Long-Term Performance of Thermal Insulating Composite Systems Based on Water Resistance and Surface Multifunctionality
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. ETICS
2.1.2. Multifunctional Coatings
2.2. Methods
2.2.1. Moisture Transport Properties
2.2.2. Photocatalytic Efficacy and Color Change
2.2.3. Biological Colonization
2.2.4. Accelerated Aging Procedure
2.2.5. DRIFT and SEM-EDS Analyses
3. Results
3.1. Moisture Transport Properties
3.2. Color Change and Photocatalytic Efficacy
3.2.1. Chromatic Coordinates
3.2.2. Photocatalysis Evaluation
3.3. Biological Colonization
3.4. Chemical and Morphological Analyses
3.4.1. SEM-EDS
3.4.2. DRIFT
4. Discussion
5. Conclusions
- HW (silane/siloxane emulsion) ensured good water repellency and durability on acrylic ETICS, but showed reduced performance on lime- and silicate-based systems and limited self-cleaning;
- NS (ethyl silicate with TiO2) offered initial photocatalytic and self-cleaning properties, but a negative performance in terms of biocidal effect, durability, and effectiveness over time;
- AQ (acrylic dispersion with TiO2) combined acceptable durability and moderate self-cleaning on acrylic systems but increased drying resistance and showed weak performance on mineral-based ETICS.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| θ | Contact Angle |
| ΔE | Color Change |
| a* | Color Coordinate (red-green) |
| AQ | Hydrophobic, Self-cleaning and Biocidal Coating |
| b* | Color Coordinate (yellow-blue) |
| BC | Base Coat |
| Cc | Capillary Water Absorption Coefficient |
| DI | Drying Index |
| DRIFT | Diffuse Reflectance Infrared Fourier Transform |
| EDS | Energy Dispersive Spectroscopy |
| EIFS | Exterior Insulation Finishing System |
| EPS | Expanded Polystyrene |
| ETA | European Technical Approval |
| ETICS | External Thermal Insulation Composite Systems |
| EWI | Exterior Wall Insulation Systems |
| FC | Finishing Coat |
| HT | Hygrothermal Cycles |
| HW | Hydrophobic and Biocidal Coating |
| ICB | Insulation Cork Board |
| L* | Lightness |
| MC | Multifunctional Coatings |
| MW | Mineral Wool |
| NHL | Natural Hydraulic Lime |
| NS | Photocatalytic Self-Cleaning and Antimicrobial Coating |
| Ref | Without Protective Treatment |
| RH | Relative Humidity |
| RhB | Rhodamine B |
| S1 | System 1 |
| S2 | System 2 |
| S3 | System 3 |
| S4 | System 4 |
| SCI | Specular Component Included |
| SEM | Scanning Electron Microscope |
| SO2 | Air Pollutant Cycles |
| T | Temperature |
| UV | Ultraviolet Radiation Cycles |
References
- Malanho, S.; Veiga, M.R. Bond strength between layers of ETICS—Influence of the characteristics of mortars and insulation materials. J. Build. Eng. 2020, 28, 101021. [Google Scholar] [CrossRef]
- Amaro, B.; Saraiva, D.; De Brito, J.; Flores-Colen, I. Inspection and diagnosis system of ETICS on walls. Constr. Build. Mater. 2013, 47, 1257–1267. [Google Scholar] [CrossRef]
- EAD 040083-00-0404; External Thermal Insulation Composite Systems (ETICS) with Rendering. EOTA (European Organisation for Technical Approvals): Brussels, Belgium, 2020; pp. 1–88.
- Barreira, E.; de Freitas, V.P. Experimental study of the hygrothermal behaviour of External Thermal Insulation Composite Systems (ETICS). Build. Environ. 2013, 63, 31–39. [Google Scholar] [CrossRef]
- Xu, H.; Wang, H.; Huo, Q.; Qin, Y.; Zhou, H. Comparative study of Chinese, European and ISO external thermal insulation composite system (ETICS) standards and technical recommendations. J. Build. Eng. 2023, 68, 105687. [Google Scholar] [CrossRef]
- Malanho, S.; Veiga, R.; Farinha, C. Global Performance of Sustainable Thermal Insulating Systems with Cork for Building Facades. Buildings 2021, 11, 83. [Google Scholar] [CrossRef]
- Parracha, J.L.; Borsoi, G.; Flores-Colen, I.; Veiga, R.; Nunes, L.; Dionísio, A.; Gomes, M.G.; Faria, P. Performance parameters of ETICS: Correlating water resistance, bio-susceptibility and surface properties. Constr. Build. Mater. 2021, 272, 121956. [Google Scholar] [CrossRef]
- Zhou, X.; Kubilay, A.; Derome, D.; Carmeliet, J. Comparison of wind-driven rain load on building facades in the urban environment and open field: A case study on two buildings in Zurich, Switzerland. Build. Environ. 2023, 233, 110038. [Google Scholar] [CrossRef]
- Bauer, E.; Souza, A.L.R. Failure patterns associated with facade zones and anomalies in the initiation and propagation of degradation. Constr. Build. Mater. 2022, 347, 128563. [Google Scholar] [CrossRef]
- Ren, H.; Koshy, P.; Chen, W.F.; Qi, S.; Sorrell, C.C. Photocatalytic materials and technologies for air purification. J. Hazard. Mater. 2017, 325, 340–366. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa, J.M.; Miller, A.Z.; Pozo-Antonio, J.S.; González-Pérez, J.A.; Jiménez-Morillo, N.T.; Dionisio, A. Assessing the effects of UVA photocatalysis on soot-coated TiO2-containing mortars. Sci. Total Environ. 2017, 605–606, 147–157. [Google Scholar] [CrossRef]
- Amaro, B.; Saraiva, D.; De Brito, J.; Flores-Colen, I. Statistical survey of the pathology, diagnosis and rehabilitation of ETICS in walls. J. Civ. Eng. Manag. 2014, 20, 511–526. [Google Scholar] [CrossRef]
- Parracha, J.L.; Borsoi, G.; Veiga, R.; Flores-Colen, I.; Nunes, L.; Garcia, A.R.; Ilharco, M.; Dionísio, A.; Faria, P. Effects of hygrothermal, UV and SO2 accelerated ageing on the durability of ETICS in urban environments. Build. Environ. 2021, 204, 108151. [Google Scholar] [CrossRef]
- Veigas, C.A.; Borsoi, G.; Moreira, L.M.; Parracha, J.L.; Nunes, L.; Malanho, S.; Veiga, R.; Flores-Colen, I. Diversity and distribution of microbial communities on the surface of External Thermal Insulation Composite Systems (ETICS) facades in residential buildings. Int. Biodeterior. Biodegrad. 2023, 184, 105658. [Google Scholar] [CrossRef]
