Polypyrrole Hybrid Nanocomposite Electrode Materials with Outstanding Specific Capacitance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Processing of Hybrid Nanocomposites
2.2.1. Fabrication of Polyimide/Single-Walled Carbon Nanotube (PI/SWCNT) Hybrid Nanocomposites
2.2.2. Electrodeposition and Doping of Polypyrrole (PPy)
3. Characterization
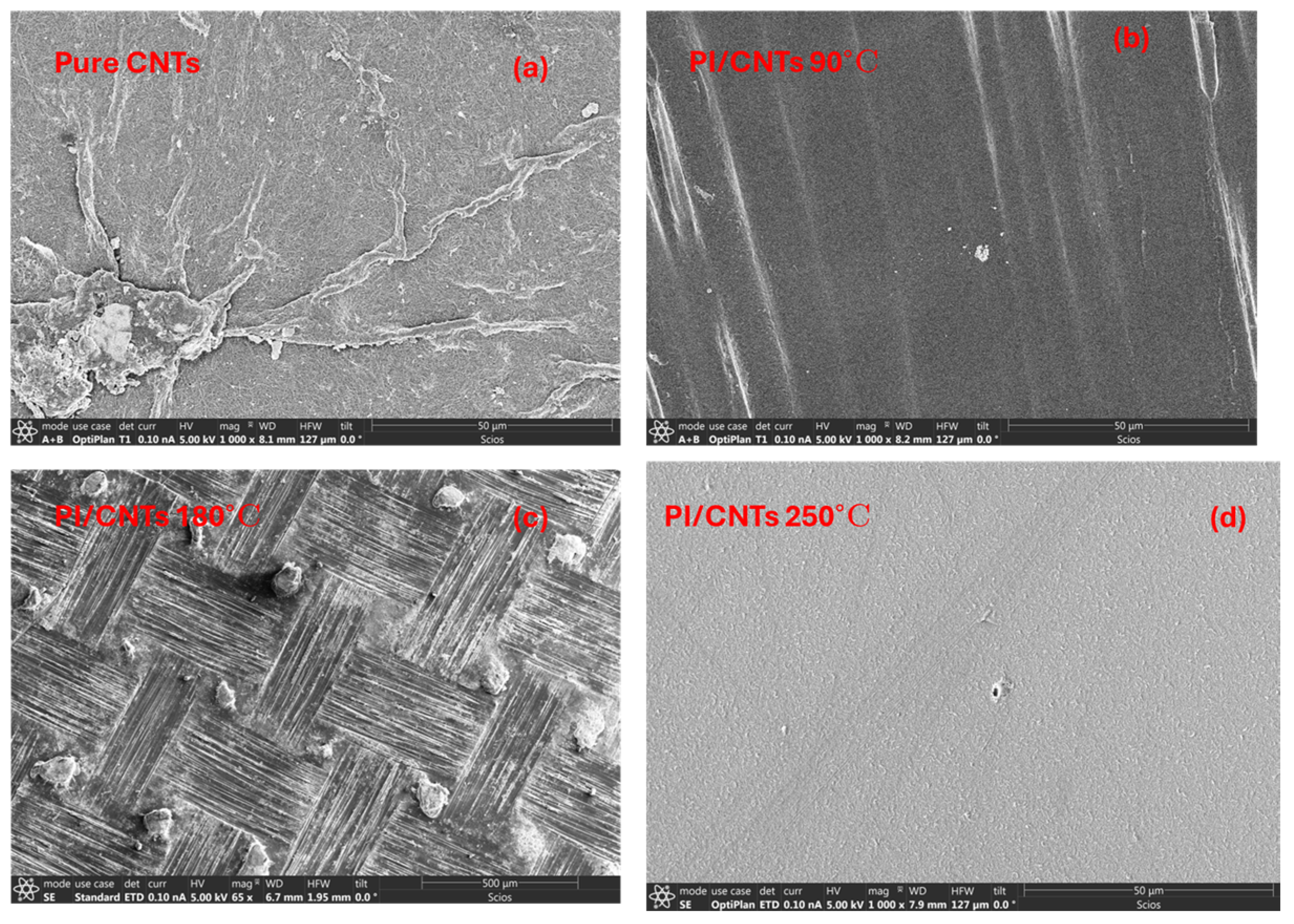
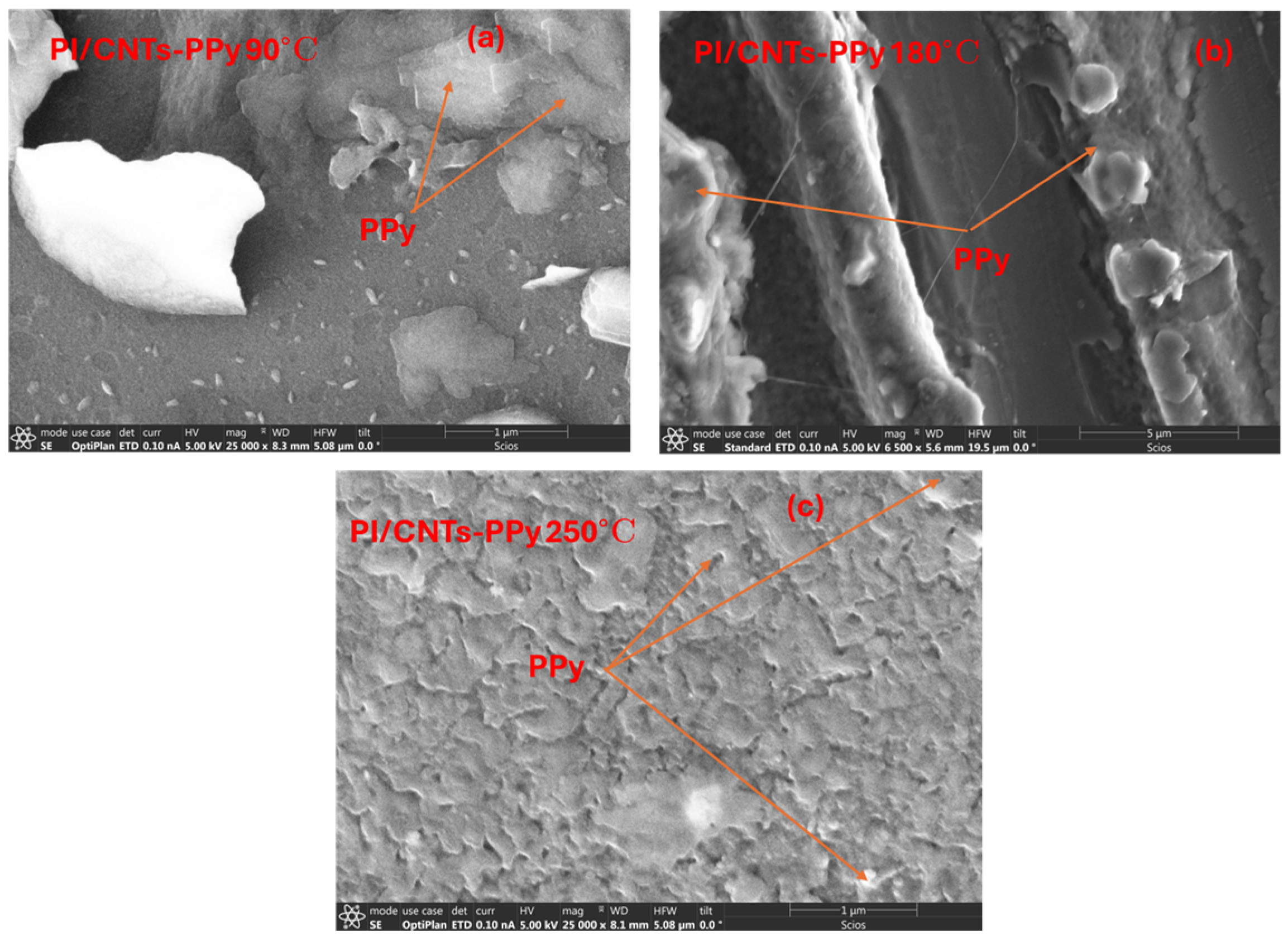
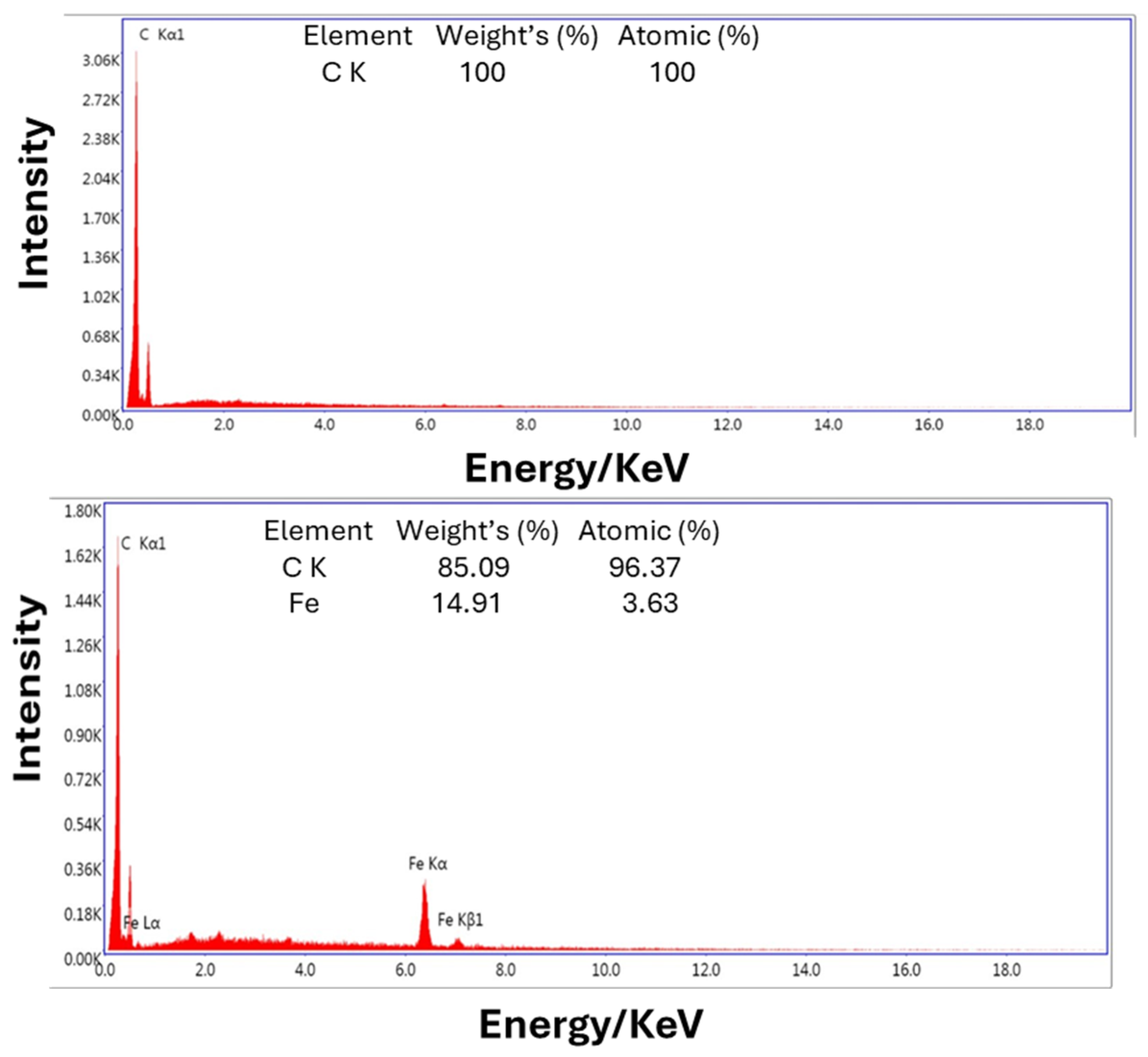
3.1. Morphological Characterization: Using Scanning Electron Microscopy
3.2. Cyclic Voltammetry
3.3. Gravimetric Cyclic Charge/Discharge
3.4. Electrochemical Impedance Spectroscopy
4. Results and Discussions
4.1. Scanning Electron Microscopy (SEM)
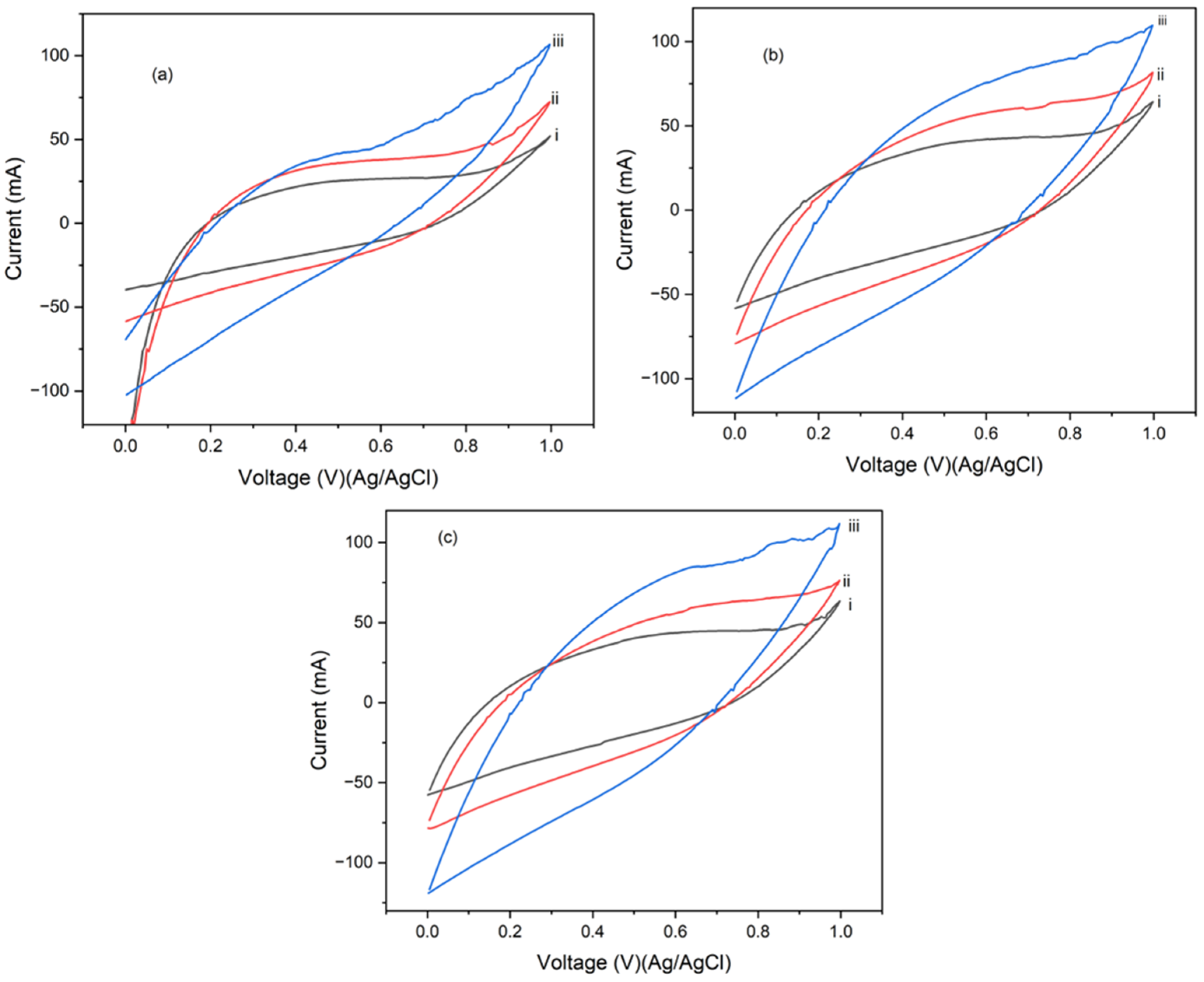
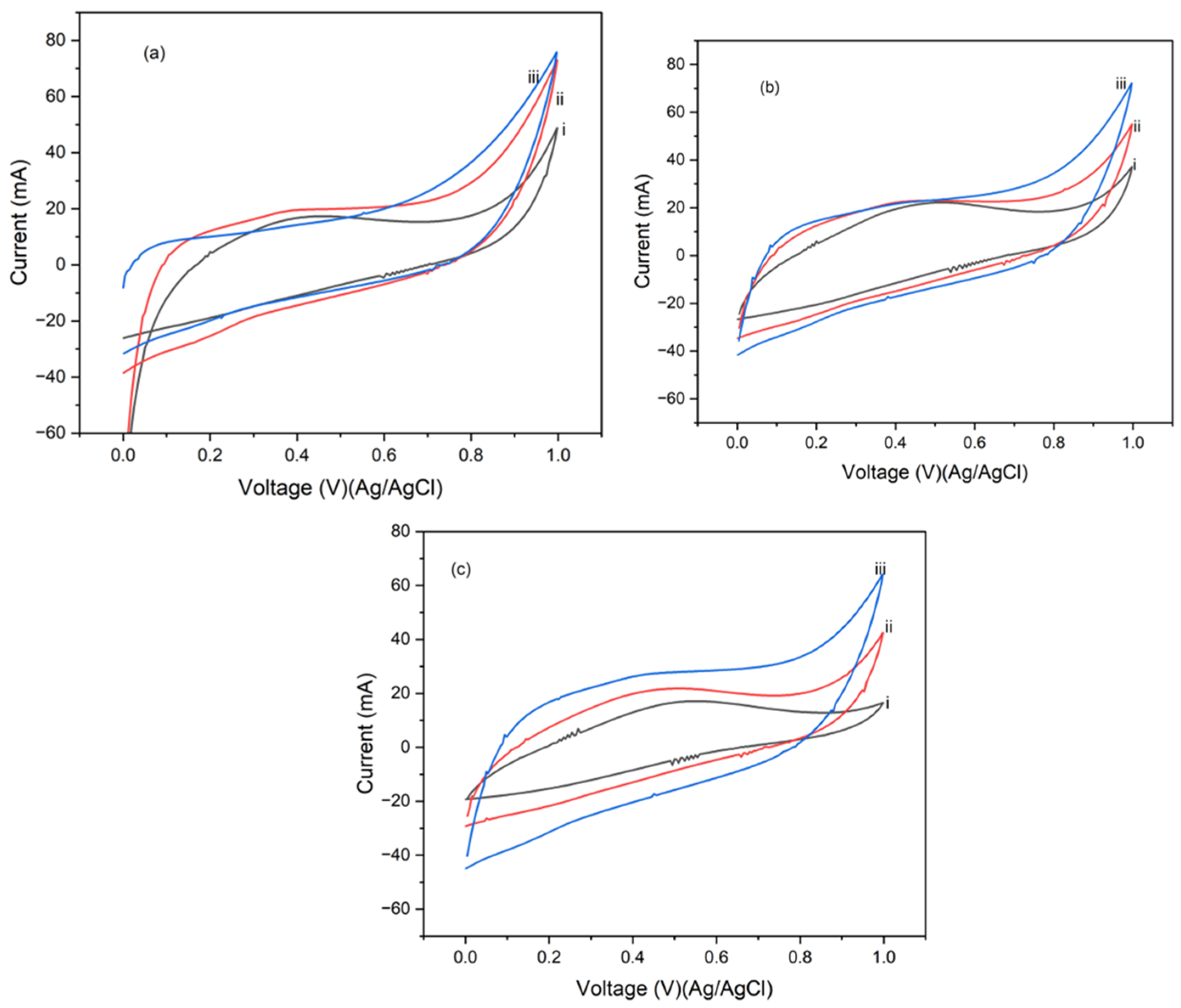
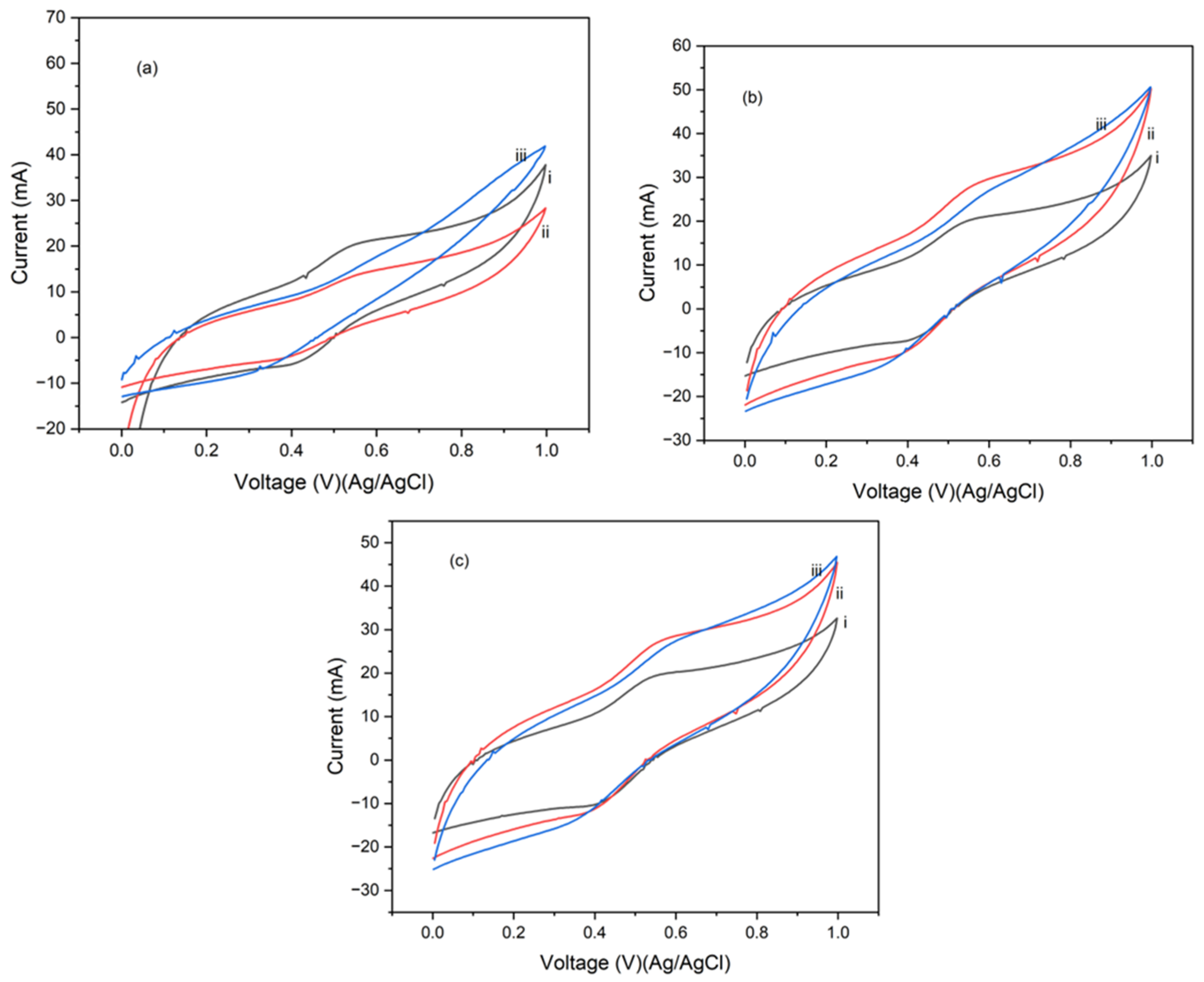
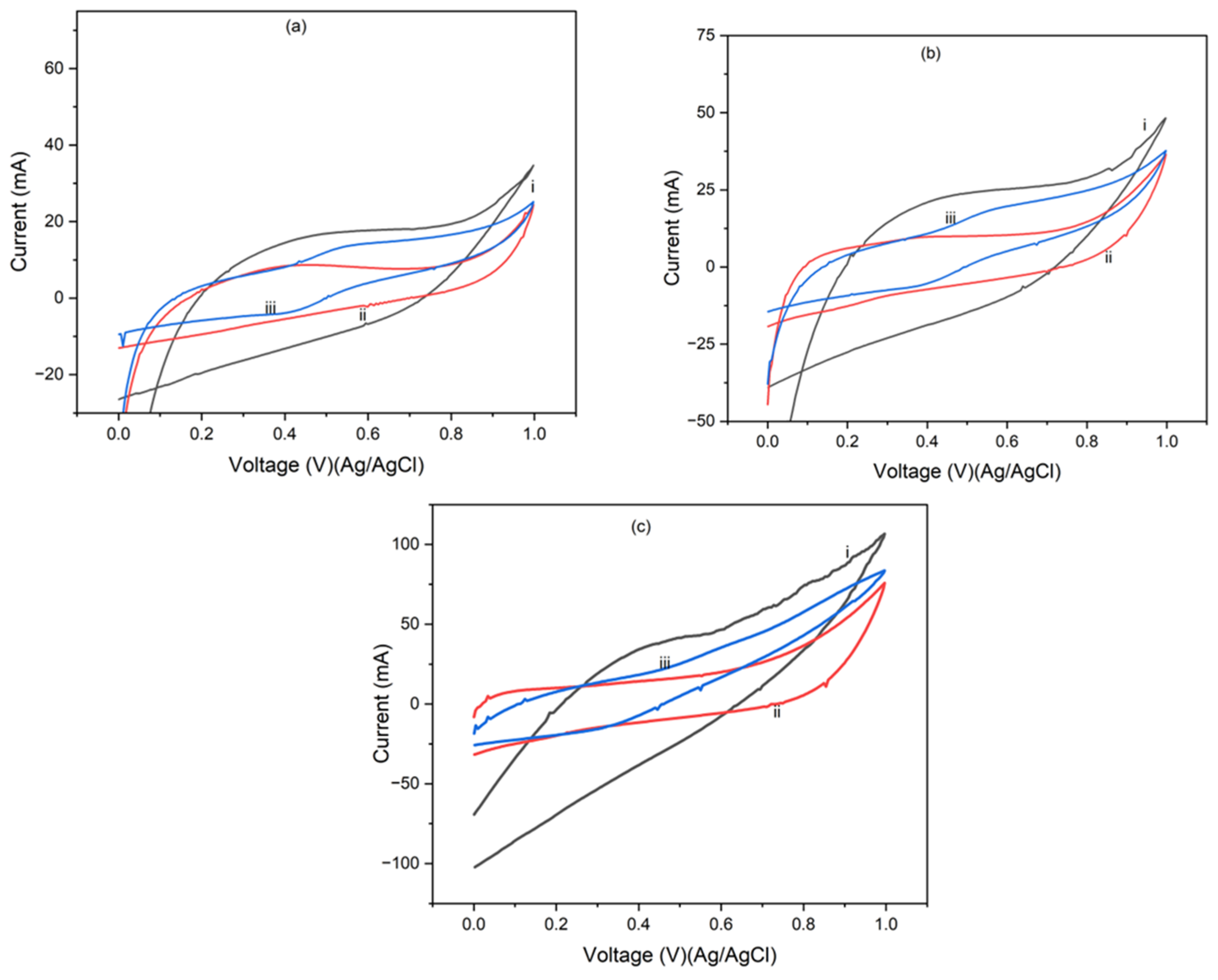
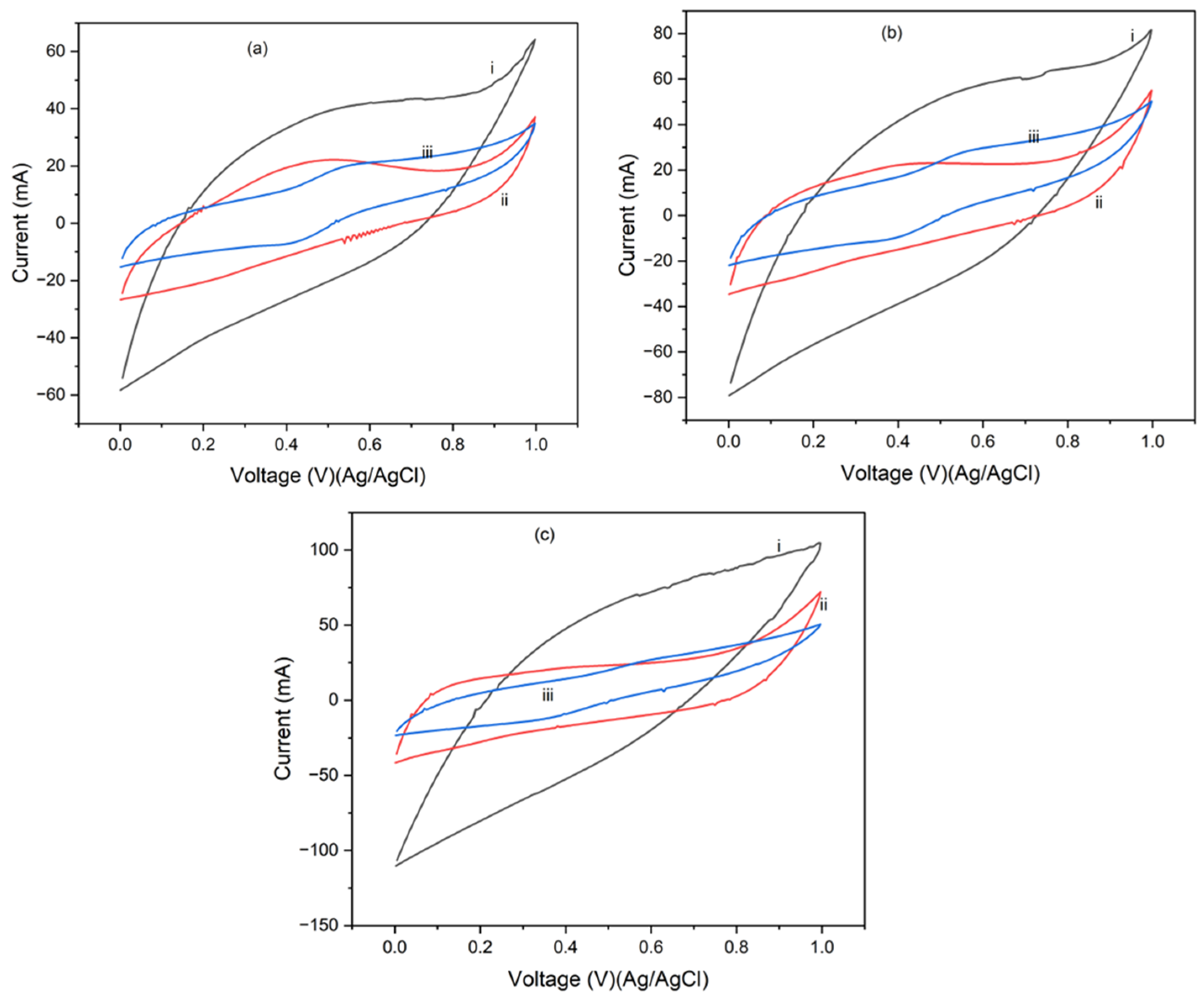
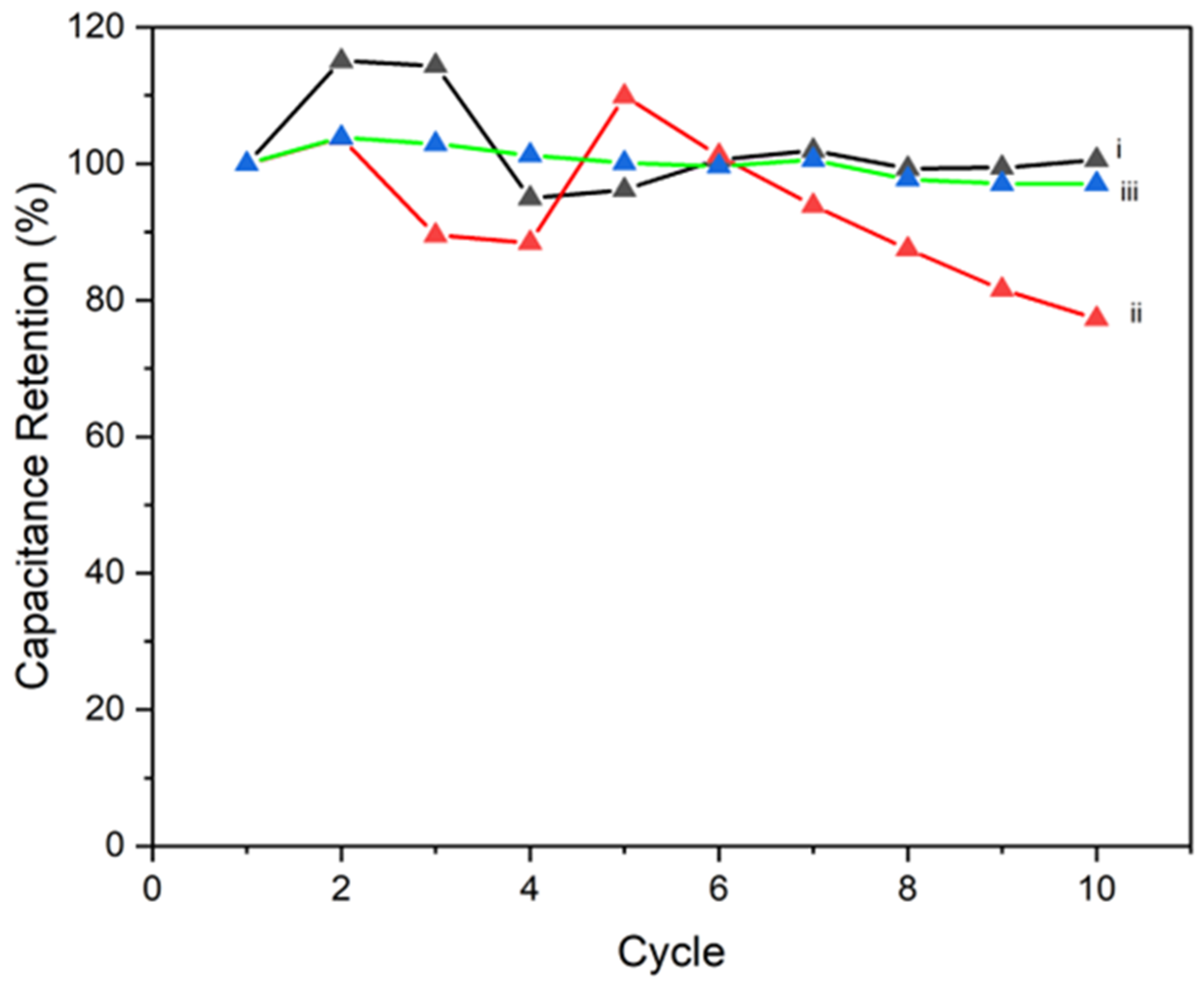
4.2. Cyclic Voltammetry
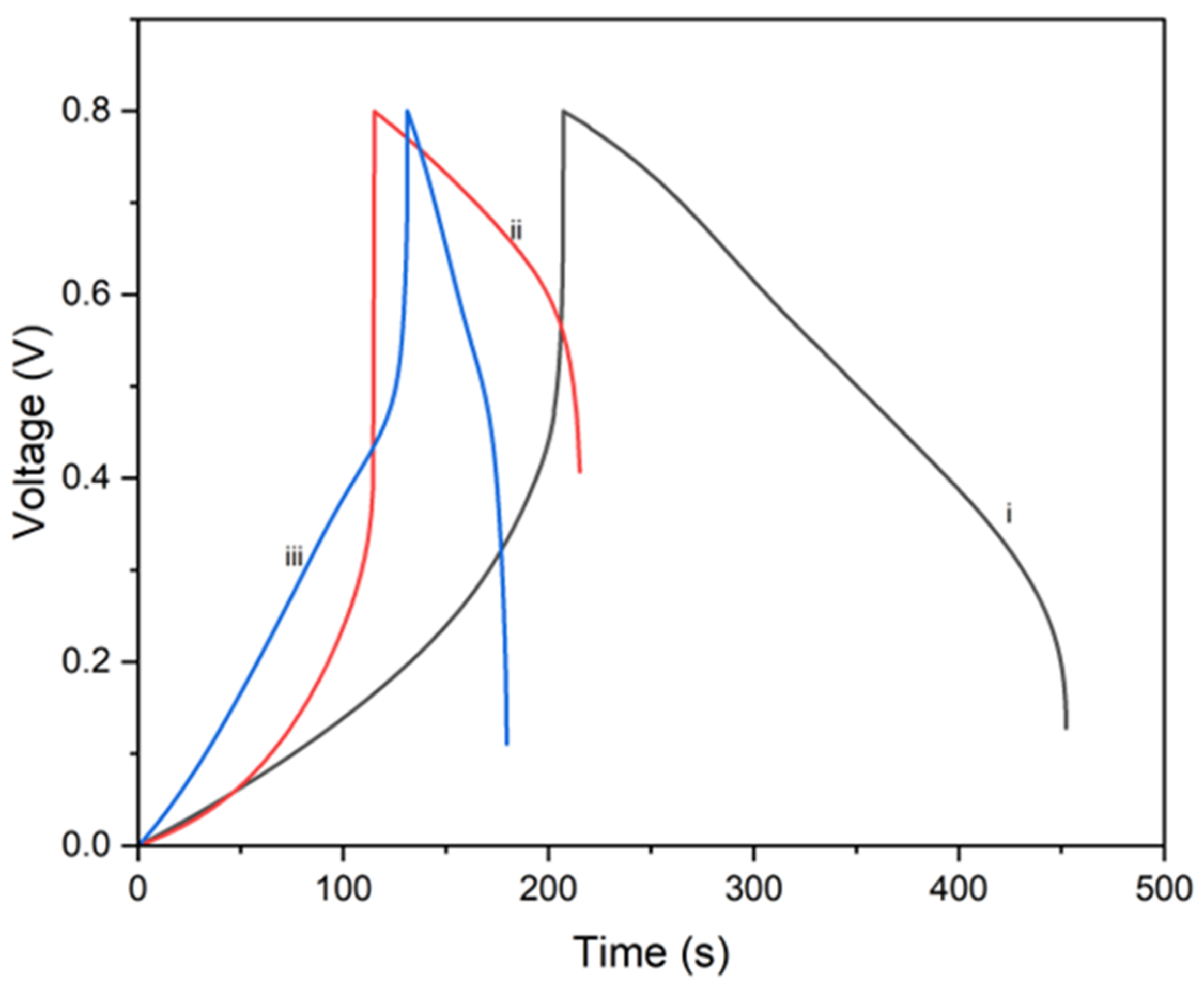
4.3. Galvanostatic Charge/Discharge
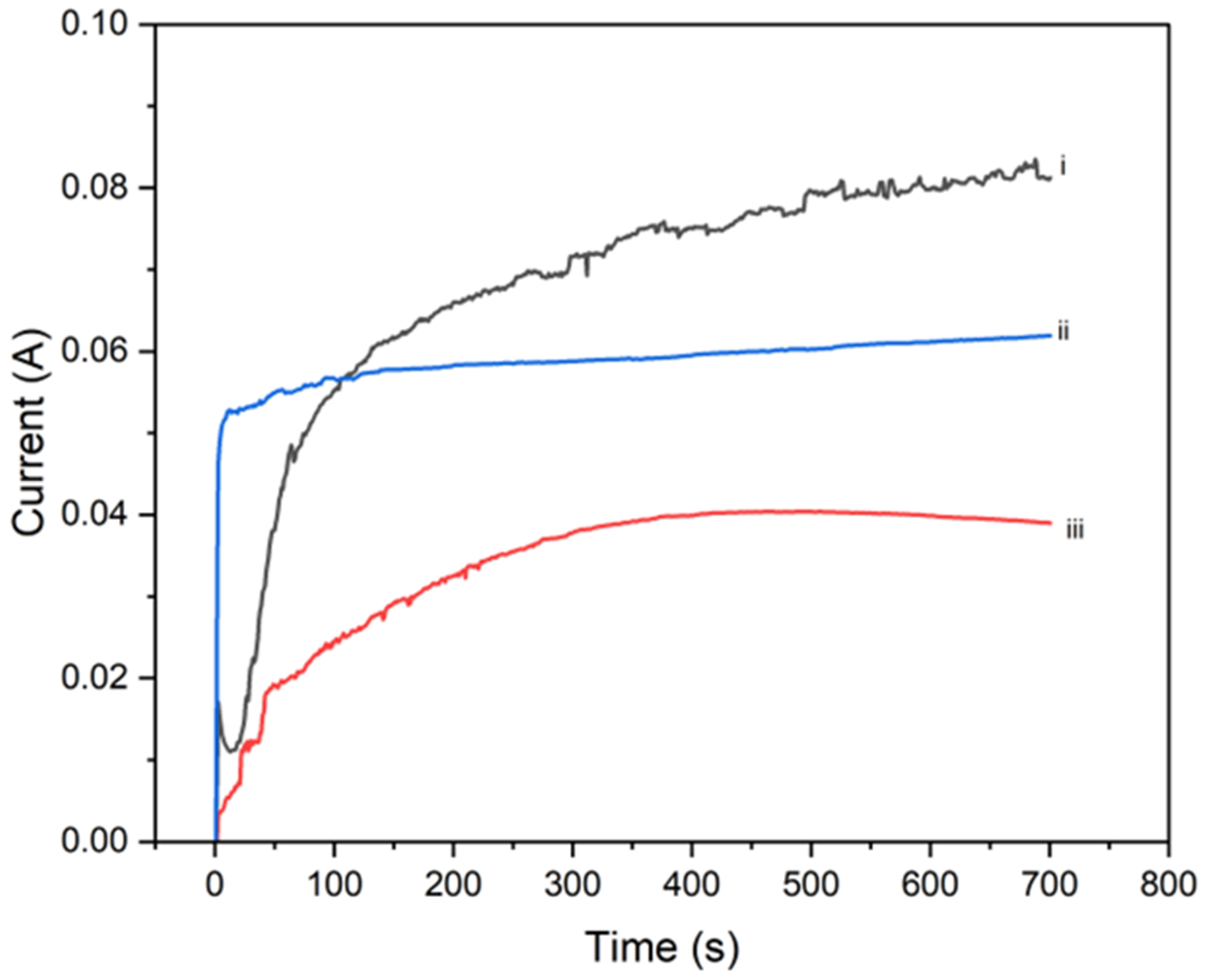
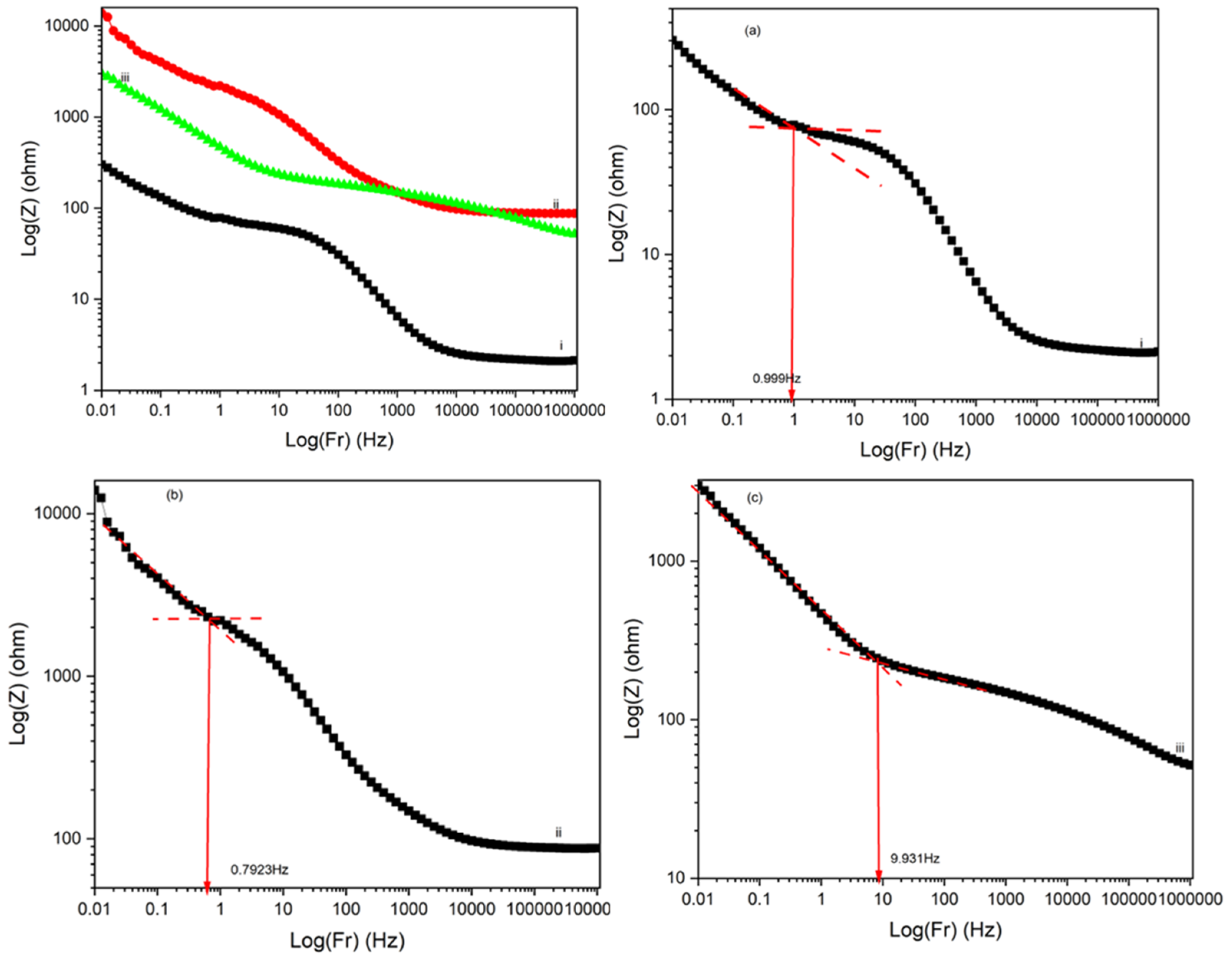
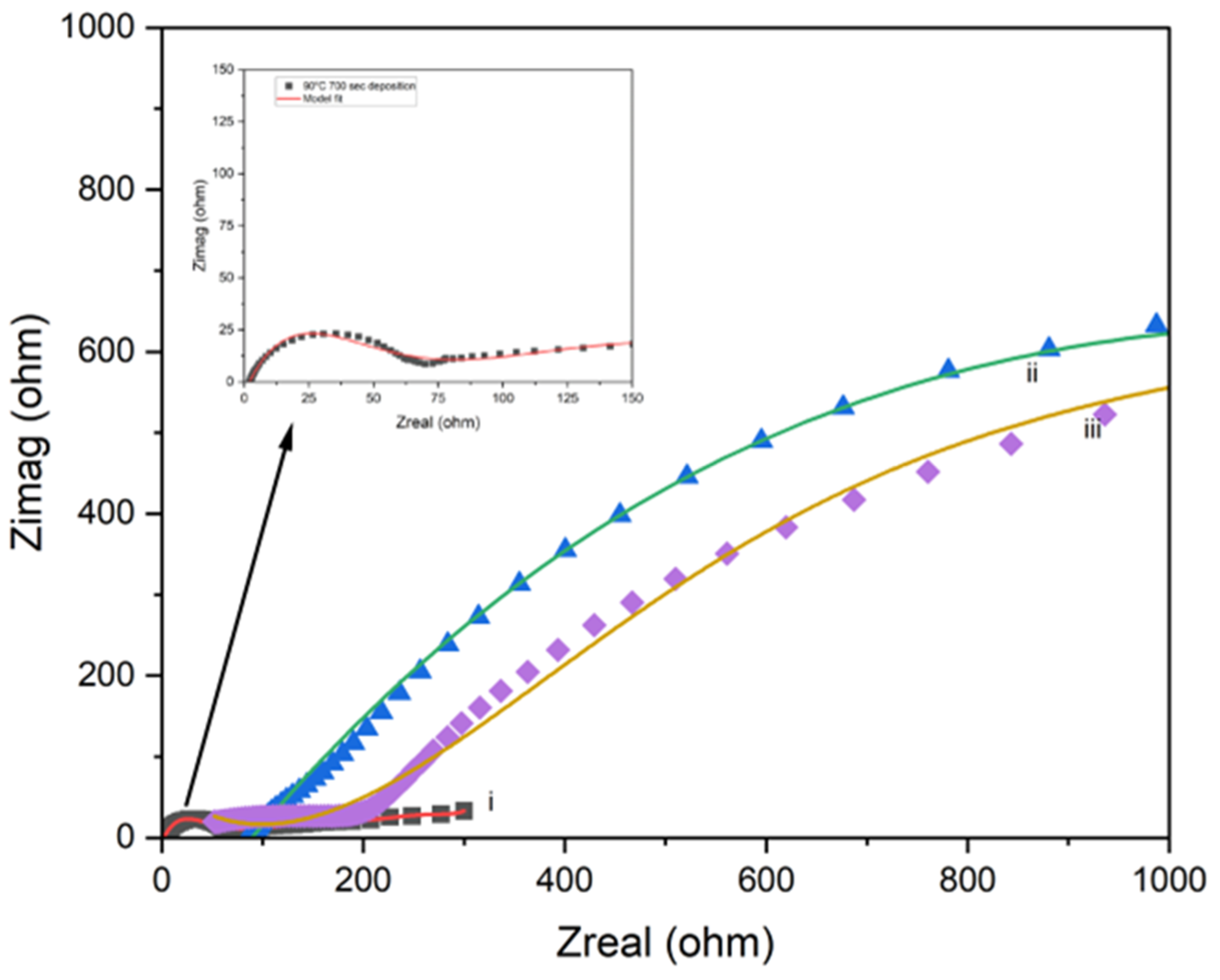
4.4. Electrochemical Impedance Spectroscopy (EIS) Analysis
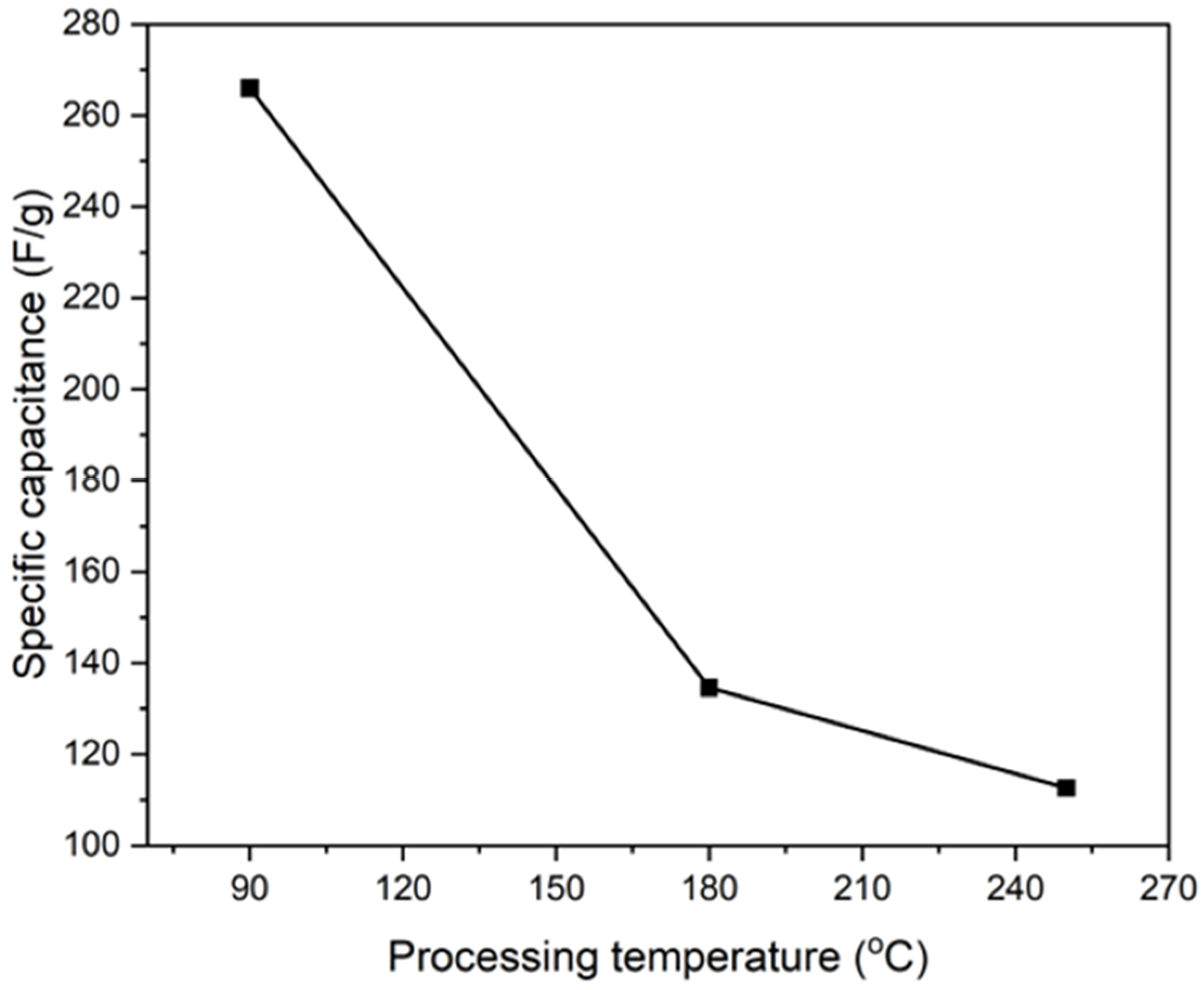
4.5. Impact of Processing Temperature on Polymerization and Performance
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, C.; Li, F.; Ma, L.P.; Cheng, H.M. Advanced materials for energy storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef] [PubMed]
