Pyrolysis Characteristics of Empty Fruit Bunches at Different Temperatures and Heating Rates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
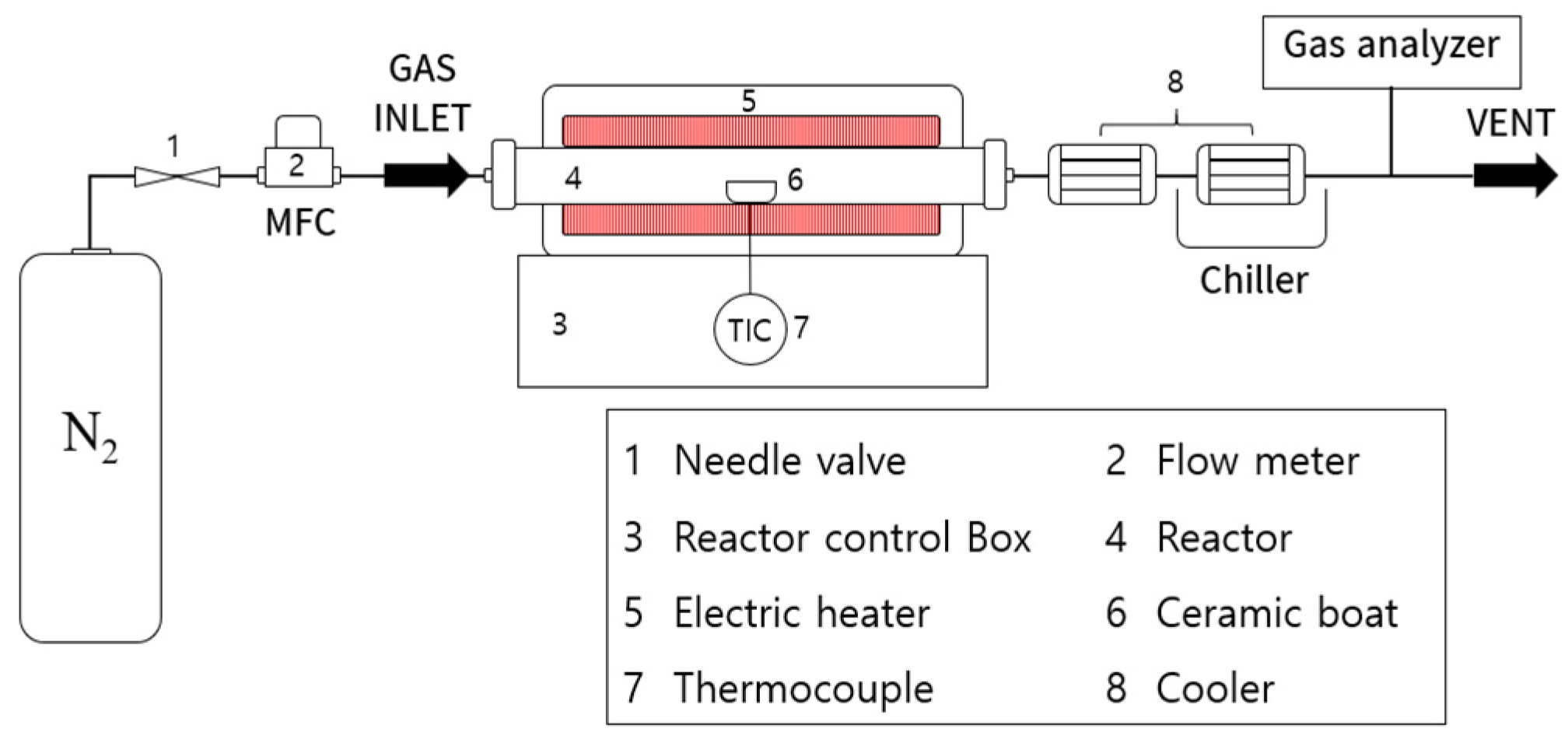
2.2. Experimental Methods
2.2.1. Thermogravimetric Analysis
2.2.2. Gas Product Analysis
2.2.3. Gas Chromatography–Mass Spectrometry Analysis of Liquid Products
2.2.4. Fourier Transform Infrared and X-Ray Diffraction of Biochar
2.3. Kinetic Analysis
- A: pre-exponential factor (min−1)
- E: apparent activation energy (kJ/mol)
- n: apparent order of reactionR: gas constant (8.3136 J/mol·K)
- T: absolute temperature (K)
- t: time (min)
- α: degree of conversion
- mo: original mass of the sample
- mi: instantaneous mass at any time
3. Results and Discussion
3.1. Thermogravimetric Analysis of EFB
3.2. Kinetic Analysis of EFB
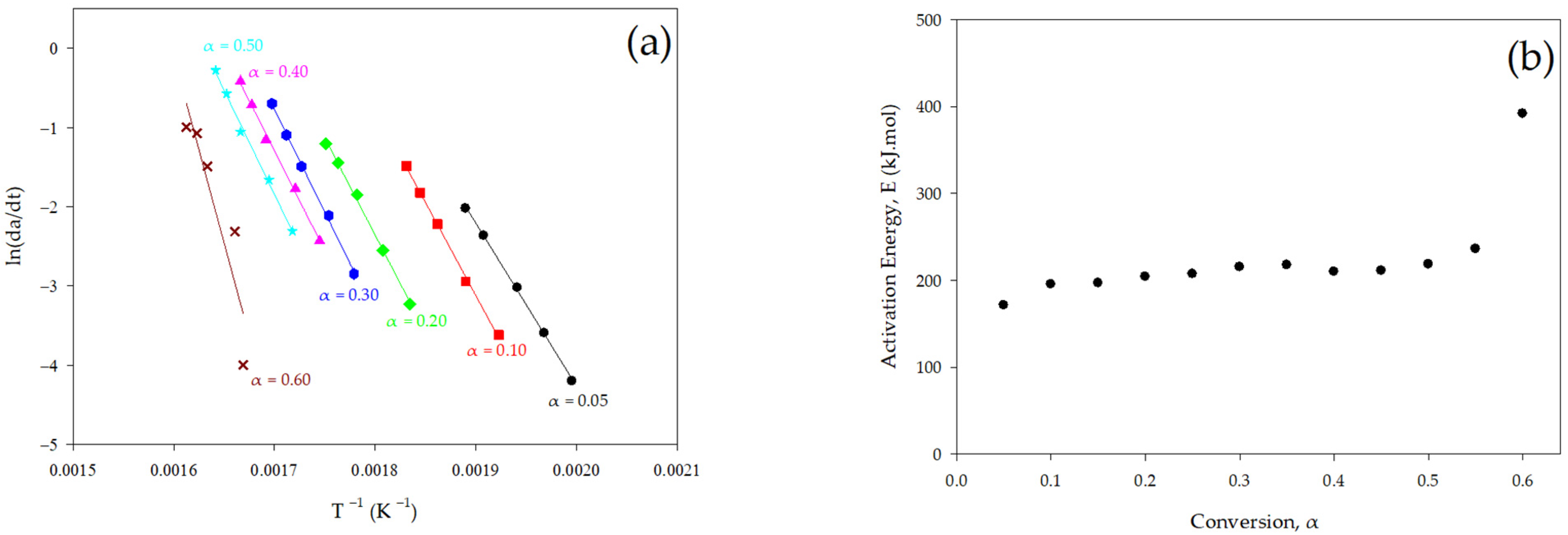
3.2.1. Differential Method
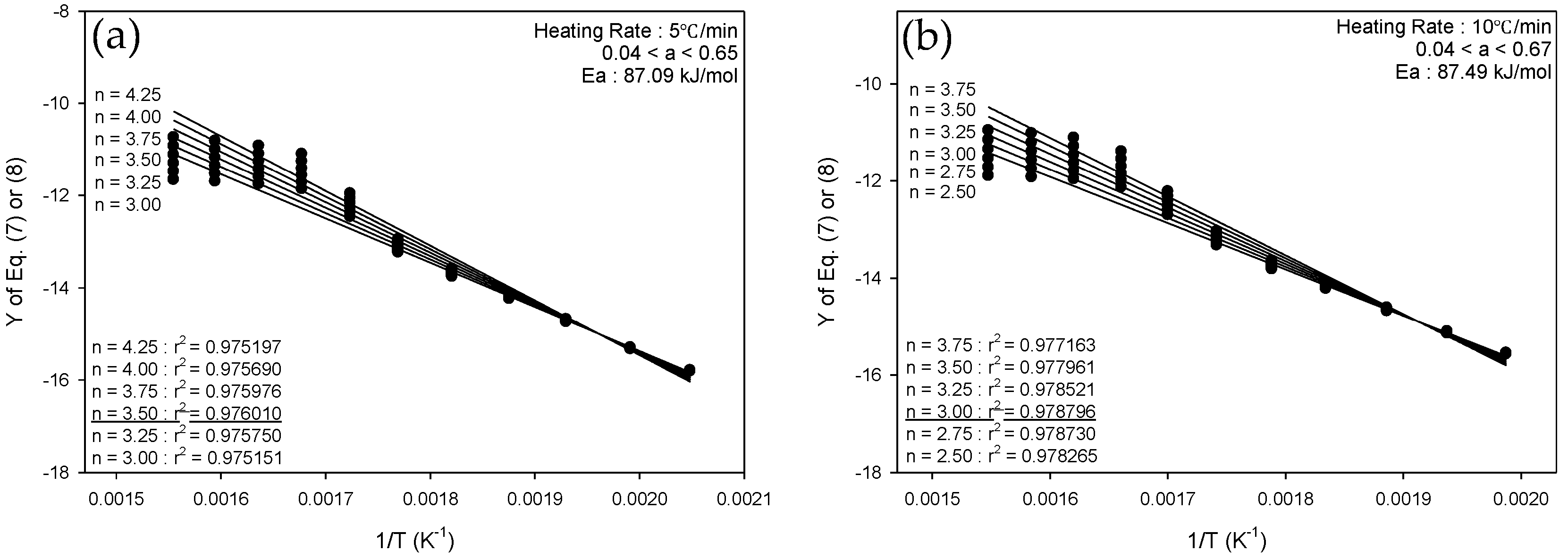
3.2.2. Integral Method
3.3. Product Analysis
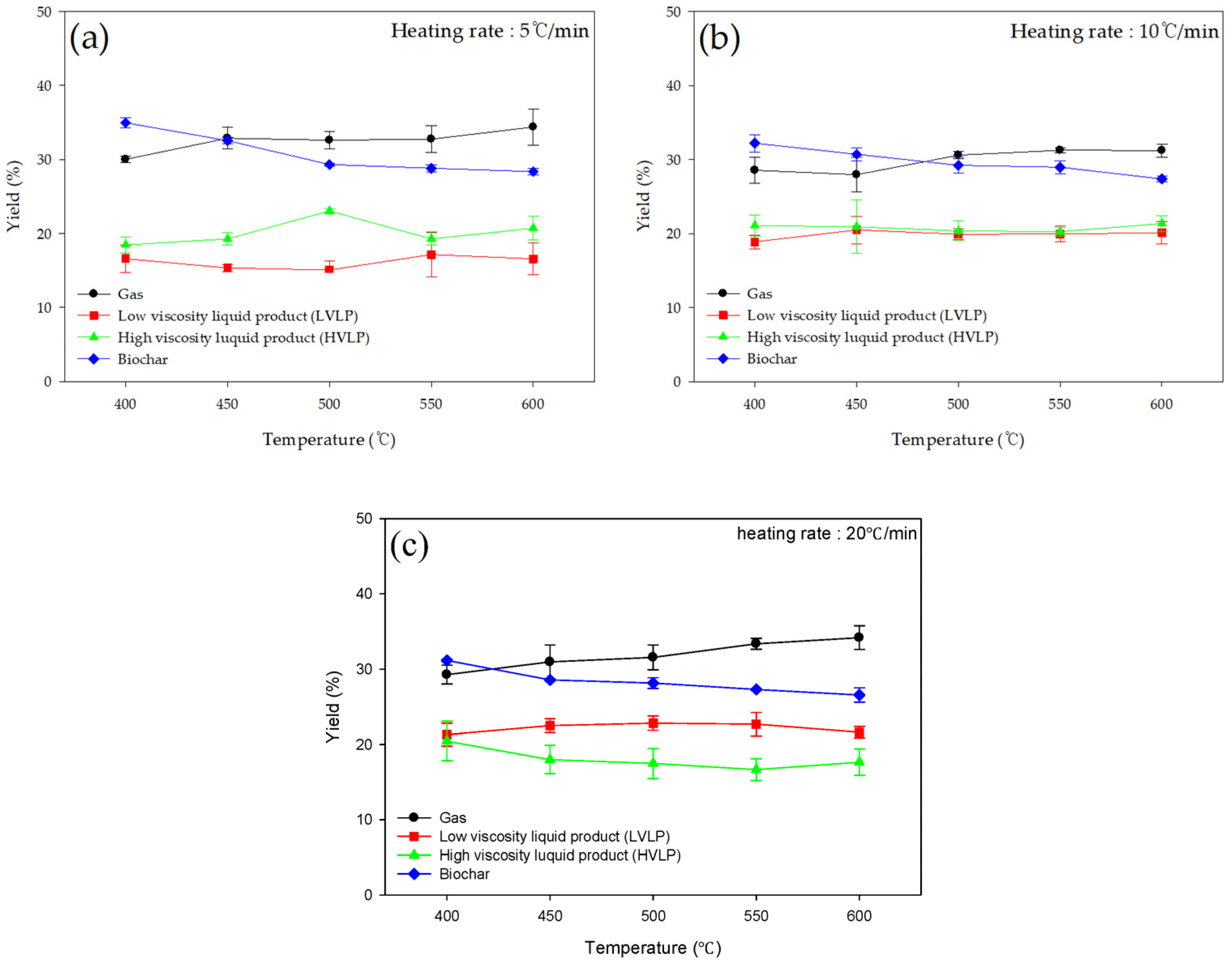
3.3.1. Product Yield
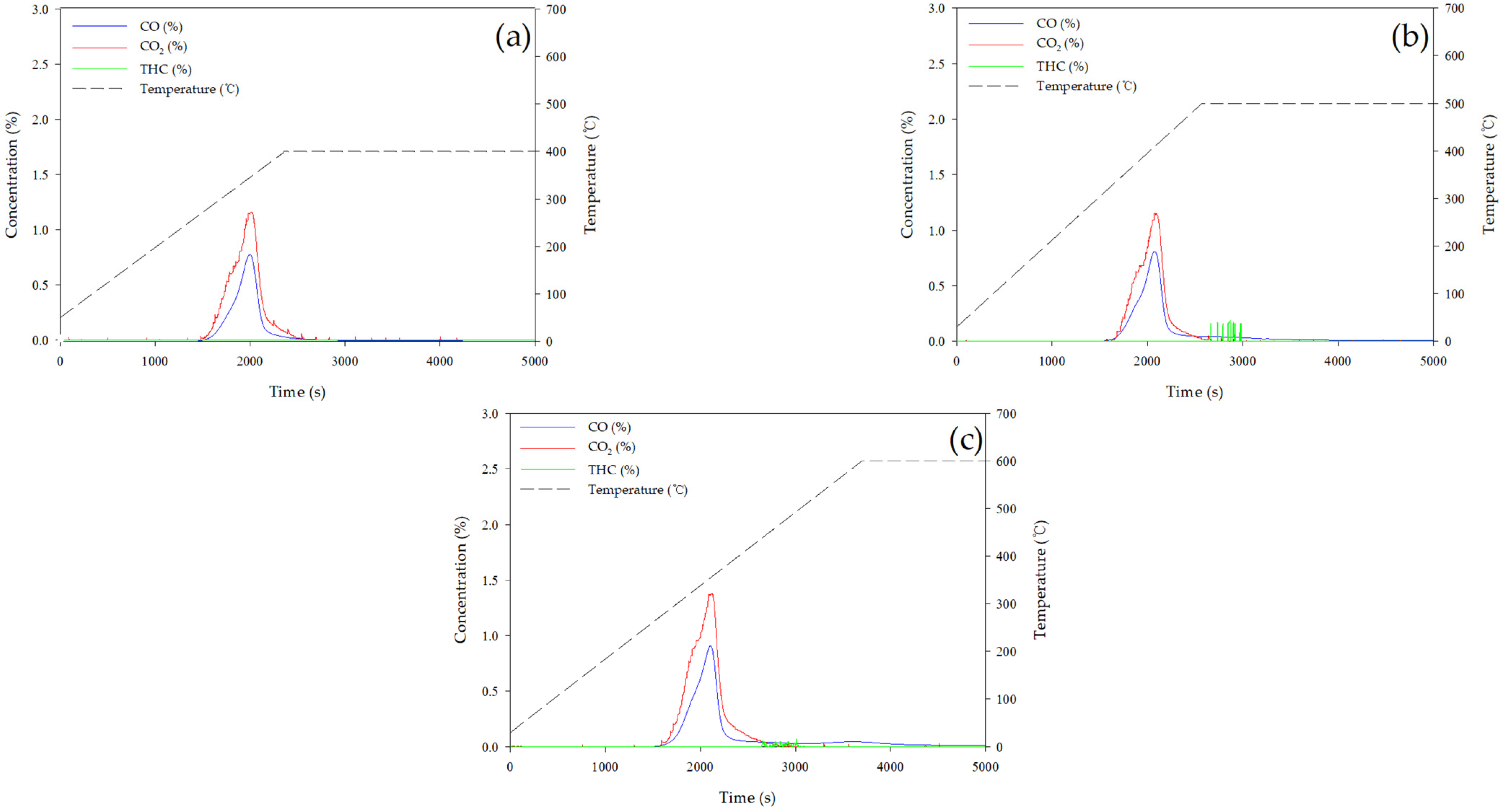
3.3.2. Gas Product Analysis of EFB
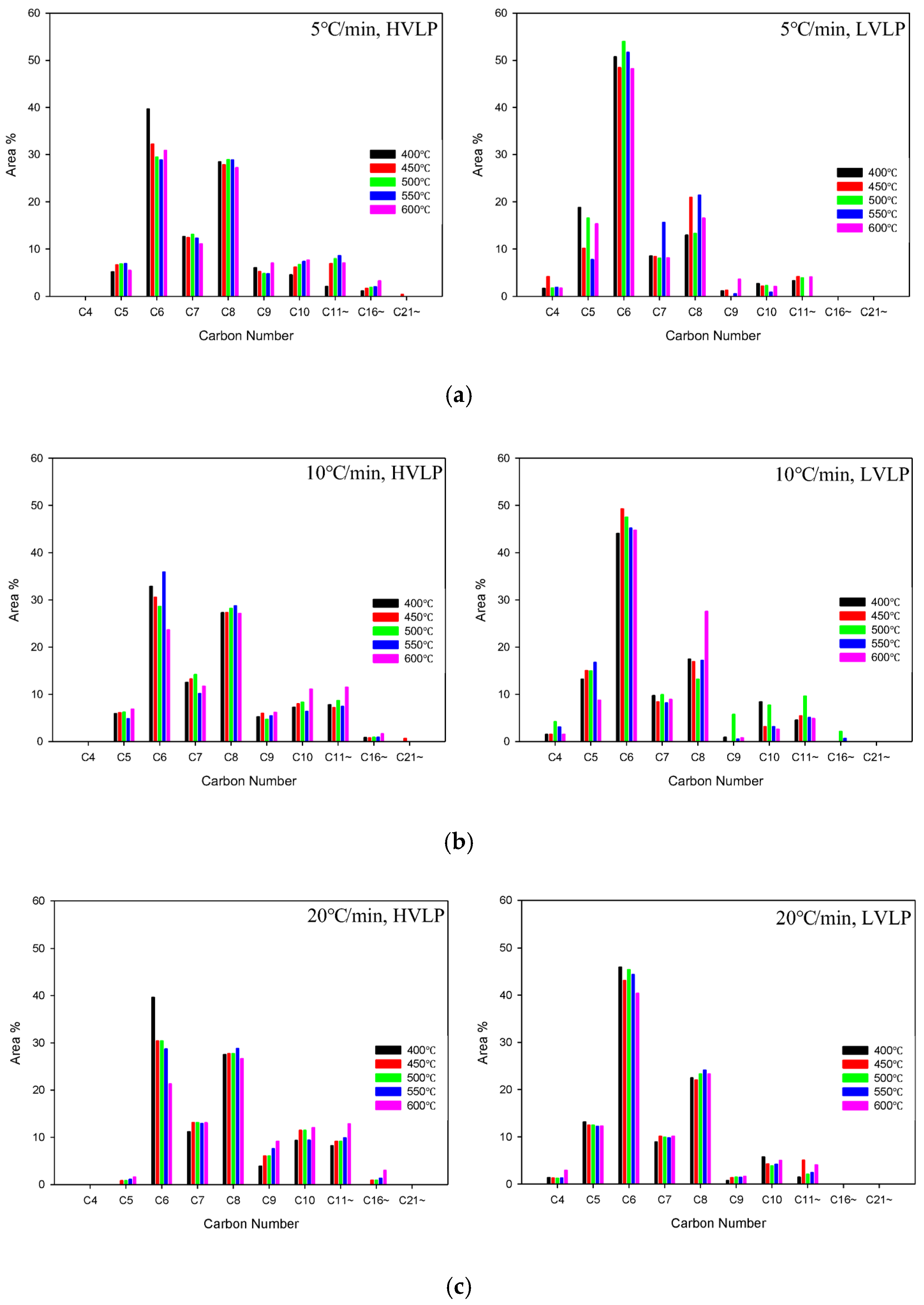
3.3.3. Liquid Product Analysis
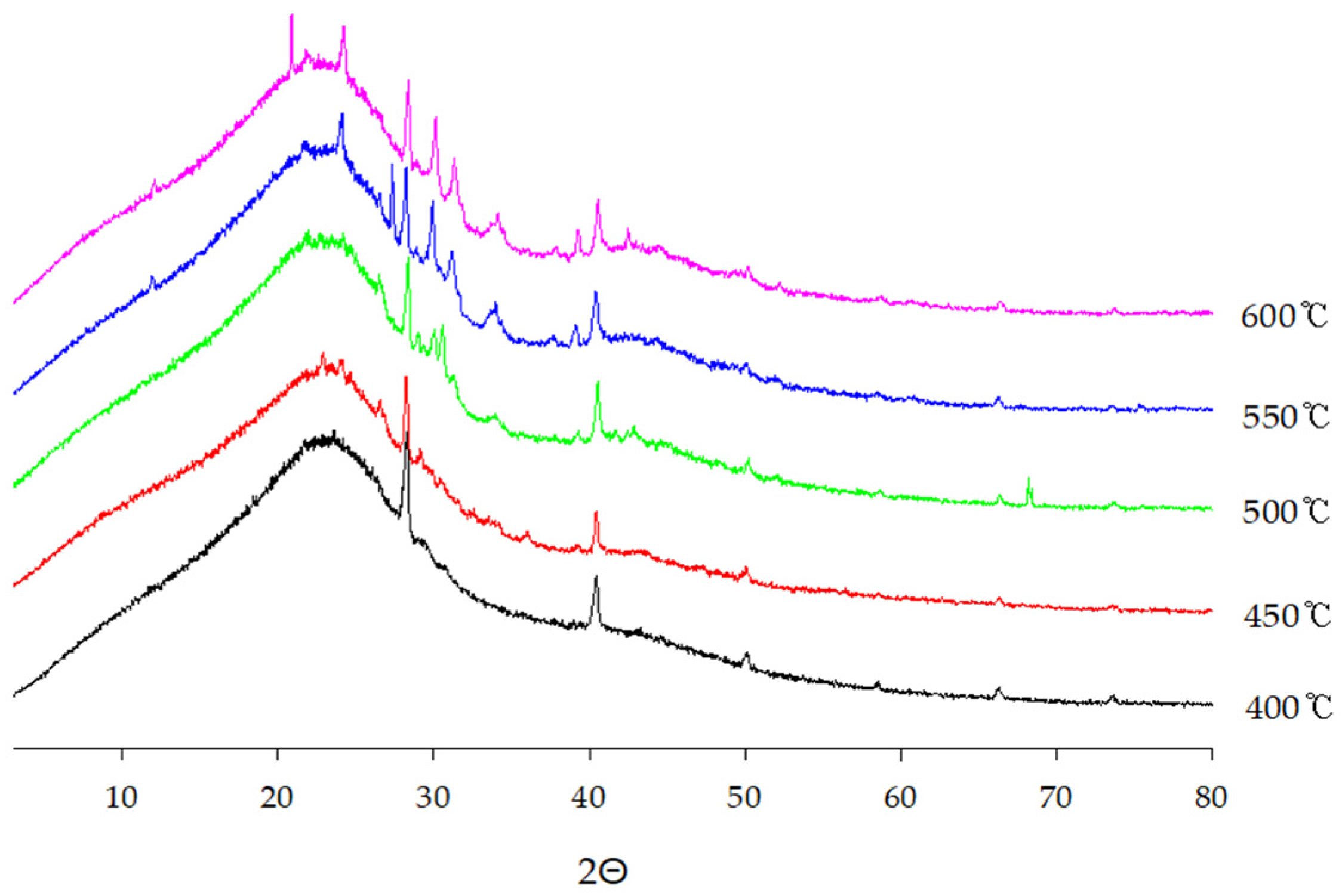
3.3.4. Biochar Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bilgili, M.; Tumse, S.; Nar, S. Comprehensive Overview on the Present State and Evolution of Global Warming, Climate Change, Greenhouse Gasses and Renewable Energy. Arab. J. Sci. Eng. 2024, 49, 14503–14531. [Google Scholar] [CrossRef]
- Azarpour, A.; Suhaimi, S.; Zahedi, G.; Bahadori, A. A review on the drawbacks of renewable energy as a promising energy source of the future. Arab. J. Sci. Eng. 2013, 38, 317–328. [Google Scholar] [CrossRef]
- Vamvuka, D. Bio-oil, solid and gaseous biofuels from biomass pyrolysis processes—An overview. Int. J. Energy Res. 2011, 35, 835–862. [Google Scholar] [CrossRef]
- Samiran, N.A.; Jaafar, M.N.M.; Ng, J.; Lam, S.S.; Chong, C.T. Progress in biomass gasification technique–with focus on Malaysian palm biomass for syngas production. Renew. Sustain. Energy Rev. 2016, 62, 1047–1062. [Google Scholar] [CrossRef]