- Vega-Garcia, P.; Lok, C.S.C.; Marhoon, A.; Schwerd, R.; Johann, S.; Helmreich, B. Modelling the environmental fate and behavior of biocides used in façades covered with mortars and plasters and their transformation products. Build. Environ. 2022, 216, 108991. [Google Scholar] [CrossRef]
- Silva, A.S.; Borsoi, G.; Parracha, J.L.; Flores-Colen, I.; Veiga, R.; Faria, P.; Dionísio, A. Evaluating the effectiveness of self-cleaning products applied on external thermal insulation composite systems (ETICS). J. Coat. Technol. Res. 2022, 19, 1437–1448. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Wang, F.; Liu, Z.; Su, W.; Chen, Z.; Jiang, J. Investigation on the effects of polyaniline/lignin composites on the performance of waterborne polyurethane coating for protecting cement-based materials. J. Build. Eng. 2023, 64, 105665. [Google Scholar] [CrossRef]
- Heib, F.; Hempelmann, R.; Munief, W.M.; Ingebrandt, S.; Fug, F.; Possart, W.; Groß, K.; Schmitt, M. High-precision drop shape analysis (HPDSA) of quasistatic contact angles on silanized silicon wafers with different surface topographies during inclining-plate measurements: Influence of the surface roughness on the contact line dynamics. Appl. Surf. Sci. 2015, 342, 11–25. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, R.; Wang, H.; Hu, F.; Jin, P. Study on PVA-siloxane mixed emulsion coatings for hydrophobic cement mortar. Prog. Org. Coat. 2020, 147, 105775. [Google Scholar] [CrossRef]
- Borsoi, G.; Esteves, C.; Flores-Colen, I.; Veiga, R. Effect of hygrothermal aging on hydrophobic treatments applied to building exterior claddings. Coatings 2020, 10, 363. [Google Scholar] [CrossRef]
- van Duijkeren, E.; Schink, A.K.; Roberts, M.C.; Wang, Y.; Schwarz, S. Mechanisms of Bacterial Resistance to Antimicrobial Agents. Microbiol. Spectr. 2018, 6, 2. [Google Scholar] [CrossRef]
- Castro-Hoyos, A.M.; Rojas Manzano, M.A.; Maury-Ramírez, A. Challenges and Opportunities of Using Titanium Dioxide Photocatalysis on Cement-Based Materials. Coatings 2022, 12, 968. [Google Scholar] [CrossRef]
- Riaz, S.; Park, S.J. An overview of TiO2-based photocatalytic membrane reactors for water and wastewater treatments. J. Ind. Eng. Chem. 2020, 84, 23–41. [Google Scholar] [CrossRef]
- Hot, J.; Dasque, A.; Topalov, J.; Mazars, V.; Ringot, E. Titanium valorization: From chemical milling baths to air depollution applications. J. Clean. Prod. 2020, 249, 119344. [Google Scholar] [CrossRef]
- Padmanabhan, N.T.; Thomas, R.M.; John, H. Antibacterial self-cleaning binary and ternary hybrid photocatalysts of titanium dioxide with silver and graphene. J. Environ. Chem. Eng. 2022, 10, 107275. [Google Scholar] [CrossRef]
- La Russa, M.F.; Ruffolo, S.A.; Rovella, N.; Belfiore, C.M.; Palermo, A.M.; Guzzi, M.T.; Crisci, G.M. Multifunctional TiO2 coatings for Cultural Heritage. Prog. Org. Coat. 2012, 74, 186–191. [Google Scholar] [CrossRef]
- Celia, E.; Darmanin, T.; Taffin de Givenchy, E.; Amigoni, S.; Guittard, F. Recent advances in designing superhydrophobic surfaces. J. Colloid. Interface Sci. 2013, 402, 1–18. [Google Scholar] [CrossRef]
- Nath, R.K.; Zain, M.F.M.; Jamil, M. An environment-friendly solution for indoor air purification by using renewable photocatalysts in concrete: A review. Renew. Sustain. Energy Rev. 2016, 62, 1184–1194. [Google Scholar] [CrossRef]
- Higashimoto, S. Titanium-dioxide-based visible-light-sensitive photocatalysis: Mechanistic insight and applications. Catalysts 2019, 9, 201. [Google Scholar] [CrossRef]
- Munafò, P.; Goffredo, G.B.; Quagliarini, E. TiO2-based nanocoatings for preserving architectural stone surfaces: An overview. Constr. Build. Mater. 2015, 84, 201–218. [Google Scholar] [CrossRef]
- Vega-Garcia, P.; Schwerd, R.; Scherer, C.; Schwitalla, C.; Johann, S.; Rommel, S.H.; Helmreich, B. Influence of façade orientation on the leaching of biocides from building façades covered with mortars and plasters. Sci. Total Environ. 2020, 734, 139465. [Google Scholar] [CrossRef]
- European Union. Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 concerning the Making Available on the Market and Use of Biocidal Products. Off. J. Eur. Union 2022, 167, 1–123. [Google Scholar]
- Schoknechta, U.; Sommerfelda, T.; Borhob, N.; Bagdab, E. Interlaboratory comparison for a laboratory leaching test procedure with façade coatings. Prog. Org. Coat. 2013, 76, 351–359. [Google Scholar] [CrossRef]
- Bollmann, U.E.; Minelgaite, G.; Schlüsener, M.; Ternes, T.; Vollertsen, J.; Bester, K. Leaching of Terbutryn and Its Photodegradation Products from Artificial Walls under Natural Weather Conditions. Environ. Sci. Technol. 2016, 50, 4289–4295. [Google Scholar] [CrossRef] [PubMed]
- La Russa, M.F.; Rovella, N.; Alvarez De Buergo, M.; Belfiore, C.M.; Pezzino, A.; Crisci, G.M.; Ruffolo, S.A. Nano-TiO2 coatings for cultural heritage protection: The role of the binder on hydrophobic and self-cleaning efficacy. Prog. Org. Coat. 2016, 91, 1–8. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Luongo, N.; Massari, S.; Lupica Spagnolo, S.; Daniotti, B.; Pedeferri, M.P. Durability of self-cleaning cement-based materials. Constr. Build. Mater. 2021, 280, 122441. [Google Scholar] [CrossRef]
- Bersch, J.D.; Flores-Colen, I.; Masuero, A.B.; Dal Molin, D.C.C. Photocatalytic TiO2-Based Coatings for Mortars on Facades: A Review of Efficiency, Durability, and Sustainability. Buildings 2023, 13, 186. [Google Scholar] [CrossRef]
- Goffredo, G.B.; Terlizzi, V.; Munafò, P. Multifunctional TiO2-based hybrid coatings on limestone: Initial performances and durability over time. J. Build. Eng. 2017, 14, 134–149. [Google Scholar] [CrossRef]
- EN 16322:2013; Conservation of Cultural Heritage—Test Methods—Determination of Drying Properties. CEN: Brussels, Belgium, 2013.
