Investigating Sodium Percarbonate for Upgrading Torrefied Spent Coffee Grounds as Alternative Solid Biofuel by Taguchi Optimization
Abstract
1. Introduction
2. Materials and Methods
2.1. TSSCG Preparation
2.2. Design of Experiment
2.3. Characterization
3. Results
3.1. Characteristics of Torrefied SCG with and Without Sodium Percarbonate Blending
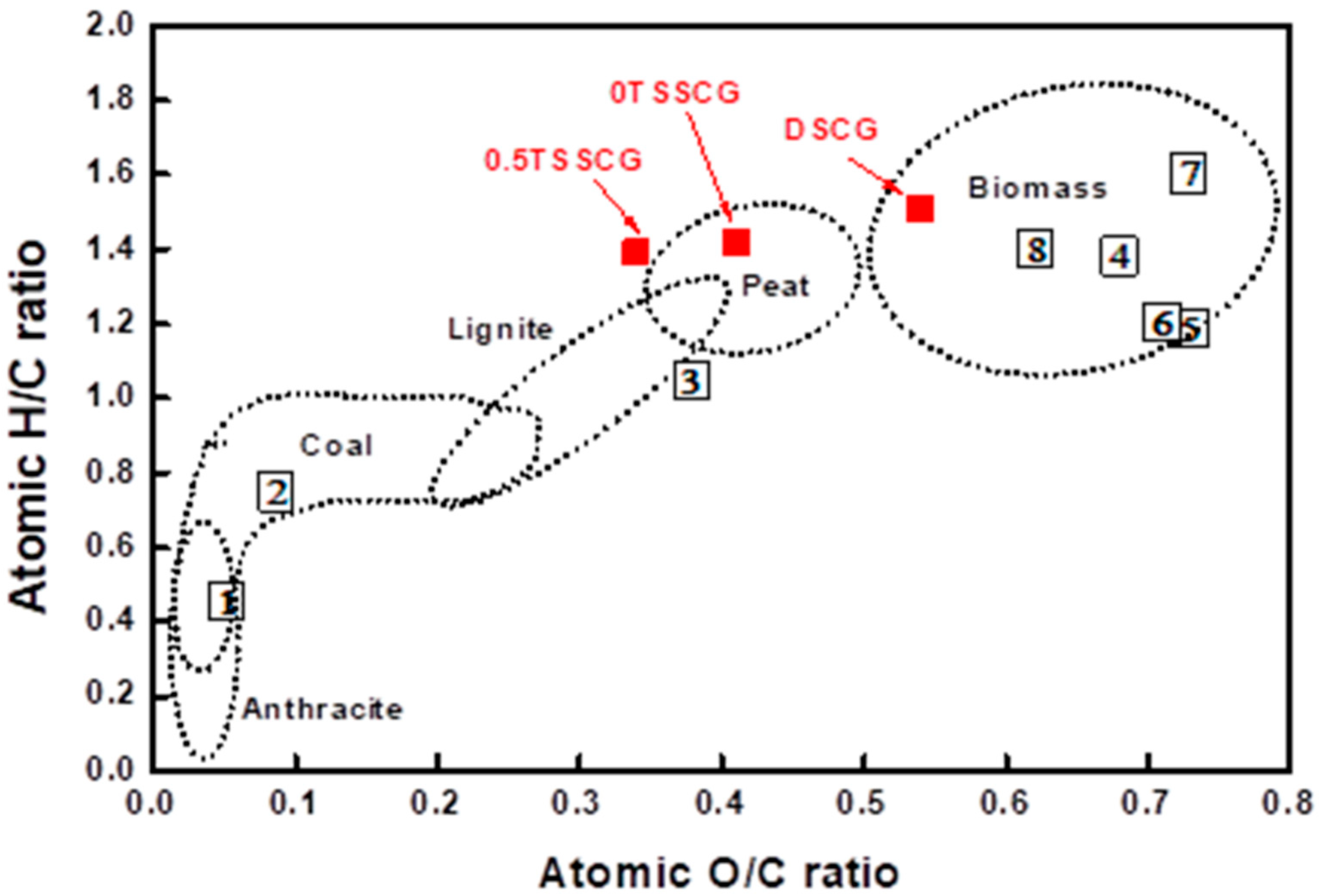

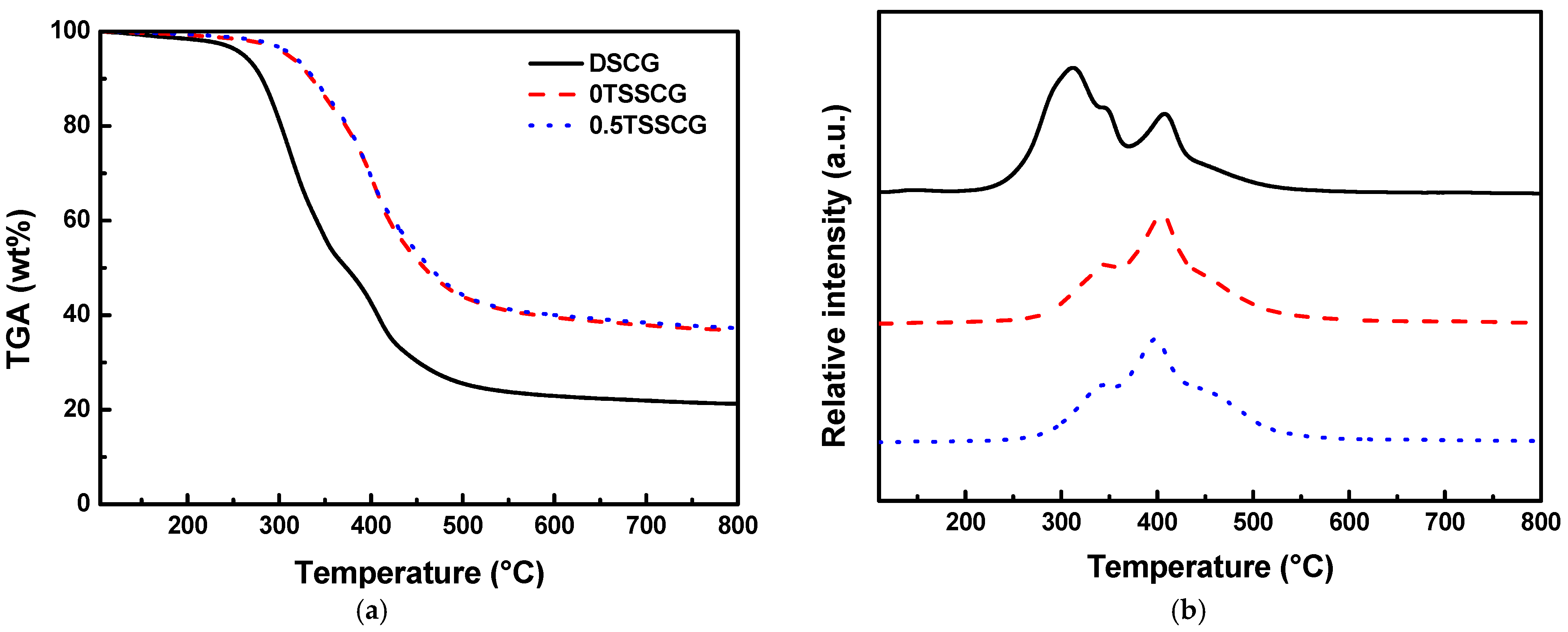
3.2. Taguchi Optimization
