The Effect of Enzymatic Disintegration Using Cellulase and Lysozyme on the Efficiency of Methane Fermentation of Sewage Sludge
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Enzymes
- Cellulase (EC 3.2.1.4), derived from Aspergillus niger, (TCI TOKYO CHEMICAL INDUSTRY CO., LTD., 16-12 Nihonbashi-kodemmacho, Chuo-ku, Tokyo 103-0001, Japan) with activity of ≥17,000 U/g,
- Lysozyme (EC 3.2.1.17), a lytic enzyme obtained from chicken egg protein with activity of ≥20,360 U/mg dry weight (Thermo Fisher Scientific, Waltham, MA, USA).
2.3. Fermentation Process
2.4. Physicochemical Analyses
- -
- pH (potentiometrically)—in accordance with the PN-EN ISO 10523:2012 standard [31].
- -
- Alkalinity (ALK)—titration to pH 4.5.
- -
- Chemical oxygen demand (COD)—NANOCOLOR® VIS II spectrophotometer from MACHEREY NAGEL GmbH & Co. KG, Düren, Germany. (The test is equivalent to EPA 410.4 and APHA 5220D methods [32].)
- -
- Volatile fatty acids (VFAs)—UDK139 Semi-Automatic Steam Distillation Apparatus, VELP, Usmate, Italy (via distillation and titration with NaOH).
- -
- Dry mass (DM)—a weight method in accordance with the PN-EN 12880:2004 standard [33].
2.5. Biogas Production and Composition
2.6. Microbiological Tests
- -
- Escherichia coli—according to ISO 4832:2007, confirmed in Brilliant Green Bile and ColiTest media [34].
2.7. Statistical Analysis
3. Results
3.1. Physical and Chemical Properties of Sewage Sludge
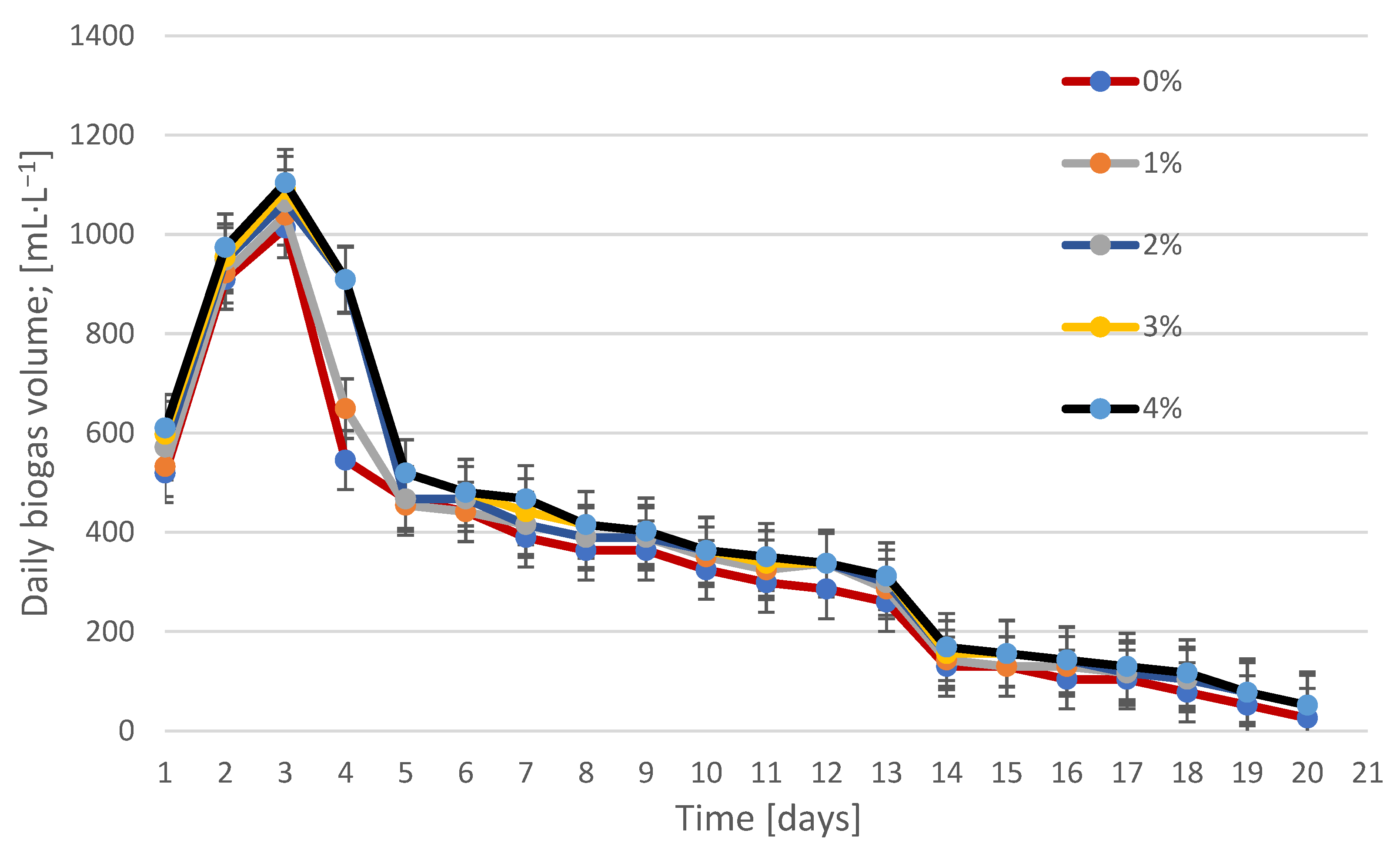
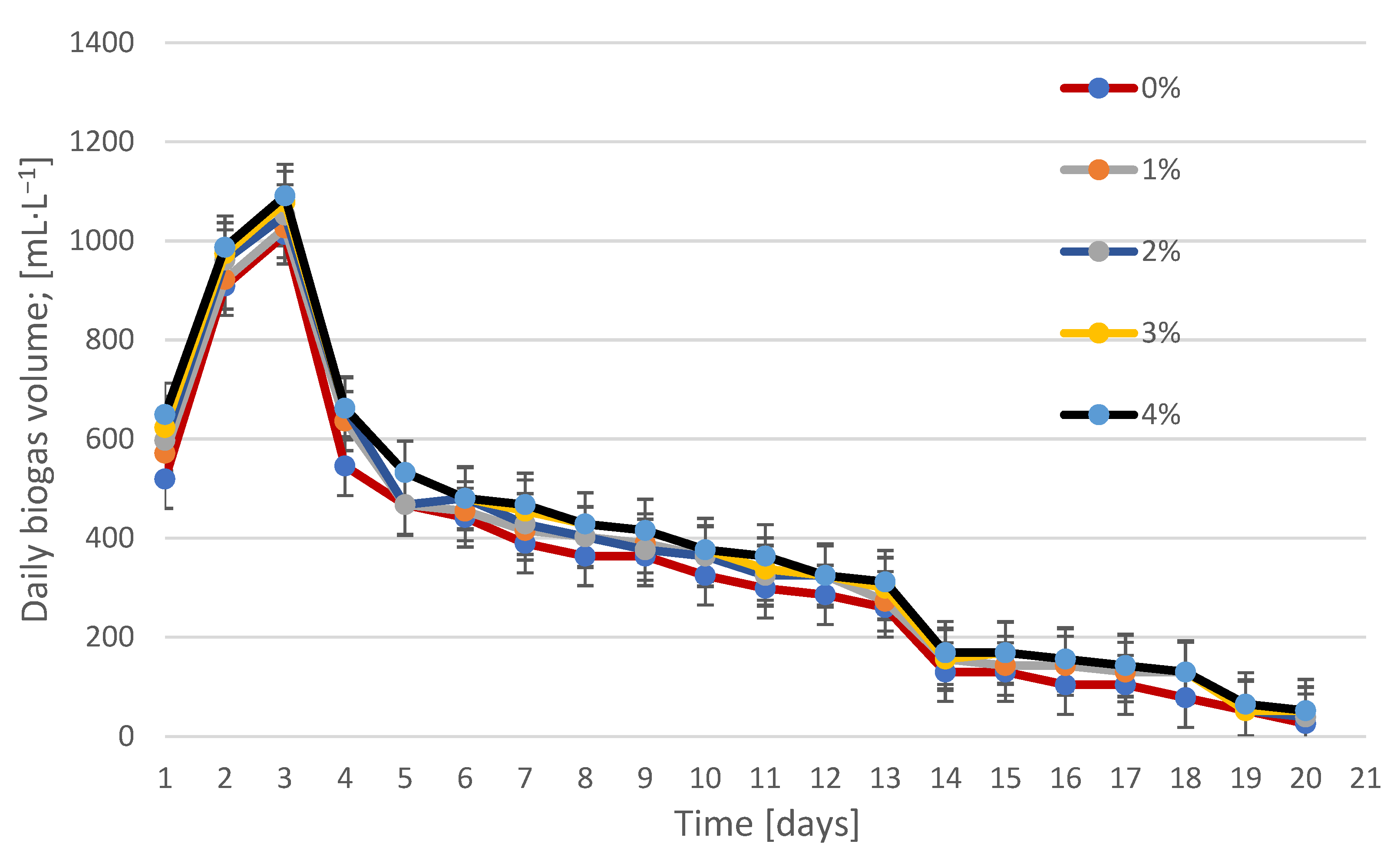
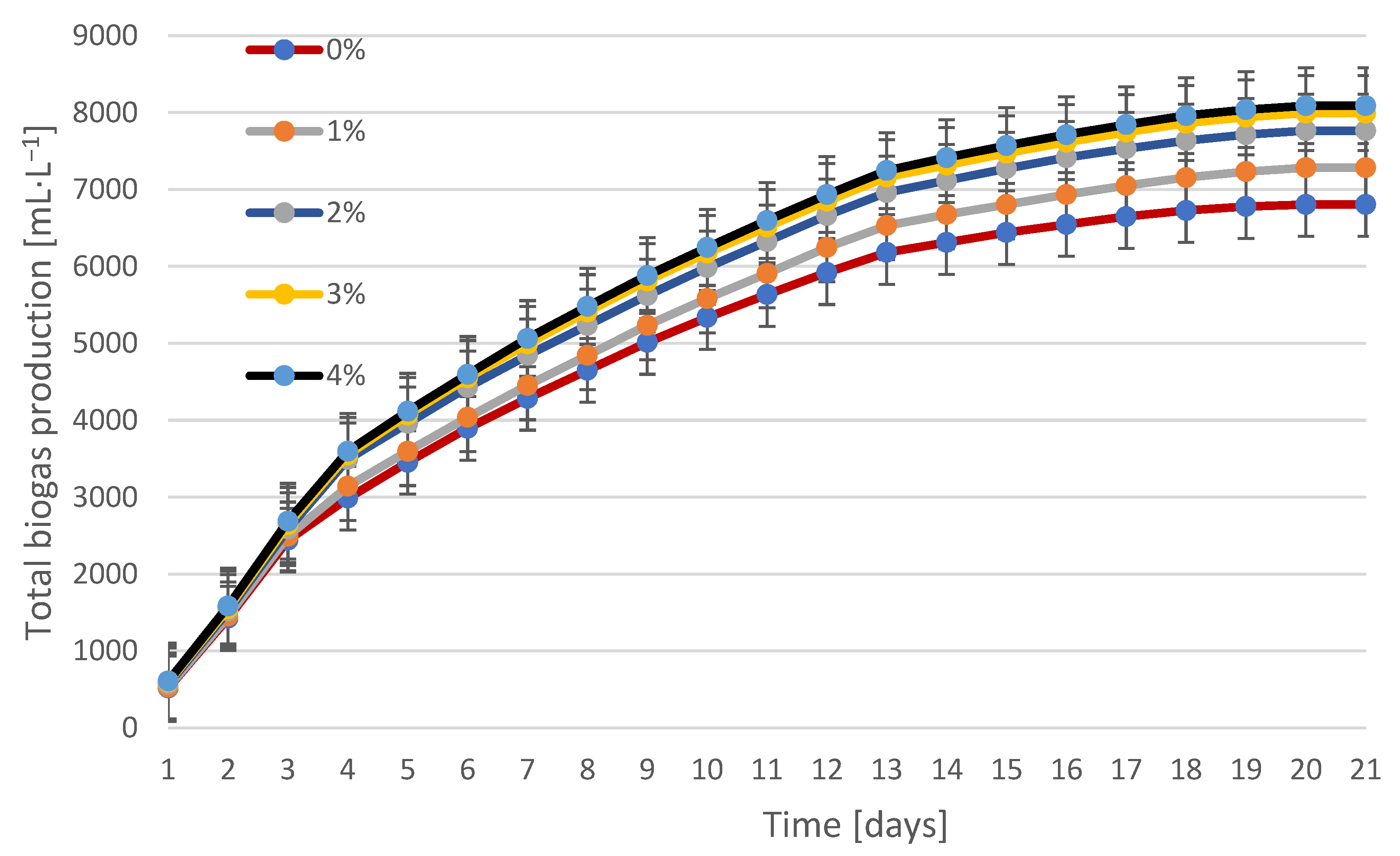
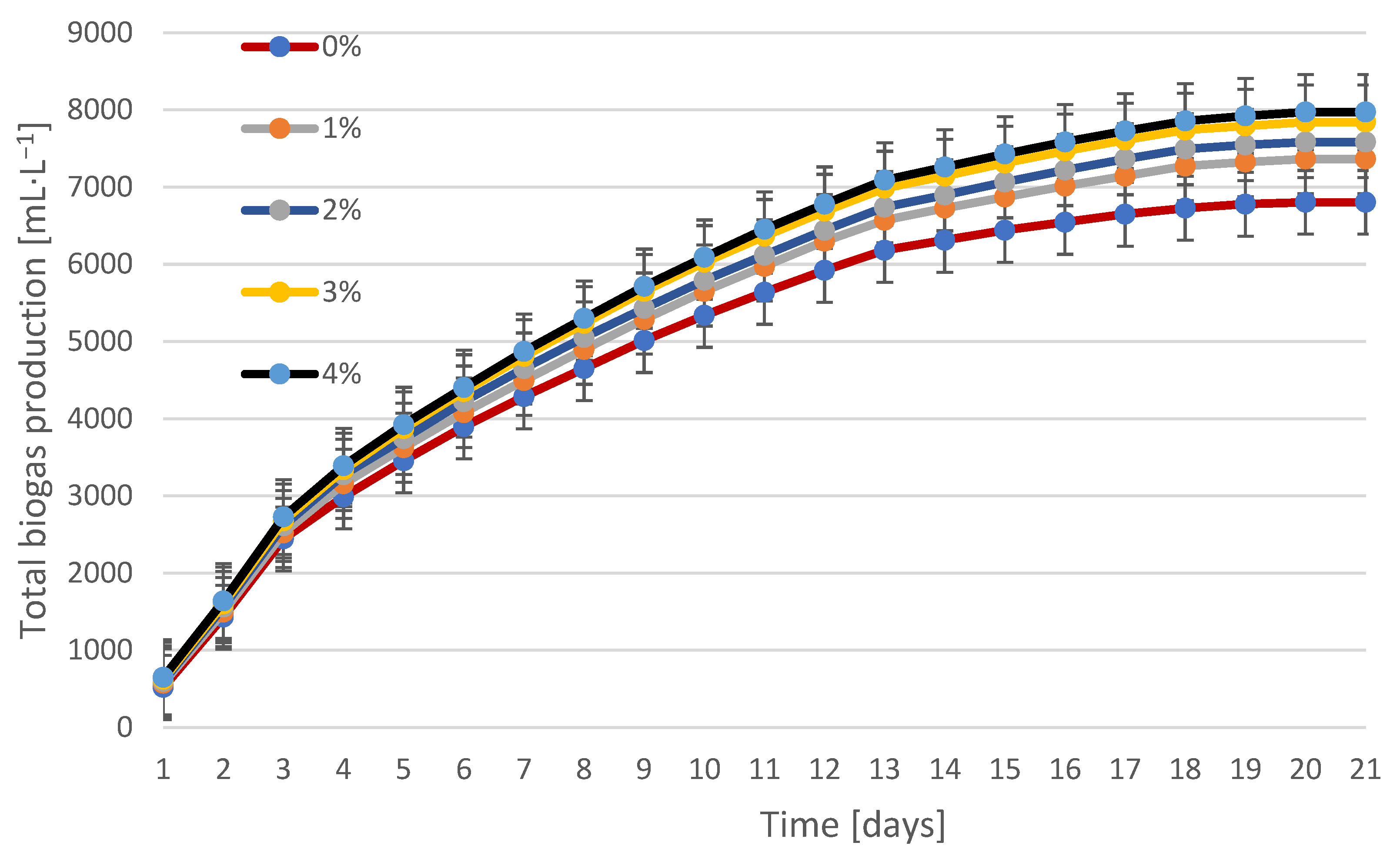
3.2. Biogas Production and Composition During Fermentation
3.3. Fermentation Process Parameters
3.4. Microbiological Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nguyen, V.K.; Chaudhary, D.K.; Dahal, R.H.; Trinh, N.H.; Kim, J.; Chang, S.W.; Hong, Y.; La, D.D.; Nguyen, X.C.; Ngo, H.H.; et al. Review on pretreatment techniques to improve anaerobic digestion of sewage sludge. Fuel 2021, 285, 119105. [Google Scholar] [CrossRef]
- Neumann, P.; Pesante, S.; Venegas, M.; Vidal, G. Developments in pre-treatment methods to improve anaerobic digestion of sewage sludge. Rev. Environ. Sci. Bio/Technol. 2016, 15, 173–211. [Google Scholar] [CrossRef]
