Abstract
Biomass plays an important role in the energy transformation aimed at carbon neutrality, with its potential estimated at 1/3rd of the entire energy mix. One of the main ways of using biomass is combustion or co-combustion, which enables the production of heat and electricity while maintaining low emissions. A promising path to utilize the combustion by-product—ash—is the possibility of using it as a natural and cheap catalyst that can effectively support the process of solid fuel gasification. This paper reviews scientific studies on the properties of biomass ash and its use to support the gasification process. The issues related to the genesis of mineral matter in plants are presented, emphasizing the importance of its transformations during biomass combustion. Particular emphasis is placed on the characterization of biomass ash, which was carried out on the basis of a comprehensive overview of the results regarding its chemical composition. An analysis of the physicochemical and surface properties relevant to the use of biomass ashes as catalysts in the gasification process was performed. In addition, a review of studies on catalytic gasification of solid fuels using biomass ash was conducted, taking into account the impact of biomass ash on the most important parameters characterizing the course of the gasification reaction, i.e., reactivity, quality of the gaseous products, and the kinetics reaction. The summary compares the most important advantages and disadvantages of using biomass ashes in the gasification process along with recommendations for future research.
1. Introduction
Growing energy consumption (which in 2023 reached 619.63 EJ—an increase of 82.15 EJ over 10 years [1]) as well as environmental protection are the main factors determining legislative changes (including the Paris Agreement concluded on 12 December 2015 [2]), the aims of which are carbon neutrality and CO2 emission reduction [3,4]. This requires an energy transformation, and the use of fossil fuels responsible for CO2 emissions will be limited (the share of energy sources, i.e., coal, crude oil, and natural gas in the energy mix in 2023 was approximately 81.47% [1]) which will be replaced by environmentally friendly renewable energy sources including biomass [4,5]. Today, biomass is one of the most frequently used renewable energy sources. Between 2000 and 2019, the global primary energy consumption from biomass increased from 41.6 to 57.0 EJ, which represented approximately 10% of the total primary energy consumption [6], thus increasing their role in the global energy sector to a projected share of bioenergy in the energy mix of 25 to 33% in 2050 [7]. Its advantages result primarily from almost unlimited and universal access, its attractive price, and neutrality in the context of carbon dioxide emissions [8]. The sources of bioenergy are mainly wood, green and aquatic biomass, as well as animal and waste origin biomass [9]. For the generation of energy from biomass, the used methods focus on three main processes: biochemical, physicochemical, and thermochemical [10]. Biochemical processes are carried out through fermentation under specially selected process conditions, leading to the production of liquid (alcohols, alkanes) and gaseous (hydrogen, methane) biofuels [11]. Physicochemical methods focus mainly on hydrolysis or extraction leading to the liquefaction of biomass [10,12]. In turn, among the thermochemical methods of biomass processing, the main ones are combustion and co-combustion, although other processes such as torrefaction, pyrolysis, gasification, or hydrothermal processing are becoming increasingly important [13]. The biomass with energy potential amounts to approximately 4.1 to 6.1 billion Mg, which includes 3 billion Mg of biomass from forest residues, as well as 1.1 to 3.1 billion Mg of biomass in the form of agricultural residues [14]. Considering the fact that approximately 95% of the energy produced from biomass is obtained through combustion or co-combustion, this confirms that this is the main method of thermochemical biomass utilization. Ash is produced as a solid by-product of the oxidation reactions of organic material contained in biomass either in the fly or in the bottom form. According to annual estimates worldwide, around 170 to even 476 million Mg of ash is produced during biomass combustion [15,16]. Several applications have been found for biomass ash: (i) in the construction industry (e.g., roads and buildings) and in the production of geopolymers (e.g., concrete mixtures); (ii) as fertilizers and compounds for the remediation of post-mining areas; and (iii) as a source material for recovering important elements, including P, Na, K, and others [17,18,19,20,21,22,23]. One of the potential and interesting methods of biomass ash utilization is their use as natural catalysts in the gasification process. The catalytic potential of biomass ash components has been confirmed in numerous studies on the co-gasification of different raw materials (mainly coal) with biomass [24,25,26,27,28,29] and with biomass char [30,31,32,33]. The results of the research clearly indicate an improvement in reactivity with increasing biomass/biomass char content in the co-gasified mixture by reducing the time needed to achieve maximum conversion degree and increasing the reactivity indices Rx. The evaluation of co-gasification products indicates an increase in gas fraction yields with a decrease in liquid and char yields. Among gaseous products, there has been an increase in H2 and CO2 yields. In addition, an increase in the values of constant reaction rates and a decrease in the values of kinetic parameters—activation energy and pre-exponential factors—are observed. The approach based on co-gasification of biomass/biomass char with other raw materials has been extensively verified and has been the subject of numerous scientific review publications [34,35,36,37,38,39,40,41,42,43]. A different approach, which appears much less frequently in scientific publications, is the direct use of biomass ash as a catalyst in the gasification of coal raw materials. The use of biomass ash in catalytic gasification technology for solid fuels contributes to the diversification of applications for biomass combustion by-products, as well as expanding the scope of the circular economy. Considering that 83.6% of the total thermal power of syngas obtained in industrial gasifiers was generated using coal and waste, this creates a huge area for the utilization of biomass ash [42,44]. Furthermore, the need for preparation of a catalyst based on biomass ash is of great importance from an economic as well as an environmental point of view. This is because it reduces the need to use new chemical reagents and dispose of used ones as well as to conduct energy-consuming physicochemical processes [45,46].
The main advantage of biomass ash in the context of catalytic gasification is the high content of catalytically active compounds (K, Na, Ca, Mg, Fe) and at the same time the low content of gasification reaction inhibitors (Si and Al). Combined with the above-mentioned advantages of general availability and low price, they can be an attractive material, improving the course of the gasification process. Considering its catalytic potential and the above-mentioned advantages, biomass ash may be an attractive solution for the catalytic gasification of solid fuels. Increased efficiency, combined with lower process temperatures and qualitative and quantitative improvements in the gas obtained, assuming low costs associated with the acquisition and preparation of catalysts, are the main factors enabling the difficulties associated with the commercialisation of catalytic gasification of solid fuels to be overcome [47,48]. To the best of the authors’ knowledge, the literature on catalytic gasification of solid fuels does not contain an up-to-date assessment of the state of knowledge on the use of biomass ash as a catalyst in gasification processes and is based mainly on the results of individual studies. The literature review presented in this paper aims, based on an analysis of the research results obtained to date, to systematize information on the physicochemical properties of ash from combustion in the context of solid fuel gasification technology, assess the catalytic efficiency of biomass ash in this process, and identify current and future challenges related to the use of biomass ash as catalysts and propose directions for further research.
Considering the above, the aim of this review paper is to present the state of knowledge on the possibility of using ash from biomass combustion as a catalyst in the gasification process. In order to provide a comprehensive assessment of this issue, the following aspects were analyzed: (i) the genesis of mineral matter contained in biomass; (ii) its transformation during combustion; (iii) properties of biomass ash in the context of the key parameters related to catalytic gasification; and (iv) catalytic activity of ash during the gasification reaction. The analysis of catalytic activity was based on the influence of the presence of biomass ash on the most important parameters during the study of the gasification process of solid fuels, i.e., the reactivity, quality, and composition of the obtained process gas as well as the kinetics of the process. This review summarizes the most significant features of ash catalysts, taking into account their advantages and disadvantages as well as providing recommendations for areas of future research.
2. Mineral Matter in Biomass
Scientific studies focusing on the assessment of the physicochemical properties of ash from biomass combustion indicate their great diversity in the context of qualitative and quantitative composition. This factor is therefore of key importance because it determines to the greatest extent the prospects for their further development. In order to understand the physicochemical nature of biomass ash and its qualitative and quantitative variability, the entire biomass transformation cycle, which begins with the growth of the plant and ends with obtaining biomass ash, should be presented.
2.1. Genesis of Mineral Matter in Biomass
The presence of the inorganic fraction in the structure of the plant is the result of the mineral nutrition process that takes place during vegetation. Mineral matter absorption takes place in the plant–soil system according to the reaction presented in the following diagram [49,50]:
where M is a nutrient ion of inorganic origin.
M(solid)↔M(solution)↔M(plant root)↔M(plant top)
As a source of mineral elements contained in the soil, long-term changes in the abiotic nature occur, including weathering and leaching of rocks, as well as in the biotic nature, including the decomposition of the organic matter of animals, plants, and microbes. The presence of water in the soil layer causes these components to dissolve, which enables their absorption and assimilation through the root system of the plant from where they are distributed to the upper parts of the plant [50]. This process requires energy supplied by solar radiation [49]. Some mineral elements, i.e., K, Ca, Fe, Mg, B, Zn, Cu, Mo, Ni, Cl, and P, are necessary for the metabolism and functioning of the plant. In turn, elements such as Na, Si, Co, or Al are not essential, but their presence contributes to the improvement of life processes during the vegetation stage. Research conducted at the end of the nineteenth century showed that from the point of view of qualitative and quantitative variability of mineral elements, plants do not have the ability to selectively absorb minerals contained in the soil [50]. Plants can therefore also absorb and accumulate mineral elements that are not essential for their proper development, including elements that have a negative impact on themselves and on the surrounding environment. Taking into account the complexity of the vegetation process and the fact that greater mineral diversity is observed in biomass compared to conventional solid fuels (e.g., hard coal or lignite), a greater number of variables influencing its mineral structure can be expected [51,52,53]. Table 1 presents the main factors determining differences in plants’ absorption and accumulation of elements that make up mineral matter.

Table 1.
Factors determining the amount and composition of mineral matter in plants [9,50,54,55,56,57].
The factors shown in Table 1 can be divided into two main groups: natural and anthropogenic. Natural factors depend on genetic and environmental conditions in the plant–soil system, and exert their influence during the growth and development of the plant. The main role is played by the genus and species of plants dictated by their physiology as well as genetics, which change through evolution, determining heritable traits. The functional diversity of their outer parts (leaves, flowers, stems, branches, or bark), as well as the vegetation phase in which the plant is present, are also important. The environmental aspect has an equally significant impact in the context of the physicochemical conditions of the substrate on which vegetation occurs. The most important soil factors include pH, surface charge, soil texture and structure, hydration, as well as fertility. Plant growth is also determined by a group of atmospheric and geographical conditions that include the availability of sunlight, air humidity, climate or season, and geographical location, as well as altitude above sea level [50,54,55,56]. Anthropogenic factors constitute the second group that influences the composition of plant mineral matter, the source of which is human activity. Among these variables, the most important is the addition of substances supporting the proper growth of plants (fertilizers) and substances ensuring their protection against the harmful effects of insects, rodents, weeds, and fungi (e.g., with the use of pesticides). Subsequently, it is also possible to indicate the inclusion of mineral pollutants entering during harvesting, as well as during transport, storage, or processing. In this group other factors of lesser importance due to their limited range can also be indicated, including distance from roads, cities, factories, or mines, as well as cultivation conditions, i.e., their type, density, or care [9,57].
2.2. Mechanism of Ash Formation from Mineral Matter
The changes that occur during biomass combustion are an extremely important factor that significantly determines the physicochemical properties of biomass ash. The elements that make up the mineral matter of plants, which are chemically bound to the organic structure, are known as inherent ash. These elements occur in the form of ionically bonded salts or as organically or inorganically bonded compounds with a carbon skeleton, and their origin arises from natural and anthropogenic factors. A characteristic feature of inherent ash is its homogeneous distribution in the plant and high mobility, which cause this part of the inorganic fraction to undergo intensive transformations as a result of combustion. On the other hand, all types of contaminants delivered to the plant during its harvest and subsequent management that are not chemically related to its structure are called foreign ash [58,59].
In order to organize and give a clear meaning to the issues discussed in this paper, it is necessary to indicate the differences between the concepts of mineral matter and ash. The use of the identical terms mineral or inorganic elements/matter/fraction refers to inorganic or organometallic elements as well as compounds contained in plants. In turn, ash can be defined as the solid residue remaining after oxidation of the organic fraction contained in a given portion of biomass. According to such definitions, the difference can be defined as the mass loss resulting from physicochemical changes occurring during biomass combustion, which constitute the essence of the ash formation mechanism, which will be presented in an illustrative manner later in this subchapter.
Combustion and co-combustion of biomass are commonly used and well-known processes in its thermochemical conversion [60]. Energy is obtained as a result of oxidation of the material constituting the organic fraction of biomass, while the mineral material undergoes transformations that lead to the formation of ash (fly and bottom) or slag, which are by-products of the combustion process. These transformations occur in different ways depending on process conditions (e.g., temperature, total pressure, partial pressure of oxygen, reactor type, etc.) as well as feedstock factors (e.g., chemical composition of the mineral matter of the combusted biomass, particle size, etc.) [61]. Depending on the nature of these changes, the following can be distinguished:
- Chemical transformations—as a series of primary and secondary reactions taking place between the reagents present in the reactor;
- Physical changes—related to the change in the properties of mineral matter as a result of the formation of new phases generated by the interaction of solids, liquids, and gases.
Chemical transformations consist of a very broad spectrum of reactions occurring during the thermochemical transformation of mineral matter, which makes their detailed identification difficult. For this reason and with regard to their practical systematization, primary and secondary reactions are distinguished [61]. Primary reactions are the first stage of reactions that occur during the combustion of biomass in the element-oxygen system. The research results indicate that the course of primary reactions is largely influenced by the relative thermodynamical stability of the ash-forming element oxides defined as the Gibbs free energy for the reactions of oxide formation as a function of the temperature. It turns out that elements such as Ca, Mg, Si, or P whose thermodynamic stability is below the lines drawn for organic combustion products, i.e., CO, CO2, and H2O, will remain stable regardless of combustion conditions in the form of oxides. On the other hand, the elements form less stable oxides—whose thermodynamic stability is above the lines drawn for organic combustion products—that can be reduced to metal vapours or react with steam to form hydroxides (K or Na) and also be released in gaseous form or in the form of hydrides (S and Cl) [61]. Secondary reactions are defined as those occurring between elements and their forms produced during primary reactions [58,61]. The secondary reaction pathways are very diverse and their prediction is almost impossible due to the fact that they are determined by a large number of parameters, i.e., feedstock reactivity, element concentration, physical state (solid, liquid, gas), temperature, total pressure, particle size/dispersion, heat and mass transfer, as well as the course of primary reactions [61,62,63]. Research on these transformations allowed for a classification according to which the most common directions of transformations were specified, depending on the chemical group of a given compound. The high volatility of phosphorus, sulphur, and chlorine can lead to interactions with other elements of ash consequently creating thermal stable condensed phases or their evaporation into the gas phase [61]. Silicates and aluminosilicates, sulphates, carbonates and bicarbonates, phosphates, oxalates, chlorides, oxides and hydroxides, nitrates, and other salts are also formed [61,62,63,64,65]. The stability of these forms is determined mainly by temperature. Chlorides are usually products of secondary reactions, but due to their high volatility, they are released into the gas phase and precipitate at lower temperatures [61]. Carbonates are mainly secondary phases that contain the elements Ca, Mg, K, and Na that undergo numerous decomposition or dehydration changes during heating [63]. A similar origin was attributed to sulphates that form compounds with alkali metal, alkaline earth element, and aluminum, which often undergo decomposition or crystallization/recrystallization reactions [63]. Nitrates similar to chlorides are formed in secondary reactions and are characterized by high volatility in the gas phase; therefore their presence in ashes is relatively low. Oxalates are mainly in the transitional state in the formation of other forms, i.e., carbonates, sulphates, silicates, or aluminosilicates [63]. The genesis of phosphates is mainly attributed to secondary reactions, but some may still be of primary origin. Phosphates are generally present in large amounts in biomass ashes such as P2O5 or apatites and are chemically bound to Al, Ca, Fe, K, Mg, Mn, Na, or Zn [63]. For parts of oxides and hydroxides, the origin can be attributed to primary reactions, but hydroxides can also undergo further chemical transformations, e.g., decomposition, creating secondary reaction products to more stable thermal forms [61,63]. Silicates and aluminosilicates attribute their origin mainly to secondary reactions having a high tendency to interact with alkali and alkaline earth metals (AAEMs) present in biomass ash [63]. Elements that constitute trace amounts in the total content in mineral matter are also subject to chemical transformations, among which are especially heavy metal elements that potentially pose a serious threat to the surrounding environment. Studies on the transformation of trace elements and their oxide forms indicate that, due to their low melting points, they can be partially released into the gas phase [66,67]. As, Zn, Cd, Pb, or Hg have a particular affinity for the gas phase, with higher concentrations reported in fly ash, confirming the volatilization ability [68]. In contrast, for V, Mn, Cr, or Co, higher contents are recorded in the bottom residue/slag, suggesting that they are more chemically bound to coarse particles [68]. Trace elements can also participate in secondary reactions. Positive affinity for silicates has been reported for Ag, As, Au, Be, Cd, Er, Eu, Gd, Ge, Ho, Lu, Sb, Sc, Se, Sm, Ta, Tm, and W. However, for the elements B, Ba, Bi, Ce, Co, Cs, Cu, Ga, Hf, La, Li, Nb, Nd, Pb, Pr, Sn, Th, U, Zn, and Zr, affinity towards phosphates was recorded. Particular affinity towards carbonates, oxides, and hydroxides was obtained for B, Ba, Bi, Co, Cs, Cu, Ga, Hf, Li, Pb, Rb, Sn, Sr, Y, and Yb. In addition, a positive affinity for Co, Cr, Cu, Mo, Ni, Rb, Sr, U, and V was observed for sulphates, while an attachment to chloride formation was noted for B, Co, Sr, U, and V [69].
The most widely used research technique for tracking chemical transformations in biomass ash as well as to determine the factors influencing their course is X-ray diffraction (XRD) analysis. Figure 1 lists the main crystalline phases by group of compounds that have been identified in biomass ashes, with the aim of showing the complexity of the reactions and transformation mechanisms occurring in mineral matter during biomass combustion.
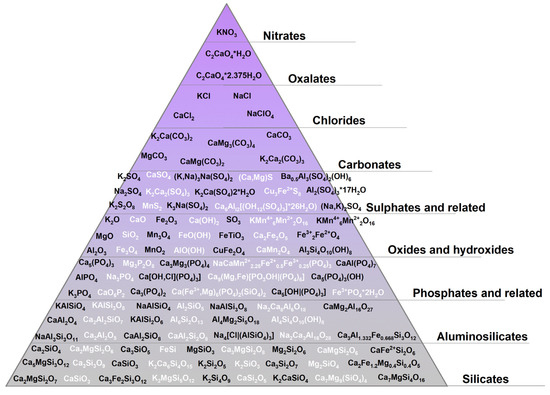
Figure 1.
Minerals in biomass ashes [58,61,62,64,65,70,71,72,73,74,75,76,77].
Figure 1.
Minerals in biomass ashes [58,61,62,64,65,70,71,72,73,74,75,76,77].
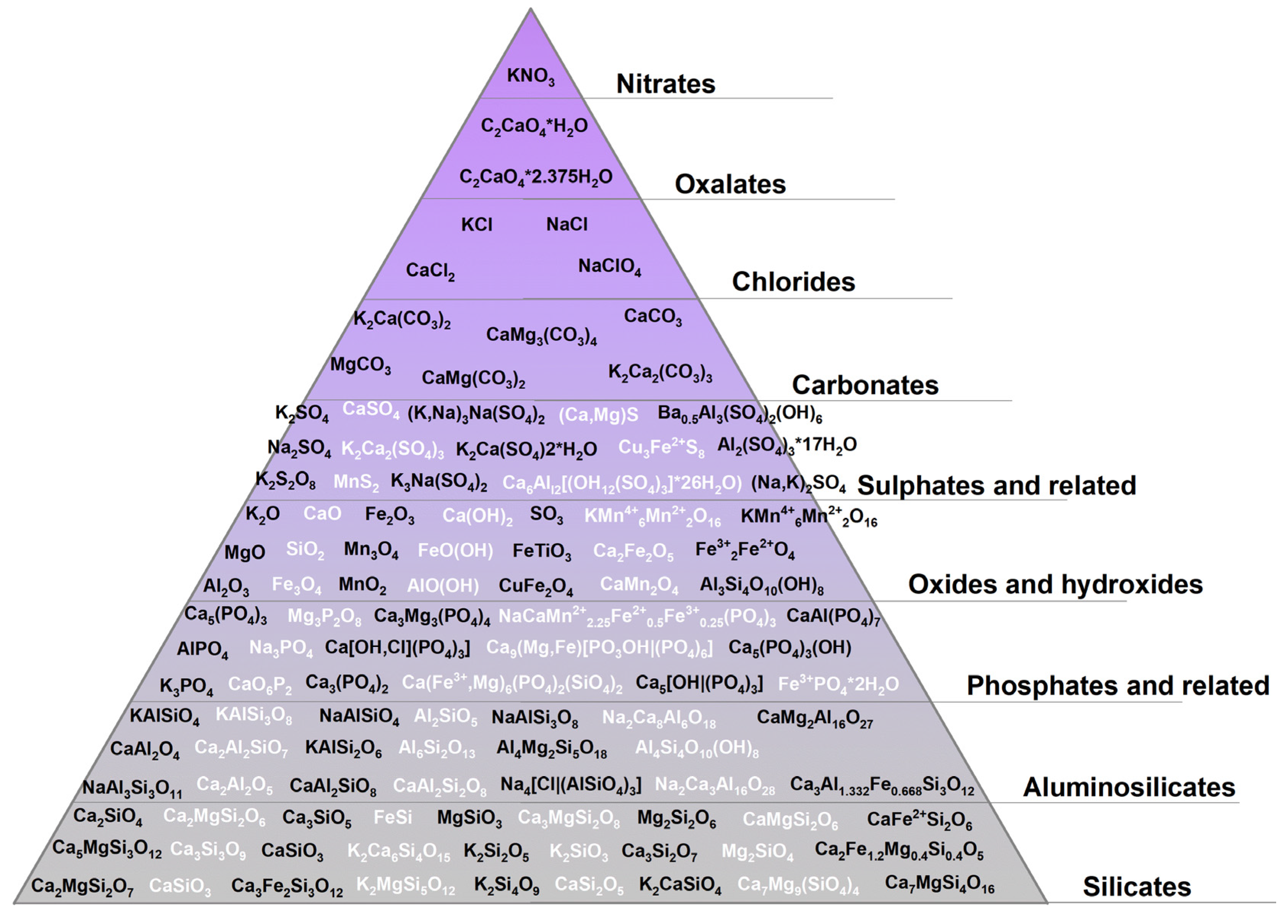
The wide range of crystalline minerals confirmed by the XRD method in biomass ash demonstrates the complex nature of the chemical transformations of mineral matter occurring during biomass combustion. In addition, the data presented prove that the elements that form the core of mineral matter include Na, K, Mg, Ca, Si, Al, and Fe. Elements of alkali metals (K, Na) and alkaline earth metals (Ca, Mg) have an affinity towards the formation of silicates, aluminosilicates, phosphates, sulphates, carbonates, oxides, and chlorides, yet trace amounts of oxalates or nitrates can be formed. The most stable forms found in biomass ash are oxides, silicates, and aluminosilicates. Chlorides, nitrates, and oxalates make up only a small part of the total, which decomposes or evaporates into a gaseous form at high temperatures.
