Evaluating Fuel Properties of Strained Polycycloalkanes for High-Performance Sustainable Aviation Fuels
Abstract
1. Introduction
2. Computational and Predictive Methods
2.1. Target Polycycloalkanes and Strain Energy Calculation
2.2. Machine Learning for Fuel Property Prediction
3. Results and Discussion
3.1. Strain Energy in the Polycycloalkanes
3.2. Boiling Point and Flash Point Estimate
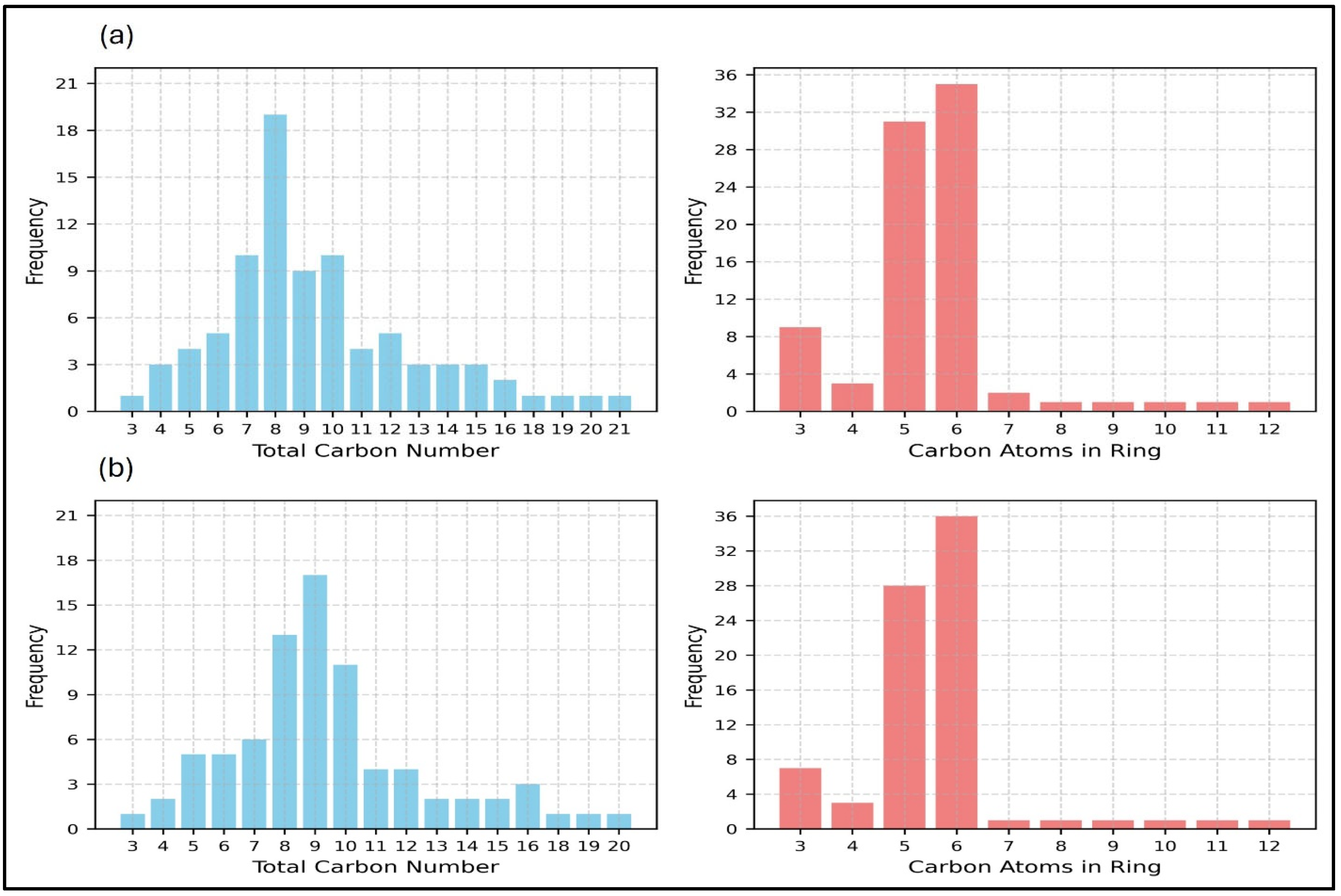
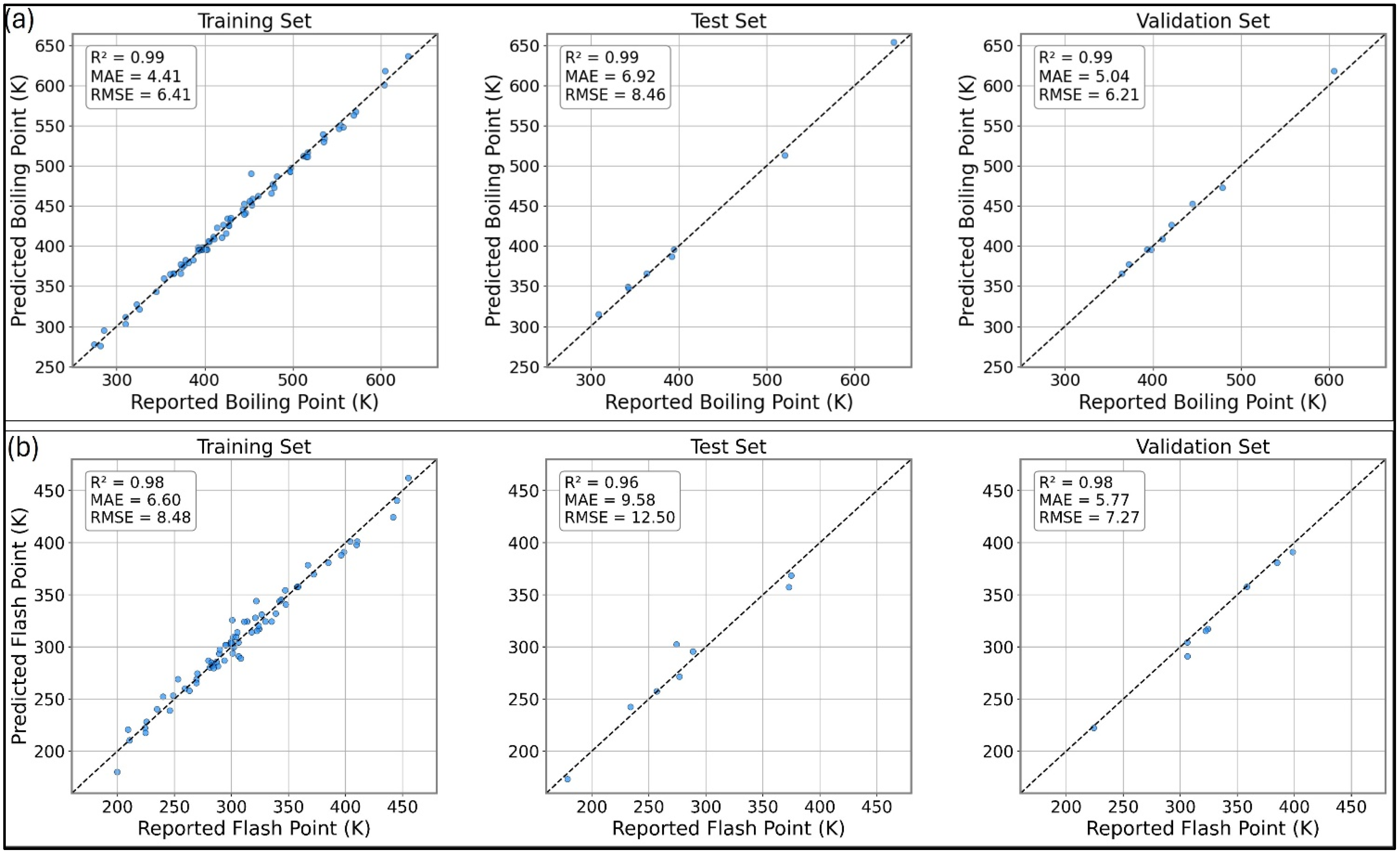
3.2.1. Property Dataset and ML Model Performance
