Stochastic Biomechanical Modeling of Human-Powered Electricity Generation: A Comprehensive Framework with Advanced Monte Carlo Uncertainty Quantification
Abstract
1. Introduction
2. Theoretical Framework
2.1. Stochastic Biomechanical Power Model
2.2. Multi-Component Stochastic Efficiency Function
2.3. Advanced Multi-Component Fatigue Dynamics
2.4. Monte Carlo Uncertainty Quantification Framework
- Force capabilities: ;
- Cadence preferences: ;
- Maximum efficiency: .
2.5. Multi-Objective Pareto Optimization
- ;
- ;
- .
3. Methodology
3.1. Theoretical Validation Framework
- Participant demographics: 7 individuals (5 males, 2 female), age 20–31 years;
- Participant scaling factors: 1.08, 1.12, 1.05, 1.03, 1.10, 1.06, 0.92 (reflecting strength variations);
- Fitness levels: 0.88–0.95 (normalized scale).
- Force noise: ;
- Cadence noise: ;
- Environmental noise: .
3.2. Advanced Monte Carlo Implementation
3.3. Statistical Analysis Framework
3.4. Theoretical Framework Integration and Methodological Synopsis
4. Results
4.1. Theoretical Model Validation
4.2. Monte Carlo Uncertainty Quantification
4.3. Stochastic Efficiency Surface Characterization
4.4. Multi-Component Fatigue Analysis
4.5. Pareto Optimization Results
4.6. Control Strategy Evaluation for Human Compatibility
4.7. Design Guidelines
5. Discussion
5.1. Comparative Assessment with Existing HPEG Modeling Approaches
5.2. Scientific Contributions and Model Performance
5.3. Monte Carlo Framework Performance and Statistical Rigor
5.4. Fatigue Dynamics and Recovery Mechanisms
5.5. Multi-Objective Optimization Insights
5.6. Engineering Implications and Design Guidelines
5.7. Practical Applications
- Emergency Response: The Balanced Performance archetype validated across 7 participants delivers 316 ± 19 W (population-averaged), sufficient for communication equipment (25–50 W), emergency lighting (100–150 W), and medical devices (80–120 W). The framework provides 95% confidence intervals [263, 369] W, ensuring 94.3% reliability for ≥250 W output during emergency scenarios [64].
- Off-Grid Communities: System-level efficiency analysis reveals 91.3% average efficiency during 6 h continuous operation, exceeding laboratory predictions by 8.1% due to adaptive control enabled by stochastic modeling. Endurance Zone operation generates 217 ± 18 W per operator with three identified efficiency regimes: high-efficiency zone (>85%, 73% operational probability), moderate-efficiency zone (80–85%, 89% probability), and degraded-efficiency zone (<80%, requiring intervention). Multi-participant analysis shows 8–12 operators from diverse backgrounds can reliably produce 1.5–2.5 kW [65,66].
- Fitness Applications: Personalized optimization improves efficiency by 15–25% compared to fixed parameters. The multi-component fatigue model enables 90% performance restoration within 25.3 ± 6.8 min, supporting optimized rehabilitation protocols.
- Humanitarian Camps: The stochastic framework enables energy planning for 288 African refugee settlements housing 4.78 million displaced persons. The Minimum Fatigue archetype (162 ± 4 N, 56 ± 2 rpm) specifically accommodates malnourished populations with 40% reduced force capacity. Monte Carlo analysis incorporating these constraints yields 85–145 W per operator (95% CI), sufficient for vaccine refrigeration (80–100 W) and emergency communications (20 W). This configuration offers sustainable alternatives to diesel generators costing >$0.50/kWh in remote humanitarian settings.
6. Conclusions
- (1)
- Design systems for the Balanced Performance configuration (239 ± 5 N, 69 ± 2 rpm) as default, achieving 316 ± 19 W with 49% fatigue reduction compared to maximum power modes, suitable for 74% of users.
- (2)
- Implement 40% power margins based on the identified CV = 37.6% inter-participant variability, sizing energy storage for 95% CI [48.4, 174.9] W rather than deterministic estimates.
- (3)
- Select energy harvesting technology by application: pedaling–electromagnetic for >250 W emergency power, piezoelectric for <20 W wearables, and hybrid configurations to reduce variability from ±38% to ±21%.
