Experimental Study on the Thin-Film Evaporation of Organic Solvent Droplets on Metal Surfaces
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Equipment
2.2. Preparation of Samples
2.2.1. Preparation and Characterization of Surfaces with Different Roughness
2.2.2. Preparation and Characterization of Surfaces with Different Wettability
2.3. Experimental Preparations
2.3.1. Temperature Calibration and Temperature Uniformity Verification
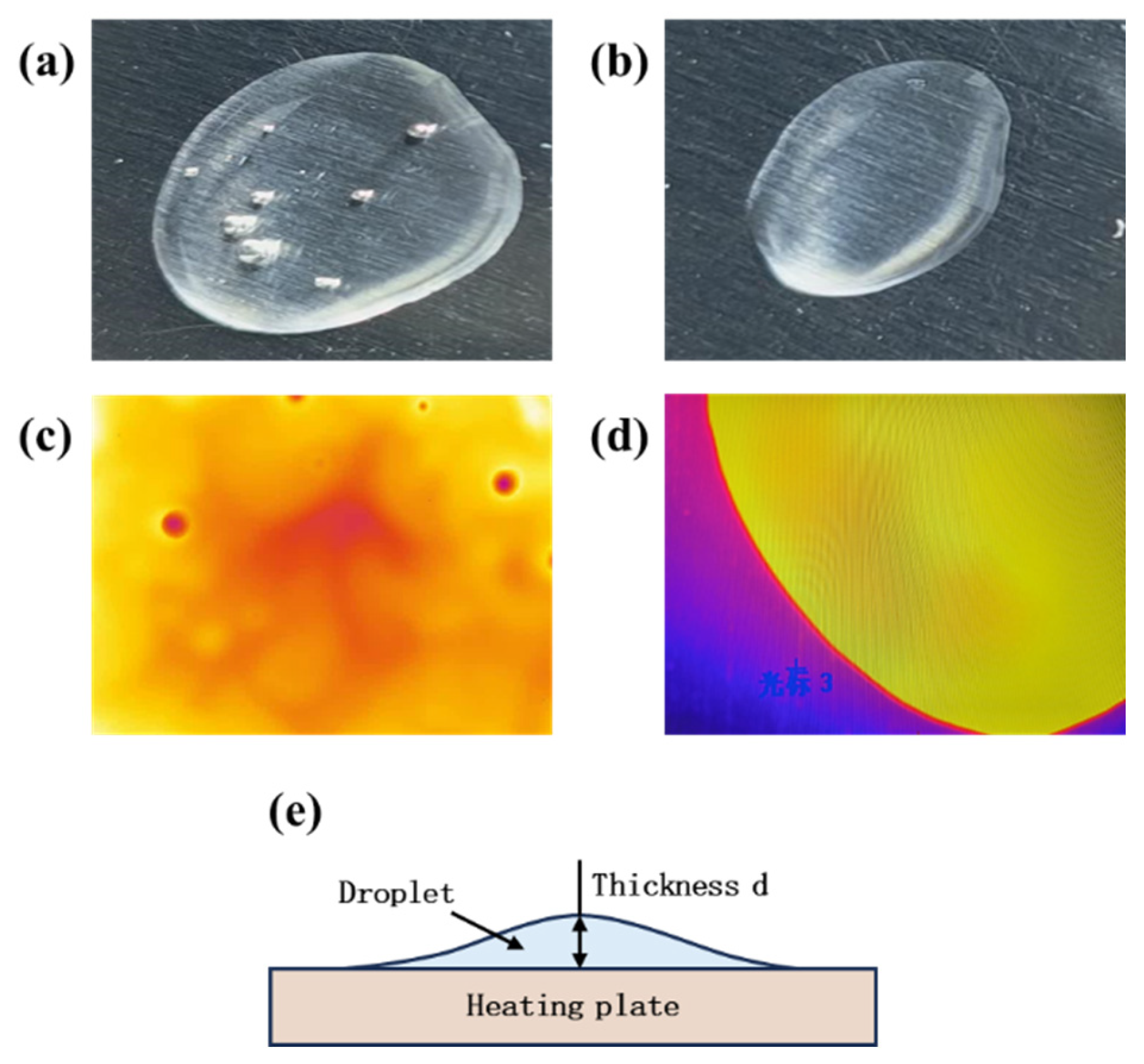
2.3.2. Measurement of the Thickness and Surface Temperature of Droplets During the Thin Film Evaporation
3. Results and Discussion
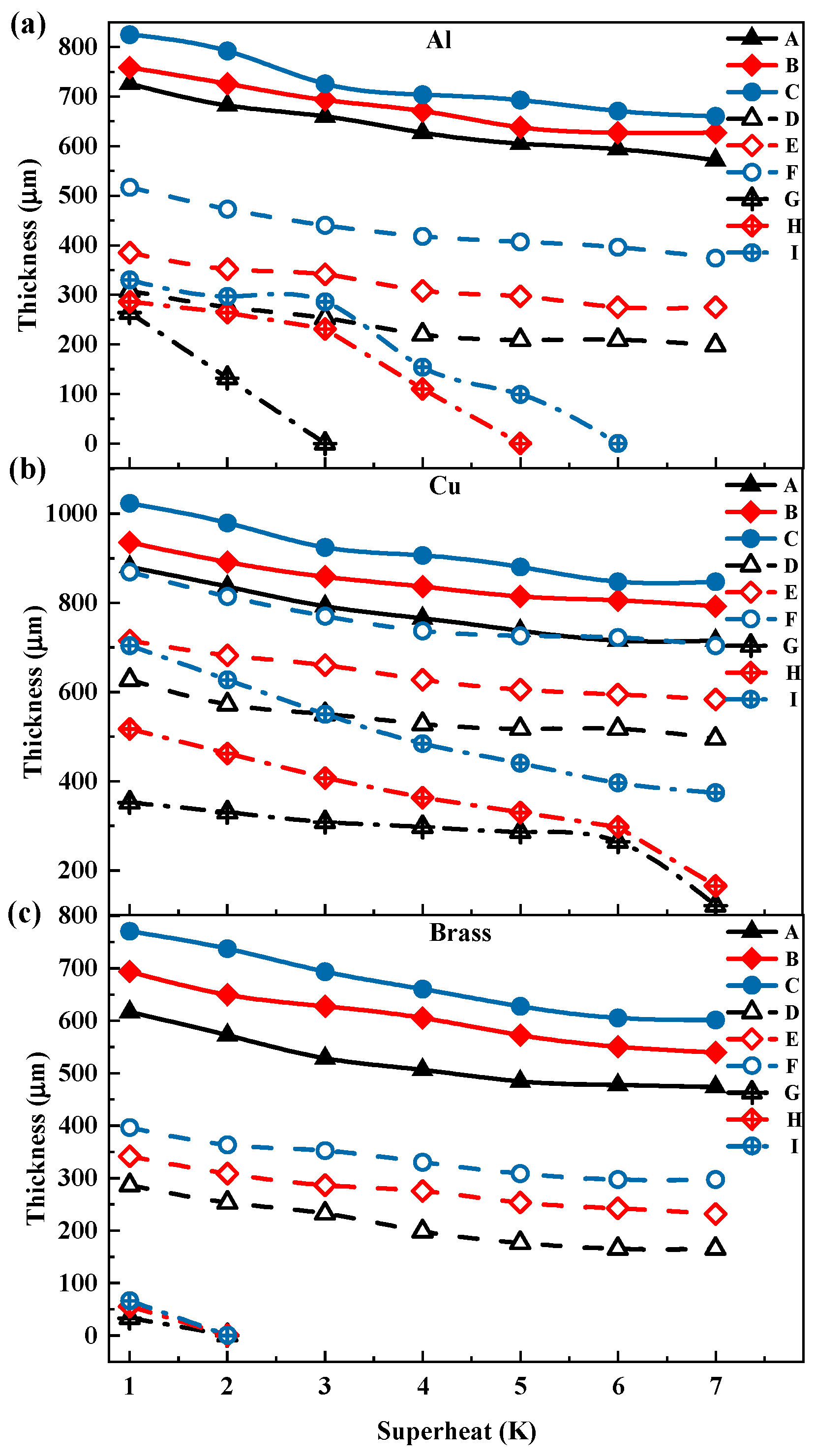
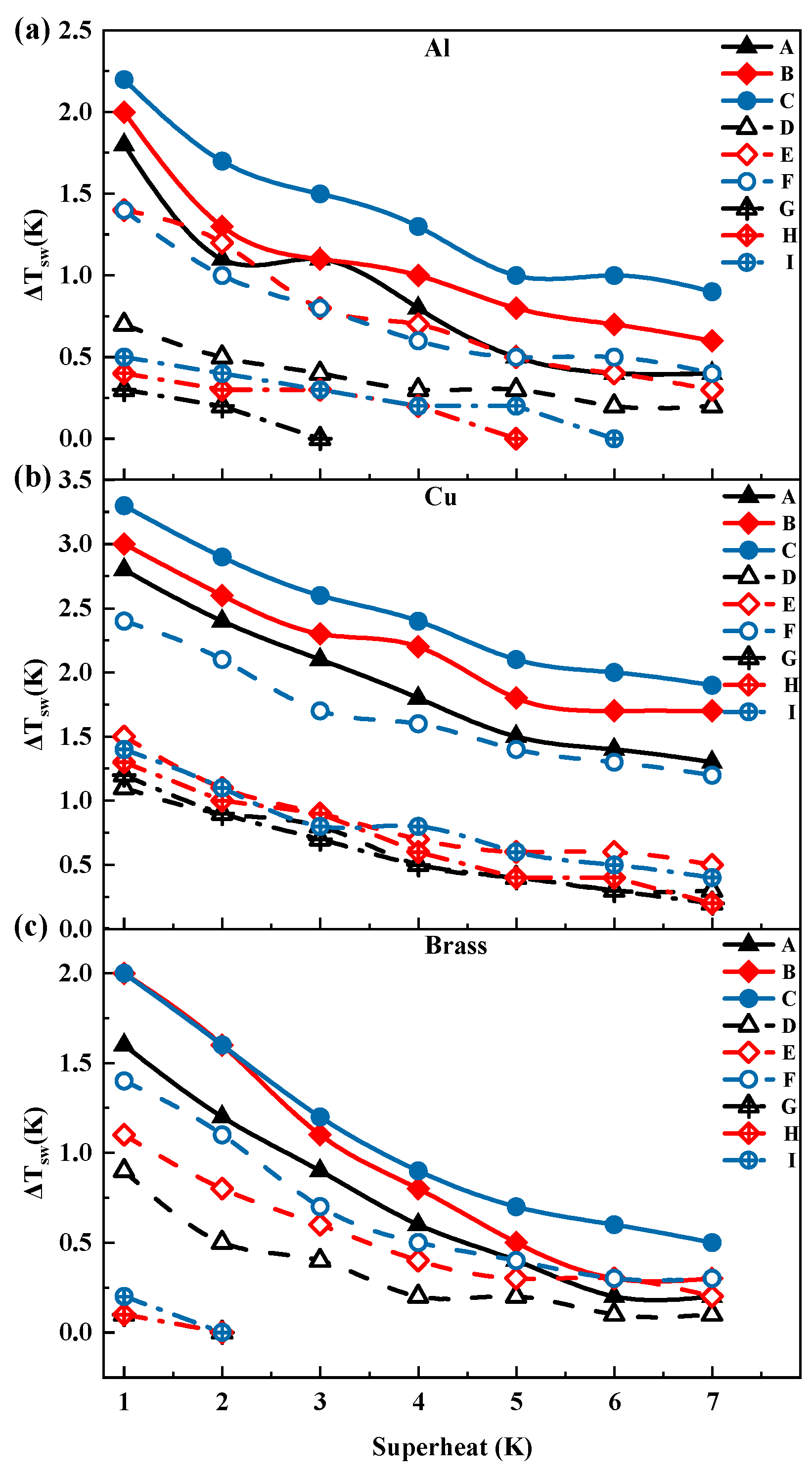
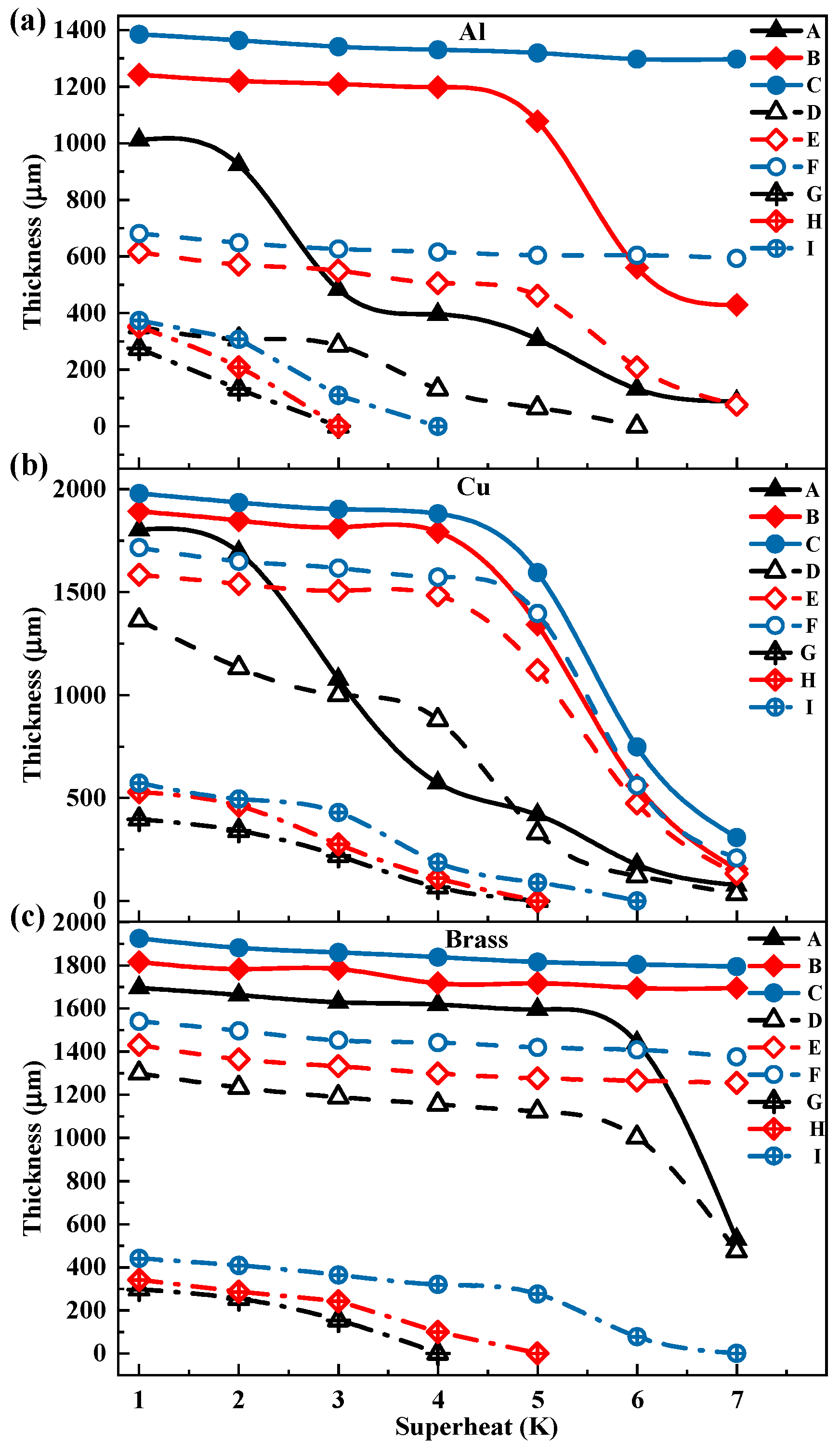
3.1. Thickness and Surface Temperature of Propylene Glycol Droplets During Film Evaporation
| Symbol | Surface Treatment | |
|---|---|---|
| A | Original | 1.51 |
| B | 0.72 | |
| C | 0.65 | |
| D | Hydrophilic | 1.51 |
| E | 0.72 | |
| F | 0.65 | |
| G | Hydrophobic | 1.51 |
| H | 0.72 | |
| I | 0.65 |
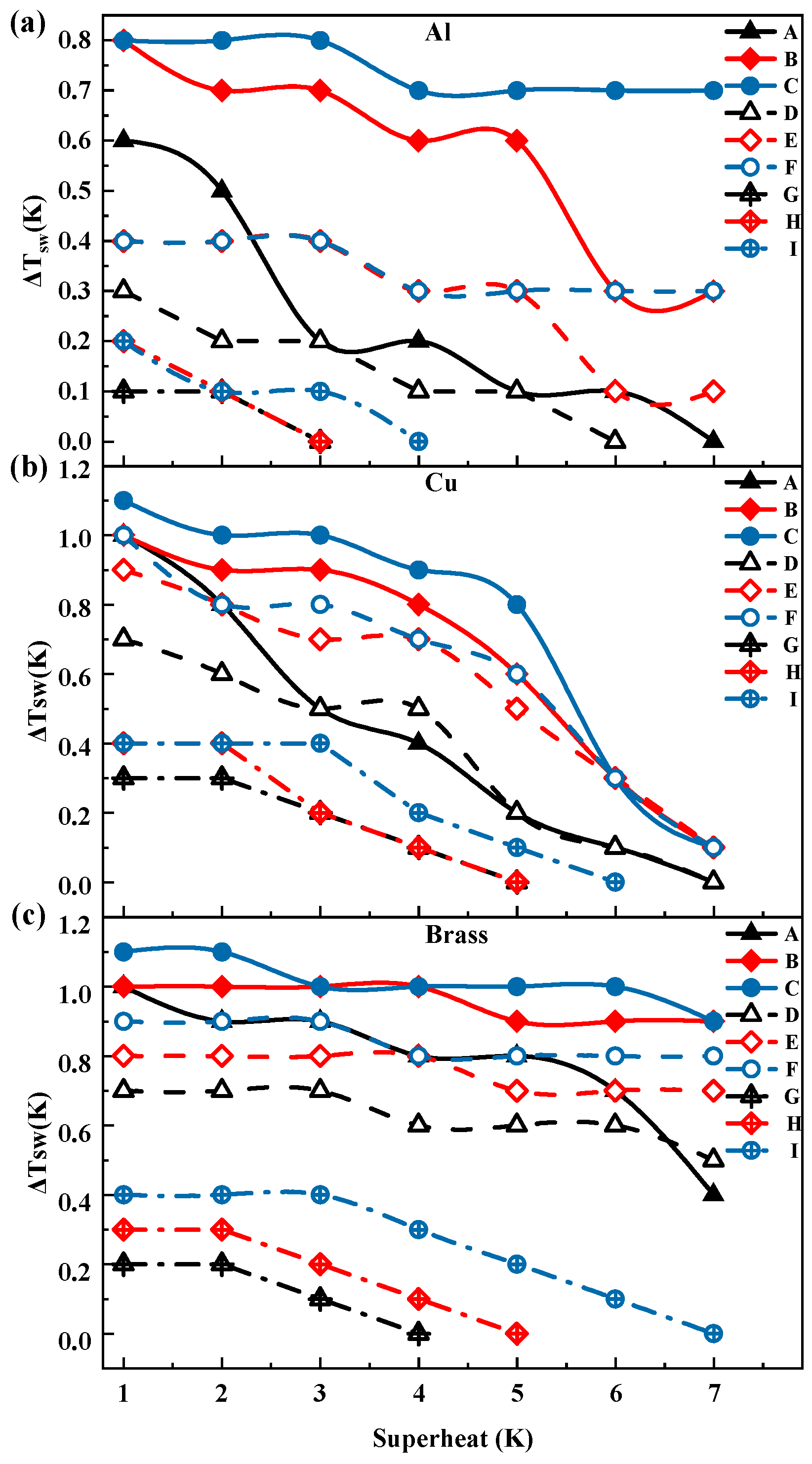
3.2. Thickness and Surface Temperature of Ethanol Droplets During Film Evaporation
4. Conclusions
- (1)
- With the gradual increase in the degree of superheating, the droplet undergoes three distinct stages during the film evaporation process. In the initial stage, as the degree of superheating increases, the reduction in droplet thickness is relatively slow. In the intermediate stage, the droplet thickness decreases sharply as the degree of superheating further increases. In the final stage, as the droplet thickness reaches a relatively low level, its rapid reduction trend begins to slow down until the film evaporation phenomenon becomes difficult to observe.
