q.; Wu, L.; Cheng, L.; Huang, K.; Chen, J.; Yao, X. Optimal Electrocatalyst Design Strategies for Acidic Oxygen Evolution. Adv. Sci. 2024, 11, 2401975. [Google Scholar] [CrossRef]
- Xu, X.; Shao, Z.; Jiang, S.P. High-Entropy Materials for Water Electrolysis. Energy Technol. 2022, 10, 2200573. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Jiang, S.P.; Shao, Z. Designing single-atom catalysts toward improved alkaline hydrogen evolution reaction. Mater. Rep. Energy 2022, 2, 100144. [Google Scholar] [CrossRef]
- Khattak, Z.A.K.; Younus, H.A.; Ahmad, N.; Alomar, M.; Ullah, H.; Al-Abri, M.; Al-Hajri, R.; Kao, C.-M.; Verpoort, F. Tuning CoPi stability in electrochemical water oxidation via surface modification with an organic ligand shell. Int. J. Hydrogen Energy 2024, 76, 141–151. [Google Scholar] [CrossRef]
- Zhang, P.; Hong, S.; Song, N.; Han, Z.; Ge, F.; Dai, G.; Dong, H.; Li, C. Alloy as advanced catalysts for electrocatalysis: From materials design to applications. Chin. Chem. Lett. 2024, 35, 109073. [Google Scholar] [CrossRef]
- Wang, W.; He, T.; Yang, X.; Liu, Y.; Wang, C.; Li, J.; Xiao, A.; Zhang, K.; Shi, X.; Jin, M. General synthesis of amorphous PdM (M = Cu, Fe, Co, Ni) alloy nanowires for boosting HCOOH dehydrogenation. Nano Lett. 2021, 21, 3458–3464. [Google Scholar] [CrossRef]
- Guo, H.; Fang, Z.; Li, H.; Fernandez, D.; Henkelman, G.; Humphrey, S.M.; Yu, G. Rational design of rhodium–iridium alloy nanoparticles as highly active catalysts for acidic oxygen evolution. ACS Nano 2019, 13, 13225–13234. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, Y.; Furukawa, S. Catalysis of alloys: Classification, principles, and design for a variety of materials and reactions. Chem. Rev. 2022, 123, 5859–5947. [Google Scholar] [CrossRef]
- Du, M.; Li, X.; Pang, H.; Xu, Q. Alloy electrocatalysts. EnergyChem 2023, 5, 100083. [Google Scholar] [CrossRef]
- Niyitanga, T.; Kim, H. Synergistic effect of trimetallic-based CuxMox/Co1−xO nanoparticles/reduced graphene oxide as high efficient electrocatalyst for oxygen evolution reaction. Chem. Eng. J. 2023, 451, 138649. [Google Scholar] [CrossRef]
- Hu, X.; Han, P.; Zhang, W.; Li, T.; Liu, M.; Wu, Y. Catalytic property and synergistic effect of the multi-active sites Pd/PdO/Er2O3/Cu2O/PdCu3 formed in situ on the surface of the tri-organometallic Pd(II)/Er(III)/Cu(II) film. Mol. Catal. 2024, 568, 114486. [Google Scholar] [CrossRef]
- Luo, M.; Guo, S. Strain-controlled electrocatalysis on multimetallic nanomaterials. Nat. Rev. Mater. 2017, 2, 17059. [Google Scholar] [CrossRef]
- Guan, Y.; Dong, L.; Qiu, L.; Yan, P.; Yu, X.Y. 3D Self-Supported Catalyst with Multiple Active Components for Efficient Neutral Hydrogen Evolution Reaction. ChemCatChem 2023, 15, e202300845. [Google Scholar] [CrossRef]
- Hu, Q.; Gao, K.; Wang, X.; Zheng, H.; Cao, J.; Mi, L.; Huo, Q.; Yang, H.; Liu, J.; He, C. Subnanometric Ru clusters with upshifted D band center improve performance for alkaline hydrogen evolution reaction. Nat. Commun. 2022, 13, 3958. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, S.; Wang, J.; Zhong, W. Modulating the D-Band Center of Electrocatalysts for Enhanced Water Splitting. Chem. Eur. J. 2024, 30, e202402725. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.-T.; Xu, Q.; Zhu, Q.-L. Hierarchically Ordered Pore Engineering of Metal–Organic Framework-Based Materials for Electrocatalysis. Adv. Mater. 2024, 36, 2401926. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, J.; Liu, D.; Xu, W.; Wang, Y.; Yao, J.; Tan, H.T.; Dinh, K.N.; Wu, C.; Kuang, M.; et al. Tuning the Electronic Structures of Multimetal Oxide Nanoplates to Realize Favorable Adsorption Energies of Oxygenated Intermediates. ACS Nano 2020, 14, 17640–17651. [Google Scholar] [CrossRef]
