Abstract
The integrated production of ethanol fuel through the simultaneous use of various by-products and waste materials is an intriguing concept, as it maximizes the raw material potential while addressing the challenge of managing waste biomass from different technological processes. The efficient utilization of lignocellulosic waste depends on employing a pretreatment method that enhances the susceptibility of structural polysaccharides to hydrolysis. The aim of the study was to assess the possibility of the simultaneous use of corn stillage biomass and beet molasses as raw materials for the production of ethanol fuel. The research focused on optimizing the process conditions for the acid pretreatment of stillage biomass and the enzymatic hydrolysis of cellulose and evaluating the effectiveness of two fermentation strategies: SHF (Separate Hydrolysis and Fermentation) and SSF (Simultaneous Saccharification and Fermentation). The highest hydrolysis susceptibility was observed in biomass pretreated with 2% v/v H3PO4 for 30 min at 121 °C. The maximum glucose concentration of about 12 g/L (hydrolysis efficiency about 35.5%) was achieved even with the lowest enzyme dose, i.e., 7.5 FPU per gram of biomass. The yeast also showed high fermentation activity in media prepared from stillage biomass and molasses, producing about 50 g/L of ethanol regardless of the fermentation strategy used. The complete fermentation of carbohydrates assimilated by yeast confirmed the complementarity of the two raw materials used to prepare fermentation media, emphasizing the high potential of the proposed technological solution for ethanol fuel production.
1. Introduction
The growing environmental pollution caused by the use of conventional energy sources, along with the depletion of fossil fuel resources, drives the search for effective technologies to produce energy carriers from renewable raw materials [1,2]. Bioethanol obtained in the fermentation process is an interesting alternative to liquid fossil fuels. Given the increased demand for ethyl alcohol, the primary issue to address is the potential rise in the prices of carbohydrate-rich raw materials, which are also used competitively in food and feed production [3,4]. A potential solution to this problem is the production of ethanol fuel from waste generated by the agri-food industry. However, the use of raw waste materials after pretreatment, often containing toxic substances such as furfural or 5-hydrxymethylfurfural, poses a serious technological challenge, which can be solved by using the detoxification process [5]. Lignocellulosic biomass is a waste material with significant potential as a source of carbohydrates for ethanol production, offering an alternative to starch-based food raw materials [6]. However, despite many years of research into the use of this raw material, its efficient processing into bioethanol remains a challenge. One of the challenges is to develop an effective lignocellulose pretreatment method that will ensure the susceptibility of the biomass to enzymatic hydrolysis [7]. Another problem is obtaining a fermentation medium with a high carbohydrate content that would provide ethanol concentrations above 40 g/L, thereby making the distillation process profitable [8]. One solution to these challenges is the use of highly effective lignocellulose decomposition methods, often involving expensive solvents or energy-intensive physicochemical techniques, in combination with active cellulase preparations to achieve a high level of cellulose depolymerization [9,10,11]. The use of various available methods is sometimes associated with increased costs in the production of cellulosic ethanol. An interesting solution to the identified problems, gaining increasing attention from the biofuel industry, is the intensification of ethanol production through the simultaneous use of raw materials that were traditionally used separately for producing first- and second-generation ethanol. The raw materials used can be considered both as a source of carbohydrates essential for the ethanol biosynthesis process and as a fuel for energy production through direct combustion, which is necessary to carry out technological operations. This comprehensive use of biomass enhances the energy balance and improves the economics of the bioethanol production process [12,13,14,15]. Several concepts have been proposed for integrating first- and second-generation ethanol production processes through the comprehensive utilization of both storage and structural carbohydrates. Some of them use sugarcane as a raw material for bioethanol production. In every method involving this raw material, the first step is extracting the sucrose-containing juice and separating the stems and leaves. One approach involves cleaning and concentrating the juice while simultaneously preprocessing the solid waste. The carbohydrates released through cellulose pretreatment and hydrolysis are then combined with the concentrated juice and subjected to alcoholic fermentation. Isolated lignins are used to produce electricity and steam necessary for the distillation and concentration of juice [12]. In an alternative approach, the purified and concentrated sugarcane juice and the hydrolysate from the solid fraction of sugarcane waste are fermented separately, with integration occurring at the distillation stage [13]. Other technological approaches focus solely on using sugarcane juice in the ethanol biosynthesis process, while the solid residue (bagasse) is utilized to generate the electricity and steam required for the technological process [16,17]. Attempts have also been made to integrate the production of first- and second-generation ethanol from plant-based raw materials containing starch. In the simplest approach, corn grain is milled and enzymatically hydrolyzed using amylolytic enzymes, while the remaining corn stover is simultaneously pretreated and hydrolyzed. The resulting hydrolysates are combined at the alcoholic fermentation stage [18]. More complex concepts involve, in addition to the previously described solution, the separate application of SSF (Simultaneous Saccharification and Fermentation) technology for both starch and cellulose hydrolysates or the use of the cellulose hydrolysate for fungal biomass production. The remaining solid fraction is a substrate for the production of feed for farm animals [14,19,20]. An interesting attempt to integrate the production of first- and second-generation bioethanol is the use of distillery stillage, obtained during ethanol production from starch raw materials, as a source of lignocellulose for second-generation ethanol production [21,22,23].
A concept that has been insufficiently explored is the production of ethanol fuel using two different raw materials, by-products or technological wastes, as sources of sucrose and lignocellulose. Beet molasses, a by-product of sugar production from sugar beet, can be used as a culture medium for yeast biomass production, the production of organic acids using various bacterial species, and ethanol production [24,25,26,27]. The usefulness of beet molasses in fermentation processes depends on the complementarity of its composition, which is influenced by various factors, including environmental conditions (e.g., fertilization intensity during sugar beet cultivation) and technological aspects related to the sugar production process itself. Unfortunately, effective use of this raw material often requires supplementation of the fermentation medium with minerals [28]. Problems with the complete bioconversion of carbohydrates in molasses worts led to research on the potential use of another waste raw material in combination with molasses, which is then treated as a supplementary source of trophic substances to improve the efficiency of the fermentation process. One such waste that can be used in combination with molasses is distillery stillage from the fermentation industry, which can be effectively utilized as a source of lignocellulose for second-generation ethanol production. Our previous studies have shown that hydrolysates obtained from the biomass of distillery stillages of various origins can serve as a complementary substrate in cellulosic ethanol production. However, the main limitation of their use is the relatively low concentration of carbohydrates derived from lignocellulose decomposition [29]. The solution to this problem may be the combined use of these hydrolysates together with another waste raw material rich in carbohydrates, e.g., beet molasses.
