High-Efficiency, Lightweight, and Reliable Integrated Structures—The Future of Fuel Cells and Electrolyzers
Abstract
1. Introduction
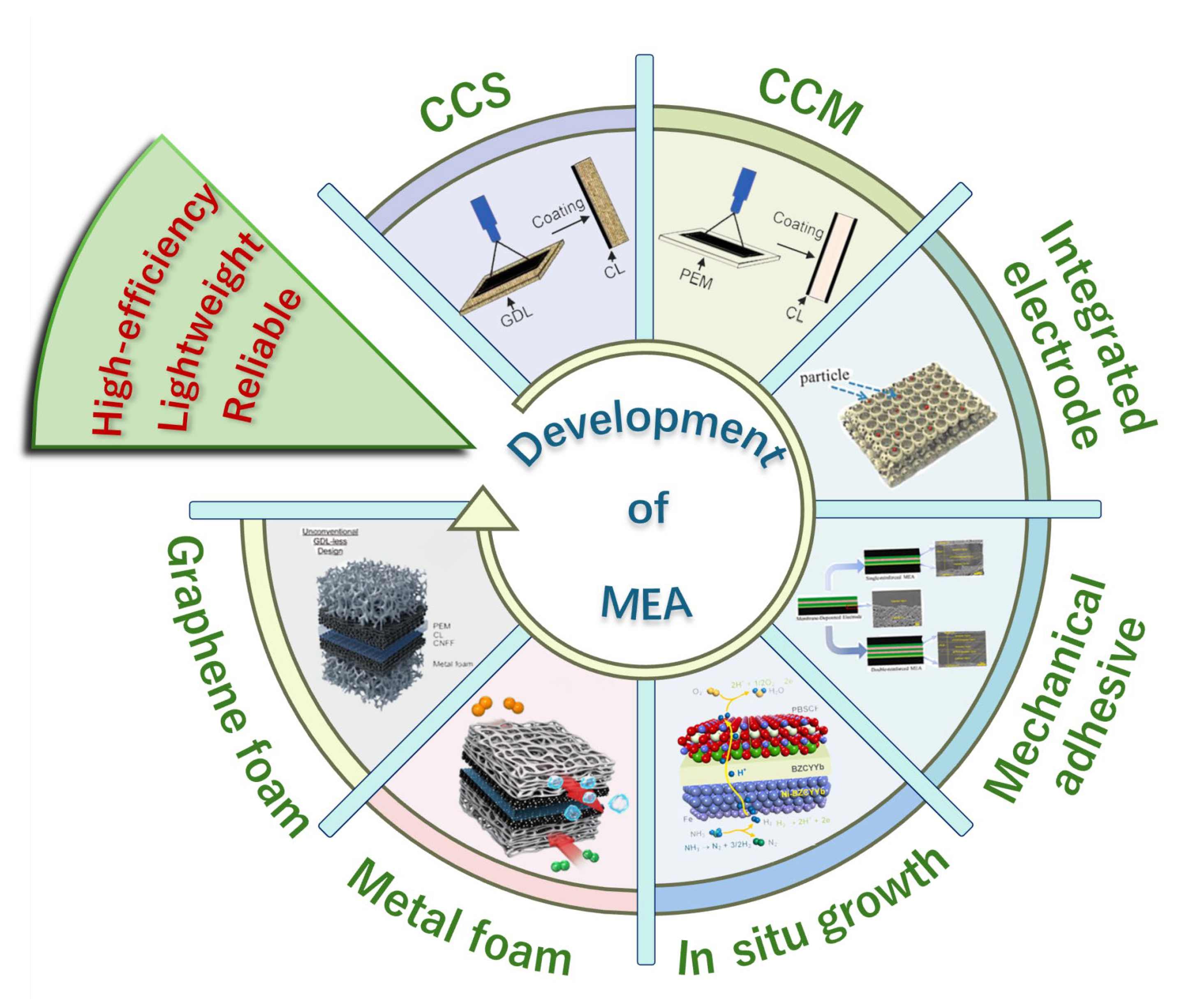
2. Integrated MEAs
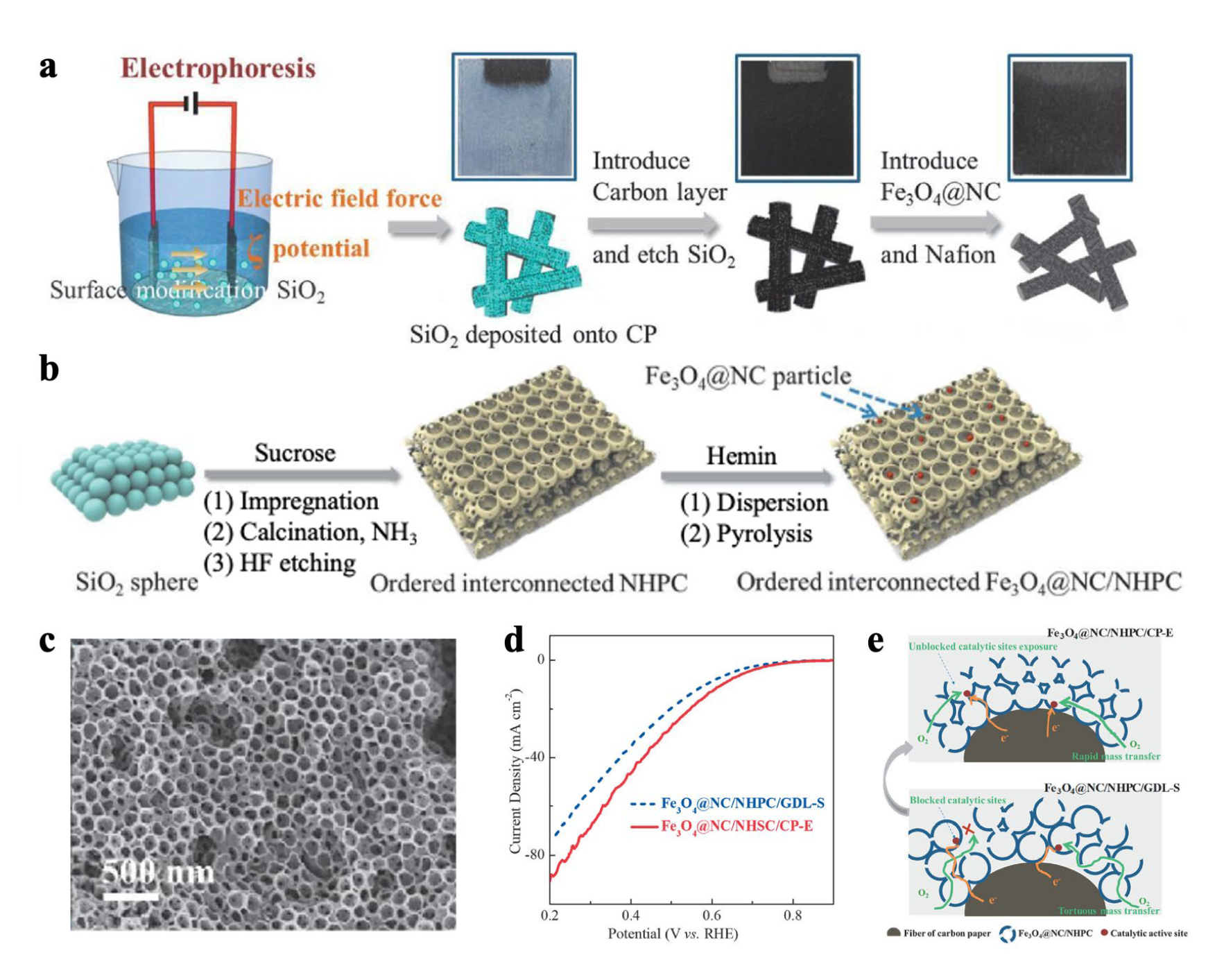
2.1. Integrated Electrode
2.2. Integrated Membrane Electrode Assemblies
2.2.1. Mechanical Adhesive Process
2.2.2. In Situ Growth
2.3. Integrated Bipolar Plate–Electrode
3. Summary and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rekker, S.; Chen, G.W.; Heede, R.; Ives, M.C.; Wade, B.; Greig, C. Evaluating fossil fuel companies’ alignment with 1.5 °C climate pathways. Nat. Clim. Change 2023, 13, 927–934. [Google Scholar] [CrossRef]
- Bergh, J.V.D.; Beers, C.V.; King, L.C. Climate activists–rethink fossil–fuel subsidy cuts. Nature 2023, 617, 465. [Google Scholar] [CrossRef] [PubMed]
- Schrotenboer, A.H.; Veenstra, A.A.; Broek, M.A.U.H.; Ursavas, E. A Green Hydrogen Energy System: Optimal control strategies for integrated hydrogen storage and power generation with wind energy. Renew. Sustain. Energy Rev. 2022, 168, 112744. [Google Scholar] [CrossRef]
- Qu, E.; Hao, X.; Xiao, M.; Han, D.; Huang, S.; Huang, Z.; Wang, S.; Meng, Y. Proton exchange membranes for high temperature proton exchange membrane fuel cells: Challenges and perspectives. J. Power Sources 2022, 533, 231386. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Ming, J.; Chen, L.; Shu, C.; Qu, T.; Tan, Q.; Liu, Y.; Asefa, T. Harvesting waste heat energy by promoting H+-ion concentration difference with a fuel cell structure. Nano Energy 2019, 57, 101–107. [Google Scholar] [CrossRef]
- Jaouen, F. Enabling low-cost and sustainable fuel cells. Nat. Mater. 2022, 21, 733–735. [Google Scholar] [CrossRef]
- Introducing the All–New Toyota MIRAI. Toyota Europe Newsroom. 2020. Available online: https://newsroom.toyota.eu/introducing–the–all–new–toyota–MIRAI/ (accessed on 2 January 2025).