- Cao, G.; Yang, S.; Ren, J.-C.; Liu, W. Electronic descriptors for designing high-entropy alloy electrocatalysts by leveraging local chemical environments. Nat. Commun. 2025, 16, 1251. [Google Scholar] [CrossRef]
- Lopes, P.P.; Li, D.; Lv, H.; Wang, C.; Tripkovic, D.; Zhu, Y.; Schimmenti, R.; Daimon, H.; Kang, Y.; Snyder, J.; et al. Eliminating dissolution of platinum-based electrocatalysts at the atomic scale. Nat. Mater. 2020, 19, 1207–1214. [Google Scholar] [CrossRef]
- Patel, P.P.; Hanumantha, P.J.; Datta, M.K.; Velikokhatnyi, O.I.; Hong, D.; Poston, J.A.; Manivannan, A.; Kumta, P.N. Cobalt based nanostructured alloys: Versatile high performance robust hydrogen evolution reaction electro-catalysts for electrolytic and photo-electrochemical water splitting. Int. J. Hydrogen Energy 2017, 42, 17049–17062. [Google Scholar] [CrossRef]
- Zhang, W.; Cui, L.; Liu, J. Recent advances in cobalt-based electrocatalysts for hydrogen and oxygen evolution reactions. J. Alloys Compd. 2020, 821, 153542. [Google Scholar] [CrossRef]
- Turmine, M.; Xie, Y.; Miche, A.; Vivier, V. Electrodeposition of Ni-Co Alloys from Ionic Liquids and Their Electrocatalytic Properties. In Electrochemical Society Meeting Abstracts 243; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2023; p. 2373. [Google Scholar]
- Karimzadeh, A.; Aliofkhazraei, M.; Walsh, F.C. A review of electrodeposited Ni-Co alloy and composite coatings: Microstructure, properties and applications. Surf. Coat. Technol. 2019, 372, 463–498. [Google Scholar] [CrossRef]
- Gao, M.; Yang, C.; Zhang, Q.; Yu, Y.; Hua, Y.; Li, Y.; Dong, P. Electrochemical fabrication of porous Ni-Cu alloy nanosheets with high catalytic activity for hydrogen evolution. Electrochim. Acta 2016, 215, 609–616. [Google Scholar] [CrossRef]
- Ngamlerdpokin, K.; Tantavichet, N. Electrodeposition of nickel–copper alloys to use as a cathode for hydrogen evolution in an alkaline media. Int. J. Hydrogen Energy 2014, 39, 2505–2515. [Google Scholar] [CrossRef]
- Maurya, A.; Suman, S.; Bhardwaj, A.; Mohapatra, L.; Kushwaha, A.K. Substrate dependent electrodeposition of Ni–Co alloy for efficient hydrogen evolution reaction. Electrocatalysis 2023, 14, 68–77. [Google Scholar] [CrossRef]
- Gomez, M.J.; Franceschini, E.A.; Lacconi, G.I. Ni and Ni x Co y Alloys Electrodeposited on Stainless Steel AISI 316L for Hydrogen Evolution Reaction. Electrocatalysis 2018, 9, 459–470. [Google Scholar] [CrossRef]
- Goranova, D.; Lefterova, E.; Rashkov, R. Electrocatalytic activity of Ni-Mo-Cu and Ni-Co-Cu alloys for hydrogen evolution reaction in alkaline medium. Int. J. Hydrogen Energy 2017, 42, 28777–28785. [Google Scholar] [CrossRef]
- Saha, S.; Ramanujachary, K.V.; Lofland, S.E.; Ganguli, A.K. Cu-Co-Ni alloys: An efficient and durable electrocatalyst in acidic media. Mater. Res. Express 2016, 3, 016501. [Google Scholar] [CrossRef]