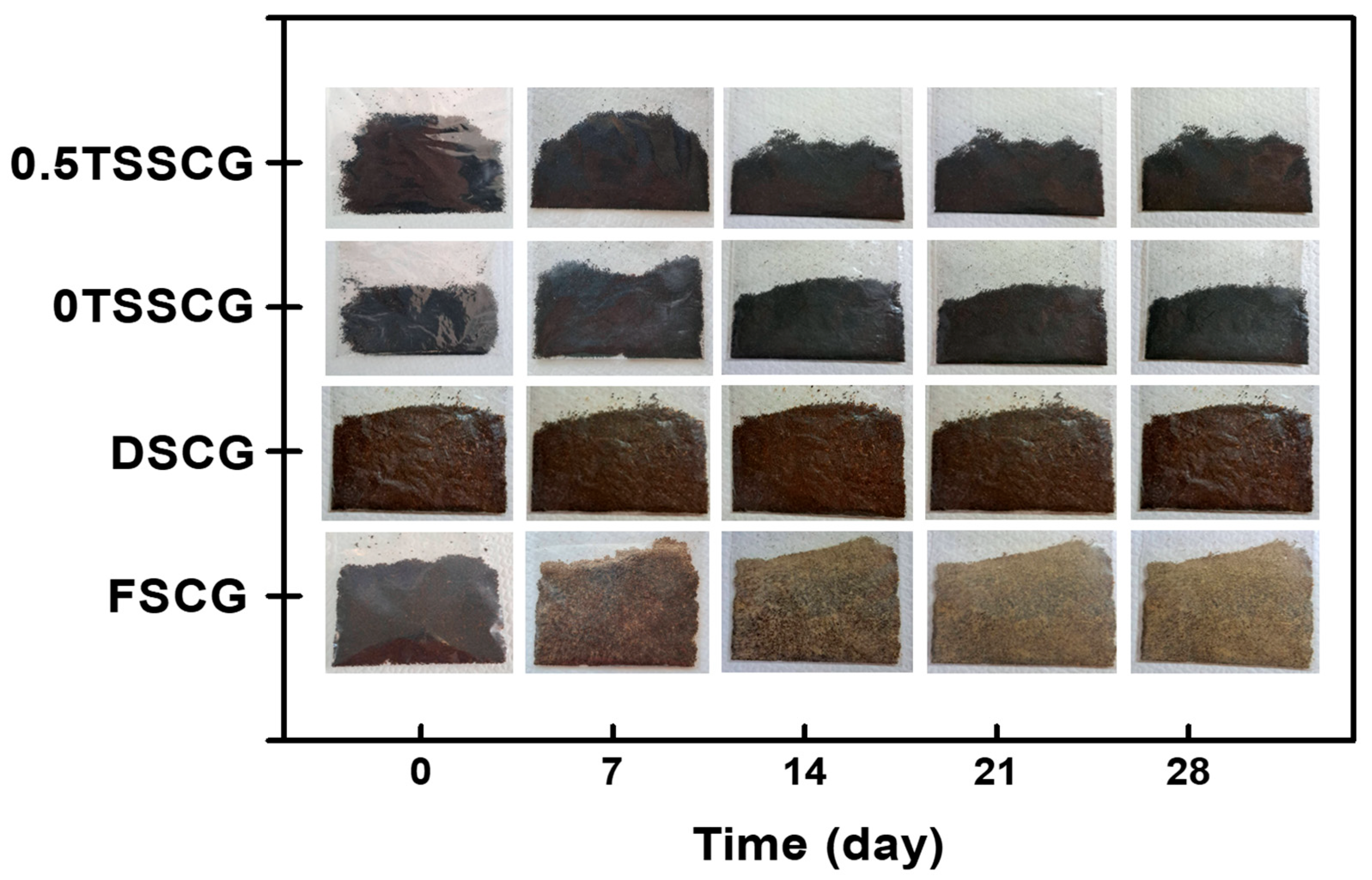
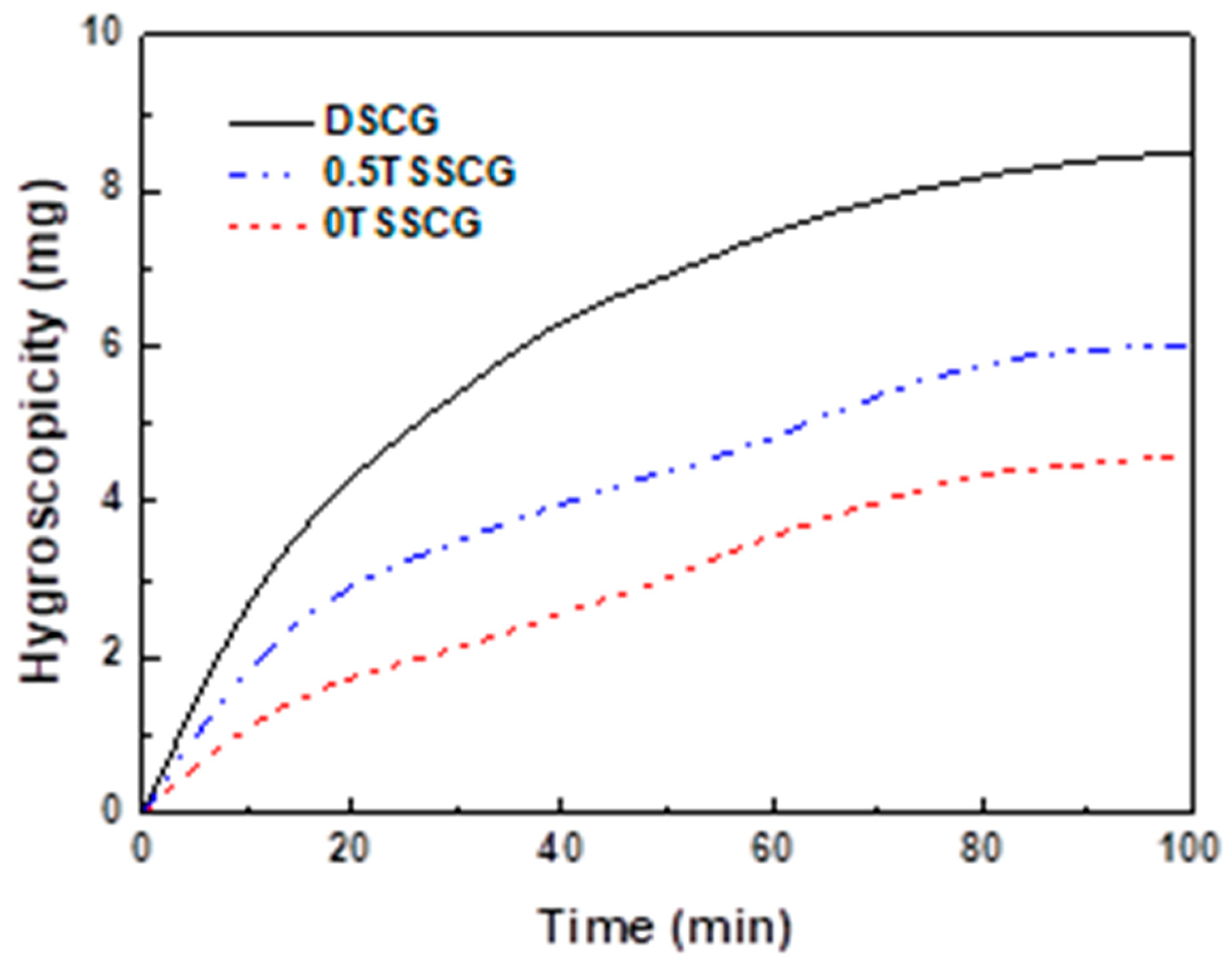
3.3. Storage Stability
3.4. Fuel Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCGs | Spent coffee grounds |
| 0TSSCG | Torrefied SCGs without sodium percarbonate |
| 0.5TSSCG | Torrefied SCGs blended with 0.5 wt% sodium percarbonate |
| HHV | Higher heating value |
| FSCG | Fresh spent coffee grounds (before drying) |
| DSCG | Dried spent coffee grounds |
| S/N | Signal-to-noise |
| VM | Volatile matter |
| FC | Fixed carbon |
References
- IEA. Global Energy Review 2025; IEA: Paris, France, 2025. [Google Scholar]
- IEA. Coal Mid-Year Update-July 2024; IEA: Paris, France, 2024. [Google Scholar]
- Budget, G.C. Fossil fuel CO2 emissions increase again in 2024. ScienceDaily, 12 November 2024. Available online: www.sciencedaily.com/releases/2024/11/241112191227.htm (accessed on 4 August 2025).