- (2)
- The effects of the heating-plate material, roughness, and wettability on droplet thickness were further investigated. First, the droplet thickness was greatest on the copper plate, while the thickness was smaller on brass or aluminum plates. Second, increase in roughness led to decrease in the overall droplet thickness. Additionally, changes in wettability also affected droplet thickness. Hydrophilic and hydrophobic treatments reduced droplet thickness, with the reduction being more pronounced under hydrophobic treatment.
- (3)
- Under different superheat conditions, the thickness of ethanol droplets and propylene glycol droplets exhibited distinct trends. In the low superheat region, the thickness of ethanol droplets was higher, whereas the opposite trend was observed in the high superheat region, indicating that ethanol is more sensitive to changes in superheat compared to propylene glycol. Additionally, under certain superheat conditions, the droplet thickness may experience a sudden drop, where an increase of 1 K in superheat can result in a thickness reduction of up to 50%.
- (4)
- When entering the thin-film evaporation state, the droplet surface temperature gradually approaches the wall temperature, exhibiting a trend consistent with the thickness variation, indicating an intrinsic correlation. Increasing surface roughness slightly elevated the overall temperature level. Compared to propylene glycol droplets, ethanol droplets exhibited a more stable variation in the surface-to-wall temperature difference in the low superheat region, but showed more pronounced changes in the high superheat region.