- Full Specs of Toyota MIRAI 2021. Toyota USA. 2020. Available online: https://www.toyota.com/MIRAI/features/mileage_estimates/3002/3003 (accessed on 2 January 2025).
- Li, Y.; Zhu, E.; Yu, J.; Yu, J.; Yang, J.; Liu, W.; Cui, X.; Yang, X.; Zhang, Y.; Xu, M. Pt–Ni nanoparticles electrodeposited on rGO/CFP as high-performance integrated electrode for methanol oxidation. Int. J. Hydrogen Energy 2022, 47, 23957–23970. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, M.M.; Wang, K.; Chen, J.W.; Yu, T.W.; Song, S.Q. Fe3O4@N–doped interconnected hierarchical porous carbon and its 3D integrated electrode for oxygen reduction in acidic media. Adv. Sci. 2020, 7, 2000407. [Google Scholar]
- Yasutake, M.; Kawachino, D.; Noda, Z.; Matsuda, J.; Lyth, S.; Ito, K.; Hayashi, A.; Sasaki, K. Catalyst–integrated gas diffusion electrodes for polymer electrolyte membrane water electrolysis: Porous titanium sheets with nanostructured TiO2 surfaces decorated with Ir electrocatalysts. J. Electrochem. Soc. 2020, 167, 124523. [Google Scholar] [CrossRef]
- Wang, C.; Lee, K.; Liu, C.P.; Kulkarni, D.; Atanassov, P.; Peng, X.; Zenyuk, I.V. Design of PEM water electrolysers with low iridium loading. Int. Mater. Rev. 2024, 69, 3–18. [Google Scholar] [CrossRef]
- Ferner, K.J.; Litster, S. Composite anode for PEM water electrolyzers: Lowering iridium loadings and reducing material costs with a conductive addi-tive. ACS Appl. Energy Mater. 2024, 7, 8124–8135. [Google Scholar] [CrossRef] [PubMed]
- Delikaya, Ö.; Bevilacqua, N.; Eifert, L.; Kunz, U.; Zeis, R.; Roth, C. Porous electrospun carbon nanofibers network as an integrated electrode@gas diffusion layer for high temperature polymer electrolyte membrane fuel cells. Electrochim. Acta 2020, 345, 136192. [Google Scholar] [CrossRef]
- Xing, Y.; Liu, L.; Fu, Z.; Li, Y.; Li, H. Reinforced integrated membrane electrode assembly based on ionomer wet-binding and ePTFE reinforcement strategy for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2023, 50, 79–90. [Google Scholar] [CrossRef]
