Enhancing the Adsorption Performance of HKUST-1 by Adding NH4F During Room-Temperature Synthesis for Desulfurization of Fuel Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solvothermal Synthesis of HKUST-1-S
2.3. Room-Temperature Synthesis of HKUST-1-x with the Addition of NH4F
2.4. Batch Adsorption Test
2.5. Cycle Test
2.6. Characterization
3. Results and Discussion
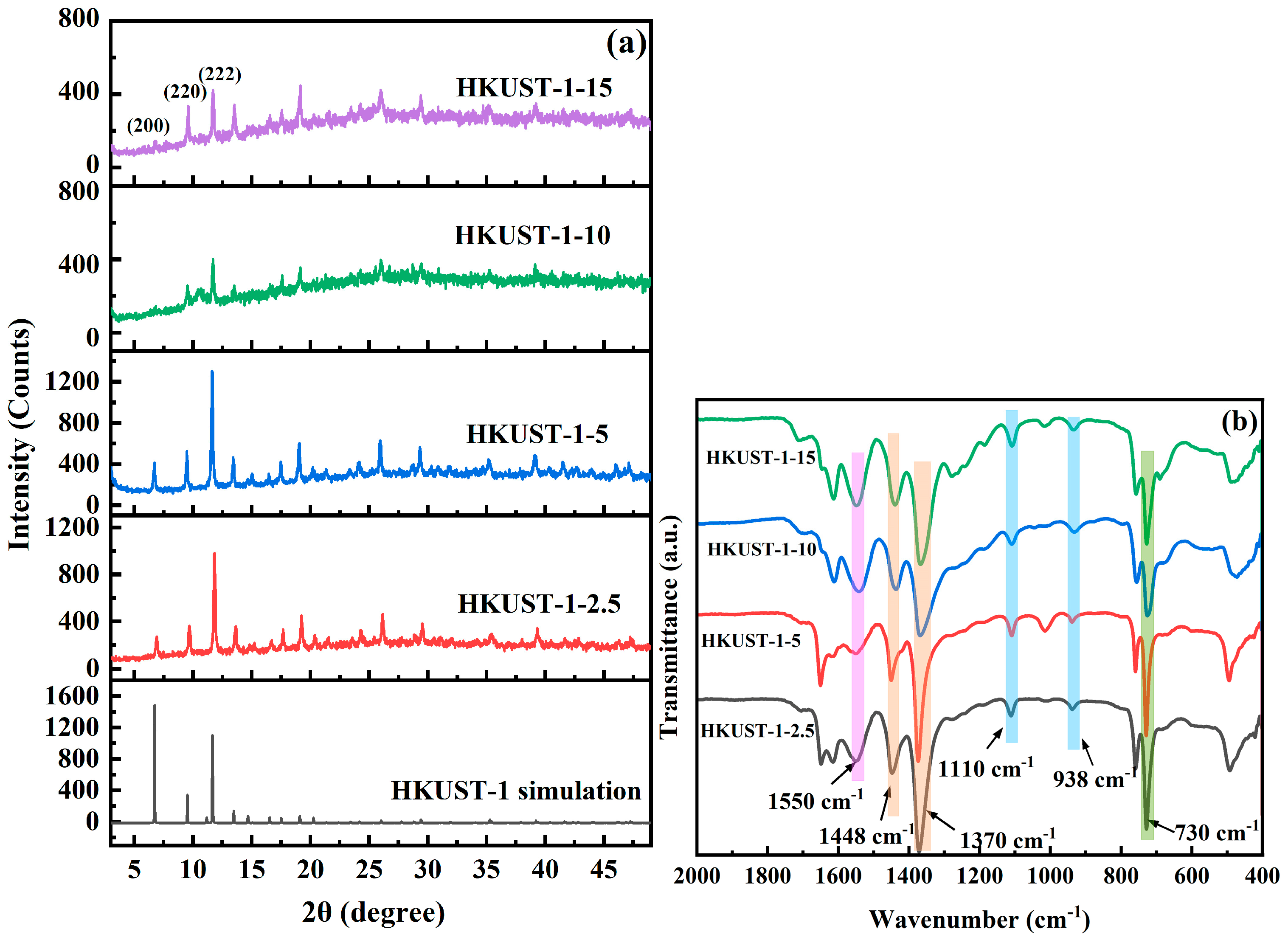
3.1. Structural Characterization
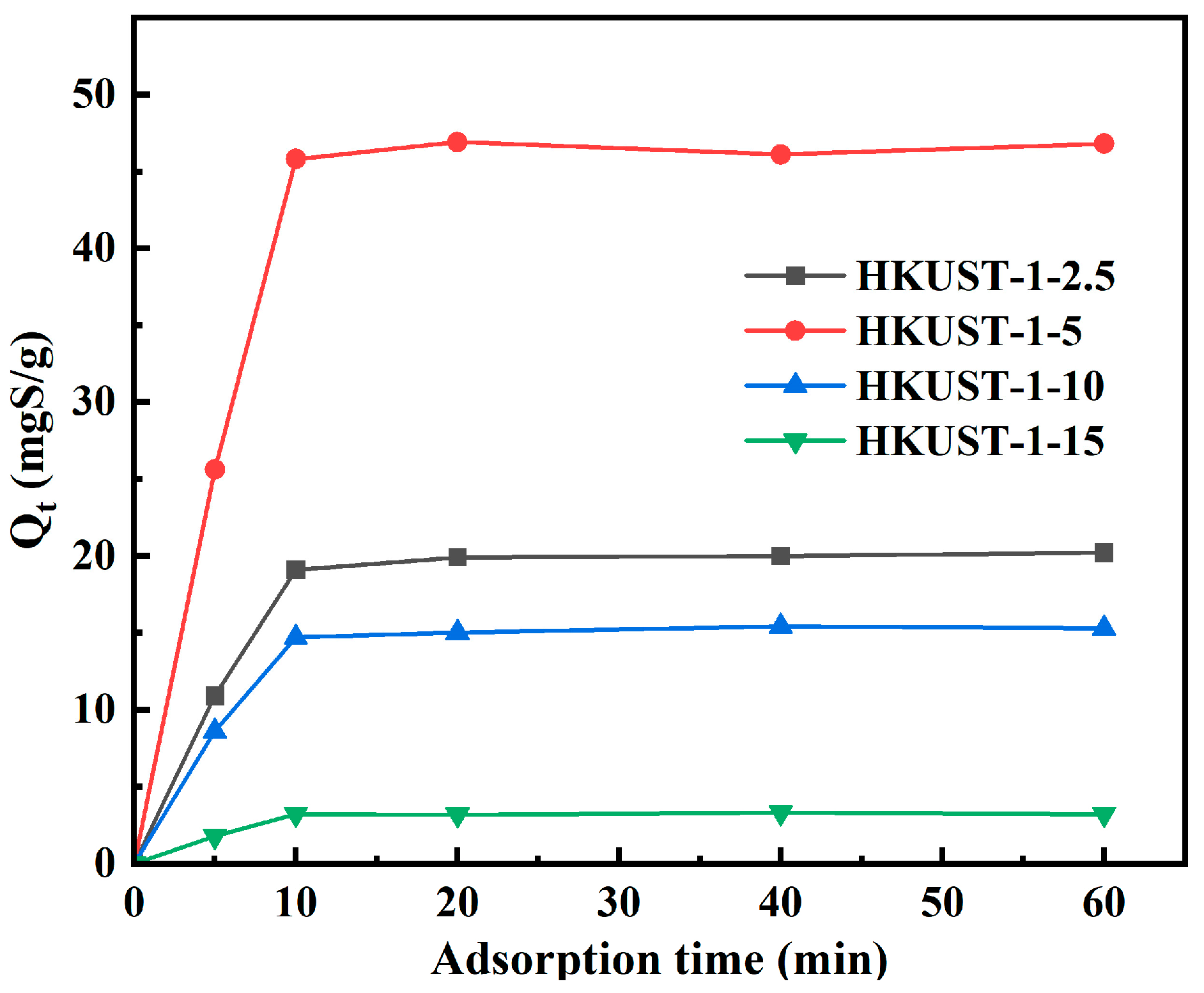
3.2. Evaluation of Adsorption Desulfurization Performance
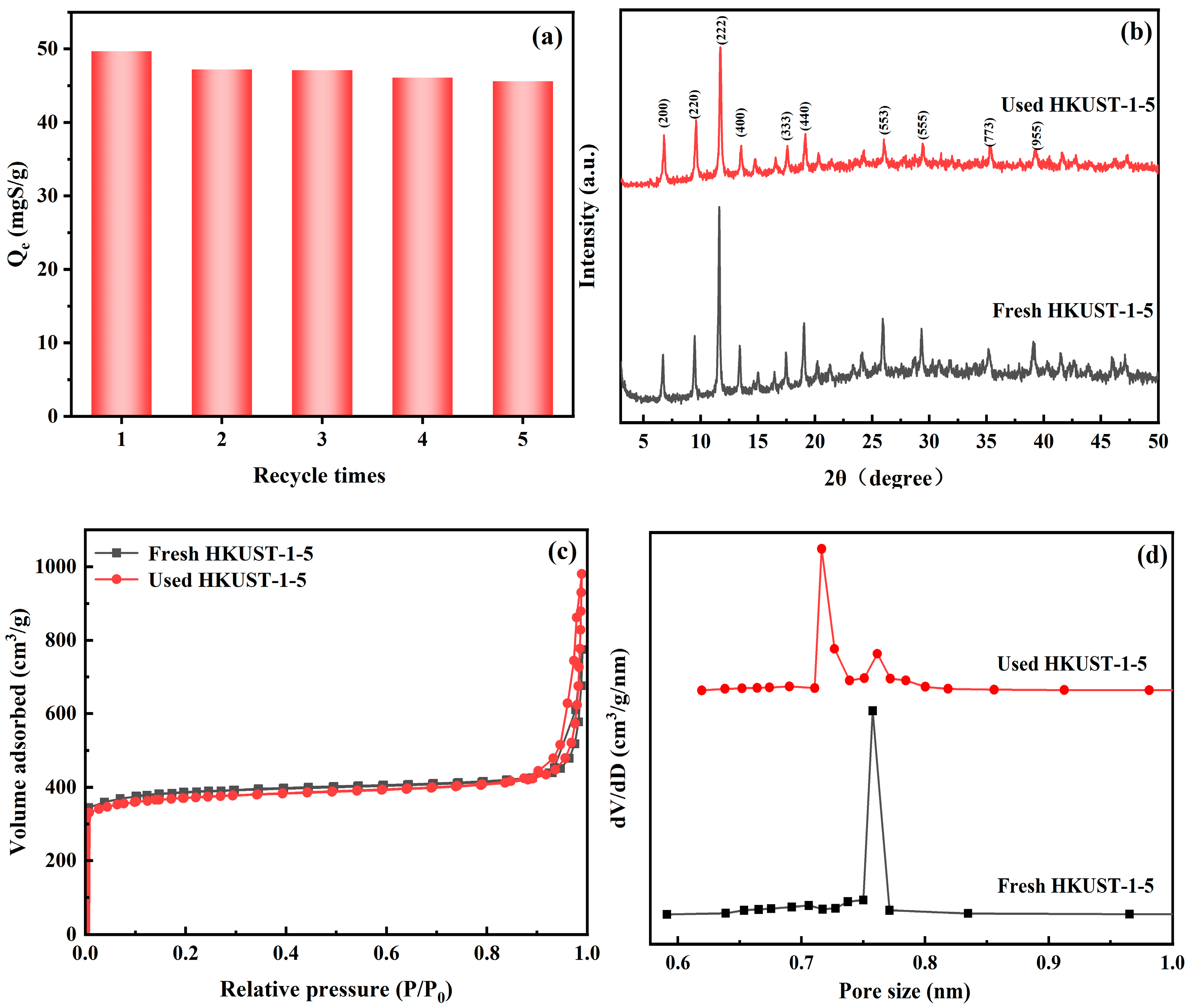
3.3. Relationship Between Adsorption Desulfurization Performance and Structure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niquille-Röthlisberger, A.; Prins, R. Hydrodesulfurization of 4,6-Dimethyldibenzothiophene and Dibenzothiophene over Alumina-Supported Pt, Pd, and Pt-Pd Catalysts. J. Catal. 2006, 242, 207–216. [Google Scholar] [CrossRef]
- Moustafa, T.M.; Froment, G.F. Kinetic Modeling of Coke Formation and Deactivation in the Catalytic Cracking of Vacuum Gas Oil. Ind. Eng. Chem. Res. 2003, 42, 14–25. [Google Scholar] [CrossRef]
- Rajendran, A.; Cui, T.Y.; Fan, H.X.; Yang, Z.F.; Feng, J.; Li, W.Y. A Comprehensive Review on Oxidative Desulfurization Catalysts Targeting Clean Energy and Environment. J. Mater. Chem. A 2020, 8, 2246–2285. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, Y.; Zhu, J.; Liu, F.; Cheng, K.; Cheng, H.; Zhu, W. Modulating Hydrogen Spillover Effect to Boost Ultradeep Hydrodesulfurization of 4,6-Dimethyldibenzothiophene over Pt-Ni2P/Al2O3. Sep. Purif. Technol. 2024, 351, 128095. [Google Scholar] [CrossRef]
- Ye, G.; Wang, H.; Zeng, X.; Wang, L.; Wang, J. Defect-Rich Bimetallic UiO-66(Hf-Zr): Solvent-free Rapid Synthesis and Robust Ambient-temperature Oxidative Desulfurization Performance. Appl. Catal. B—Environ. Energy 2021, 299, 120659. [Google Scholar] [CrossRef]