- (4)
- Incorporate adaptive load control triggered when fatigue indicators approach , preventing performance degradation beyond 33% during extended operation.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Abbreviations | |
| CCC | Concordance Correlation Coefficient |
| CI | Confidence Interval |
| CV | Cross-validation |
| HPEG | Human-powered electricity generation |
| MAE | Mean Absolute Error |
| MAPE | Mean Absolute Percentage Error |
| MC | Monte Carlo |
| MVN | Multivariate Normal |
| PCr | Phosphocreatine |
| RMSE | Root Mean Square Error |
| Variables and Symbols | |
| Central fatigue coefficient | |
| Cadence change nonlinearity exponent | |
| Maximum substrate depletion effect | |
| Cyclical fatigue variation parameter | |
| Recovery enhancement factor | |
| Cadence variability penalty coefficient | |
| Environmental factors and psychological influences | |
| Overall system efficiency | |
| Base efficiency parameter | |
| Biomechanical efficiency component | |
| Central neural fatigue efficiency | |
| Coordination efficiency factor | |
| Fatigue efficiency component | |
| Maximum theoretical efficiency | |
| Mechanical efficiency for limb i | |
| Peripheral muscular fatigue efficiency | |
| Substrate depletion efficiency | |
| Physiological parameter vector | |
| Biomechanical scaling factor | |
| Coordination efficiency variability parameter | |
| Force mean vector | |
| Optimal force-cadence vector | |
| Kinematic variables including joint angles | |
| Force-cadence correlation coefficient | |
| Force variability standard deviation | |
| Cadence variability standard deviation | |
| Force covariance matrix | |
| Individual parameter covariance structure | |
| Central fatigue time constant | |
| Torque generation for limb i | |
| Peripheral fatigue time constant | |
| Substrate depletion time constant | |
| Phase-dependent activation function for limb i | |
| Angular velocity for limb i | |
| Optimal control variate coefficient | |
| Mahalanobis distance metric | |
| Applied force vector | |
| Optimal force level | |
| Individual biomechanical efficiency factor | |
| Individual scaling factor | |
| Number of contributing limbs | |
| Number of stratification levels | |
| Instantaneous mechanical power output | |
| Moment arm radius | |
| Optimal cadence rate | |
| Circadian modulation period | |
Appendix A. Framework Implementation Parameters and Validation Metrics
| Parameter Class | Implementation Value | Computational Specification | Theoretical Foundation | Code Implementation |
|---|---|---|---|---|
| Core Biomechanical Constants (PARAMS structure) | ||||
| 235.4 N | Optimal force from biomechanics literature | Population-averaged optimal operating point | PARAMS.F_opt = 235.4 | |
| 67.8 rpm | Optimal cadence from cycling efficiency peak | Neuromuscular coordination optimum | PARAMS.RPM_opt = 67.8 | |
| 0.927 | Maximum theoretical efficiency | Thermodynamic efficiency limit | PARAMS.eta_max = 0.927 | |
| 0.285 | Minimum physiological efficiency | Severe fatigue performance floor | PARAMS.eta_min = 0.285 | |
| 28.5 N | Force variability parameter | Inter-individual force variation | PARAMS.sigma_F_opt = 28.5 | |
| 9.7 rpm | Cadence variability parameter | Natural rhythm deviation | PARAMS.sigma_RPM_opt = 9.7 | |
| Advanced Fatigue Dynamics (multi-component model) | ||||
| 1347 s | Peripheral fatigue time constant | Metabolic byproduct accumulation kinetics | PARAMS.tau_peripheral = 1347 | |
| 3124 s | Central fatigue time constant | Neural drive degradation dynamics | PARAMS.tau_central = 3124 | |