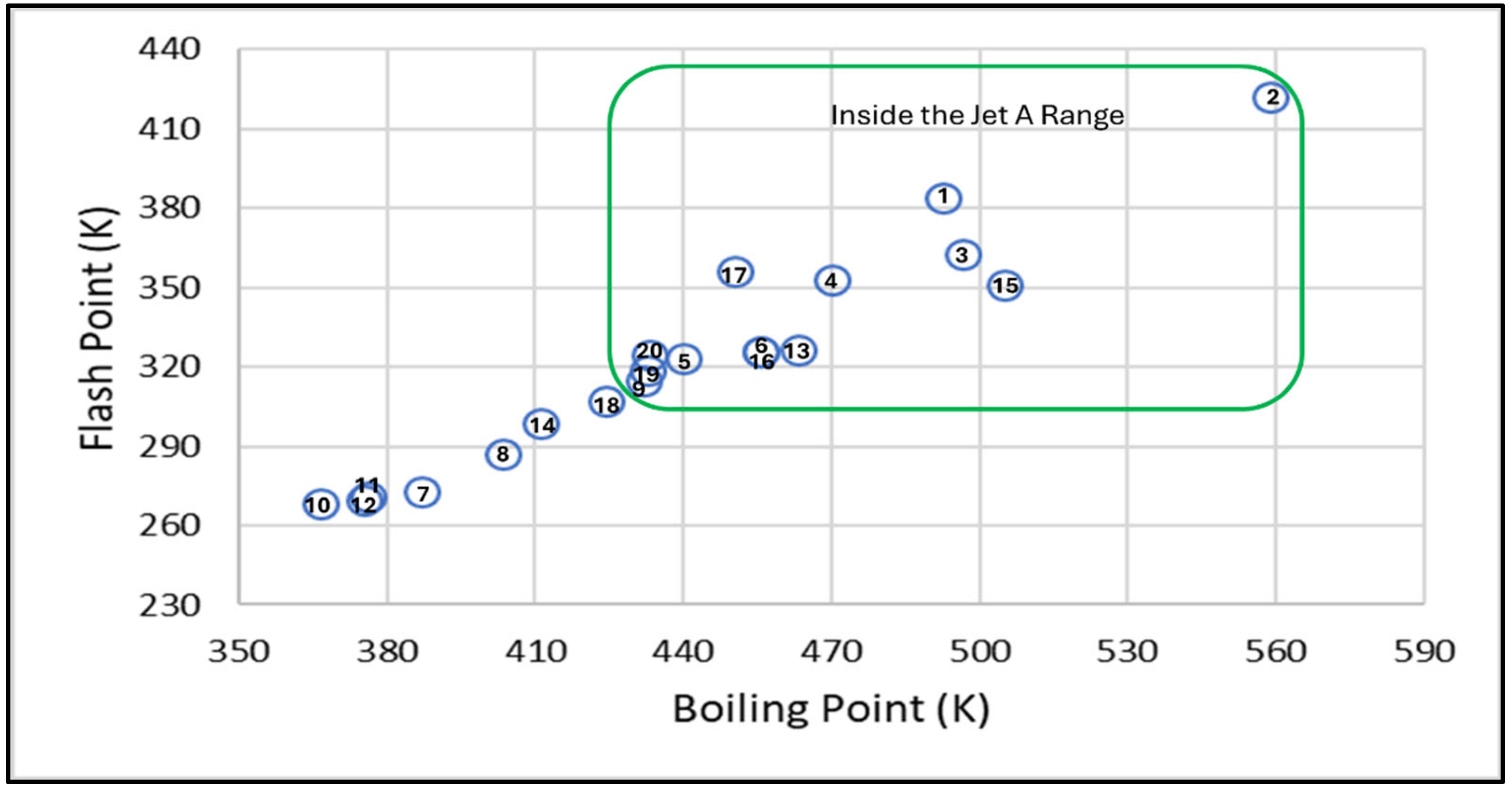
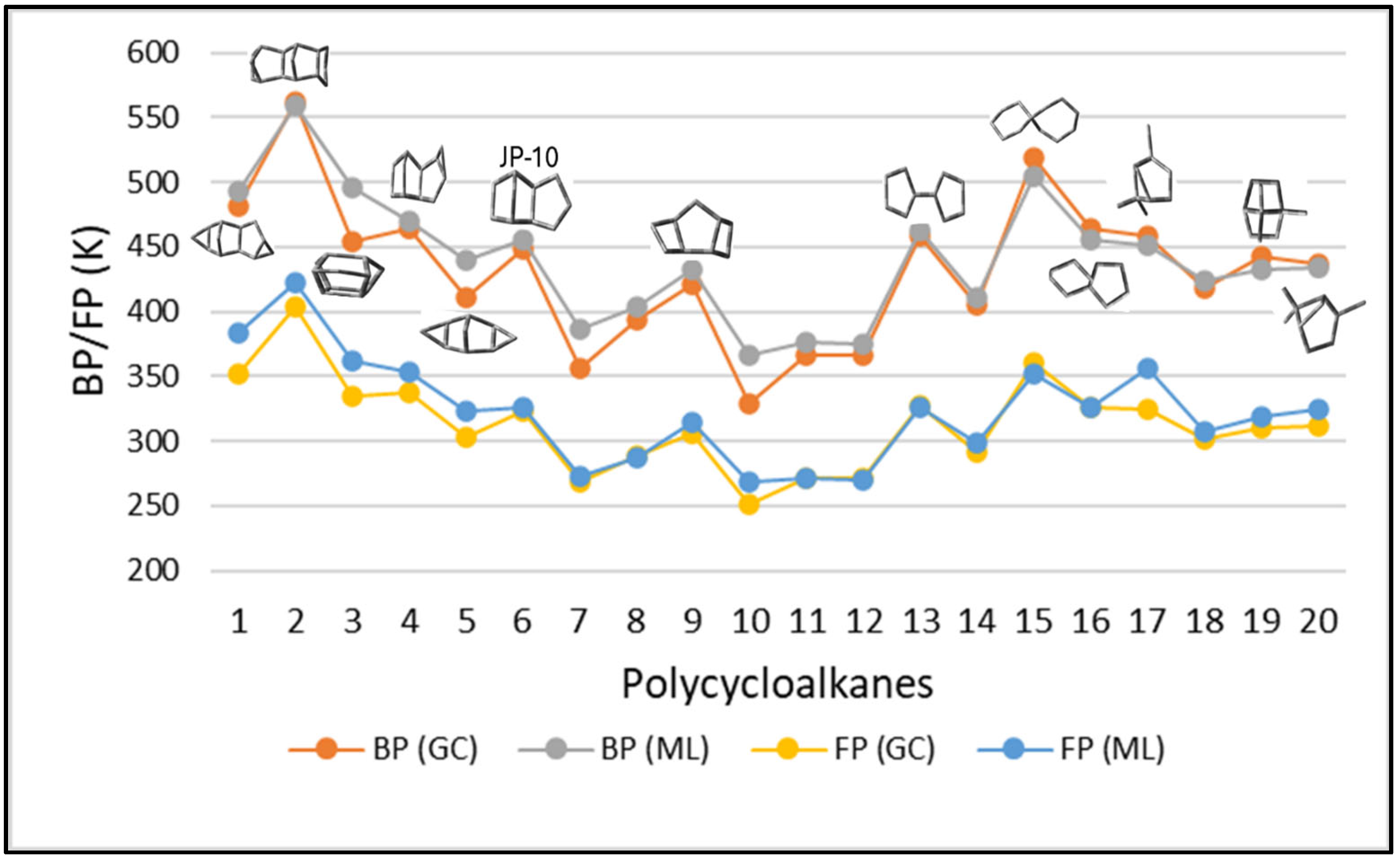
3.2.2. Structure-Property Trends in Polycycloalkanes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schapova, N. Electric Aeroplanes Are Already in Our Skies, So When Will They Become the Norm? Available online: https://www.abc.net.au/news/2024-05-05/electric-aeroplanes-aviation-industry-shrink-carbon-emissions/103796074 (accessed on 20 March 2025).