- Nilavu, M.C.; Leelasree, T.; Aggarwal, H.; Rajesh, N. Cobalt-Based Metal–Organic Framework for Desulfurization of Thiophene as a Model Fuel. Sustain. Energy Fuels 2024, 8, 1679–1690. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Lin, Q.J.; Gao, D.L.; Liu, Y.X.; Qin, N.; Xue, Y.X.; Yang, Q. The Introduction of Nitrogen-Containing Heterocyclic Compounds (NHC) and Cu2+ in Co-MOF Further Improves the Removal of Dibenzothiophene from Model Fuels. Sep. Purif. Technol. 2024, 335, 126096. [Google Scholar] [CrossRef]
- Liu, J.; Fu, Z.; Tan, P.; Li, M.; Liu, X.Q.; Sun, L.B. Constructing Light-Insensitive Ag (I) Sites by in situ Integration of Nanoheaters for Efficient Adsorptive Desulfurization. Chem. Eng. J. 2024, 484, 149717. [Google Scholar] [CrossRef]
- Mansour, E.A.; Taha, M.; Mahmoud, R.K.; Shehata, N.; Abdelhameed, R.M. A Combined Experimental and Computational Studies on Thiophene Adsorption from Liquid Fuels over ZIF-67@ZnFe LDH Composites. Mater. Chem. Phys. 2025, 334, 130499. [Google Scholar] [CrossRef]
- Yu, Q.; Zhan, T.; Xia, Z.; Lu, G.; Ma, N.; Dai, W. Well-Construction of Hollow-Pocket Shaped Zn-based Metal-Organic Framework for Boosting the Capture Ability towards Thiophene Sulfur. Particuology 2025, 99, 116–127. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Wong-Foy, A.G.; Matzger, A.J. Liquid Phase Adsorption by Microporous Coordination Polymers: Removal of Organosulfur Compounds. J. Am. Chem. Soc. 2008, 130, 6938–6939. [Google Scholar] [CrossRef]
- Achmann, S.; Hagen, G.; Hämmerle, M.; Malkowsky, I.M.; Kiener, C.; Moos, R. Sulfur Removal from Low-sulfur Gasoline and Diesel Fuel by Metal-Organic Frameworks. Chem. Eng. Technol. 2010, 33, 275–280. [Google Scholar] [CrossRef]
- Peralta, D.; Chaplais, G.; Simon-Masseron, A.; Barthelet, K.; Pirngruber, G.D. Metal-Organic Framework Materials for Desulfurization by Adsorption. Energy Fuels 2012, 26, 4953–4960. [Google Scholar] [CrossRef]
