Freezing Method Assists Peracetic Acid Oxidation for Promoting the Methane Production from Sludge Anaerobic Digestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate and Oxidant Used
2.2. Sludge Pretreatment Procedure and Anaerobic Digestion Experiment
2.3. Kinetic Model-Based Analysis
2.3.1. Modified Gompertz Model
2.3.2. First-Order Kinetic Model
2.4. Analytical Methods
3. Results and Discussion
3.1. Determination of the Optimal Parameters of Combined Freezing and PAA Pretreatment
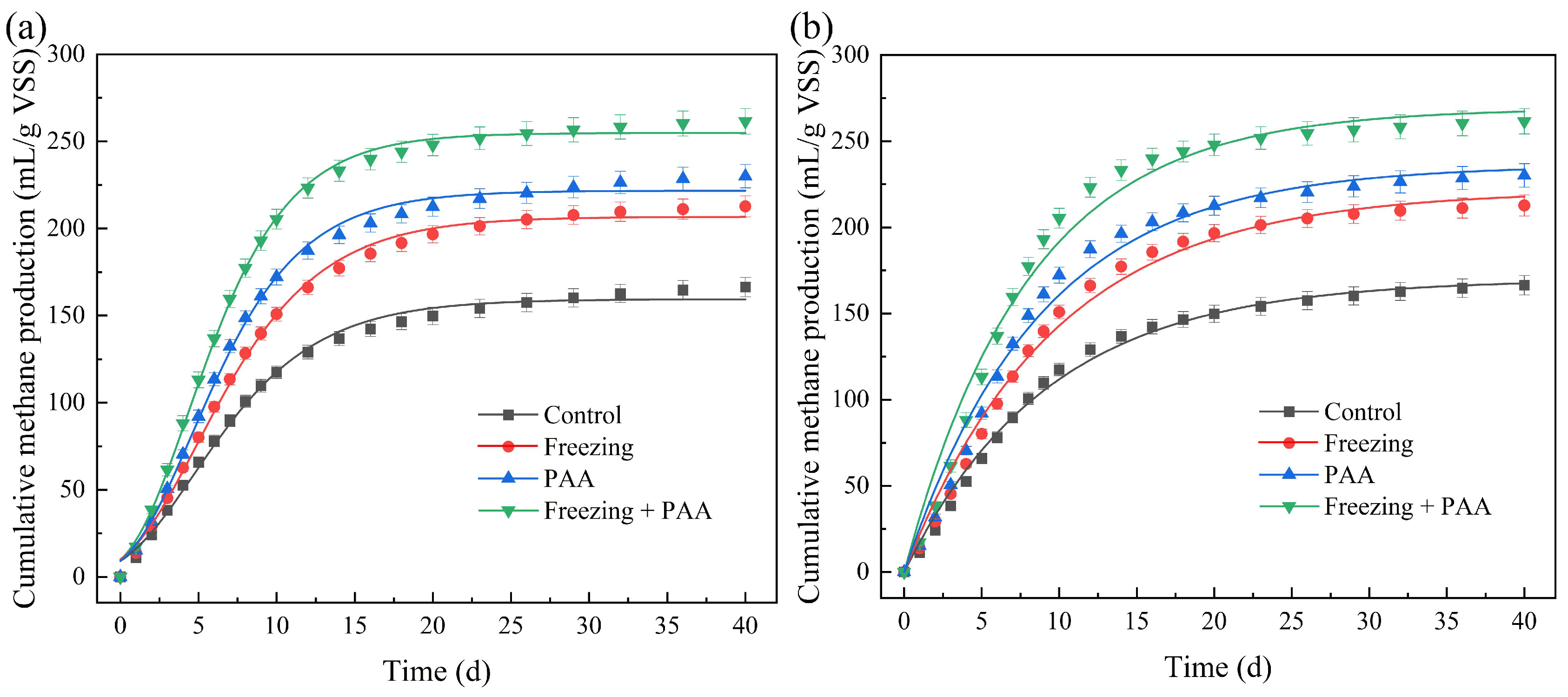
3.2. The Synergistic Effect of Combined Freezing and PAA Pretreatment on Methane Generation
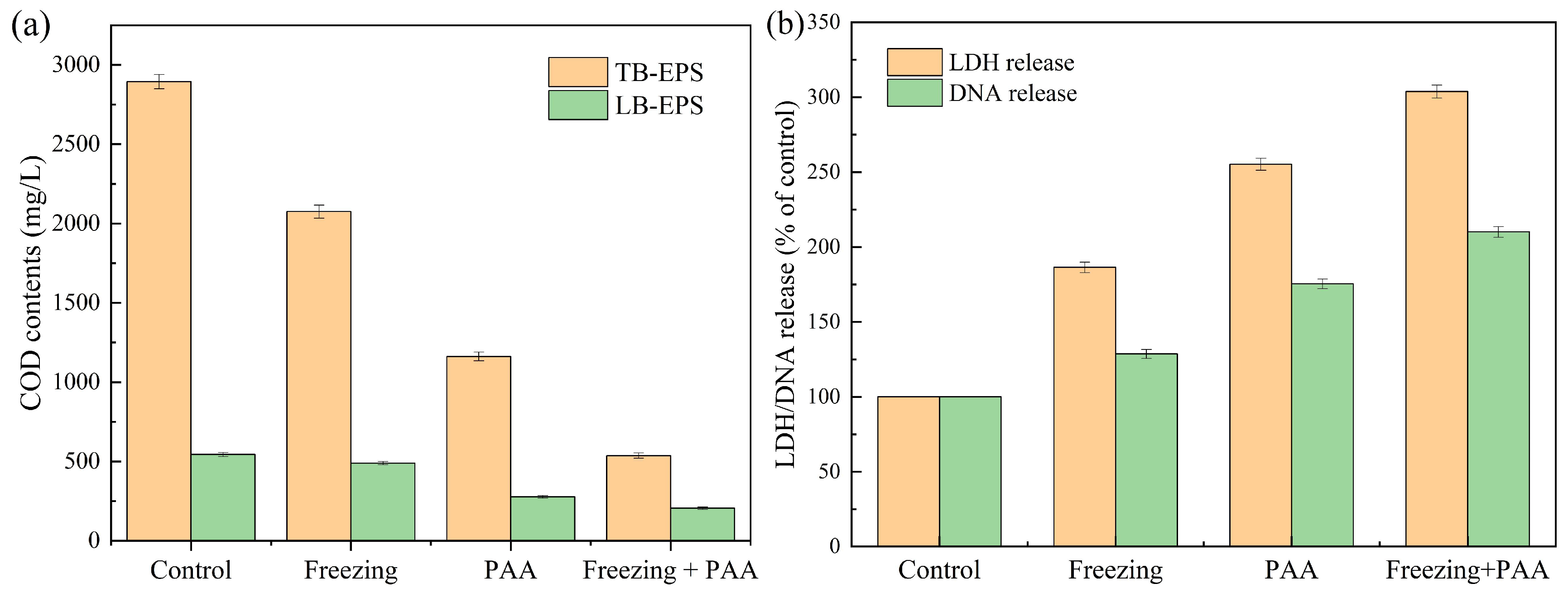
3.3. Effect of Combined Freezing and PAA Pretreatment on Sludge Hydrolysis Efficiency
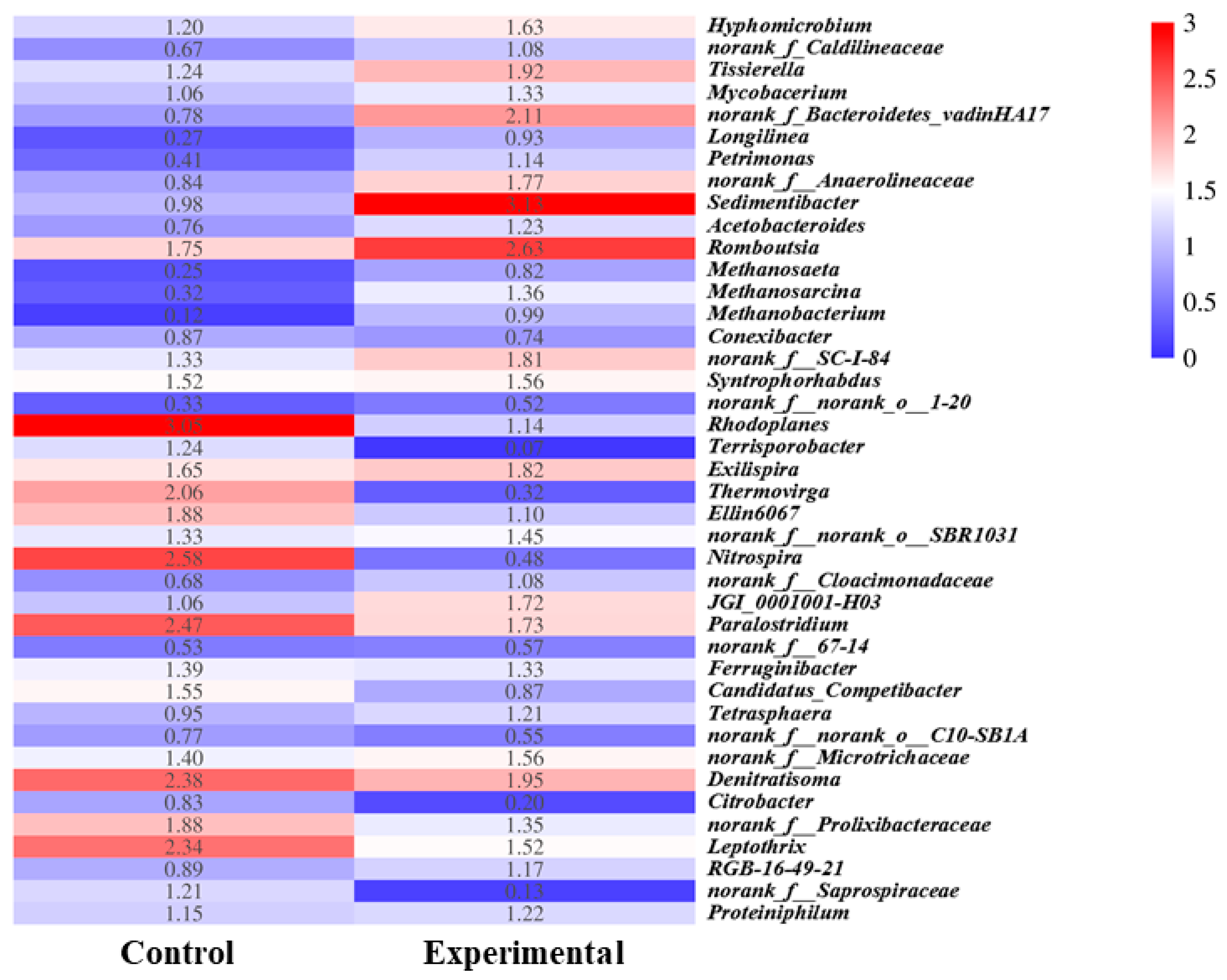
3.4. Influence of Combined Freezing and PAA Pretreatment on Microbial Community
3.5. Economic Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Liew, C.S.; Yunus, N.M.; Chidi, B.S.; Lam, M.K.; Goh, P.S.; Mohamad, M.; Sin, J.C.; Lam, S.M.; Lim, J.W.; Lam, S.S. A review on recent disposal of hazardous sewage sludge via anaerobic digestion and novel composting. J. Hazard. Mater. 2022, 423, 126995. [Google Scholar] [CrossRef]
- Wei, J.; Hao, X.; van Loosdrecht, M.C.M.; Li, J. Feasibility analysis of anaerobic digestion of excess sludge enhanced by iron: A review. Renew. Sust. Energ. Rev. 2018, 89, 16–26. [Google Scholar] [CrossRef]
- Drewnowski, J.; Makinia, J. Modeling Hydrolysis of Slowly Biodegradable Organic Compounds in Biological Nutrient Removal Activated Sludge Systems. Water Sci. Technol. 2013, 67, 2067–2074. [Google Scholar] [CrossRef] [PubMed]
- Miron, Y.; Zeeman, G.; van Lier, J.B.; Lettinga, G. The role of sludge retention time in the hydrolysis and acidification of lipids, carbohydrates and proteins during digestion of primary sludge in CSTR systems. Water Res. 2000, 34, 1705–1713. [Google Scholar] [CrossRef]
- Wang, X.; Gao, C.; Qi, X.; Zhang, Y.; Chen, T.; Xie, Y.; Zhang, A.; Gao, J. Enhancing sludge fermentation and anaerobic digestion by mechanical cutting pretreatment. J. Water Process Eng. 2021, 40, 101812. [Google Scholar] [CrossRef]