- Parracha, J.L.; Borsoi, G.; Veiga, R.; Flores-Colen, I.; Nunes, L.; Viegas, C.A.; Moreira, L.M.; Dionísio, A.; Glória Gomes, M.; Faria, P. Durability assessment of external thermal insulation composite systems in urban and maritime environments. Sci. Tot. Environ. 2022, 849, 157828. [Google Scholar] [CrossRef]
- UNI 11259; Determination of the Photocatalytic Activity of Hydraulic Binders—Rhodamine Method (in Italian). UNI: Milan, Italy, 2008.
- Krishnan, P.; Zhang, M.H.; Yu, L.; Feng, H. Photocatalytic degradation of particulate pollutants and self-cleaning performance of TiO2-containing silicate coating and mortar. Constr. Build. Mater. 2013, 44, 309–316. [Google Scholar] [CrossRef]
- ASTM-D2244; Standard Practice for Calculation of Color Tolerances and Color Differences from Instrumentally Measured Color Coordinates. ASTM International: West Conshohocken, PA, USA, 2022.
- Munafò, P.; Quagliarini, E.; Goffredo, G.B.; Bondioli, F.; Licciulli, A. Durability of nano-engineered TiO2 self-cleaning treatments on limestone. Constr. Build. Mater. 2014, 65, 218–231. [Google Scholar] [CrossRef]
- ASTM C1338-19; Standard Test Method for Determining Fungi Resistance of Insulation Materials and Facings. ASTM: West Conshohocken, PA, USA, 2019.
- ASTM D5590-17; Standard Test Method for Determining the Resistance of Paint Films and Related Coatings to Fungal Defacement by Accelerated Four-Week Agar Plate Assay. ASTM: West Conshohocken, PA, USA, 2017.
- Parracha, J.L.; Borsoi, G.; Flores-Colen, I.; Veiga, R.; Nunes, L. Impact of natural and artificial ageing on the properties of multilayer external wall thermal insulation systems. Constr. Build. Mater. 2022, 317, 125834. [Google Scholar] [CrossRef]
- ISO 16474-3; Paints and Varnishes—Methods of Exposure to Laboratory Light Sources—Part 3, Fluorescent UV Lamps. ISO: Geneva, Switzerland, 2013.
- Diamanti, M.V.; Paolini, R.; Rossini, M.; Aslan, A.B.; Zinzi, M.; Poli, T.; Pedeferri, M.P. Long term self-cleaning and photocatalytic performance of anatase added mortars exposed to the urban environment. Constr. Build. Mater. 2015, 96, 270–278. [Google Scholar] [CrossRef]