The aim of the study was to assess the efficiency of the alcohol fermentation process in media prepared from beet molasses and distillery stillage. The simultaneous use of these two raw materials aimed to produce a complete fermentation medium rich in carbohydrates and other trophic substances essential for yeast. A similar concept was previously examined by the authors, although it concentrated exclusively on cellulose hydrolysis during the fermentation process [30]. This study conducted a comprehensive evaluation of various inorganic acids for the pretreatment of stillage biomass, assessed the impact of cellulose hydrolysis conditions on glucose yield, and investigated the effects of simultaneously using molasses and corn stillage biomass. Hydrolysis was carried out according to the principles of SHF (Separate Hydrolysis and Fermentation) and SSF (Simultaneous Saccharification and Fermentation).
2. Materials and Methods
2.1. Raw Materials
Beet molasses, containing 49.70 ± 1.21% sucrose and an extract of 81.30 ± 0.40°Brix, was used to prepare the fermentation medium; corn stillage biomass, obtained after centrifugation, served as the lignocellulosic component. The stillage was sourced from the Radzicz Distillery (Poland), which employs barothermic technology, subjecting cereal grain to high pressure and temperature (160 °C, 6 atm) for approximately 50 min. The stillage, with a moisture content of 80%, was immediately frozen at –20 °C until use. The stillage contained 32.2 ± 1.4% cellulose, 20.9 ± 1.2% hemicellulose, and 3.2 ± 1.9% lignins, all expressed on a dry weight (DW) basis.
2.2. Cellulolytic Enzymes
The study utilized Laminex Super 3G from Danisco (DuPont Industrial Biosciences, Archimedesweg 30, Leiden, Netherlands), a cellulase complex with a concentration of 10–15% and enzymatic activity of 35.9 FPU/mL at pH 5 and 50 °C, capable of hydrolyzing β-glucans and non-starch polysaccharides. FPU (Filter Paper Units) activity was determined following the NREL protocol outlined in Technical Report NREL/TP-510-42628.
2.3. Yeast
Fermentation was performed using Saccharomyces cerevisiae yeast, Ethanol Red strain (Lesaffre Advanced Fermentations), provided as an active dry yeast preparation. The yeast was applied in the form of yeast milk (1.25 ± 0.12 x 109 CFU/mL, viability 94.3 ± 08%) obtained by suspending 5 g of the yeast preparation in 30 mL of sterile 0.9% w/v NaCl and rehydrating for 30 min (following the manufacturer’s instructions). The yeast milk was added to the fermentation medium at a dose of 3 mL per liter of medium.
2.4. Research Stages
The research was divided into three stages, with the first focusing on determining the optimal conditions for acid pretreatment of the stillage biomass to maximize cellulose susceptibility to hydrolysis. In the next stage, the optimal conditions for enzymatic cellulose hydrolysis were determined to achieve the highest glucose concentration. The final stage involved conducting the alcoholic fermentation of media prepared from beet molasses and distillery stillage biomass, using two technologies, SHF and SSF, with media made from molasses alone serving as the control.
2.4.1. Selection of Conditions for the Pretreatment of Corn Stillage Biomass
The acid pretreatment conditions involved the use of various inorganic acids (H2SO4, H3PO4, HNO3) at concentrations of 1% and 2% v/v, applied for 30 or 60 min at 121 °C. For pretreatment, 40 mL of the appropriate acid solution was added to 1 g DW of stillage biomass. The resulting suspension was autoclaved at 121 °C for either 30 or 60 min. The pretreatment conditions were selected based on our previous research [29]. The pretreated solution was cooled, neutralized to pH 5.5 using 30% w/v NaOH solution, and the volume was adjusted to 50 mL with distilled water. The content of sugars and pretreatment by-products in the obtained solution was analyzed using the HPLC-RID and HPLC-DAD methods, respectively. Then, to evaluate the effect of biomass pretreatment conditions on its susceptibility to enzymatic hydrolysis, a cellulase preparation was added to the obtained solution at a concentration of 5 FPU/g DW. The mixture was incubated at 50 °C for 72 h with shaking at 120 rpm. After the hydrolysis, the concentration of the obtained sugars was analyzed using the HPLC-RID method. Based on the obtained results, a pretreatment variant was selected and used in the subsequent stages of the research.
2.4.2. Selection of Enzymatic Hydrolysis Conditions
The evaluation of the effect of enzymatic hydrolysis conditions on the amount of glucose obtained was performed for biomass after pretreatment carried out under the following conditions: 2% v/v H3PO4, 30 min, 121 °C. In order to optimize the hydrolysis conditions, the effect of different biomass concentrations (2%, 6%, 10% w/v) and enzyme doses (7.5, 15, 22.5 FPU/g DW) was analyzed. For enzymatic hydrolysis, 80 mL of 2% v/v H3PO4 was added to 2, 6, or 10 g DW of biomass, and the mixture was subjected to the pretreatment procedure. After pretreatment, the obtained solution was neutralized to pH 5.5 using 30% w/v NaOH and 1M NaOH, and the volume was adjusted to 100 mL. The appropriate dose of cellulolytic enzyme was then added, and the mixture was incubated at 50 °C for 72 h with shaking at 120 rpm. Upon completion of hydrolysis, the glucose concentration was analyzed using the HPLC-RID method. Based on the concentration of glucose, the yield of the hydrolysis process was calculated from the following formula:
where CGlu is the concentration of glucose (grams per liter) in the sample, CCel is the cellulose content in biomass (per 1 g of dry weight), and CBiom is the initial biomass content (grams per liter) [31].