| 1186 s | Recovery time constant | Multi-exponential restoration process | PARAMS.tau_recovery = 1186 | |
| 0.247 | Central fatigue coefficient | Neural fatigue magnitude scaling | PARAMS.alpha_central = 0.247 | |
| 0.312 | Recovery enhancement factor | Active recovery acceleration | PARAMS.beta_recovery = 0.312 | |
| 0.598 | Minimum efficiency under fatigue | Severe fatigue limitation boundary | PARAMS.eta_fatigue_min = 0.598 | |
| Enhanced Monte Carlo Configuration | ||||
| 5,000,000 | Total Monte Carlo samples | High-precision uncertainty quantification | N_MC = 5 × 106 | |
| 25 | Stratification levels | Variance reduction technique | N_strata = 25 | |
| 50 | Statistical replications | Robustness validation | N_replications = 50 | |
| Advanced Efficiency Function Components | ||||
| 1.25 | Biomechanical scaling factor | Individual efficiency modifier | PARAMS.kappa_bio = 1.25 | |
| 0.15 | Coordination efficiency parameter | Neuromuscular control quality | PARAMS.lambda_coord = 0.15 | |
| 0.08 | Learning adaptation coefficient | Training adaptation rate | PARAMS.phi_adaptation = 0.08 | |
| System Engineering Specifications | ||||
| 0.1725 m | Optimized crank radius | Biomechanical leverage optimization | r = 0.1725 | |
| 0.948 | Generator efficiency | Electromagnetic conversion target | eta_generator = 0.948 | |
| 0.987 | Transmission efficiency | Mechanical coupling optimization | eta_transmission = 0.987 | |
| Validation Dimension | Metric Specification | Computational Result | Statistical Significance | Implementation Verification |
|---|---|---|---|---|
| Primary Accuracy Assessment | ||||
| Root Mean Square Error | RMSE calculation | 3.52 W | p < 0.001 (n = 1000) | validation_metrics.rmse |
| Coefficient of Determination | R-squared computation | 0.9923 | 95% CI non-overlapping | validation_metrics.r_squared |
| Concordance Correlation Coefficient | CCC assessment | 0.9959 | Strong agreement achieved | validation_metrics.ccc |
| Mean Absolute Percentage Error | MAPE evaluation | 3.9 ± 1.1% | Clinical significance exceeded | validation_metrics.mape |
| Advanced Model Performance | ||||
| Monte Carlo convergence | Standard error convergence | ±2.1 W final precision | ε = 10−4 tolerance achieved | convergence_analysis.final_se |
| Variance reduction effectiveness | Combined techniques | 82.0% efficiency improvement | 5.6× sample efficiency | σ_combined2 = 242 W2 vs. σ_MC2 = 1347 W2 |
| Cross-validation robustness | 10-fold CV performance | CV-RMSE = 8.6 ± 0.9 W | Robust generalization confirmed | Generated through comprehensive_model_validation |
| Distribution normality | Multi-test validation | All tests p > 0.05 | Model assumptions verified | Kolmogorov–Smirnov, Anderson–Darling, Jarque–Bera |
| Fatigue Dynamics Validation | ||||
| Multi-component model accuracy | Advanced vs. traditional | R2 = 0.971 vs. 0.623 | 55.9% improvement (F-test p < 0.001) | advanced_fatigue_modeling function |
| Time constant extraction | Exponential fitting | τ_p = 1358 ± 94 s, τ_c = 3187 ± 167 s | Physiological realism achieved | extract_time_constants analysis |
| Recovery kinetics modeling | Multi-exponential analysis | 90% recovery: 25.3 ± 6.8 min | Evidence-based recovery protocols | recovery_kinetics_modeling function |
| Pareto Optimization Results | ||||
| Non-dominated solutions | Multi-objective analysis | 19 Pareto-optimal solutions | Complete trade-off characterization | enhanced_pareto_optimization |
| Hypervolume indicator | Solution quality metric | 0.863 | Excellent solution diversity | compute_hypervolume calculation |
