Simplified Mechanisms of Nitrogen Migration Paths for Ammonia-Coal Co-Combustion Reactions
Abstract
1. Introduction
2. Materials and Methods
2.1. Parameters for Zero-Dimensional Reaction Model for Ammonia–Coal Co-Combustion
2.2. Parameters for Entrained Flow Reaction Model for Ammonia–Coal Co-Combustion
3. Results
3.1. Nitrogen Containing Products of Ammonia–Coal Co-Combustion Based on Closed 0D Reactor
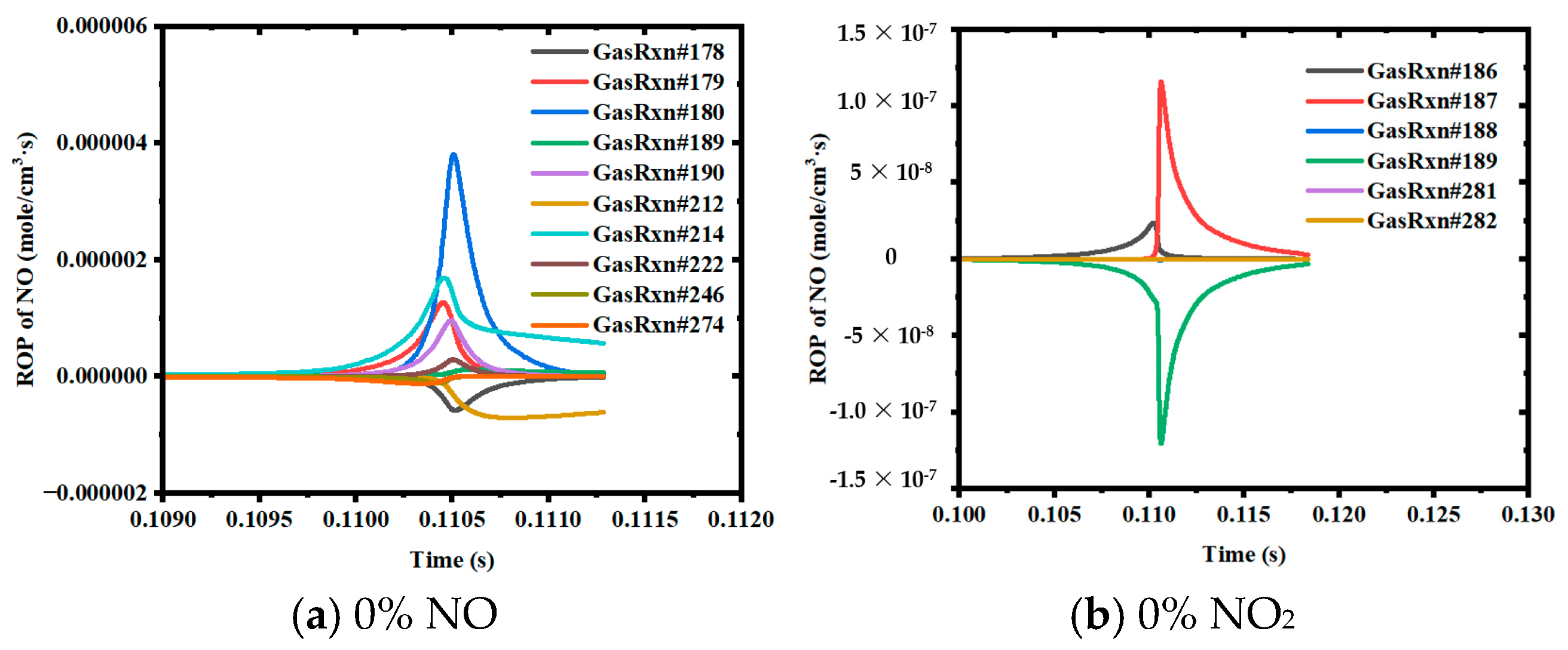
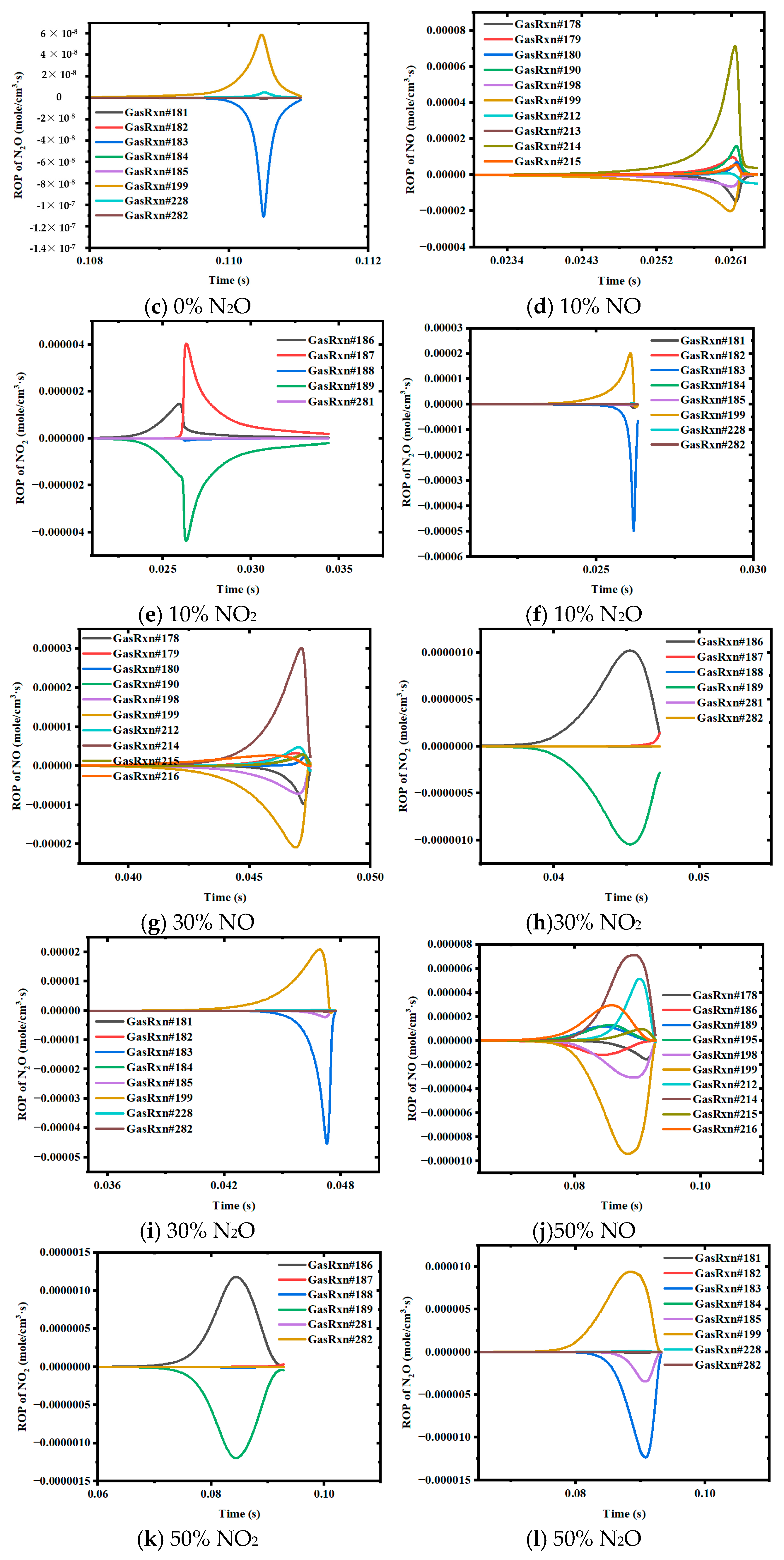
3.2. ROP Analysis of Ammonia–Coal Reaction Products Based on Closed 0D Homogeneous Reactor
3.3. Nitrogen Containing Products of Ammonia–Coal Co-Combustion Based on Entrained Flow Reactor
