Enhancing the Structural and Electrochemical Properties of Lithium Iron Phosphate via Titanium Doping During Precursor Synthesis
Abstract
1. Introduction
2. Experimental
2.1. Material Synthesis
2.1.1. Preparation of Ferrous Sulfate Solution
2.1.2. Preparation of Ammonium Dihydrogen Phosphate Solution
2.1.3. Preparation of Titanium-Doped Iron Phosphate with Different Titanium Concentrations
2.1.4. Preparation of Titanium-Doped Lithium Iron Phosphate
2.2. Materials Characterization
2.3. Electrochemical Measurements
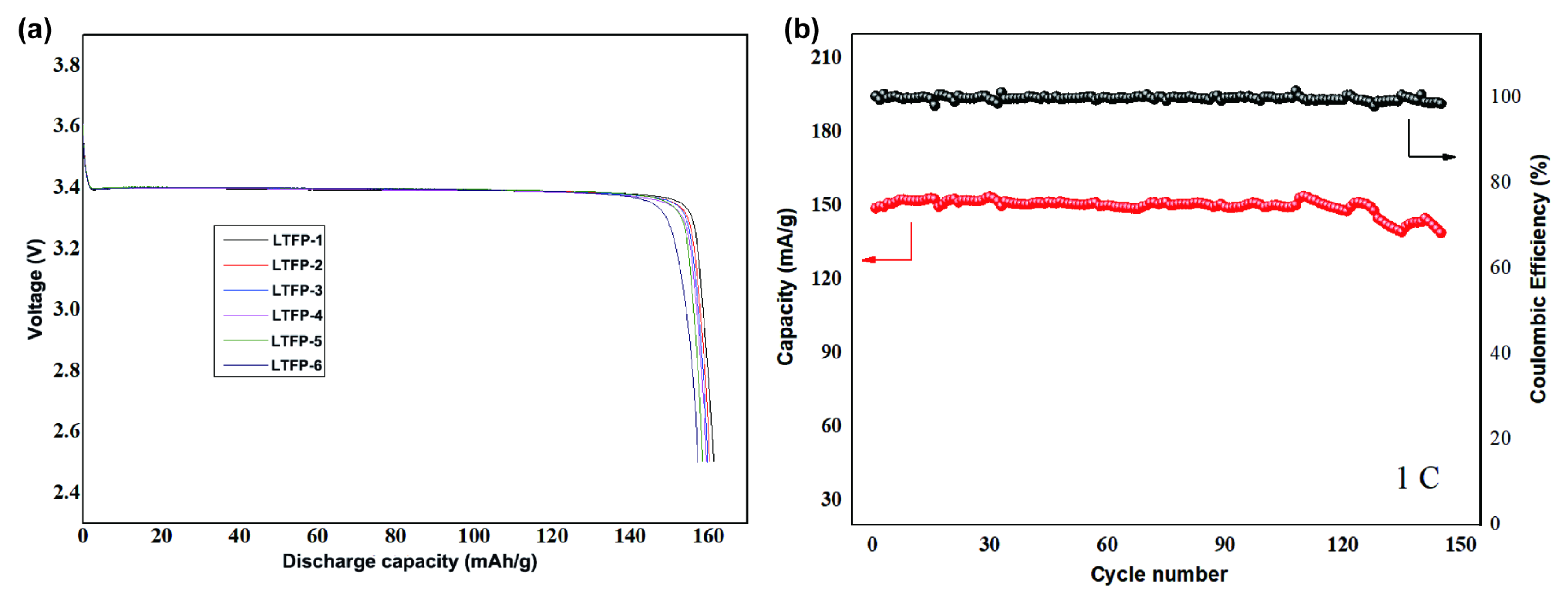
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cao, Z.; Ma, B.; Jing, Q.; Xing, P.; Liu, B.; Wang, C. Facile and inexpensive preparation method of iron phosphate from laterite residue. Ceram. Int. 2020, 46, 11304–11310. [Google Scholar] [CrossRef]
- Yang, S.; Zavalij, P.Y.; Whittingham, M.S. Stanley Whittingham. Hydrothermal synthesis of lithium iron phosphate cathodes. Electrochem. Commun. 2001, 3, 505–508. [Google Scholar] [CrossRef]
- Sugiyama, S.; Ichii, T.; Fujisawa, M.; Kawashiro, K.; Tomida, T.; Shigemoto, N.; Hayashi, H. Heavy metal immobilization in aqueous solution using calcium phosphate and calcium hydrogen phosphates. J. Colloid Interface Sci. 2003, 259, 408–410. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; He, W.; Wang, M.; Zhang, X.; Li, P.; Yan, S.; Tian, X.; Sun, X.; Han, X. Biosynthesis and characterization of layered iron phosphate. Smart Mater. Struct. 2008, 17, 065034. [Google Scholar] [CrossRef]
- Syutkin, S.A.; Pervushin, V.Y.; Kuznetsov, G.F.; Kleshchev, D.G.; Zherebtsov, D.A.; German, V.A.; Viktorov, V.V.; Kolmogorov, A.M.; Serikov, A.S. Transformations of hydrated titanium dioxide in hydrothermal treatment. Russ. J. Appl. Chem. 2010, 83, 1209–1214. [Google Scholar] [CrossRef]
- Davidson, M.; Ngo, C.; Gage, S.H.; Pylypenko, S.; Trewyn, B.G. Direct synthesis of Fe rich SBA-15 at low pH by in-situ formation of iron phosphate phase. Microporous Mesoporous Mater. 2019, 276, 270–279. [Google Scholar] [CrossRef]
- Dean, J.A. Lange’s Handbook of Chemistry. Mater. Manuf. Process. 1990, 5, 687–688. [Google Scholar] [CrossRef]
- Deng, L.; Ma, G.; Chen, Q. Preparation of Iron Phosphate Battery Materials from Industrial Ferrous Sulfate Waste by Liquid Phase Method. Integr. Ferroelectr. 2023, 234, 67–78. [Google Scholar] [CrossRef]