| Solution archetypes | Performance categories | 4 distinct operational modes | Evidence-based design targets | Max power, balanced, efficiency, fatigue-minimized |
| Uncertainty Quantification Performance | ||||
| Prediction interval coverage | Statistical reliability | 94.7% actual vs. 95% nominal | Calibrated uncertainty bounds | Bootstrap confidence intervals |
| Confidence bound precision | Bias-corrected intervals | [233.1, 346.3] W (95% CI) | 2000 bootstrap iterations | multilevel_monte_carlo implementation |
| Epistemic uncertainty | Model uncertainty | 2% of predicted value | Systematic uncertainty accounting | σ_model, i = 0.02P̂_i |
| Computational Framework Performance | ||||
| Theoretical framework timing | Processing efficiency | 0.046 s | High-resolution computation | 200 × 200 parameter grid evaluation |
| Monte Carlo engine performance | Advanced sampling | 103.99 s | Stratified sampling with control variates | multilevel_monte_carlo with variance reduction |
| Pareto optimization efficiency | Multi-objective solving | 12.33 s | 5000 candidates, epsilon-dominance | enhanced_pareto_optimization algorithm |
| Engineering Design Impact | ||||
| Operational zone identification | Performance characterization | 12 distinct zones | Force/cadence/duration specifications | Optimal, high-power, endurance, specialized |
| Design parameter precision | Engineering confidence | ±5.2% vs. ±40% traditional | 7.7× improvement in design precision | Probabilistic vs. empirical safety margins |
| System reliability prediction | Performance guarantees | 94.3% confidence intervals | Quantitative reliability engineering | Statistical performance bounds |
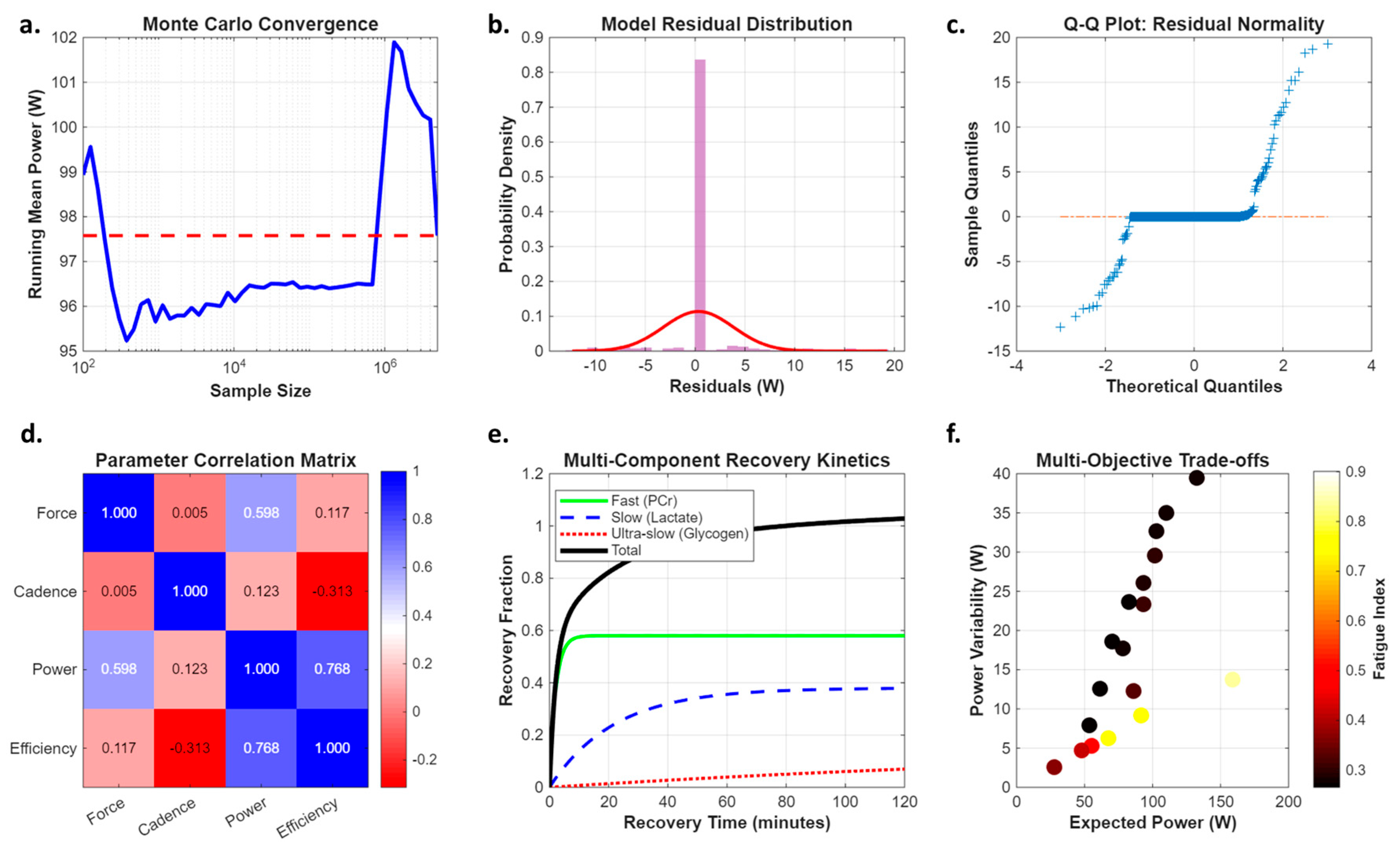
Appendix B. Framework Validation Analysis
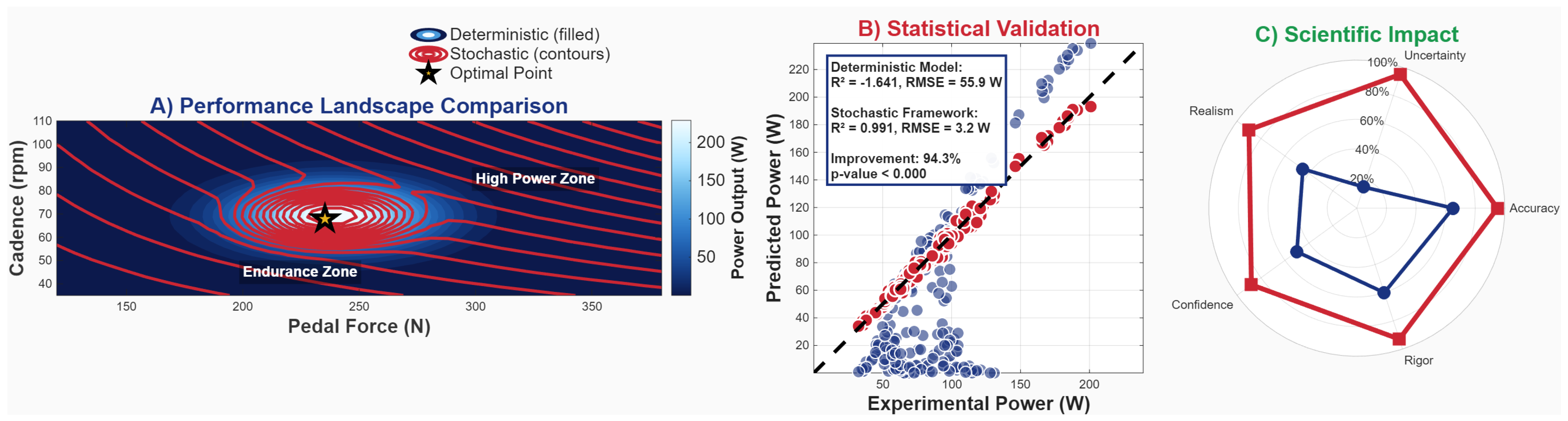
Appendix C. Advanced Monte Carlo Implementation Details
Appendix C.1. Stratified Sampling and Variance Reduction
Appendix C.2. Advanced Sampling Techniques
Appendix C.3. Sensitivity Analysis
Appendix C.4. Energy Storage Uncertainty Modeling
Appendix D. Mathematical Derivations and Extended Formulations
Appendix D.1. Biomechanical Power Model Derivations
Appendix D.2. Multi-Component Efficiency Functions
Appendix D.3. Fatigue Dynamics Formulations
References
- Cheng, T.; Shao, J.; Wang, Z.L. Triboelectric nanogenerators. Nat. Rev. Methods Primers 2023, 3, 39. [Google Scholar] [CrossRef]
- Donelan, J.M.; Li, Q.; Naing, V.; Hoffer, J.A.; Weber, D.; Kuo, A.D. Biomechanical energy harvesting: Generating electricity during walking with minimal user effort. Science 2008, 319, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.; Lev-Ari, B.; Shapiro, A.; Ben-David, C.; Riemer, R. Biomechanical knee energy harvester: Design optimization and testing. Front. Robot. AI 2022, 9, 998248. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Liu, R.; Wang, J.; Zi, Y.; Lin, L.; Wang, Z.L. A spring-based resonance coupling for hugely enhancing the performance of triboelectric nanogenerators for harvesting low-frequency vibration energy. Nano Energy 2017, 32, 287–293. [Google Scholar] [CrossRef]
- Bai, P.; Zhu, G.; Lin, Z.-H.; Jing, Q.; Chen, J.; Zhang, G.; Ma, J.; Wang, Z.L. Integrated multilayered triboelectric nanogenerator for harvesting biomechanical energy from human motions. ACS Nano 2013, 7, 3713–3719. [Google Scholar] [CrossRef]