- Duan, M.; Wang, Y.; Fan, H.; Su, Z.; Yang, C.; Kou, J.; Shangguan, J. Revealing the Contribution of Cu(II) and Cu(I) Inherent in MOF-199 for Efficient Thiophene Removal at Room Temperature. Sep. Purif. Technol. 2024, 349, 127922. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.J.; Fan, H.L.; Shangguan, J.; Wang, H.; Mi, J. Removal of Sulfur Compounds by a Copper-based Metal Organic Framework under Ambient Conditions. Energy Fuels 2015, 29, 298–304. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, T.; Cai, K.; Chen, P.; Liu, F.; Tao, D.J. Rapid Mechanochemical Construction of HKUST-1 with Enhancing Water Stability by Hybrid Ligands Assembly Strategy for Efficient Adsorption of SF6. Chem. Eng. J 2022, 437, 135364. [Google Scholar] [CrossRef]
- Duan, C.; Li, F.; Luo, S.; Xiao, J.; Li, L.; Xi, H. Facile Synthesis of Hierarchical Porous Metal-Organic Frameworks with Enhanced Catalytic Activity. Chem. Eng. J. 2018, 334, 1477–1483. [Google Scholar] [CrossRef]
- Duan, Y.; Li, H.; Shi, X.; Ji, C.; Imbrogno, J.; Zhao, D. Stability of Metal–Organic Frameworks in Organic Media with Acids and Bases. Ind. Eng. Chem. Res. 2025, 64, 5372–5382. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Huang, E.; Kong, L.; Zeng, Y. Efficient Adsorption Desulfurization via Encapsulation of CeO2 Nanoparticles in Hierarchical Porous HKUST-1. J. Colloid. Interface Sci. 2025, 689, 137237. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.C.; Qian, X.Y.; He, Q.X.; Miao, K.J.; Jiang, Y.; Tan, P.; Liu, X.Q.; Sun, L.B. Generation of Hierarchical Porosity in Metal–Organic Frameworks by the Modulation of Cation Valence. Angew. Chem. Int. Ed. 2019, 58, 10104–10109. [Google Scholar] [CrossRef]
- Chen, M.; Chen, J.; Liu, Y.; Liu, J.; Li, L.; Yang, B.; Ma, L. Enhanced Adsorption of Thiophene with the GO-modified Bimetallic Organic Framework Ni-MOF-199. Colloid. Surf. A—Physicochem. Eng. Asp. 2019, 578, 123553. [Google Scholar] [CrossRef]