- Choi, J.M.; Han, S.K.; Lee, C.Y. Enhancement of methane production in anaerobic digestion of sewage sludge by thermal hydrolysis pretreatment. Bioresour. Technol. 2018, 259, 207–213. [Google Scholar] [CrossRef]
- Ziemiński, K.; Frąc, M. Methane fermentation process as anaerobic digestion of biomass: Transformations, stages and microorganisms. Afr. J. Biotechnol. 2012, 11, 4127–4139. [Google Scholar] [CrossRef]
- Mustapha, N.A.; Hu, A.; Yu, C.P.; Sharuddin, S.S.; Ramli, N.; Shirai, Y.; Maeda, T. Seeking key microorganisms for enhancing methane production in anaerobic digestion of waste sewage sludge. Appl. Microbiol. Biotechnol. 2018, 102, 5323–5334. [Google Scholar] [CrossRef]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Li, Q.; Qiao, W.; Wang, X.; Takayanagi, K.; Shofie, M.; Li, Y.Y. Kinetic characterization of thermophilic and mesophilic anaerobic digestion for coffee grounds and waste activated sludge. Waste Manag. 2015, 36, 77–85. [Google Scholar] [CrossRef]
- Carrère, H.; Dumas, C.; Battimelli, A.; Batstone, D.J.; Delgenes, J.P.; Steyer, J.P.; Ferrer, I. Pretreatment methods to improve sludge anaerobic degradability: A review. J. Hazard. Mater. 2010, 183, 1–15. [Google Scholar] [CrossRef]
- Pilli, S.; Bhunia, P.; Yan, S.; LeBlanc, R.J.; Tyagi, R.D.; Surampalli, R.Y. Ultrasonic pretreatment of sludge: A review. Ultrason. Sonochem. 2011, 18, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Dauknys, R.; Mažeikienė, A.; Paliulis, D. Effect of ultrasound and high voltage disintegration on sludge digestion process. J. Environ. Manag. 2020, 270, 110833. [Google Scholar] [CrossRef] [PubMed]
- Barjenbruch, M.; Kopplow, O. Enzymatic, mechanical and thermal pre-treatment of surplus sludge. Adv. Environ. Res. 2003, 7, 715–720. [Google Scholar] [CrossRef]
- Elsamadony, M. Enrich waste activated sludge digestibility via natural enzyme supplementation. E3S Web Conf. 2019, 83, 01012. [Google Scholar] [CrossRef]
- Myszograj, S.; Płuciennik-Koropczuk, E. Thermal disintegration of sewage sludge as a method of improving the biogas potential. Energies 2023, 16, 559. [Google Scholar] [CrossRef]