- Barbara, G.; Daniel, G.; Bogumi, K. Effects of processing parameters on hydrolysis of TiOSO4. Pol. J. Chem. Technol. 2009, 11, 15–21. [Google Scholar]
- Hsieh, C.-T.; Chen, I.L.; Chen, W.-Y.; Wang, J.-P. Synthesis of iron phosphate powders by chemical precipitation route for high-power lithium iron phosphate cathodes. Electrochim. Acta 2012, 83, 202–208. [Google Scholar] [CrossRef]
- Jiang, T.; Tu, Y.; Su, Z.; Lu, M.; Liu, S.; Liu, J.; Gu, F.; Zhang, Y. A novel value-added utilization process for pyrite cinder: Selective recovery of Cu/Co and synthesis of iron phosphate. Hydrometallurgy 2020, 193, 105314. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, G.; Zhou, K.; Peng, C.; Salih, K.A.M.; Zhou, H.; Wu, Y.; Chen, W. Sequential separation and recovery of phosphorus and lithium from lithium phosphate slag by selective extraction-precipitation. Sep. Purif. 2024, 333, 125907. [Google Scholar] [CrossRef]
- Kim, J.; Li, W.; Philips, B.L.; Grey, C.P. Phosphate adsorption on the iron oxyhydroxides goethite (α-FeOOH), akaganeite (β-FeOOH), and lepidocrocite (γ-FeOOH): A 31P NMR Study. Energy Environ. Sci. 2011, 4, 4298–4305. [Google Scholar] [CrossRef]
- Lou, W.-B.; Zhang, Y.; Zhang, Y.; Zheng, S.-L.; Sun, P.; Wang, X.-J.; Li, J.-Z.; Qiao, S.; Zhang, Y.; Wenzel, M.; et al. Leaching performance of Al-bearing spent LiFePO4 cathode powder in H2SO4 aqueous solution. Trans. Nonferrous Met. Soc. 2021, 31, 817–831. [Google Scholar] [CrossRef]
- Jin, J.; Liu, X.; Yuan, S.; Gao, P.; Li, Y.; Zhang, H.; Meng, X. Innovative utilization of red mud through co-roasting with coal gangue for separation of iron and aluminum minerals. J. Ind. Eng. Chem. 2021, 98, 298–307. [Google Scholar] [CrossRef]
- Jiang, Y.; Peng, C.; Zhou, K.; Hu, Z.; Zhang, G.; Wu, Y.; Zhang, J.; Chen, W. Recovery of iron from titanium white waste for the preparation of LiFePO4 battery. J. Clean. Prod. 2023, 415, 137817. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, T.-A.; Cai, L.; Lv, G.; Cao, X. Preparation of Doped Iron Phosphate by Selective Precipitation of Iron from Titanium Dioxide Waste Acid. Metals 2020, 10, 789. [Google Scholar] [CrossRef]
- Zherebtsov, D.A.; Syutkin, S.A.; Pervushin, V.Y.; Kuznetsov, G.F.; Kleshchev, D.G.; German, V.A.; Viktorov, V.V.; Kolmogortsev, A.M.; Serikov, A.S. Characteristics of the hydrous titanium dioxide-anatase phase transformation during hydrothermal treatment in aqueous solutions. Russ. J. Inorg. Chem. 2010, 55, 1197–1201. [Google Scholar] [CrossRef]