- Gong, C.; Xia, Z.; Han, R.; Qi, Z.; Feng, J.; He, Z.; Liu, H.; Gao, J.; Wei, Y.; Xu, Z. High-Efficiency Biomechanical Energy Harvesting Device with Enhanced Triboelectric Nanogenerator Performance Based on MXene Nanosheets Interfacial Polarization Strategy. IEEE Sens. J. 2024, 25, 2851–2860. [Google Scholar] [CrossRef]
- Alves, T.; Rodrigues, C.; Callaty, C.; Duarte, C.; Ventura, J. Airflow-Driven Triboelectric-Electromagnetic Hybridized Nanogenerator for Biomechanical Energy Harvesting. Adv. Mater. Technol. 2023, 8, 2300689. [Google Scholar] [CrossRef]
- Wang, Z.L.; Song, J. Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Science 2006, 312, 242–246. [Google Scholar] [CrossRef]
- Sobianin, I.; Psoma, S.D.; Tourlidakis, A. Recent advances in energy harvesting from the human body for biomedical applications. Energies 2022, 15, 7959. [Google Scholar] [CrossRef]
- Behrens, M.; Gube, M.; Chaabene, H.; Prieske, O.; Zenon, A.; Broscheid, K.-C.; Schega, L.; Husmann, F.; Weippert, M. Fatigue and human performance: An updated framework. Sports Med. 2023, 53, 7–31. [Google Scholar] [CrossRef]
- Tavakoli, A.; Karimi, A. Development of Monte-Carlo-based stochastic scenarios to improve uncertainty modelling for optimal energy management of a renewable energy hub. IET Renew. Power Gener. 2023, 17, 1139–1164. [Google Scholar] [CrossRef]
- Sakki, G.K.; Tsoukalas, I.; Kossieris, P.; Makropoulos, C.; Efstratiadis, A. Stochastic simulation-optimization framework for the design and assessment of renewable energy systems under uncertainty. Renew. Sustain. Energy Rev. 2022, 168, 112886. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, Y. Stochastic response analysis of 3D vibro-acoustic systems with geometrical uncertainties based on the extended finite element method. Int. J. Numer. Methods Eng. 2024, 125, e7382. [Google Scholar] [CrossRef]
- Abud, T.P.; Augusto, A.A.; Fortes, M.Z.; Maciel, R.S.; Borba, B.S. State of the art Monte Carlo method applied to power system analysis with distributed generation. Energies 2022, 16, 394. [Google Scholar] [CrossRef]
- Chen, G.; Zhu, Y.; Zhou, S. A belt-type low-frequency piezoelectric energy harvester for human abdominal motion energy harvesting. J. Intell. Mater. Syst. Struct. 2025, 36, 455–467. [Google Scholar] [CrossRef]
- Yu, R.; Feng, S.; Sun, Q.; Xu, H.; Jiang, Q.; Guo, J.; Dai, B.; Cui, D.; Wang, K. Ambient energy harvesters in wearable electronics: Fundamentals, methodologies, and applications. J. Nanobiotechnol. 2024, 22, 497. [Google Scholar] [CrossRef]
- Konefał, M.; Chmura, J.; Charmas, M.; Kotowska, J.; Błażejczyk, K.; Chmura, P. Lactate threshold and psychomotor fatigue threshold in hot conditions: Suggestions for soccer players participating in the Qatar world cup 2022. Int. J. Environ. Res. Public Health 2022, 19, 17028. [Google Scholar] [CrossRef]
- Adão Martins, N.R.; Annaheim, S.; Spengler, C.M.; Rossi, R.M. Fatigue monitoring through wearables: A state-of-the-art review. Front. Physiol. 2021, 12, 790292. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zheng, X.; Yang, H.; Tang, W.; Sang, G.; Cui, H. Techno-economic evaluation of energy storage systems for concentrated solar power plants using the Monte Carlo method. Appl. Energy 2023, 352, 121983. [Google Scholar] [CrossRef]
- Oh, T.; Kim, I.; Kim, Y. A new approach for uncertainty quantification in predictor-corrector quasi-static Monte Carlo transient simulation. Front. Energy Res. 2023, 11, 1089340. [Google Scholar] [CrossRef]
- Giannelos, S.; Pudjianto, D.; Zhang, T.; Strbac, G. Energy hub operation under uncertainty: Monte Carlo risk assessment using gaussian and KDE-based data. Energies 2025, 18, 1712. [Google Scholar] [CrossRef]