- Demirbas, A. Correlations between carbon dioxide emissions and carbon contents of fuels. Energy Sources Part B 2006, 1, 421–427. [Google Scholar] [CrossRef]
- Demirbas, A. Carbon dioxide emissions and carbonation sensors. Energy Sources Part A Recovery Util. Environ. Eff. 2007, 30, 70–78. [Google Scholar] [CrossRef]
- Santojanni, F.B.; Miner, H.; Hain, H.; Sutton, G. The Impact of Climate Change on Biodiversity in Coastal Ecosystems. J. Ilmu Pendidik. Hum. 2023, 12, 167–182. [Google Scholar] [CrossRef]
- World Health Organization. Ambient (Outdoor) Air Pollution; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Malik, K.; Capareda, S.C.; Kamboj, B.R.; Malik, S.; Singh, K.; Arya, S.; Bishnoi, D.K. Biofuels production: A review on sustainable alternatives to traditional fuels and energy sources. Fuels 2024, 5, 157–175. [Google Scholar] [CrossRef]
- El-Araby, R. Biofuel production: Exploring renewable energy solutions for a greener future. Biotechnol. Biofuels Bioprod. 2024, 17, 129. [Google Scholar] [CrossRef] [PubMed]
- Ibitoye, S.E.; Mahamood, R.M.; Jen, T.-C.; Loha, C.; Akinlabi, E.T. An overview of biomass solid fuels: Biomass sources, processing methods, and morphological and microstructural properties. J. Bioresour. Bioprod. 2023, 8, 333–360. [Google Scholar] [CrossRef]
- Natural Resources Canada. Solid Biomass Fuels (Solid Biofuels Bulletin; No. 1); Natural Resources Canada: Ottawa, ON, Canada, 2016.
- Khan, S.; Irshad, S.; Mehmood, K.; Hasnain, Z.; Nawaz, M.; Rais, A.; Gul, S.; Wahid, M.; Hashem, A.; Abd_Allah, E. Biochar production and characteristics, its impacts on soil health, crop production, and yield enhancement: A review. Plants 2024, 13, 166. [Google Scholar] [CrossRef]
- Duque-Acevedo, M.; Lancellotti, I.; Andreola, F.; Barbieri, L.; Belmonte-Ureña, L.J.; Camacho-Ferre, F. Management of agricultural waste biomass as raw material for the construction sector: An analysis of sustainable and circular alternatives. Environ. Sci. Eur. 2022, 34, 70. [Google Scholar] [CrossRef]
- Lee, K.-T.; Shih, Y.-T.; Rajendran, S.; Park, Y.-K.; Chen, W.-H. Spent coffee ground torrefaction for waste remediation and valorization. Environ. Pollut. 2023, 324, 121330. [Google Scholar] [CrossRef]
- Go, A.W.; Pham, T.Y.N.; Ju, Y.-H.; Agapay, R.C.; Angkawijaya, A.E.; Quijote, K.L. Extraction of lipids from post-hydrolysis spent coffee grounds for biodiesel production with hexane as solvent: Kinetic and equilibrium data. Biomass Bioenergy 2020, 140, 105704. [Google Scholar] [CrossRef]
- Johnson, K.; Liu, Y.; Lu, M. A review of recent advances in spent coffee grounds upcycle technologies and practices. Front. Chem. Eng. 2022, 4, 838605. [Google Scholar] [CrossRef]
- Mordor Intelligence. Coffee Market Report; Mordor Intelligence: Hyderabad, India, 2025. [Google Scholar]
- Franca, A.S.; Oliveira, L.S. Potential uses of spent coffee grounds in the food industry. Foods 2022, 11, 2064. [Google Scholar] [CrossRef]
- Chen, W.-H.; Cheng, C.-L.; Show, P.-L.; Ong, H.C. Torrefaction performance prediction approached by torrefaction severity factor. Fuel 2019, 251, 126–135. [Google Scholar] [CrossRef]
- Smith, E.W.; Aniza, R.; Petrissans, A.; Quirino, R.L.; Colin, B.; Petrissans, M.; Chen, W.-H.J. Synergistic and Antagonistic Effects of Catalytic Torrefaction-Pyrolysis of Woody Biomass in a Carbon Dioxide Atmosphere for Biofuel Production. Green Energy Fuel Res. 2025, 2, 152–173. [Google Scholar] [CrossRef]
- Ibitoye, S.E.; Loha, C.; Mahamood, R.M.; Jen, T.-C.; Alam, M.; Sarkar, I.; Das, P.; Akinlabi, E.T. An overview of biochar production techniques and application in iron and steel industries. Bioresour. Bioprocess. 2024, 11, 65. [Google Scholar] [CrossRef]
- Kabir, E.; Kim, K.-H.; Kwon, E.E. Biochar as a tool for the improvement of soil and environment. Front. Environ. Sci. 2023, 11, 1324533. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Z.; Hu, Z.; Zhang, J. Recent advances in biochar application for water and wastewater treatment: A review. PeerJ 2020, 8, e9164. [Google Scholar] [CrossRef] [PubMed]