- Ji, Z.; Huang, Y.; Chen, Q.; An, J.; Hao, D.; Shen, Z.; Ma, C.; Wang, Y. The fabrication of integrated proton exchange membrane fuel cells: Excellent mechanical strength and electrochemical performance. Int. J. Hydrogen Energy 2024, 65, 158–163. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Liu, Y.; Kou, W.; Shen, W.; He, G. Promoting opposite diffusion and efficient conversion of polysulfides in Trap FexC–Doped asymmetric porous membranes as integrated electrodes. Chem. Eng. J. 2020, 382, 122858. [Google Scholar] [CrossRef]
- Zeng, L.; Zhao, T. Integrated inorganic membrane electrode assembly with layered double hydroxides as ionic conductors for anion exchange membrane water electrolysis. Nano Energy 2015, 11, 110–118. [Google Scholar] [CrossRef]
- Wan, L.; Pang, M.; Le, J.; Xu, Z.; Zhou, H.; Xu, Q.; Wang, B. Oriented intergrowth of the catalyst layer in membrane electrode assembly for alkaline water electrolysis. Nat. Commun. 2022, 13, 7956. [Google Scholar] [CrossRef]
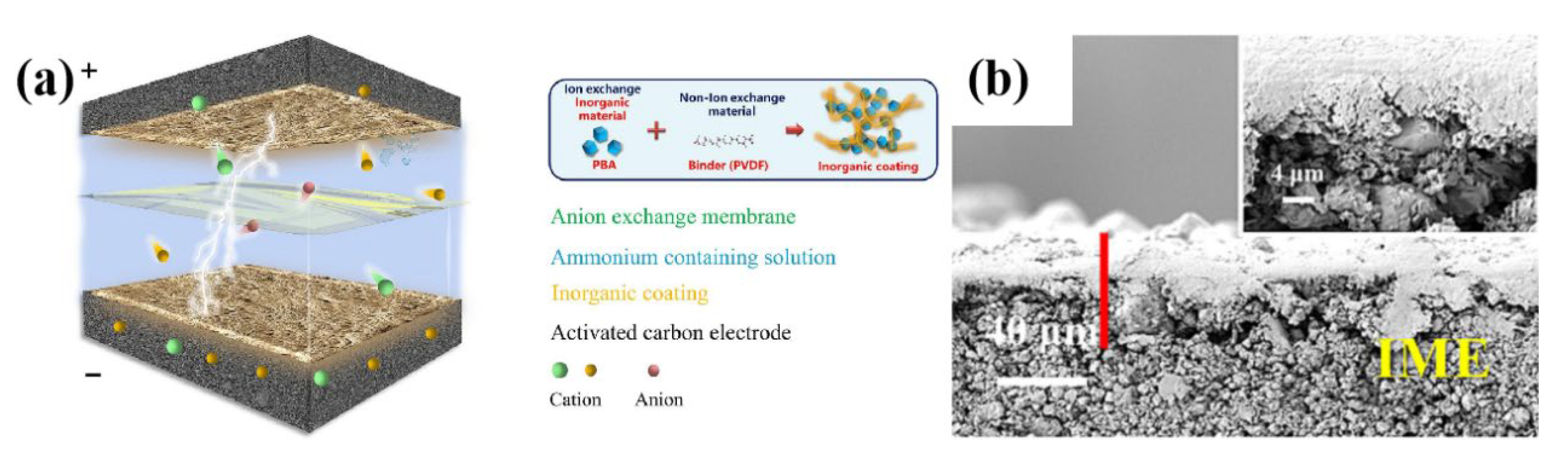
- Wang, Q.; Wu, Q.; Zhao, M.; Lu, S.; Liang, D. Prussian blue analogue based integrated membrane electrodes for desalination and selective removal of ammonium ions in a rocking-chair capacitive deionization. Chem. Eng. J. 2024, 482, 148923. [Google Scholar] [CrossRef]
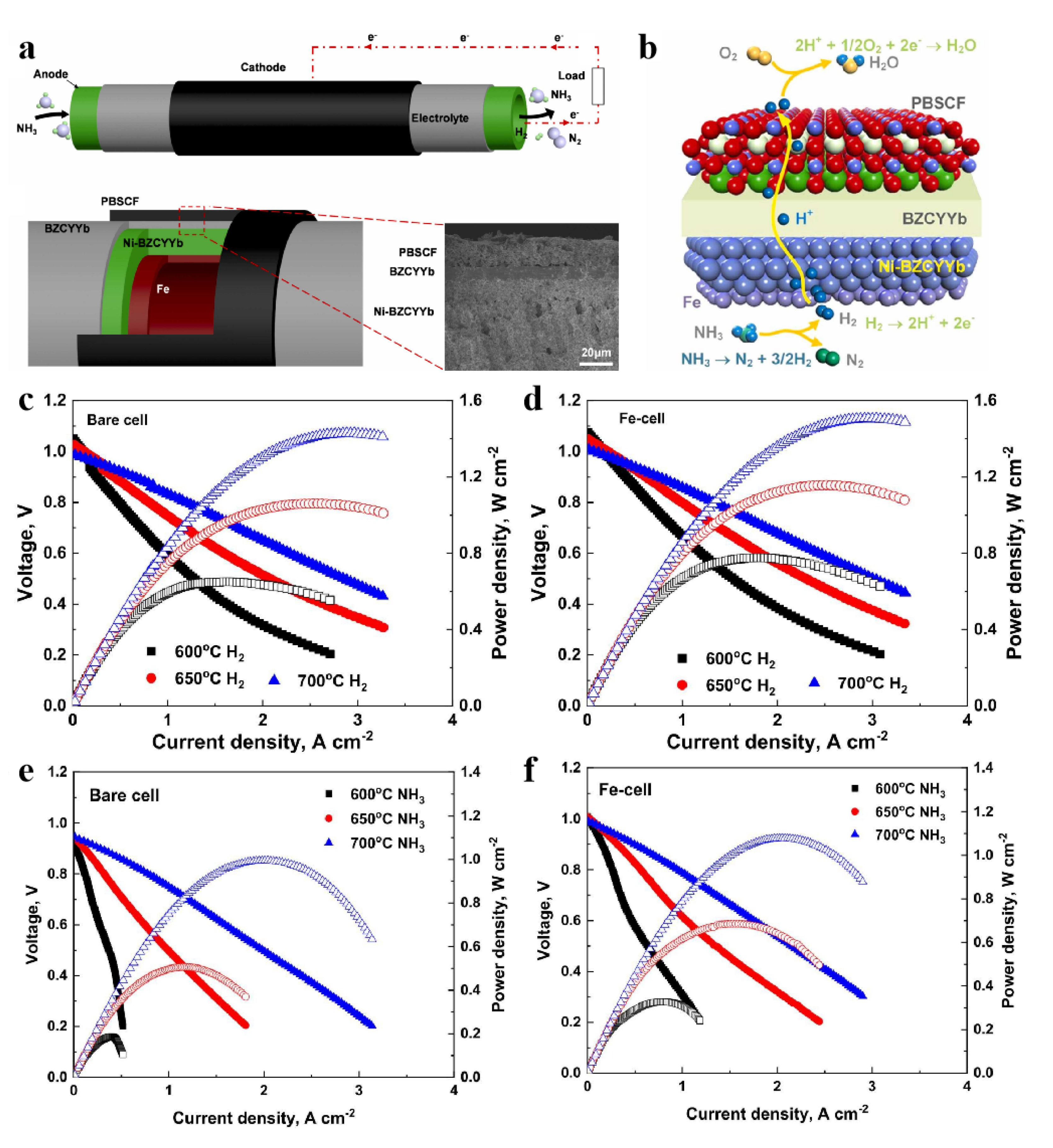
- Pan, Y.; Zhang, H.; Xu, K.; Zhou, Y.; Zhao, B.; Yuan, W.; Sasaki, K.; Choi, Y.; Chen, Y.; Liu, M. A high-performance and durable direct NH3 tubular protonic ceramic fuel cell integrated with an internal catalyst layer. Appl. Catal. B Environ. 2022, 306, 121071. [Google Scholar] [CrossRef]
- Srouji, A.; Zheng, L.; Dross, R.; Turhan, A.; Mench, M. Ultra-high current density water management in polymer electrolyte fuel cell with porous metallic flow field. J. Power Sources 2013, 239, 433–442. [Google Scholar] [CrossRef]