- Graziani, L.; Quagliarini, E.; Bondioli, F.; D’Orazio, M. Durability of self-cleaning TiO2 coatings on fired clay brick façades: Effects of UV exposure and wet & dry cycles. Build. Environ. 2014, 71, 193–203. [Google Scholar] [CrossRef]
- Roncon, R.; Borsoi, G.; Parracha, J.L.; Flores-Colen, I.; Veiga, R.; Nunes, L. Impact of water-repellent products on the moisture transport properties and mould susceptibility of external thermal insulation composite systems. Coatings 2021, 11, 554. [Google Scholar] [CrossRef]
- Ghaee, A.; Ghadimi, A.; Sadatnia, B.; Ismail, A.F.; Mansourpour, Z.; Khosravi, M. Synthesis and characterization of poly(vinylidene fluoride) membrane containing hydrophobic silica nanoparticles for CO2 absorption from CO2/N2 using membrane contactor. Chem. Eng. Res. Des. 2017, 120, 47–57. [Google Scholar] [CrossRef]
- Quéré, D. Non-sticking drops. Rep. Prog. Phys. 2005, 68, 2495–2532. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Ershad-Langroudi, A. Polymeric coatings for protection of historic monuments: Opportunities and challenges. J. Appl. Polym. Sci. 2009, 112, 2535–2551. [Google Scholar] [CrossRef]
- Feltes, J.; Borsoi, G.; Caiado, P.; Dionísio, A.; Parracha, J.; Flores-Colen, I. Graffiti removal on external thermal insulation composite systems through chemical-mechanical methods: A feasible protocol? J. Build. Eng. 2023, 66, 105872. [Google Scholar] [CrossRef]
- Lu, T.; Solis-Ramos, E.; Yi, Y.; Kumosa, M. UV degradation model for polymers and polymer matrix composites. Polym. Degrad. Stab. 2018, 154, 203–210. [Google Scholar] [CrossRef]
- Gil, B.C.; Borsoi, G.; Parracha, J.L.; Dionísio, A.; Veiga, R.; Flores-Colen, I. Effectiveness and durability of anti-graffiti products applied on ETICS: Towards a compatible and sustainable graffiti removal protocol. Environ. Sci. Pollut. Res. 2023, 30, 65160–65176. [Google Scholar] [CrossRef] [PubMed]
- Folli, A.; Pade, C.; Hansen, T.B.; De Marco, T.; MacPhee, D.E. TiO2 photocatalysis in cementitious systems: Insights into self-cleaning and depollution chemistry. Cem. Concr. Res. 2012, 42, 539–548. [Google Scholar] [CrossRef]