- Zhu, Y.-M.; Ruan, Z.-W.; Tang, S.-Z.; Thangadurai, V. Research status in preparation of FePO4: A review. Ionics 2014, 20, 1501–1510. [Google Scholar] [CrossRef]
- Lundager Madsen, H.E.; Koch, C.B. Kinetics of solution crystal growth of strengite, FePO4,2H2O. J. Cryst. Growth 2018, 482, 9–14. [Google Scholar] [CrossRef]
- Xiao, C.; Xiao, L.; Gao, C.; Zeng, L. Thermodynamic study on removal of magnesium from lithium chloride solutions using phosphate precipitation method. Sep. Purif. 2015, 156, 582–587. [Google Scholar] [CrossRef]
- He, L.; Xu, W.; Song, Y.; Liu, X.; Zhao, Z. Selective removal of magnesium from a lithium-concentrated anolyte by magnesium ammonium phosphate precipitation. Sep. Purif. 2017, 187, 214–220. [Google Scholar] [CrossRef]
- Kumar, N.; Bhardwaj, N.K.; Chakrabarti, S.K.; Kumar, S. Synthesis and application of calcium sulphate pigment for paper coating: Potential and prospects. Powder Technol. 2012, 218, 40–45. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Liu, J.; Banis, M.N.; Xiao, B.; Lushington, A.; Xiao, W.; Li, R.; Sham, T.-K.; Liang, G.; et al. Origin of phase inhomogeneity in lithium iron phosphate during carbon coating. Nano Energy 2018, 45, 52–60. [Google Scholar] [CrossRef]
- Zhao, C.X.; Liu, J.N.; Li, B.Q.; Ren, D.; Chen, X.; Yu, J.; Zhang, Q. Multiscale Construction of Bifunctional Electrocatalysts for Long-Lifespan Rechargeable Zinc–Air Batteries. Adv. Funct. 2020, 30, 2003619. [Google Scholar] [CrossRef]
- Xiao, C.; Zeng, L.; Wei, J.; Xiao, L.; Zhang, G. Thermodynamic analysis for the separation of tungsten and aluminium in alkaline medium using solvent extraction. Hydrometallurgy 2017, 174, 91–96. [Google Scholar] [CrossRef]
- Zou, W.; Feng, X.; Wang, R.; Wei, W.; Luo, S.; Zheng, R.; Yang, D.; Mi, H.; Chen, H. High-efficiency core-shell magnetic heavy-metal absorbents derived from spent-LiFePO4 Battery. J. Hazard. Mater. 2021, 402, 123583. [Google Scholar] [CrossRef]
- Chen, T.; Qiu, C.; Zhang, X.; Wang, H.; Song, J.; Zhang, K.; Yang, T.; Zuo, Y.; Yang, Y.; Gao, C.; et al. An Ultrasmall Ordered High-Entropy Intermetallic with Multiple Active Sites for the Oxygen Reduction Reaction. J. Am. Chem. Soc. 2023, 146, 1174–1184. [Google Scholar] [CrossRef]
- PDF#29-0715; Iron Phosphate. International Center for Diffraction Data: Newtown Square, PA, USA, 2016.