- Yuan, W.; Tang, Y.; Yang, X.; Wan, Z. Porous metal materials for polymer electrolyte membrane fuel cells—A review. Appl. Energy 2012, 94, 309–329. [Google Scholar] [CrossRef]
- Suo, M.; Sun, K.; Chen, R.; Che, Z.; Zeng, Z.; Li, Q.; Tao, X.; Wang, T. Oxygen transport in proton exchange membrane fuel cells with metal foam flow fields. J. Power Sources 2022, 521, 230937. [Google Scholar] [CrossRef]
- Wan, Z.; Yan, H.; Sun, Y.; Yang, C.; Chen, X.; Kong, X.; Chen, Y.; Tu, Z.; Wang, X. Thermal management improvement of air-cooled proton exchange membrane fuel cell by using metal foam flow field. Appl. Energy 2023, 333, 120642. [Google Scholar] [CrossRef]
- Sun, Y.; Lin, Y.; Wan, Z.; Wang, Q.; Yang, C.; Yin, W.; Qiu, T. Water management and performance enhancement in proton exchange membrane fuel cell through metal foam flow field with hierarchical pore structure. Chem. Eng. J. 2024, 494, 152944. [Google Scholar] [CrossRef]
- Zhang, G.; Qu, Z.; Wang, Y. Proton exchange membrane fuel cell of integrated porous bipolar plate–gas diffusion layer structure: Entire morphology simulation. eTransportation 2023, 17, 100250. [Google Scholar] [CrossRef]
- Jiao, K.; Xuan, J.; Du, Q.; Bao, Z.; Xie, B.; Wang, B.; Zhao, Y.; Fan, L.; Wang, H.; Hou, Z.; et al. Designing the next generation of proton-exchange membrane fuel cells. Nature 2021, 595, 361–369. [Google Scholar] [CrossRef]
- Tanaka, S.; Shudo, T. Corrugated mesh flow channel and novel microporous layers for reducing flooding and resistance in gas diffusion layer-less polymer electrolyte fuel cells. J. Power Sources 2014, 268, 183–193. [Google Scholar] [CrossRef]
- Park, J.E.; Lim, J.; Lim, M.S.; Kim, S.; Kim, O.-H.; Lee, D.W.; Lee, J.H.; Cho, Y.-H.; Sung, Y.-E. Gas diffusion layer/flow-field unified membrane-electrode assembly in fuel cell using graphene foam. Electrochim. Acta 2019, 323, 134808. [Google Scholar] [CrossRef]