2.4.3. Preparation of the Fermentation Media
The fermentation media prepared based on molasses alone included two variants of the molasses dose: 111 g/L and 222 g/L (control variants). The same doses of molasses were used to prepare media based on corn stillage biomass, following the assumptions of either the SHF technology or the SSF technology. The preparation of control media began by adding 555 mL of 2% v/v H3PO4 to 111 or 222 g of beet molasses. The resulting solution was autoclaved at 121 °C for 30 min to replicate the lignocellulose pretreatment conditions used in the experimental variants. After cooling the solution, the pH was adjusted to 5.5 with 30% w/v NaOH and 1M NaOH, and the volume was adjusted to 1000 mL. The prepared fermentation medium was inoculated with 3 mL/l of yeast milk and incubated at 35 °C for 72 h. The composition of the fermentation medium was analyzed using the HPLC-RID technique both before and after alcoholic fermentation. Preparation of the fermentation media according to the SHF technology began by adding 555 mL of 2% v/v H3PO4 and 111 or 222 g of beet molasses to 67 g DW of corn stillage biomass. The resulting suspension was autoclaved at 121 °C for 30 min to pretreat the lignocellulose. After cooling the solution, the pH was adjusted to 5.5 with 30% w/v NaOH and 1M NaOH, and the volume was adjusted to 1000 mL. Next, the cellulolytic preparation was added at a dose of 7.5 FPU/g DW of corn stillage biomass and hydrolyzed at 50 °C for 72 h with shaking at 120 rpm. After completing the enzymatic hydrolysis, the solution was cooled, inoculated with yeast milk at a dose of 3 mL/L, and incubated at 35 °C for 72 h. The composition of the fermentation medium was analyzed using HPLC-RID after pretreatment, enzymatic hydrolysis, and alcoholic fermentation. Preparation of the fermentation media according to the assumptions of SSF technology began by adding 555 mL of 2% v/v H3PO4 and 111 or 222 g of beet molasses to 67 g DW of corn stillage biomass. The resulting suspension was autoclaved at 121 °C for 30 min to pretreat the lignocellulose. After cooling the solution, the pH was adjusted to 5.5 with 30% w/v NaOH and 1M NaOH, and the volume was adjusted to 1000 mL. Next, a cellulolytic preparation was added at a dose of 7.5 FPU/g DW of corn stillage biomass. The mixture was inoculated with yeast milk (3 mL/L) and incubated at 35 °C for 72 h. The composition of the fermentation medium was analyzed using HPLC-RID after pretreatment and alcoholic fermentation. All fermentation media were prepared in triplicate. Following fermentation, all substrates were distilled using a glass column equipped with 20 cap-type glass shelves. The distillate was then analyzed by capillary gas chromatography to determine the content of volatile by-products from alcoholic fermentation.
2.5. Analytical Methods
2.5.1. Analysis of Lignocellulose Component Concentrations
The content of cellulose, hemicellulose, and lignin in biomass was determined using a Fibertec 8000® device from FOSS. The analysis included the extraction of NDF (Neutral Detergent Fiber), ADF (Acid Detergent Fiber), and ADL (Acid Detergent Lignin), conducted according to the manufacturer’s methodology and the standards outlined in [32,33].
2.5.2. HPLC-RID Analysis
The concentrations of glucose, fructose, xylose, galactose, glycerol, and ethanol in the pretreatment solutions, hydrolysates, and fermentation media were analyzed using the HPLC-RID method on an Agilent 1220 system (Agilent Technologies, Palo Alto, CA, USA), equipped with a thermostat module, RID detector, and Hi-Plex H column (7.7 mm × 300 mm, 8 μm). The column operating temperature was 60 °C, while the RID detector was set to operate at 50 °C. Chromatographic separation was carried out using 5 mM H2SO4 as the mobile phase, at a flow rate of 0.6 mL/min, with a 20 µL injection volume, following the manufacturer’s recommendations. Quantitative determinations were conducted using the external standard method (ESTD). Before analysis, samples were centrifuged for 10 min at 7000× g and 20 °C. The supernatant was then diluted and filtered through a 0.45 µm membrane filter (PES). The chromatographic column used does not permit the direct determination of sucrose, the primary sugar in molasses; however, it enables its indirect determination by measuring the combined concentrations of glucose and fructose [29]. The separation properties of the chromatographic column used do not permit the distinct determination of fructose, xylose, and galactose concentrations due to their similar retention times. Therefore, the concentration of these compounds was reported as the sum of their individual concentrations.
2.5.3. HPLC-DAD Analysis
The concentrations of pretreatment by-products, such as sugar dehydration products (furfural and 5-hydroxymethylfurfural) and lignin depolymerization products (1,2-dihydrobenzene, vanillic acid, syringaldehyde, ferulic acid, vanillin, guaiacol, sinapyl alcohol, coumaric acid), in the pretreated solutions under various process conditions were analyzed using an Agilent Technologies® model 1220 liquid chromatograph equipped with a diode array detector (HPLC-DAD). Chromatographic separation was performed using a ZORBAX Eclipse Plus C18 column (4.6 mm × 100 mm, 3.5 µm) (Agilent Technologies®), with a mobile phase consisting of 0.3% acetic acid (70%) and methanol (30%) at a flow rate of 0.5 mL/min and a column temperature of 30 °C, with an injection volume of 5 µL. Detection was performed at wavelengths of 280 and 320 nm [34]. Quantitative analysis was carried out using the external standard method (ESTD).
2.5.4. Analysis of the Composition of Volatile By-Products by Capillary GC
The distillate samples obtained by multiple distillation were analyzed by capillary gas chromatography using an Agilent 7890 chromatograph (Agilent Technologies, USA) equipped with an FID detector and a CP-Wax 57 CB column (50 m × 0.32 mm, 0.2 µm). The detailed conditions for chromatographic separation are provided in a previous publication [35].
2.6. Statistical Methods
All laboratory analyses were performed in triplicate. Statistical analysis was carried out using the Statistica software ver. 13.3 (analysis of variance, determination of SD). HSD Tukey’s test was applied at the significance level of α < 0.05.
3. Results and Discussion
3.1. The Influence of Corn Stillage Biomass Pretreatment Conditions on the Amount of Sugars Obtained and the Formation of By-Products
In the first stage of the study, the impact of barothermic pretreatment using different inorganic acids, varying concentrations, and exposure times was assessed in terms of sugar release, biomass susceptibility to enzymatic hydrolysis, and by-product formation. Previous studies by other authors did not compare the effects of different acids, such as H2SO4, H3PO4, and HNO3, on the decomposition of corn stillage biomass. The effect of different inorganic acids used in corn stillage biomass pretreatment was evaluated by analyzing the concentrations of carbohydrates released during both pretreatment and enzymatic hydrolysis (Table 1). Sulfuric acid and nitric acid were found to produce the highest sugar concentration after pretreatment. The highest glucose concentration (76.75 ± 2.35 mg/g biomass) and combined galactose and xylose concentration (107.10 ± 3.50 mg/g biomass) were achieved using 2% v/v nitric acid with a 30-min exposure time (Table 1). The lowest carbohydrate concentration from pretreatment alone was observed when phosphoric acid was used. Using this acid at a concentration of 1% v/v for 30 min yielded glucose concentrations of just 4.6 mg/g biomass and galactose and xylose concentrations of 13 mg/g biomass (Table 1). The amount of glucose obtained after pretreatment was influenced by both the type and concentration of the acid used. At higher acid concentrations, an increased release of glucose from biomass was observed, particularly with sulfuric and phosphoric acids at 60-min exposure and with nitric acid at 30-min exposure. After applying 2% v/v nitric acid, the increase in the amount of released glucose compared to the lower concentration was as high as 16 mg/g of biomass. Only in the case of phosphoric acid did extending the exposure time of biomass to the acid result in an increase in the amount of released glucose. Increasing the exposure time from 30 to 60 min resulted in an increase in glucose concentration from 13 to 16 mg/g of biomass, depending on the concentration of H3PO4 used.