- Di Lecce, D.; Verrelli, R.; Hassoun, J. Lithium-ion batteries for sustainable energy storage: Recent advances towards new cell configurations. Green Chem. 2017, 19, 3442–3467. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abbas, Q.; Al Makky, A. and Abdelkareem, M.A. Supercapacitors as next generation energy storage devices: Properties and applications. Energy 2022, 248, 123617. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Siwal, S.S.; Zhang, Q.; Devi, N.; Thakur, V.K. Carbon-based polymer nanocomposite for high-performance energy storage applications. Polymers 2020, 12, 505. [Google Scholar] [CrossRef]
- Yao, F.; Pham, D.T.; Lee, Y.H. Carbon-based materials for lithium-ion batteries, electrochemical capacitors, and their hybrid devices. ChemSusChem 2015, 8, 2284–2311. [Google Scholar] [CrossRef]
- Nathawat, R.; Rathore, S.S.; Kharangarh, P.R.; Devi, R.; Kumari, A. Synthesis and application of carbon-based nanocomposite. In Carbon Nanomaterials and Their Nanocomposite-Based Chemiresistive Gas Sensors; Elsevier: Amsterdam, The Netherlands, 2023; pp. 169–203. [Google Scholar]
- Iqbal, S.; Khatoon, H.; Pandit, A.H.; Ahmad, S. Recent development of carbon based materials for energy storage devices. Mater. Sci. Energy Technol. 2019, 2, 417–428. [Google Scholar] [CrossRef]
- Ansaldo, A.; Bondavalli, P.; Bellani, S.; Del Rio Castillo, A.E.; Prato, M.; Pellegrini, V.; Pognon, G.; Bonaccorso, F. High-Power Graphene–Carbon Nanotube Hybrid Supercapacitors. ChemNanoMat 2017, 3, 436–446. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, D.; Yin, Z.; Yan, Q.; Zhang, H. Graphene and graphene-based materials for energy storage applications. Small 2014, 10, 3480–3498. [Google Scholar] [CrossRef]