- Rijal, D.; Vasilyev, V.; Wang, F. Advancing Sustainable Aviation Fuel Design: Machine Learning for High-Energy-Density Liquid Polycyclic Hydrocarbons. Energy Fuels 2025, 39, 3243–3255. [Google Scholar] [CrossRef]
- Wang, F.; Rijal, D. Sustainable Aviation Fuels for Clean Skies: Exploring the Potential and Perspectives of Strained Hydrocarbons. Energy Fuels 2024, 38, 4904–4920. [Google Scholar] [CrossRef]
- Hart, A.; Onwudili, J.A.; Yildirir, E.; Hashemnezhad, S.E. Energy-dense sustainable aviation fuel-range hydrocarbons from cyclohexanone as a biomass-derived feedstock via sequential catalytic aldol condensation and hydrodeoxygenation. Chem. Eng. J. 2025, 509, 161494. [Google Scholar] [CrossRef]
- Kramer, S.; Andac, G.; Heyne, J.; Ellsworth, J.; Herzig, P.; Lewis, K.C. Perspectives on fully synthesized sustainable aviation fuels: Direction and opportunities. Front. Energy Res. 2022, 9, 782823. [Google Scholar] [CrossRef]
- ASTM D7566-22; Standard Specification for Aviation Turbine Fuel Containing Synthesized Hydrocarbons. ASTM International: West Conshohocken, PA, USA, 2022.