- Tongsh, C.; Wu, S.; Jiao, K.; Huo, W.; Du, Q.; Park, J.W.; Xuan, J.; Wang, H.; Brandon, N.P.; Guiver, M.D. Fuel cell stack redesign and component integration radically increase power density. Joule 2023, 8, 175–192. [Google Scholar] [CrossRef]
- Chen, Y.; Wan, X.; Li, G.; Ye, J.; Gao, J.; Wen, D. Metal hydrogel–based integrated wearable biofuel cell for self–powered epidermal sweat biomarker monitoring. Adv. Funct. Mater. 2024, 34, 2404329. [Google Scholar] [CrossRef]
- Ahmad Kamaroddin, M.F.; Sabli, N.; Tuan Abdullah, T.A.; Siajam, S.I.; Abdullah, L.C.; Abdul Jalil, A.; Ahmad, A. Mem-brane-Based Electrolysis for Hydrogen Production: A Review. Membranes 2021, 11, 810. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pang, Y.; Xu, H.; Martinez, A.; Chen, K.S. PEM Fuel cell and electrolysis cell technologies and hydrogen infrastructure development—A review. Energy Environ. Sci. 2022, 15, 2288–2328. [Google Scholar] [CrossRef]
- Badgett, A.; Brauch, J.; Thatte, A.; Rubin, R.; Skangos, C.; Wang, X.; Ahluwalia, R.; Pivovar, B.; Ruth, M. Updated Manufactured Cost Analysis for Proton Exchange Membrane Water Electrolyzers; No. NREL/TP-6A20-87625; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2024. [Google Scholar]
- Chen, L.; Wang, Z.; Sun, C.; Zhu, H.; Xia, Y.; Hu, G.; Fang, B. Water management capacity of metal foam flow field for PEMFC under flooding situation. Micromachines 2023, 14, 1224. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Chen, M.; Zhao, Z.; Zhang, Z.; Ye, S.; Xu, S.; Wang, H.; Li, H. Bridging the gap between highly active oxygen reduction reaction catalysts and effective catalyst layers for proton exchange membrane fuel cells. Nat. Energy 2021, 6, 475–486. [Google Scholar] [CrossRef]
- Lim, K.H.; Lee, A.S.; Atanasov, V.; Kerres, J.; Park, E.J.; Adhikari, S.; Maurya, S.; Manriquez, L.D.; Jung, J.; Fujimoto, C.; et al. Protonated phosphonic acid electrodes for high power heavy-duty vehicle fuel cells. Nat. Energy 2022, 7, 248–259. [Google Scholar] [CrossRef]