Chemical transformations classified as both primary and secondary reactions are accompanied by a number of physical transformations. Their complexity is also a factor that makes it difficult to identify the unambiguous formation pathways of the compounds that make up biomass ash. The parameters that influence physical transformations are primarily the concentration and dispersion of elements, the temperature and pressure (total and partial), the reactivity of the phases produced, and the heat and mass transfer. The main physical transformations of the mineral fraction accompanying biomass combustion include evaporation, temperature decomposition, melting, crystallization/recrystallization, agglomeration, aggregation, and sintering [63,64].
The detailed characterization of the transformation of biomass ash during combustion is beyond the scope of this paper, and this aspect is described in more detail in the publications [63,69].
2.3. Physicochemical Properties of Biomass Ash
2.3.1. Chemical Composition
The results of ash composition analysis (mainly using the XRD method) give very valuable results in determining the quality and systematizing the type of compounds that form biomass ash. However, most scientific papers concerning, i.e., the chemical characterization of biomass ash, present summaries of their composition in elemental or oxide form, which makes it possible to evaluate them qualitatively and quantitatively. In order to show the wide spectrum of diversity in biomass ash, the contents of elements of a dominant nature (whose average concentration in biomass samples exceeds 0.1%—see Figure 2) and of a trace nature (whose average content is less than 0.1%—see Figure 3) are summarized. The analysis is divided into green biomass (GBA), woody biomass (WBA), and other biomass (OBA—mixed, waste, animal biomass). Dominant inorganic elements (>0.1%) include elements such as Na, K, Mg, Ca, Fe, Si, Al, Ti, Mn, and Zn as well as S, P, and Cl, due to their significant content as well as their significant influence on the final properties of the ash. Moreover, in the review of scientific publications performed, the authors very often report the composition of ash in oxide form, which, as shown in the previous section, is not consistent with the real state. For this reason, a calculation is made for the more universal form of the elemental content, which defines the chemical properties to a greater extent. A detailed summary of the dominant and trace element contents of biomass ash is summarized in Appendix A (Table A1 and Table A2, respectively).
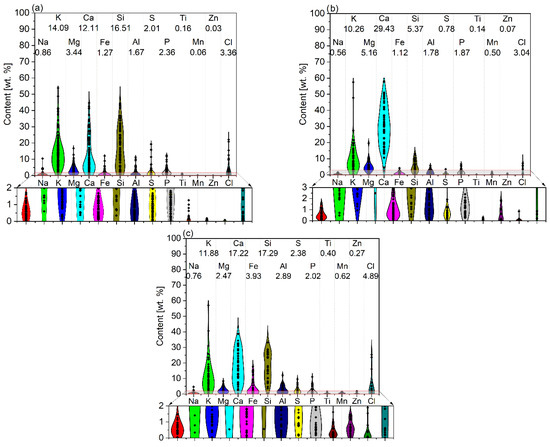
Figure 2.
Dominant element content with average values in ashes from (a) green biomass (GBA), (b) woody biomass (WBA), and (c) other biomass (OBA). Own analysis based on [17,72,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101].
Figure 2.
Dominant element content with average values in ashes from (a) green biomass (GBA), (b) woody biomass (WBA), and (c) other biomass (OBA). Own analysis based on [17,72,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101].
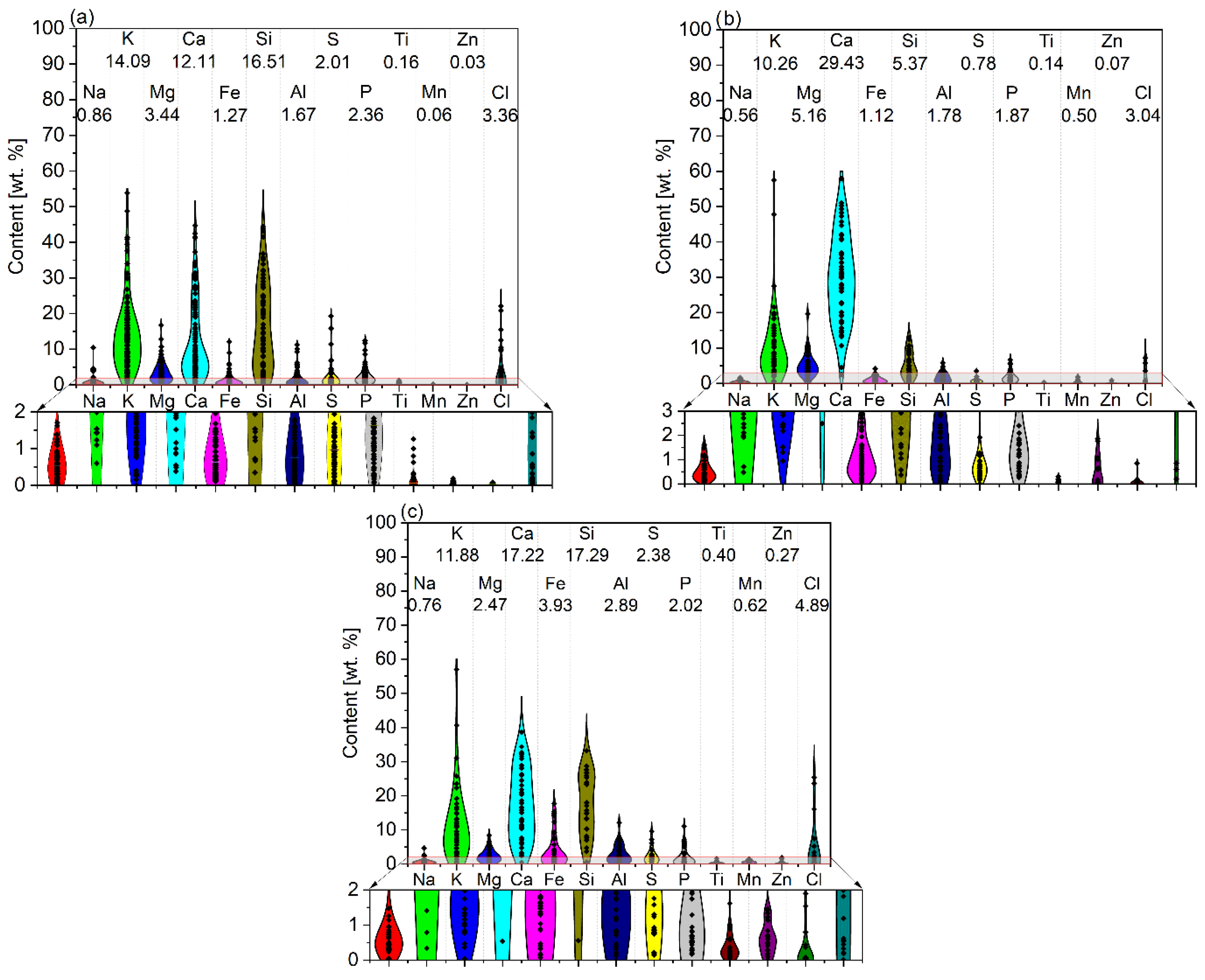
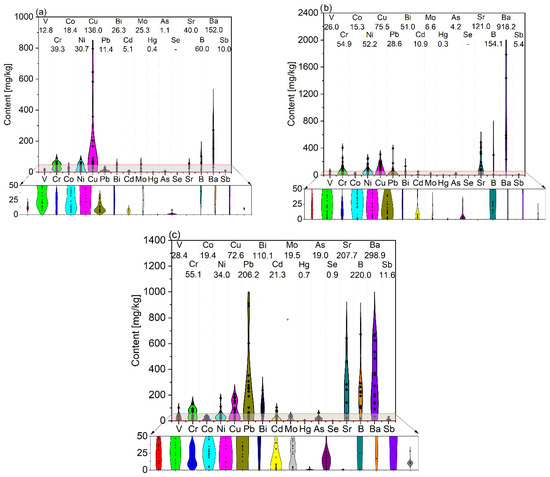
Figure 3.
Trace element content with average values in ashes from (a) green biomass (GBA), (b) woody biomass (WBA), and (c) other biomass (OBA). Own analysis based on [79,80,82,83,84,85,86,90,93,97,102].
Figure 3.
Trace element content with average values in ashes from (a) green biomass (GBA), (b) woody biomass (WBA), and (c) other biomass (OBA). Own analysis based on [79,80,82,83,84,85,86,90,93,97,102].
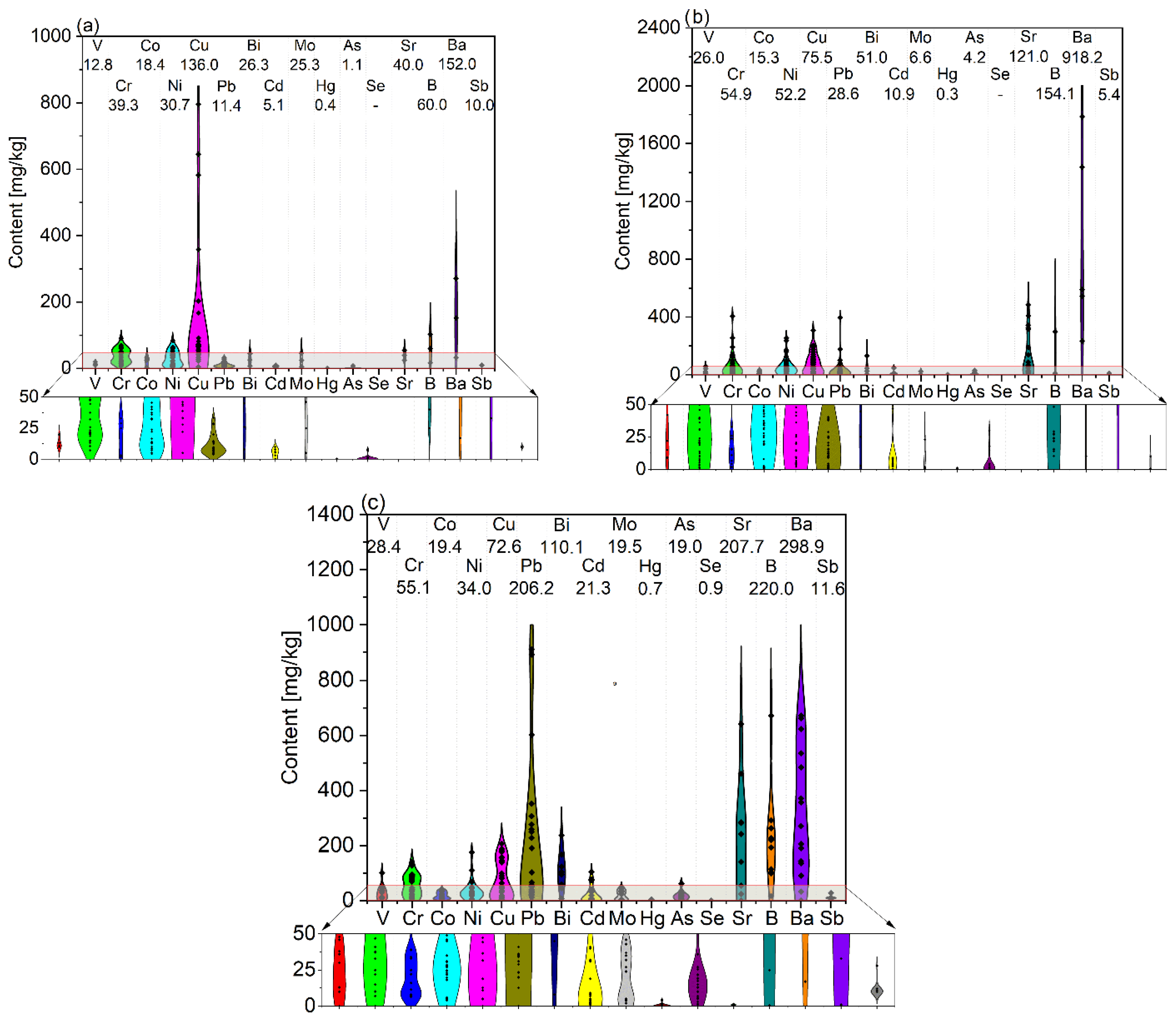
The content of dominant elements (>0.1%) present in biomass ash varies both within the main types (green, woody, and other biomass), but also between them. Analyzing the compiled data, it can be seen that calcium, potassium, and silicon play a major role. In the case of Ca, its highest average content was recorded for WBA (29.43%), which is nearly double that of the other two biomass types. For silicon, on the other hand, similar averaged contents (16.51% and 17.29%) were obtained for GBA and OBA, respectively, while WBA had a nearly three-times-lower average content of this element (5.37%). The smallest differences between biomass types were observed for potassium, with amounts averaging from 10.26% (WBA) to 14.09% (GBA). The average contents of the aforementioned elements within the types of biomass ash vary widely from tenths of a percent to as much as tens per centum. Elements that play a catalytic role in gasification processes, in addition to K or Ca, are magnesium, sodium, and iron. The highest average Mg content was recorded for WBA (5.16%), while the lowest was recorded for OBA (2.47%). The average amount of Na did not exceed 1%, ranging from 0.56% for WBA to 0.86% for GBA. In the case of Fe, its average content varied slightly, ranging from 1.12 to 1.27% (WBA and GBA, respectively) to 3.93% (OBA). Interestingly, the most significant variation in the average amount of this ash component was recorded just for OBA. Among the elements that showed a negative effect during the gasification reactions, it is important to note the significant silicon average content (from 5.37% for WBA to 16.51% for GBA and 17.29% for OBA), while the average aluminum content seemed to be insignificant, ranging from 1.67% for GB to 2.89% for OBA. Concerning the physicochemical properties and potential use of biomass ash, the content of chlorine and sulphur was also important. The average amount of Cl in the ash was similar, ranging from 3.04% for WBA to 4.89% for OB, with the greatest variation for OB. The average sulphur content ranged from 0.78% (WBA) to 2.38% (OBA), following an analogous trend. However, compared to chlorine, sulphur had far less variation, especially in WBA and OBA. The average phosphorus content was at a very similar level and did not depend much on the type of biomass ash. The average amounts of titanium, manganese, and zinc were the lowest among the elements discussed in this group, and the highest amounts of these components were recorded in OBA ash (0.40, 0.62, and 0.27%, respectively).
The content of trace elements, although only being a small share of the total weight of ash, is important from the point of view of its properties as well as its safe use in terms of negative environmental impact. As with dominant elements, trace elements are characterized by considerable variation. Among trace elements, barium plays a major role, with an average content ranging from 152.0 mg/kg of GBA to 918.2 mg/kg of WBA. Significant averaging was also observed for boron and strontium, with the lowest amounts of these constituents recorded in GBA (60.0 and 40.0 mg/kg, respectively) and the highest for OBA (207.7 and 220.0 mg/kg). In the case of copper, the highest average amount was observed for GBA (136.0 mg/kg), while lead and bismuth were observed in OBA (206.2 and 110.1 mg/kg, respectively). The contents of other trace elements were significantly lower compared to those of the above-mentioned elements, for which the average amounts of elements such as vanadium, chromium, cobalt, and nickel were in the range of tens of mg/kg and did not depend on the type of biomass. The lowest average amounts of trace elements were recorded for selenium, mercury, arsenic, cadmium, and molybdenum, the contents of which generally did not exceed a few mg/kg.
In the context of discussing the chemical characteristics of biomass ash, as well as its potential for application, Vassiliev et al. suggest an interesting interpretation of the chemical composition in a graphical form, as shown in Figure 4 [63]. The presented division in terms of chemical composition is made on the basis of the analysis of correlations between ash-forming elements and their associations as presented in the quoted paper. The chemical classification aims to divide the biomass ash in terms of the dominant phase-mineral forms. In this aspect, three main areas are distinguished [15,63]:
- The upper corner, in which Si, Al, Fe, Na, or Ti play a dominant role, and their main forms of occurrence in biomass ashes are glasses, silicates, and oxyhydroxides;
- Left bottom corner, in which elements such as Ca, Mg, or Mn predominate, occurring mainly in the form of carbonates, oxyhydroxides, glasses, silicates, phosphates, and sulphates;
- Bottom right corner, the area dominated by K, P, S, or Cl occurring as phosphates, sulphates, chlorides, glasses, silicates, and carbonates.
At this point, it should be recalled that these are certainly not the only forms of occurrence of these elements in biomass ash due to the complex nature of the physical and chemical transformations occurring during the thermal conversion of biomass.
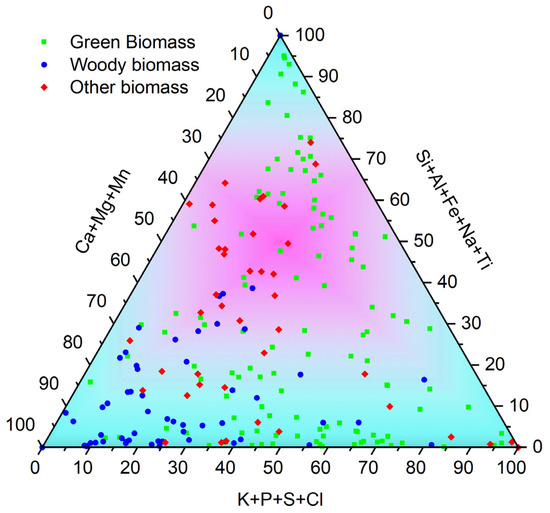
Figure 4.
Areas of biomass ashes in the chemical classification system. Own analysis based on [17,72,77,78,79,80,81,83,84,85,86,87,88,89,90,91,92,94,95,96,98,99,100,101].
Figure 4.
Areas of biomass ashes in the chemical classification system. Own analysis based on [17,72,77,78,79,80,81,83,84,85,86,87,88,89,90,91,92,94,95,96,98,99,100,101].
In the context of the division of biomass ash, it can be observed that GBA, as well as OBA, are characterized by a large variation, almost evenly covering the surface of the entire graph. However, when ash from WBA is evaluated, one can see a thickening within the lower left corner, suggesting that carbonate, phosphate, sulphate, silicate, oxyhydroxide, and glass forms may be dominant. In addition, WBA, as well as OBA, may have an increased susceptibility to leaching, low-temperature transformation, emission of volatile components, and sludge during combustion. Other parts of green biomass ash can also contribute to increased erosion phenomena, as well as to the formation of low-temperature eutectics [103].
2.3.2. Melting Temperatures
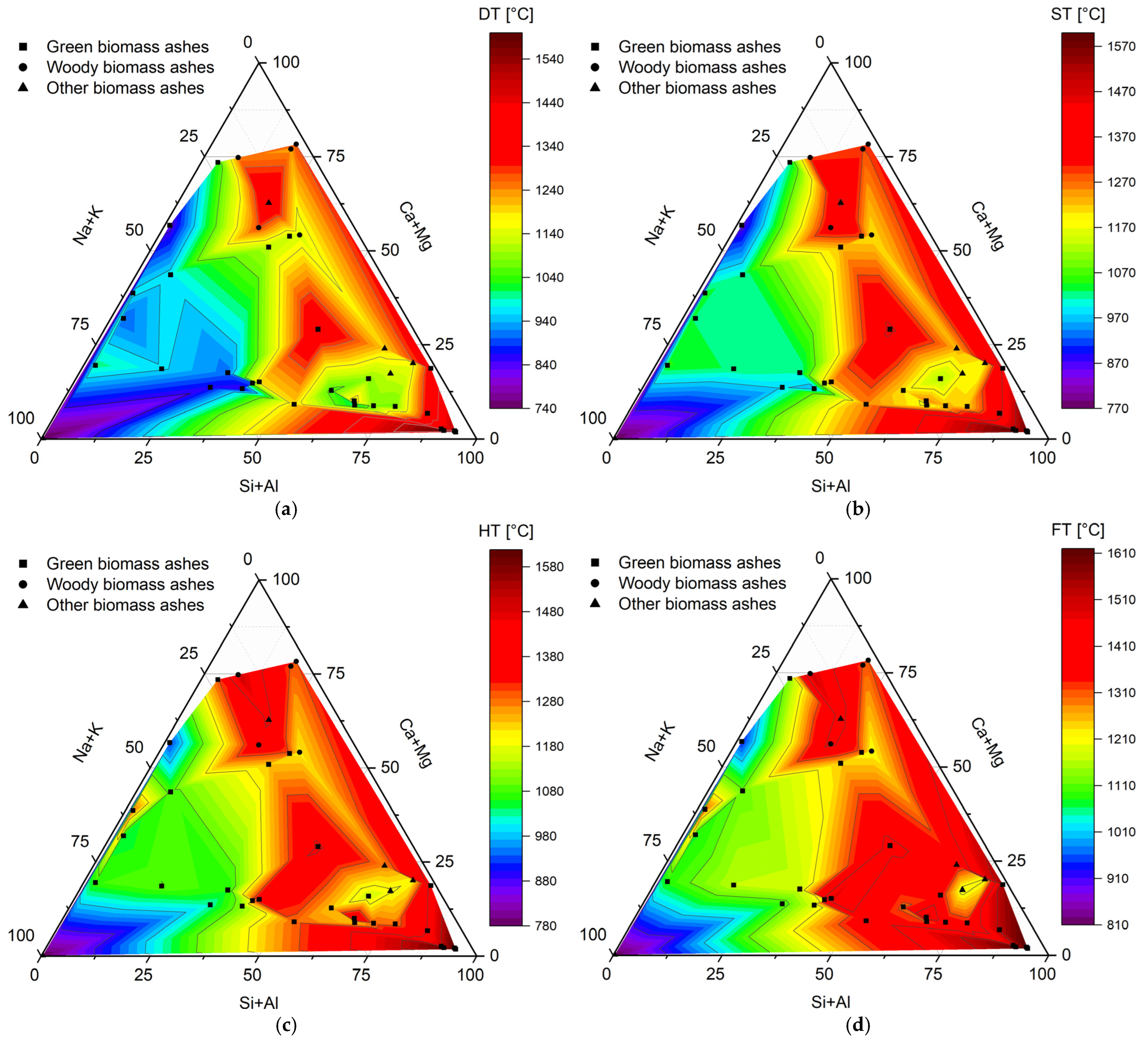
Analysis of the composition of biomass ash is of utmost importance from the point of view of its performance during thermochemical conversion. To evaluate it, Figure 5 graphically shows the values of melting temperatures (DT—deformation temperature, ST—softening temperature, HT—hemispherical temperature, FT—flow temperature) depending on the content of the main ash components (Na + K, Ca + Mg, and Si + Al).