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, X.S.; Hu, M.H.; Caneau, C.G.; Bhat, R.; Zah, C.E. Thermal management strategies for high power semiconductor pump lasers. IEEE Trans. Compon. Packag. Technol. 2006, 29, 268–276. [Google Scholar] [CrossRef]
- Zeng, J.; Wang, Q.Y.; Shi, Y.; Liu, P.; Chen, R.K. Osmotic Pumping and Salt Rejection by Polyelectrolyte Hydrogel for Continuous Solar Desalination. Adv. Energy Mater. 2019, 9, 1900552. [Google Scholar] [CrossRef]
- Gukeh, M.J.; Bao, C.B.; Mukhopadhyay, A.; Damoulakis, G.; Mazumder, S.K.; Megaridis, C.M. Air-cooled hybrid vapor chamber for thermal management of power electronics. Appl. Therm. Eng. 2023, 224, 120081. [Google Scholar] [CrossRef]
- Wang, J.X.; Yu, B.B.; Qian, C.Y.; Shi, J.Y.; Chen, J.P. Experimental study on the boiling heat transfer characteristics of a pump-driven two-phase cooling loop system for high heat flux avionics. Therm. Sci. Eng. Prog. 2023, 45, 102150. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; Wang, G.; Wei, J. Experimental study of mechanical-capillary driven phase-change loop for heat dissipation of electronic devices and batteries. Appl. Therm. Eng. 2022, 210, 118350. [Google Scholar] [CrossRef]
- Tang, H.; Tang, Y.; Wan, Z.P.; Li, J.; Yuan, W.; Lu, L.S.; Li, Y.; Tang, K.R. Review of applications and developments of ultra-thin micro heat pipes for electronic cooling. Appl. Energy 2018, 223, 383–400. [Google Scholar] [CrossRef]