- Petit, C.; Burress, J.; Bandosz, T.J. The Synthesis and Characterization of Copper-based Metal–Organic Framework/Graphite Oxide Composites. Carbon 2011, 49, 563–572. [Google Scholar] [CrossRef]
- Bazzi, L.; Boukayouht, K.; Mansouri, S.; El Hankari, S. Eco-friendly and Cost-effective Synthesis of Hierarchical Porous HKUST-1 from Thin Waste Electric Cables for Enhanced Cationic Dye Removal. Process Saf. Environ. Prot. 2024, 191, 750–759. [Google Scholar] [CrossRef]
- Yañez-Aulestia, A.; Trejos, V.M.; Esparza-Schulz, J.M.; Ibarra, I.A.; Sánchez-González, E. Chemically Modified HKUST-1(Cu) for Gas Adsorption and Separation: Mixed-metal and Hierarchical Porosity. ACS Appl. Mater. Interfaces 2024, 16, 65581–65591. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, J.M.; Jin, L.N.; Sun, W.Y. Metal Ion Induced Porous HKUST-1 Nano/Microcrystals with Controllable Morphology and Size. Crystengcomm 2016, 18, 4127–4132. [Google Scholar] [CrossRef]
- Khan, N.A.; Jun, J.W.; Jeong, J.H.; Jhung, S.H. Remarkable Adsorptive Performance of a Metal–Organic Framework, Vanadium-Benzenedicarboxylate (MIL-47), for Benzothiophene. Chem. Commun. 2011, 47, 1306–1308. [Google Scholar] [CrossRef]
- Han, L.; Zhang, J.; Mao, Y.; Zhou, W.; Xu, W.; Sun, Y. Facile and Green Synthesis of MIL-53 (Cr) and its Excellent Adsorptive Desulfurization Performance. Ind. Eng. Chem. Res. 2019, 58, 15489–15496. [Google Scholar] [CrossRef]
- Khan, N.A.; Jhung, S.H. Low-temperature Loading of Cu+ Species over Porous Metal-Organic Frameworks (MOFs) and Adsorptive Desulfurization with Cu+-Loaded MOFs. J. Hazard. Mater. 2012, 237, 180–185. [Google Scholar] [CrossRef]