3.4. ROP Analysis of Ammonia–Coal Reaction Products Based on Entrained Flow Reactor
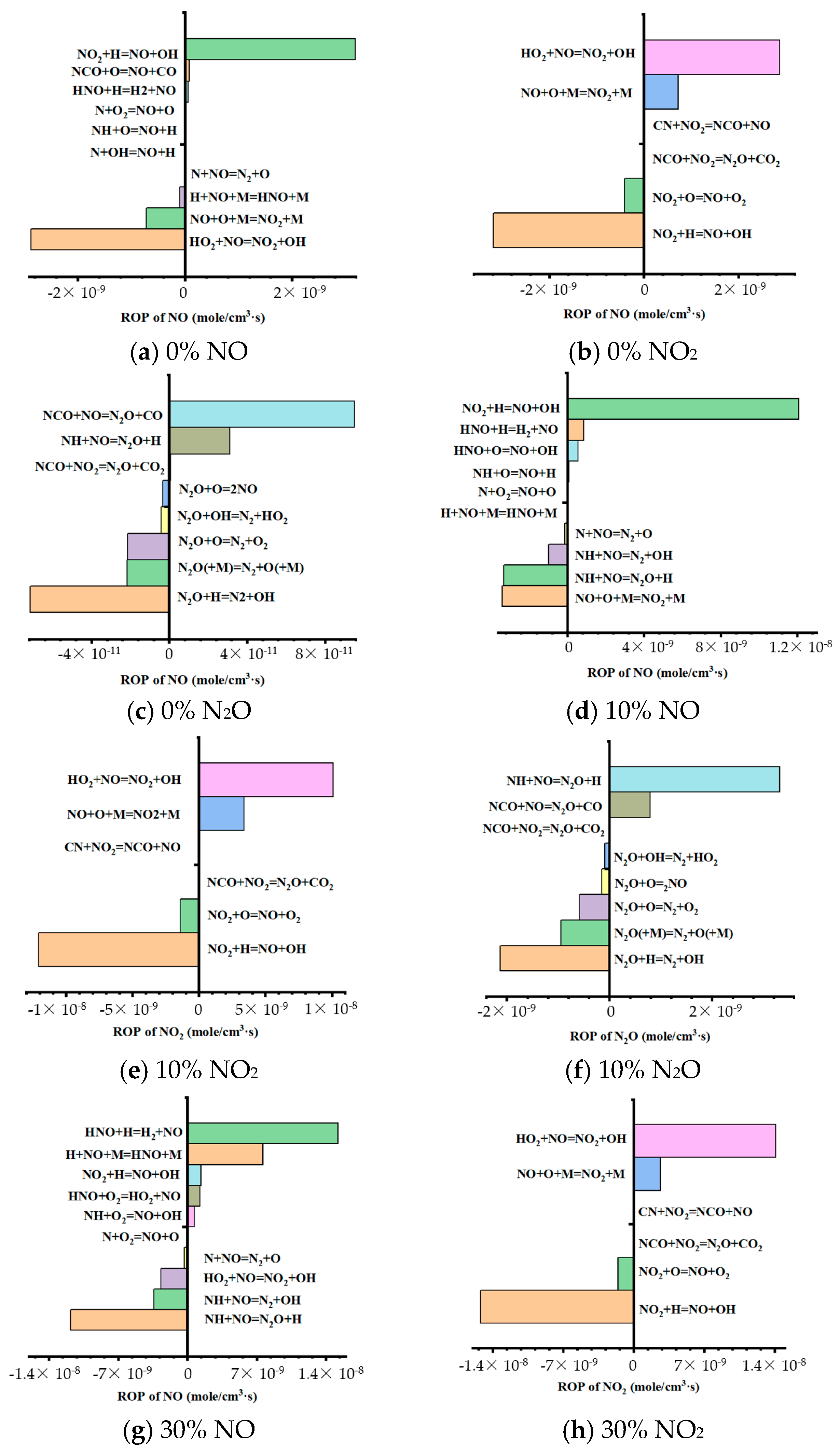
3.5. Simplified NOx Formation Mechanisms of Ammonia–Coal Co-Combustion
4. Conclusions
- (1)
- The co-firing ratio shows a significant effect on the formation of NOx in the ammonia–coal co-firing processes. In the zero-dimensional homogeneous reaction process, with the increase in co-firing ratio, the molar concentrations of NO2 and N2O increase, whereas the molar concentration of NO increases first and then decreases. In the entrained flow reaction process, the total emission of NOx increases with the rise in co-firing ratio, where the stable emission concentration of NO increases first and then decreases, reaching the maximum at 30% co-firing ratio. However, the stable emission concentration of N2O continues to increase monotonously.
- (2)
- The formation of N2O mainly depends on the reduction in NH and NO. With the increase in co-firing ratio, the unreacted NH promotes the reduction in NO to N2O, while the H radical produced by the reaction hinders the formation of NO. At 1273 K, N2O is mainly derived from the reduction in NO, where NH + NO = N2O + H is the primary formation reaction of N2O.