- Chhokar, C.; Ashouri, M.; Bahrami, M. Modeling the thermal and hydrodynamic performance of grooved wick flat heat pipes. Appl. Therm. Eng. 2024, 257, 124281. [Google Scholar] [CrossRef]
- Bae, K.; Kang, G.; Cho, S.K.; Park, W.; Kim, K.; Padilla, W.J. Flexible thin-film black gold membranes with ultrabroadband plasmonic nanofocusing for efficient solar vapour generation. Nat. Commun. 2015, 6, 10103. [Google Scholar] [CrossRef]
- Peng, G.L.; Ding, H.R.; Sharshir, S.W.; Li, X.J.; Liu, H.C.; Ma, D.K.; Wu, L.R.; Zang, J.F.; Liu, H.; Yu, W.; et al. Low-cost high-efficiency solar steam generator by combining thin film evaporation and heat localization: Both experimental and theoretical study. Appl. Therm. Eng. 2018, 143, 1079–1084. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Zhao, F.; Guo, Y.H.; Zhang, Y.; Yu, G.H. A hydrogel-based antifouling solar evaporator for highly efficient water desalination. Energy Environ. Sci. 2018, 11, 1985–1992. [Google Scholar] [CrossRef]
- Guo, Y.H.; Zhao, F.; Zhou, X.Y.; Chen, Z.C.; Yu, G.H. Tailoring Nanoscale Surface Topography of Hydrogel for Efficient Solar Vapor Generation. Nano Lett. 2019, 19, 2530–2536. [Google Scholar] [CrossRef]
- Chen, X.F.; Zhu, Y.B.; Yu, H.; Liu, J.Z.; Easton, C.D.; Wang, Z.Y.; Hu, Y.X.; Xie, Z.L.; Wu, H.A.; Zhang, X.W.; et al. Ultrafast water evaporation through graphene membranes with subnanometer pores for desalination. J. Membr. Sci. 2021, 621, 118934. [Google Scholar] [CrossRef]