- Gwenzi, W.; Chaukura, N.; Wenga, T.; Mtisi, M. Biochars as media for air pollution control systems: Contaminant removal, applications and future research directions. Sci. Total Environ. 2021, 753, 142249. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Remón, J.; Jiang, Z.; Ding, W. Recent advances in the preparation and application of biochar derived from lignocellulosic biomass: A mini review. Polymers 2024, 16, 851. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio/Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Azzi, E.S.; Li, H.; Cederlund, H.; Karltun, E.; Sundberg, C. Modelling biochar long-term carbon storage in soil with harmonized analysis of decomposition data. Geoderma 2024, 441, 116761. [Google Scholar]
- Al-Awadhi, Y.M.; Pradhan, S.; McKay, G.; Al-Ansari, T.; Mackey, H.R. Coffee waste biochar: A widely available and low-cost biomass for producing carbonaceous water treatment adsorbents. Chem. Eng. Trans. 2022, 92, 319–324. [Google Scholar]
- Tan, X.; Zhang, F.; Wang, H.; Ho, S.-H. The magic of algae-based biochar: Advantages, preparation, and applications. Bioengineered 2023, 14, 2252157. [Google Scholar] [CrossRef]
- Mukherjee, A.; Patra, B.R.; Podder, J.; Dalai, A.K. Synthesis of biochar from lignocellulosic biomass for diverse industrial applications and energy harvesting: Effects of pyrolysis conditions on the physicochemical properties of biochar. Front. Mater. 2022, 9, 870184. [Google Scholar] [CrossRef]
- Parikh, J.; Channiwala, S.; Ghosal, G. A correlation for calculating HHV from proximate analysis of solid fuels. Fuel 2005, 84, 487–494. [Google Scholar] [CrossRef]
- Guo, S.; Liu, L.; Zhao, D.; Zhao, C.; Li, X.; Li, G. Optimization of briquette fuels by co-torrefaction of residual biomass and plastic waste using response surface methodology. Molecules 2023, 28, 2568. [Google Scholar] [CrossRef]
- Fu, Y.; Shen, Y.; Zhang, Z.; Ge, X.; Chen, M. Activated bio-chars derived from rice husk via one-and two-step KOH-catalyzed pyrolysis for phenol adsorption. Sci. Total Environ. 2019, 646, 1567–1577. [Google Scholar]
- Chen, W.-H.; Du, J.-T.; Lee, K.-T.; Ong, H.C.; Park, Y.-K.; Huang, C.-C. Pore volume upgrade of biochar from spent coffee grounds by sodium bicarbonate during torrefaction. Chemosphere 2021, 275, 129999. [Google Scholar] [CrossRef]
- Mikutta, R.; Kleber, M.; Kaiser, K.; Jahn, R. Organic matter removal from soils using hydrogen peroxide, sodium hypochlorite, and disodium peroxodisulfate. Soil Sci. Soc. Am. J. 2005, 69, 120–135. [Google Scholar] [CrossRef]
- Ksibi, M. Chemical oxidation with hydrogen peroxide for domestic wastewater treatment. Chem. Eng. J. 2006, 119, 161–165. [Google Scholar] [CrossRef]
- Sanderson, W.R. Cleaner industrial processes using hydrogen peroxide. Pure Appl. Chem. 2000, 72, 1289–1304. [Google Scholar] [CrossRef]
- Falagas, M.; Thomaidis, P.; Kotsantis, I.; Sgouros, K.; Samonis, G.; Karageorgopoulos, D. Airborne hydrogen peroxide for disinfection of the hospital environment and infection control: A systematic review. J. Hosp. Infect. 2011, 78, 171–177. [Google Scholar] [CrossRef]
- Li, L.; Niu, X.; Zhang, D.; Ye, X.; Zhang, Z.; Liu, Q.; Ding, L.; Chen, K.; Chen, Y.; Chen, K.; et al. A systematic review on percarbonate-based advanced oxidation processes in wastewater remediation: From theoretical understandings to practical applications. Water Res. 2024, 259, 121842. [Google Scholar] [CrossRef]
- Ke, D.; Kengla, C.; Lee, S.J.; Yoo, J.J.; Zhu, X.; Murphy, S.V. Release kinetics and in vitro characterization of sodium percarbonate and calcium peroxide to oxygenate bioprinted tissue models. Int. J. Mol. Sci. 2022, 23, 6842. [Google Scholar] [CrossRef]
- Lee, K.-T.; Gabriela, S.; Chen, W.-H.; Rajendran, S.; Selvarajoo, A. Optimizing dispersion of torrefied spent coffee grounds for enhanced biochar-EVA composite properties through sodium percarbonate-assisted treatment. J. Ind. Eng. Chem. 2025, 150, 758–770. [Google Scholar] [CrossRef]
- ASTM D1102; Standard Test Method for Ash in Wood. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM E872; Standard Test Method for Volatile Matter in the Analysis of Particulate Wood Fuels. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM E1534; Standard Test Method for Determination of Ash Content of Particulate Wood Fuels. ASTM International: West Conshohocken, PA, USA, 2021.
- Adebiyi, F.M.; Ore, O.T.; Aderibigbe, O.E. Thermogravimetric characteristics and predictive modelling of oil sand bitumen pyrolysis using artificial neural network. Discov. Energy 2024, 4, 17. [Google Scholar] [CrossRef]
- ASTM E871; Standard Test Method for Moisture Analysis of Particulate Wood Fuels. ASTM International: West Conshohocken, PA, USA, 2021.