Table 1.
Effect of acid pretreatment conditions of stillage biomass on carbohydrate yield and enzymatic hydrolysis efficiency.
Enzymatic hydrolysis of corn stillage biomass pretreated with sulfuric and nitric acids, using a cellulase preparation, resulted in an increase in glucose concentration by ca. 60–70 mg/g of biomass, regardless of the acid concentration or exposure time (Table 1). Biomass pretreated with phosphoric acid exhibited significantly higher susceptibility to enzymatic hydrolysis. Depolymerization of H3PO4-pretreated biomass using a cellulase complex led to an increase in glucose concentration of up to 150 mg/g biomass, compared to the concentration achieved through pretreatment alone. The highest susceptibility of corn stillage biomass to enzymatic hydrolysis was observed after pretreatment with 2% v/v phosphoric acid for 30 min (Table 1). Enzymatic hydrolysis using the enzyme preparation had no effect on the concentrations of galactose, xylose, and arabinose obtained from the depolymerization of biomass pretreated with sulfuric and nitric acids (Table 1). Based on the glucose concentrations obtained from the enzymatic hydrolysis of biomass, the pretreatment method using 2% v/v H3PO4 for 30 min was selected for further studies.
Studies by other authors indicate a positive effect of higher sulfuric acid concentrations on the amount of sugars obtained after pretreatment [36,37,38]. Studies on the use of whole wheat stillage as a lignocellulose source also highlighted the effectiveness of phosphoric acid during pretreatment. The use of phosphoric acid at concentrations of 1% and 1.5% v/v in whole stillage pretreatment ensured high biomass susceptibility to enzymatic hydrolysis, with the number of fermentable sugars obtained showing no statistically significant differences compared to sulfuric acid pretreatment [21]. Pretreatment of lignocellulosic biomass with phosphoric acid leads to the esterification of hydroxyl groups in cellulose, forming cellulose phosphate. This process has been shown to enhance the removal of crystalline regions in cellulose, thereby increasing its susceptibility to enzymatic hydrolysis [39,40].
Despite the use of acid pretreatment of biomass at elevated temperatures, the resulting levels of toxic by-products were relatively low (Table 2). The use of strongly dehydrating inorganic acids promotes the dehydration of sugars, leading to the formation of furfural from xylose and 5-hydroxymethylfurfural from glucose [41]. As a result of pretreatment, phenolic compounds, which are products of lignin depolymerization, may also appear in the hydrolysates [42]. In this study, the concentration of by-products in the solutions obtained after pretreatment was low, owing to the low biomass load during pretreatment (20 g DW/l) and the use of biomass with high water content. The study used distillery stillage biomass directly after centrifugation in an agricultural distillery, with a moisture content of approximately 80%, which contributed to the additional hydration of the obtained hydrolysate. The highest degree of monosaccharide dehydration was observed with sulfuric and nitric acids, despite the high hydration level of the fermentation medium. Furan aldehydes (5-HMF and furfural) were detected in the solutions after pretreatment with 2% H2SO4 and 1% HNO3 (Table 2). The highest concentrations of 5-HMF (over 3 mg/L) and furfural (over 15 mg/L) were observed in the medium after pretreatment with 1% HNO3. The presence of lignin depolymerization products in the solutions after pretreatment was primarily influenced by the type of inorganic acid used. The highest concentration of phenolic compounds, primarily syringaldehyde and ferulic acid, was observed with phosphoric acid pretreatment, though its concentration had no significant impact on the presence of these compounds (Table 2).

Table 2.
Effect of acid pretreatment conditions of stillage biomass on the formation of toxic by-products.
However, pretreatment with sulfuric acid at both concentrations resulted in an increased 1,2-dihydrobenzene concentration, reaching approximately 11–13 mg/L. Samples treated with 1% v/v sulfuric and nitric acids showed higher concentrations of vanillic acid, syringaldehyde, and ferulic acid compared to those treated with 2% v/v acids (Table 2). Other authors using sulfuric acid at similar concentrations (0.5% and 1% v/v) and 120 °C reported 5-HMF concentrations of up to 1.3 mg/g DW, with no detection of furfural [43]. Small amounts of furfural were observed during the pretreatment of corn fiber with sulfuric acid at 120 °C [38]. To the best of our knowledge, no studies have been published on the impact of different inorganic acids on the yield of products released during lignin degradation. Until now, research has primarily focused on the effects of using sulfuric acid for the pretreatment of lignocellulosic biomass. However, it is important to note that the effects of using different strong inorganic acids depend on the biomass’s origin, moisture content, and degree of processing. The results of this study highlight the effectiveness of acid pretreatment at elevated temperatures in preparing corn stillage biomass as a substrate for bioethanol production.
3.2. The Influence of the Enzymatic Hydrolysis Conditions of Acid-Pretreated Corn Stillage Biomass on the Glucose Concentration
In the next stage of the research, after selecting the optimal biomass pretreatment conditions, the effect of selected hydrolysis parameters, such as biomass concentration and enzyme dosage, on the amount of glucose released during cellulose depolymerization was evaluated. Three biomass concentrations (20, 60, and 100 g DW/L) and three cellulolytic enzyme complex doses (7.5, 15, and 22.5 FPU/g biomass) were tested. The highest glucose concentration, ca. 12 g/L, was obtained for the highest biomass concentration of 100 g DW/L (Figure 1). It is worth noting that the cellulase dose had no effect on the amount of glucose obtained. This clearly indicates that the high efficiency of the enzymatic hydrolysis process was achieved with the lowest enzyme dose, i.e., 7.5 FPU/g of biomass (Figure 1).