- Żubrowska-Sudoł, M.; Podedworna, J.; Sytek-Szmeichel, K.; Bisak, A.; Krawczyk, P.; Garlicka, A. The effects of mechanical sludge disintegration to enhance full-scale anaerobic digestion of municipal sludge. Therm. Sci. Eng. Prog. 2018, 5, 289–295. [Google Scholar] [CrossRef]
- Gao, Q.; Li, L.; Zhang, Y.; Zhou, H.; Jiang, J.; Wei, L.; Wang, G.; Ding, J.; Zhao, Q. Advanced oxidation processes (AOPs)-based sludge pretreatment techniques for enhanced short-chain fatty acids production: A critical review. Chem. Eng. J. 2024, 489, 151496. [Google Scholar] [CrossRef]
- Tulun, S.; Bilgin, M. Enhancement of anaerobic digestion of waste activated sludge by chemical pretreatment. Fuel 2019, 254, 115671. [Google Scholar] [CrossRef]
- Bhat, A.P.; Gogate, P.R. Cavitation-based pre-treatment of wastewater and waste sludge for improvement in the performance of biological processes: A review. J. Environ. Chem. Eng. 2021, 9, 104743. [Google Scholar] [CrossRef]
- Banach, J.L.; van Bokhorst-van de Veen, H.; van Overbeek, L.S.; van der Zouwen, P.S.; Zwietering, M.H.; van der Fels-Klerx, H.J. Effectiveness of a peracetic acid solution on Escherichia coli reduction during fresh-cut lettuce processing at the laboratory and industrial scales. Int. J. Food Microbiol. 2020, 321, 108537. [Google Scholar] [CrossRef]
- Nicolau-Lapeña, I.; Abadias, M.; Viñas, I.; Bobo, G.; ás Lafarg, T.; Ribas-Agustí, A.; Aguiló-Aguayo, I. Water UV-C treatment alone or in combination with peracetic acid: A technology to maintain safety and quality of strawberries. Int. J. Food Microbiol. 2020, 335, 108887. [Google Scholar] [CrossRef]
- Appels, L.; Assche, A.V.; Willems, K.; Degreve, J.; Impe, J.V.; Dewil, R. Peracetic acid oxidation as an alternative pre-treatment for the anaerobic digestion of waste activated sludge. Bioresour. Technol. 2011, 102, 4124–4130. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, A.; Liu, H.; Wang, S.; Liu, W.; Wang, A.; Yue, X. Extracellular polymeric substance decomposition linked to hydrogen recovery from waste activated sludge: Role of peracetic acid and free nitrous acid co-pretreatment in a prefermentation-bioelectrolysis cascading system. Water Res. 2020, 176, 115724. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.Y.; Sun, J.P.; Li, S.F.; Sun, J.; Liu, X.M.; Wang, A.Q. Effect of microwaves combined with peracetic acid to improve the dewatering performance of residual sludge. Environ. Sci. Pollut. Res. 2024, 31, 44885–44899. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, X.; Du, M.; Yang, J.; Lu, Q.; Fu, Q.; He, D.; Zhao, J.; Wang, D. Peracetic acid promotes biohydrogen production from anaerobic dark fermentation of waste activated sludge. Sci. Total Environ. 2022, 844, 156991. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, H.; Li, X.; Chen, X.; Peng, L.; Zhu, T.; Sun, P.; Liu, Y. Peracetic acid (PAA)-based pretreatment effectively improves medium-chain fatty acids (MCFAs) production from sewage sludge. Env. Sci. Ecotechnol. 2024, 20, 100355. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Qiao, M.; Xu, Y.; Ma, C.; Zhang, X. Pretreatment of Waste Activated Sludge by Peracetic Acid Oxidation for Enhanced Anaerobic Digestion. Environ. Prog. Sustain. Energy 2018, 37, 2058–2062. [Google Scholar] [CrossRef]
- Ren, W.; Zhang, Y.; Liu, X.; Li, S.; Li, H.; Zhai, Y. Peracetic acid pretreatment improves biogas production from anaerobic digestion of sewage sludge by promoting organic matter release, conversion and affecting microbial community. J. Environ. Manag. 2024, 349, 119427. [Google Scholar] [CrossRef]
- Hu, K.; Jiang, J.-Q.; Zhao, Q.-L.; Lee, D.-J.; Wang, K.; Qiu, W. Conditioning of wastewater sludge using freezing and thawing: Role of curing. Water Res. 2011, 45, 5969–5976. [Google Scholar] [CrossRef] [PubMed]
- Phalakornkule, C.; Nuchdang, S.; Khemkhao, M.; Mhuantong, W.; Wongwilaiwalin, S.; Tangphatsornruang, S.; Champreda, V.; Kitsuwan, J.; Vatanyoopaisarn, S. Effect of freeze-thaw process on physical properties, microbial activities and population structures of anaerobic sludge. J. Biosci. Bioeng. 2017, 123, 474–481. [Google Scholar] [CrossRef]