- Oboirien, B.; Thulari, V.; North, B. Enrichment of trace elements in bottom ash from coal oxy-combustion: Effect of coal types. Appl. Energy 2016, 177, 81–86. [Google Scholar] [CrossRef]
- Li, F.; Jiang, Z.; Ji, W.; Chen, Y.; Ma, J.; Gui, X.; Zhao, J.; Zhou, C. Effects of hydrothermal carbonization temperature on carbon retention, stability, and properties of animal manure-derived hydrochar. Int. J. Agric. Biol. Eng. 2022, 15, 124–131. [Google Scholar] [CrossRef]
- Seki, Y.; Kıilıinç, A.Ç.; Dalmıiş, R.; Köktaş, S.; Çelik, E. Characterization of flax, jute, and sisal fibers after sodium perborate modification. AATCC J. Res. 2019, 6, 25–31. [Google Scholar] [CrossRef]
- Fujitani, K.; Mizutani, T.; Oida, T.; Kawase, T. Oxidative cleavage with hydrogen peroxide: Preparation of polycarboxylic acids from cyclic olefins. J. Oleo Sci. 2009, 58, 37–42. [Google Scholar] [CrossRef][Green Version]
- Huang, Z.; Jiang, S.; Guo, J.; Wang, X.; Tan, M.; Xiong, R.; Wang, Z.; Wu, Z.; Li, H. Oxidative torrefaction of phragmites australis: Gas-pressurized effects and correlation analysis based on color value. Energy Fuels 2020, 34, 11073–11082. [Google Scholar] [CrossRef]
- Lozano, D.C.P.; Jones, H.E.; Reina, T.R.; Volpe, R.; Barrow, M.P. Unlocking the potential of biofuels via reaction pathways in van Krevelen diagrams. Green Chem. 2021, 23, 8949–8963. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Jayakumar, N.S.; Sahu, J.N.; Ganesan, P.; Bhutto, A.W.; Mubarak, N.M. Hydrothermal carbonization of oil palm shell. Korean J. Chem. Eng. 2015, 32, 1789–1797. [Google Scholar] [CrossRef]
- Fengel, D. Characterization of cellulose by deconvoluting the OH valency range in FTIR spectra. Holzforschung 1992, 46, 283–288. [Google Scholar] [CrossRef]
- Tahir, M.H.; Zhao, Z.; Ren, J.; Naqvi, M.; Ahmed, M.S.; Shah, T.-U.-H.; Shen, B.; Elkamel, A.; Irfan, R.M.; Rahman, A.U. Fundamental investigation of the effect of functional groups on the variations of higher heating value. Fuel 2019, 253, 881–886. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Ragadhita, R.; Fiandini, M. Interpretation of Fourier transform infrared spectra (FTIR): A practical approach in the polymer/plastic thermal decomposition. Indones. J. Sci. Technol. 2023, 8, 113–126. [Google Scholar]
- Mawhinney, D.B.; Yates, J.T., Jr. FTIR study of the oxidation of amorphous carbon by ozone at 300 K—Direct COOH formation. Carbon 2001, 39, 1167–1173. [Google Scholar] [CrossRef]
- Tavadi, A.R.; Basheer, D.; Melese, K.G.; Kumaresan, K.; Mohan, N.; Kerur, M.R.H.; Shashidhara, L. Experimental analysis of mechanical properties and FTIR analysis of areca fiber-reinforced epoxy composites incorporating Al2O3. J. Ind. Text. 2025, 55, 15280837241313217. [Google Scholar] [CrossRef]
- Chen, W.-H.; Ho, K.-Y.; Lee, K.-T.; Ding, L.; Lin, K.-Y.A.; Rajendran, S.; Singh, Y.; Chang, J.-S. Dual pretreatment of mixing H2O2 followed by torrefaction to upgrade spent coffee grounds for fuel production and upgrade level identification of H2O2 pretreatment. Environ. Res. 2022, 215, 114016. [Google Scholar] [CrossRef] [PubMed]
- Demirbaş, A. Relationships between lignin contents and heating values of biomass. Energy Convers. Manag. 2001, 42, 183–188. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, Y.; Jia, J. Analysis of gas phase products and free radical formation mechanism in anthracite spontaneous combustion process. Thermochim. Acta 2025, 748, 179981. [Google Scholar] [CrossRef]
- Okuyama, N.; Komatsu, N.; Shigehisa, T.; Kaneko, T.; Tsuruya, S. Hyper-coal process to produce the ash-free coal. Fuel Process. Technol. 2004, 85, 947–967. [Google Scholar] [CrossRef]
- Diao, W.; Zhu, L.; Yao, Q.; Li, X.; Gong, C.; Wang, H.; Huang, Z. Enhancing biomethan generation of lignite with blue-green algae as an additive. Fuel 2025, 384, 133961. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, W.; Jiang, Z.; Mi, B.; Fei, B. Investigating combustion behaviors of bamboo, torrefied bamboo, coal and their respective blends by thermogravimetric analysis. Renew. Energy 2016, 87, 346–352. [Google Scholar] [CrossRef]
- Chen, W.-H.; Du, S.-W.; Tsai, C.-H.; Wang, Z.-Y. Torrefied biomasses in a drop tube furnace to evaluate their utility in blast furnaces. Bioresour. Technol. 2012, 111, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, D.T.; Machin, E.B.; Cabrera-Barjas, G.; Flores, M.; Urra, H.G.; De Carvalho, F.S.; Santos, M.I.S.d.; Machin, A.B.; Canettieri, E.V.; Perez, N.P.; et al. Sugarcane bagasse torrefaction for fluidized bed gasification. Appl. Sci. 2021, 11, 6105. [Google Scholar] [CrossRef]
- Fadhil, A.B.; Alhayali, M.A.; Saeed, L.I. Date (Phoenix dactylifera L.) palm stones as a potential new feedstock for liquid bio-fuels production. Fuel 2017, 210, 165–176. [Google Scholar] [CrossRef]
- Alauddin, Z.A.B.Z.; Lahijani, P.; Mohammadi, M.; Mohamed, A.R. Gasification of lignocellulosic biomass in fluidized beds for renewable energy development: A review. Renew. Sustain. Energy Rev. 2010, 14, 2852–2862. [Google Scholar] [CrossRef]
- Rashid, K.M.J. Optimize the Taguchi method, the signal-to-noise ratio, and the sensitivity. Int. J. Stat. Appl. Math. 2023, 8, 64–70. [Google Scholar] [CrossRef]
- Wzorek, M.; Król, A.; Junga, R.; Małecka, J.; Yilmaz, E.; Kolasa-Więcek, A. Effect of storage conditions on lignocellulose biofuels properties. Sci. Rep. 2024, 14, 15192. [Google Scholar] [CrossRef]
- Franca, A.S.; Oliveira, L.S. Coffee processing solid wastes: Current uses and future perspectives. Agric. Wastes 2009, 9, 155–189. [Google Scholar]
- Tapia, M.S.; Alzamora, S.M.; Chirife, J. Effects of Water Activity (aw) on Microbial Stability as a Hurdle in Food Preservation; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; pp. 323–355. [Google Scholar]
- Glass, S.; Zelinka, S.L. Moisture Relations and Physical Properties of Wood; U.S. Department of Agriculture Forest Service: Washington, DC, USA, 2021; pp. 4-1–4-22.