- (3)
- The formation of intermediates such as NNH and NCO is essential to the transformation of NOx. In order to increase the conversion efficiency of nitrogen source to N2 and inhibit the formation of NOx, the formation of NNH intermediates should be promoted as much as possible, while the formation of NCO intermediates should be reduced. In addition, in order to reduce the formation of HCN, the dominant precursor of NOx, the decoupling combustion method can be used to reduce the contact between volatiles and ammonia.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Li, C.Q.; Fang, Y.J.; Liu, P.; Peng, T.D. The paradigm shift in China’s energy consumption: A multi-timescale interpretation of coal consumption fluctuations. Energy Convers. Manag. 2025, 341, 120074. [Google Scholar] [CrossRef]
- Zhang, W.Z.; Liu, X.; Zhang, C.Q.; Li, M.; Niu, T.; Xie, Y.; Wang, H.Y. Industrial scale testing on the combustion and NOx emission characteristics of ammonia cofiring in a 40 MWth coal-fired boiler. Fuel 2024, 359, 130471. [Google Scholar] [CrossRef]
- Sun, M.W.; Ling, Z.Q.; Mao, J.N.; Zeng, X.Y.; Yuan, D.K.; Liu, M.S. Ammonia-based clean energy systems: A review of recent progress and key challenges. Energies 2025, 18, 2845. [Google Scholar] [CrossRef]
- Hu, F.; Li, P.; Wang, K.; Li, W.H.; Guo, J.J.; Liu, L.; Liu, Z.H. Evaluation, development, and application of a new skeletal mechanism for fuel-NO formation under air and oxy-fuel combustion. Fuel Process. Technol. 2020, 199, 106256. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Y.; Liu, X.; Yu, R.H.; Xie, Z.C.; Zhang, K.; Xu, J.Y.; Xu, M.H. Experimental and numerical study on the effects of coal on ammonia-N conversion behavior during ammonia-coal co-firing. Fuel 2024, 364, 131032. [Google Scholar] [CrossRef]
- Chen, P.; Wang, Y.; Wang, P.; Gu, M.Y.; Jiang, B.Y.; Luo, K.; Fan, J.R.; Wang, Y. Oxidation mechanism of ammonia-N/coal-N during ammonia-coal co-combustion. Int. J. Hydrogen Energy 2022, 47, 35498–35514. [Google Scholar] [CrossRef]
- Wargadalam, V.J.; Löffler, G.; Winter, F.; Hofbauer, H. Homogeneous formation of NO and N2O from the oxidation of HCN and NH3 at 600–1000 °C. Combust. Flame 2000, 120, 465–478. [Google Scholar] [CrossRef]
- Hong, D.K.; Yuan, L.; Wang, C.B. Competition between NH3-O2 reaction and char-O2 reaction and its influence on NO generation and reduction during char/NH3 co-combustion: Reactive molecular dynamic simulations. Fuel 2022, 324, 124666. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, J.G.; Lyu, Q.G.; Liu, J.Z.; Pan, F.; Zhang, J.H. The ultra-low NOx emission characteristics of pulverized coal combustion after high temperature preheating. Fuel 2020, 277, 118050. [Google Scholar] [CrossRef]
- Cai, L.G.; Shang, X.; Gao, S.Q.; Wang, Y.; Dong, L.; Xu, G.W. Low-NOx coal combustion via combining decoupling combustion and gas reburningLow-NOx coal combustion via combining decoupling combustion and gas reburning. Fuel 2013, 112, 695–703. [Google Scholar] [CrossRef]
- Li, C.Z.; Tan, L.L. Formation of NOx and SOx precursors during the pyrolysis of coal and biomass. Part III. Further discussion on the formation of HCN and NH3 during pyrolysis. Fuel 2000, 79, 1899–1906. [Google Scholar] [CrossRef]