- Macherzyński, B.; Popowska-Nowak, E.; Włodarczyk-Makuła, M.; Bień, B.; Wszelaka-Rylik, M. Intensification of Energy Production in the Anaerobic Digestion Process of Sewage Sludge Using Enzymatic Disintegration. Energies 2025, 18, 11. [Google Scholar] [CrossRef]
- Macherzyński, B. Effects of Enzymatic Disintegration on the Decomposition of Organic Compounds During Methane Fer-mentation of Sewage Sludge. Catalysts 2025, 15, 75. [Google Scholar] [CrossRef]
- Bartłomiej, M.; Małgorzata, W.R.; Paweł, G.; Aleksandra, K. Precipitation of Struvite from Supernatants Separated from Enzymatically Disintegrated Digested Sewage Sludge. Catalysts 2025, 15, 361. [Google Scholar] [CrossRef]
- Romano, R.T.; Zhang, R.; Teter, S.; McGarvey, J.A. The effect of enzyme addition on anaerobic digestion of Jose Tall Wheat Grass. Bioresour. Technol. 2009, 100, 4564–4571. [Google Scholar] [CrossRef]
- Jiang, X.; Lyu, Q.; Bi, L.; Liu, Y.; Xie, Y.; Ji, G.; Huan, G.C.; Xu, L.; Yan, Z. Improvement of sewage sludge anaerobic digestion through synergistic effect combined trace elements enhancer with enzyme pretreatment and microbial community response. Chemosphere 2022, 286, 131356. [Google Scholar] [CrossRef]
- Xu, N.; Liu, S.; Xin, F.; Zhou, J.; Jia, H.; Xu, J.; Jiang, M.; Dong, W. Biomethane production from lignocellulose: Biomass recalcitrance and its impacts on anaerobic digestion. Front. Bioeng. Biotechnol. 2019, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Liew, Y.X.; Chan, Y.J.; Manickam, S.; Chong, M.F.; Chong, S.; Tiong, T.J.; Lim, J.W.; Pan, G.T. Enzymatic pretreatment to enhance anaerobic bioconversion of high strength wastewater to biogas: A review. Sci. Total Environ. 2020, 713, 136373. [Google Scholar] [CrossRef]
- Kim, S.; Woo, S.G.; Lee, J.; Lee, D.H.; Hwang, S. Evaluation of feasibility of using the bacteriophage t4 lysozyme to improve the hydrolysis and biochemical methane potential of secondary sludge. Energies 2019, 12, 3644. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.I.; Hwang, S. Enhancement of hydrolysis efficiency and biogas production by treatment of secondary sludge with bacteriophage lysozymes. Sustain. Energy Technol. Assess. 2022, 54, 102897. [Google Scholar] [CrossRef]