- Descals, A.; Gaveau, D.L.; Wich, S.; Szantoi, Z.; Meijaard, E. Global mapping of oil palm planting year from 1990 to 2021. Earth Syst. Sci. Data Discuss. 2024, 16, 5111–5129. [Google Scholar] [CrossRef]
- Dolah, R.; Karnik, R.; Hamdan, H. A comprehensive review on biofuels from oil palm empty bunch (EFB): Current status, potential, barriers and way forward. Sustainability 2021, 13, 10210. [Google Scholar] [CrossRef]
- Chiew, Y.L.; Iwata, T.; Shimada, S. System analysis for effective use of palm oil waste as energy resources. Biomass Bioenergy 2011, 35, 2925–2935. [Google Scholar] [CrossRef]
- Okarda, B.; Purnomo, H.; Juniyanti, L.; Kusumadewi, S.D.; Nadhira, S. Indonesian palm oil towards sustainability: A system dynamic approach. IOP Conf. Ser. Earth Environ. Sci. 2024, 1379, 012037. [Google Scholar] [CrossRef]
- Sutrisno, B.; Hidayat, A. Pyrolysis of palm empty fruit bunch: Yields and analysis of bio-oil. MATEC Web Conf. 2018, 154, 01036. [Google Scholar] [CrossRef]
- Razali, W.A.W.; Baharuddin, A.S.; Talib, A.T.; Sulaiman, A.; Naim, M.; Hassan, M.A.; Shirai, Y. Degradation of oil palm empty fruit bunches (OPEFB) fiber during composting process using in-vessel composter. BioResources 2012, 7, 4786–4805. [Google Scholar]
- Kong, S.; Loh, S.; Bachmann, R.T.; Rahim, S.A.; Salimon, J. Biochar from oil palm biomass: A review of its potential and challenges. Renew. Sustain. Energy Rev. 2014, 39, 729–739. [Google Scholar] [CrossRef]
- Windiastuti, E.; Bindar, Y.; Hasanudin, U. Identification of potential application of oil palm empty fruit bunches (EFB): A review. IOP Conf. Ser. Earth Environ. Sci. 2022, 1063, 012024. [Google Scholar] [CrossRef]
- Umana, U.S.; Ebong, M.S.; Godwin, E.O. Biomass production from oil palm and its value chain. J. Hum. Earth Future 2020, 1, 30–38. [Google Scholar] [CrossRef]
- Sumathi, S.; Chai, S.P.; Mohamed, A.R. Utilization of oil palm as a source of renewable energy in Malaysia. Renew. Sustain. Energy Rev. 2008, 12, 2404–2421. [Google Scholar] [CrossRef]
- Hardianto, T.; Wenas, A.A.; Juangsa, F.B. Upgrading process of palm empty fruit bunches as alternative solid fuel: A review. Clean Energy 2023, 7, 1173–1188. [Google Scholar] [CrossRef]
- Jamil, K.; Hayashi, J.; Li, C. Pyrolysis of a Victorian brown coal and gasification of nascent char in CO2 atmosphere in a wire-mesh reactor. Fuel 2004, 83, 833–843. [Google Scholar] [CrossRef]