- Chen, P.; Fang, Y.; Wang, P.P.; Gu, M.Y.; Luo, K.; Fan, J.R. The effect of ammonia co-firing on NO heterogeneous reduction in the high-temperature reduction zone of coal air-staging combustion: Experimental and quantum chemistry study. Combust. Flame 2022, 237, 111857. [Google Scholar] [CrossRef]
- Jiao, A.Y.; Zhou, Z.N.; Yang, X.C.; Xu, H.T.; Liu, F.; Liao, X.W.; Liu, J.X.; Jiang, X.M. The crucial role of oxygen in NO heterogeneous reduction with NH3 at high temperature. Energy 2023, 284, 129272. [Google Scholar] [CrossRef]
- Du, B.; Huang, W.; Kuai, N.; Yuan, J.J.; Li, Z.S.; Gan, Y. Experimental investigation on inerting mechanism of dust explosion. Procedia Eng. 2012, 43, 338–342. [Google Scholar] [CrossRef]
- Jia, M.C.; Su, S.; He, L.M.; Chen, Y.F.; Xu, K.; Jiang, L.; Xu, J.; Wang, Y.; Hu, S.; Xiang, J. Experimental and density functional theory study on role of calcium in NO reduction by NH3 on char surface during ammonia co-firing with pulverized coal. Energy 2023, 285, 129484. [Google Scholar] [CrossRef]
- Ding, X.; Li, W.; Liu, P.; Kang, Z.Z. Numerical calculation on combustion process and NO transformation behavior in a coal-fired boiler blended ammonia: Effects of the injection position and blending ratio. Int. J. Hydrogen Energy 2023, 48, 29771–29785. [Google Scholar] [CrossRef]
- Chen, P.; Gong, C.; Hua, C.; Wang, P.; Gu, M.; Luo, K.; Fan, J.; Wang, Y. Experimental and mechanism study on NO formation characteristics and N chemical reaction mechanism in ammonia-coal co-firing. Fuel 2024, 360, 130539. [Google Scholar] [CrossRef]
- Dai, L.; Wang, C.A.; Zhang, T.; Li, Y.; Wang, C.; Liu, J.; Gao, X.; Che, D. Simulation study on nitrogen transformation characteristics of NH3/coal co-firing under deeply air-staged condition. J. Energy Inst. 2024, 114, 101613. [Google Scholar] [CrossRef]
- Cai, Z.H.; Huang, M.M.; Wei, G.F.; Liu, Z.; Zhang, H.; Hao, Q.; Yu, Z. Theoretical study on the inherent relationship between laminar burning velocity, ignition delay time, and NO emission of ammonia-syngas-air mixtures under gas turbine conditions. Int. J. Hydrogen Energy 2024, 50, 690–705. [Google Scholar] [CrossRef]
- Watanabe, J.; Yamamoto, K. Flamelet model for pulverized coal combustion. Proceed. Combust. Inst. 2015, 35, 2315–2322. [Google Scholar] [CrossRef]
- Wen, X.; Luo, Y.; Luo, K.; Jin, H.; Fan, J. LES of pulverized coal combustion with a multi-regime flamelet model. Fuel 2017, 188, 661–671. [Google Scholar] [CrossRef]
- Gas Research Institute. GRI-Mech 3.0: An Optimized Detailed Chemical Reaction Mechanism for Methane Combustion. 2002. Available online: http://combustion.berkeley.edu/gri-mech/version30/files30/grimech30.dat (accessed on 30 October 2002).
- Zhang, X.Q.; Zhao, S.W.; Zhang, Q.S.; Wang, Y.J.; Zhang, J. A review of ammonia combustion reaction mechanism and emission reduction strategies. Energies 2025, 18, 1707. [Google Scholar] [CrossRef]