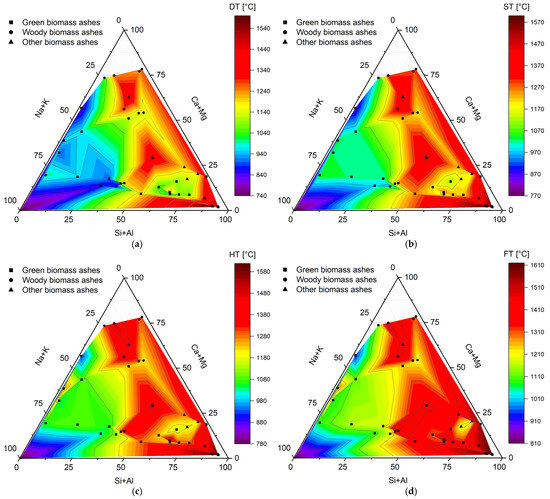
Figure 5.
Melting temperatures ((a) DT—deformation temperature, (b) ST—softening temperature, (c) HT—hemispherical temperature, (d) FT—flow temperature) of biomass ashes from green biomass (GBA), woody biomass (WBA), and other biomass (OBA). Own analysis based on [62,65,70,71,72,74,76,92,96,104,105].
Analyzing Figure 5, it can be seen that they were characterized by quite a wide range of temperatures within a given type, as there were almost twofold differences between the extreme values. In addition, a clear and unambiguous trend was observed in the content of the ash components, the composition of which was significantly correlated with the values of the melting points. The highest DT, ST, HT, and FT values were always recorded with the highest Si + Al content (>50%), as well as the lowest Na + K content (<50%). It also seems that the Ca + Mg content did not determine such a significant effect, but for the area below 50% of the content of these elements dominated by the presence of silicon and aluminum as well as the slight presence of potassium and sodium, ash melting occurred at much higher temperatures. Thus, the results presented here prove that alkali metal elements are mainly responsible for the low-melting character of biomass ash, while silicates and aluminosilicates promote an increase in characteristic melting temperatures. The presence of calcium and magnesium does not play such a key role, because as shown in Figure 1, it forms silicates and aluminosilicates with higher thermal resistance. Considering also the division into types of biomass ash, one can find confirmation of the relationships indicated earlier. As shown in Figure 2, green biomass with the highest K and Na contents generally has the lowest melting temperatures. In contrast, for ash from other types of biomass with the highest Si and Al contents, melting occurs for significantly higher temperature values.
2.3.3. Surface Properties
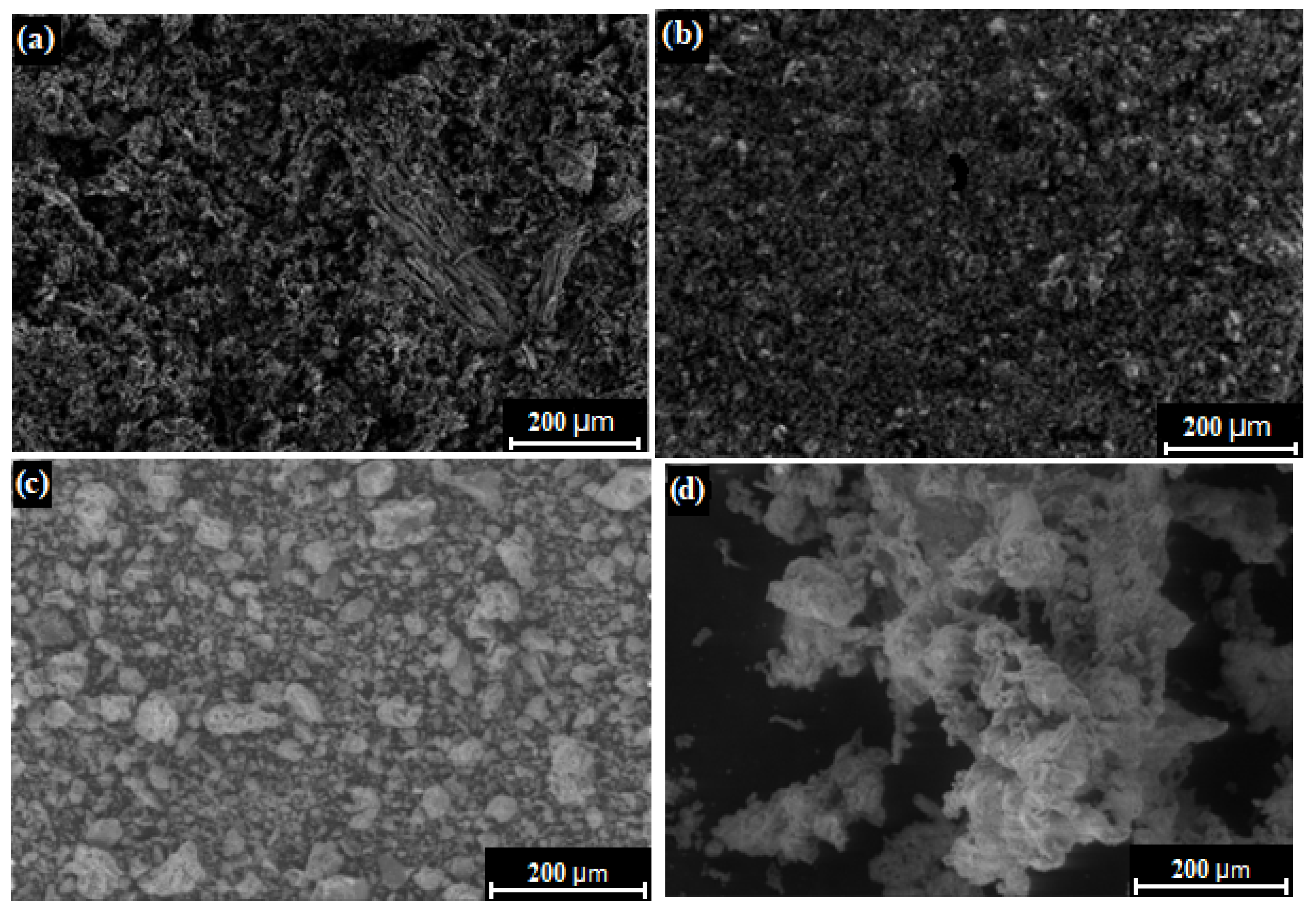
Properties characterizing the surfaces of biomass ash, although rarely studied by researchers, are nevertheless an important factor in terms of the performance of biomass ash. Figure 6 shows SEM images of four sample ashes from green biomass (sunflower husk, beet pulp, corn cob) and woody biomass (beech chips) to determine the morphological properties. In turn, Table 2 summarizes the most important parameters that describe the texture of biomass ash.
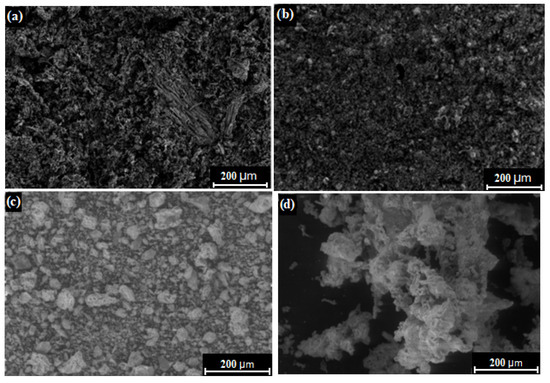
Figure 6.
SEM of biomass ashes from (a) sunflower husk, (b) beet pulp, (c) beech chips, and (d) corn cob. Own source.

Table 2.
Surface properties of biomass ash. Own analysis based on [81,83,84,92,98,106,107,108].
The SEM images shown in Figure 6 indicate that the biomass ash has a very different irregular shape. In the case of the sunflower husk ash sample, an uneven structure is observed, resembling a sponge, having many hollow randomly distributed spaces. Beet pulp ash, on the other hand, has a more condensed and monolithic structure. In the case of beech chip ash, high-grained, irregularly shaped grains are observed, whereas corn cob ash appears to be composed of larger agglomerates with highly differentiated, irregular shapes. The differences in the morphologies of the biomass ash were not due to differences in the values of the prevailing process conditions, since the procedure and apparatus used were identical for each type of biomass. Therefore, the sources of differences should be sought in the properties of raw materials, more specifically, the chemical compositions of the raw materials, which apparently also affect the morphological parameters.
The values of the parameters defining the surface of ash, presented in Table 2, confirm the high variation observed in the analysis of SEM images. The value of the BET area of biomass ash varies in a wide range from 0.18 m2/g to values more than two hundred and fifty times higher (46.00 m2/g). However, a high BET area is relatively rare, as it averages 6.39 m2/g. High variation is also observed for the total pore volume (from 0.01 to 0.04 cm3/g) and the average pore diameter (from 3.00 to 10.24 nm), indicating that mesopores play a dominant role in the texture of biomass ash. The same relationship, consistent with the observations made during SEM evaluation, is also observed for the particle size, which ranges from 22.00 to 40.00 μm, with an average of 28.40 μm. The total density of biomass ash ranges from 1.31 to 2.74 g/cm3. On the other hand, the bulk density is a parameter that ranges from 0.10 to 0.78 g/cm3, with an average of 0.35 g/cm3.
All of the physicochemical and surface parameters collected and described so far are extremely relevant to the use of biomass ash. The next section of the paper discusses the application method of biomass ash as a catalyst in the gasification process.
3. Biomass Ash as a Catalyst in the Gasification Processes
With the projected increasing use of biomass, especially through combustion or co-combustion, new directions should be sought to diversify ash management. One way to dispose of it efficiently is to be able to use biomass ash as a catalyst in the gasification of solid fuels. The evaluation of this issue was carried out based on the physicochemical properties presented in Chapter Two, which confirmed the catalytic potential of biomass ashes. A literature review was conducted on the use of ash from biomass combustion as a catalyst in gasification reactions. The analysis included the effect of the ash additives on three key aspects, namely, improving reactivity, the quality of the resulting gas, and the kinetics of the process.
Gasification technology involves the thermochemical conversion of solid fuels to syngas using a gasification agent, i.e., steam, carbon dioxide, oxygen, air, or mixtures thereof. As a result of the reactions, gaseous products (mainly H2, CO, CO2, CH4, and light hydrocarbons) are obtained with a wide range of utility potential [109]. Due to the nature of the products obtained, this process is not only one of the methods that allows for energy recovery (generation of heat and electricity), but is also considered for material recovery, because syngas can be used to produce substitutes for conventional fuels, as well as syntheses of chemicals [110]. Gasification is one of the well-studied fuel conversion processes, but the idea of catalyzing the reactions is still the subject of much scientific research, as evidenced by the growing number of scientific publications on this subject. The purpose of using a catalyst for the gasification reaction is to reduce the activation energy, which leads to an increase in the reaction rate and in the maximum conversion degree, as well as a reduction in the reaction time, an increase in the yield of gaseous products, and a decrease in the process temperature, which reduces the cost of the process while increasing its efficiency [110,111,112]. However, it is worth remembering that catalytic activity depends on a large number of factors, i.e., the type of active compounds, their concentration and dispersion, particle size and surface properties, and the method of feed to the fuel [110,113]. The continuously problematic aspects of catalytic gasification include the possibility of recovering the catalyst and its activity in the next cycle of operation, which is, in fact, an important subject of research on the process. Another important area of study is the search for new catalytic materials which, in view of the problems associated with catalyst regeneration, will make it possible to provide a single-use catalytic material that is easily accessible and affordable.
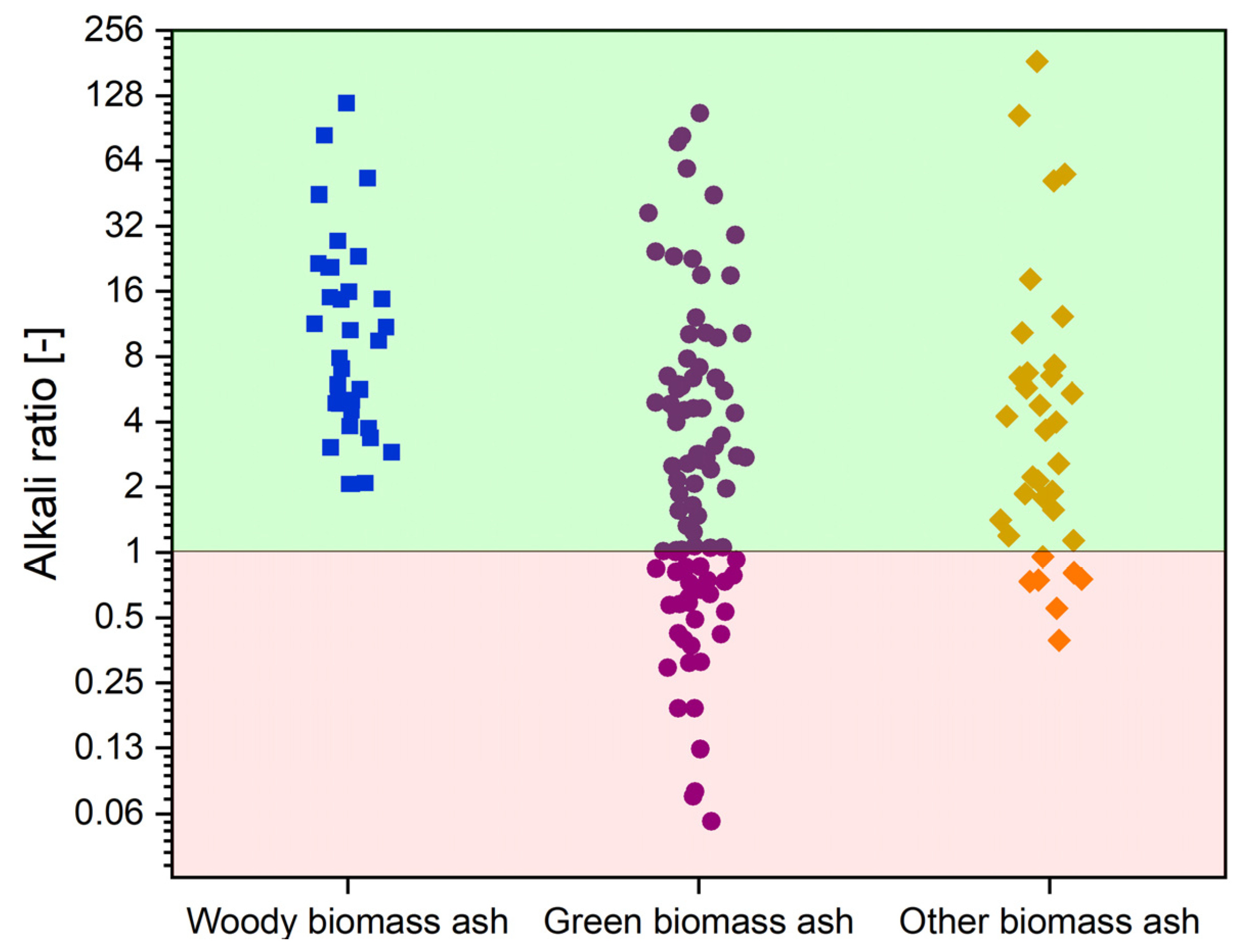
With the benefits thus defined and the problems arising from the use of catalysts in gasification technology, it is worth presenting the key aspect supporting the use of biomass ash as a catalytic material, namely its chemical composition. The components catalytically active in gasification reactions mainly include alkali metal (Na, K), alkaline earth (Mg, Ca), or some metals, such as Fe [110,114]. Their presence contributes most to the improvement of the values of parameters that describe the course of the gasification reactions. When considering a given material as a catalyst, it is also worth noting the content of components that have negative effects on the gasification process, such as Si or Al [110,114]. To demonstrate the catalytic potential of biomass ash, Figure 7 summarizes the values of the alkali ratio, calculated as the ratio of the content of catalytic active elements to the content of inhibitory elements for the three types of biomass, based on the data collected in Table A1, which is an appendix to the paper.
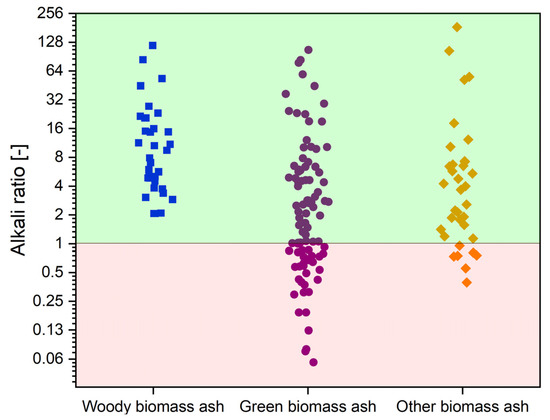
Figure 7.
Alkali ratio values for green biomass ash (GBA), woody biomass ash (WBA), and other biomass ash (OBA). Own analyzed based on [17,72,77,78,79,80,81,83,84,85,86,87,88,89,90,91,92,94,95,96,98,99,100,101].
As shown in Figure 7, the area is divided into two zones. In the red zone, where the alkali ratio takes values from ~0 to 1, are the biomass ashes in which elements that negatively affect catalytic activity play a dominant role. The green zone with a range of 1 to 256 contains biomass ashes characterized by a predominance of catalytically active elements. It can be concluded that most of the ashes presented have a predominance of active elements. This group includes WBA and the bulk of OBA. In the case of GBA, the most varied values of the alkali index are observed, from ashes with clearly dominant Si and Al content to ashes with a favourable composition. For this reason, most biomass ashes can be considered an attractive material for catalyzing gasification reactions.
In order to evaluate the impact of the use of biomass ash on the gasification process, Table 3, based on the review of the literature, collects the results of studies on the catalytic gasification process of various solid raw materials, taking into account the type and amount of ash and the operating conditions of the process and reactor type as well as a brief description characterizing the studies carried out in the publication.

Table 3.
Studies on using biomass ashes as catalysts during gasification.
As indicated by the data presented in Table 3, it can be seen that studies of the catalytic gasification process using biomass ash have been conducted for a wide range of solid fuels ranging from highly reactive biomass to coals and char obtained by pyrolysis of biomass, solid petroleum products, or used tyres. The most commonly used method of gasification examinations is thermogravimetric analysis, although fluidized bed and fixed bed reactors have also been used. The presented studies mainly assess the effect on the process of temperature and, less frequently, total pressure, type, and amount of catalytic additive. Of the gasifying agents used, steam and carbon dioxide are used most frequently, while air is used less often. In the conducted studies, green biomass ash dominates as a catalytic additive, and its amount varies widely from 0.01 to 80.00%.
3.1. Reactivity Analysis
Reactivity is a key factor determining the course of the gasification process, as it enables the assessment of its efficiency as well as cost effectiveness; hence it is an introduction to the analysis of catalytic gasification of solid fuels with biomass ash. Brown et al. [115] conducted thermogravimetric studies of CO2 gasification of coal char in the presence of 0, 10, 25, 50, and 75% of two materials—switchgrass ash and quartz sand. The authors observed that compared to the process with the use of quartz sand, the gasification rate was increased by increasing the amount of switchgrass ash. In addition, they performed co-gasification tests of coal char and switchgrass char, which also proved to increase the reaction rate. The authors found the highest gasification rate, ranging from 3%/min (10% ash) to 7.5%/min (75% ash), was obtained with switchgrass ash, demonstrating that switchgrass ash was the most effective additive as compared to quartz sand, for which the reaction rate did not exceed 3%/min, regardless of the amount of additive. An analogous catalytic effect was reported by He et al. [127], who studied the thermogravimetric gasification of petroleum coke in the presence of cotton straw ash using CO2 as a gasifying agent. In addition, the authors also noted that compared to methods based on the washing or leaching of active compounds from ash, the method of physical mixing of biomass ash and petroleum coke gives the best results in terms of gasification rate. Also, Li et al. [125] confirmed an increase in reaction rates for catalytic measurements with a growing share of biomass ash (corn stalk and poplar sawdust) during thermogravimetric gasification of anthracite with CO2. A better result was obtained for corn stalk ash, which has lower amounts of SiO2 and Al2O3, as well as higher amounts of K2O, Na2O, and MgO compared to poplar sawdust ash. On the other hand, Lahijani et al. [116], subjecting palm shell char to thermogravimetric carbon dioxide gasification in the presence of palm empty fruit bunch ash (5.0, 7.5, 10.0, and 12.5%), observed an increase in the maximum of the carbon conversion degree from about 90% for the noncatalytic sample to 100% with ash. This effect was achieved regardless of the amount of catalyst additive. In addition, the presence of biomass ash also resulted in shorter reaction times compared to the noncatalytic process, and the most favourable results were obtained for 10% ash addition. A similar trend was obtained by Yu et al. [126] when studying the thermogravimetric method and using CO2 gasification of petcoke in the presence of rice straw ash and cotton straw ash [102]. They confirmed a significant catalytic effect observed by higher conversion rates, resulting in a shorter reaction time. Better catalytic activity was recorded for ash with a higher alkali ratio, i.e., cotton straw (~38). Qin et al. [124] showed that CO2 gasification by the TGA method of coal char before and after demineralization of the mineral matter, using rice straw as a catalytic additive, resulted in a greater catalytic effect for the coal char sample without the inorganic fraction. Moreover, for the demineralized sample, the catalytic effect occurred with less catalyst, suggesting that the ash components present in the coal char ash may have had an inhibitory effect on the process. Czerski et al. [104] studied the gasification of tyre char using 5, 10, and 15% of selected biomass ashes (sunflower husk, beech chips, beet pulp, and corn cobs) as catalytic additives by the thermogravimetric method in a CO2 atmosphere and showed that the presence of ash contributed to a shift in TGA conversion curves toward lower temperatures, regardless of the additive amounts. Moreover, the amount of catalyst improved the reactivity indicators (decrease in the temperature of 50% carbon conversion and increase in the constant rate of CO2 gasification reaction), while the most favourable values were achieved for 15% corn cob ash. In turn, Wei et al. [105], gasifying bituminous coal char and anthracite char with the addition of 10% rice straw ash using a thermogravimetric method and in a carbon dioxide atmosphere, showed an increase in the reactivity index value R0.9 for the ash-catalyzed process compared to the noncatalytic gasification. The catalytic effect occurred regardless of the type of raw material that was gasified, and the difference in favour of the catalytic measurements increased as the process temperature rose. The catalytic properties of biomass ash were also observed during thermogravimetric gasification studies of pinewood char with biomass ash in a steam atmosphere performed by Nanou et al. [117]. They indicated an increase in the maximum conversion degree from about 15% for the raw sample to 100% for ash-catalyzed samples. In addition, as the catalyst share increased from 14.1 to 55.5%, the reaction time decreased to values of 1180 s and 720 s, respectively. Śpiewak et al. [129,130,133] performed steam gasification of char from tyre pyrolysis with 5, 10, and 15% ashes from sunflower husk, beet pulp, beech chips, and corn cobs in a fixed bed reactor using a thermovolumetric method at pressures of 0.5 and 1.0 MPa and temperatures ranging from 800 to 1000 °C. The authors showed that adding a catalyst and increasing its loading up to 15 wt% improved the reactivity of the tyre char (higher maximum carbon conversion with lower half time conversion) at temperatures up to 850 °C. At the highest temperature, however, the catalytic effect was insignificant and was observed mainly for 5 or 10 wt% of catalysts. In addition, the more significant catalytic effect of the presence of biomass ashes was demonstrated at higher total pressure (higher values of R0.5 and the maximum conversion degree). Vilches et al. [120] subjected biomass char to gasification under a steam atmosphere in a fluidized bed reactor. They used olivine as well as olivine coated with ash from the gasification of biomass char as catalysts. The results indicate that olivine coated with biomass ash shows higher reactivity than when olivine alone is used, because the gasification rate is about twice as high.