- Li, X.; Zhi, L. Graphene hybridization for energy storage applications. Chem. Soc. Rev. 2018, 47, 3189–3216. [Google Scholar] [CrossRef]
- Shao, Q.; Wu, Z.S.; Chen, J. Two-dimensional materials for advanced Li-S batteries. Energy Storage Mater. 2019, 22, 284–310. [Google Scholar] [CrossRef]
- Ni, J.; Li, Y. Carbon nanomaterials in different dimensions for electrochemical energy storage. Adv. Energy Mater. 2016, 6, 1600278. [Google Scholar] [CrossRef]
- Kothandam, G.; Singh, G.; Guan, X.; Lee, J.M.; Ramadass, K.; Joseph, S.; Benzigar, M.; Karakoti, A.; Yi, J.; Kumar, P.; et al. Recent Advances in Carbon-Based Electrodes for Energy Storage and Conversion. Adv. Sci. 2023, 10, 2301045. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Owusu, K.A.; Xu, X.; Zhu, T.; Zhang, G.; Yang, W.; Mai, L. 1D Carbon-based nanocomposites for electrochemical energy storage. Small 2019, 15, 1902348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, T.; Feng, M.; Cui, Y.; Zhang, T.; Zhang, Y.; Feng, Y.; Zhang, Y.; Chi, Q.; Liu, X. Significantly improved energy storage performance of PVDF ferroelectric films by blending PMMA and filling PCBM. ACS Sustain. Chem. Eng. 2021, 9, 16291–16303. [Google Scholar] [CrossRef]
- Ye, L.; Ran, C.; Xie, Z.; Zhang, J.; Ma, S. Significantly Enhanced Energy Density of Polyvinylidene Fluoride/Polyimide-Based Nanocomposites by Core–Shell BaTiO3@ SiO2. Langmuir 2024, 40, 7710–7722. [Google Scholar] [CrossRef]