- Alnasif, A.; Mashruk, S.; Hayashi, M.; Jójka, J.; Shi, H.; Hayakawa, A.; Valera-Medina, A. Performance investigation of currently available reaction mechanisms in the estimation of NO measurements: A comparative study. Energies 2023, 16, 3847. [Google Scholar] [CrossRef]
- Grassi, G.; Vervisch, L.; Domingo, P. Reduced chemistry for numerical combustion of NH3/H2 fuel blend. Combust. Flame 2025, 279, 114287. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, X.; Yu, J.; Ni, Y.; Lin, R.; Wang, X.; Feng, H. Combustion characteristics and nitrogen conversion mechanism in ammonia/coal co-firing process. Int. J. Hydrogen Energy 2024, 69, 317–330. [Google Scholar] [CrossRef]
- Zhou, S.K.; Cui, B.C.; Yang, W.J.; Tan, H.; Wang, J.; Dai, H.; Li, L.; Rahman, Z.U.; Wang, X.; Deng, S.; et al. An experimental and kinetic modeling study on NH3/air, NH3/H2/air, NH3/CO/air, and NH3/CH4/air premixed laminar flames at elevated temperature. Combust. Flame 2023, 248, 112536. [Google Scholar] [CrossRef]
- Guo, L.; Yu, C.Y.; Sun, W.C.; Zhang, H.; Cheng, P.; Yan, Y.; Lin, S.; Zeng, W.; Zhu, G.; Jiang, M. Study on effects of ethylene or acetylene addition on the stability of ammonia laminar diffusion flame by optical diagnostics and chemical kinetics. Appl. Energy 2024, 362, 123032. [Google Scholar] [CrossRef]
- Miller, J.A.; Glarborg, P. Modeling the thermal de-NOx process: Closing in on a final solution. Int. J. Chem. Kinet. 1999, 31, 757–765. [Google Scholar] [CrossRef]
- Chen, P.; Hua, C.; Jiang, B.; Gu, M.; Ge, Z.; Zhang, M.; Luo, K.; Fan, J.; Wang, Y. Influence mechanism of ammonia mixing on NO formation characteristics of pulverized coal combustion and N oxidation in ammo-nia-N/coal-N. Fuel 2023, 336, 126813. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, H.; Zheng, J.F.; Yang, X.; Marias, F.; Zhang, Y.; Wang, F. Role of coal ash minerals and unburned carbon in NH3; conversion during ammonia-coal co-firing: Mechanistic insights into reactions with O2 and NO. Chem. Eng. J. 2025, 521, 166573. [Google Scholar] [CrossRef]
- Chen, P.; Jiang, B.Y.; Gu, M.Y.; Luo, K.; Fan, J.; Wang, Y. Studying the mechanism and impact of H2O-rich atmosphere on N oxidation during ammonia combustion and ammonia-coal co-combustion. Fuel 2023, 352, 129092. [Google Scholar] [CrossRef]
- Chen, P.; Gong, C.; Hua, C.H.; Gu, M.; Jiang, B.; Fan, J.; Wang, Y. Mechanism analysis of fuel-N oxidation during ammonia-coal co-combustion: Influence of H2O. Fuel 2023, 342, 127747. [Google Scholar] [CrossRef]
- Gui, R.R.; Yan, Q.H.; Xue, T.S.; Gao, Y.; Li, Y.; Zhu, T.; Wang, Q. The promoting/inhibiting effect of water vapor on the selective catalytic reduction of NOx. J. Hazard. Mater. 2022, 439, 129665. [Google Scholar] [CrossRef] [PubMed]



| Co-Firing Ratios | Ar | C2H2 | CH4 | CO | HCN | NH3 | O2 |
|---|---|---|---|---|---|---|---|
| 0% | 0.95 | 0.0033 | 0.011 | 0.0037 | 0.00018 | 0.000076 | 0.030 |
| 10% | 0.95 | 0.0030 | 0.010 | 0.0034 | 0.00016 | 0.0033 | 0.029 |
| 30% | 0.95 | 0.0023 | 0.0081 | 0.0026 | 0.00012 | 0.0098 | 0.028 |
| 50% | 0.95 | 0.0016 | 0.0058 | 0.0019 | 0.000090 | 0.016 | 0.027 |
| Co-Firing Ratios | Ar | C2H2 | CH4 | CO | HCN | NH3 | O2 |
|---|---|---|---|---|---|---|---|
| 0% | 0.95 | 0.0033 | 0.011 | 0.0037 | 0.00018 | 0.000076 | 0.035 |