In conclusion, regardless of the material tested, the gasification agent, or the reactor used, the addition of biomass ash had a beneficial effect on improving reactivity during gasification, noted by reducing the process time, increasing the conversion degree, or improving the reactivity indices.
3.2. Analysis of the Quality and Quantity of Gaseous Products
Characterizing the quality and composition of the gas obtained from the gasification reactions is also an important factor in assessing the course of this process, because it determines its possible applications. The requirements for biomass catalysts in the context of process gas quality focus on increasing the yield of gaseous products; increasing the selectivity of the reactions, primarily towards hydrogen formation; and obtaining gas with precisely defined parameters (Higher Heating Value—HHV, H2/CO, and CO/CO2 ratios). Table 4 presents the shares of gaseous products obtained during noncatalytic and catalytic gasification examinations of various carbon materials using biomass ash as well as the calculated parameters characterizing process gas quality.

Table 4.
Quality and quantity of the gases obtained during catalytic gasification with biomass ash.
Analyzing the data presented in Table 4, it can be seen that the addition of biomass ash significantly affects the composition of the gaseous products of gasification. Rizkiana et al. [118] performed steam gasification of brown coal in a fixed bed reactor with three types of biomass ash: brown seaweed, rice straw, and eel grass. Evaluation of the yields of gas products indicated significant improvements in efficiency, especially H2 and CO2. Regardless of the set temperature value (550, 650, and 750 °C), the most favourable catalytic effect was achieved in the presence of brown seaweed ash. As a result, an increase in the hydrogen and carbon monoxide shares was obtained for each temperature, with a decrease in the methane shares. As a consequence, the addition of biomass ashes increased the H2/CO as well as the CO/CO2 ratio and decreased HHV in particular at 550 and 650 °C. At the highest temperature, the values of the HHV, as well as hydrogen to carbon monoxide ratio and carbon monoxide to carbon dioxide ratio, were varied similarly during the catalyzing process compared to noncatalytic gasification. The authors attributed the high activity of brown seaweed ash to its potassium content (at 17.7%, the highest content of the various ashes) and the absence of Si and Al (0.0%) in it. The authors also showed that brown seaweed ash and eel grass ash had good stability under gasification conditions. This is evidenced by the fact that, despite a thrice-repeated regeneration, the yield of gaseous products still exceeded the yield of components obtained by the noncatalytic process. However, a direct comparison of the two ashes showed some differences in catalytic activity. In the subsequent regeneration cycle, eel grass ash provided similar gas product yields but demonstrated greater stability. Researchers attributed this to differences in potassium content, as this component is highly volatile, which could have reduced the activity of brown seaweed ash in the next operation cycle. Śpiewak et al. [130], studying the steam gasification of tyre char in the presence of 5, 10, and 15% ash from sunflower husks, beet pulp, beech chips, and corn cobs, noted that the greatest catalytic effect of biomass ash was achieved in the low-temperature range (800–900 °C), resulting in an increase in CO yields (800 and 900 °C), as well as H2 and CO2 (800–850 °C). The highest hydrogen and carbon dioxide yields were generally observed for beech chip ash addition, suggesting the highest water gas shift reaction (WGSR) activity. In the case of carbon monoxide, the highest yields were achieved in the presence of sunflower husk ash, suggesting significant activity toward the conversion reaction. As the analysis of biomass ash composition showed, sunflower husk ash and beech chip ash have the highest alkali ratio values (34.8 and 16.2, respectively). These relationships are confirmed by the shares of gas products shown in Table 4. As the amount of catalytic additive increased, a decrease in the value of the H2/CO ratio and an increase in the CO/CO2 ratio was observed. In contrast, HHV values were very similar regardless of the amount of ash catalyst used. Increased yields of hydrogen and carbon dioxide and thus the shares of these gas products in the presence of biomass ash have also been observed in other research works [131,132]. Furthermore, Guo et al. [128], when gasifying corn straw in the presence of varying amounts of corn straw ash and in an air atmosphere, observed that the addition of a catalyst resulted in an increase in the shares of CO, H2, and CO2 compared to the noncatalytic process, as a result of which the calorific value of the obtained gas increased. It is also worth noting that improvement in the quantities of the main gasification products was observed with catalyst content of 2, 5, 10, and 15%. At the highest amount of catalyst used of 20%, the authors noted a decrease in the shares of the above reaction products, which resulted in an increase in tar yield and a decrease in gas yield and calorific value. In general, studies that evaluate the catalytic potential of ash in the gasification of solid fuels have reported a decrease in the hydrocarbon yields, which is particularly important in the context of using the gasification products as a synthesis gas or hydrogen source. Pissot et al. [121] and Vilches et al. [122], gasifying different carbonaceous materials in a fluidized bed reactor, observed that ash-coated olivine contributes to the reduction in tar yields due to its steam reforming. The studies presented above show that biomass ash effectively contributes to catalyzing heterogeneous reactions (especially conversion reactions) and homogeneous reactions (especially WGSR) and improves the quality of the resulting gas by increasing the hydrogen shares and as a consequence increasing the H2/CO ratio. Simultaneously, the authors note the transformation of the physicochemical structure of biomass ash, affecting the catalytic activity. Gomes et al. [132] studied steam gasification of residual forest biomass in the presence of biomass fly ash and reported a decrease in carbon monoxide yields with a simultaneous increase in CO2 and H2 yields. As a result, an increase in the H2/CO ratio was obtained. The authors attributed the observed effect to an increase in the significance of the WGSR due to the presence of catalytically active CaO and CaCO3. An additional advantage of using biomass fly ash was the adsorption of CO2 by calcium oxide, which led to the enrichment of the obtained gas with hydrogen.
In summary, regardless of the material tested, the catalytic additive, the type of reactor, and the values of the process parameters, the presence of biomass ash promotes an increase in the efficiency of the conversion reaction and WGSR, resulting in increased yields of CO, CO2, and H2 as well as H2/CO ratio, while simultaneously reducing the yield of hydrocarbons.
3.3. Analysis of Kinetics
The gasification process is a complex series of heterogeneous and homogeneous reactions influenced by a number of technological and material factors. For these reasons, the kinetics of the process are an important factor in assessing the catalytic efficiency of biomass ash. Yu et al. [126], analyzing the kinetics of the gasification process of petcoke in the presence of rice straw ash and cotton straw ash using the random pore model (RPM) and the Shrinking Core Model (SCM), noted an increase in the values of the reaction rate constants compared to the noncatalytic process, regardless of the set temperature value. Czerski et al. [104] obtained a similar relationship for all biomass ashes analyzed (sunflower husk, beet pulp, beech chips, and corn cobs) during CO2 gasification of char from tyres using the n-order Coats and Redfern method. Furthermore, it has been demonstrated that as the biomass ash content increases from 5% to 15%, the value of the constant reaction rate increases, which indicates an improvement in the efficiency of the CO2 gasification process. In general, a reduction in the calculated values of activation energy and the pre-exponential factor was also obtained in comparison with the noncatalytic process. Lahijani et al. [116] determined the kinetics of the CO2 gasification process using the RPM of palm shell char in the presence of palm empty fruit bunch ash in amounts of 5.0, 7.5, 10.0, and 12.5%, where they observed a decrease in activation energy from 268.11 kJ/mol to 158.75 kJ/mol compared to the no catalyst process. The same effect of using biomass ash from cotton straw on the activation energy value during gasification of petroleum coke was also noted by He et al. [127] using the modified random pore model (MRPM). In turn, Brown et al. [115] pointed out that with reduction in the activation energy caused by the presence of switchgrass ash during coal char gasification, the value of the pre-exponential factor decreased as well. In a study conducted by Yu et al. [105,126] comparing the effects of rice straw ash and cotton straw ash addition on gasification, lower kinetic parameters were obtained for ash with higher potassium content (cotton straw). They also proved that high catalytic activity occurred regardless of the progress of the reaction. In turn, Wei et al. [105,126] confirmed the effectiveness of a rice straw ash catalyst rich in potassium compounds in improving the kinetics of the gasification process compared to the noncatalytic process. Śpiewak et al. [129,130] conducted catalytic steam gasification of tyre char with the addition of various types and amounts of biomass ash and obtained a decrease in activation energy calculated using the grain model (GM) and the random pore model (RPM). It is also interesting that as the amount of the catalytic additive increases from 5 to 15%, the kinetic parameters, i.e., activation energy and pre-exponential factor, generally increase. Previous analysis of the reactivity and quality of the gas obtained showed a noticeable catalytic effect with increasing amounts of biomass ash, and the increase in activation energy was compensated by the increasing value of the pre-exponential factor. This unusual effect has been attributed to two phenomena. The first is the catalyst moving on the surface of the solid fuel to reach the next active sites, which requires some additional energy. The second factor identified is the channelling and pitting action of the catalyst, which is necessary to disrupt the aromatic structure of carbon. A similar compensation effect for these materials was also noted in the aforementioned publication by Czerski et al. [104].
In summary, it can be concluded that the use of various biomass ash materials as catalysts during the gasification process affects the kinetic parameters. An improvement in constant reaction rates was observed, while different results were obtained regarding their impact on the activation energy and the pre-exponential factor. However, a comprehensive analysis of these parameters showed that biomass ash had a beneficial effect on the gasification reaction process.
3.4. Biomass Ash as a Composite Catalyst
Biomass ash can be considered as a composite catalyst containing several active components. Studies on the catalytic gasification of solid fuels using single-component additives has often indicated the deactivation of this component and, consequently, of the catalyst. In the case of alkali metals, a strong tendency toward melting was observed, which limited the surface area available for the gasifying agent and caused evaporation in the reaction area [135,136,137,138,139]. Alkaline earth metals, however, show special abilities in terms of deactivation through sintering and agglomeration, as well as interaction with silicates and aluminosilicates [135]. Despite their activity, transition metal elements are rarely used as catalysts in the gasification process because of their high price. For these reasons, iron is used mainly in studies on catalytic gasification. The disadvantages of iron catalysts include low dispersion and a tendency to form mesoporous structures, limiting the surface area available for the gasification agent [135]. The biomass ashes are treated as composite catalysts, generally containing more than one active component, as evidenced by the data compiled in Figure 2, where the average contents of various elements confirm the presence of up to five catalytically active components (K, Na, Ca, Mg, and Fe). Studies on the use of composite catalysts during the gasification process have shown that their activity is higher compared to that of single-component catalysts [114,140,141]. Furthermore, the presence of more than one active component is intended to overcome the negative effects associated with the performance of a single component and to ensure a synergistic effect [114].
3.5. Deactivation of Biomass Ash Catalyst
Despite the multicomponent composition of chemically active elements, biomass ash can also be deactivated during gasification reactions. Kryca et al. [119] and Gomes et al. [132] observed the possibility of deactivation of ash catalysts coating the surface of olivine, which forms the fluidized bed in gasifiers. The formation of carbon deposits on the surface of the catalyst and its contamination with sulphur or nitrogen compounds were identified as the main causes. As a way to limit catalyst deactivation, the authors suggested bed circulation between the gasification reactor and the combustion reactor, where the active layer is regenerated by burning the impurities covering the surface. The positive effect of recirculation on the performance of the ash catalyst was also reported by Pissot et al. [121]. The temperature of the process is of significant importance in the deactivation of active components. The results of a study conducted by Wei et al. [105] during the gasification of lignite and anthracite in the presence of 10% rice straw ash, which is particularly rich in potassium, proved that the vaporization capacity of AAEM increases as the process temperature is increased up to 1000 °C. However, the results obtained by Śpiewak et al. [130] show that potassium, in particular, has a high ability to melt and clog pores on the surface of the raw material and evaporate. Calcium, on the other hand, shows a high affinity for forming larger agglomerates. In addition, elements of the first and second groups of the periodic table can form inactive silicates and aluminosilicates [118,129]. However, according to the authors of the papers presented above, the negative effects associated with the deactivation of some components of biomass ashes do not rule out the possibility of their use in the gasification process, but their use, however, requires the selection of appropriate processes and technological conditions.
3.6. Advantages and Disadvantages of Biomass Ash as a Catalyst in Gasification Processes and Recommendations for Future Studies
To conclude the potential for using biomass ash in the catalytic gasification of solid fuels, Table 5 summarizes the advantages and disadvantages of this technological solution.

Table 5.
Advantages and disadvantages of the use of biomass ash catalysts in gasification technology.
Considering the advantages summarized, it is undoubtedly worth emphasizing the low costs associated with the use of biomass ash resulting from both the wide availability and waste character of the material, as well as the simplicity of preparation in the case of use in fluidized bed reactors or the lack of any preparation when used by physical mixing in fixed bed reactors. In addition to the low operating cost, the most important factor is favourable chemical composition, as biomass ashes generally have high contents of catalytically active elements, i.e., K, Na, Ca, Mg, and Fe, while having low contents of Si and Al, considered to be reaction inhibitors. The diversity of catalytic components means that biomass ash can be classified as a composite catalyst, showing properties that are more favourable compared to those of single-component catalysts. Unfortunately, a characteristic of biomass is the variability in the composition of the mineral matter in it, which can cause differences in the proportions of active compounds and inhibitors. In addition, biomass ash also has elements that are considered toxic, which can also contribute to an increase in their emissions as well as the contamination of gaseous products. Of the disadvantages, low melting points should also be noted, especially in the situation of a predominant content of the elements such as K and Na, which can become a reason for deactivation by means of melting or evaporation. Nevertheless, in the vast majority of literature reports, ash catalysts are characterized by high catalytic activity improving parameters that characterize reactivity, the quality of the process gas, and the kinetics of the gasification process. In addition, they are catalytically active in both heterogeneous and homogeneous reactions, leading to increased H2 yields, among other things. In the case of fluidized bed reactors, there is also the possibility of regenerating the catalysts, which makes it possible to reuse them.
Thus, it can be concluded with certainty that biomass ash can be an attractive catalyst during gasification of diverse materials, especially low-reactivity ones. Their disadvantages notwithstanding, they also have a number of advantages, which, in the opinion of the authors, will enable their effective application in the gasification process to improve its efficiency and the quality of the obtained gas. However, in order to implement such a solution, it is necessary to deepen our knowledge in the aspect of catalytic gasification using biomass ash, especially in terms of the following:
- Expanding the range of potential catalysts to include new, untested biomass ash along with gasified feedstocks;
- Verification of physicochemical properties and catalytic activity for the same type of biomass with different origins;
- Studies on physicochemical transformations during reactions in a reducing atmosphere (gasification agent) under variable pressure and temperature conditions with special emphasis on activity, deactivation capacity, and emission of toxic elements;
- Developing the optimal form and method of catalyst feed;
- Estimating the cost of implementing such a technology solution.
4. Conclusions
Due to its energy potential and carbon neutrality, biomass is one of the most promising solid fuels categorized as a renewable energy source. The by-product of biomass combustion and co-combustion—ash—can be effectively used to improve the efficiency and quality of the gasification process, which is an alternative to the existing ash disposal methods. This paper demonstrates the potential of biomass ash as natural catalysts for solid fuel gasification technologies. The catalytic potential of biomass ash can significantly contribute to overcoming the main limitations associated with the commercialisation of catalytic gasification of solid fuels. Based on an analysis of the physicochemical properties of biomass ash as well as the results of research on the catalytic gasification of solid fuels, biomass ash can be classified as a highly attractive process-supporting material. The addition of ash catalysts affects the gasification process by improving reactivity, gas quality and process kinetics. During the conducted studies, an increase in the maximum conversion degrees and a reduction in the gasification reaction time were observed, as well as a reduction in process temperature and an improvement in parameter values, i.e., reactivity indices and fractional conversion times. An increase in the yield of gaseous products, in particular H2 and CO, has been shown, which proves the catalytic effect of biomass ash during the conversion and the water–gas shift reactions. There is also an improvement in the constant rate of the gasification reaction and a positive change in kinetic parameter values that has been noted. The benefits of using biomass ash in gasification technology seem to outweigh the disadvantages presented. The implementation of this solution requires further studies related to the search for new biomass ashes, verification of physicochemical properties and catalytic activity, as well as the development of a feeding system. At the same time, solutions should be sought to limit the disadvantages of catalysts based on biomass ash, i.e., primarily their deactivation and the presence of hazardous elements.
Author Contributions
Conceptualization, P.S. and G.C.; methodology, P.S. and G.C.; formal analysis, P.S. and G.C.; writing—original draft preparation, P.S. and G.C.; writing—review and editing, G.C.; visualization, P.S.; supervision, G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by AGH University of Krakow, Faculty of Energy and Fuels, Research Subsidy, No. 16.16.210.476.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
Appendix A.1

Table A1.
Dominant element content with average values in ashes from (a) green biomass (GBA), (b) woody biomass (WBA), and (c) other biomass (OBA). Own analysis based on: [17,72,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101].
Table A1.