- Behera, B.C.; Sethi, B.K.; Mishra, R.R.; Dutta, S.K.; Thatoi, H.N. Microbial cellulases–Diversity & biotechnology with reference to mangrove environment: A review. J. Genet. Eng. Biotechnol. 2017, 15, 197–210. [Google Scholar] [CrossRef]
- Ejaz, U.; Sohail, M.; Ghanemi, A. Cellulases: From bioactivity to a variety of industrial applications. Biomimetics 2021, 6, 44. [Google Scholar] [CrossRef]
- Nawaz, N.; Wen, S.; Wang, F.; Nawaz, S.; Raza, J.; Iftikhar, M.; Usman, M. Lysozyme and its application as antibacterial agent in food industry. Molecules 2022, 27, 6305. [Google Scholar] [CrossRef]
- Primo, E.D.; Otero, L.H.; Ruiz, F.; Klinke, S.; Giordano, W. The disruptive effect of lysozyme on the bacterial cell wall explored by an in-silico structural outlook. Biochem. Mol. Biol. Educ. 2018, 46, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Christy, P.M.; Gopinath, L.; Divya, D. A review on anaerobic decomposition and enhancement of biogas production through enzymes and microorganisms. Renew. Sustain. Energy Rev. 2014, 34, 167–173. [Google Scholar] [CrossRef]
- Liu, S.; Wu, J.; Hu, Z.; Jiang, M. Changes in microbial community during hydrolyzed sludge reduction. Front. Microbiol. 2023, 14, 1239218. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Luo, K.; Li, X.M.; Wang, D.B.; Zheng, W.; Zeng, G.M.; Liu, J.J. Enhanced efficiency of biological excess sludge hydrolysis under anaerobic digestion by additional enzymes. Bioresour. Technol. 2010, 101, 2924–2930. [Google Scholar] [CrossRef]
- Liu, G.; Wang, K.; Li, X.; Ma, L.; Ma, X.; Chen, H. Enhancement of excess sludge hydrolysis and decomposition with different lysozyme dosage. J. Hazard. Mater. 2019, 366, 395–401. [Google Scholar] [CrossRef]
- PN-EN ISO 10523:2012; Jakość Wody—Oznaczanie pH. Polski Komitet Normalizacyjny: Warszawa, Poland, 2012.
- Standard Methods 5220D: Chemical Oxygen Demand, Closed Reflux, Colorimetric Method. Available online: https://www.nemi.gov/methods/method_summary/5716/ (accessed on 10 August 2025).
- PN-EN 12880:2004; Charakterystyka Osadów Ściekowych—Oznaczanie Suchej Pozostałości i Zawartości Wody. Polski Komitet Normalizacyjny: Warszawa, Poland, 2004.