- Calia, A.; Lettieri, M.; Masieri, M. Durability assessment of nanostructured TiO2 coatings applied on limestones to enhance building surface with self-cleaning ability. Build. Environ. 2016, 110, 1–10. [Google Scholar] [CrossRef]
- Solovyeva, M.; Selishchev, D.; Cherepanova, S.; Stepanov, G.; Zhuravlev, E.; Richter, V.; Kozlov, D. Self-Cleaning Photoactive Cotton Fabric Modified with Nanocrystalline TiO2 for Efficient Degradation of Volatile Organic Compounds and DNA Contaminants. Chem. Eng. J. 2020, 388, 124167. [Google Scholar] [CrossRef]
- Guo, M.-Z.; Ling, T.-C.; Poon, C.-S. Nano-TiO2-based architectural mortar for NO removal and bacteria inactivation: Influence of coating and weathering conditions. Cem. Concr. Compos. 2013, 36, 101–108. [Google Scholar] [CrossRef]
- Maury-Ramirez, A.; De Muynck, W.; Stevens, R.; Demeestere, K.; De Belie, N. Titanium dioxide based strategies to prevent algal fouling on cementitious materials. Cem. Concr. Compos. 2013, 36, 93–100. [Google Scholar] [CrossRef]
- Hegyi, A.; Grebenişan, E.; Lăzărescu, A.; Stoian, V.; Szilagyi, H. Influence of TiO2 Nanoparticles on the Resistance of Cementitious Composite Materials to the Action of Fungal Species. Materials. 2021, 14, 4442. [Google Scholar] [CrossRef] [PubMed]
- Borsoi, G.; Veiga, R.; Santos Silva, A. Effect of nanostructured lime-based and silica-based products on the consolidation of historical renders. In Proceedings of the 3rd Historic Mortars Conference, Glasgow, Scotland, 11–14 September 2013. [Google Scholar]
- Kim, J.; Kim, D.; Yun, T.S. Containment of sulfate in leachate as gypsum (CaSO4·2H2O) mineral formation in bio-cemented sand via enzyme-induced carbonate precipitation. Sci. Rep. 2023, 13, 10938. [Google Scholar] [CrossRef]
- Kistanova, N.S.; Chashchukhina, A.D.; Kudryashova, O.S.; Khayrulina, E.A. Influence of polyacrylamide on the precipitation of gypsum in sodium chloride solutions. Environ. Earth Sci. 2023, 82, 565. [Google Scholar] [CrossRef]
- İnceoğlu, F.; Mermer, N.K.; Kırmızı, V.; Tombaş, G. Influence of cement with different calcium sulfate phases on cementitious tile adhesive mortars: Microstructure and performance aspects. Int. J. Adhes. Adhes. 2021, 104, 102744. [Google Scholar] [CrossRef]