Dominant element content with average values in ashes from (a) green biomass (GBA), (b) woody biomass (WBA), and (c) other biomass (OBA). Own analysis based on: [17,72,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101].
| Types of Ashes | Content of Component [wt. %] | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na | K | Mg | Ca | Fe | Si | Al | S | P | Ti | Mn | Zn | Cl | |
| Green biomass | |||||||||||||
| [78] | |||||||||||||
| Vineyard pellet ash | 0.46 | 24.98 | 6.30 | 30.30 | 0.50 | 1.22 | 0.48 | 1.58 | 3.21 | 0.04 | 0.12 | ||
| [81] | |||||||||||||
| Soybean plant ash | 0.44 | 15.99 | 9.58 | 13.38 | 4.54 | 3.47 | 1.02 | 0.61 | 5.01 | 0.30 | 0.10 | 0.03 | |
| Mustard plant ash | 1.10 | 13.76 | 4.44 | 22.41 | 1.18 | 1.54 | 0.36 | 3.09 | 4.88 | 0.07 | 0.04 | 2.72 | |
| Maize plant ash | 0.54 | 5.58 | 4.22 | 8.14 | 2.19 | 18.94 | 0.84 | 0.61 | 4.93 | 0.14 | 0.06 | 1.34 | |
| Groundnut plant ash | 0.55 | 6.28 | 8.43 | 12.83 | 9.02 | 10.19 | 3.40 | 0.51 | 5.76 | 0.63 | 0.14 | 0.03 | |
| Cotton plant ash | 0.93 | 20.35 | 3.14 | 21.83 | 1.25 | 1.46 | 0.49 | 1.10 | 3.06 | 0.09 | 0.09 | 4.56 | |
| Wheat plant ash | 0.31 | 5.00 | 1.36 | 2.97 | 1.96 | 27.21 | 0.47 | 0.56 | 1.13 | 0.11 | 0.05 | 1.85 | |
| Pigeon pea plant ash | 0.39 | 10.12 | 7.06 | 15.67 | 3.16 | 2.81 | 0.74 | 0.41 | 12.46 | 0.18 | 0.09 | 0.14 | |
| Groundnut shell ash | 0.58 | 4.25 | 3.89 | 8.01 | 12.15 | 12.95 | 5.61 | 0.86 | 2.32 | 0.80 | 0.19 | 0.13 | |
| [82] | |||||||||||||
| Virginia mallow ash | 13.92 | 27.66 | 0.54 | 1.30 | 1.68 | 0.09 | 0.02 | ||||||
| Jerusalem artichoke ash | 15.44 | 19.33 | 0.25 | 0.79 | 0.32 | 0.01 | 0.05 | ||||||
| Multiflorous rose ash | 13.94 | 21.73 | 0.19 | 0.63 | 2.62 | 0.08 | 0.04 | ||||||
| Miscanthus giganteus ash | 8.75 | 4.65 | 0.25 | 0.75 | 2.48 | 0.06 | 0.02 | ||||||
| Miscanthus sacchariflorus ash | 4.97 | 3.64 | 0.45 | 0.25 | 2.13 | 0.05 | 0.05 | ||||||
| Prairie cordgrass ash | 3.26 | 7.89 | 0.48 | 0.14 | 2.29 | 0.07 | 0.03 | ||||||
| Common reed ash | 4.15 | 5.51 | 0.32 | 0.11 | 1.16 | 0.11 | 0.06 | ||||||
| Switch grass ash | 5.36 | 4.84 | 0.54 | 0.22 | 1.49 | 0.12 | 0.05 | ||||||
| Wheat straw ash | 6.93 | 4.21 | 0.49 | 0.60 | 1.01 | 0.03 | 0.02 | ||||||
| Triticale straw ash | 18.76 | 0.38 | 0.77 | 0.78 | 2.62 | 0.05 | 0.02 | ||||||
| Oat straw ash | 9.22 | 4.62 | 0.20 | 0.34 | 1.64 | 0.03 | 0.01 | ||||||
| Barley straw ash | 10.86 | 8.42 | 0.14 | 0.79 | 0.22 | 0.03 | 0.04 | ||||||
| Buckwheat straw ash | 13.11 | 7.80 | 0.27 | 0.69 | 2.27 | 0.03 | 0.02 | ||||||
| Hay ash | 17.52 | 6.55 | 0.27 | 0.84 | 2.48 | 0.07 | 0.04 | ||||||
| Cherry pit ash | 19.37 | 9.17 | 0.93 | 1.45 | 0.95 | 0.07 | 0.08 | ||||||
| Sunflower husk ash | 14.18 | 19.03 | 0.31 | 0.97 | 2.78 | 0.04 | 0.01 | ||||||
| Rape pod ash | 12.95 | 27.73 | 0.16 | 0.61 | 1.42 | 0.03 | 0.00 | ||||||
| Apple pomace ash | 14.37 | 1.84 | 0.17 | 19.31 | 3.32 | 0.03 | 0.05 | ||||||
| Hazelnut shell ash | 18.45 | 10.91 | 0.21 | 0.92 | 1.57 | 0.01 | 0.03 | ||||||
| Walnut shell ash | 18.50 | 7.28 | 0.11 | 0.44 | 1.20 | 0.01 | 0.01 | ||||||
| [83] | |||||||||||||
| Straw ash | 0.60 | 48.80 | 0.40 | 1.90 | 0.11 | 0.07 | 4.18 | 0.72 | 0.01 | 0.03 | 20.80 | ||
| [142] | |||||||||||||
| Straw winter wheat ash | 0.40 | 14.50 | 4.40 | 7.80 | 2.20 | 0.01 | |||||||
| Cereals triticale ash | 0.50 | 14.20 | 4.30 | 7.10 | 9.80 | 0.01 | |||||||
| [86] | |||||||||||||
| Olive cake ash | 0.45 | 26.81 | 1.81 | 7.36 | 0.63 | 5.18 | 0.64 | 0.96 | 2.18 | ||||
| Bagasse ash | 0.15 | 3.07 | 0.90 | 2.07 | 2.10 | 22.97 | 3.60 | 0.32 | 0.61 | ||||
| [88] | |||||||||||||
| Groundnut ash | 4.15 | 7.98 | 3.68 | 7.73 | 2.77 | 19.93 | 5.67 | 1.83 | 0.33 | 1.44 | |||
| Bagasse ash | 0.04 | 1.44 | 1.97 | 1.97 | 0.34 | 30.05 | 0.26 | 0.50 | 0.05 | 0.12 | |||
| Arecanut ash | 0.15 | 15.70 | 0.29 | 0.88 | 2.94 | 19.62 | 2.04 | 3.18 | 0.04 | 3.70 | |||
| Cashew shell ash | 3.89 | 17.98 | 6.43 | 5.37 | 1.44 | 3.78 | 1.62 | 6.40 | 0.07 | 1.99 | |||
| Rice husk ash | 0.30 | 1.99 | 0.28 | 0.49 | 0.36 | 43.22 | 0.01 | 0.46 | 0.02 | 0.11 | |||
| [89] | |||||||||||||
| Bambusa blumeana ash | 0.58 | 20.89 | 1.67 | 2.68 | 0.55 | 25.13 | 0.95 | ||||||
| Eucalyptus skin ash I | 0.57 | 6.65 | 5.15 | 41.47 | 0.13 | 0.35 | 0.16 | ||||||
| Miscanthus floridulus ash | 0.50 | 16.10 | 2.44 | 4.22 | 0.92 | 24.56 | 3.63 | ||||||
| Dendrocalamus latiflorus ash | 0.30 | 12.25 | 1.44 | 3.72 | 0.21 | 30.91 | 0.40 | ||||||
| Eucalyptus skin ash II | 1.09 | 2.72 | 3.09 | 19.17 | 3.76 | 18.94 | 5.17 | ||||||
| Palm skin ash | 0.33 | 1.24 | 3.45 | 42.47 | 0.45 | 5.59 | 2.48 | ||||||
| Pine needle ash | 0.54 | 6.56 | 7.06 | 23.62 | 2.97 | 10.29 | 4.07 | ||||||
| Broussonetia papyrifera ash | 0.09 | 14.48 | 3.52 | 30.20 | 0.78 | 7.99 | 0.67 | ||||||
| Eucalyptus skin ash III | 1.39 | 19.31 | 3.60 | 22.85 | 1.18 | 5.56 | 2.72 | ||||||
| Corn straw ash I | 0.79 | 34.05 | 3.93 | 8.56 | 0.63 | 10.41 | 0.52 | ||||||
| Corn straw ash II | 0.60 | 8.15 | 6.65 | 9.81 | 1.06 | 23.13 | 1.57 | ||||||
| Corn straw ash III | 0.32 | 21.79 | 4.74 | 6.84 | 0.43 | 20.13 | 0.60 | ||||||
| Corn straw ash IV | 0.79 | 34.05 | 3.93 | 8.56 | 0.63 | 10.41 | 0.52 | ||||||
| Corn straw ash V | 0.91 | 9.14 | 1.57 | 7.02 | 2.45 | 20.14 | 4.93 | ||||||
| Corn straw ash VI | 0.62 | 9.24 | 12.82 | 13.99 | 1.12 | 13.22 | 1.55 | ||||||
| Corn straw ash VII | 0.31 | 20.89 | 8.60 | 17.02 | 0.70 | 7.95 | 0.61 | ||||||
| Corn cob ash | 0.88 | 31.37 | 20.68 | 2.12 | 0.71 | 12.89 | 1.09 | ||||||
| Corn straw ash | 0.37 | 7.13 | 6.75 | 29.59 | 2.93 | 10.40 | 3.12 | ||||||
| Banana stem ash | 0.17 | 53.79 | 0.40 | 3.15 | 0.30 | 5.36 | 0.31 | ||||||
| Banana leaf ash | 0.39 | 13.78 | 1.89 | 34.38 | 1.20 | 9.39 | 1.26 | ||||||
| Straw ash | 0.70 | 14.19 | 2.36 | 4.67 | 0.30 | 28.14 | 0.17 | ||||||
| Wheat straw ash | 0.61 | 6.95 | 1.74 | 7.81 | 1.69 | 28.95 | 3.14 | ||||||
| Bambusa blumeana ash | 0.58 | 20.89 | 1.67 | 2.68 | 0.55 | 25.13 | 0.73 | ||||||
| Masson pine leaf ash | 0.12 | 3.75 | 5.07 | 33.44 | 2.70 | 6.11 | 3.05 | ||||||
| Camellia ash | 0.17 | 10.14 | 5.23 | 21.67 | 1.08 | 3.17 | 11.24 | ||||||
| Wheat straw ash | 0.59 | 8.97 | 2.11 | 9.58 | 1.31 | 27.53 | 3.53 | ||||||
| Fungus bag ash | 0.23 | 3.60 | 1.30 | 37.28 | 1.50 | 14.37 | 1.73 | ||||||
| Corn cob ash | 2.20 | 24.40 | 1.67 | 4.06 | 2.49 | 15.95 | 2.79 | ||||||
| Dry turf ash | 0.78 | 2.46 | 1.12 | 1.16 | 5.92 | 28.99 | 10.05 | ||||||
| Flue-cured tobacco rod ash | 0.61 | 37.59 | 1.67 | 30.67 | 0.38 | 0.70 | 0.21 | ||||||
| Pepper rod ash | 0.29 | 12.64 | 4.70 | 16.07 | 3.46 | 12.33 | 5.62 | ||||||
| Takesue ash | 0.31 | 9.21 | 1.15 | 1.85 | 0.70 | 35.12 | 0.59 | ||||||
| [91] | |||||||||||||
| Olive cake ash | 0.45 | 26.81 | 1.81 | 7.36 | 0.63 | 5.18 | 0.64 | 0.96 | 2.18 | 0.06 | 0.07 | ||
| [92] | |||||||||||||
| Rice husk ash I | 0.29 | 1.54 | 0.33 | 0.54 | 0.60 | 43.81 | 0.19 | 0.04 | 0.10 | 0.08 | |||
| Rice husk ash II | 0.09 | 1.43 | 0.26 | 0.55 | 0.24 | 44.36 | 0.17 | 0.03 | 0.07 | 0.01 | |||
| Rice straw ash I | 0.69 | 11.17 | 1.29 | 3.19 | 0.34 | 33.86 | 0.13 | 0.97 | 0.85 | 0.57 | |||
| Rice straw ash II | 0.60 | 8.99 | 1.01 | 3.32 | 0.22 | 35.59 | 0.12 | 1.06 | 0.73 | 0.13 | |||
| Corn cob ash I | 4.58 | 22.87 | 2.90 | 4.00 | 0.58 | 14.71 | 1.46 | 1.94 | 1.27 | 12.56 | |||
| Corn cob ash II | 4.32 | 20.00 | 4.48 | 4.35 | 0.45 | 15.83 | 1.29 | 2.06 | 1.73 | 9.95 | |||
| [93] | |||||||||||||
| Miscanthus ash | 0.54 | 0.07 | |||||||||||
| Sunflower husk ash | 0.57 | 0.06 | |||||||||||
| Wheat straw ash | 0.53 | 0.03 | |||||||||||
| [69] | |||||||||||||
| Rice husk ash | 0.17 | 3.45 | 0.30 | 1.00 | 0.29 | 41.53 | 0.55 | 0.52 | 0.26 | 0.01 | 0.17 | ||
| Switchgrass ash | 0.08 | 15.58 | 2.53 | 5.50 | 0.29 | 29.07 | 0.47 | 0.87 | 1.06 | 0.02 | 0.19 | ||
| Corn cob ash | 0.21 | 39.49 | 1.19 | 1.52 | 1.05 | 16.95 | 0.43 | 1.38 | 2.10 | 0.04 | 0.56 | ||
| Plum pit ash | 0.59 | 13.52 | 2.52 | 31.41 | 1.22 | 5.55 | 1.36 | 3.09 | 4.44 | 0.10 | 0.22 | ||
| Marine macroalgae ash | 10.44 | 6.28 | 4.64 | 15.77 | 0.48 | 2.59 | 0.52 | 11.41 | 0.42 | 0.02 | 9.67 | ||
| Beech wood chip ash | 0.08 | 14.94 | 4.52 | 44.73 | 0.25 | 2.53 | 0.26 | 0.31 | 1.09 | 0.02 | 0.07 | ||
| Walnut shell ash | 0.09 | 40.95 | 2.29 | 27.23 | 0.28 | 1.31 | 0.28 | 0.81 | 1.03 | 0.03 | 0.31 | ||
| Sunflower shell ash | 0.16 | 41.37 | 7.16 | 11.13 | 0.92 | 0.67 | 0.06 | 4.56 | 2.85 | 0.01 | 1.31 | ||
| [94] | |||||||||||||
| Cereal corps ash | 1.63 | 15.77 | 1.69 | 4.36 | 1.12 | 23.57 | 1.01 | 1.32 | 1.40 | 0.07 | 4.74 | ||
| Sugarcane ash | 0.82 | 6.97 | 1.87 | 3.79 | 5.59 | 24.96 | 5.82 | 1.32 | 1.05 | 1.26 | 0.33 | ||
| Soyabean ash | 1.26 | 17.43 | 3.38 | 12.15 | 1.47 | 9.71 | 1.80 | 1.20 | 2.09 | 0.05 | |||
| Woody corps ash | 0.89 | 16.60 | 3.86 | 30.02 | 0.60 | 1.99 | 0.74 | 1.04 | 4.06 | 0.06 | 0.51 | ||
| Grass ash | 0.43 | 18.26 | 2.41 | 4.57 | 0.49 | 24.50 | 0.37 | 0.84 | 1.75 | 0.19 | 3.35 | ||
| [95] | |||||||||||||
| Apple pomace ash | 1.17 | 7.94 | 1.17 | 6.64 | 5.70 | 21.20 | 9.25 | 0.95 | 1.47 | 0.23 | |||
| Sunflower husk ash | 0.19 | 5.68 | 0.16 | 0.86 | 1.12 | 35.75 | 6.27 | 0.08 | 0.17 | 0.02 | |||
| Walnut shell ash | 0.75 | 8.68 | 0.84 | 5.05 | 2.05 | 32.16 | 0.48 | 1.35 | 1.43 | 0.02 | |||
| [97] | |||||||||||||
| Corn straw ash I | 0.74 | 29.86 | 5.02 | 2.66 | 0.31 | 8.23 | 0.13 | 0.81 | 8.61 | 10.47 | |||
| Corns straw ash II | 4.18 | 16.89 | 6.60 | 3.75 | 0.68 | 11.25 | 0.25 | 0.60 | 11.79 | 2.40 | |||
| [98] | |||||||||||||
| Barley straw | 0.37 | 5.50 | 2.03 | 6.02 | 0.80 | 33.92 | 0.92 | 0.52 | 1.45 | 0.09 | 0.02 | ||
| Wheat straw | 0.22 | 13.47 | 2.15 | 4.75 | 0.16 | 31.88 | 0.38 | 0.58 | 0.79 | 0.01 | 0.03 | ||
| Sunflower seed husks | 1.20 | 31.14 | 10.72 | 14.91 | 0.18 | 0.73 | 0.26 | 3.10 | 4.56 | 1.00 | 0.02 | ||
| Soy straw | 0.07 | 12.34 | 16.74 | 27.40 | 0.20 | 1.53 | 0.42 | 1.36 | 4.89 | 0.02 | 0.01 | ||
| [17] | |||||||||||||
| Eudicot straw ash | 0.30 | 23.24 | 1.51 | 14.37 | 0.21 | 1.94 | 0.16 | 1.52 | 2.57 | 0.06 | |||
| Grass straw ash | 0.37 | 11.87 | 1.45 | 6.22 | 0.49 | 23.43 | 0.42 | 1.00 | 1.27 | 0.06 | |||
| [99] | |||||||||||||
| Corn stalk ash I | 8.38 | 4.40 | 7.29 | 0.91 | 14.74 | 1.16 | 1.44 | 1.31 | 22.10 | ||||
| Wheat stalk ash I | 1.99 | 2.41 | 7.79 | 1.54 | 22.42 | 1.38 | 1.56 | 0.00 | 15.52 | ||||
| Cotton branch ash I | 12.62 | 6.03 | 19.80 | 1.26 | 3.93 | 0.00 | 1.48 | 3.58 | 4.10 | ||||
| Corn stalk ash II | 15.77 | 5.49 | 9.65 | 1.68 | 20.66 | 1.48 | 1.68 | 1.61 | |||||
| Wheat stalk ash II | 7.22 | 2.77 | 10.29 | 1.96 | 28.15 | 2.12 | 0.64 | 0.00 | |||||
| Cotton branch ash II | 11.87 | 8.62 | 23.30 | 1.40 | 5.78 | 0.00 | 2.04 | 4.97 | |||||
| [72] | |||||||||||||
| Straw ash I | 0.81 | 12.66 | 1.57 | 4.82 | 0.32 | 29.89 | 0.15 | 1.04 | 0.96 | 0.02 | 3.72 | ||
| Straw ash II | 0.76 | 6.16 | 0.76 | 3.34 | 0.37 | 36.77 | 0.11 | 1.19 | 0.60 | 0.01 | 0.48 | ||
| Pine sawdust ash I | 0.33 | 1.10 | 0.96 | 25.64 | 2.05 | 5.47 | 1.04 | 15.79 | 0.31 | 0.10 | 0.16 | ||
| Pine sawdust ash II | 0.21 | 0.60 | 1.10 | 26.82 | 2.59 | 6.03 | 0.90 | 15.93 | 0.28 | 0.12 | 0.07 | ||
| Chinese Parasol Tree leaf ash I | 0.83 | 9.61 | 3.58 | 20.28 | 1.25 | 11.53 | 0.99 | 6.74 | 1.59 | 0.08 | 3.97 | ||
| Chinese Parasol Tree leaf ash II | 0.79 | 6.72 | 4.07 | 21.10 | 1.26 | 13.19 | 0.88 | 6.95 | 1.68 | 0.08 | 0.86 | ||
| [101] | |||||||||||||
| Straw ash | 1.71 | 9.25 | 0.81 | 2.18 | 4.70 | ||||||||
| Corn ash | 0.04 | 29.80 | 1.48 | 4.73 | 4.06 | ||||||||
| Eucalyptus ash | 1.38 | 7.71 | 6.58 | 41.27 | 1.03 | ||||||||
| Miscanthus ash | 0.04 | 9.55 | 0.00 | 7.15 | 0.70 | ||||||||
| Woody biomass | |||||||||||||
| [82] | |||||||||||||
| Birch wood ash | 0.71 | 13.26 | 0.65 | 0.56 | 2.09 | 1.76 | 0.02 | ||||||
| Pine wood ash | 11.64 | 20.11 | 0.37 | 0.71 | 1.86 | 1.07 | 0.02 | ||||||
| Oak wood ash | 5.73 | 15.67 | 0.93 | 0.51 | 0.53 | 1.01 | 0.02 | ||||||
| Hornbeam wood ash | 6.99 | 2.49 | 0.86 | 0.40 | 1.65 | 1.86 | 0.02 | ||||||
| Ash wood ash | 7.04 | 27.98 | 0.58 | 0.31 | 1.80 | 1.05 | 0.02 | ||||||
| Poplar wood ash | 6.50 | 17.39 | 0.46 | 0.50 | 0.64 | 0.05 | 0.01 | ||||||
| Willow ash | 3.73 | 13.60 | 0.27 | 0.47 | 0.33 | 0.09 | 0.04 | ||||||
| Acacia wood ash | 3.88 | 22.72 | 0.62 | 0.18 | 0.27 | 0.08 | 0.02 | ||||||
| Beech bark ash | 2.88 | 35.37 | 0.46 | 0.24 | 1.14 | 0.05 | 0.01 | ||||||
| Oak bark ash | 2.11 | 32.25 | 0.38 | 0.96 | 0.40 | 0.04 | 0.01 | ||||||
| Hornbeam bark ash | 2.47 | 27.76 | 0.11 | 0.00 | 0.35 | 0.03 | 0.02 | ||||||
| Ash bark ash | 2.32 | 31.14 | 0.09 | 0.33 | 0.51 | 0.03 | 0.01 | ||||||
| Acacia bark ash | 1.99 | 30.29 | 0.15 | 0.17 | 0.72 | 0.06 | 0.03 | ||||||
| [83] | |||||||||||||
| Wood ash | 1.60 | 16.50 | 1.30 | 17.10 | 0.70 | 0.70 | 3.51 | 0.78 | 0.60 | 0.85 | 7.20 | ||
| [142] | |||||||||||||
| Bark spruce ash | 0.80 | 5.10 | 6.50 | 42.20 | 1.70 | 0.06 | |||||||
| Wood chip spruce ash | 0.60 | 6.70 | 4.80 | 44.70 | 3.60 | 0.04 | |||||||
| [85] | |||||||||||||
| White wood ash | 1.11 | 7.64 | 2.83 | 17.72 | 1.61 | 12.52 | 2.43 | 0.64 | 0.87 | ||||
| [89] | |||||||||||||
| Sawdust ash I | 0.21 | 3.21 | 6.01 | 36.90 | 2.84 | 6.86 | 2.89 | ||||||
| Stripe wood ash | 0.44 | 13.53 | 4.93 | 14.97 | 2.97 | 12.89 | 4.74 | ||||||
| Pine branch ash | 0.40 | 2.71 | 6.13 | 26.08 | 2.36 | 4.90 | 2.82 | ||||||
| Fir branch ash | 0.43 | 5.91 | 5.50 | 34.23 | 2.19 | 6.01 | 2.09 | ||||||
| Camphor tree branch ash | 0.26 | 11.12 | 5.98 | 27.12 | 1.24 | 4.28 | 5.82 | ||||||
| Sawdust ash II | 0.46 | 3.34 | 3.73 | 34.11 | 4.18 | 9.00 | 3.22 | ||||||
| Wood crumbs | 1.48 | 8.70 | 6.23 | 36.68 | 0.99 | 3.99 | 0.96 | ||||||
| Sawdust material | 0.26 | 6.10 | 1.52 | 41.92 | 1.45 | 8.23 | 2.31 | ||||||
| Masson pine | 0.22 | 1.92 | 10.93 | 40.68 | 0.85 | 1.62 | 0.92 | ||||||
| Chinese fir | 0.32 | 18.36 | 6.18 | 31.57 | 0.73 | 1.53 | 0.56 | ||||||
| Sweet gum | 0.46 | 14.87 | 19.65 | 22.62 | 0.55 | 2.27 | 0.55 | ||||||
| Birch glutinosa | 0.22 | 21.52 | 9.87 | 17.07 | 1.13 | 3.88 | 1.39 | ||||||
| Oil palm | 0.59 | 47.76 | 3.17 | 4.50 | 0.43 | 9.79 | 0.09 | ||||||
| Bark | 0.17 | 6.86 | 5.43 | 40.65 | 1.17 | 3.16 | 3.73 | ||||||
| Sawdust III | 0.45 | 8.60 | 4.26 | 29.87 | 2.70 | 8.71 | 3.30 | ||||||
| Timber ash | 0.75 | 15.78 | 4.99 | 19.13 | 2.54 | 9.21 | 3.57 | ||||||
| Shrub ash | 0.13 | 6.93 | 4.81 | 50.29 | 0.40 | 1.06 | 0.34 | ||||||
| Blue Japanese oak ash | 0.18 | 4.90 | 3.71 | 48.24 | 1.94 | 2.92 | 1.10 | ||||||
| Black locust ash | 0.19 | 5.32 | 2.18 | 49.39 | 1.44 | 3.70 | 1.47 | ||||||
| Cryptomeria ash | 0.05 | 0.48 | 0.95 | 57.92 | 1.34 | 2.96 | 1.08 | ||||||
| Black locust ash II | 0.09 | 10.50 | 3.37 | 47.26 | 0.23 | 0.38 | 0.14 | ||||||
| Toona sinensis ash | 0.18 | 11.93 | 4.50 | 51.06 | 0.18 | 0.57 | 0.24 | ||||||
| [91] | |||||||||||||
| White wood ash | 1.11 | 7.64 | 2.83 | 17.72 | 1.61 | 12.52 | 2.43 | 0.64 | 0.87 | 0.18 | 1.08 | ||
| [93] | |||||||||||||
| Oak ash | 0.41 | 0.03 | |||||||||||
| Pine ash | 0.43 | 0.04 | |||||||||||
| Willow ash | 0.16 | 0.09 | |||||||||||
| [94] | |||||||||||||
| Tropical hardwood ash | 0.96 | 19.92 | 2.83 | 19.30 | 1.54 | 6.47 | 1.43 | 1.28 | 2.40 | 0.20 | 3.59 | ||
| Temperate hardwood ash | 0.39 | 14.11 | 4.16 | 27.16 | 1.05 | 5.08 | 1.59 | 1.16 | 3.58 | 0.08 | 0.60 | ||
| Softwood ash | 0.70 | 8.30 | 2.95 | 22.87 | 2.24 | 10.63 | 2.17 | 1.92 | 1.22 | 0.31 | 0.20 | ||
| Temperate hardwood bark ash | 1.19 | 4.90 | 4.58 | 45.74 | 0.84 | 3.14 | 0.74 | 0.64 | 0.87 | 0.06 | 5.80 | ||
| Softwood bark ash | 1.48 | 4.98 | 3.14 | 42.17 | 1.61 | 3.14 | 1.91 | 0.80 | 1.40 | 0.08 | |||
| [97] | |||||||||||||
| Wood biomass I | 0.66 | 0.19 | |||||||||||
| Wood biomass II | 0.17 | 0.03 | |||||||||||
| Wood biomass III | 0.09 | 0.01 | |||||||||||
| [17] | |||||||||||||
| Hardwood ash | 0.67 | 12.20 | 2.47 | 26.73 | 0.35 | 1.25 | 0.58 | 0.92 | 4.06 | 0.06 | |||