- Zhang, G.; Feng, Q.; Hu, J.; Sun, G.; Evrendilek, F.; Liu, H.; Liu, J. Performance and mechanism of bamboo residues pyrolysis: Gas emissions, by-products, and reaction kinetics. Sci. Total Environ. 2022, 838, 156560. [Google Scholar] [CrossRef]
- Varma, A.K.; Thakur, L.S.; Shankar, R.; Mondal, P. Pyrolysis of wood sawdust: Effects of process parameters on products yield and characterization of products. Waste Manag. 2019, 89, 224–235. [Google Scholar] [CrossRef]
- Biswas, B.; Singh, R.; Kumar, J.; Singh, R.; Gupta, P.; Krishna, B.B.; Bhaskar, T. Pyrolysis behavior of rice straw under carbon dioxide for production of bio-oil. Renew. Energy 2018, 129, 686–694. [Google Scholar] [CrossRef]
- Abnisa, F.; Arami-Niya, A.; Daud, W.W.; Sahu, J.N.; Noor, I.M. Utilization of oil palm tree residues to produce bio-oil and bio-char via pyrolysis. Energy Convers. Manag. 2013, 76, 1073–1082. [Google Scholar] [CrossRef]
- Rahayu, D.E.; Karnaningroem, N.; Altway, A.; Slamet, A. Utilization of oil palm empty fruit bunches biomass through slow pyrolysis process. IOP Conf. Ser. Earth Environ. Sci. 2021, 913, 012018. [Google Scholar] [CrossRef]
- Sukiran, M.A.; Kheang, L.S.; Bakar, N.A.; May, C.Y. Production and characterization of bio-char from the pyrolysis of empty fruit bunches. Am. J. Appl. Sci. 2011, 8, 984. [Google Scholar] [CrossRef]
- Mohamed, A.R.; Hamzah, Z.; Daud, M.Z.M.; Zakaria, Z. The effects of holding time and the sweeping nitrogen gas flowrates on the pyrolysis of EFB using a fixed–bed reactor. Procedia Eng. 2013, 53, 185–191. [Google Scholar] [CrossRef]
- Lim, X.; Sanna, A.; Andrésen, J.M. Influence of red mud impregnation on the pyrolysis of oil palm biomass-EFB. Fuel 2014, 119, 259–265. [Google Scholar] [CrossRef]
- Bridgwater, A.V.; Meier, D.; Radlein, D. An overview of fast pyrolysis of biomass. Org. Geochem. 1999, 30, 1479–1493. [Google Scholar] [CrossRef]
- Cooney, J.D.; Day, M.; Wiles, D.M. Thermal degradation of poly (ethylene terephthalate): A kinetic analysis of thermogravimetric data. J. Appl. Polym. Sci. 1983, 28, 2887–2902. [Google Scholar] [CrossRef]