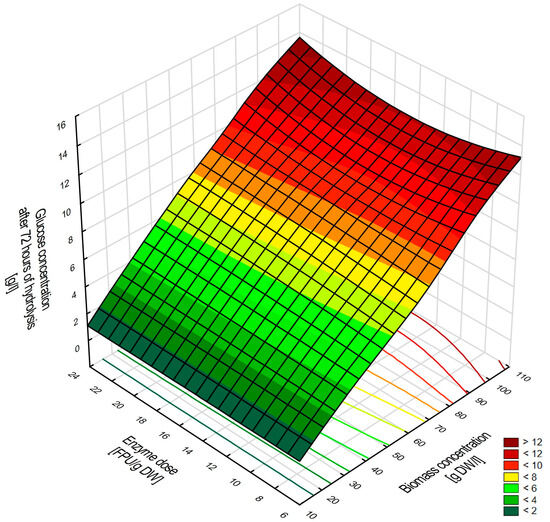
Figure 1.
The influence of biomass concentration and cellulase dose on glucose yield during 72 h of enzymatic hydrolysis.
The enzymatic hydrolysis efficiency of cellulose was approximately 35%, irrespective of the biomass concentration or enzyme dose. This efficiency was lower than in our previous studies, likely due to the catalytic specificity of the cellulase preparation employed [29]. The results suggest that increasing the enzyme preparation dose is not a viable solution to this issue, as the higher FPU/g biomass doses used in the studies did not improve cellulose hydrolysis efficiency. In studies on the hydrolysis process of lignocellulosic biomass, it is most often observed that hydrolysis efficiency increases with a higher dose of the enzyme preparation [42]. However, in the bioethanol production process, enzyme preparations constitute a significant portion of raw material costs, which is why the goal is to achieve maximum hydrolysis efficiency with the lowest possible enzyme doses. Therefore, an important finding of this research is the achievement of maximum glucose concentration even with the lowest dose of the enzyme preparation. The authors’ research demonstrated that the primary factor determining the amount of glucose released is the concentration of stillage biomass. Similar observations have also been reported by other authors [38]. The obtained results allowed for the selection of the conditions for enzymatic hydrolysis of cellulose in the acid-pretreated corn stillage biomass, which was used in the next stage of the research.
3.3. Efficiency of Bioethanol Production Using Beet Molasses and Corn Stillage Biomass Simultaneously Under SHF and SSF Conditions
The final stage of the research investigated the effects of using acid-pretreated corn stillage biomass combined with beet molasses as a substrate for ethanol fuel production under two fermentation strategies: SHF and SSF technologies. One of the primary challenges in cellulosic ethanol production is that alcoholic fermentation of lignocellulosic substrates yields low ethanol concentrations (below 40 g/L), falling short of the profitability threshold for distillation [8]. A potential solution to this issue is integrating the bioethanol production process by simultaneously utilizing various waste materials or by-products as additional sugar sources [20,44,45].
Therefore, this study utilized acid-pretreated corn stillage biomass and beet molasses simultaneously. The fermentation media consisted of either molasses alone (control) or a combination of stillage biomass and molasses, applied in both SHF and SSF technologies (Table 3). Molasses was added at two different concentrations. Studies demonstrated that using molasses as the sole substrate in the alcoholic fermentation process was effective only at a lower concentration (111 g/L). At this concentration, complete fermentation of carbohydrates was achieved, yielding an ethanol concentration of 21.4 g/L (Figure 2A,B).

Table 3.
Characteristics of fermentation media variants.
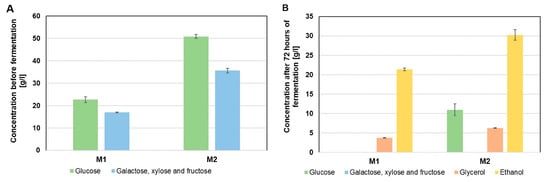
Figure 2.
Composition of molasses media before alcoholic fermentation (A) and after fermentation (B).
When a higher concentration of molasses (222 g/L) was used, incomplete carbohydrate fermentation occurred, with approximately 10 g/L of residual glucose remaining. This result confirmed that molasses alone, due to its incomplete composition, is not a suitable substrate for implementing a highly efficient alcoholic fermentation process (Figure 2B) [28]. A solution to this problem may be the use of molasses in combination with another by-product of the fermentation industry, complementary in composition, to provide a fermentation medium with a complete nutrient profile. The fermentation media was prepared from corn stillage biomass pretreated with 2% v/v H3PO4 for 30 min, followed by enzymatic hydrolysis (enzyme dose of 7.5 FPU) for 72 h and supplemention with molasses, and had a carbohydrate concentration 16 g/L higher than the medium prepared from molasses alone, regardless of the molasses concentration used (Figure 2A and Figure 3B). Using SHF technology, fermentation media with molasses and corn stillage biomass containing carbohydrates at concentrations of over 56 g/L (SHF MS + M1) and 102 g/L (SHF MS + M2) were obtained. In all media obtained using SHF technology, full utilization of fermentable sugars was observed during 72 h of fermentation. The remaining mixture of fructose, galactose, and xylose at a concentration of about 2 g/L was due to the non-metabolization of xylose by S. cerevisiae yeast (Figure 3C). Alcoholic fermentation of the media obtained from stillage biomass and molasses at a higher dose (222 g/L, SHF MS + M2) resulted in a high final ethanol concentration of 51.5 g/L (Figure 3B).
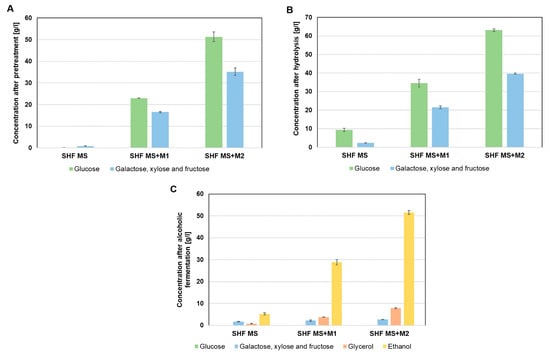
Figure 3.
Composition of media derived from molasses and acid-pretreated stillage biomass in SHF technology: after pretreatment (A), after enzymatic hydrolysis (B), and after alcoholic fermentation (C).