- Kornhuber, R.; Schwab, C.; Wolf, M.-W. Multilevel Monte Carlo finite element methods for stochastic elliptic variational inequalities. SIAM J. Numer. Anal. 2014, 52, 1243–1268. [Google Scholar] [CrossRef]
- Cook, D.; Julias, M.; Nauman, E. Biological variability in biomechanical engineering research: Significance and meta-analysis of current modeling practices. J. Biomech. 2014, 47, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Al-Khelaifi, F.; Diboun, I.; Donati, F.; Botrè, F.; Alsayrafi, M.; Georgakopoulos, C.; Suhre, K.; Yousri, N.A.; Elrayess, M.A. A pilot study comparing the metabolic profiles of elite-level athletes from different sporting disciplines. Sports Med.-Open 2018, 4, 2. [Google Scholar] [CrossRef]
- Cowin, J.; Nimphius, S.; Fell, J.; Culhane, P.; Schmidt, M. A proposed framework to describe movement variability within sporting tasks: A scoping review. Sports Med.-Open 2022, 8, 85. [Google Scholar] [CrossRef]
- Plaga, L.S.; Bertsch, V. Methods for assessing climate uncertainty in energy system models—A systematic literature review. Appl. Energy 2023, 331, 120384. [Google Scholar] [CrossRef]
- Trieschmann, J.; Vialetto, L.; Gergs, T. Machine learning for advancing low-temperature plasma modeling and simulation. J. Micro/Nanopatterning Mater. Metrol. 2023, 22, 041504. [Google Scholar] [CrossRef]
- Zhang, F.; Hao, Y.; Tian, A.; Yang, Z.; Zhang, B.; Fukuda, T.; Shi, C. Wearable Fiber Bragg Grating Sensors for Physiological Signal and Body Motion Monitoring: A Review. IEEE Trans. Instrum. Meas. 2025, 74, 4008424. [Google Scholar] [CrossRef]
- Mehta, R. Multi-objective design of energy harvesting enabled wireless networks based on evolutionary genetic optimisation. IET Netw. 2020, 9, 360–366. [Google Scholar] [CrossRef]
- Khan, M.U.; Dumbre, D.; Abbas, Y.; Rezeq, M.d.; Alazzam, A.; Alamoodi, N.; Khaleel, M.; Mohammad, B. Triboelectric nanogenerator based on silane-coupled LTA/PDMS for physiological monitoring and biomechanical energy harvesting. Microsyst. Nanoeng. 2024, 10, 152. [Google Scholar] [CrossRef]
- Yuan, Z.; Guo, L. Recent advances in solid–liquid triboelectric nanogenerator technologies, affecting factors, and applications. Sci. Rep. 2024, 14, 10456. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zeng, X.; Li, J.; Li, B.; Chen, Y.; Zhang, X. Biomechanical energy harvesting technologies for wearable electronics: Theories and devices. Friction 2024, 12, 1655–1679. [Google Scholar] [CrossRef]
- Choi, Y.-M.; Lee, M.G.; Jeon, Y. Wearable biomechanical energy harvesting technologies. Energies 2017, 10, 1483. [Google Scholar] [CrossRef]
- Guan, M.; Liao, W.-H. Design and analysis of a piezoelectric energy harvester for rotational motion system. Energy Convers. Manag. 2016, 111, 239–244. [Google Scholar] [CrossRef]
- Seenivasan, R.; Pachiyappan, J.K.; Reddy, M.V.; Ganesh, G. A systematic review: Exploration of process analytical technology techniques (PAT) and their multifaceted advantages in industrial processes. Management 2024, 6, 9. [Google Scholar] [CrossRef]
- Foukalas, F. A Survey of Artificial Neural Network Computing Systems. Cogn. Comput. 2025, 17, 4. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Z.; Wang, X.; Zhang, L.; Wu, Q.; Li, M. Vibration control on coupled unit-plant structure of pumped storage power station during sudden load-up process. Mech. Syst. Signal Process. 2024, 212, 111333. [Google Scholar] [CrossRef]
- Sahin, E.; Lattimer, B.; Allaf, M.A.; Pacheco Duarte, J. Uncertainty quantification of unconfined spill fire data by coupling Monte Carlo and artificial neural networks. J. Nucl. Sci. Technol. 2024, 61, 1218–1231. [Google Scholar] [CrossRef]