- Coats, A.W.; Redfern, J.P. Kinetic parameters from thermogravimetric data. Nature 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Zheng, C.; Lee, D.H.; Liang, D.T. In-depth investigation of biomass pyrolysis based on three major components: Hemicellulose, cellulose and lignin. Energy Fuels 2006, 20, 388–393. [Google Scholar] [CrossRef]
- Brachi, P.; Santes, V.; Torres-Garcia, E. Pyrolytic degradation of spent coffee ground: A thermokinetic analysis through the dependence of activation energy on conversion and temperature. Fuel 2021, 302, 120995. [Google Scholar] [CrossRef]
- Torres-García, E.; Ramírez-Verduzco, L.F.; Aburto, J. Pyrolytic degradation of peanut shell: Activation energy dependence on the conversion. Waste Manag. 2020, 106, 203–212. [Google Scholar] [CrossRef]
- Yeo, J.Y.; Chin, B.L.F.; Tan, J.K.; Loh, Y.S. Comparative studies on the pyrolysis of cellulose, hemicellulose, and lignin based on combined kinetics. J. Energy Inst. 2019, 92, 27–37. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, M.; Zhang, D. A Thermogravimetric study of the characteristics of pyrolysis of cellulose isolated from selected biomass. Appl. Energy 2018, 220, 87–93. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Sundaram, E.G.; Natarajan, E. Pyrolysis of coconut shell: An experimental investigation. J. Eng. Res. [TJER] 2009, 6, 33–39. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.S. A review on operating parameters for optimum liquid oil yield in biomass pyrolysis. Renew. Sustain. Energy Rev. 2012, 16, 5101–5109. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Yan, M.; Hantoko, D.; Susanto, H.; Ardy, A.; Waluyo, J.; Weng, Z.; Lin, J. Hydrothermal treatment of empty fruit bunch and its pyrolysis characteristics. Biomass Convers. Biorefinery 2019, 9, 709–717. [Google Scholar] [CrossRef]
- Li, C.; Hayashi, J.; Sun, Y.; Zhang, L.; Zhang, S.; Wang, S.; Hu, X. Impact of heating rates on the evolution of function groups of the biochar from lignin pyrolysis. J. Anal. Appl. Pyrolysis 2021, 155, 105031. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman Jr, C.U.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Demirbas, A. The influence of temperature on the yields of compounds existing in bio-oils obtained from biomass samples via pyrolysis. Fuel Process Technol 2007, 88, 591–597. [Google Scholar] [CrossRef]