- Chen, D.; Liu, T.; Zhou, X.; Tjiu, W.C.; Hou, H. Electrospinning fabrication of high strength and toughness polyimide nanofiber membranes containing multiwalled carbon nanotubes. J. Phys. Chem. B 2009, 113, 9741–9748. [Google Scholar] [CrossRef]
- You, L.; Liu, B.; Hua, H.; Jiang, H.; Yin, C.; Wen, F. Energy storage performance of polymer-based dielectric composites with two-dimensional fillers. Nanomaterials 2023, 13, 2842. [Google Scholar] [CrossRef]
- Li, W.; Jiang, L.; Zhang, X.; Shen, Y.; Nan, C.W. High-energy-density dielectric films based on polyvinylidene fluoride and aromatic polythiourea for capacitors. J. Mater. Chem. A 2014, 2, 15803–15807. [Google Scholar] [CrossRef]
- Li, J.; Yin, J.; Yang, C.; Li, N.; Feng, Y.; Liu, Y.; Zhao, H.; Li, Y.; Zhu, C.; Yue, D.; et al. Enhanced dielectric performance and energy storage of PVDF-HFP-based composites induced by surface charged Al2O3. J. Polym. Sci. Part B Polym. Phys. 2019, 57, 574–583. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Sharma, J.D.; Chand, S.; Raina, K.K.; Kumar, R. Enhanced Electroactive Phases in Bi3.3La0.7Ti3O12-poly (vinylidene fluoride) composites with Improved Dielectric Properties. Solid State Commun. 2021, 326, 114176. [Google Scholar] [CrossRef]
- Okafor, P.A.; Huxel, B.; Iroh, J.O. Electrochemical behavior of multifunctional graphene–polyimide nanocomposite film in two different electrolyte solutions. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Oktay, B.; Türker, S.; Karataş, S.; Apohan, N. Multi-walled carbon nanotube reinforced polyimide composites. J. Turk. Chem. Soc. Sect. A Chem. 2018, 5, 283–294. [Google Scholar] [CrossRef][Green Version]