- She, Y.; Hong, J.; Zhang, Q.; Chen, B.-Y.; Wei, W.; Xin, X. Revealing microbial mechanism associated with volatile fatty acids production in anaerobic acidogenesis of waste activated sludge enhanced by freezing/thawing pretreatment. Bioresour. Technol. 2020, 302, 122869. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chu, S.; Su, P.; Guo, R.; Duan, Y.; Wang, Y. Freezing method assists calcium hypochlorite for synergistically promoting methane production from sludge anaerobic digestion. J. Environ. Manag. 2022, 324, 116243. [Google Scholar] [CrossRef]
- Hu, J.; Li, Z.; Wu, Z.; Tao, W. Potassium ferrate coupled with freezing method enhances methane production from sludge anaerobic digestion. Bioresour. Technol. 2021, 332, 125112. [Google Scholar] [CrossRef]
- Wu, Y.; Song, K.; Sun, X.; Ngo, H.; Guo, W.; Nghiem, L.; Wang, Q. Mechanisms of free nitrous acid and freezing co-pretreatment enhancing short-chain fatty acids production from waste activated sludge anaerobic fermentation. Chemosphere 2019, 230, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, X.; Wu, Y.; Xu, Q.; Du, M.; Wang, D.; Yang, Q.; Liu, Y.; Ni, B.; Yang, G.; et al. Activation of nitrite by freezing process for anaerobic digestion enhancement of waste activated sludge: Performance and mechanisms. Chem. Eng. J. 2020, 387, 124147. [Google Scholar] [CrossRef]
- Xu, Q.X.; Luo, T.Y.; Wu, R.L.; Wei, W.; Sun, J.; Dai, X.H.; Ni, B.J. Rhamnolipid pretreatment enhances methane production from two-phase anaerobic digestion of waste activated sludge. Water Res. 2021, 194, 116909. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Gliniewicz, K.; Mendes-Soares, H. Comparative analysis of microbial community of novel lactic acid fermentation inoculated with different undefined mixed cultures. Bioresour. Technol. 2015, 179, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Guo, B.; Li, Z.; Eshtiaghi, N.; Tao, W. Revealing the mechanisms for potassium ferrate affecting methane production from anaerobic digestion of waste activated sludge. Bioresour. Technol. 2020, 317, 124022. [Google Scholar] [CrossRef] [PubMed]
- Dhar, B.R.; Nakhla, G.; Ray, M.B. Techno-economic evaluation of ultrasound and thermal pretreatments for enhanced anaerobic digestion of municipal waste activated sludge. Waste Manag. 2012, 32, 542–549. [Google Scholar] [CrossRef]