| 10% | 0.94 | 0.0030 | 0.010 | 0.0034 | 0.00016 | 0.0033 | 0.035 |
| 30% | 0.94 | 0.0023 | 0.0081 | 0.0026 | 0.00012 | 0.0098 | 0.034 |
| 50% | 0.94 | 0.0016 | 0.0058 | 0.0019 | 0.000090 | 0.016 | 0.033 |
| Co-Firing Ratios | NO | NO2 | N2O | Conversion Efficiency of NOx |
|---|---|---|---|---|
| 0% | 0.000203 | 5.39 × 10−8 | 9.23 × 10−7 | 0.804 |
| 10% | 0.00226 | 4.15 × 10−6 | 0.000766 | 0.499 |
| 30% | 0.00202 | 8.93 × 10−6 | 0.00218 | 0.233 |
| 50% | 0.00152 | 1.92 × 10−5 | 0.00346 | 0.178 |
| Reaction Number | Free Radical Reaction | A | B | E (cal) |
|---|---|---|---|---|
| 178 | N + NO = N2 + O | 2.70 × 1013 | 0 | 355 |
| 179 | N + O2 = NO + O | 9.00 × 109 | 1 | 6500 |
| 180 | N + OH = NO + H | 3.36 × 1013 | 0 | 385 |
| 181 | N2O + O = N2 + O2 | 1.40 × 1012 | 0 | 10,810 |
| 182 | N2O + O = 2NO | 2.90 × 1013 | 0 | 23,150 |
| 183 | N2O + H = N2 + OH | 3.87 × 1014 | 0 | 18,880 |
| 184 | N2O + OH = N2 + HO2 | 2.00 × 1012 | 0 | 21,060 |
| 185 | N2O(+M) = N2 + O(+M) | 7.91 × 1010 | 0 | 56,020 |
| 186 | HO2 + NO = NO2 + OH | 2.11 × 1012 | 0 | −480 |
| 187 | NO + O+M = NO2 + M | 1.06 × 1020 | −1.41 | 0 |
| 188 | NO2 + O = NO + O2 | 3.90 × 1012 | 0 | −240 |
| 189 | NO2 + H = NO + OH | 1.32 × 1014 | 0 | 360 |
| 190 | NH + O = NO + H | 4.00 × 1013 | 0 | 0 |
| 191 | NH + H = N + H2 | 3.20 × 1013 | 0 | 330 |
| 195 | NH + O2 = NO + OH | 1.28 × 106 | 1.5 | 100 |
| 198 | NH + NO = N2 + OH | 2.16 × 1013 | −0.23 | 0 |
| 199 | NH + NO = N2O + H | 3.65 × 1014 | −0.45 | 0 |
| 212 | H + NO + M = HNO + M | 4.48 × 1019 | −1.32 | 740 |
| 213 | HNO + O = NO + OH | 2.50 × 1013 | 0 | 0 |
| 214 | HNO + H = H2 + NO | 9.00 × 1011 | 0.72 | 660 |
| 215 | HNO + OH = NO + H2O | 1.30 × 107 | 1.9 | −950 |
| 216 | HNO + O2 = HO2 + NO | 1.00 × 1013 | 0 | 13,000 |
| 222 | NCO + O = NO + CO | 2.35 × 1013 | 0 | 0 |
| 228 | NCO + NO = N2O + CO | 1.90 × 1017 | −1.52 | 740 |
| 246 | CH + NO = HCN + O | 4.10 × 1013 | 0 | 0 |
| 274 | HCCO + NO = HCNO + CO | 9.00 × 1012 | 0 | 0 |
| 281 | CN + NO2 = NCO + NO | 6.16 × 1015 | −0.752 | 345 |
| 282 | NCO + NO2 = N2O + CO2 | 3.25 × 1012 | 0 | −705 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Wu, F.; Chen, G.; Cheng, W.; Han, B.; Zuo, K.; Gao, X.; Liu, J.; Liu, J. Simplified Mechanisms of Nitrogen Migration Paths for Ammonia-Coal Co-Combustion Reactions. Energies 2025, 18, 5325. https://doi.org/10.3390/en18195325
Hu Y, Wu F, Chen G, Cheng W, Han B, Zuo K, Gao X, Liu J, Liu J. Simplified Mechanisms of Nitrogen Migration Paths for Ammonia-Coal Co-Combustion Reactions. Energies. 2025; 18(19):5325. https://doi.org/10.3390/en18195325
Chicago/Turabian StyleHu, Yun, Fang Wu, Guoqing Chen, Wenyu Cheng, Baoju Han, Kexiang Zuo, Xinglong Gao, Jianguo Liu, and Jiaxun Liu. 2025. "Simplified Mechanisms of Nitrogen Migration Paths for Ammonia-Coal Co-Combustion Reactions" Energies 18, no. 19: 5325. https://doi.org/10.3390/en18195325
APA StyleHu, Y., Wu, F., Chen, G., Cheng, W., Han, B., Zuo, K., Gao, X., Liu, J., & Liu, J. (2025). Simplified Mechanisms of Nitrogen Migration Paths for Ammonia-Coal Co-Combustion Reactions. Energies, 18(19), 5325. https://doi.org/10.3390/en18195325