- PN-ISO 4832; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coliforms—Colony-Count Technique. ISO: Geneva, Switzerland, 2007.
- Junior, I.V.; de Almeida, R.; Cammarota, M.C. A review of sludge pretreatment methods and co-digestion to boost biogas production and energy self-sufficiency in wastewater treatment plants. J. Water Process Eng. 2021, 40, 101857. [Google Scholar] [CrossRef]
- Baksi, S.; Sarkar, U.; Villa, R.; Basu, D.; Sengupta, D. Conversion of biomass to biofuels through sugar platform: A review of enzymatic hydrolysis highlighting the trade-off between product and substrate inhibitions. Sustain. Energy Technol. Assess. 2023, 55, 102963. [Google Scholar] [CrossRef]
- Kardos, L.; Juhasz, A.; Palko, G.Y.; Olah, J.; Barkacs, K.; Zaray, G.Y. Comparing of mesophilic and thermophilic anaerobic fermented sewage sludge based on chemical and biochemical tests. Appl. Ecol. Environ. Res. 2011, 9, 293–302. [Google Scholar] [CrossRef]
- Macherzyński, B.; Włodarczyk-Makuła, M. Evaluation of the possibility of disposal of Coke sludge in the co-fermentation process. Annu. Set Environ. Prot. 2015, 17, 1142–1161. [Google Scholar]
- Becker, T.; Ogez, J.R.; Builder, S.E. Downstream processing of proteins. Biotechnol. Adv. 1983, 1, 247–261. [Google Scholar] [CrossRef]
- Ohno, N.; Morrison, D.C. Lipopolysaccharide interaction with lysozyme. J. Biol. Chem. 1989, 264, 4434–4441. [Google Scholar] [CrossRef]
- Elliason, D.J.; Tatini, S.R. Enhanced inactivation of Salmonella typhimurium and verotoxigenic Escherichia coli by nisin at 6·5 °C. Food Microbiol. 1999, 16, 257–267. [Google Scholar] [CrossRef]
- Sakakibara, Y.; Yanagisawa, H. Techniques for the separation of proteins by isoelectric point column chromatography. Bull. Aichi Univ. Educ. 2007, 56, 45–49. [Google Scholar]
- Sedov, S.A.; Belogurova, N.G.; Shipovskov, S.; Levashov, V.A.; Levasho, P.A. Lysis of Escherichia coli cells by lysozyme: Discrimination between adsorption and enzyme action. Colloids Surf. B Biointerfaces 2011, 88, 131–133. [Google Scholar] [CrossRef]