- Rosales Calderon, O.; Tao, L.; Abdullah, Z.; Moriarty, K.; Smolinski, S.; Milbrandt, A.; Talmadge, M.; Bhatt, A.; Zhang, Y.; Ravi, V. Sustainable Aviation Fuel (SAF) State-of-Industry Report: State of SAF Production Process; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2024. [Google Scholar] [CrossRef]
- Romero-Izquierdo, A.G.; Guzmán-Martínez, C.E.; Lara-Montaño, O.D.; Hernández, S.; Gutiérrez-Antonio, C. Advanced Biorefineries to Produce Sustainable Aviation Fuel. In Sustainable Aviation Fuels: Recent Advances and Future Challenges; Springer: Berlin/Heidelberg, Germany, 2025; pp. 229–249. [Google Scholar]
- John, S.U.; Onu, C.E.; Ezechukwu, C.M.-J.; Nwokedi, I.C.; Onyenanu, C.N. Multi-product biorefineries for biofuels and value-added products: Advances and future perspectives. Acad. Green Energy 2025, 2. [Google Scholar] [CrossRef]
- Kosir, S.; Heyne, J.; Graham, J. A machine learning framework for drop-in volume swell characteristics of sustainable aviation fuel. Fuel 2020, 274, 117832. [Google Scholar] [CrossRef]
- Landera, A.; Bambha, R.P.; Hao, N.; Desai, S.P.; Moore, C.M.; Sutton, A.D.; George, A. Building structure-property relationships of cycloalkanes in support of their use in sustainable aviation fuels. Front. Energy Res. 2022, 9, 771697. [Google Scholar] [CrossRef]
- Ismael, M.A.; El-Adawy, M.; Farooqi, A.S.; Hamdy, M.; Shahid, M.Z.; Elserfy, Z.; Nemitallah, M.A. Sustainable Aviation Fuel: Operational Challenges, Techno-economics, and Life Cycle Analysis. Energy Fuels 2025, 39, 13848–13878. [Google Scholar] [CrossRef]
- Battin-Leclerc, F.; Simmie, J.M.; Blurock, E. Cleaner Combustion. Developing Detailed Chemical Kinetic Models; Series: Green Energy and Technology; Springer International Publishing AG: Cham, Switzerland, 2013. [Google Scholar]
- Muldoon, J.A.; Harvey, B.G. Bio-based cycloalkanes: The missing link to high-performance sustainable jet fuels. ChemSusChem 2020, 13, 5777–5807. [Google Scholar] [CrossRef]
- Wang, F. Determination of Outer Valence Space of Norbornadiene: A Pathway to Understanding Strained Hydrocarbon Fuels. Energy Fuels 2025, 39, 3508–3516. [Google Scholar] [CrossRef]
- Wang, F.; Chong, D.P. Polycycloalkanes at the Helm: Exploring high energy density eFuel with norbornyl derivatives. Mater. Today Chem. 2024, 41, 102264. [Google Scholar] [CrossRef]
- Fang, Z.; Wen, C.; Zhang, X.; Chen, L.; Zhang, Q.; Liu, J.; Ma, L. Advance, challenge, and outlook of carbon-increasing strategies for producing sustainable high-energy-density jet fuels from lignocellulosic derivatives. Innov. Energy 2025, 2, 100093. [Google Scholar] [CrossRef]
- Rijal, D.; Vasilyev, V.; Yang, Y.; Wang, F. Insights of Density Functional Theory into JP-10 Tetrahydrodicyclopentadiene Fuel Properties. Processes 2025, 13, 543. [Google Scholar] [CrossRef]
- ASTM D4054-19; Practice for Qualification and Approval of New Aviation Turbine Fuels and Fuel Additives. ASTM International: West Conshohocken, PA, USA, 2019.