| Softwood ash | 0.59 | 7.06 | 2.95 | 22.87 | 1.47 | 9.29 | 2.17 | 0.72 | 1.27 | 0.18 | |||
| [99] | |||||||||||||
| Poplar ash I | 6.89 | 9.17 | 26.16 | 0.77 | 2.08 | 0.00 | 0.76 | 5.50 | 0.86 | ||||
| Poplar ash II | 5.48 | 10.55 | 30.73 | 1.05 | 3.00 | 0.00 | 1.24 | 6.68 | |||||
| [101] | |||||||||||||
| Pine ash | 0.82 | 18.26 | 6.39 | 27.80 | 3.05 | ||||||||
| Spruce ash | 0.45 | 57.53 | 2.35 | 10.65 | 1.61 | ||||||||
| Oak ash | 0.37 | 10.13 | 3.56 | 36.38 | 1.66 | ||||||||
| Beech ash | 0.30 | 27.56 | 8.80 | 14.37 | 2.09 | ||||||||
| Willow ash | 1.19 | 19.43 | 2.43 | 32.94 | 5.68 | ||||||||
| Poplar ash | 0.21 | 15.55 | 7.91 | 40.97 | 0.37 | ||||||||
| Other biomass | |||||||||||||
| [78] | |||||||||||||
| Biomass mix ash I | 0.30 | 12.35 | 5.21 | 32.71 | 1.55 | 8.18 | 1.44 | 0.93 | 1.94 | 0.10 | 0.21 | ||
| Biomass mix ash I | 0.29 | 13.11 | 4.61 | 34.43 | 1.38 | 7.08 | 1.16 | 1.30 | 1.75 | 0.11 | 0.57 | ||
| Biomass mix ash I | 0.28 | 16.13 | 6.64 | 28.97 | 0.89 | 7.03 | 1.22 | 1.76 | 1.90 | 0.10 | 0.54 | ||
| [79] | |||||||||||||
| Biomass ash I | 0.37 | 3.07 | 2.06 | 6.16 | 1.77 | 14.97 | 3.29 | 1.24 | 0.56 | 0.18 | 0.45 | ||
| Biomass ash II | 0.30 | 15.03 | 4.11 | 16.47 | 1.29 | 13.36 | 1.15 | 2.84 | 0.52 | 0.25 | 1.82 | ||
| Biomass ash III | 0.45 | 11.37 | 3.40 | 15.10 | 2.03 | 15.76 | 2.41 | 4.01 | 0.63 | 0.16 | 1.19 | ||
| [80] | |||||||||||||
| Biomass ash I | 1.14 | 4.63 | 2.70 | 21.83 | 1.81 | 1.76 | 0.21 | 0.06 | |||||
| Biomass ash II | 0.86 | 2.96 | 1.98 | 7.67 | 1.82 | 3.59 | 0.28 | 0.05 | |||||
| Biomass ash III | 0.33 | 5.61 | 0.99 | 6.37 | 1.64 | 2.05 | 0.56 | 0.03 | |||||
| Biomass ash IV | 0.68 | 3.74 | 0.79 | 5.90 | 1.83 | 2.26 | 0.31 | 0.03 | |||||
| Biomass ash V | 0.34 | 2.43 | 1.33 | 4.75 | 1.66 | 2.63 | 0.34 | 0.01 | |||||
| [82] | |||||||||||||
| Biomass ash mix I | 6.91 | 20.39 | 0.34 | 0.15 | 0.18 | 0.69 | 0.02 | ||||||
| Biomass ash mix II | 10.81 | 24.51 | 0.47 | 0.85 | 3.20 | 0.28 | 0.03 | ||||||
| [83] | |||||||||||||
| Biomass ash mix I | 0.33 | 4.72 | 1.27 | 15.80 | 1.32 | 1.91 | 0.80 | 0.69 | 0.84 | 0.37 | 0.62 | ||
| Biomass ash mix II | 0.80 | 31.10 | 0.47 | 5.93 | 0.32 | 0.21 | 9.62 | 0.67 | 0.41 | 1.90 | 3.44 | ||
| Biomass ash mix III | 0.48 | 19.20 | 3.00 | 23.10 | 0.86 | 0.84 | 4.28 | 1.29 | 1.46 | 0.80 | 2.50 | ||
| Biomass ash mix IV | 4.70 | 17.70 | 1.16 | 12.50 | 1.04 | 0.72 | 2.10 | 0.83 | 0.68 | 1.54 | 23.67 | ||
| Biomass ash mix V | 2.67 | 14.80 | 1.75 | 28.10 | 0.41 | 0.46 | 1.58 | 0.81 | 0.29 | 0.06 | 7.46 | ||
| Biomass ash mix VI | 0.51 | 11.80 | 0.37 | 31.60 | 0.19 | 0.13 | 2.58 | 0.44 | 0.10 | 0.44 | 5.23 | ||
| Biomass ash mix VII | 0.74 | 57.00 | 0.04 | 0.54 | 0.15 | 0.14 | 6.15 | 0.94 | 0.01 | 0.02 | 16.10 | ||
| Biomass ash mix VIII | 0.49 | 40.70 | 0.77 | 3.28 | 0.07 | 0.00 | 2.21 | 0.52 | 0.00 | 0.03 | 25.42 | ||
| [84] | |||||||||||||
| Biomass ash I | 0.05 | 9.55 | 2.59 | 7.15 | 2.12 | 27.68 | 0.79 | 0.75 | 0.70 | 0.05 | 0.68 | 0.07 | 0.33 |
| Biomass ash II | 0.03 | 8.88 | 0.84 | 2.38 | 1.81 | 33.23 | 2.18 | 0.20 | 0.50 | 0.06 | 0.11 | 0.01 | 0.19 |
| Biomass ash III | 0.03 | 7.64 | 1.04 | 2.69 | 4.26 | 23.52 | 4.82 | 0.78 | 0.55 | 0.15 | 0.18 | 0.08 | 1.99 |
| [85] | |||||||||||||
| Biomass ash I | 0.95 | 3.35 | 2.91 | 38.68 | 1.62 | 10.32 | 2.64 | 0.35 | 0.50 | 0.01 | |||
| Biomass ash II | 0.86 | 3.48 | 5.63 | 12.86 | 3.46 | 23.34 | 4.21 | 0.58 | 0.71 | 0.03 | |||
| Biomass ash III | 0.96 | 3.44 | 4.41 | 11.12 | 2.91 | 26.84 | 3.80 | 0.35 | 0.49 | 0.01 | |||
| [87] | |||||||||||||
| Biomass ash | 0.36 | 25.78 | 3.19 | 10.34 | 1.38 | 7.76 | 0.82 | 7.19 | 1.19 | ||||
| [90] | |||||||||||||
| Wood waste with bark ash | 0.24 | 0.79 | 1.47 | 13.20 | 1.51 | 0.56 | 2.00 | 2.93 | 0.67 | ||||
| Biomass ash mix I | 0.01 | ||||||||||||
| [94] | |||||||||||||
| Recovered wood ash | 1.48 | 2.16 | 8.44 | 11.44 | 3.98 | 18.02 | 5.03 | 2.32 | 0.29 | 1.62 | |||
| Paper sludge ash | 0.36 | 0.34 | 2.83 | 18.58 | 1.47 | 17.10 | 12.17 | 0.22 | 0.17 | 0.90 | |||
| Sewage sludge ash | 1.26 | 1.41 | 1.45 | 10.72 | 9.08 | 14.79 | 6.35 | 0.88 | 6.11 | 0.59 | |||
| [77] | |||||||||||||
| Biomass ash I | 0.33 | 22.36 | 2.72 | 26.28 | 9.28 | 25.37 | 6.54 | 4.99 | 0.25 | 1.14 | |||
| Biomass ash II | 0.43 | 10.99 | 1.35 | 26.00 | 14.90 | 26.73 | 7.48 | 6.71 | 0.36 | 1.34 | |||
| Biomass ash III | 0.66 | 7.09 | 4.34 | 32.21 | 17.74 | 23.99 | 4.97 | 7.14 | 0.23 | 0.60 | |||
| Biomass ash IV | 0.42 | 13.26 | 2.16 | 28.81 | 5.71 | 28.73 | 6.93 | 11.10 | 0.28 | 1.23 | |||
| Biomass ash V | 0.63 | 23.55 | 2.18 | 20.98 | 15.39 | 27.58 | 5.61 | 3.30 | 0.22 | 0.47 | |||
| Biomass ash VI | 2.40 | 16.66 | 2.54 | 30.89 | 7.74 | 25.91 | 6.39 | 5.54 | 0.24 | 1.43 | |||
| [100] | |||||||||||||
| Cattle manure ash I | 6.38 | 16.57 | 14.09 | 4.77 | 0.38 | 1.02 | |||||||
| Cattle manure ash I | 8.37 | 0.03 | 20.91 | 12.33 | 3.72 | 0.32 | 0.89 | ||||||
| Cattle manure ash I | 0.72 | 6.35 | 1.05 | 17.91 | 7.29 | 13.24 | 1.73 | 0.62 | |||||
Appendix A.2

Table A2.
Trace element contents with average values in ashes from (a) green biomass (GBA), (b) woody biomass (WBA), and (c) other biomass (OBA). Own analysis based on [79,80,82,83,84,85,86,90,93,97,102].
Table A2.
Trace element contents with average values in ashes from (a) green biomass (GBA), (b) woody biomass (WBA), and (c) other biomass (OBA). Own analysis based on [79,80,82,83,84,85,86,90,93,97,102].
| Type of Biomass | Content of Component [mg/kg] | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V | Cr | Co | Ni | Cu | Pb | Bi | Cd | Mo | Hg | As | Se | Sr | B | Ba | Sb | |
| Green biomass | ||||||||||||||||
| [82] | ||||||||||||||||
| Virginia mallow ash | 47.51 | 83.89 | 67.89 | 28.19 | 0.93 | |||||||||||
| Jerusalem artichoke ash | 50.61 | 35.83 | 72.62 | 6.43 | 0.32 | |||||||||||
| Multiflorous rose ash | 23.14 | 52.58 | 33.00 | 6.11 | 0.36 | |||||||||||
| Miscanthus giganteus ash | 60.87 | 45.65 | 70.70 | 9.23 | 0.31 | |||||||||||
| Miscanthus sacchariflorus ash | 63.77 | 51.98 | 81.55 | 7.27 | 0.39 | |||||||||||
| Prairie cordgrass ash | 50.29 | 32.73 | 54.99 | 13.03 | 0.65 | |||||||||||
| Common reed ash | 44.48 | 63.10 | 44.27 | 14.65 | 0.13 | |||||||||||
| Switch grass ash | 64.57 | 32.18 | 69.11 | 28.73 | 0.73 | |||||||||||
| Wheat straw ash | 60.47 | 7.38 | 27.84 | 6.82 | 0.18 | |||||||||||
| Triticale straw ash | 90.85 | 12.84 | 43.03 | 7.32 | 0.14 | |||||||||||
| Oat straw ash | 14.58 | 13.14 | 22.06 | 7.69 | 0.09 | |||||||||||
| Barley straw ash | 20.62 | 8.84 | 64.88 | 4.39 | 0.18 | |||||||||||
| Buckwheat straw ash | 47.94 | 8.24 | 38.78 | 3.90 | 0.08 | |||||||||||
| Hay ash | 69.56 | 37.30 | 51.31 | 33.33 | 0.07 | |||||||||||
| Cherry pit ash | 65.53 | 40.23 | 795.33 | 12.35 | 1.58 | |||||||||||
| Sunflower husk ash | 21.18 | 22.23 | 91.72 | 4.42 | 0.09 | |||||||||||
| Rape pod ash | 18.82 | 23.76 | 33.18 | 3.82 | 0.09 | |||||||||||
| Apple pomace ash | 10.67 | 64.33 | 582.00 | 13.82 | 1.72 | |||||||||||
| Hazelnut shell ash | 37.62 | 61.64 | 645.00 | 7.56 | 0.13 | |||||||||||
| Walnut shell ash | 50.93 | 42.04 | 202.75 | 6.51 | 0.12 | |||||||||||
| [83] | ||||||||||||||||
| Straw ash | 11.00 | 15.00 | 29.00 | 5.00 | 5.00 | 20.00 | 26.00 | 6.00 | 25.00 | 0.40 | 8.00 | 40.00 | 60.00 | 152.00 | 10.00 | |
| [142] | ||||||||||||||||
| Straw winter wheat ash | 10.10 | 13.22 | 2.00 | 4.20 | 167.00 | 5.20 | 0.20 | |||||||||
| Cereals triticale ash | 20.00 | 20.00 | 3.00 | 10.20 | 46.00 | 4.50 | 0.20 | |||||||||
| [90] | ||||||||||||||||
| Wheat straw ash | 10.00 | 22.00 | 25.00 | 5.00 | 5.00 | 20.00 | 8.00 | 3.00 | 5.00 | 0.30 | 7.00 | 55.00 | 17.00 | 271.00 | 10.00 | |
| Wheat straw ash | 13.00 | 7.00 | 33.00 | 5.00 | 5.00 | 20.00 | 45.00 | 8.00 | 46.00 | 0.40 | 1.00 | 25.00 | 103.00 | 33.00 | 10.00 | |
| [93] | ||||||||||||||||
| Miscanthus ash | 25.70 | 16.40 | 58.77 | 9.69 | 7.68 | |||||||||||
| Sunflower husk ash | 50.09 | 59.37 | 358.00 | 6.78 | 9.93 | |||||||||||
| Wheat straw ash | 32.25 | 14.17 | 72.54 | 8.52 | 5.42 | |||||||||||
| Woody biomass | ||||||||||||||||
| [82] | ||||||||||||||||
| Birch wood ash | 39.07 | 34.91 | 97.10 | 40.48 | 1.01 | |||||||||||
| Pine wood ash | 62.04 | 45.84 | 196.00 | 28.89 | 1.59 | |||||||||||
| Oak wood ash | 89.87 | 125.67 | 190.67 | 54.49 | 1.91 | |||||||||||
| Hornbeam wood ash | 10.65 | 158.67 | 140.67 | 40.20 | 1.13 | |||||||||||
| Ash wood ash | 30.66 | 24.84 | 121.00 | 15.31 | 0.78 | |||||||||||
| Poplar wood ash | 20.57 | 26.19 | 96.92 | 9.65 | 0.18 | |||||||||||
| Willow ash | 45.97 | 32.00 | 123.50 | 8.93 | 0.34 | |||||||||||
| Acacia wood ash | 36.31 | 59.72 | 158.00 | 15.83 | 0.49 | |||||||||||
| Beech bark ash | 20.65 | 99.07 | 104.00 | 9.08 | 0.12 | |||||||||||
| Oak bark ash | 31.06 | 48.07 | 202.67 | 24.18 | 0.85 | |||||||||||
| Hornbeam bark ash | 12.49 | 68.57 | 25.21 | 11.23 | 0.13 | |||||||||||
| Ash bark ash | 13.94 | 42.95 | 19.83 | 9.71 | 0.11 | |||||||||||
| Acacia bark ash | 20.07 | 64.70 | 61.00 | 14.03 | 0.98 | |||||||||||
| [83] | ||||||||||||||||
| Wood ash | 22.00 | 64.00 | 33.00 | 20.00 | 113.00 | 396.00 | 131.00 | 47.00 | 23.00 | 1.20 | 30.00 | 342.00 | 298.00 | 234.00 | 10.00 | |
| [142] | ||||||||||||||||
| Bark spruce ash | 58.40 | 132.60 | 23.90 | 94.10 | 87.80 | 25.30 | 3.90 | |||||||||
| Wood chip spruce ash | 42.00 | 54.10 | 15.30 | 61.50 | 126.80 | 25.40 | 4.80 | |||||||||
| [90] | ||||||||||||||||
| Wood ash | 76.40 | 28.00 | 89.60 | 13.10 | 2.38 | 0.03 | 3.34 | |||||||||
| Biomass mix I | 41.00 | 175.00 | 177.00 | 9.00 | 0.20 | 18.00 | ||||||||||
| Biomass mix I | 50.00 | 48.00 | 39.00 | 7.60 | 0.30 | 15.00 | ||||||||||
| Beech wood chip ash | 8.90 | 24.00 | 4.200 | 36.00 | 98.00 | 38.70 | 49.00 | 2.06 | 4.50 | 324.00 | 10.26 | 1786.00 | 0.71 | |||
| Bark and wood chip ash | 15.10 | 20.10 | 6.700 | 34.10 | 70.10 | 20.70 | 4.50 | 1.00 | 1.60 | |||||||
| Wood chip ash | 9.50 | 21.10 | 2.000 | 8.00 | 44.20 | 18.50 | 0.20 | 0.40 | 1.90 | |||||||
| [93] | ||||||||||||||||
| Oak ash | 22.41 | 19.17 | 307.00 | 12.21 | 8.26 | |||||||||||
| Pine ash | 18.87 | 21.69 | 203.00 | 12.91 | 8.06 | |||||||||||
| Willow ash | 39.93 | 75.51 | 190.00 | 10.23 | 7.50 | |||||||||||
| [97] | ||||||||||||||||
| Wood biomass ash I | 50.00 | 11.20 | 79.60 | 84.40 | 79.90 | 44.80 | 3.10 | 408.50 | 588.80 | |||||||
| Wood biomass ash II | 123.00 | 26.00 | 170.30 | 79.70 | 101.40 | 2.80 | 2.60 | 0.01 | 314.70 | 1437.00 | ||||||
| Wood biomass ash III | 61.70 | 15.80 | 35.20 | 56.10 | 37.80 | 25.20 | 6.00 | 0.19 | 485.00 | 545.00 | ||||||
| [102] | ||||||||||||||||
| Aspen ash | 0.10 | 2.10 | 14.10 | |||||||||||||
| Bird cherry ash | 254.70 | 254.70 | 36.30 | 3.50 | 27.80 | |||||||||||
| Black alder ash | 1.00 | 0.50 | 9.50 | 1.20 | 26.80 | |||||||||||
| Blackthorn ash | 1.40 | 0.90 | 6.20 | 0.20 | 87.40 | |||||||||||
| Common laburnum ash | 3.70 | 0.80 | 3.90 | 0.40 | 22.10 | |||||||||||
| Common lilac ash | 1.50 | 37.60 | 3.40 | 0.60 | 24.00 | |||||||||||
| Common plum ash | 78.70 | 44.60 | 20.80 | 1.20 | 66.30 | |||||||||||
| European ash | 407.00 | 236.70 | 41.60 | 2.70 | 64.10 | |||||||||||
| European crab apple ash | 9.90 | 1.70 | 6.70 | 1.80 | 189.60 | |||||||||||
| Grey alder ash | 191.70 | 92.30 | 26.70 | 4.50 | 14.30 | |||||||||||
| Hawthorn ash | 8.20 | 2.00 | 14.80 | 4.60 | 48.40 | |||||||||||
| Norway maple ash | 3.30 | 1.90 | 6.10 | 0.80 | 29.30 | |||||||||||
| Rowan ash | 6.90 | 2.70 | 9.60 | 0.80 | 21.70 | |||||||||||
| Silver birch ash | 59.90 | 31.00 | 5.20 | 0.40 | 10.50 | |||||||||||
| White willow ash | 0.30 | 0.20 | 4.60 | 0.10 | 24.20 | |||||||||||
| Wych elm ash | 1.80 | 1.20 | 8.60 | 0.60 | 138.30 | |||||||||||
| Common juniper | 5.30 | 1.20 | 4.00 | 3.10 | 15.40 | |||||||||||
| Norway spruce | 98.50 | 45.30 | 14.30 | 0.70 | 188.90 | |||||||||||
| Scots pine | 138.20 | 91.40 | 14.40 | 0.20 | 15.70 | |||||||||||
| Other biomass | ||||||||||||||||
| [79] | ||||||||||||||||
| Biomass ash I | 0.02 | 0.01 | 0.01 | |||||||||||||
| Biomass ash II | 0.02 | 0.02 | ||||||||||||||
| Biomass ash III | 0.05 | 0.03 | 0.01 | 0.02 | 0.06 | |||||||||||
| [80] | ||||||||||||||||
| Biomass ash I | 93.76 | 22.15 | 24.73 | 82.58 | 276.80 | 3.90 | 5.09 | 21.72 | 1.37 | |||||||
| Biomass ash II | 37.32 | 8.13 | 21.78 | 47.45 | 66.70 | 2.18 | 2.02 | 0.19 | 10.04 | 0.84 | ||||||
| Biomass ash III | 139.63 | 7.95 | 30.57 | 44.32 | 34.30 | 2.54 | 3.79 | 0.24 | 17.47 | 0.40 | ||||||
| Biomass ash IV | 32.91 | 7.07 | 19.76 | 64.39 | 102.99 | 2.58 | 4.67 | 0.13 | 19.31 | 1.25 | ||||||
| Biomass ash V | 37.73 | 7.58 | 18.20 | 36.58 | 31.05 | 4.14 | 4.00 | 0.16 | 0.77 | |||||||
| [82] | ||||||||||||||||
| Biomass ash mix I | 95.64 | 110.33 | 188.00 | 50.67 | 1.44 | |||||||||||
| Biomass ash mix II | 25.00 | 176.33 | 181.00 | 12.69 | 0.13 | |||||||||||
| [83] | ||||||||||||||||
| Biomass ash mix I | 36.00 | 92.00 | 39.00 | 31.00 | 11.00 | 352.00 | 95.00 | 19.00 | 24.00 | 0.80 | 19.00 | 284.00 | 263.00 | 484.00 | 10.00 | |
| Biomass ash mix II | 38.00 | 76.00 | 39.00 | 23.00 | 140.00 | 602.00 | 237.00 | 105.00 | 43.00 | 0.10 | 36.00 | 242.00 | 221.00 | 91.00 | 12.00 | |
| Biomass ash mix III | 10.00 | 87.00 | 25.00 | 27.00 | 145.00 | 250.00 | 124.00 | 41.00 | 5.00 | 1.70 | 27.00 | 641.00 | 671.00 | 191.00 | 10.00 | |
| Biomass ash mix IV | 10.00 | 70.00 | 25.00 | 4.00 | 156.00 | 892.00 | 167.00 | 77.00 | 5.00 | 4.20 | 62.00 | 283.00 | 292.00 | 136.00 | 10.00 | |
| Biomass ash mix V | 30.00 | 15.00 | 34.00 | 31.00 | 5.00 | 53.00 | 59.00 | 9.00 | 27.00 | 0.05 | 15.00 | 461.00 | 227.00 | 357.00 | 10.00 | |
| Biomass ash mix VI | 10.00 | 41.00 | 34.00 | 6.00 | 5.00 | 228.00 | 104.00 | 32.00 | 32.00 | 0.10 | 19.00 | 141.00 | 114.00 | 144.00 | 11.00 | |
| Biomass ash mix VII | 10.00 | 22.00 | 25.00 | 5.00 | 5.00 | 20.00 | 8.00 | 3.00 | 5.00 | 0.30 | 7.00 | 55.00 | 17.00 | 271.00 | 10.00 | |
| Biomass ash mix VIII | 13.00 | 7.00 | 33.00 | 5.00 | 5.00 | 20.00 | 45.00 | 8.00 | 46.00 | 0.40 | 10.00 | 25.00 | 103.00 | 33.00 | 10.00 | |
| [84] | ||||||||||||||||
| Biomass ash I | 46.00 | 82.00 | 8.00 | 45.00 | 158.00 | 41.00 | 5.00 | 35.00 | 3.00 | 0.58 | 220.00 | 0.86 | 10.00 | |||
| Biomass ash II | 48.00 | 41.00 | 8.00 | 23.00 | 51.00 | 35.00 | 2.00 | 37.00 | 13.00 | 0.43 | 99.00 | 0.85 | 10.00 | |||
| Biomass ash III | 101.00 | 90.00 | 16.00 | 45.00 | 90.00 | 57.00 | 7.00 | 43.00 | 6.00 | 0.46 | 193.00 | 1.04 | 28.00 | |||
| [85] | ||||||||||||||||
| Biomass ash I | 6.40 | 24.50 | 18.90 | 23.40 | 1.90 | 0.01 | 206.40 | |||||||||
| Biomass ash II | 11.50 | 68.30 | 31.60 | 36.10 | 2.30 | 0.01 | 671.40 | |||||||||
| Biomass ash III | 6.70 | 28.50 | 12.80 | 29.00 | 1.10 | 0.01 | 534.90 | |||||||||
| [90] | ||||||||||||||||
| Biomass ash mix I | 36.00 | 92.00 | 39.00 | 31.00 | 11.00 | 352.00 | 95.00 | 19.00 | 24.00 | 0.80 | 19.00 | 284.00 | 484.00 | 10.00 | ||
| Biomass ash mix II | 10.00 | 70.00 | 25.00 | 4.00 | 156.00 | 892.00 | 167.00 | 77.00 | 5.00 | 4.20 | 62.00 | 283.00 | 136.00 | 10.00 | ||
| Forest residues ash I | 10.10 | 6.40 | 24.50 | 18.90 | 23.40 | 1.90 | 0.01 | 5.10 | 206.40 | |||||||
| Forest residues ash II | 128.10 | 11.50 | 68.30 | 31.600 | 36.10 | 2.30 | 0.01 | 25.60 | 671.40 | |||||||
| Wood chips from forest residue ash I | 46.00 | 145.0 | 259.00 | 40.00 | 662.00 | |||||||||||
| Wood chips from forest residue ash II | 49.00 | 177.00 | 307.00 | 31.00 | 623.00 | |||||||||||
| Wood chips from forest residue ash III | 47.00 | 28.00 | 207.00 | 911.00 | 75.00 | 371.00 | ||||||||||
| Forest waste ash | 35.00 | 99.00 | 191.00 | 1.00 | 1.00 | |||||||||||
References
- Energy Institute. Statistical Review of World Energy; Energy Institute: London, UK, 2024. [Google Scholar]
- United Nations Treaty Collection Paris Agreement. Available online: https://treaties.un.org/pages/viewdetails.aspx?src=treaty&mtdsg_no=xxvii-7-d&chapter=27&clang=_en (accessed on 29 September 2025).