- Khan, N.A.; Hasan, Z.; Jhung, S.H. Ionic Liquids Supported on Metal-Organic Frameworks: Remarkable Adsorbents for Adsorptive Desulfurization. Chem. Eur. J. 2014, 20, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Jafarinasab, M.; Akbari, A.; Omidkhah, M.; Shakeri, M. An efficient Co-based Metal–Organic Framework Nanocrystal (Co-ZIF-67) for Adsorptive Desulfurization of Dibenzothiophene: Impact of the Preparation Approach on Structure Tuning. Energy Fuels 2020, 34, 12779–12791. [Google Scholar] [CrossRef]
- Zhang, X.F.; Wang, Z.; Feng, Y.; Zhong, Y.; Liao, J.; Wang, Y.; Yao, J. Adsorptive Desulfurization from the Model Fuels by Functionalized UiO-66(Zr). Fuel 2018, 234, 256–262. [Google Scholar] [CrossRef]
- Khan, N.A.; Bhadra, B.N.; Jhung, S.H. Heteropoly Acid-loaded Ionic Liquid@Metal–Organic Frameworks: Effective and Reusable Adsorbents for the Desulfurization of a Liquid Model Fuel. Chem. Eng. J. 2018, 334, 2215–2221. [Google Scholar] [CrossRef]
- Wei, S.; He, H.; Cheng, Y.; Yang, C.; Zeng, G.; Qiu, L. Performances, Kinetics and Mechanisms of Catalytic Oxidative Desulfurization from Oils. RSC Adv. 2016, 6, 103253–103269. [Google Scholar] [CrossRef]
- Huang, G.; Sun, Z.; Yu, Z.; Liu, Y.Y.; Wang, Y.; Wang, W.; Wang, A.; Hu, Y. Supported Ni2P Catalysts Derived from Nickel Phyllosilicate with Enhanced Hydrodesulfurization Performance. J. Catal. 2023, 419, 37–48. [Google Scholar] [CrossRef]
- Vigdorowitsch, M.; Pchelintsev, A.; Tsygankova, L.; Tanygina, E. Freundlich Isotherm: An Adsorption Model Complete Framework. Appl. Sci. 2021, 11, 8078. [Google Scholar] [CrossRef]
- Guo, Y.; Feng, C.; Wang, S.; Xie, Y.; Guo, C.; Liu, Z.; Wang, J. Construction of Planar-type Defect-engineered Metal–Organic Frameworks with both Mixed-valence Sites and Copper-ion Vacancies for Photocatalysis. J. Mater. Chem. A 2020, 8, 24477–24485. [Google Scholar] [CrossRef]
- Xie, H.; Yi, D.; Shi, L.; Meng, X. High Performance of CuY Zeolite for Catalyzing Acetylene Carbonylation and the Effect of Copper Valence States on Catalyst. Chem. Eng. J. 2017, 313, 663–670. [Google Scholar] [CrossRef]