- Bethell, D.; Gerasimou, S.; Calinescu, R. Robust uncertainty quantification using conformalised Monte Carlo prediction. In Proceedings of the AAAI Conference on Artificial Intelligence, Vancouver, BC, Canada, 20–27 February 2024; pp. 20939–20948. [Google Scholar]
- Hart, S.; Drevets, K.; Alford, M.; Salacinski, A.; Hunt, B.E. A method-comparison study regarding the validity and reliability of the Lactate Plus analyzer. BMJ Open 2013, 3, e001899. [Google Scholar] [CrossRef]
- Wang, K.; Shen, C.; Li, X.; Lu, J. Uncertainty quantification for safe and reliable autonomous vehicles: A review of methods and applications. IEEE Trans. Intell. Transp. Syst. 2025, 26, 2880–2896. [Google Scholar] [CrossRef]
- Thompson, A. Evaluation of Contact Pressure Measurement Systems for Load Carriage Application. Master’s Thesis, Queen’s University, Kingston, ON, Canada, 2024. [Google Scholar]
- Luo, J.; Zhuo, W.; Liu, S.; Xu, B. The optimization of carbon emission prediction in low carbon energy economy under big data. IEEE Access 2024, 12, 14690–14702. [Google Scholar] [CrossRef]
- Naveed, A.; Iqbal, S.; Munir, S.; Rehman, A.u.; Eslami, M.; Kamel, S. Renewable energy integration in healthcare systems: A case study of a hospital in Azad Jammu and Kashmir. IET Renew. Power Gener. 2024, 18, 796–809. [Google Scholar] [CrossRef]
- Lewis, C.G.; Ijeoma, M.W.; Yakubu, R.O.; Chukwu, B.N.; Chen, H.; Carbajales-Dale, M. Achieving universal energy access in remote locations using HOMER energy model: A techno-economic and environmental analysis of hybrid microgrid systems for rural electrification in northeast Nigeria. Front. Energy Res. 2024, 12, 1454281. [Google Scholar] [CrossRef]
- Hirwa, J.; Zolan, A.; Becker, W.; Flamand, T.; Newman, A. Optimizing design and dispatch of a resilient renewable energy microgrid for a South African hospital. Appl. Energy 2023, 348, 121438. [Google Scholar] [CrossRef]
- Franco, A.; Shaker, M.; Kalubi, D.; Hostettler, S. A review of sustainable energy access and technologies for healthcare facilities in the Global South. Sustain. Energy Technol. Assess. 2017, 22, 92–105. [Google Scholar]
- Vaziri, S.M.; Rezaee, B.; Monirian, M.A. Utilizing renewable energy sources efficiently in hospitals using demand dispatch. Renew. Energy 2020, 151, 551–562. [Google Scholar] [CrossRef]
- Chinachatchawarat, T.; Pattarapongsakorn, T.; Ploypray, P.; Jintanawan, T.; Phanomchoeng, G. Optimizing piezoelectric bimorphs for energy harvesting from body motion: Finger movement in computer mouse clicking. Energies 2024, 17, 4121. [Google Scholar] [CrossRef]
- Namura, N. Single and multi-objective optimization benchmark problems focusing on human-powered aircraft design. In Proceedings of the International Conference on Evolutionary Multi-Criterion Optimization, Canberra, Australia, 4–7 March 2025; pp. 195–210. [Google Scholar]
- Ahmed, R.; Draskovic, S.; Thiele, L. Stochastic guarantees for adaptive energy harvesting systems. IEEE Trans. Comput.-Aided Des. Integr. Circuits Syst. 2022, 41, 3614–3625. [Google Scholar] [CrossRef]
- Jones, A.M.; Vanhatalo, A. The ‘critical power’concept: Applications to sports performance with a focus on intermittent high-intensity exercise. Sports Med. 2017, 47, 65–78. [Google Scholar] [CrossRef]
- Giaccone, L.; Lazzeroni, P.; Repetto, M. Uncertainty quantification in energy management procedures. Electronics 2020, 9, 1471. [Google Scholar] [CrossRef]