- Younus, H.A.; Al Hinai, M.; Al Abri, M.; Al Hajri, R. Copper-promoted growth of hierarchical Cu–Co–Ni alloys on copper nanodendrites for enhanced hydrogen evolution in a wide pH range. Int. J. Hydrogen Energy 2024, 92, 247–259. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, Y.; He, H.; Tan, W. Potentiostatic electrodeposition of Ni–Se–Cu on nickel foam as an electrocatalyst for hydrogen evolution reaction. J. Colloid Interface Sci. 2020, 578, 555–564. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, C.-Y.; Chang, T.-F.M.; Luo, X.; Yamane, D.; Sone, M. Electrodeposition of Ni-Co Alloys and Their Mechanical Properties by Micro-Vickers Hardness Test. Electrochem 2021, 2, 1–9. [Google Scholar] [CrossRef]
- Schweckandt, D.S.; del Carmen Aguirre, M. Electrodeposition of Ni-Co alloys. Determination of properties to be used as coins. Procedia Mater. Sci. 2015, 8, 91–100. [Google Scholar] [CrossRef]
- Zhao, H.; Du, Z.; Liu, D.; Lai, Y.; Yang, T.; Wang, M.; Ning, Y.; Yin, F.; Zhao, B. Preparation of self-supported Ni-based ternary alloy catalysts for superior electrocatalytic hydrogen evolution. Mol. Catal. 2022, 520, 112163. [Google Scholar] [CrossRef]
- Zou, P.; Li, J.; Zhang, Y.; Liang, C.; Yang, C.; Fan, H.J. Magnetic-field-induced rapid synthesis of defect-enriched Ni-Co nanowire membrane as highly efficient hydrogen evolution electrocatalyst. Nano Energy 2018, 51, 349–357. [Google Scholar] [CrossRef]
- Qi, Y.; Qi, H.; Li, J.; Lu, C. Synthesis, microstructures and UV–vis absorption properties of β-Ni(OH)2 nanoplates and NiO nanostructures. J. Cryst. Growth 2008, 310, 4221–4225. [Google Scholar] [CrossRef]
- Yuan, H.; Kong, B.; Liu, Z.; Cui, L.; Wang, X. Dealloying-derived nanoporous Sn-doped copper with prior selectivity toward formate for CO2 electrochemical reduction. Chem. Commun. 2024, 60, 184–187. [Google Scholar] [CrossRef]
- Shen, Y.; Guo, S.-G.; Du, F.; Yuan, X.-B.; Zhang, Y.; Hu, J.; Shen, Q.; Luo, W.; Alsaedi, A.; Hayat, T. Prussian blue analogue-derived Ni and Co bimetallic oxide nanoplate arrays block-built from porous and hollow nanocubes for the efficient oxygen evolution reaction. Nanoscale 2019, 11, 11765–11773. [Google Scholar] [CrossRef]
- Wan, X.; Wu, R.; Deng, J.; Nie, Y.; Chen, S.; Ding, W.; Huang, X.; Wei, Z. A metal–organic framework derived 3D hierarchical Co/N-doped carbon nanotube/nanoparticle composite as an active electrocatalyst for oxygen reduction in alkaline electrolyte. J. Mater. Chem. A 2018, 6, 3386–3390. [Google Scholar] [CrossRef]
- Xu, C.; Li, Y.; Adams, R.A.; Pol, V.G.; Xiao, Y.; Varma, A.; Chen, P. One-step combustion synthesis of carbon-coated NiO/Ni composites for lithium and sodium storage. J. Alloys Compd. 2021, 884, 160927. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhong, X.; Jia, X.; Yao, J. Bimetallic Ni–Co nanoparticles confined within nitrogen defective carbon nitride nanotubes for enhanced photocatalytic hydrogen production. Environ. Res. 2022, 203, 111844. [Google Scholar] [CrossRef]
- Li, Z.; Ning, S.; Xu, J.; Zhu, J.; Yuan, Z.; Wu, Y.; Chen, J.; Xie, F.; Jin, Y.; Wang, N.; et al. In situ electrochemical activation of Co(OH)2@Ni(OH)2 heterostructures for efficient ethanol electrooxidation reforming and innovative zinc–ethanol–air batteries. Energy Environ. Sci. 2022, 15, 5300–5312. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Jiang, D.; Chu, Z.; Peng, J.; Luo, J.; Mao, Y.; Yang, P.; Jin, W. One-step synthesis of three-dimensional Co(OH)2/rGO nano-flowers as enzyme-mimic sensors for glucose detection. Electrochim. Acta 2018, 270, 147–155. [Google Scholar] [CrossRef]