- Galán, G.; Taifouris, M.; Martín, M.; Grossmann, I.E. Multiscale Analysis through the Use of Biomass Residues and CO2 towards Energetic Security at Country Scale via Methane Production. Energy 2025, 318, 134890. [Google Scholar] [CrossRef]
- Meisel, K.; Jordan, M.; Dotzauer, M.; Schröder, J.; Lenz, V.; Naumann, K.; Cyffka, K.-F.; Dögnitz, N.; Schindler, H.; Daniel-Gromke, J.; et al. Quo Vadis, Biomass? Long-Term Scenarios of an Optimal Energetic Use of Biomass for the German Energy Transition. Int. J. Energy Res. 2024, 2024, 6687376. [Google Scholar] [CrossRef]
- Pietrzak, M.B.; Olczyk, M.; Kuc-Czarnecka, M.E. Assessment of the Feasibility of Energy Transformation Processes in European Union Member States. Energy 2022, 15, 661. [Google Scholar] [CrossRef]
- Kalak, T. Potential Use of Industrial Biomass Waste as a Sustainable Energy Source in the Future. Energy 2023, 16, 1783. [Google Scholar] [CrossRef]
- Sertolli, A.; Gabnai, Z.; Lengyel, P.; Bai, A. Biomass Potential and Utilization in Worldwide Research Trends—A Bibliometric Analysis. Sustainability 2022, 14, 5515. [Google Scholar] [CrossRef]
- Hupa, M.; Karlström, O.; Vainio, E. Biomass Combustion Technology Development—It Is All about Chemical Details. Proc. Combust. Inst. 2017, 36, 113–134. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An Overview of the Chemical Composition of Biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Adams, P.; Bridgwater, T.; Lea-Langton, A.; Ross, A.; Watson, I. Biomass Conversion Technologies. In Greenhouse Gases Balances of Bioenergy Systems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 107–139. [Google Scholar]
- Gouveia, L.; Passarinho, P.C. Biomass Conversion Technologies: Biological/Biochemical Conversion of Biomass. In Biorefineries. Lecture Notes in Energy; Springer International Publishing: Cham, Switzerland, 2017; pp. 99–111. [Google Scholar]
- Vaithyanathan, V.K.; Goyette, B.; Rajagopal, R. A Critical Review of the Transformation of Biomass into Commodity Chemicals: Prominence of Pretreatments. Environ. Chall. 2023, 11, 100700. [Google Scholar] [CrossRef]
- Ramos, A.; Monteiro, E.; Rouboa, A. Biomass Pre-Treatment Techniques for the Production of Biofuels Using Thermal Conversion Methods—A Review. Energy Convers. Manag. 2022, 270, 116271. [Google Scholar] [CrossRef]
- Belviso, C. State-of-the-Art Applications of Fly Ash from Coal and Biomass: A Focus on Zeolite Synthesis Processes and Issues. Prog. Energy Combust. Sci. 2018, 65, 109–135. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An Overview of the Composition and Application of Biomass Ash.: Part 2. Potential Utilisation, Technological and Ecological Advantages and Challenges. Fuel 2013, 105, 19–39. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, J.; Xu, D.; Zhang, S.; Lv, P.; Jiang, Y.; Song, X.; Merime Bkangmo Kontchouo, F.; Jiao, Y.; Li, B.; et al. Synergistic Effect Mechanism of Biomass Ash-Derived K-Ca-Si Catalytic System on Syngas Production and Reactivity Characteristics of High-Sulfur Petroleum Coke Gasification. Fuel 2024, 365, 131224. [Google Scholar] [CrossRef]
- Zhai, J.; Burke, I.T.; Mayes, W.M.; Stewart, D.I. New Insights into Biomass Combustion Ash Categorisation: A Phylogenetic Analysis. Fuel 2021, 287, 119469. [Google Scholar] [CrossRef]
- Oleszek, S.; Shiota, K.; Chen, M.; Takaoka, M. Effective Separation and Recovery of Manganese and Potassium from Biomass Ash by Solvent Extraction. ACS Omega 2022, 7, 20155–20164. [Google Scholar] [CrossRef]
- Yang, S.; Song, G.; Na, Y.; Qi, X.; Yang, Z. Experimental Study on the Recovery of Sodium in High Sodium Fly Ash from Thermochemical Conversion of Zhundong Coal. Fuel 2018, 229, 22–33. [Google Scholar] [CrossRef]
- Ma, D.; Sun, J.; Zhang, Y.; Sun, Z.; Wang, X.; Zi, J.; Stojiljković, D.; Manić, N.; Tan, H.; Rahman, Z.; et al. Evaluation of Ash/Slag Heavy Metal Characteristics and Potassium Recovery of Four Biomass Boilers. Biomass Bioenergy 2023, 173, 106770. [Google Scholar] [CrossRef]
- Przygocka-Cyna, K.; Barłóg, P.; Spiżewski, T.; Grzebisz, W. Bio-Fertilizers Based on Digestate and Biomass Ash as an Alternative to Commercial Fertilizers—The Case of Tomato. Agronomy 2021, 11, 1716. [Google Scholar] [CrossRef]
- Maresca, A.; Hyks, J.; Astrup, T.F. Long-Term Leaching of Nutrients and Contaminants from Wood Combustion Ashes. Waste Manag. 2018, 74, 373–383. [Google Scholar] [CrossRef]
- Ardhira, P.J.; Shukla, S.K.; Sathyan, D. Synthesis of Geopolymer Mortar from Biomass Ashes and Forecasting Its Compressive Strength Behaviour. Case Stud. Constr. Mater. 2024, 21, e03581. [Google Scholar] [CrossRef]
- Hu, J.; Shao, J.; Yang, H.; Lin, G.; Chen, Y.; Wang, X.; Zhang, W.; Chen, H. Co-Gasification of Coal and Biomass: Synergy, Characterization and Reactivity of the Residual Char. Bioresour. Technol. 2017, 244, 1–7. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, J.; Gu, Y.; Zhong, W.; Gao, K. Experimental Study on CO2 Co-Gasification Characteristics of Biomass and Waste Plastics: Insight into Interaction and Targeted Regulation Method. Energy 2024, 292, 130509. [Google Scholar] [CrossRef]
- Ajorloo, M.; Ghodrat, M.; Scott, J.; Strezov, V. Experimental Analysis of the Effects of Feedstock Composition on the Plastic and Biomass Co-Gasification Process. Renew. Energy 2024, 231, 120960. [Google Scholar] [CrossRef]
- Rizkiana, J.; Guan, G.; Widayatno, W.B.; Hao, X.; Huang, W.; Tsutsumi, A.; Abudula, A. Effect of Biomass Type on the Performance of Cogasification of Low Rank Coal with Biomass at Relatively Low Temperatures. Fuel 2014, 134, 414–419. [Google Scholar] [CrossRef]
- Qin, Y.; Han, Q.; Zhao, Z.; Du, Z.; Feng, J.; Li, W.; Vassilev, S.V.; Vassileva, C.G. Impact of Biomass Addition on Organic Structure and Mineral Matter of Char during Coal-Biomass Co-Gasification under CO2 Atmosphere. Fuel 2017, 202, 556–562. [Google Scholar] [CrossRef]
- Song, Y.; Feng, J.; Ji, M.; Ding, T.; Qin, Y.; Li, W. Impact of Biomass on Energy and Element Utilization Efficiency during Co-Gasification with Coal. Fuel Process. Technol. 2013, 115, 42–49. [Google Scholar] [CrossRef]
- Magalhães, D.; Akgül, A.; Kazanç, F.; Costa, M. Interactions during CO2 Co-Gasification of Biomass and Coal Chars Obtained from Fast Pyrolysis in a Drop Tube Furnace. Energy Fuels 2021, 35, 7065–7076. [Google Scholar] [CrossRef]
- Li, X.; He, J.; Liu, M.; Bai, J.; Bai, Z.; Li, W. Interaction between Coal and Biomass during Co-Gasification: A Perspective Based on the Separation of Blended Char. Processes 2022, 10, 286. [Google Scholar] [CrossRef]
- Liang, W.; Ning, X.; Wang, G.; Zhang, J.; Li, R.; Chang, W.; Wang, C. Influence Mechanism and Kinetic Analysis of Co-Gasification of Biomass Char and Semi-Coke. Renew. Energy 2021, 163, 331–341. [Google Scholar] [CrossRef]
- Wei, J.; Guo, Q.; Song, X.; Ding, L.; Mosqueda, A.; Liu, Y.; Yoshikawa, K.; Yu, G. Effect of Hydrothermal Carbonization Temperature on Reactivity and Synergy of Co-Gasification of Biomass Hydrochar and Coal. Appl. Therm. Eng. 2021, 183, 116232. [Google Scholar] [CrossRef]
- Raj, R.; Singh, D.K.; Tirkey, J.V. Co-Gasification of Plastic Waste Blended with Coal and Biomass: A Comprehensive Review. Environ. Technol. Rev. 2023, 12, 614–642. [Google Scholar] [CrossRef]
- Emami-Taba, L.; Irfan, M.F.; Wan Daud, W.M.A.; Chakrabarti, M.H. Fuel Blending Effects on the Co-Gasification of Coal and Biomass—A Review. Biomass Bioenergy 2013, 57, 249–263. [Google Scholar] [CrossRef]
- Emami Taba, L.; Irfan, M.F.; Wan Daud, W.A.M.; Chakrabarti, M.H. The Effect of Temperature on Various Parameters in Coal, Biomass and CO-Gasification: A Review. Renew. Sustain. Energy Rev. 2012, 16, 5584–5596. [Google Scholar] [CrossRef]
- Wei, J.; Wang, M.; Xu, D.; Shi, L.; Li, B.; Bai, Y.; Yu, G.; Bao, W.; Xu, J.; Zhang, H.; et al. Migration and Transformation of Alkali/Alkaline Earth Metal Species during Biomass and Coal Co-Gasification: A Review. Fuel Process. Technol. 2022, 235, 107376. [Google Scholar] [CrossRef]
- Mallick, D.; Mahanta, P.; Moholkar, V.S. Co-Gasification of Coal and Biomass Blends: Chemistry and Engineering. Fuel 2017, 204, 106–128. [Google Scholar] [CrossRef]
- Block, C.; Ephraim, A.; Weiss-Hortala, E.; Minh, D.P.; Nzihou, A.; Vandecasteele, C. Co-Pyrogasification of Plastics and Biomass, a Review. Waste Biomass Valorization 2019, 10, 483–509. [Google Scholar] [CrossRef]
- Ramos, A.; Monteiro, E.; Silva, V.; Rouboa, A. Co-Gasification and Recent Developments on Waste-to-Energy Conversion: A Review. Renew. Sustain. Energy Rev. 2018, 81, 380–398. [Google Scholar] [CrossRef]
- Wang, M.; Wan, Y.; Guo, Q.; Bai, Y.; Yu, G.; Liu, Y.; Zhang, H.; Zhang, S.; Wei, J. Brief Review on Petroleum Coke and Biomass/Coal Co-Gasification: Syngas Production, Reactivity Characteristics, and Synergy Behavior. Fuel 2021, 304, 121517. [Google Scholar] [CrossRef]
- Wei, J.; Wang, M.; Wang, F.; Song, X.; Yu, G.; Liu, Y.; Vuthaluru, H.; Xu, J.; Xu, Y.; Zhang, H.; et al. A Review on Reactivity Characteristics and Synergy Behavior of Biomass and Coal Co-Gasification. Int. J. Hydrog. Energy 2021, 46, 17116–17132. [Google Scholar] [CrossRef]
- Shahbaz, M.; Al-Ansari, T.; Inayat, M.; Sulaiman, S.A.; Parthasarathy, P.; McKay, G. A Critical Review on the Influence of Process Parameters in Catalytic Co-Gasification: Current Performance and Challenges for a Future Prospectus. Renew. Sustain. Energy Rev. 2020, 134, 110382. [Google Scholar] [CrossRef]
- Santos, S.M.; Assis, A.C.; Gomes, L.; Nobre, C.; Brito, P. Waste Gasification Technologies: A Brief Overview. Waste 2022, 1, 140–165. [Google Scholar] [CrossRef]
- Kiwfo, K.; Suteerapataranon, S.; McKelvie, I.D.; Meng Woi, P.; Kolev, S.D.; Saenjum, C.; Christian, G.D.; Grudpan, K. A New Need, Quality, and Sustainability (NQS) Index for Evaluating Chemical Analysis Procedures Using Natural Reagents. Microchem. J. 2023, 193, 109026. [Google Scholar] [CrossRef]
- Goh, H.Y.; Wong, W.W.C.; Ong, Y.Y. A Study to Reduce Chemical Waste Generated in Chemistry Teaching Laboratories. J. Chem. Educ. 2020, 97, 87–96. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Alam, T. Gasification of Solid Fuels (Coal, Biomass and MSW): Overview, Challenges and Mitigation Strategies. Energy 2022, 15, 4444. [Google Scholar] [CrossRef]
- Alptekin, F.M.; Celiktas, M.S. Review on Catalytic Biomass Gasification for Hydrogen Production as a Sustainable Energy Form and Social, Technological, Economic, Environmental, and Political Analysis of Catalysts. ACS Omega 2022, 7, 24918–24941. [Google Scholar] [CrossRef] [PubMed]
- Fried, M. Dynamics of the Soil-Plant System. In The Soil-Plant System: In Relation to Inorganic Nutrition; 212AD; Elsevier: Amsterdam, The Netherlands, 2012; pp. 3–5. [Google Scholar]
- Pandey, R. Mineral Nutrition of Plants. In Plant Biology and Biotechnology; Springer: New Delhi, India, 2015; pp. 499–538. [Google Scholar]
- Liu, B.; He, Q.; Jiang, Z.; Xu, R.; Hu, B. Relationship between Coal Ash Composition and Ash Fusion Temperatures. Fuel 2013, 105, 293–300. [Google Scholar] [CrossRef]
- Liu, G.; Vassilev, S.V.; Gao, L.; Zheng, L.; Peng, Z. Mineral and Chemical Composition and Some Trace Element Contents in Coals and Coal Ashes from Huaibei Coal Field, China. Energy Convers. Manag. 2005, 46, 2001–2009. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, H.; Gao, L.; Zheng, L.; Peng, Z. Petrological and Mineralogical Characterizations and Chemical Composition of Coal Ashes from Power Plants in Yanzhou Mining District, China. Fuel Process. Technol. 2004, 85, 1635–1646. [Google Scholar] [CrossRef]
- Tao, G.; Geladi, P.; Lestander, T.A.; Xiong, S. Biomass Properties in Association with Plant Species and Assortments. II: A Synthesis Based on Literature Data for Ash Elements. Renew. Sustain. Energy Rev. 2012, 16, 3507–3522. [Google Scholar] [CrossRef]
- Monti, A.; Di Virgilio, N.; Venturi, G. Mineral Composition and Ash Content of Six Major Energy Crops. Biomass Bioenergy 2008, 32, 216–223. [Google Scholar] [CrossRef]
- Humphries, E.C. Mineral Components and Ash Analysis. In Moderne Methoden der Pflanzenanalyse/Modern Methods of Plant Analysis; Springer: Berlin/Heidelberg, Germany, 1956; pp. 468–502. [Google Scholar]
- Kenney, K.L.; Smith, W.A.; Gresham, G.L.; Westover, T.L. Understanding Biomass Feedstock Variability. Biofuels 2013, 4, 111–127. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Matias, J.C.O.; Catalão, J.P.S. Biomass Combustion Systems: A Review on the Physical and Chemical Properties of the Ashes. Renew. Sustain. Energy Rev. 2016, 53, 235–242. [Google Scholar] [CrossRef]
- Doshi, V.; Vuthaluru, H.B.; Korbee, R.; Kiel, J.H.A. Development of a Modeling Approach to Predict Ash Formation during Co-Firing of Coal and Biomass. Fuel Process. Technol. 2009, 90, 1148–1156. [Google Scholar] [CrossRef]
- Luo, Z.; Zhou, J. Thermal Conversion of Biomass. In Handbook of Climate Change Mitigation and Adaptation; Springer International Publishing: Cham, Switzerland, 2022; pp. 965–1021. [Google Scholar]
- Boström, D.; Skoglund, N.; Grimm, A.; Boman, C.; Öhman, M.; Broström, M.; Backman, R. Ash Transformation Chemistry during Combustion of Biomass. Energy Fuels 2012, 26, 85–93. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, K.; Yao, X.; Li, J. Mineral Transformations and Molten Mechanism during Combustion of Biomass Ash. Renew. Energy 2023, 216, 119113. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Vassileva, C.G. An Overview of the Behaviour of Biomass during Combustion: Part I. Phase-Mineral Transformations of Organic and Inorganic Matter. Fuel 2013, 112, 391–449. [Google Scholar] [CrossRef]
- Magdziarz, A.; Gajek, M.; Nowak-Woźny, D.; Wilk, M. Mineral Phase Transformation of Biomass Ashes—Experimental and Thermochemical Calculations. Renew. Energy 2018, 128, 446–459. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, S.; Li, Y.; Gao, J. Physico-Chemical Characteristics and Mineral Transformation Behavior of Ashes from Crop Straw. Energy Fuels 2009, 23, 5144–5150. [Google Scholar] [CrossRef]
- Chen, C.; Luo, Z.; Yu, C. Release and Transformation Mechanisms of Trace Elements during Biomass Combustion. J. Hazard. Mater. 2019, 380, 120857. [Google Scholar] [CrossRef]
- Jiménez, S.; Ballester, J. A Comparative Study of Different Methods for the Sampling of High Temperature Combustion Aerosols. Aerosol Sci. Technol. 2005, 39, 811–821. [Google Scholar] [CrossRef][Green Version]
- Xu, S.; Zhou, C.; Fang, H.; Zhu, W.; Shi, J.; Liu, G. Characteristics of Trace Elements and Potential Environmental Risks of the Ash from Agricultural Straw Direct Combustion Biomass Power Plant. Chemosphere 2023, 333, 138989. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Baxter, D. Trace Element Concentrations and Associations in Some Biomass Ashes. Fuel 2014, 129, 292–313. [Google Scholar] [CrossRef]
- Reinmöller, M.; Schreiner, M.; Laabs, M.; Scharm, C.; Yao, Z.; Guhl, S.; Neuroth, M.; Meyer, B.; Gräbner, M. Formation and Transformation of Mineral Phases in Biomass Ashes and Evaluation of the Feedstocks for Application in High-Temperature Processes. Renew. Energy 2023, 210, 627–639. [Google Scholar] [CrossRef]
- Assad Munawar, M.; Hussain Khoja, A.; Hassan, M.; Liaquat, R.; Raza Naqvi, S.; Taqi Mehran, M.; Abdullah, A.; Saleem, F. Biomass Ash Characterization, Fusion Analysis and Its Application in Catalytic Decomposition of Methane. Fuel 2021, 285, 119107. [Google Scholar] [CrossRef]