- Chi, Y.Z.; Li, Y.Y.; Fei, X.N.; Wang, S.P.; Yuan, H.Y. Enhancement of thermophilic anaerobic digestion of thickened waste activated sludge by combined microwave and alkaline pretreatment. J. Environ. Sci. 2011, 23, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; De Wilde, V. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef]
- Choi, J.; Han, S.; Lee, C. Enhancement of methane production in anaerobic digestion of sewage sludge by thermal hydrolysis pretreatment. Bioresour. Technol. 2018, 259, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Lee, J. Ultrasonic sludge disintegration for enhanced methane production in anaerobic digestion: Effects of sludge hydrolysis efficiency and hydraulic retention time. Bioprocess Biosyst. Eng. 2012, 35, 289–296. [Google Scholar] [CrossRef]
- Tang, Y.; Dai, X.; Dong, B.; Guo, Y.; Dai, L. Humification in extracellular polymeric substances (EPS) dominates methane release and EPS reconstruction during the sludge stabilization of high-solid anaerobic digestion. Water Res. 2020, 175, 115686. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Zhu, M.J. Influences of bisphenol A on hydrogen production from food waste by thermophilic dark fermentation. Environ. Res. 2024, 260, 119625. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, T.; Huang, Z.; Liu, Y.; Hong, J. Sulfur-Doped Graphene-Activated Perdisulfate for Synergetic Destruction of Bisphenol A and Complex Microbial Flora. Catal. Lett. 2023, 153, 2057–2073. [Google Scholar] [CrossRef]
- Zhang, W.J.; Cao, B.B.; Wang, D.S.; Ma, T.; Xia, H.; Yu, D.H. Influence of wastewater sludge treatment using combined peroxyacetic acid oxidation and inorganic coagulants re-flocculation on characteristics of extracellular polymeric substances (EPS). Water Res. 2016, 88, 728–739. [Google Scholar] [CrossRef]
- Davis, K.J.; Lu, S.; Barnhart, E.P.; Parker, A.E.; Fields, M.W.; Gerlach, R. Type and amount of organic amendments affect enhanced biogenic methane production from coal and microbial community structure. Fuel 2018, 211, 600–608. [Google Scholar] [CrossRef]
- Maspolim, Y.; Zhou, Y.; Guo, C.; Xiao, K.; Ng, W.J. The effect of pH on solubilization of organic matter and microbial community structures in sludge fermentation. Bioresour. Technol. 2015, 190, 289–298. [Google Scholar] [CrossRef]