- Karton, A.; De Oliveira, M.T. Good Practices in Database Generation for Benchmarking Density Functional Theory. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2025, 15, e1737. [Google Scholar] [CrossRef]
- Rijal, D. Predicting the properties of sustainable aviation fuels. Nat. Rev. Clean Technol. 2025, 1, 312. [Google Scholar] [CrossRef]
- Sundararajan, S. Python for Data Analytics. In Multivariate Analysis and Machine Learning Techniques; Springer: Berlin/Heidelberg, Germany, 2025; pp. 29–80. [Google Scholar]
- Joback, K.G.; Reid, R.C. Estimation of pure-component properties from group-contributions. Chem. Eng. Commun. 1987, 57, 233–243. [Google Scholar] [CrossRef]
- Alibakhshi, A.; Mirshahvalad, H.; Alibakhshi, S. A modified group contribution method for accurate prediction of flash points of pure organic compounds. Ind. Eng. Chem. Res. 2015, 54, 11230–11235. [Google Scholar] [CrossRef]
- Dragojlovic, V. Conformational analysis of cycloalkanes. ChemTexts 2015, 1, 14. [Google Scholar] [CrossRef]
- Wiberg, K.B. Strained Hydrocarbons: Structures, Stability, and Reactivity; Wiley Online Library: Hoboken, NJ, USA, 2004; pp. 717–740. [Google Scholar]
- Khoury, P.R.; Goddard, J.D.; Tam, W. Ring strain energies: Substituted rings, norbornanes, norbornenes and norbornadienes. Tetrahedron 2004, 60, 8103–8112. [Google Scholar] [CrossRef]
- Rablen, P.R. A Procedure for Computing Hydrocarbon Strain Energies Using Computational Group Equivalents, with Application to 66 Molecules. Chemistry 2020, 2, 347–360. [Google Scholar] [CrossRef]
- Hearn, J.C.; Rice, B.M.; Barnes, B.C.; Chung, P.W. Predicting Hydrocarbon Strain Energy via a Group Equivalent Machine Learning Approach. J. Phys. Chem. A 2024, 128, 7489–7497. [Google Scholar] [CrossRef]
- Ponomarev, D.; Takhistov, V. What are isodesmic reactions? J. Chem. Educ. 1997, 74, 201. [Google Scholar] [CrossRef]
- Chan, B.; Collins, E.; Raghavachari, K. Applications of isodesmic-type reactions for computational thermochemistry. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2021, 11, e1501. [Google Scholar] [CrossRef]
- Dutra, F.R.; Custodio, R. Comparative assessment of the direct and isodesmic methods for pKa calculation of monocarboxylic acids using density functional theory. Comput. Theor. Chem. 2024, 1237, 114629. [Google Scholar] [CrossRef]
- Shiekh, B.A. Hierarchy of commonly used DFT methods for predicting the thermochemistry of Rh-mediated chemical transformations. ACS Omega 2019, 4, 15435–15443. [Google Scholar] [CrossRef]
- Expanding the Limit of Computation Chemistry. Gaussian 16. Available online: https://gaussian.com/gaussian16/ (accessed on 15 March 2025).
- Aviation Fuels Technical Review; Chevron Corp. 2007. Available online: https://www.chevron.com/-/media/chevron/operations/documents/aviation-tech-review.pdf (accessed on 20 March 2025).
- Rücker, G.; Rücker, C. On topological indices, boiling points, and cycloalkanes. J. Chem. Inf. Comput. Sci. 1999, 39, 788–802. [Google Scholar] [CrossRef]
- Li, R.; Herreros, J.M.; Tsolakis, A.; Yang, W. Machine learning-quantitative structure property relationship (ML-QSPR) method for fuel physicochemical properties prediction of multiple fuel types. Fuel 2021, 304, 121437. [Google Scholar] [CrossRef]
- Rumble, J. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- NIST Chemistry WebBook. Available online: http://webbook.nist.gov (accessed on 20 March 2025).
- Yaws, C.L.; Gabbula, C. Yaws" Handbook of Thermodynamic and Physical Properties of Chemical Compounds; Knovel: New York, NY, USA, 2003. [Google Scholar]
- Landrum, G. RDKit: Open-Source Cheminformatics. Available online: http://www.rdkit.org (accessed on 20 March 2025).