By using SSF technology, which reduces the energy costs associated with the separate hydrolysis process, a similar level of carbohydrate fermentation was achieved as in the media obtained with SHF technology, regardless of the molasses concentration used. The ethanol concentration in media obtained using SSF technology also did not show any statistically significant differences compared to the SHF media (Figure 4B). The maximum ethanol concentration in SSF media was approximately 50 g/L, achieved with a higher molasses concentration (SSF MS + M2) (Figure 4B). The obtained results clearly indicate that the combined use of corn stillage biomass and molasses in fermentation media, regardless of the technology used (SHF or SSF), significantly increases the amount of fermentable sugars and ensures a complete composition of the medium, guaranteeing a high attenuation of the fermentation process.
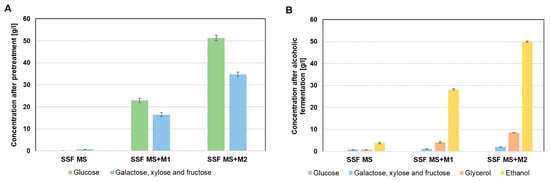
Figure 4.
Composition of media derived from molasses and acid-pretreated stillage biomass in SSF technology: after pretreatment (A) and after alcoholic fermentation (B).
The high fermentation activity of yeast is a key factor in achieving a high final ethanol concentration. This can only be attained with media containing all necessary trophic substances, including carbon and nitrogen sources, as well as essential minerals and biologically active compounds [46]. The effectiveness of the proposed solution, which involves the simultaneous use of two raw materials, one of which is lignocellulosic biomass, was also demonstrated by other authors. When corn grain and preprocessed corn straw were used as substrates (in a 20:10% ratio) for bioethanol production, high fermentation efficiency (over 80%) was observed [18]. Notably, aside from the studies mentioned above, no experimental data have been reported on enhancing the fermentation process by integrating first- and second-generation ethanol production exclusively from waste materials. Other authors primarily focused on process integration simulations to assess the cost-effectiveness of bioethanol production from sugarcane and explore opportunities for improving the energy balance [16,17,18,42,47,48].
This study not only introduces an integration method for ethanol production but also offers a comprehensive qualitative and quantitative analysis of volatile by-products in the resulting distillates (Table 4). When analyzing the composition of distillates obtained from media with a higher concentration of molasses (222 g/L), it should be noted that the addition of distillery stillage, regardless of the technology used (SHF or SSF), resulted in a reduction in the concentrations of acetaldehyde, isobutanol, and 2-methyl-1-butanol. It should be noted that as a result of the fermentation of media containing stillage and molasses in higher concentrations, in addition to a high ethanol yield, a significant reduction in acetaldehyde concentration was also observed, indicating a high activity of alcohol dehydrogenase [49]. The reduced concentrations of the higher alcohols, isobutanol and 2-methyl-1-butanol, indicated that the addition of the distiller’s stillage provided an adequate nitrogen source in the fermentation medium. Isobutanol and 2-methyl-1-butanol are formed from valine and isoleucine, respectively. Their accumulation in the fermentation medium suggests limited availability of nitrogen sources for the yeast [50,51]. Analysis of the obtained results clearly indicates that the combined use of acid-pretreated distillery stillage biomass and beet molasses enhanced both fermentation efficiency and the composition of volatile by-products.

Table 4.
Composition of volatile by-products of the alcoholic fermentation process in the obtained distillates.
4. Conclusions
Fermentation media composed of acid-pretreated corn stillage biomass supplemented with beet molasses were analyzed to assess their efficiency in producing fuel bioethanol. Pretreatment of biomass with 2% v/v H3PO4 was shown to enhance its susceptibility to enzymatic hydrolysis. The fermentation strategy, which utilized beet molasses combined with stillage biomass as a lignocellulose source in the fermentation media, achieved a final ethanol concentration exceeding 50 g/L when a higher molasses dose (222 g/L) was applied, irrespective of the technology used (SHF or SSF). The fermentation strategy, incorporating enzymatic hydrolysis of cellulose from pretreated stillage biomass during fermentation, offers a technological solution that ensures full attenuation of carbohydrates assimilated by S. cerevisiae yeast, resulting in high fermentation efficiency and good distillate quality. The presented results demonstrate the potential for the effective production of fuel bioethanol from corn stillage biomass enriched with beet molasses. The high fermentation efficiency (regardless of whether SHF or SSF technology is used) of the media derived from the combination of these two raw materials highlights the completeness of the fermentation medium’s composition in terms of essential trophic substances for the yeast, which is more challenging to achieve when using only beet molasses. The obtained results indicate the high application potential of the proposed technological solution, which provides a high concentration of bioethanol with a relatively low level of volatile fermentation by-products. The presented concept offers the potential for the effective management of by-products from the agri-food industry, such as distillery stillage and beet molasses.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/en18020312/s1: Statistical analysis data (S1.–S3.).
Author Contributions
Conceptualization, D.M. and G.K.; methodology, K.O. and D.M.; formal analysis, K.O. and D.M.; investigation, K.O. and D.M.; data curation, K.O. and D.M.; writing—original draft preparation, K.O. and D.M.; writing—review and editing, G.K.; supervision, D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no funding.