- PDF#40-1499; Lithium Iron Phosphate. International Center for Diffraction Data: Newtown Square, PA, USA, 2016.
- Zhang, C.; Liang, Y.; Yao, L.; Qiu, Y. Synthesis and characterization of LiFePO4-carbon nanofiber with Ti4+ substitution by electrospinning and thermal treatment. Solid State Ionics 2014, 267, 74–79. [Google Scholar] [CrossRef]
- Li, L.; Li, X.; Wang, Z.; Wu, L.; Zheng, J.; Guo, H. Stable cycle-life properties of Ti-doped LiFePO4 compounds synthesized by co-precipitation and normal temperature reduction method. J. Phys. Chem. Solids 2009, 70, 238–242. [Google Scholar] [CrossRef]




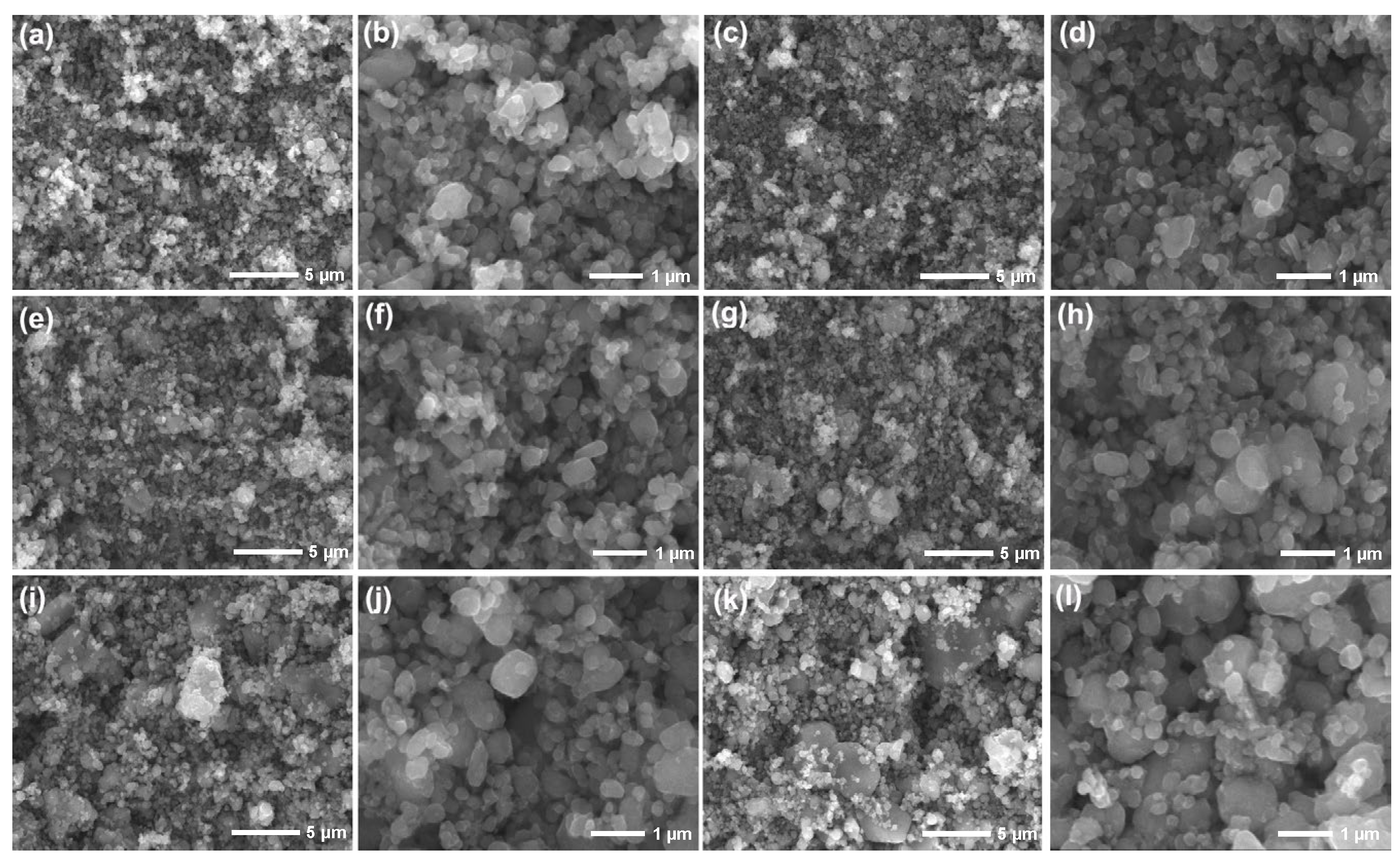
| Sample | TFP-1 | TFP-2 | TFP-3 | TFP-4 | TFP-5 | TFP-6 | |