- Plawsky, J.L.; Fedorov, A.G.; Garimella, S.V.; Ma, H.B.; Maroo, S.C.; Chen, L.; Nam, Y. Nano- and Microstructures for Thin-Film Evaporation—A Review. Nanosc. Microsc. Therm. 2014, 18, 251–269. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Shi, Y.; Chen, R.K. Transition between thin film boiling and evaporation on nanoporous membranes near the kinetic limit. Int. J. Heat Mass Transf. 2020, 154, 119673. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, Y. Effect of Photolithographic Biomimetic Surface Microstructure on Wettability and Droplet Evaporation Process. Biomimetics 2024, 9, 724. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.F.; Xu, S.S.; Lee, Y.C.; Yang, R.G. Capillary-driven liquid film boiling heat transfer on hybrid mesh wicking structures. Nano Energy 2018, 51, 373–382. [Google Scholar] [CrossRef]
- Xia, G.D.; Wang, J.H.; Zhou, W.B.; Ma, D.D.; Wang, J. Orientation effects on liquid-vapor phase change heat transfer on nanoporous membranes. Int. Commun. Heat Mass Transf. 2020, 119, 104934. [Google Scholar] [CrossRef]
- Hanks, D.F.; Lu, Z.M.; Sircar, J.; Salamon, T.R.; Antao, D.S.; Bagnall, K.R.; Barabadi, B.; Wang, E.N. Nanoporous membrane device for ultra high heat flux thermal management. Microsyst. Nanoeng. 2018, 4, 1. [Google Scholar] [CrossRef]
- Xiao, R.; Maroo, S.C.; Wang, E.N. Negative pressures in nanoporous membranes for thin film evaporation. Appl. Phys. Lett. 2013, 102, 123103. [Google Scholar] [CrossRef]
- Cattani, L.; Bozzoli, F.; Ayel, V.; Romestant, C.; Bertin, Y. Experimental estimation of the local heat transfer coefficient for thin liquid film evaporation in a capillary tube. Appl. Therm. Eng. 2023, 219, 119482. [Google Scholar] [CrossRef]