- Córdova, O.; Chamy, R.; Guerrero, L.; Sánchez-Rodríguez, A. Assessing the Effect of Pretreatments on the Structure and Functionality of Microbial Communities for the Bioconversion of Microalgae to Biogas. Front. Microbiol. 2018, 9, 1388. [Google Scholar] [CrossRef]
- Bahreini, G.; Nazari, L.; Ho, D.; Flannery, C.; Elbeshbishy, E.; Santoro, D.; Nakhla, G. Enzymatic pre-treatment for enhancement of primary sludge fermentation. Bioresour. Technol. 2020, 305, 123071. [Google Scholar] [CrossRef]
- Wu, D.; Li, L.; Zhen, F.; Liu, H.; Xiao, F.; Sun, Y.; Peng, X.; Li, Y.; Wang, X. Thermodynamics of volatile fatty acid degradation during anaerobic digestion under organic overload stress: The potential to better identify process stability. Water Res. 2022, 214, 118187. [Google Scholar] [CrossRef]
- Manai, I.; Miladi, B.; El Mselmi, A.; Hamdi, M.; Bouallagui, H. Improvement of activated sludge resistance to shock loading by fungal enzyme addition during textile wastewater treatment. Environ. Technol. 2016, 38, 880–890. [Google Scholar] [CrossRef]
- Nasar, N.; Pizzagalli, G.; Coulon, F.; Bajón-Fernández, Y. Enzymes targeting distinct hydrolysis blind-spots of thermal and biological pre-treatments significantly uplift biogas production. Bioresour. Technol. 2025, 426, 132353. [Google Scholar] [CrossRef] [PubMed]
- Parawira, W. Enzyme research and applications in biotechnological intensification of biogas production. Crit. Rev. Biotechnol. 2012, 32, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, Z.; Fattah, M.; Shamkhy, A. Investigation of Solid Waste Generation Rate and Biogas Production. Results Eng. 2024, 23, 102531. [Google Scholar] [CrossRef]
- Luo, K.; Yang, Q.; Li, X.; Tang, Y.; Luo, Z.; Liu, J. Enhanced hydrolysis of excess sludge by external enzymes. Huan Jing Ke Xue = Huanjing Kexue 2010, 31, 763–767. [Google Scholar] [PubMed]
- Dz.U. 2019 poz. 1311. Rozporządzenie Ministra Gospodarki Morskiej i Żeglugi Śródlądowej z Dnia 12 lipca 2019 r. w Sprawie Substancji Szczególnie Szkodliwych dla Środowiska Wodnego Oraz Warunków, Jakie Należy Spełnić Przy Wprowadzaniu do Wód Lub do Ziemi Ścieków, a Także Przy Odprowadzaniu Wód Opadowych Lub Roztopowych do Wód lub do Urządzeń Wodnych. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20190001311 (accessed on 10 August 2025).