- Biedunkova, O.; Kuznietsov, P.; Gandziure, V. Behaviour of dissolved inorganic salts in the cooling water of a nuclear power plant open recirculation system and formation of water discharge. R. Soc. Open Sci. 2024, 11, 240492. [Google Scholar] [CrossRef]
- Borsoi, G.; Tavares, M.; Veiga, R.; Santos Silva, A. Microstructural characterization of consolidant products for historical renders: An innovative nanostructured lime dispersion and a more traditional ethyl silicate limewater solution. Microsc. Microanal. 2012, 18, 1181–1189. [Google Scholar] [CrossRef]
- Zendri, E.; Biscontin, G.; Nardini, I.; Rialto, S. Characterization and reactivity of silicatic consolidants. Constr. Build. Mater. 2007, 21, 1098–1106. [Google Scholar] [CrossRef]
- Hashim, H.; Dias, L.; Martins, S.; Pires, V.; Costa, M.; Barrulas, P. Optimization of the Application of Commercial Hydrophobic Coatings for Natural Stone Protection and Preservation. Heritage 2024, 7, 3495–3510. [Google Scholar] [CrossRef]
- Hoyos-Montilla, A.A.; Puertas, F.; Molina Mosquera, J.; Tobón, J.I. Infrared spectra experimental analyses on alkali-activated fly ash-based binders. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 269, 120698. [Google Scholar] [CrossRef]
- Shanti, R.; Hadi, A.N.; Salim, Y.S.; Chee, S.Y.; Ramesh, S.; Ramesha, K. Degradation of ultra-high molecular weight poly(methyl methacrylate-co-butyl acrylate-coacrylic acid) under ultra violet irradiation. RSC Adv. 2017, 7, 112. [Google Scholar] [CrossRef]
- Olabarrieta, J.; Zorita, S.; Peña, I.; Rioja, N.; Monzón, O.; Benguria, P.; Scifo, L. Aging of photocatalytic coatings under a water flow: Long run performance and TiO2 nanoparticles release. Appl. Catal. B 2012, 123–124, 182–192. [Google Scholar] [CrossRef]
- Carmona-Quiroga, P.M.; Martínez-Ramírez, S.; Viles, H.A. Efficiency and durability of a self-cleaning coating on concrete and stones under both natural and artificial ageing trials. Appl. Surf. Sci. 2018, 433, 312–320. [Google Scholar] [CrossRef]
- Kalinowski, M.; Chilmon, K.; Kuziak, J.; Łukowski, P. Photocatalytically Induced Degradation of Nano-TiO2-Modified Paint Coatings Under Low-Radiation Conditions. Coatings. 2025, 15, 281. [Google Scholar] [CrossRef]