- Lakew, E.; Sarchami, A.; Giustini, G.; Kim, H.; Bellur, K. Thin Film Evaporation Modeling of the Liquid Microlayer Region in a Dewetting Water Bubble. Fluids 2023, 8, 126. [Google Scholar] [CrossRef]
- Pang, B.; Yang, G.; Liu, X.; Huang, Y.; Li, W.; He, Y.; Shi, Z.; Yang, Z.; Dong, T. Experimental Study of Evaporation Characteristics of Acoustically Levitated Fuel Droplets at High Temperatures. Energies 2024, 17, 271. [Google Scholar] [CrossRef]
- Wu, G.; Nie, W.; Yang, C.; Zhou, S.; Wang, H. Experimental Study of the Effect of the Initial Droplet Diameter on the Evaporation Characteristics of Unsymmetrical Dimethylhydrazine Droplets in a Subcritical Environment. Aerospace 2024, 11, 297. [Google Scholar] [CrossRef]
- Wu, S.; Chen, L.; Jiang, S.; Guo, F.; Tao, Z. Droplet migration and early deposition of Mist CVD. Electron. Compon. Mater. 2024, 43, 454–460,467. [Google Scholar] [CrossRef]
- Dhavaleswarapu, H.K.; Garimella, S.V.; Murthy, J.Y. Microscale Temperature Measurements Near the Triple Line of an Evaporating Thin Liquid Film. J. Heat Transf. 2009, 131, 061501. [Google Scholar] [CrossRef]
- Migliaccio, C.P.; Dhavaleswarapu, H.K.; Garimella, S.V. Temperature measurements near the contact line of an evaporating meniscus V-groove. Int. J. Heat Mass Transf. 2011, 54, 1520–1526. [Google Scholar] [CrossRef]
- Ojha, M.; Chatterjee, A.; Dalakos, G.; Wayner, P.C.; Plawsky, J.L. Role of solid surface structure on evaporative phase change from a completely wetting corner meniscus. Phys. Fluids 2010, 22, 052101. [Google Scholar] [CrossRef]