- Evans, R.J.; Milne, T.A. Molecular characterization of the pyrolysis of biomass. Energy Fuels 1987, 1, 123–137. [Google Scholar] [CrossRef]
- Wang, F.; Zheng, Y.; Huang, Y.; Yang, X.; Liu, C.; Kang, J.; Zheng, Z. Effect of Temperature on Characteristics of Bio-Oil and Bio-Char During Pyrolysis of Yunnan Pine. J. Biobased Mater. Bioenergy 2016, 10, 81–89. [Google Scholar] [CrossRef]
- Cai, W.; Zhu, X.; Kumar, R.; Zhu, Z.; Ye, J.; Zhao, J. Catalytic pyrolysis of biomass waste using montmorillonite-supported ultrafine iron nanoparticles for enhanced bio-oil yield and quality. Green Energy Resour. 2024, 2, 100085. [Google Scholar] [CrossRef]
- Gollakota, A.R.; Reddy, M.; Subramanyam, M.D.; Kishore, N. A review on the upgradation techniques of pyrolysis oil. Renew. Sustain. Energy Rev. 2016, 58, 1543–1568. [Google Scholar] [CrossRef]
- Wang, D.; Li, D.; Liu, Y.; Lv, D.; Ye, Y.; Zhu, S.; Zhang, B. Study of a new complex method for extraction of phenolic compounds from bio-oils. Sep. Purif. Technol. 2014, 134, 132–138. [Google Scholar] [CrossRef]
- Abel, S.E.R.; Loh, S.K.; Wahab, N.A.; Masek, O.; Tanimu, M.I.; Bachmann, R.T. Effect of operating temperature on physicochemical properties of empty fruit bunch cellulose-derived biochar. J. Oil Palm Res. 2021, 33, 643–652. [Google Scholar]
- Sukiran, M.A.; Daud, W.M.A.W.; Abnisa, F.; Nasrin, A.B.; Aziz, A.A.; Loh, S.K. A comprehensive study on torrefaction of empty fruit bunches: Characterization of solid, liquid and gas products. Energy 2021, 230, 120877. [Google Scholar] [CrossRef]
- Rosli, N.S.; Harun, S.; Jahim, J.M.; Othaman, R. Chemical and physical characterization of oil palm empty fruit bunch. Malays. J. Anal. Sci. 2017, 21, 188–196. [Google Scholar]
- Kasim, N.N.; Ismail, K.; Mohamed, A.R.; Ishak, M.A.M.; Ahmad, R.; Ismail, W. Characteristic, thermochemical behaviors and kinetic of demineralized and torrefied empty fruit bunches (EFB). Adv. Sci. Technol. Eng. Syst. J. 2018, 3, 365–373. [Google Scholar] [CrossRef]
- Lani, N.S.; Ngadi, N.; Johari, A.; Jusoh, M. Isolation, characterization, and application of nanocellulose from oil palm empty fruit bunch fiber as nanocomposites. J. Nanomater. 2014, 2014, 702538. [Google Scholar] [CrossRef]