- Casella, I.G.; Guascito, M.R.; Sannazzaro, M.G. Voltammetric and XPS investigations of nickel hydroxide electrochemically dispersed on gold surface electrodes. J. Electroanal. Chem. 1999, 462, 202–210. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Cano, E.; López, M.F.; Simancas, J.; Bastidas, J. X-ray photoelectron spectroscopy study on the chemical composition of copper tarnish products formed at low humidities. J. Electrochem. Soc. 2001, 148, E26. [Google Scholar] [CrossRef]
- McCrum, I.T.; Janik, M.J. pH and Alkali Cation Effects on the Pt Cyclic Voltammogram Explained Using Density Functional Theory. J. Phys. Chem. C 2016, 120, 457–471. [Google Scholar] [CrossRef]
- Xue, S.; Garlyyev, B.; Watzele, S.; Liang, Y.; Fichtner, J.; Pohl, M.D.; Bandarenka, A.S. Influence of alkali metal cations on the hydrogen evolution reaction activity of Pt, Ir, Au, and Ag electrodes in alkaline electrolytes. ChemElectroChem 2018, 5, 2326–2329. [Google Scholar] [CrossRef]
- Arumugam, S.; Toku, Y.; Ju, Y. Fabrication of γ-Fe2O3 nanowires from abundant and low-cost Fe plate for highly effective electrocatalytic water splitting. Sci. Rep. 2020, 10, 5407. [Google Scholar] [CrossRef]
- Patel, M.; Joshi, K.K.; Modi, K.H.; Pataniya, P.M.; Siraj, S.; Sahatiya, P.; Sumesh, C. CuS nanoparticles: An efficient electrocatalyst for hydrogen evolution reaction in a wide pH range. Electrochim. Acta 2023, 441, 141740. [Google Scholar] [CrossRef]
- Liu, S.-Q.; Wen, H.-R.; Zhu, Y.-W.; Fu, X.-Z.; Sun, R.; Wong, C.-P. Amorphous Ni(OH)2 encounter with crystalline CuS in hollow spheres: A mesoporous nano-shelled heterostructure for hydrogen evolution electrocatalysis. Nano Energy 2018, 44, 7–14. [Google Scholar] [CrossRef]
- Burungale, V.V.; Bae, H.; Cha, A.-N.; Heo, J.; Ryu, S.-W.; Kang, S.-H.; Ha, J.-S. Self-standing Co decorated Cu2O/CuS-based porous electrocatalyst for effective hydrogen evolution reaction in basic media. Int. J. Hydrogen Energy 2023, 48, 4193–4206. [Google Scholar] [CrossRef]
- Bodnarova, R.; Latyshev, V.; Vorobiov, S.; Lisnichuk, M.; You, H.; Komanicky, V. Improved catalytic activity of MoxNiyAlz thin films as electrocatalyst for hydrogen evolution reaction in alkaline media. Int. J. Hydrogen Energy 2024, 49, 506–517. [Google Scholar] [CrossRef]
- Du, Y.; Li, Q.; Han, L.; Yang, P.; Xin, L.; Jin, W.; Xiao, W.; Li, Z.; Wang, J.; Wu, Z.; et al. Metallic Mo, Ru coupled with graphitic carbon dots to activate inertness 1D copper-based nanowires as efficient electrocatalyst for pH-universal hydrogen evolution reaction. Appl. Catal. B Environ. 2024, 344, 123617. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Q.; Cheng, N.; Asiri, A.M.; Sun, X. Self-supported Cu3P nanowire arrays as an integrated high-performance three-dimensional cathode for generating hydrogen from water. Angew. Chem. 2014, 126, 9731–9735. [Google Scholar] [CrossRef]