- Tang, H.; Geng, K.; Wu, L.; Liu, J.; Chen, Z.; You, W.; Yan, F.; Guiver, M.D.; Li, N. Fuel cells with an operational range of −20 °C to 200 °C enabled by phosphoric acid-doped in-trinsically ultramicroporous membranes. Nat. Energy 2022, 7, 153–162. [Google Scholar] [CrossRef]
- Lee, S.; Seong, J.G.; Jo, Y.; Hwang, S.J.; Gwak, G.; Park, Y.; Kim, Y.C.; Lim, K.H.; Park, H.Y.; Jang, J.H.; et al. Self-assembled network polymer electrolyte membranes for application in fuel cells at 250 °C. Nat. Energy 2024, 9, 849–861. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Setzler, B.P.; Rojas-Carbonell, S.; Ben Yehuda, C.; Amel, A.; Page, M.; Wang, L.; Hu, K.; Shi, L.; et al. Poly(aryl piperidinium) membranes and ionomers for hydroxide exchange membrane fuel cells. Nat. Energy 2019, 4, 392–398. [Google Scholar] [CrossRef]
- Adabi, H.; Shakouri, A.; Ul Hassan, N.; Varcoe, J.R.; Zulevi, B.; Serov, A.; Regalbuto, J.R.; Mustain, W.E. High-performing commercial Fe–N–C cathode electrocatalyst for anion-exchange membrane fuel cells. Nat. Energy 2021, 6, 834–843. [Google Scholar] [CrossRef]
- Zhan, R.; Wang, Y.; Ni, M.; Zhang, G.; Du, Q.; Jiao, K. Three-dimensional simulation of solid oxide fuel cell with metal foam as cathode flow distributor. Int. J. Hydrogen Energy 2020, 45, 6897–6911. [Google Scholar] [CrossRef]


| Category | Key Method | Fabrication/Integration Approach | Electrochemical Performance | Advantages | Limitations |
|---|---|---|---|---|---|
| Integrated Electrode | Pt–Ni/rGO/CFP electrodeposited [9]; FeO4 @N–carbon 3D electrode [10]; Porous CNF network electrode [14] | Electrochemical deposition; templated growth; electrospinning+carbonization | ORR activity improved, current densities > 100 mA/cm2 in acidic media; enhanced methanol oxidation performance | High surface area, catalyst utilization, improved mass transport | Complex synthesis, scaling challenges, durability under cycling |