- Kim, B.S.; Bae, S.H.; Park, Y.H.; Kim, J.H. Polyimide/carbon nanotubes composite films: A potential for FPCB. In Proceedings of the 2006 International Conference on Nanoscience and Nanotechnology, Brisbane, Australia, 3–7 July 2006. [Google Scholar]
- Iroh, J.O.; Levine, K.; Shah, K.; Zhu, Y.; Donley, M.; Mantz, R.; Johnson, J.; Voevodin, N.N.; Balbyshev, V.N.; Khramov, A.N. Electrochemical behaviour of conducting polymer/polyimide composite. Surf. Eng. 2004, 20, 93–98. [Google Scholar] [CrossRef]
- So, H.H.; Cho, J.W.; Sahoo, N.G. Effect of carbon nanotubes on mechanical and electrical properties of polyimide/carbon nanotubes nanocomposites. Eur. Polym. J. 2007, 43, 3750–3756. [Google Scholar] [CrossRef]
- Thuau, D.; Koutsos, V.; Cheung, R. Electrical and mechanical properties of carbon nanotube-polyimide composites. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 2009, 27, 3139–3144. [Google Scholar] [CrossRef]
- Cerrada, M.L.; Arranz-Andrés, J.; Caballero-González, A.; Blázquez-Blázquez, E.; Pérez, E. The β form in PVDF nanocomposites with carbon nanotubes: Structural features and properties. Polymers 2023, 15, 1491. [Google Scholar] [CrossRef]
- Andezai, A.; Iroh, J.O. Influence of the Processing Conditions on the Rheology and Heat of Decomposition of Solution Processed Hybrid Nanocomposites and Implication to Sustainable Energy Storage. Energies 2024, 17, 3930. [Google Scholar] [CrossRef]