- Marhani; Sahur, A.; Laban, S.; Musa, Y. Characterization of biochar empty fruit bunches OPEFB at various temperatures and burning time. J. Exp. Biol. Agric. Sci. 2022, 10, 599–606. [Google Scholar] [CrossRef]
- Kim, P.; Johnson, A.; Edmunds, C.W.; Radosevich, M.; Vogt, F.; Rials, T.G.; Labbé, N. Surface functionality and carbon structures in lignocellulosic-derived biochars produced by fast pyrolysis. Energy Fuels 2011, 25, 4693–4703. [Google Scholar] [CrossRef]
- Luo, Q.; Deng, Y.; Li, Y.; He, Q.; Wu, H.; Fang, X. Effects of pyrolysis temperatures on the structural properties of straw biochar and its adsorption of tris-(1-chloro-2-propyl) phosphate. Sci. Rep. 2024, 14, 25711. [Google Scholar] [CrossRef] [PubMed]
- Waqas, M.; Aburiazaiza, A.S.; Miandad, R.; Rehan, M.; Barakat, M.A.; Nizami, A.S. Development of biochar as fuel and catalyst in energy recovery technologies. J. Clean. Prod. 2018, 188, 477–488. [Google Scholar] [CrossRef]



| Elements (%) | C | 46.58 |
| H | 5.94 | |
| N | 0.54 | |
| S | 0.04 | |
| O | 43.55 | |
| Others | 3.55 | |
| Volatile (%) | 71.91 | |
| Fixed Carbon (%) | 26.45 | |
| Ash (%) | 1.65 | |
| Item | Condition |
|---|---|
| Column over temp | 40.0 °C |
| Injection temperature | 250.0 °C |
| Injection mode | Split |
| Flow control mode | Linear velocity |
| Pressure | 60.1 kPa |
| Total flow | 579.2 mL/min |
| Column flow (He) | 1.15 mL/min |
| Linear velocity | 38.6 cm/s |
| Purge flow | 3.0 mL/min |
| Split ratio | 500.0 |
| Conversion (α) | Temperature and Heating Rate | Activation Energy (kJ/mol) |
|---|---|---|
| 0.05 | 228 °C (5 °C/min)–256 °C (40 °C/min) | 171.32 |
| 0.10 | 247 °C (5 °C/min)–273 °C (40 °C/min) | 195.35 |
| 0.15 | 260 °C (5 °C/min)–287 °C (40 °C/min) | 196.83 |
| 0.20 | 272 °C (5 °C/min)–298 °C (40 °C/min) | 203.92 |
| 0.25 | 281 °C (5 °C/min)–308 °C (40 °C/min) | 207.12 |
| 0.30 | 289 °C (5 °C/min)–316 °C (40 °C/min) | 215.31 |
| 0.35 | 295 °C (5 °C/min)–322 °C (40 °C/min) | 217.57 |
| 0.40 | 300 °C (5 °C/min)–327 °C (40 °C/min) | 209.72 |
| 0.45 | 305 °C (5 °C/min)–332 °C (40 °C/min) | 211.03 |
| 0.50 | 309 °C (5 °C/min)–336 °C (40 °C/min) | 218.45 |
| 0.55 | 314 °C (5 °C/min)–341 °C (40 °C/min) | 235.98 |