- Sanmartín, P.; Noya-Pintos, D.; Fuentes, E.; Pozo-Antonio, J.S. Cracks in consolidants containing TiO2 as a habitat for biological colonization: A case of quaternary bioreceptivity. Mat. Sci. Eng. C 2021, 124, 112058. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowski, K.; Skowera, E.; Pietniewicz, E.; Zukowska, G.Z.; Van Der Ven, L.G.J.; Korczagin, I.; Malanowski, P. UV stability of polymeric binder films used in waterborne facade paints. Prog. Org. Coat. 2014, 77, 298–304. [Google Scholar] [CrossRef]
- Fukaya, N.; Ogi, S.; Sotome, H.; Fujimoto, K.J.; Yanai, T.; Bäumer, N.; Fernández, G.; Miyasaka, H.; Yamaguch, S. Impact of Hydrophobic/Hydrophilic Balance on Aggregation Pathways, Morphologies, and Excited-State Dynamics of Amphiphilic Diketopyrrolopyrrole Dyes in Aqueous Media. J. Am. Chem. Soc. 2022, 144, 22479–22492. [Google Scholar] [CrossRef]
- Colangiuli, D.; Calia, A.; Bianco, N. Novel multifunctional coatings with photocatalytic and hydrophobic properties for the preservation of the stone building heritage. Const. Build. Mat. 2015, 93, 189–196. [Google Scholar] [CrossRef]
- Ribeiro, T.; Baleizão, C.; Farinha, J.P. Functional Films from Silica/Polymer Nanoparticles. Materials 2014, 7, 3881–3900. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.; Barrelas, J.; Silva, A.; De Brito, J.; Dias, I.S. Impact of Environmental Exposure Conditions on the Maintenance of Facades’ Claddings. Buildings 2021, 11, 138. [Google Scholar] [CrossRef]

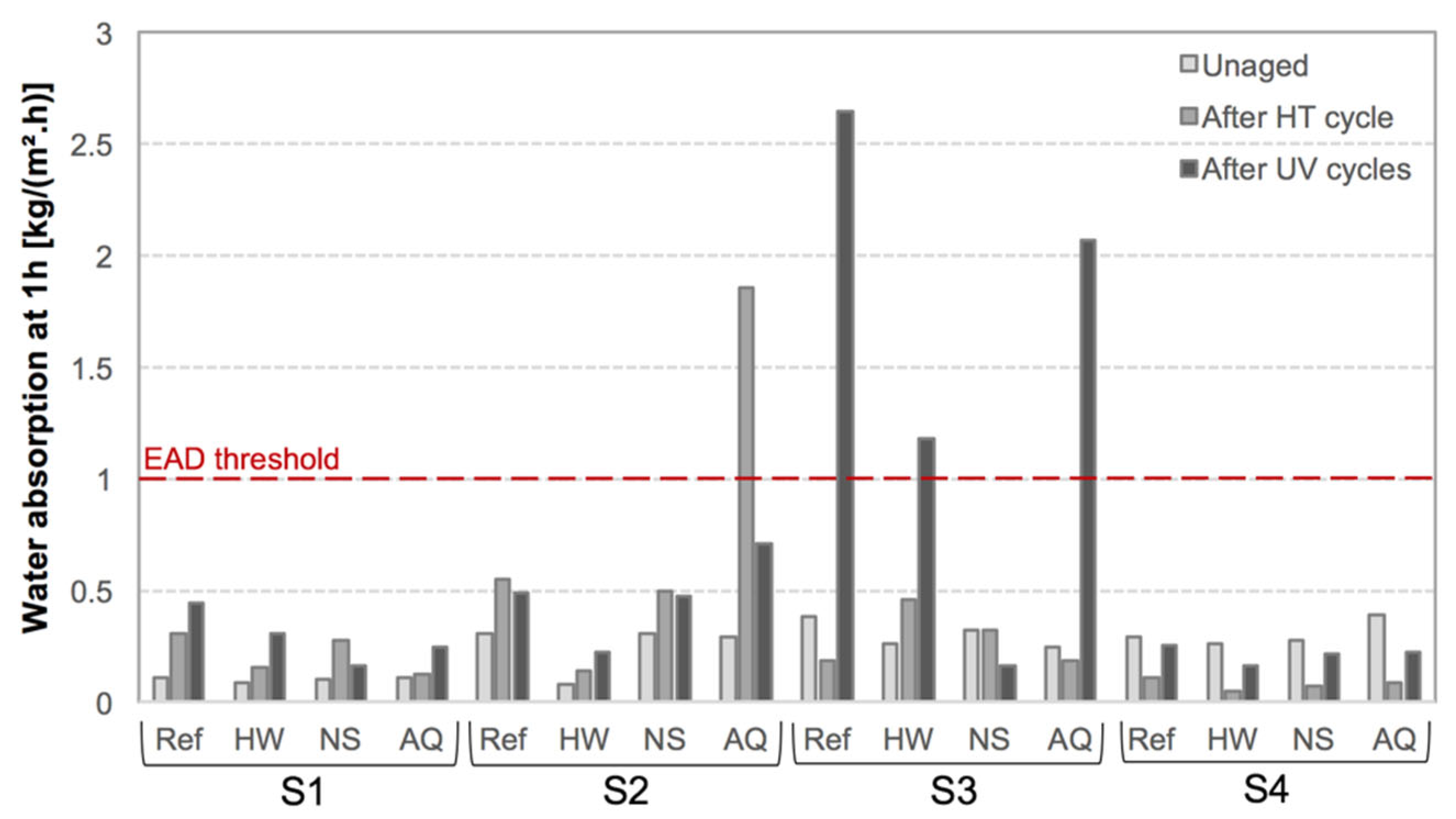

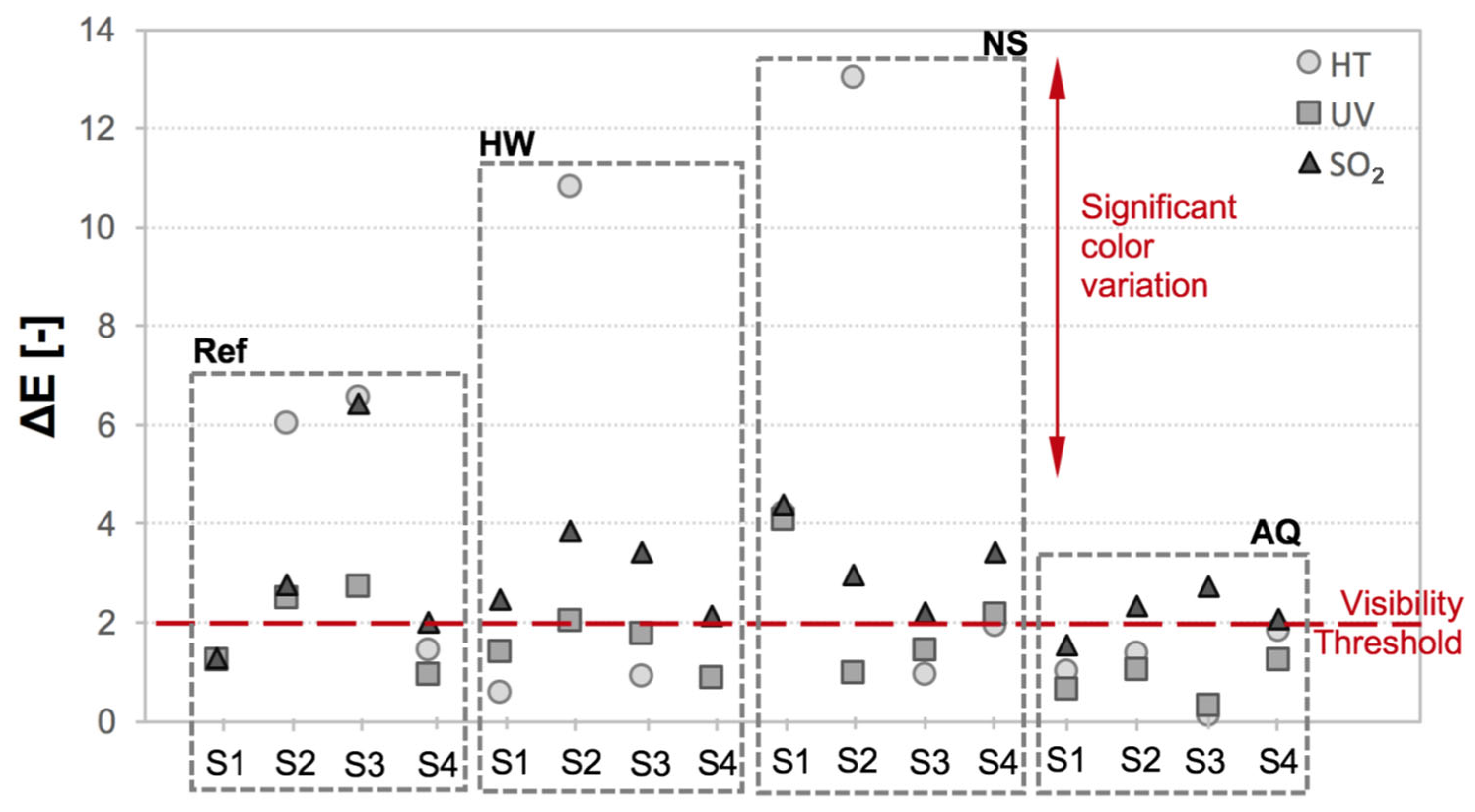
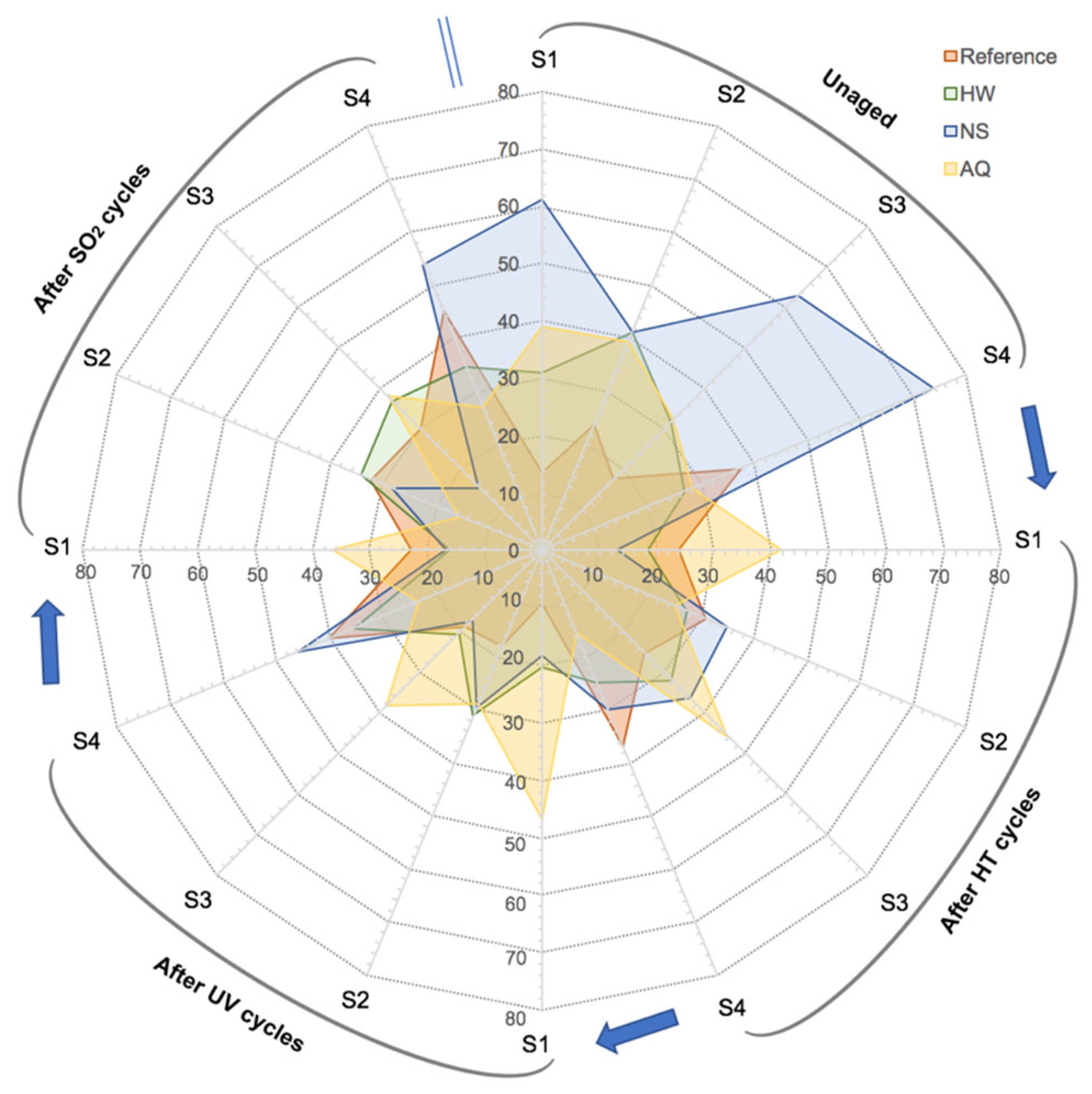

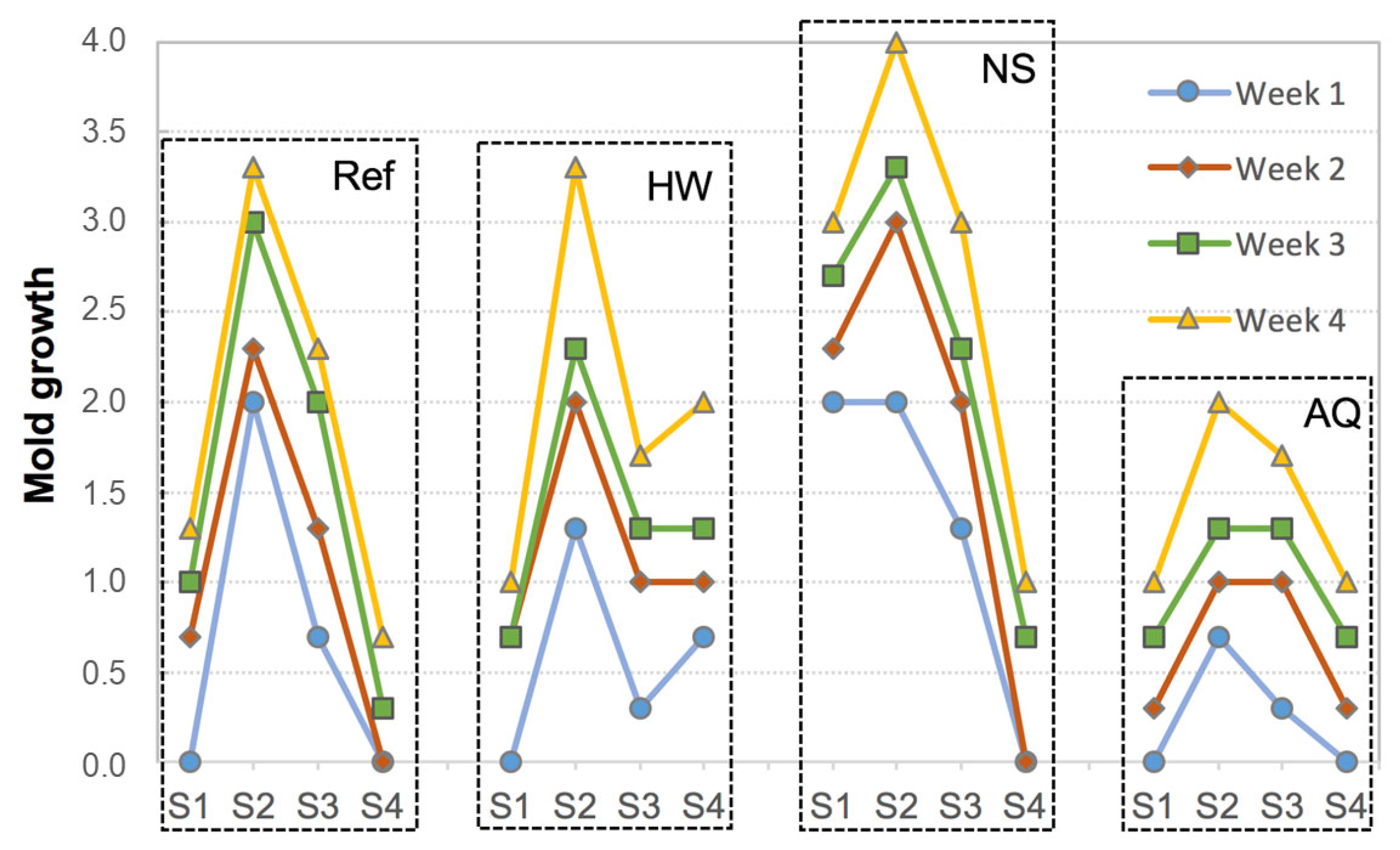
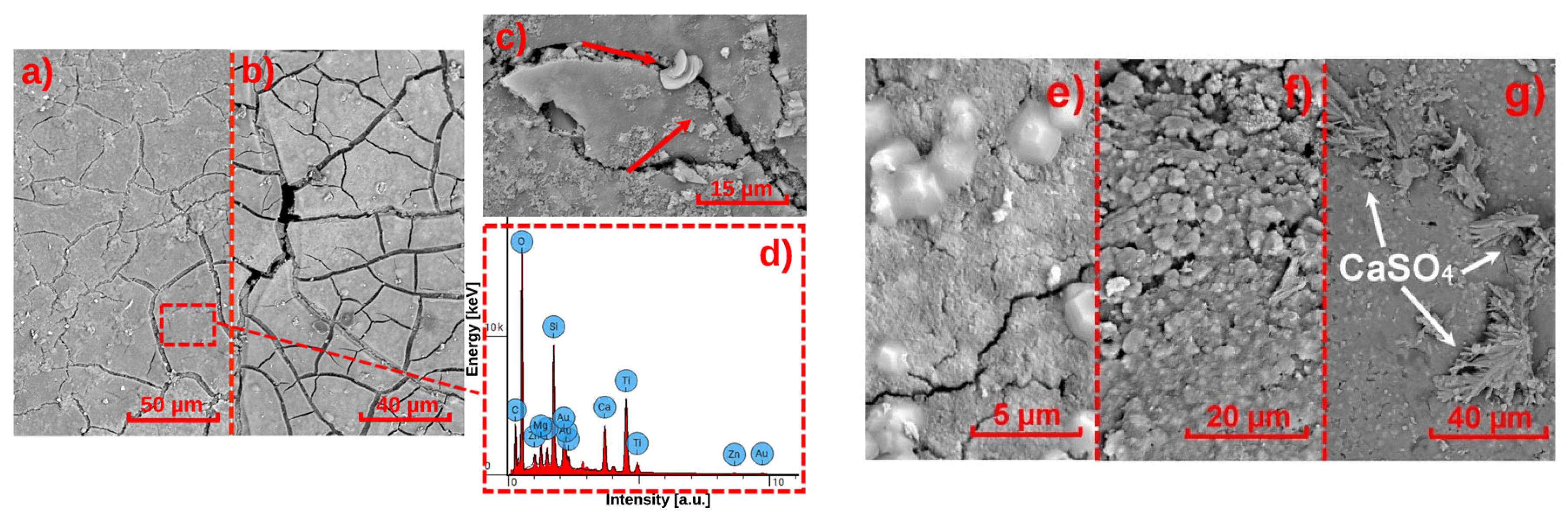

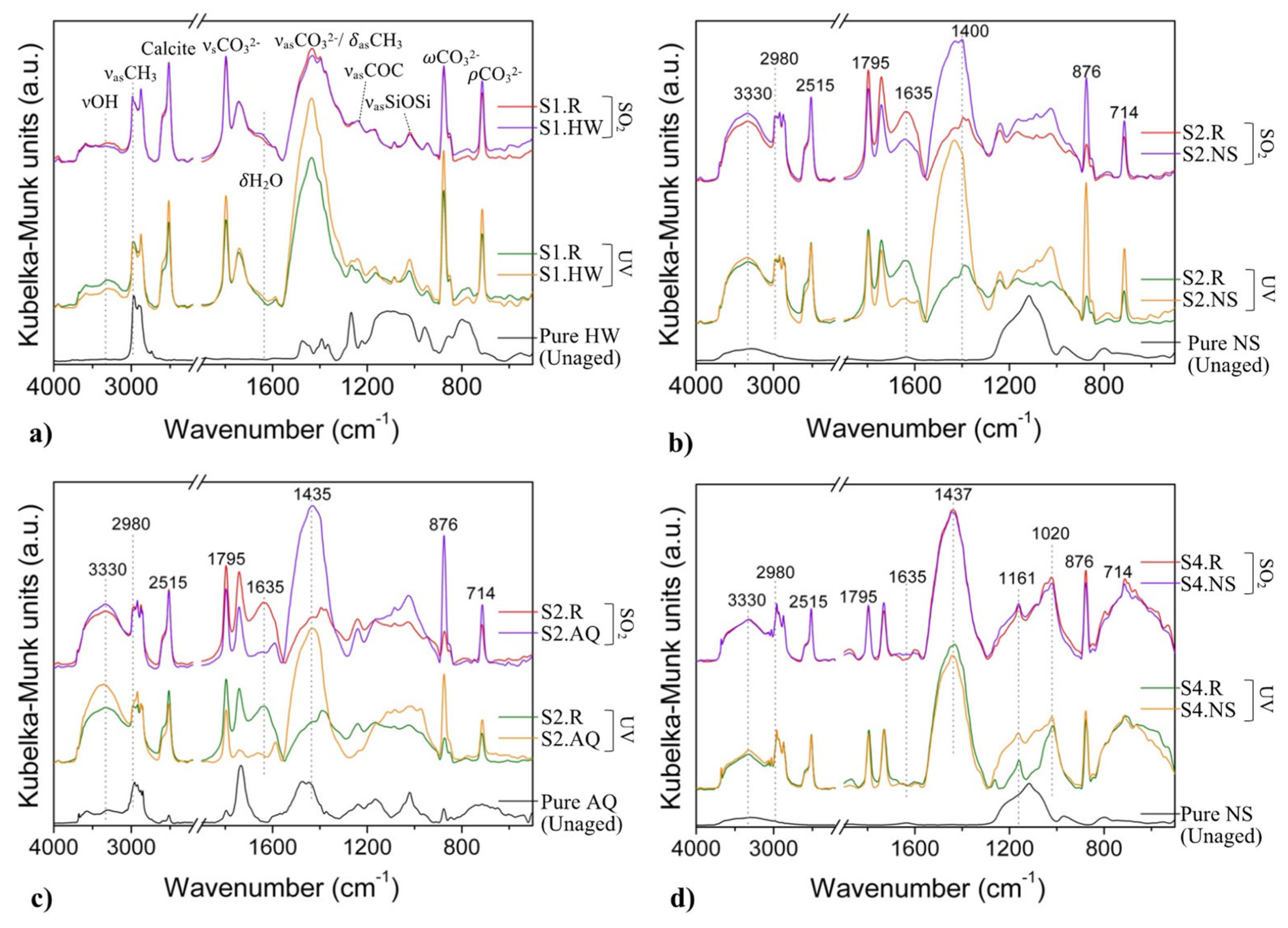
| System Acronym | Thermal Insulation | Base Coat (BC) 1 | Finishing Coat (FC) | Thickness (mm) | |
|---|---|---|---|---|---|
| Key-Coat | Finishing | ||||
| S1 | EPS | Cement, synthetic resins, mineral additives | Water-based acrylic dispersion | Water-based acrylic co-polymer, pigments, marble powder, and additives | 40.6 |
| S2 | ICB | NHL, cement, mineral fillers, resins, and synthetic fibers | Air lime, hydraulic binder, and organic additives | 65.8 | |