|---|---|---|---|---|---|---|---|
| Fe % | 36.25 | 36.26 | 36.24 | 36.21 | 36.20 | 36.18 | |
| P % | 20.86 | 20.8 | 20.76 | 20.66 | 20.67 | 20.65 | |
| Fe/P | 0.9638 | 0.9668 | 0.9681 | 0.9720 | 0.9711 | 0.9717 | |
| Ti ppm | 948.4 | 2560 | 3599 | 4589 | 5409 | 6740 | |
| S ppm | 136 | 109 | 81 | 187 | 81 | 96 | |
| specific surface area (m2/g) | 5.661 | 6.836 | 6.759 | 5.527 | 5.265 | 6.389 | |
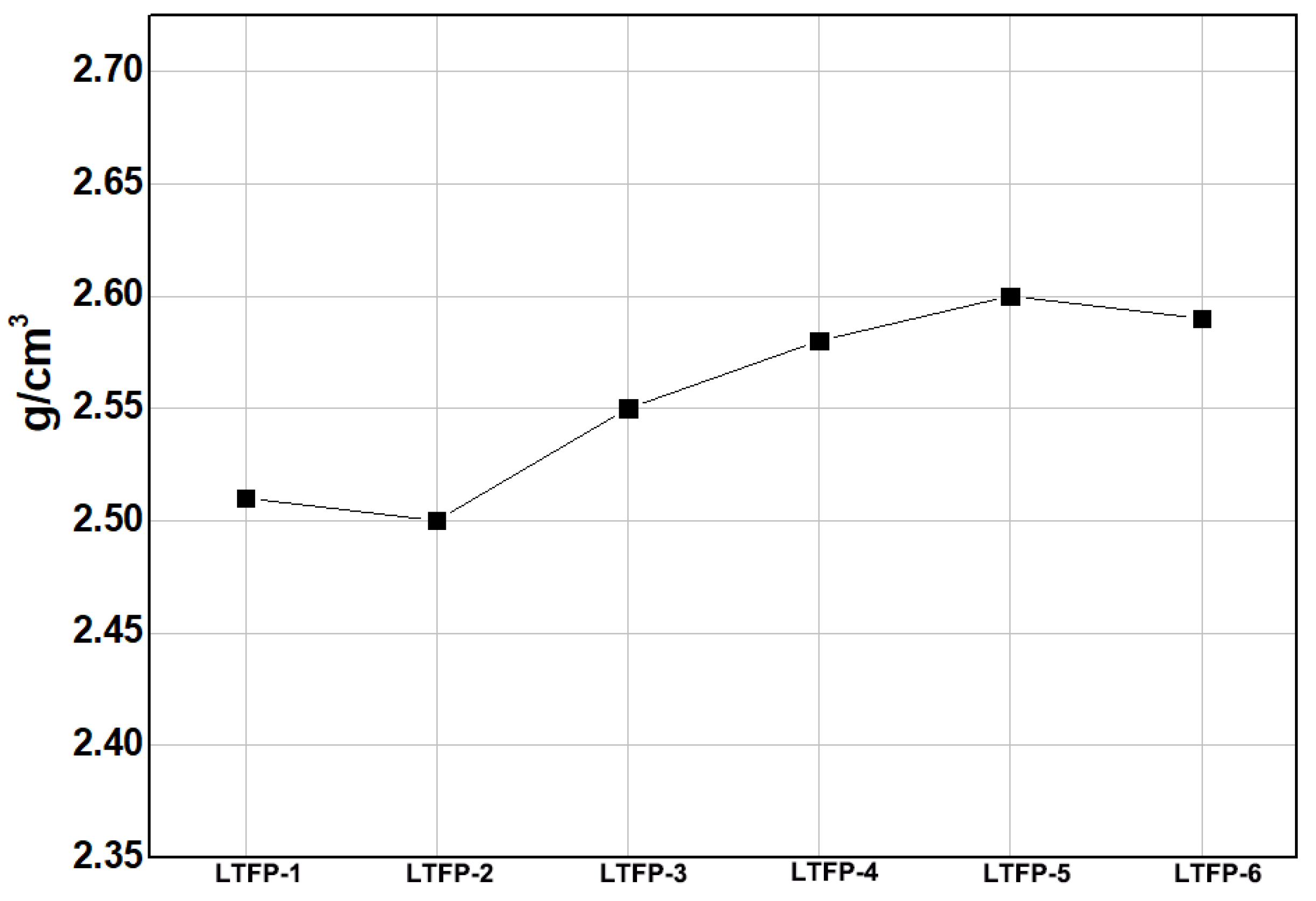
| tap density (g/cm3) | 0.58 | 0.57 | 0.64 | 0.73 | 0.74 | 0.72 | |
| pH | 3.29 | 3.02 | 2.94 | 3.02 | 3.23 | 3.3 | |
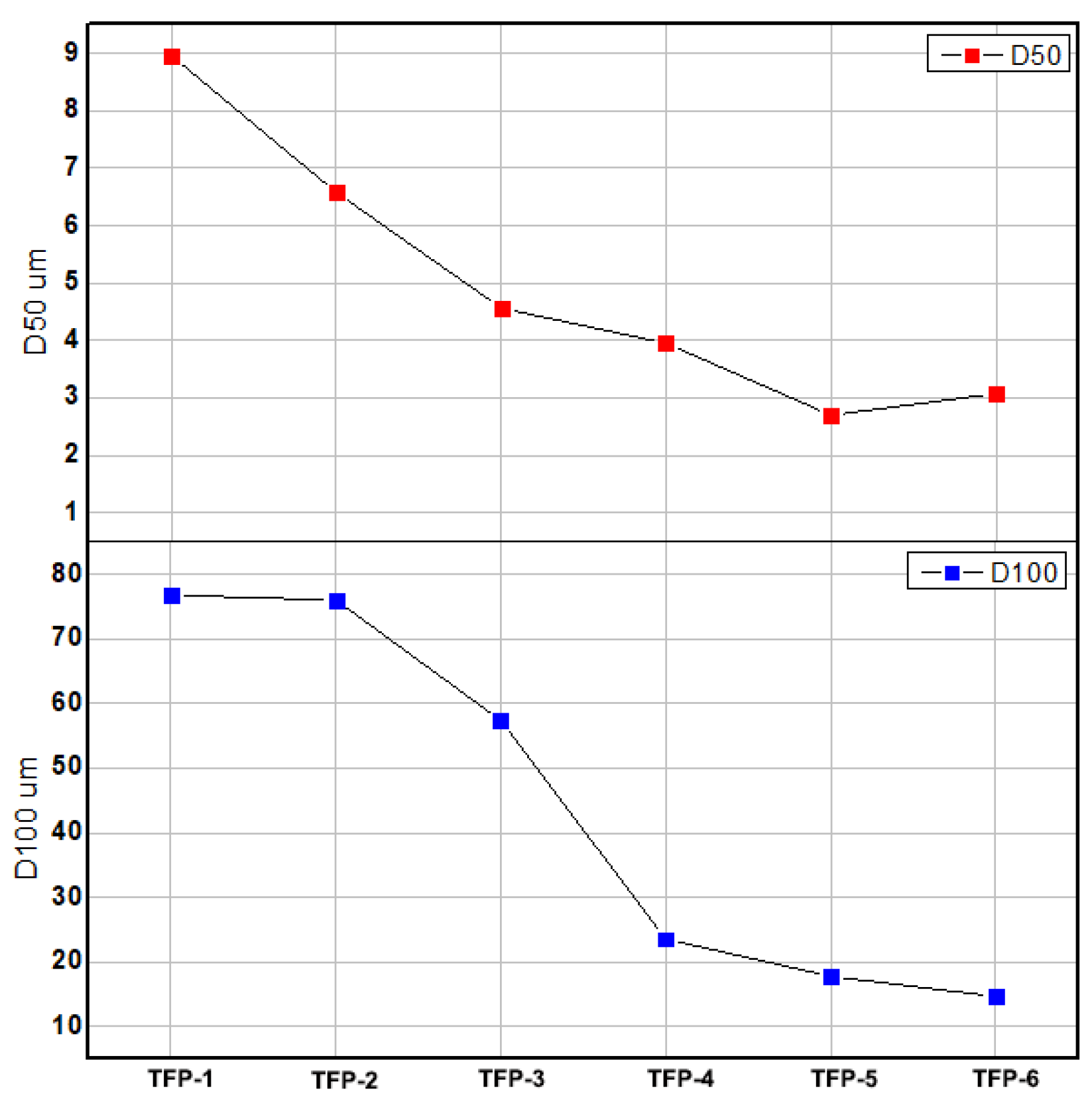
| particle size (um) | D50 | 8.97% | 6.58% | 4.567% | 3.945% | 2.7% | 3.07% |
| D100 | 76.787% | 76.007% | 57.366% | 23.48% | 17.69% | 14.629% | |