- Moriwaki, H.; Tian, Y.-S.; Kawashita, N.; Takagi, T. Mordred: A molecular descriptor calculator. J. Cheminform. 2018, 10, 4. [Google Scholar] [CrossRef]
- Comesana, A.E.; Huntington, T.T.; Scown, C.D.; Niemeyer, K.E.; Rapp, V.H. A systematic method for selecting molecular descriptors as features when training models for predicting physiochemical properties. Fuel 2022, 321, 123836. [Google Scholar] [CrossRef]
- Dodge, Y. The Concise Encyclopedia of Statistics; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Zhong, Y.; Liu, F.; Huang, G.; Zhang, J.; Li, C.; Ding, Y. Thermogravimetric experiments-based prediction of biomass pyrolysis behavior: A comparison of typical machine learning regression models in Scikit-learn. Mar. Pollut. Bull. 2024, 202, 116361. [Google Scholar] [CrossRef]
- Üstün, C.E.; De Freitas, R.D.S.M.; Okafor, E.C.; Shahbakhti, M.; Jiang, X.; Paykani, A. Machine Learning Applications for Predicting Fuel Ignition and Flame Properties: Current Status and Future Perspectives. Energy Fuels 2025, 39, 13281–13314. [Google Scholar] [CrossRef]
- Sun, Y.; Ding, S.; Zhang, Z.; Jia, W. An improved grid search algorithm to optimize SVR for prediction. Soft Comput. 2021, 25, 5633–5644. [Google Scholar] [CrossRef]
- Sontakke, S.A.; Lohokare, J.; Dani, R.; Shivagaje, P. Classification of cardiotocography signals using machine learning. In Proceedings of SAI Intelligent Systems Conference; Springer: Berlin/Heidelberg, Germany, 2018; pp. 439–450. [Google Scholar] [CrossRef]
- Tian, W.; Song, J.; Li, Z.; de Wilde, P. Bootstrap techniques for sensitivity analysis and model selection in building thermal performance analysis. Appl. Energy 2014, 135, 320–328. [Google Scholar] [CrossRef]
- Hetland, M.L.; Nelli, F. Activity 1: Data Analysis with Pandas, Matplotlib, and Seaborn. In Beginning Python: From Novice to Professional; Springer: Berlin/Heidelberg, Germany, 2024; pp. 487–504. [Google Scholar]
- Hall, H.; Smith, C.; Baldt, J. Enthalpies of formation of nortricyclene, norbornene, norbornadiene, and quadricyclane. J. Am. Chem. Soc. 1973, 95, 3197–3201. [Google Scholar] [CrossRef]
- Politzer, P.; Seminario, J.M. Computational determination of the structures and some properties of tetrahedrane, prismane, and some of their aza analogs. J. Phys. Chem. 1989, 93, 588–592. [Google Scholar] [CrossRef]
- Consumer Chemistry. Available online: https://wou.edu/chemistry/courses/online-chemistry-textbooks/ch105-consumer-chemistry/ch105-chapter-7/ (accessed on 20 March 2025).
- Wen, L.; Shan, S.; Lai, W.; Shi, J.; Li, M.; Liu, Y.; Liu, M.; Zhou, Z. Accelerating the Design of High-Energy-Density Hydrocarbon Fuels by Learning from the Data. Molecules 2023, 28, 7361. [Google Scholar] [CrossRef]
- Ye, Y.; Cai, J.; Tang, W.; Li, Y.; Li, D.; Li, X.; Xu, M.; Zhang, C.; Zou, J.; Yue, C. An experimental and kinetic study of quadricyclane autoignition at high temperatures. Combust. Flame 2025, 271, 113813. [Google Scholar] [CrossRef]
- Descriptor List. Available online: http://mordred-descriptor.github.io/documentation/master/descriptors.html (accessed on 25 March 2025).
- Bonchev, D.; Trinajstić, N. Information theory, distance matrix, and molecular branching. J. Chem. Phys. 1977, 67, 4517–4533. [Google Scholar] [CrossRef]
- Owens, C.B.; Mathew, N.; Olaveson, T.W.; Tavenner, J.P.; Kober, E.M.; Tucker, G.J.; Hart, G.L.; Homer, E.R. Feature engineering descriptors, transforms, and machine learning for grain boundaries and variable-sized atom clusters. npj Comput. Mater. 2025, 11, 21. [Google Scholar] [CrossRef]
- Guidechem. Cycloundecane. Available online: https://www.guidechem.com/encyclopedia/cycloundecane-dic285156.html#Properties (accessed on 20 August 2025).