- Hu, J.; Hu, S.; Zhang, J.; Xu, X.; Wu, J. Thermal hydrolysis assists calcium hypochlorite pretreatment to enhance anaerobic digestion performance of waste activated sludge. J. Water Process Eng. 2025, 70, 107094. [Google Scholar] [CrossRef]
- Jabari, L.; Gannoun, H.; Cayol, J.L.; Hedi, A.; Sakamoto, M.; Falsen, E.; Ohkuma, M.; Hamdi, M.; Fauque, G.; Ollivier, B.; et al. Macellibacteroides fermentans gen. nov., sp nov., a member of the family Porphyromonadaceae isolated from an upflow anaerobic filter treating abattoir wastewaters. Int. J. Syst. Evol. Microbiol. 2012, 62, 2522–2527. [Google Scholar] [CrossRef]
- Liu, X.; Du, M.; Yang, J.; Wu, Y.; Xu, Q.; Wang, D.; Yang, Q.; Yang, G.; Li, X. Sulfite serving as a pretreatment method for alkaline fermentation to enhance short-chain fatty acid production from waste activated sludge. Chem. Eng. J. 2020, 385, 123991. [Google Scholar] [CrossRef]
- Wang, R.; Li, C.; Lv, N.; Pan, X.; Cai, G.; Ning, J.; Zhu, G. Deeper insights into effect of activated carbon and nano-zero-valent iron addition on acidogenesis and whole anaerobic digestion. Bioresour. Technol. 2021, 324, 124671. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, C. Response of a continuous biomethanation process to transient organic shock loads under controlled and uncontrolled pH conditions. Water Res. 2015, 73, 68–77. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, A.; Duan, Y.; Wang, S.; Gao, Y.; Chen, X.; Cui, Z.; Guo, Z.; Yue, X. Unraveling the behavior of nitrite on promoting short-chain fatty acids accumulation from waste activated sludge by peracetic acid pretreatment: Extracellular polymeric substance decomposition and underlying mechanism. Sci. Total Environ. 2022, 841, 156793. [Google Scholar] [CrossRef]
- Breitenstein, A.; Wiegel, J.; Haertig, C.; Weiss, N.; Andreesen, J.R.; Lechner, U. Reclassifcation of 4. Clostridium hydroxybenzoicum as Sedimentibacter hydroxybenzoicus gen. nov., comb. nov., and description of Sedimentibacter saalensis sp nov. Int. J. Syst. Evol. Microbiol. 2002, 52, 801–807. [Google Scholar] [CrossRef]