| S3 | MW | Cement, synthetic resins, mineral fillers, and additives | Water-based acrylic co-polymer and mineral additives | Water-based acrylic paint, mineral aggregates, pigments, and additives | 61.3 |
| S4 | ICB | NHL, cement, mixed binders, and cork aggregates | Water-based dispersion of silicate | Water-based silicate paint, organic additives, and pigments | 43.9 |
| Product Identification | Color | Density (g/cm3) at T = 20 °C/RH% = 60 | pH | Drying Residue (g/L) | Application Yield (L/m2) | Flash Point (°C) |
|---|---|---|---|---|---|---|
| HW | Whitish | 0.99 ± 0.02 | 9.3 ± 0.5 | 59 ± 6 | 0.166–0.25 (no dilution) | 64 |
| NS | Whitish | 0.98 ± 0.05 | 9.2 ± 0.5 | 30 ± 2 | 0.1–0.125 (no dilution) | >23 |
| AQ | Transparent (opal) | 1.31 ± 0.09 | 8.5 ± 0.5 | 718 ± 21 | 0.26–0.27 (10% dilution in water) | >100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borsoi, G.; Parracha, J.L.; Bersch, J.D.; Garcia, A.R.; Dionísio, A.; Faria, P.; Veiga, R.; Flores-Colen, I. Long-Term Performance of Thermal Insulating Composite Systems Based on Water Resistance and Surface Multifunctionality. Energies 2025, 18, 5008. https://doi.org/10.3390/en18185008
Borsoi G, Parracha JL, Bersch JD, Garcia AR, Dionísio A, Faria P, Veiga R, Flores-Colen I. Long-Term Performance of Thermal Insulating Composite Systems Based on Water Resistance and Surface Multifunctionality. Energies. 2025; 18(18):5008. https://doi.org/10.3390/en18185008
Chicago/Turabian StyleBorsoi, Giovanni, João L. Parracha, Jéssica D. Bersch, Ana R. Garcia, Amélia Dionísio, Paulina Faria, Rosário Veiga, and Inês Flores-Colen. 2025. "Long-Term Performance of Thermal Insulating Composite Systems Based on Water Resistance and Surface Multifunctionality" Energies 18, no. 18: 5008. https://doi.org/10.3390/en18185008
APA StyleBorsoi, G., Parracha, J. L., Bersch, J. D., Garcia, A. R., Dionísio, A., Faria, P., Veiga, R., & Flores-Colen, I. (2025). Long-Term Performance of Thermal Insulating Composite Systems Based on Water Resistance and Surface Multifunctionality. Energies, 18(18), 5008. https://doi.org/10.3390/en18185008