- Mohd Nurazzi, N.; Asyraf, M.M.; Khalina, A.; Abdullah, N.; Sabaruddin, F.A.; Kamarudin, S.H.; Ahmad, S.B.; Mahat, A.M.; Lee, C.L.; Aisyah, H.A.; et al. Fabrication, functionalization, and application of carbon nanotube-reinforced polymer composite: An overview. Polymers 2021, 13, 1047. [Google Scholar] [CrossRef]
- Martínez-Hernández, A.L.; Velasco-Santos, C.; Castano, V. Carbon nanotubes composites: Processing, grafting and mechanical and thermal properties. Curr. Nanosci. 2010, 6, 12–39. [Google Scholar] [CrossRef]
- Chazot, C.A.; Jons, C.K.; Hart, A.J. In situ interfacial polymerization: A technique for rapid formation of highly loaded carbon nanotube-polymer composites. Adv. Funct. Mater. 2020, 30, 2005499. [Google Scholar] [CrossRef]
- Taha, T.A.M.; Alanazi, S.S.; El-Nasser, K.S.; Alshammari, A.H.; Ismael, A. Structure–property relationships in PVDF/SrTiO3/CNT nanocomposites for optoelectronic and solar cell applications. Polymers 2024, 16, 736. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhai, J.; Wen, D.; Dong, S. Graphene oxide/polypyrrole nanocomposites: One-step electrochemical doping, coating and synergistic effect for energy storage. J. Mater. Chem. 2012, 22, 6300–6306. [Google Scholar] [CrossRef]
- Shah, S.S.; Niaz, F.; Ehsan, M.A.; Das, H.T.; Younas, M.; Khan, A.S.; Rahman, H.U.; Nayem, S.A.; Oyama, M.; Aziz, M.A. Advanced strategies in electrode engineering and nanomaterial modifications for supercapacitor performance enhancement: A comprehensive review. J. Energy Storage 2024, 79, 110152. [Google Scholar] [CrossRef]