- Su, X.L.; Tian, Q.; Zhang, J.; Yuan, X.Z.; Shi, X.S.; Guo, R.B.; Qiu, Y.L. Acetobacteroides hydrogenigenes gen. nov., sp nov., an anaerobic hydrogen-producing bacterium in the family Rikenellaceae isolated from a reed swamp. Int. J. Syst. Evol. Microbiol. 2014, 64, 2986–2991. [Google Scholar] [CrossRef]
- Narayanan, N.; Krishnakumar, B.; Anupama, V.N.; Manilal, V.B. Methanosaeta sp., the major archaeal endosymbiont of Metopus es. Res. Microbiol. 2009, 160, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Wu, Q.; Shapiro, B.M.; McKernan, S.E. Limited Mechanistic Link Between the Monod Equation and Methanogen Growth: A Perspective from Metabolic Modeling. Microbiol. Spectr. 2022, 10, e02259-21. [Google Scholar] [CrossRef]




| Parameters | Measurement Methods | WAS | Inoculum |
|---|---|---|---|
| pH | pH meter | 6.8 ± 0.1 | 7.7 ± 0.1 |
| TSS (total suspended solids) (mg/L) | Weight method | 21,063 ± 185 | 18,640 ± 154 |
| VSS (volatile suspended solids) (mg/L) | Weight method | 13,796 ± 161 | 7882 ± 105 |
| TCOD (total chemical oxygen demand) (mg/L) | Dichromate titration | 20,849 ± 133 | 11,074 ± 78 |
| SCOD (soluble chemical oxygen demand) (mg/L) | Dichromate titration | 87 ± 4 | 255 ± 6 |
| Reactors | PAA Pretreatment | Freezing Pretreatment | Methane Yield (mL/g VSS) | ||
|---|---|---|---|---|---|
| Dosage (g/g TSS) | Time (h) | Temperature (°C) | Time (h) | ||
| 1 | 0 | 24 | 4 | 8 | 166.3 ± 3.8 |
| 2 | 0.02 | 24 | −5 | 8 | 197.3 ± 4.0 |
| 3 | 0.04 | 24 | −5 | 8 | 214.2 ± 4.3 |
| 4 | 0.06 | 24 | −5 | 8 | 229.3 ± 4.3 |
| 5 | 0.08 | 24 | −5 | 8 | 237.8 ± 5.1 |
| 6 | 0.1 | 24 | −5 | 8 | 212.3 ± 4.4 |
| 7 | 0.02 | 24 | −10 | 8 | 205.7 ± 4.2 |
| 8 | 0.04 | 24 | −10 | 8 | 220.4 ± 4.5 |
| 9 | 0.06 | 24 | −10 | 8 | 241.6 ± 5.4 |
| 10 | 0.08 | 24 | −10 | 8 | 259.0 ± 5.7 |
| 11 | 0.1 | 24 | −10 | 8 | 207.2 ± 4.1 |
| 12 | 0.02 | 24 | −15 | 8 | 211.7 ± 4.6 |
| 13 | 0.04 | 24 | −15 | 8 | 228.5 ± 4.8 |
| 14 | 0.06 | 24 | −15 | 8 | 247.5 ± 5.3 |
| 15 | 0.08 | 24 | −15 | 8 | 251.3 ± 5.5 |
| 16 | 0.1 | 24 | −15 | 8 | 205.6 ± 4.5 |
| 17 | 0.02 | 24 | −20 | 8 | 214.7 ± 4.2 |
| 18 | 0.04 | 24 | −20 | 8 | 233.4 ± 4.7 |
| 19 | 0.06 | 24 | −20 | 8 | 251.7 ± 5.0 |