- Hu, H.; Weibel, J.A.; Garimella, S.V. Role of nanoscale roughness in the heat transfer characteristics of thin film evaporation. Int. J. Heat Mass Transf. 2020, 150, 119306. [Google Scholar] [CrossRef]
- Xiaohuan, Z.; Xianbing, J.; Ye, W.; Jinliang, X. Pool boiling heat transfer on superhydrophilic and superhydrophobic surfaces. Chem. Prog. 2016, 35, 3793–3798. [Google Scholar] [CrossRef]
- Qian, B.T.; Shen, Z.Q. Fabrication of superhydrophobic surfaces by dislocation-selective chemical etching on aluminum, copper, and zinc substrates. Langmuir 2005, 21, 9007–9009. [Google Scholar] [CrossRef]
- Gregory, J. The calculation of Hamaker constants. Adv. Colloid Interface Sci. 1970, 2, 396–417. [Google Scholar] [CrossRef]
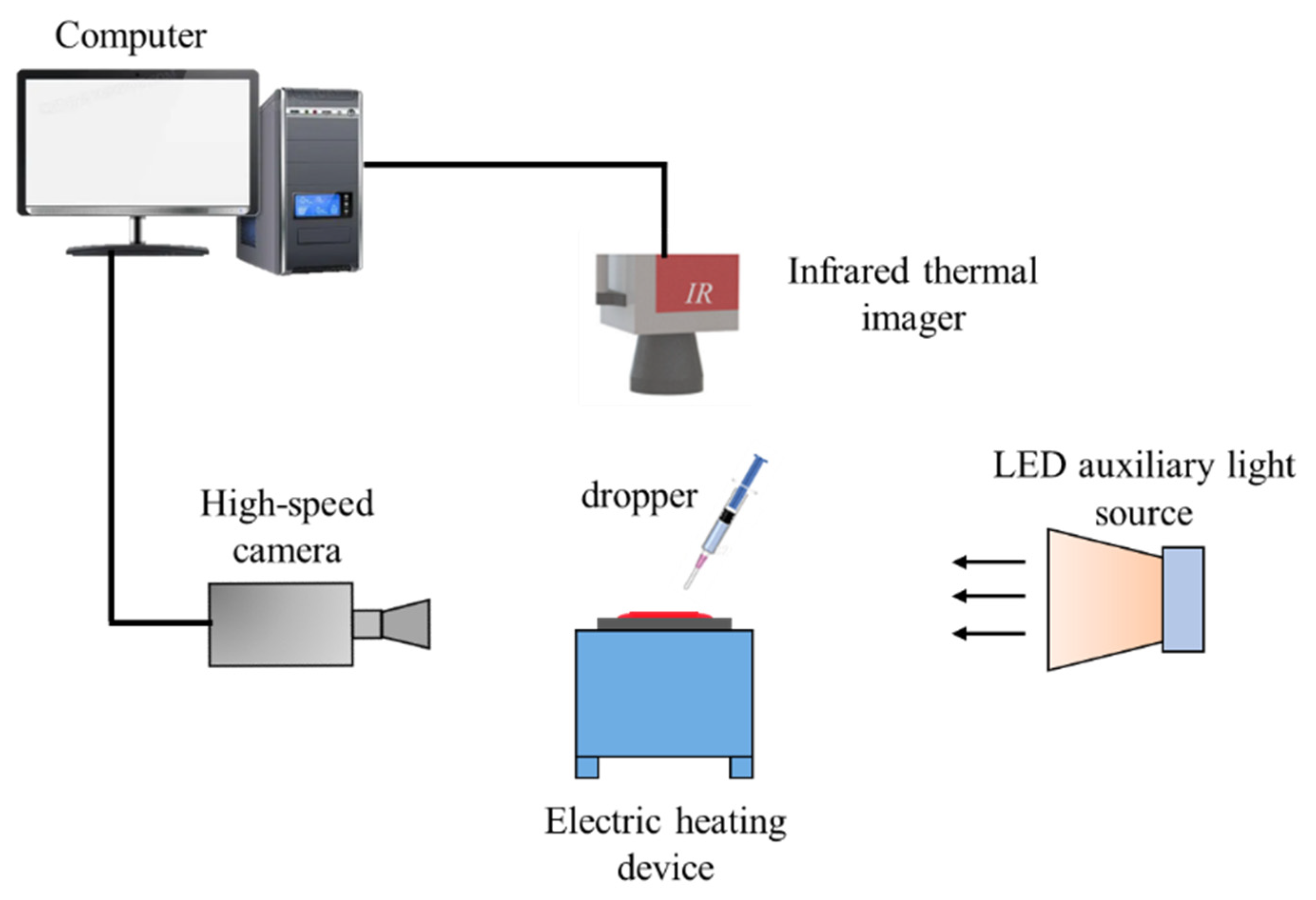
| Experimental Equipment | Equipment Type | Manufacturer | Key Parameters |
|---|---|---|---|
| Electric heating device | JF966-1010 | JFTOOLS, Dongguan, China | 300W |
| High-speed camera | dimax.HS1 | PCO AG, Kelheim, Germany | 1.8 × 105 fps |
| LED auxiliary light source | RF-200W | Lan Yihe, Dongguan, China | 200 W |
| Contact-angle measuring instrument | SDC-200S | Sindin, Dongguan, China | 0.001° |
| Infrared thermal imager | A655sc | Teledyne FLIR, Wilsonville, OR, USA | −40~650 °C |
| high-depth-of-field microscope | VHX-7000 | Keyence, Osaka, Japan | 1222 W pixels |
| Temperature data acquisition system | LR8431-30 | HIOKI E.E., Nagano, Japan | 0.1 °C |
| Ultrasonic cleaning apparatus | VGT-2120QTD | GTSONIC, Guangdong, China | 20–80 kHz |