- Gooneratne, R.; Iroh, J.O. Polypyrrole Modified Carbon Nanotube/Polyimide Electrode Materials for Supercapacitors and Lithium-ion Batteries. Energies 2022, 15, 9509. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, F.; Zhang, L.; Lu, Y.; Zhang, Y.; Yang, X.; Ma, Y.; Huang, Y. High energy density Li-ion capacitor assembled with all graphene-based electrodes. Carbon 2015, 92, 106–118. [Google Scholar] [CrossRef]
- Yang, C.; Ji, X.; Fan, X.; Gao, T.; Suo, L.; Wang, F.; Sun, W.; Chen, J.; Chen, L.; Han, F.; et al. Flexible aqueous Li-ion battery with high energy and power densities. Adv. Mater. 2017, 29, 1701972. [Google Scholar] [CrossRef]
- Ajuria, J.; Arnaiz, M.; Botas, C.; Carriazo, D.; Mysyk, R.; Rojo, T.; Talyzin, A.V.; Goikolea, E. Graphene-based lithium ion capacitor with high gravimetric energy and power densities. J. Power Sources 2017, 363, 422–427. [Google Scholar] [CrossRef]








| SWCNTs/PI-PPy Electrode | Specific Capacitance (F/g) | ||
|---|---|---|---|
| 5 mV/s | 10 mV/s | 25 mV/s | |
| 90 °C | 655.34 | 446.48 | 277.69 |
| 180 °C | 370.99 | 272.95 | 162.68 |
| 250 °C | 234.42 | 162.78 | 69.02 |
| SWCNTs/PI-PPy Electrode | Specific Capacities (mAh/g) | ||
|---|---|---|---|
| 5 mV/s | 10 mV/s | 25 mV/s | |
| 90 °C | 182.04 | 124.02 | 77.14 |
| 180 °C | 103.05 | 75.82 | 45.19 |
| 250 °C | 65.12 | 45.22 | 19.17 |
| PI/CNTs–PPy Electrode Material | Specific Capacitance (F/g) 0.5 A/g | Specific Capacity (mAh/g) |
|---|---|---|
| 90 °C | 265.98 | 73.88 |