| 20 | 0.08 | 24 | −20 | 8 | 238.8 ± 5.2 |
| 21 | 0.1 | 24 | −20 | 8 | 202.5 ± 4.0 |
| Pretreatment Conditions | Kinetic Model Parameters | |||
|---|---|---|---|---|
| Mm (mL/g VSS) | Rm (mL/g VSS/d) | λ (d) | R2 | |
| Control | 159.4 ± 1.6 | 13.15 ± 0.53 | 0.23 ± 0.24 | 0.9940 |
| Freezing | 206.8 ± 1.6 | 17.18 ± 0.52 | 0.47 ± 0.18 | 0.9967 |
| PAA | 221.8 ± 1.8 | 20.67 ± 0.69 | 0.62 ± 0.18 | 0.9961 |
| Freezing + PAA | 254.9 ± 1.4 | 25.69 ± 0.64 | 0.67 ± 0.12 | 0.9979 |
| Pretreatment Conditions | B0 (mL/g VSS) | k (d−1) | R2 |
|---|---|---|---|
| Control | 169.8 ± 2.4 | 0.104 ± 0.005 | 0.9931 |
| Freezing | 221.3 ± 4.0 | 0.108 ± 0.004 | 0.9896 |
| PAA | 236.0 ± 4.6 | 0.114 ± 0.006 | 0.9860 |
| Freezing + PAA | 269.0 ± 5.3 | 0.125 ± 0.007 | 0.9835 |
| Pretreatment Conditions | Cost b (USD) | Increase in Methane Production (USD) | Dewatering, Transportation, and Landfill Costs | Net Saving Compared to the Control d (USD) | |
|---|---|---|---|---|---|
| Amount of Solid c (ton) | Decrease in Cost (USD) | ||||
| Freezing | 31.64 | 8.22 | 0.59 | 58.24 | 34.82 |
| PAA | 50.76 | 11.32 | 0.56 | 66.64 | 27.20 |
| Freezing + PAA | 82.40 | 16.89 | 0.43 | 103.04 | 37.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.-W.; Chen, Y.-Q.; Liu, Z.-S.; Wang, S.-W. Freezing Method Assists Peracetic Acid Oxidation for Promoting the Methane Production from Sludge Anaerobic Digestion. Energies 2025, 18, 731. https://doi.org/10.3390/en18030731
Liu Z-W, Chen Y-Q, Liu Z-S, Wang S-W. Freezing Method Assists Peracetic Acid Oxidation for Promoting the Methane Production from Sludge Anaerobic Digestion. Energies. 2025; 18(3):731. https://doi.org/10.3390/en18030731
Chicago/Turabian StyleLiu, Zhen-Wei, Yan-Qiu Chen, Zhi-Shuai Liu, and Sheng-Wu Wang. 2025. "Freezing Method Assists Peracetic Acid Oxidation for Promoting the Methane Production from Sludge Anaerobic Digestion" Energies 18, no. 3: 731. https://doi.org/10.3390/en18030731
APA StyleLiu, Z.-W., Chen, Y.-Q., Liu, Z.-S., & Wang, S.-W. (2025). Freezing Method Assists Peracetic Acid Oxidation for Promoting the Methane Production from Sludge Anaerobic Digestion. Energies, 18(3), 731. https://doi.org/10.3390/en18030731