| 0.60 | 326 °C (5 °C/min)–347 °C (40 °C/min) | 391.65 |
| Heating Rate | Conversion (α) | Activation Energy (kJ/mol) |
|---|---|---|
| 5 °C/min | 0.04 < α < 0.65 | 87.09 |
| 10 °C/min | 0.04 < α < 0.67 | 87.49 |
| 20 °C/min | 0.03 < α < 0.69 | 87.00 |
| 30 °C/min | 0.03 < α < 0.68 | 105.96 |
| 40 °C/min | 0.02 < α < 0.68 | 110.44 |
| Heating Rate | Temperature (°C) | Gas (%) | High Viscosity Liquid Product (%) | Low Viscosity Liquid Product (%) | Biochar (%) |
|---|---|---|---|---|---|
| 5 °C/min | 400 | 30.00 | 18.45 | 16.60 | 34.95 |
| 450 | 32.86 | 19.26 | 15.34 | 32.54 | |
| 500 | 32.60 | 23.04 | 15.07 | 29.31 | |
| 550 | 32.74 | 19.26 | 17.16 | 28.81 | |
| 600 | 34.37 | 20.71 | 16.56 | 28.35 | |
| 10 °C/min | 400 | 28.54 | 21.07 | 18.87 | 32.19 |
| 450 | 27.94 | 20.89 | 20.48 | 30.69 | |
| 500 | 30.58 | 20.35 | 19.89 | 29.21 | |
| 550 | 31.24 | 20.24 | 19.92 | 28.95 | |
| 600 | 31.20 | 21.33 | 20.10 | 27.37 | |
| 20 °C/min | 400 | 29.26 | 20.46 | 21.29 | 31.16 |
| 450 | 30.97 | 17.99 | 22.48 | 28.56 | |
| 500 | 31.56 | 17.49 | 22.81 | 28.15 | |
| 550 | 33.38 | 16.66 | 22.68 | 27.29 | |
| 600 | 34.19 | 17.65 | 21.61 | 26.55 |
| Heating Rate | Temp. (°C) | Saturated Components (%) | Non-Saturated Components (%) | Aliphatic Components (%) | Aromatic Components (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| HVLP | LVLP | HVLP | LVLP | HVLP | LVLP | HVLP | LVLP | ||
| 5 °C/min | 400 | 20.97 | 18.38 | 79.03 | 81.62 | 23.81 | 38.10 | 76.19 | 61.90 |
| 450 | 16.88 | 22.6 | 83.12 | 77.4 | 23.66 | 44.68 | 76.34 | 55.32 | |
| 500 | 16.63 | 19.64 | 83.37 | 80.36 | 23.49 | 41.97 | 76.51 | 58.03 | |
| 550 | 19.86 | 17.83 | 80.14 | 82.17 | 20.86 | 37.70 | 79.14 | 62.30 | |
| 600 | 16.46 | 18.2 | 83.54 | 81.8 | 19.62 | 42.75 | 80.38 | 57.25 | |
| 10 °C/min | 400 | 14.62 | 24.23 | 85.38 | 75.77 | 17.07 | 48.83 | 82.93 | 51.17 |
| 450 | 14.33 | 21.88 | 85.67 | 78.12 | 20.18 | 45.06 | 79.82 | 54.94 | |
| 500 | 13.88 | 30.40 | 86.12 | 69.60 | 19.58 | 45.44 | 80.42 | 54.56 | |
| 550 | 22.73 | 22.68 | 77.27 | 77.32 | 25.88 | 46.11 | 74.12 | 53.89 | |
| 600 | 15.21 | 20.98 | 84.79 | 79.02 | 20.09 | 44.27 | 79.91 | 55.73 | |
| 20 °C/min | 400 | 9.94 | 22.79 | 90.06 | 77.21 | 14.01 | 42.95 | 85.99 | 57.05 |