- Galli, C.; Mandolini, L. The role of ring strain on the ease of ring closure of bifunctional chain molecules. Eur. J. Org. Chem. 2000, 2000, 3117–3125. [Google Scholar] [CrossRef]
- Tufail, S.; Riggs, H.; Tariq, M.; Sarwat, A.I. Advancements and challenges in machine learning: A comprehensive review of models, libraries, applications, and algorithms. Electronics 2023, 12, 1789. [Google Scholar] [CrossRef]
- Rakhimbekova, A.; Madzhidov, T.I.; Nugmanov, R.I.; Gimadiev, T.R.; Baskin, I.I.; Varnek, A. Comprehensive analysis of applicability domains of QSPR models for chemical reactions. Int. J. Mol. Sci. 2020, 21, 5542. [Google Scholar] [CrossRef]
- Baran, O.; Karathanassis, I.K.; Pickett, L.M.; Manin, J.; Gavaises, M. High-Speed Optical Imaging of Cavitation and Spray Dynamics for Sustainable Aviation Fuels. Energy Fuels 2025, 39, 11912–11925. [Google Scholar] [CrossRef]
- Slovokhotov, Y.L.; Batsanov, A.S.; Howard, J.A. Molecular van der Waals symmetry affecting bulk properties of condensed phases: Melting and boiling points. Struct. Chem. 2007, 18, 477–491. [Google Scholar] [CrossRef]
- Gundekari, S.; Manupathi, A.; Chandu, S.; Varkolu, M.; Kumar, P.; Karmee, S.K. Catalytic hydroconversion of lignin-based aromatics to aviation fuels—A review. Biomass Convers. Biorefinery 2025, 15, 6557–6583. [Google Scholar] [CrossRef]
- Dong, T.; Hao, Z.; Zhang, Y.; Zhang, Y. Mechanistic insights into the chemical structural changes of lignite on potential formation of the polycyclic aromatic hydrocarbons. Chemosphere 2022, 307, 135916. [Google Scholar] [CrossRef]
- Zarezin, D.P.; Rudakova, M.A.; Bykov, V.I.; Bermeshev, M.V. Metal chlorides supported on silica as efficient catalysts for selective isomerization of endo-tetrahydrodicyclopentadiene to exo-tetrahydrodicyclopentadiene for JP-10 producing. Fuel 2021, 288, 119579. [Google Scholar] [CrossRef]
- Shi, C.; Borchardt, T.B. JRgui: A Python program of Joback and Reid method. ACS Omega 2017, 2, 8682–8688. [Google Scholar] [CrossRef]
- Cohen, C.A.; Muessig, C.W. Jet and Rocket Fuel. Google Patents 3381046, 30 April 1968. [Google Scholar]
- Wucherer, E.; Wilson, A. Chemical, Physical and Hazards Properties of Quadricyclane. NASA. 1998. (19980205776). Available online: https://apps.dtic.mil/sti/tr/pdf/ADA345589.pdf (accessed on 2 October 2025).
- ChemSrc. Available online: https://www.chemsrc.com/en/baike/675791.html (accessed on 25 June 2025).
- PubChem. Explore Chemistry. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 20 March 2025).



| Polycycloalkanes | RS | DFT-IR | Ref | ∆ (RS—Ref) % | ∆ (DFT-IR—Ref) % |
|---|---|---|---|---|---|
| 7 (quadricyclane) | 93.48 | 78.59 | 78.70 * | 18.78 | 0.14 |
| 10 (prismane) | 134.11 | 126.69 | 128.00 # | 4.77 | 1.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rijal, D.; Vasilyev, V.; Yang, Y.; Wang, F. Evaluating Fuel Properties of Strained Polycycloalkanes for High-Performance Sustainable Aviation Fuels. Energies 2025, 18, 5253. https://doi.org/10.3390/en18195253
Rijal D, Vasilyev V, Yang Y, Wang F. Evaluating Fuel Properties of Strained Polycycloalkanes for High-Performance Sustainable Aviation Fuels. Energies. 2025; 18(19):5253. https://doi.org/10.3390/en18195253
Chicago/Turabian StyleRijal, Dilip, Vladislav Vasilyev, Yunxia Yang, and Feng Wang. 2025. "Evaluating Fuel Properties of Strained Polycycloalkanes for High-Performance Sustainable Aviation Fuels" Energies 18, no. 19: 5253. https://doi.org/10.3390/en18195253
APA StyleRijal, D., Vasilyev, V., Yang, Y., & Wang, F. (2025). Evaluating Fuel Properties of Strained Polycycloalkanes for High-Performance Sustainable Aviation Fuels. Energies, 18(19), 5253. https://doi.org/10.3390/en18195253