- Wang, Z.; Ji, S.; Liu, F.; Wang, H.; Wang, X.; Wang, Q.; Pollet, B.G.; Wang, R. Highly efficient and stable catalyst based on Co (OH) 2@ Ni electroplated on Cu-metallized cotton textile for water splitting. ACS Appl. Mater. Interfaces 2019, 11, 29791–29798. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, M.; Ni, Y. Grass-like Ni/Cu nanosheet arrays grown on copper foam as efficient and non-precious catalyst for hydrogen evolution reaction. Appl. Catal. B Environ. 2020, 268, 118392. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, H.; Sun, J.; Qin, F.; Luo, D.; Xie, L.; Yu, F.; Bao, J.; Li, Y.; Yu, Y. Hierarchical Cu@ CoFe layered double hydroxide core-shell nanoarchitectures as bifunctional electrocatalysts for efficient overall water splitting. Nano Energy 2017, 41, 327–336. [Google Scholar] [CrossRef]
- Hong, C.-B.; Li, X.; Wei, W.-B.; Wu, X.-T.; Zhu, Q.-L. Nano-engineering of Ru-based hierarchical porous nanoreactors for highly efficient pH-universal overall water splitting. Appl. Catal. B Environ. 2021, 294, 120230. [Google Scholar] [CrossRef]
- Bandyopadhyay, D.; Ghosh, S.; Houben, L.; Bar-Ziv, R.; Bar-Sadan, M. Full Water Splitting Electrolyzed by Cu–Co Bimetallic Phosphides. ACS Appl. Energy Mater. 2023, 6, 10987–10995. [Google Scholar] [CrossRef]
- Asen, P.; Esfandiar, A. Facile synthesis of cotton flower like Ni–Co/Ni–Co–O–P as bifunctional active material for alkaline overall water splitting and acetaminophen sensing. Int. J. Hydrogen Energy 2022, 47, 32516–32530. [Google Scholar] [CrossRef]
- Tavallaie, M.M.; Alizadeh, M.; Pashangeh, S. Effects of substrate materials and electrodeposition parameters on hydrogen evolution reaction of Ni–Cu–Fe coatings. Int. J. Hydrogen Energy 2024, 77, 1117–1132. [Google Scholar] [CrossRef]
- Ma, W.; Ma, R.; Wang, C.; Liang, J.; Liu, X.; Zhou, K.; Sasaki, T. A superlattice of alternately stacked Ni–Fe hydroxide nanosheets and graphene for efficient splitting of water. ACS Nano 2015, 9, 1977–1984. [Google Scholar] [CrossRef]
- Chang, Z.; Zhu, L.; Zhao, J.; Chen, P.; Chen, D.; Gao, H. NiMo/Cu-nanosheets/Ni-foam composite as a high performance electrocatalyst for hydrogen evolution over a wide pH range. Int. J. Hydrogen Energy 2021, 46, 3493–3503. [Google Scholar] [CrossRef]
- Jin, H.; Wang, J.; Su, D.; Wei, Z.; Pang, Z.; Wang, Y. In situ cobalt–cobalt oxide/N-doped carbon hybrids as superior bifunctional electrocatalysts for hydrogen and oxygen evolution. J. Am. Chem. Soc. 2015, 137, 2688–2694. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Kim, J.H.; Shin, D.; Bak, G.; Jang, D.; Kim, W.B.; Hwang, Y.J. Synergistic effect of Pt-loaded CoNC electrocatalysts for hydrogen evolution reaction in alkaline conditions. Appl. Surf. Sci. 2023, 610, 155523. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Younus, H.A.; Al Hinai, M.; Al Abri, M.; Al-Hajri, R. Hierarchical Core-Shell Cu@Cu-Ni-Co Alloy Electrocatalyst for Efficient Hydrogen Evolution in Alkaline Media. Energies 2025, 18, 1515. https://doi.org/10.3390/en18061515
Younus HA, Al Hinai M, Al Abri M, Al-Hajri R. Hierarchical Core-Shell Cu@Cu-Ni-Co Alloy Electrocatalyst for Efficient Hydrogen Evolution in Alkaline Media. Energies. 2025; 18(6):1515. https://doi.org/10.3390/en18061515
Chicago/Turabian StyleYounus, Hussein A., Maimouna Al Hinai, Mohammed Al Abri, and Rashid Al-Hajri. 2025. "Hierarchical Core-Shell Cu@Cu-Ni-Co Alloy Electrocatalyst for Efficient Hydrogen Evolution in Alkaline Media" Energies 18, no. 6: 1515. https://doi.org/10.3390/en18061515
APA StyleYounus, H. A., Al Hinai, M., Al Abri, M., & Al-Hajri, R. (2025). Hierarchical Core-Shell Cu@Cu-Ni-Co Alloy Electrocatalyst for Efficient Hydrogen Evolution in Alkaline Media. Energies, 18(6), 1515. https://doi.org/10.3390/en18061515