| Heat collecting thermostatic magnetic stirrer | DF-101S | Yushen Instrument Co., Ltd., Shanghai, China | ±1 °C |
| 1000 Mesh | 2000 Mesh | 3000 Mesh | |
|---|---|---|---|
| 1.51 | 0.72 | 0.65 | |
| 9.48 | 6.07 | 5.61 |
| Material | Surface Treatment | 1.51 | 0.72 | 0.65 |
|---|---|---|---|---|
| Cu | Hydrophilic | 19.017 | 20.500 | 19.223 |
| Hydrophobic | 119.143 | 120.618 | 119.522 | |
| Unprocessed | 44.754 | 44.680 | 44.868 | |
| Al | Hydrophilic | 18.340 | 20.096 | 20.436 |
| Hydrophobic | 122.383 | 119.886 | 121.072 | |
| Unprocessed | 42.074 | 43.890 | 42.421 | |
| Brass | Hydrophilic | 19.210 | 22.835 | 18.224 |
| Hydrophobic | 122.145 | 121.862 | 122.120 | |
| Unprocessed | 42.587 | 42.421 | 43.344 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, D.; Zhang, Y.; Li, Y.; Wang, B.; Xu, M. Experimental Study on the Thin-Film Evaporation of Organic Solvent Droplets on Metal Surfaces. Energies 2025, 18, 5113. https://doi.org/10.3390/en18195113
Sun D, Zhang Y, Li Y, Wang B, Xu M. Experimental Study on the Thin-Film Evaporation of Organic Solvent Droplets on Metal Surfaces. Energies. 2025; 18(19):5113. https://doi.org/10.3390/en18195113
Chicago/Turabian StyleSun, Deji, Ying Zhang, Yi Li, Boda Wang, and Meng Xu. 2025. "Experimental Study on the Thin-Film Evaporation of Organic Solvent Droplets on Metal Surfaces" Energies 18, no. 19: 5113. https://doi.org/10.3390/en18195113
APA StyleSun, D., Zhang, Y., Li, Y., Wang, B., & Xu, M. (2025). Experimental Study on the Thin-Film Evaporation of Organic Solvent Droplets on Metal Surfaces. Energies, 18(19), 5113. https://doi.org/10.3390/en18195113