- Liu, Z.; Smith, S. Enzyme Recovery from Biological Wastewater Treatment. Waste Biomass Valorization 2020, 12, 4185–4211. [Google Scholar] [CrossRef]
| Indicator | Unit | Before the Process | ||
|---|---|---|---|---|
| Fermented Sludge | Excess Sludge | Mixture of Excess and Fermented Sludge | ||
| pH | - | 7.4 ± 0.2 | 6.1 ± 0.2 | 7.4 ± 0.2 |
| ALK | mg CaCO3·L−1 | 3295 ± 50 | 70 ± 10 | 1915 |
| COD | mg O2·L−1 | 1800 ± 100 | 268 ± 12 | 1200 ± 100 |
| VFA | mg CH3COOH·L−1 | 130 ± 12 | 37 ± 5 | 93 ± 7 |
| VFA/ALK | - | 0.04 | 0.53 | 0.05 |
| DM | g·L−1 | 28.46 ± 0.37 | 5.12 ± 0.64 | 23.78 ± 0.54 |
| Organic Substances | g·L−1 | 15.88 ± 0.33 | 2.57 ± 0.29 | 15.78 ± 0.24 |
| Indicator | Unit | 0% | Cellulase | Lysozyme | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2% | 3% | 4% | 1% | 2% | 3% | 4% | |||
| pH | - | 7.5 ± 0.1 | 7.6 ± 0.2 | 7.7 ± 0.1 | 7.7 ± 0.1 | 7.7 ± 0.2 | 7.6 ± 0.1 | 7.7 ± 0.2 | 7.7 ± 0.2 | 7.7 ± 0.2 |
| ALK | mg CaCO3·L−1 | 2800 ± 40 | 3095 ± 35 | 3225 ± 20 | 3235 ± 50 | 3235 ± 15 | 3205 ± 35 | 3210 ± 35 | 3215 ± 20 | 3235 ± 40 |
| COD | mg O2·L−1 | 893 ± 15 | 856 ± 10 | 843 ± 10 | 840 ± 20 | 835 ± 15 | 888 ± 20 | 852 ± 10 | 835 ± 15 | 820 ± 15 |
| VFA | mg CH3COOH·L−1 | 102 ± 10 | 74 ± 4 | 74 ± 5 | 74 ± 3 | 74 ± 7 | 93 ± 5 | 93 ± 4 | 93 ± 4 | 93 ± 6 |
| VFA/ALK | - | 0.04 | 0.02 | 0.02 | 0.02 | 0.02 | 0.03 | 0.03 | 0.03 | 0.03 |
| DM | g·L−1 | 12.96 ± 0.27 | 10.56 ± 0.65 | 9.48 ± 0.70 | 8.40 ± 0.76 | 8.12 ± 0.76 | 11.16 ± 0.65 | 10.76 ± 0.27 | 10.56 ± 0.28 | 10.36 ± 0.14 |
| Organic Substances | g·L−1 | 5.31 ± 0.13 | 5.26 ± 0.40 | 4.58 ± 0.04 | 3.60 ± 0.48 | 3.36 ± 0.49 | 3.38 ± 0.40 | 3.31 ± 0.37 | 3.24 ± 0.05 | 3.16 ± 0.05 |
| Parameters | Unit | 0% | 1% | 2% | 3% | 4% |
|---|---|---|---|---|---|---|
| Loading the digester with organic pollutants | g·L−1·d−1 | 0.50 | ||||
| Amount of biogas from 1 g of dry organic matter using cellulase | L·g−1 | 0.62 | 0.66 | 0.70 | 0.72 | 0.73 |
| Amount of biogas from 1 g of dry organic matter using lysozyme | 0.67 | 0.69 | 0.71 | 0.72 | ||
| Degree of decomposition of substances using cellulase | % | 66.3 | 66.7 | 71.0 | 77.2 | 78.7 |