Data Availability Statement
Dataset available on request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mani, J.; Gadissa, T.G.; Kaleab, B.G.; Selvakumar, P.; Abdisa, J.; Gurunathan, B.; Beula, I.J.; Arivalagan, P. Bioethanol production from agricultural residues as lignocellulosic biomass feedstock’s waste valorization approach: A comprehensive review. Sci. Total Environ. 2023, 879, 163158. [Google Scholar]
- Zhao, J.; Xu, Y.; Wang, W.; Griffin, J.; Roozeboom, K.; Wang, D. Bioconversion of Industrial Hemp Biomass for Bioethanol Production: A Review. Fuel 2020, 281, 118725. [Google Scholar] [CrossRef]
- Shukla, A.; Kumar, D.; Girdhar, M.; Kumar, A.; Goyal, A.; Malik, T.; Mohan, A. Strategies of pretreatment of feedstocks for optimized bioethanol production: Distinct and integrated approaches. Biotechnol. Biofuels Bioprod. 2023, 16, 44. [Google Scholar] [CrossRef]
- Dhanashri, S.P.; Minal, D.; Ashwini, P. Different pre-treatments and kinetic models for bioethanol production from lignocellulosic biomass: A review. Heliyon 2023, 9, 16604. [Google Scholar]
- Sarkar, N.; Ghosh, S.K.; Bannerjee, S.; Aikat, K. Bioethanol Production from Agricultural Wastes: An Overview. Renew. Energy 2012, 37, 19–27. [Google Scholar] [CrossRef]
- Devi, A.; Bajar, S.; Sihag, P.; Sheikh, Z.U.D.; Singh, A.; Kaur, J.; Narsi, R.B.; Pant, D. A panoramic view of technological landscape for bioethanol production from various generations of feedstocks. Bioengineered 2023, 14, 81–112. [Google Scholar] [CrossRef] [PubMed]
- Igwebuike, C.M.; Awad, S.; Andrès, Y. Renewable Energy Potential: Second-Generation Biomass as Feedstock for Bioethanol Production. Molecules 2024, 29, 1619. [Google Scholar] [CrossRef] [PubMed]
- Molaverdi, M.; Mirmohamadsadeghi, S.; Karimi, K.; Aghbashlo, M.; Tabatabaei, M. Efficient ethanol production from rice straw through cellulose restructuring and high solids loading fermentation by Mucor indicus. J. Clean. Prod. 2022, 339, 130702. [Google Scholar] [CrossRef]
- Koppram, R.; Tomás-Pejó, E.; Xiros, C.; Olsson, L. Lignocellulosic Ethanol Production at High-Gravity: Challenges and Perspectives. Trends Biotechnol. 2014, 32, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Singhvi, M.S.; Gokhale, D.V. Lignocellulosic Biomass: Hurdles and Challenges in Its Valorization. Appl. Microbiol. Biotechnol. 2019, 103, 9305–9320. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of Lignocellulosic Biomass: A Review on Recent Advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.O.S.; Junqueira, T.L.; Cavalett, O.; Pavanello, L.G.; Cunha, M.P.; Jesus, C.D.F.; Maciel Filho, R.; Bonomi, A. Biorefineries for the Production of First and Second Generation Ethanol and Electricity from Sugarcane. Appl. Energy 2013, 109, 72–78. [Google Scholar] [CrossRef]
- Macrelli, S.; Galbe, M.; Wallberg, O. Effects of Production and Market Factors on Ethanol Profitability for an Integrated First and Second Generation Ethanol Plant Using the Whole Sugarcane as Feedstock. Biotechnol. Biofuels 2014, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Hernandez, E.; Sadhukhan, J.; Campbell, G.M. Integration of Bioethanol as an In-Process Material in Biorefineries Using Mass Pinch Analysis. Appl. Energy 2013, 104, 517–526. [Google Scholar] [CrossRef]
- Bechara, R.; Gomez, A.; Saint-Antonin, V.; Schweitzer, J.M.; Maréchal, F. Methodology for the Optimal Design of an Integrated First and Second Generation Ethanol Production Plant Combined with Power Cogeneration. Bioresour. Technol. 2016, 214, 441–449. [Google Scholar] [CrossRef]
- Dias, M.O.S.; Junqueira, T.L.; Cavalett, O.; Cunha, M.P.; Jesus, C.D.F.; Rossell, C.E.V.; Maciel Filho, R.; Bonomi, A. Integrated versus Stand-Alone Second Generation Ethanol Production from Sugarcane Bagasse and Trash. Bioresour. Technol. 2012, 103, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.O.S.; Junqueira, T.L.; Rossell, C.E.V.; MacIel Filho, R.; Bonomi, A. Evaluation of Process Configurations for Second Generation Integrated with First Generation Bioethanol Production from Sugarcane. Fuel Process. Technol. 2013, 109, 84–89. [Google Scholar] [CrossRef]
- Chen, S.; Xu, Z.; Li, X.; Yu, J.; Cai, M.; Jin, M. Integrated Bioethanol Production from Mixtures of Corn and Corn Stover. Bioresour. Technol. 2018, 258, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, M.; Roozeboom, K.; Wang, D. Integrated Bioethanol Production to Boost Low-Concentrated Cellulosic Ethanol without Sacrificing Ethanol Yield. Bioresour. Technol. 2018, 250, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Lennartsson, P.R.; Erlandsson, P.; Taherzadeh, M.J. Integration of the First and Second Generation Bioethanol Processes and the Importance of By-Products. Bioresour. Technol. 2014, 165, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.B.; Kalif, M.; Ferreira, J.A.; Taherzadeh, M.J.; Lennartsson, P.R. Mild-Temperature Dilute Acid Pretreatment for Integration of First and Second Generation Ethanol Processes. Bioresour. Technol. 2017, 245, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Dawid, M.; Grzegorz, K. Microwave-Assisted Hydrotropic Pretreatment as a New and Highly Efficient Way to Cellulosic Ethanol Production from Maize Distillery Stillage. Appl. Microbiol. Biotechnol. 2021, 105, 3381–3392. [Google Scholar] [CrossRef] [PubMed]
- Mikulski, D.; Kłosowski, G. Hydrotropic Pretreatment on Distillery Stillage for Efficient Cellulosic Ethanol Production. Bioresour. Technol. 2020, 300, 122661. [Google Scholar] [CrossRef]
- Alrefaey, H.M.A.; Abdel-Rahman, M.A.; Hassan, S.E.D.; El-Din, M.N.; Azab, M.S. Sequential Optimization of the Fermentation Factors with Integrating Seed Culture Adaptation for Increased Biorefinery of Beet Molasses to Lactic Acid. Biomass Convers. Biorefin. 2021, 11, 1013–1028. [Google Scholar] [CrossRef]
- Monteagudo, J.M.; Rodríguez, L.; Rincón, J.; Fuertes, J. Optimization of the Conditions of the Fermentation of Beet Molasses to Lactic Acid by Lactobacillus delbrueckii. Acta Biotechnol. 1994, 14, 251–260. [Google Scholar] [CrossRef]
- Jiménez, A.M.; Borja, R.; Martín, A. Aerobic-Anaerobic Biodegradation of Beet Molasses Alcoholic Fermentation Wastewater. Process Biochem. 2003, 38, 1275–1284. [Google Scholar] [CrossRef]
- Monteagudo, J.M.; Rodríguez, L.; Rincón, J.; Fuertes, J. Kinetics of Lactic Acid Fermentation By Lactobacillus delbrueckii Grown on Beet Molasses. J. Chem. Technol. Biotechnol. 1997, 68, 271–276. [Google Scholar] [CrossRef]
- Cachot, T.; Pons, M.-N. Improvement of Alcoholic Fermentation on Cane and Beet Molasses by Supplementation. J. Ferment. Bioeng. 1991, 71, 24–27. [Google Scholar] [CrossRef]
- Mikulski, D.; Kłosowski, G. Efficiency of Dilute Sulfuric Acid Pretreatment of Distillery Stillage in the Production of Cellulosic Ethanol. Bioresour. Technol. 2018, 268, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Mikulski, D.; Kłosowski, G. Integration of First- and Second-Generation Bioethanol Production from Beet Molasses and Distillery Stillage After Dilute Sulfuric Acid Pretreatment. Bioenergy Res. 2022, 15, 454–465. [Google Scholar] [CrossRef]
- Rana, V.; Rana, D.; Ahring, B.K. Process Modeling of Enzymatic Hydrolysis of Wet-Exploded Corn Stover. Bioenergy Res. 2014, 7, 450–459. [Google Scholar] [CrossRef]
- ISO 13906:2008; Animal Feeding Stuffs—Determination of Acid Detergent Fibre (ADF) and Acid Detergent Lignin (ADL) Contents. International Organization for Standardization: Geneva, Switzerland, 2008.