- Berthomieu, C.; Hienerwadel, R. Fourier transform infrared (FTIR) spectroscopy. Agric. Biol. Sci. 2009, 101, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Obernberger, I.; Brunner, T.; Bärnthaler, G. Chemical properties of solid biofuels—Significance and impact. Biomass Bioenergy 2006, 30, 973–982. [Google Scholar] [CrossRef]
- Bhaskar, T.; Bhavya, B.; Singh, R.; Naik, D.V.; Kumar, A.; Goyal, H.B. Thermochemical conversion of biomass to biofuels. In Biofuels; Elsevier: Amsterdam, The Netherlands, 2011; pp. 51–77. [Google Scholar]
- Wang, Y.; Qiu, L.; Zhu, M.; Sun, G.; Zhang, T.; Kang, K. Comparative evaluation of hydrothermal carbonization and low temperature pyrolysis of eucommia ulmoides oliver for the production of solid biofuel. Sci. Rep. 2019, 9, 5535. [Google Scholar] [CrossRef] [PubMed]
- Channiwala, S.; Parikh, P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Chen, W.-H.; Cheng, W.-Y.; Lu, K.-M.; Huang, Y.-P. An evaluation on improvement of pulverized biomass property for solid fuel through torrefaction. Appl. Energy 2011, 88, 3636–3644. [Google Scholar] [CrossRef]
- Putra, H.E.; Djaenudin, D.; Damanhuri, E.; Dewi, K.; Pasek, A.D. Hydrothermal carbonization kinetics of lignocellulosic municipal solid waste. J. Ecol. Eng. 2021, 22, 188–198. [Google Scholar] [CrossRef]
- Lee, K.-T.; Gabriela, S.; Chen, W.-H.; Ong, H.C.; Rajendran, S.; Tran, K.-Q. Co-torrefaction and synergistic effect of spent coffee grounds and tea waste for sustainable waste remediation and renewable energy. Renew. Energy 2024, 233, 121181. [Google Scholar] [CrossRef]



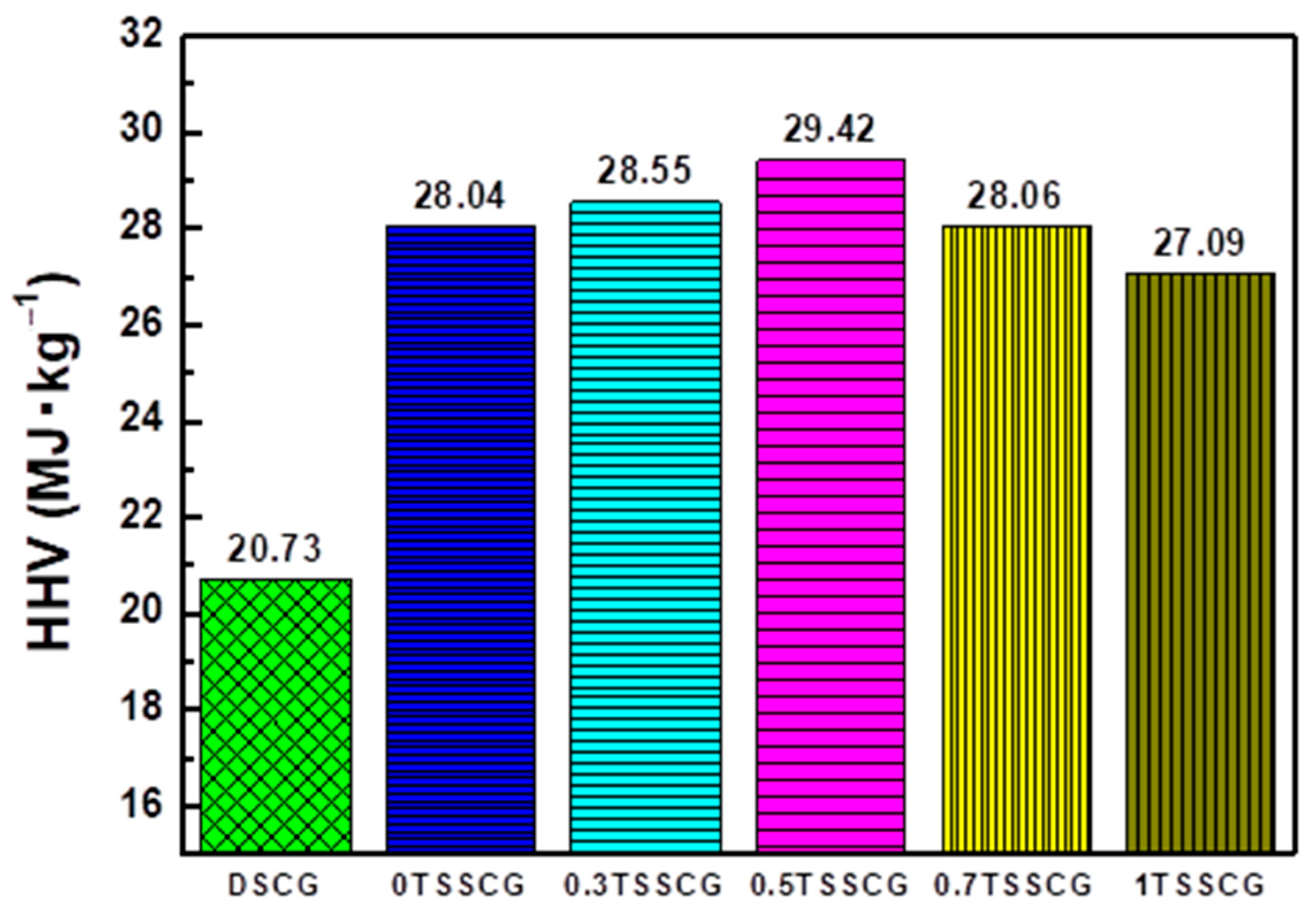
| Analyses | DSCG | 0TSSCG | 0.5TSSCG |
|---|---|---|---|
| Proximate Analysis (wt%), dry basis | |||
| Volatile matter (VM) | 84.55 | 71.34 | 71.13 |
| Fixed carbon (FC) | 13.48 | 25.54 | 24.85 |
| Ash | 1.97 | 3.13 | 4.02 |
| Elemental analysis (wt%, dry basis) | |||
| C | 52.84 | 58.38 | 61.88 |
| H | 6.67 | 6.89 | 7.18 |
| N | 2.56 | 2.83 | 3.17 |