- Lu, P.; Sun, Z.; Qi, Z.; Chen, J.; Ye, C.; Qiu, T. Molecular Engineering to Fabricating Dinuclear-crossed Secondary Building Units in Metal-Organic Frameworks for Aromatic Sulfur-containing Compounds Adsorption. Sep. Purif. Technol. 2023, 327, 124879. [Google Scholar] [CrossRef]
- Kar, A.K.; Sarkar, R.; Manal, A.K.; Kumar, R.; Chakraborty, S.; Ahuja, R.; Srivastava, R. Unveiling and Understanding the Remarkable Enhancement in the Catalytic Activity by the Defect Creation in UiO-66 during the Catalytic Transfer Hydrodeoxygenation of Vanillin with Isopropanol. Appl. Catal. B—Environ. Energy 2023, 325, 122385. [Google Scholar] [CrossRef]
- Abou-Elyazed, A.S.; Ftooh, A.I.; Sun, Y.; Ashry, A.G.; Shaban, A.K.; El-Nahas, A.M.; Yousif, A.M. Solvent-free Synthesis of HKUST-1 with Abundant Defect Sites and its Catalytic Performance in the Esterification Reaction of Oleic Acid. ACS Omega 2024, 9, 37662–37671. [Google Scholar] [CrossRef]
- Tian, L.; Song, X.; Liu, Y.; Zhang, C.; Shi, L.; Chen, Q.; Hu, T. Defect-Engineering Improves the Activity of Metal-Organic Frameworks for Catalyzing Hydroboration of Alkynes: A Combination of Experimental Investigation and Density Functional Theory Calculations. J. Colloid Interface Sci. 2024, 662, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Chui, S.S.Y.; Lo, S.M.F.; Charmant, J.P.; Orpen, A.G.; Williams, I.D. A Chemically Functionalizable Nanoporous Material [Cu3(TMA)2(H2O)3] n. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.P.; Wang, Z.Q.; Tan, H.Z.; Jing, K.Q.; Xu, Z.N.; Guo, G.C. Lewis Acid Sites in MOFs Supports Promoting the Catalytic Activity and Selectivity for CO Esterification to Dimethyl Carbonate. Catal. Sci. Technol. 2020, 10, 1699–1707. [Google Scholar] [CrossRef]
- Castillo-Villalón, P.; Ramirez, J.; Castañeda, R. Relationship Between the Hydrodesulfurization of Thiophene, Dibenzothiophene, and 4,6-Dimethyl Dibenzothiophene and the Local Structure of Co in Co–Mo–S sites: Infrared Study of Adsorbed CO. J. Catal. 2012, 294, 54–62. [Google Scholar] [CrossRef]