| impurity element (ppm) | Al | 1.35 | 3.106 | 3.154 | 1.573 | 2.266 | 1.94 |
| Ca | 4.459 | 2.859 | 3.538 | 16.35 | 17.36 | 25.17 | |
| Cr | 15.71 | 17.16 | 15.07 | 17.77 | 13.47 | 15.89 | |
| Cu | 0 | 0 | 0 | 0 | 0 | 0.717 | |
| Na | 3.962 | 0 | 0 | 0 | 0 | 5.673 | |
| K | 21.22 | 16.91 | 14.58 | 4.601 | 3.954 | 19.01 | |
| Pb | 9.692 | 11.42 | 9.002 | 10.88 | 9.237 | 11.09 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Wang, Y.; Liu, W.; Chen, T.; Liu, K. Enhancing the Structural and Electrochemical Properties of Lithium Iron Phosphate via Titanium Doping During Precursor Synthesis. Energies 2025, 18, 930. https://doi.org/10.3390/en18040930
Li P, Wang Y, Liu W, Chen T, Liu K. Enhancing the Structural and Electrochemical Properties of Lithium Iron Phosphate via Titanium Doping During Precursor Synthesis. Energies. 2025; 18(4):930. https://doi.org/10.3390/en18040930
Chicago/Turabian StyleLi, Puliang, Yang Wang, Weifang Liu, Tao Chen, and Kaiyu Liu. 2025. "Enhancing the Structural and Electrochemical Properties of Lithium Iron Phosphate via Titanium Doping During Precursor Synthesis" Energies 18, no. 4: 930. https://doi.org/10.3390/en18040930
APA StyleLi, P., Wang, Y., Liu, W., Chen, T., & Liu, K. (2025). Enhancing the Structural and Electrochemical Properties of Lithium Iron Phosphate via Titanium Doping During Precursor Synthesis. Energies, 18(4), 930. https://doi.org/10.3390/en18040930