- Xiao, R.; Chen, X.; Wang, F.; Yu, G. The Physicochemical Properties of Different Biomass Ashes at Different Ashing Temperature. Renew. Energy 2011, 36, 244–249. [Google Scholar] [CrossRef]
- Abraham, R.; George, J.; Thomas, J.; Yusuff, K.K.M. Physicochemical Characterization and Possible Applications of the Waste Biomass Ash from Oleoresin Industries of India. Fuel 2013, 109, 366–372. [Google Scholar] [CrossRef]
- Magdziarz, A.; Dalai, A.K.; Koziński, J.A. Chemical Composition, Character and Reactivity of Renewable Fuel Ashes. Fuel 2016, 176, 135–145. [Google Scholar] [CrossRef]
- Garzón, E.; Morales, L.; Martínez--Blanes, J.M.; Sánchez--Soto, P.J. Characterization of Ashes from Greenhouse Crops Plant Biomass Residues Using X--ray Fluorescence Analysis and X--ray Diffraction. X-Ray Spectrom. 2017, 46, 569–578. [Google Scholar] [CrossRef]
- Li, J.; Long, X.; Zhu, H.; Liu, Z.; Lu, X.; Zhang, D. Investigation into the Fusibility of Biomass Ashes and Their Mineral Phase Transformations at Elevated Temperatures by Using the HT-XRD Technique. Biomass Bioenergy 2023, 173, 106812. [Google Scholar] [CrossRef]
- Smołka-Danielowska, D.; Jabłońska, M. Chemical and Mineral Composition of Ashes from Wood Biomass Combustion in Domestic Wood-Fired Furnaces. Int. J. Environ. Sci. Technol. 2022, 19, 5359–5372. [Google Scholar] [CrossRef]
- Royo, J.; Canalís, P.; Quintana, D. Ash Partitioning in Combustion of Pelletized Residual Agricultural Biomass. Biomass Bioenergy 2025, 193, 107563. [Google Scholar] [CrossRef]
- Uliasz-Bocheńczyk, A.; Pawluk, A.; Pyzalski, M. Characteristics of Ash from the Combustion of Biomass in Fluidized Bed Boilers. Gospod. Surowcami Miner. 2016, 32, 149–162. [Google Scholar] [CrossRef]
- Shi, R.; Li, J.; Jiang, J.; Mehmood, K.; Liu, Y.; Xu, R.; Qian, W. Characteristics of Biomass Ashes from Different Materials and Their Ameliorative Effects on Acid Soils. J. Environ. Sci. 2017, 55, 294–302. [Google Scholar] [CrossRef]
- Trivedi, N.S.; Mandavgane, S.A.; Mehetre, S.; Kulkarni, B.D. Characterization and Valorization of Biomass Ashes. Environ. Sci. Pollut. Res. 2016, 23, 20243–20256. [Google Scholar] [CrossRef]
- Zając, G.; Szyszlak-Bargłowicz, J.; Gołębiowski, W.; Szczepanik, M. Chemical Characteristics of Biomass Ashes. Energies 2018, 11, 2885. [Google Scholar] [CrossRef]
- Lanzerstorfer, C. Chemical Composition and Physical Properties of Filter Fly Ashes from Eight Grate-Fired Biomass Combustion Plants. J. Environ. Sci. 2015, 30, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Lanzerstorfer, C. Chemical Composition and Properties of Ashes from Combustion Plants Using Miscanthus as Fuel. J. Environ. Sci. 2017, 54, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yu, C.; Bai, J.; Wang, Q.; Luo, Z. Heavy Metal Characterization of Circulating Fluidized Bed Derived Biomass Ash. J. Hazard. Mater. 2012, 233–234, 41–47. [Google Scholar] [CrossRef]
- Roberts, L.J.; Mason, P.E.; Jones, J.M.; Gale, W.F.; Williams, A.; Ellul, C. Investigating the Impact of an Al-Si Additive on the Resistivity of Biomass Ashes. Fuel Process. Technol. 2018, 178, 13–23. [Google Scholar] [CrossRef]
- Uliasz-Bochenczyk, A.; Pawluk, A. Sierka Joanna Wymywalność Zanieczyszczeń z Popiołów Lotnych Ze Spalania Biomasy. Gospod. Surowcami Miner. 2015, 31, 145–156. [Google Scholar] [CrossRef][Green Version]
- Umamaheswaran, K.; Batra, V.S. Physico-Chemical Characterisation of Indian Biomass Ashes. Fuel 2008, 87, 628–638. [Google Scholar] [CrossRef]
- Yu, L.Y.; Wang, L.W.; Li, P.S. Study on Prediction Models of Biomass Ash Softening Temperature Based on Ash Composition. J. Energy Inst. 2014, 87, 215–219. [Google Scholar] [CrossRef]
- Uliasz-Bochenczyk, A.; Mokrzycki, E. The Elemental Composition of Biomass Ashes as a Preliminary Assessment of the Recovery Potential. Gospod. Surowcami Miner. 2018, 34, 115–132. [Google Scholar]
- Roberts, L.J.; Mason, P.E.; Jones, J.M.; Gale, W.F.; Williams, A.; Hunt, A.; Ashman, J. The Impact of Aluminosilicate-Based Additives upon the Sintering and Melting Behaviour of Biomass Ash. Biomass Bioenergy 2019, 127, 105284. [Google Scholar] [CrossRef]
- Yao, X.; Xu, K.; Yan, F.; Liang, Y. The Influence of Ashing Temperature on Ash Fouling and Slagging Characteristics during Combustion of Biomass Fuels. Bioresources 2017, 12, 1593–1610. [Google Scholar] [CrossRef]
- Szczepanik, M.; Szyszlak-Bargłowicz, J.; Zając, G.; Koniuszy, A.; Hawrot-Paw, M.; Wolak, A. The Use of Multivariate Data Analysis (HCA and PCA) to Characterize Ashes from Biomass Combustion. Energy 2021, 14, 6887. [Google Scholar] [CrossRef]
- Zhai, J.; Burke, I.T.; Stewart, D.I. Beneficial Management of Biomass Combustion Ashes. Renew. Sustain. Energy Rev. 2021, 151, 111555. [Google Scholar] [CrossRef]
- Adamczyk, J.; Smołka-Danielowska, D.; Krzątała, A.; Krzykawski, T. Chemical and Mineral Composition of Bottom Ash from Agri-Food Biomass Produced under Low Combustion Conditions. Int. J. Environ. Sci. Technol. 2024, 21, 4025–4036. [Google Scholar] [CrossRef]
- Li, J.; Wei, J.; Reinmöller, M.; Liang, C.; Li, S.; Xiao, R.; Xu, J. Comparative Observation of the Flow Behavior of Low- and High-Temperature Ashes of Biomass. Fuel 2022, 327, 125232. [Google Scholar] [CrossRef]
- Carević, I.; Štirmer, N.; Trkmić, M.; Kostanić Jurić, K. Leaching Characteristics of Wood Biomass Fly Ash Cement Composites. Appl. Sci. 2020, 10, 8704. [Google Scholar] [CrossRef]
- Barišić, I.; Netinger Grubeša, I.; Hackenberger, D.K.; Palijan, G.; Glavić, S.; Trkmić, M. Multidisciplinary Approach to Agricultural Biomass Ash Usage for Earthworks in Road Construction. Materials 2022, 15, 4529. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Zheng, Y. Quantitative Chemical Composition Determination and Thermal Analysis for Typical Biomass Ashes in China. Asia-Pac. J. Chem. Eng. 2014, 9, 751–758. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, X.; Zhang, H.; Liu, H. The Influence of Combustion Temperature on Alkali Content of Cattle Manure Ash. Case Stud. Constr. Mater. 2021, 14, e00519. [Google Scholar] [CrossRef]
- Odzijewicz, J.I.; Wołejko, E.; Wydro, U.; Wasil, M.; Jabłońska-Trypuć, A. Utilization of Ashes from Biomass Combustion. Energy 2022, 15, 9653. [Google Scholar] [CrossRef]
- Sulg, M.; Konist, A.; Järvik, O. Characterization of Different Wood Species as Potential Feedstocks for Gasification. Agron. Res. 2021, 19, 276–299. [Google Scholar]
- Zhao, X.; Zhou, H.; Sikarwar, V.S.; Zhao, M.; Park, A.-H.A.; Fennell, P.S.; Shen, L.; Fan, L.-S. Biomass-Based Chemical Looping Technologies: The Good, the Bad and the Future. Energy Environ. Sci. 2017, 10, 1885–1910. [Google Scholar] [CrossRef]
- Czerski, G.; Śpiewak, K.; Grzywacz, P.; Wierońska-Wiśniewska, F. Assessment of the Catalytic Effect of Various Biomass Ashes on CO2 Gasification of Tire Char. J. Energy Inst. 2021, 99, 170–177. [Google Scholar] [CrossRef]
- Wei, J.; Gong, Y.; Ding, L.; Yu, J.; Yu, G. Influence of Biomass Ash Additive on Reactivity Characteristics and Structure Evolution of Coal Char–CO2 Gasification. Energy Fuels 2018, 32, 10428–10436. [Google Scholar] [CrossRef]
- Feng, P.; Zhou, S.; Zhao, J. Effect of Cattle Manure Ash on Workability and Mechanical Properties of Magnesium Phosphate Cement. Constr. Build. Mater. 2016, 129, 79–88. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, S.; Zhang, H. Cow Manure Ash Particle Size Distribution’s Effect on Concrete Paste Rheological Properties. Adv. Cem. Res. 2020, 32, 1–11. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, X.; Chen, X. Pozzolanic Activity of Feedlot Biomass (Cattle Manure) Ash. Constr. Build. Mater. 2012, 28, 493–498. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Li, H.; Han, X.; Zhang, M.; Sun, Y.; Fan, X.; Tu, R.; Zeng, Y.; Xu, C.C.; et al. Applications of Catalysts in Thermochemical Conversion of Biomass (Pyrolysis, Hydrothermal Liquefaction and Gasification): A Critical Review. Renew. Energy 2022, 196, 462–481. [Google Scholar] [CrossRef]
- Śpiewak, K.; Czerski, G.; Porada, S. Effect of K, Na and Ca-Based Catalysts on the Steam Gasification Reactions of Coal. Part I: Type and Amount of One-Component Catalysts. Chem. Eng. Sci. 2021, 229, 116024. [Google Scholar] [CrossRef]
- Kang, K.; Azargohar, R.; Dalai, A.K.; Wang, H. Hydrogen Production from Lignin, Cellulose and Waste Biomass via Supercritical Water Gasification: Catalyst Activity and Process Optimization Study. Energy Convers. Manag. 2016, 117, 528–537. [Google Scholar] [CrossRef]
- Sutton, D.; Kelleher, B.; Ross, J.R.H. Review of Literature on Catalysts for Biomass Gasification. Fuel Process. Technol. 2001, 73, 155–173. [Google Scholar] [CrossRef]
- Abu El-Rub, Z.; Bramer, E.A.; Brem, G. Review of Catalysts for Tar Elimination in Biomass Gasification Processes. Ind. Eng. Chem. Res. 2004, 43, 6911–6919. [Google Scholar] [CrossRef]
- Śpiewak, K.; Czerski, G.; Porada, S. Effect of K, Na and Ca-Based Catalysts on the Steam Gasification Reactions of Coal. Part II: Composition and Amount of Multi-Component Catalysts. Chem. Eng. Sci. 2021, 229, 116023. [Google Scholar] [CrossRef]
- Brown, R.C.; Liu, Q.; Norton, G. Catalytic Effects Observed during the Co-Gasification of Coal and Switchgrass. Biomass Bioenergy 2000, 18, 499–506. [Google Scholar] [CrossRef]
- Lahijani, P.; Zainal, Z.A.; Mohamed, A.R.; Mohammadi, M. Ash of Palm Empty Fruit Bunch as a Natural Catalyst for Promoting the CO2 Gasification Reactivity of Biomass Char. Bioresour. Technol. 2013, 132, 351–355. [Google Scholar] [CrossRef]
- Nanou, P.; Gutiérrez Murillo, H.E.; van Swaaij, W.P.M.; van Rossum, G.; Kersten, S.R.A. Intrinsic Reactivity of Biomass-Derived Char under Steam Gasification Conditions-Potential of Wood Ash as Catalyst. Chem. Eng. J. 2013, 217, 289–299. [Google Scholar] [CrossRef]
- Rizkiana, J.; Guan, G.; Widayatno, W.B.; Hao, X.; Li, X.; Huang, W.; Abudula, A. Promoting Effect of Various Biomass Ashes on the Steam Gasification of Low-Rank Coal. Appl. Energy 2014, 133, 282–288. [Google Scholar] [CrossRef]
- Kryca, J.; Priščák, J.; Łojewska, J.; Kuba, M.; Hofbauer, H. Apparent Kinetics of the Water-Gas-Shift Reaction in Biomass Gasification Using Ash-Layered Olivine as Catalyst. Chem. Eng. J. 2018, 346, 113–119. [Google Scholar] [CrossRef]
- Berdugo Vilches, T.; Maric, J.; Knutsson, P.; Rosenfeld, D.C.; Thunman, H.; Seemann, M. Bed Material as a Catalyst for Char Gasification: The Case of Ash-Coated Olivine Activated by K and S Addition. Fuel 2018, 224, 85–93. [Google Scholar] [CrossRef]
- Pissot, S.; Berdugo Vilches, T.; Thunman, H.; Seemann, M. Effect of Ash Circulation on the Performance of a Dual Fluidized Bed Gasification System. Biomass Bioenergy 2018, 115, 45–55. [Google Scholar] [CrossRef]
- Berdugo Vilches, T.; Seemann, M.; Thunman, H. Influence of In-Bed Catalysis by Ash-Coated Olivine on Tar Formation in Steam Gasification of Biomass. Energy Fuels 2018, 32, 9592–9604. [Google Scholar] [CrossRef]
- Pio, D.T.; Tarelho, L.A.C.; Pinto, R.G.; Matos, M.A.A.; Frade, J.R.; Yaremchenko, A.; Mishra, G.S.; Pinto, P.C.R. Low-Cost Catalysts for in-Situ Improvement of Producer Gas Quality during Direct Gasification of Biomass. Energy 2018, 165, 442–454. [Google Scholar] [CrossRef]
- Qin, Y.; He, Y.; Ren, W.; Gao, M.; Wiltowski, T. Catalytic Effect of Alkali Metal in Biomass Ash on the Gasification of Coal Char in CO2. J. Therm. Anal. Calorim. 2020, 139, 3079–3089. [Google Scholar] [CrossRef]
- Li, X.; Yang, C.; Liu, M.; Bai, J.; Li, W. Influence of Different Biomass Ash Additive on Anthracite Pyrolysis Process and Char Gasification Reactivity. Int. J. Coal Sci. Technol. 2020, 7, 464–475. [Google Scholar] [CrossRef]
- Yu, J.; Gong, Y.; Wei, J.; Ding, L.; Song, X.; Yu, G. Promoting Effect of Biomass Ash Additives on High-Temperature Gasification of Petroleum Coke: Reactivity and Kinetic Analysis. J. Energy Inst. 2020, 93, 1364–1372. [Google Scholar] [CrossRef]
- He, Q.; Yu, J.; Song, X.; Ding, L.; Wei, J.; Yu, G. Utilization of Biomass Ash for Upgrading Petroleum Coke Gasification: Effect of Soluble and Insoluble Components. Energy 2020, 192, 116642. [Google Scholar] [CrossRef]
- Guo, Q.; Yan, B.; Hu, Y.; Guo, X.; Wu, W.; Cheng, Z.; Chen, G.; Hou, L. A Novel Reutilization of Ash from Biomass Gasification Process: Feasibility and Products Improvement Analysis. Fuel 2023, 339, 127386. [Google Scholar] [CrossRef]
- Śpiewak, K.; Soprych, P.; Czerski, G. Influence of Pressure and Sunflower Husks Ash as Catalyst on Tire-Char Steam Gasification. Energy Rep. 2023, 9, 1–15. [Google Scholar] [CrossRef]
- Śpiewak, K.; Czerski, G.; Soprych, P. Steam Gasification of Tire Char Supported by Catalysts Based on Biomass Ashes. Energy 2023, 285, 129378. [Google Scholar] [CrossRef]
- Mo, Z.; He, Y.; Liu, J.; Tu, J.; Li, D.; Hu, C.; Zhang, Q.; Wang, K.; Wang, T. Biomass Steam Gasification for Hydrogen-Rich Syngas Production over Fly Ash-Based Catalyst Pretreated by Coupling of Washing and Calcination. Int. J. Hydrog. Energy 2024, 49, 164–176. [Google Scholar] [CrossRef]
- Gomes, H.G.M.F.; Lopes, D.V.; Moura, J.M.; Ribeiro, J.P.; Cruz, N.C.; Matos, M.A.A.; Tarelho, L.A.C. Biomass Fly Ash Granules as a Promising Catalyst to Promote Producer Gas Quality from Residual Forest Biomass Steam Gasification. Energy 2025, 319, 134889. [Google Scholar] [CrossRef]
- Śpiewak, K.; Soprych, P.; Czerski, G. Effect of Pressure and Biomass Ashes Addition on the Steam Gasification of Tire Char. J. Energy Inst. 2025, 123, 102282. [Google Scholar] [CrossRef]
- Kaewluan, S.; Pipatmanomai, S. Potential of Synthesis Gas Production from Rubber Wood Chip Gasification in a Bubbling Fluidised Bed Gasifier. Energy Convers. Manag. 2011, 52, 75–84. [Google Scholar] [CrossRef]
- Arnold, R.A.; Hill, J.M. Catalysts for Gasification: A Review. Sustain. Energy Fuels 2019, 3, 656–672. [Google Scholar] [CrossRef]
- Broström, M.; Enestam, S.; Backman, R.; Mäkelä, K. Condensation in the KCl–NaCl System. Fuel Process. Technol. 2013, 105, 142–148. [Google Scholar] [CrossRef]
- Li, X.; Zhi, L.; Shi, W.; Kong, L.; Bai, J.; Yu, J.; Reinmöller, M.; Guhl, S.; Meyer, B.; Li, W. Effect of K2O/Na2O on Fusion Behavior of Coal Ash with High Silicon and Aluminum Level. Fuel 2020, 265, 116964. [Google Scholar] [CrossRef]
- Li, W.; Yu, Z.; Guan, G. Catalytic Coal Gasification for Methane Production: A Review. Carbon Resour. Convers. 2021, 4, 89–99. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Ren, N.; Ma, C. Improving the Thermal Properties of NaNO3-KNO3 for Concentrating Solar Power by Adding Additives. Sol. Energy Mater. Sol. Cells 2017, 160, 263–268. [Google Scholar] [CrossRef]
- Wu, Y.; Wen, C.; Chen, X.; Jiang, G.; Liu, G.; Liu, D. Catalytic Pyrolysis and Gasification of Waste Textile under Carbon Dioxide Atmosphere with Composite Zn-Fe Catalyst. Fuel Process. Technol. 2017, 166, 115–123. [Google Scholar] [CrossRef]
- Jin, G.; Iwaki, H.; Arai, N.; Kitagawa, K. Study on the Gasification of Wastepaper/Carbon Dioxide Catalyzed by Molten Carbonate Salts. Energy 2005, 30, 1192–1203. [Google Scholar] [CrossRef]
- Obernberger, I.; Biedermann, F.; Widmann, W.; Riedl, R. Concentrations of Inorganic Elements in Biomass Fuels and Recovery in the Different Ash Fractions. Biomass Bioenergy 1997, 12, 211–224. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).