- Saha, B.; Vedachalam, S.; Dalai, A.K. Review on Recent Advances in Adsorptive Desulfurization. Fuel Process. Technol. 2021, 214, 106685. [Google Scholar] [CrossRef]
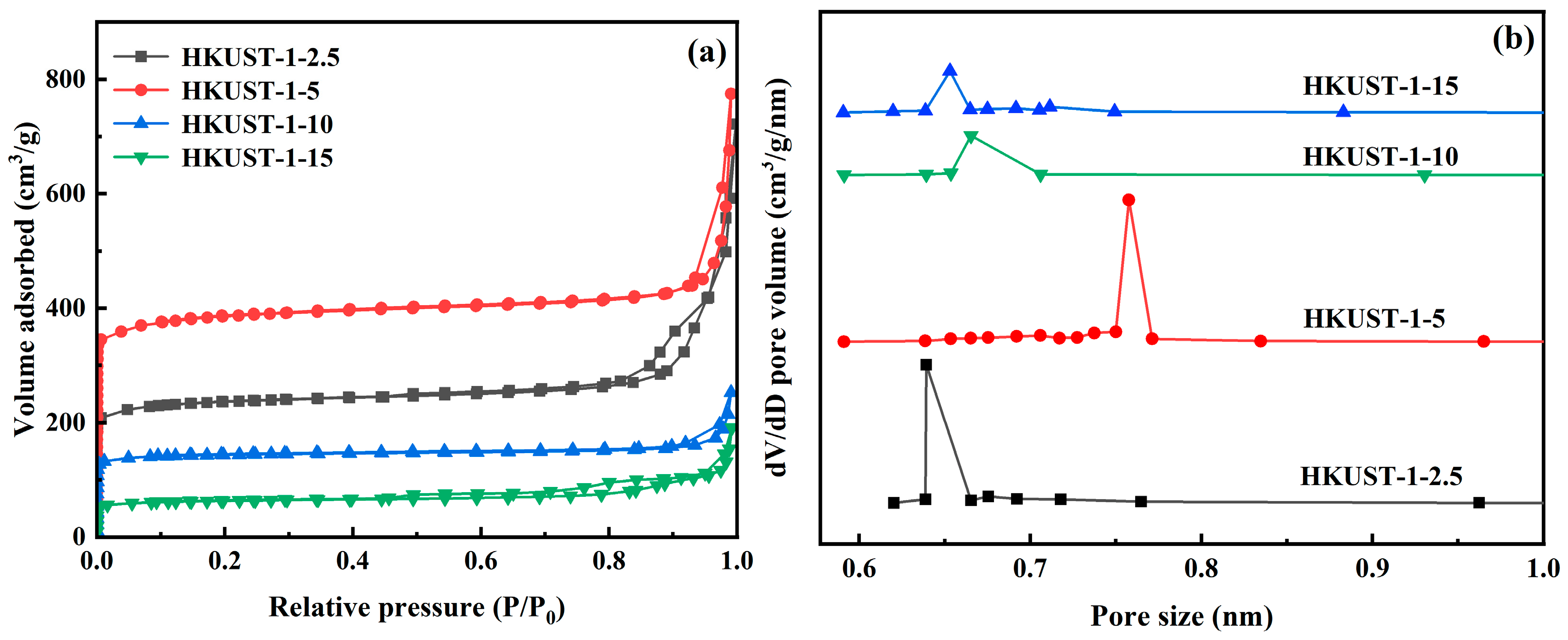









| Samples | BET Surface Area (m2/g) | Micropore Volume (mL/g) | Average Micropore Size (nm) |
|---|---|---|---|
| HKUST-1-2.5 | 927 | 0.36 | 0.68 |
| HKUST-1-5 | 1508 | 0.58 | 0.73 |
| HKUST-1-10 | 574 | 0.22 | 0.68 |
| HKUST-1-15 | 245 | 0.09 | 0.66 |
| Adsorbents | Sulfur Compounds | Sulfur Concentration (ppm) | Qe (mgS/g) | References |
|---|---|---|---|---|
| MIL-53(Al) | BT | 1000 | 4.5 | [26] |
| MIL-53(Cr) | BT | 1000 | 9.5 | |
| MIL-47 | BT | 1000 | 21.5 | |
| MIL-53(Cr)-G | DBT | 1000 | 86.4 | [27] |
| MIL-100(Fe) | BT | 1000 | 14.3 | [28] |
| MIL-101(Cr) | DBT | 1000 | 6.5 | [29] |
| Co-ZIF-67-M | DBT | 500 | 2.2 | [30] |
| UiO-66 | BT | 100 | 19.8 | [31] |
| ZIF-8 UMCM-150 | BT | 1000 | 6.6 | [32] |
| BT | 1500 | 40 | [7] | |
| DBT | 1500 | 83 | ||
| 4,6-DMDBT | 600 | 41 | ||
| HKUST-1 | BT | 1500 | 25 | |
| DBT | 1500 | 45 | ||
| 4,6-DMDBT | 600 | 16 | ||
| HKUST-1-S | BT | 1000 | 16.6 | This work |
| DBT | 1000 | 40.9 | ||
| 4,6-DMDBT | 1000 | 22.4 | ||
| HKUST-1-5 | BT | 1000 | 23.8 | |
| DBT | 1000 | 46.8 | ||
| 4,6-DMDBT | 1000 | 36.8 |
| Adsorbents | Sulfur Compounds | |||||
|---|---|---|---|---|---|---|
| BT | DBT | 4,6-DMDBT | ||||
| K1 | R2 | K1 | R2 | K1 | R2 | |
| HKUST-1-S | 0.0896 | 0.8612 | 0.1077 | 0.6082 | 0.0056 | 0.8910 |
| HKUST-1-5 | 0.0679 | 0.4103 | 0.1281 | 0.8037 | 0.0936 | 0.6381 |
| Adsorbents | Sulfur Compounds | |||||
|---|---|---|---|---|---|---|
| BT | DBT | 4,6-DMDBT | ||||
| K2 | R2 | K2 | R2 | K2 | R2 | |
| HKUST-1-S | 0.0274 | 0.9982 | 0.0084 | 0.9936 | 0.0056 | 0.9959 |
| HKUST-1-5 | 0.0297 | 0.9977 | 0.0093 | 0.9953 | 0.0077 | 0.9939 |
| Adsorbents | Sulfur Compounds | |||||