| 180 °C | 134.58 | 37.38 |
| 250 °C | 112.65 | 31.29 |
| Material | Bulk Resistance (Ω) (EIS) | Weigh Gain (%) | Specific Capacitance (F/g) (EIS) | Porosity (EIS) |
|---|---|---|---|---|
| PI/SWCNTs (90 °C) | 65.27 | 1.669 (0.003287 g) | 209.16 (23.15 Ω) | 4.59 |
| PI/SWCNTs (180 °C) | 2366.4 | 1.313 (0.002284 g) | 5.40 (1236 Ω) | 0.35 |
| PI/SWCNTs (250 °C) | 143.62 | 0.567 (0.004875 g) | 123.90 (26.35 Ω) | 1.52 |
| Material | Solution Resistance (Ω) | Charge Transfer Resistance (Ω) | Pore Resistance (Ω) | Coating Resistance (Ω) |
|---|---|---|---|---|
| PI/SWCNTs (90 °C) | 2.307 | 67.58 | 301.8 | 94 |
| PI/SWCNTs (180 °C) | 87.67 | 2465 | 13,930 | 8700 |
| PI/SWCNTs (250 °C) | 51.8 | 194.79 | 2991.9 | 400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andezai, A.; Iroh, J.O. Polypyrrole Hybrid Nanocomposite Electrode Materials with Outstanding Specific Capacitance. Energies 2025, 18, 1304. https://doi.org/10.3390/en18051304
Andezai A, Iroh JO. Polypyrrole Hybrid Nanocomposite Electrode Materials with Outstanding Specific Capacitance. Energies. 2025; 18(5):1304. https://doi.org/10.3390/en18051304
Chicago/Turabian StyleAndezai, Andekuba, and Jude O. Iroh. 2025. "Polypyrrole Hybrid Nanocomposite Electrode Materials with Outstanding Specific Capacitance" Energies 18, no. 5: 1304. https://doi.org/10.3390/en18051304
APA StyleAndezai, A., & Iroh, J. O. (2025). Polypyrrole Hybrid Nanocomposite Electrode Materials with Outstanding Specific Capacitance. Energies, 18(5), 1304. https://doi.org/10.3390/en18051304