- ISO 16472:2006; Animal Feeding Stuffs—Determination of Amylase-Treated Neutral Detergent Fibre Content (aNDF). International Organization for Standardization: Geneva, Switzerland, 2006.
- Cho, D.H.; Lee, Y.J.; Um, Y.; Sang, B.I.; Kim, Y.H. Detoxification of Model Phenolic Compounds in Lignocellulosic Hydrolysates with Peroxidase for Butanol Production from Clostridium Beijerinckii. Appl. Microbiol. Biotechnol. 2009, 83, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Kłosowski, G.; Mikulski, D. The Effect of Raw Material Contamination with Mycotoxins on the Composition of Alcoholic Fermentation Volatile By-Products in Raw Spirits. Bioresour. Technol. 2010, 101, 9723–9727. [Google Scholar] [CrossRef]
- Guo, G.L.; Chen, W.H.; Chen, W.H.; Men, L.C.; Hwang, W.S. Characterization of Dilute Acid Pretreatment of Silvergrass for Ethanol Production. Bioresour. Technol. 2008, 99, 6046–6053. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J.J. Dilute Acid Pretreatment of Rye Straw and Bermudagrass for Ethanol Production. Bioresour. Technol. 2005, 96, 1599–1606. [Google Scholar] [CrossRef]
- Noureddini, H.; Byun, J. Dilute-Acid Pretreatment of Distillers’ Grains and Corn Fiber. Bioresour. Technol. 2010, 101, 1060–1067. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, B.; Zhang, J.; Liu, L.L.S.; Ouyang, P. Effect of phosphoric acid pretreatment on enzymatic hydrolysis of microcrystalline cellulose. Biotechnol. Adv. 2010, 28, 613–619. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Lin, L.; Chen, T.; Zhang, J.; Liu, S.; Li, Z.; Ouyang, P. Dissolution of Microcrystalline Cellulose in Phosphoric Acid—Molecular Changes and Kinetics. Molecules 2009, 14, 5027–5041. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimian, F.; Denayer, J.F.M.; Mohammadi, A.; Khoshnevisan, B.; Karimi, K. A Critical Review on Pretreatment and Detoxification Techniques Required for Biofuel Production from the Organic Fraction of Municipal Solid Waste. Bioresour. Technol. 2023, 368, 128316. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Ragauskas, A.J.; Yuan, J.S. Lignin Conversion: Opportunities and Challenges for the Integrated Biorefinery. Ind. Biotechnol. 2016, 12, 161–167. [Google Scholar] [CrossRef]
- Zheng, Y.; Lee, C.; Yu, C.; Cheng, Y.S.; Zhang, R.; Jenkins, B.M.; VanderGheynst, J.S. Dilute Acid Pretreatment and Fermentation of Sugar Beet Pulp to Ethanol. Appl. Energy 2013, 105, 1–7. [Google Scholar] [CrossRef]
- Miret, C.; Chazara, P.; Montastruc, L.; Negny, S.; Domenech, S. Design of Bioethanol Green Supply Chain: Comparison between First and Second Generation Biomass Concerning Economic, Environmental and Social Criteria. Comput. Chem. Eng. 2016, 85, 16–35. [Google Scholar] [CrossRef]
- MacRelli, S.; Mogensen, J.; Zacchi, G. Techno-Economic Evaluation of 2 Nd Generation Bioethanol Production from Sugar Cane Bagasse and Leaves Integrated with the Sugar-Based Ethanol Process. Biotechnol. Biofuels 2012, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Bafrncová, P.; Šmogrovičová, D.; Sláviková, I.; Pátková, J.; Dömény, Z. Improvement of Very High Gravity Ethanol Fermentation by Media Supplementation Using Saccharomyces Cerevisiae. Biotechnol. Lett. 1999, 21, 337–341. [Google Scholar] [CrossRef]
- Dias, M.O.S.; Da Cunha, M.P.; MacIel Filho, R.; Bonomi, A.; Jesus, C.D.F.; Rossell, C.E.V. Simulation of Integrated First and Second Generation Bioethanol Production from Sugarcane: Comparison between Different Biomass Pretreatment Methods. J. Ind. Microbiol. Biotechnol. 2011, 38, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Furlan, F.F.; Costa, C.B.B.; de Castro Fonseca, G.; de Pelegrini Soares, R.; Secchi, A.R.; da Cruz, A.J.G.; de Campos Giordano, R. Assessing the Production of First and Second Generation Bioethanol from Sugarcane through the Integration of Global Optimization and Process Detailed Modeling. Comput. Chem. Eng. 2012, 43, 1–9. [Google Scholar] [CrossRef]
- Jackowetz, J.N.; Dierschke, S.; de Orduña, R.M. Multifactorial Analysis of Acetaldehyde Kinetics during Alcoholic Fermentation by Saccharomyces cerevisiae. Food Res. Int. 2011, 44, 310–316. [Google Scholar] [CrossRef]
- Barbosa, C.; Mendes-Faia, A.; Mendes-Ferreira, A. The Nitrogen Source Impacts Major Volatile Compounds Released by Saccharomyces Cerevisiae during Alcoholic Fermentation. Int. J. Food Microbiol. 2012, 160, 87–93. [Google Scholar] [CrossRef]
- Albers, E.; Larsson, C.; Lidé, N.G.; Niklasson, C.; Gustafsson, L. Influence of the Nitrogen Source on Saccharomyces cerevisiae Anaerobic Growth and Product Formation. Appl. Environ. Microbiol. 1996, 62, 3187–3195. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).