|---|---|---|---|---|---|---|
| BT | DBT | 4,6-DMDBT | ||||
| Qmax (mgS/g) | R2 | Qmax (mgS/g) | R2 | Qmax (mgS/g) | R2 | |
| HKUST-1-S | 29.1 | 0.9931 | 56.8 | 0.9887 | 29.9 | 0.9854 |
| HKUST-1-5 | 125 | 0.9969 | 60.2 | 0.9885 | 45.7 | 0.9798 |
| Adsorbents | Sulfur Compounds | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| BT | DBT | 4,6-DMDBT | |||||||
| KF | n | R2 | KF | n | R2 | KF | n | R2 | |
| HKUST-1-S | 0.298 | 1.168 | 0.9989 | 1.791 | 2.024 | 0.9945 | 1.201 | 2.281 | 0.9989 |
| HKUST-1-5 | 0.064 | 1.120 | 0.9993 | 2.939 | 2.259 | 0.9995 | 2.964 | 2.576 | 0.9975 |
| Adsorbents | BET Surface Area (m2/g) | Micropore Volume (mL/g) | Average Pore Size (nm) |
|---|---|---|---|
| Fresh HKUST-1-5 | 1508 | 0.58 | 0.73 |
| Used HKUST-1-5 | 1450 | 0.51 | 0.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, J.; Liu, X.; Kong, Y.; Zhao, R.; Sun, Y.; Abou-Elyazed, A.S. Enhancing the Adsorption Performance of HKUST-1 by Adding NH4F During Room-Temperature Synthesis for Desulfurization of Fuel Oil. Energies 2025, 18, 5344. https://doi.org/10.3390/en18205344
Fu J, Liu X, Kong Y, Zhao R, Sun Y, Abou-Elyazed AS. Enhancing the Adsorption Performance of HKUST-1 by Adding NH4F During Room-Temperature Synthesis for Desulfurization of Fuel Oil. Energies. 2025; 18(20):5344. https://doi.org/10.3390/en18205344
Chicago/Turabian StyleFu, Jiawei, Xinchun Liu, Yuqing Kong, Ruyu Zhao, Yinyong Sun, and Ahmed S. Abou-Elyazed. 2025. "Enhancing the Adsorption Performance of HKUST-1 by Adding NH4F During Room-Temperature Synthesis for Desulfurization of Fuel Oil" Energies 18, no. 20: 5344. https://doi.org/10.3390/en18205344
APA StyleFu, J., Liu, X., Kong, Y., Zhao, R., Sun, Y., & Abou-Elyazed, A. S. (2025). Enhancing the Adsorption Performance of HKUST-1 by Adding NH4F During Room-Temperature Synthesis for Desulfurization of Fuel Oil. Energies, 18(20), 5344. https://doi.org/10.3390/en18205344