| O (by difference) | 37.93 | 31.90 | 27.77 |
| Atomic H/C ratio | 1.51 | 1.42 | 1.39 |
| Atomic O/C ratio | 0.54 | 0.41 | 0.34 |
| (a) | |||||||
| Factors | Parameters | Levels | |||||
| 1 | 2 | 3 | |||||
| A | Torrefaction temperature (°C) | 200 | 250 | 300 | |||
| B | Torrefaction time (min) | 30 | 60 | 90 | |||
| C | Blending ratio (wt%) | 0 | 0.5 | 1 | |||
| (b) | |||||||
| Run | Factors | HHV (MJ∙kg−1) | |||||
| A | B | C | |||||
| 1 | 1 | 1 | 1 | 22.61 | |||
| 2 | 1 | 2 | 2 | 23.68 | |||
| 3 | 1 | 3 | 3 | 22.85 | |||
| 4 | 2 | 1 | 2 | 25.05 | |||
| 5 | 2 | 2 | 3 | 25.06 | |||
| 6 | 2 | 3 | 1 | 25.07 | |||
| 7 | 3 | 1 | 3 | 27.72 | |||
| 8 | 3 | 2 | 1 | 26.06 | |||
| 9 | 3 | 3 | 2 | 29.42 | |||
| Formulas of HHV (MJ∙kg−1) | DSCG | 0TSSCG | 0.5TSSCG |
|---|---|---|---|
| HHV a | 22.30 | 25.09 | 27.06 |
| HHV b | 17.93 | 20.13 | 19.84 |
| HHV c | 20.73 | 28.04 | 29.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.-H.; Lee, K.-T.; Sung, J.-N.; Hu, N.-Y.; Xu, Y.-S. Investigating Sodium Percarbonate for Upgrading Torrefied Spent Coffee Grounds as Alternative Solid Biofuel by Taguchi Optimization. Energies 2025, 18, 5384. https://doi.org/10.3390/en18205384
Chen W-H, Lee K-T, Sung J-N, Hu N-Y, Xu Y-S. Investigating Sodium Percarbonate for Upgrading Torrefied Spent Coffee Grounds as Alternative Solid Biofuel by Taguchi Optimization. Energies. 2025; 18(20):5384. https://doi.org/10.3390/en18205384
Chicago/Turabian StyleChen, Wei-Hsin, Kuan-Ting Lee, Ji-Nien Sung, Nai-Yun Hu, and Yun-Sen Xu. 2025. "Investigating Sodium Percarbonate for Upgrading Torrefied Spent Coffee Grounds as Alternative Solid Biofuel by Taguchi Optimization" Energies 18, no. 20: 5384. https://doi.org/10.3390/en18205384
APA StyleChen, W.-H., Lee, K.-T., Sung, J.-N., Hu, N.-Y., & Xu, Y.-S. (2025). Investigating Sodium Percarbonate for Upgrading Torrefied Spent Coffee Grounds as Alternative Solid Biofuel by Taguchi Optimization. Energies, 18(20), 5384. https://doi.org/10.3390/en18205384