| Catalyst-integrated GDEs | Ti-sheet with nanostructured TiO–Ir catalyst [11]; Graphene foam GDL/flow-field unified MEA [30] | Catalyst directly integrated with porous metallic or carbon scaffold | PEM water electrolysis: >2 A/cm2 at ~1.8–2.0 V; PEMFC: ~0.7 W/cm2 | Reduced interfacial resistance, robust structure | Ir/Pt loading high; cost; long-term stability |
| Integrated MEAs (membrane–electrode) | ePTFE reinforced wet-bonding MEA [15]; Mechanical-strengthened integrated PEMFC [16]; Inorganic LDH-based AEM MEA [18]; Catalyst intergrowth MEA for AEM electrolysis [19] | Wet-bonding ionomer + ePTFE reinforcement; direct deposition; LDH as ionic conductor; in-situ catalyst layer growth | PEMFCs: peak power ~1.0–1.5 W/cm2; AEM electrolysis: >1 A/cm2 at ~1.9 V | Strong interfacial bonding, reduced ohmic loss, improved durability | Process reproducibility, complex layer control, scale-up barriers |
| Integrated Functional Membranes/Hybrid Electrodes | Trap FeₓC porous membrane as electrode [17]; Prussian blue analogue integrated MEA for desalination [20]; NH3 ceramic fuel cell with internal catalyst [21] | Asymmetric porous membranes; electrochemical intercalation electrodes; internal catalyst layer | High selectivity (desalination); NH3 ceramic FC current > 0.5 A/cm2 at 600 °C | Multi-function integration, reduced system complexity | Application-specific, materials stability, limited scalability |
| Integrated Bipolar Plate–Electrode/Flow-field | Porous metal foams as flow field [22,23,24,25,26,43]; Porous bipolar plate–GDL unified structure [27]; Corrugated mesh + MPL [29] | Metal foams, porous alloys, mesh with hierarchical pores; co-design with GDL | PEMFC: power density > 1.0 W/cm2 with improved water/thermal management; enhanced flooding mitigation | Better mass/heat transport, lighter stack, simplified design | Cost of porous metals, mechanical durability, corrosion |