| Degree of decomposition of substances using lysozyme | 78.6 | 79.0 | 79.5 | 80.0 | ||
| Parameter | Cellulase | Lysozyme | ||||||
|---|---|---|---|---|---|---|---|---|
| 1% | 2% | 3% | 4% | 1% | 2% | 3% | 4% | |
| Total biogas production | 3.018 | 10.723 | 9.873 | 11.524 | 2.845 | 11.308 | 11.842 | 11.984 |
| Degree of decomposition of organic substances | 3.845 | 12.393 | 12.954 | 14.541 | 2.574 | 10.541 | 11.654 | 11.845 |
| Dry matter loss | 1.854 | 2.007 | 4.945 | 5.412 | 2.458 | 2.741 | 2.954 | 3.684 |
| Methane content in biogas | 1.012 | 1.215 | 1.547 | 1.001 | 1.274 | 1.365 | 1.412 | 1.247 |
| Amount of biogas produced from 1 g of dry organic matter introduced into the reactor | 3.841 | 4.505 | 5.087 | 5.423 | 2.984 | 4.505 | 5.874 | 6.954 |
| Bacteria | Before the Process | After the Process | ||||
|---|---|---|---|---|---|---|
| 0% | Lysozyme 2% | Lysozyme 3% | Cellulase 2% | Cellulase 3% | ||
| E. coli | 6.7 ± 0.58·103 | n.d. | n.d. | n.d. | n.d. | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macherzyński, B.; Wszelaka-Rylik, M.; Marszałek, A.; Popowska-Nowak, E. The Effect of Enzymatic Disintegration Using Cellulase and Lysozyme on the Efficiency of Methane Fermentation of Sewage Sludge. Energies 2025, 18, 5597. https://doi.org/10.3390/en18215597
Macherzyński B, Wszelaka-Rylik M, Marszałek A, Popowska-Nowak E. The Effect of Enzymatic Disintegration Using Cellulase and Lysozyme on the Efficiency of Methane Fermentation of Sewage Sludge. Energies. 2025; 18(21):5597. https://doi.org/10.3390/en18215597
Chicago/Turabian StyleMacherzyński, Bartłomiej, Małgorzata Wszelaka-Rylik, Anna Marszałek, and Elżbieta Popowska-Nowak. 2025. "The Effect of Enzymatic Disintegration Using Cellulase and Lysozyme on the Efficiency of Methane Fermentation of Sewage Sludge" Energies 18, no. 21: 5597. https://doi.org/10.3390/en18215597
APA StyleMacherzyński, B., Wszelaka-Rylik, M., Marszałek, A., & Popowska-Nowak, E. (2025). The Effect of Enzymatic Disintegration Using Cellulase and Lysozyme on the Efficiency of Methane Fermentation of Sewage Sludge. Energies, 18(21), 5597. https://doi.org/10.3390/en18215597