| 450 | 9.89 | 21.64 | 90.11 | 78.36 | 14.66 | 45.58 | 85.34 | 54.42 | |
| 500 | 12.14 | 22.09 | 87.86 | 77.91 | 19.99 | 42.36 | 80.01 | 53.44 | |
| 550 | 13.14 | 21.09 | 86.86 | 78.91 | 16.88 | 46.71 | 83.12 | 53.29 | |
| 600 | 15.85 | 23.21 | 84.15 | 76.79 | 23.1 | 47.19 | 76.9 | 52.81 | |
| Category | C (%) | H (%) | N (%) | S (%) | O (%) | Others (%) | |
|---|---|---|---|---|---|---|---|
| EFB (raw) | 46.58 | 5.94 | 0.54 | 0.04 | 43.55 | 3.55 | |
| 5 °C/min | 400 °C | 73.63 | 3.96 | 0.79 | 0.08 | 16.91 | 4.63 |
| 450 °C | 73.71 | 3.27 | 0.70 | 0.05 | 11.29 | 10.98 | |
| 500 °C | 74.42 | 2.52 | 0.69 | 0.06 | 11.33 | 10.98 | |
| 550 °C | 76.53 | 2.20 | 0.68 | 0.10 | 11.27 | 9.22 | |
| 600 °C | 77.00 | 2.02 | 0.55 | 0.04 | 10.21 | 10.18 | |
| 10 °C/min | 400 °C | 73.06 | 4.18 | 0.76 | 0.11 | 15.38 | 6.51 |
| 450 °C | 82.36 | 3.43 | 0.64 | 0.08 | 9.88 | 3.61 | |
| 500 °C | 78.23 | 3.04 | 0.72 | 0.09 | 11.29 | 6.63 | |
| 550 °C | 79.84 | 3.10 | 0.79 | 0.06 | 10.36 | 5.85 | |
| 600 °C | 83.40 | 2.30 | 0.56 | 0.05 | 9.13 | 4.56 | |
| 20 °C/min | 400 °C | 77.92 | 3.82 | 0.67 | 0.07 | 13.64 | 3.88 |
| 450 °C | 80.47 | 3.46 | 0.64 | 0.05 | 11.84 | 3.54 | |
| 500 °C | 81.45 | 2.66 | 0.63 | 0.05 | 11.67 | 3.54 | |
| 550 °C | 80.59 | 2.74 | 0.73 | 0.07 | 9.20 | 6.67 | |
| 600 °C | 85.06 | 2.78 | 0.75 | 0.07 | 9.64 | 1.70 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, H.; Lee, M.; Sen, R.; Choi, J.; Oh, S. Pyrolysis Characteristics of Empty Fruit Bunches at Different Temperatures and Heating Rates. Energies 2025, 18, 1404. https://doi.org/10.3390/en18061404
Ko H, Lee M, Sen R, Choi J, Oh S. Pyrolysis Characteristics of Empty Fruit Bunches at Different Temperatures and Heating Rates. Energies. 2025; 18(6):1404. https://doi.org/10.3390/en18061404
Chicago/Turabian StyleKo, Hyeongtak, Myeongjong Lee, Rumduol Sen, Jeongwoo Choi, and Seacheon Oh. 2025. "Pyrolysis Characteristics of Empty Fruit Bunches at Different Temperatures and Heating Rates" Energies 18, no. 6: 1404. https://doi.org/10.3390/en18061404
APA StyleKo, H., Lee, M., Sen, R., Choi, J., & Oh, S. (2025). Pyrolysis Characteristics of Empty Fruit Bunches at Different Temperatures and Heating Rates. Energies, 18(6), 1404. https://doi.org/10.3390/en18061404