| System-level Integration | Toyota Mirai stack design [7,8,28]; Joule (2024) stack redesign for high power density [37] | Compact integration of MEA, BPP, manifold redesign; thinner membranes, reduced inactive volume | Toyota Mirai: ~3.1 kW/L stack power density; Joule (2024): >6 kW/L | High stack-level efficiency, reduced system footprint | Manufacturing complexity, reliability, thermal/water management |
| Special Applications | Wearable hydrogel-integrated biofuel cell [38] | Metal hydrogel integrated with bioelectrodes | Powering biosensors continuously from sweat metabolites | Flexible, biocompatible, self-powered | Low absolute power density, niche application |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Deng, R.; Wang, Y.; Ma, C.; Shen, Z.; Shen, Y.; Holmes, S.M.; Ji, Z. High-Efficiency, Lightweight, and Reliable Integrated Structures—The Future of Fuel Cells and Electrolyzers. Energies 2025, 18, 5319. https://doi.org/10.3390/en18195319
Zhang J, Deng R, Wang Y, Ma C, Shen Z, Shen Y, Holmes SM, Ji Z. High-Efficiency, Lightweight, and Reliable Integrated Structures—The Future of Fuel Cells and Electrolyzers. Energies. 2025; 18(19):5319. https://doi.org/10.3390/en18195319
Chicago/Turabian StyleZhang, Jun, Runjin Deng, Yanyan Wang, Conggan Ma, Zhaojie Shen, Yitao Shen, Stuart M. Holmes, and Zhaoqi Ji. 2025. "High-Efficiency, Lightweight, and Reliable Integrated Structures—The Future of Fuel Cells and Electrolyzers" Energies 18, no. 19: 5319. https://doi.org/10.3390/en18195319
APA StyleZhang, J., Deng, R., Wang, Y., Ma, C., Shen, Z., Shen, Y., Holmes, S. M., & Ji, Z. (2025). High-Efficiency, Lightweight, and Reliable Integrated Structures—The Future of Fuel Cells and Electrolyzers. Energies, 18(19), 5319. https://doi.org/10.3390/en18195319