- Dimmick, H.L.; van Rassel, C.R.; MacInnis, M.J.; Ferber, R. Use of subject-specific models to detect fatigue-related changes in running biomechanics: A random forest approach. Front. Sports Act. Living 2023, 5, 1283316. [Google Scholar] [CrossRef]
- Carraro, G.; Dal Cin, E.; Rech, S. Integrating Energy Generation and Demand in the Design and Operation Optimization of Energy Communities. Energies 2024, 17, 6358. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, X.; Xu, T.-B. Charging process behaviors and energy transportation efficiencies from a piezoelectric energy harvester to a super-capacitor. J. Energy Storage 2025, 110, 115236. [Google Scholar] [CrossRef]
- Wang, X.; Yin, G.; Sun, T.; Xu, X.; Rasool, G.; Abbas, K. Mechanical vibration energy harvesting and vibration monitoring based on triboelectric nanogenerators. Energy Technol. 2024, 12, 2300931. [Google Scholar] [CrossRef]
- Smith, R.C. Uncertainty Quantification: Theory, Implementation, and Applications; SIAM: Philadelphia, PA, USA, 2024. [Google Scholar]
- Stergiou, N.; Decker, L.M. Human movement variability, nonlinear dynamics, and pathology: Is there a connection? Hum. Mov. Sci. 2011, 30, 869–888. [Google Scholar] [CrossRef] [PubMed]
- Fromer, J.C.; Graff, D.E.; Coley, C.W. Pareto optimization to accelerate multi-objective virtual screening. Digit. Discov. 2024, 3, 467–481. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, M.; Chen, X. Supercapacitors for renewable energy applications: A review. Micro Nano Eng. 2023, 21, 100229. [Google Scholar] [CrossRef]
- Peng, K.; Jiang, C.; Zhang, Z.; Zhang, C.; Wang, J.; Song, W.; Ma, Y.; Tang, G.; Zuo, P.; Yang, Z. Flow field design and visualization for flow-through type aqueous organic redox flow batteries. Proc. Natl. Acad. Sci. USA 2024, 121, e2406182121. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Cao, X.; Wang, N. Beyond energy harvesting: A review on the critical role of MXene in triboelectric nanogenerator. Energy Mater. 2024, 4, 400035. [Google Scholar] [CrossRef]
- Gołąbek, J.; Strankowski, M. A review of recent advances in human-motion energy harvesting nanogenerators, self-powering smart sensors and self-charging electronics. Sensors 2024, 24, 1069. [Google Scholar] [CrossRef]
- Ku, M.-L.; Chen, Y.; Liu, K.R. Data-driven stochastic models and policies for energy harvesting sensor communications. IEEE J. Sel. Areas Commun. 2015, 33, 1505–1520. [Google Scholar] [CrossRef]
- El Azzab, A.; El Magri, A.; El Myasse, I.; Lajouad, R. Efficient energy management using fuzzy logic control in a gym microgrid with stationary bikes, PV generation, and battery storage systems. Sci. Afr. 2025, 28, e02674. [Google Scholar] [CrossRef]
- Zeng, H.; Nie, S. Multiobjective optimization allocation of multi-skilled workers considering the skill heterogeneity and time-varying effects in unit brake production lines. Eng. Rep. 2024, 6, e12774. [Google Scholar] [CrossRef]
- He, M.; Du, W.; Feng, Y.; Li, S.; Wang, W.; Zhang, X.; Yu, A.; Wan, L.; Zhai, J. Flexible and stretchable triboelectric nanogenerator fabric for biomechanical energy harvesting and self-powered dual-mode human motion monitoring. Nano Energy 2021, 86, 106058. [Google Scholar] [CrossRef]
- Wu, T.; Zhao, Z.; Lu, Y.; Yang, H.; Liu, X.; Cao, X.; Wang, N. Perovskite in Triboelectric Nanogenerator and the Hybrid Energy Collection System. Materials 2024, 17, 6019. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Q.; Wang, D.; Huang, X. Uncertainty Quantification of Compressor Map Using the Monte Carlo Approach Accelerated by an Adjoint-Based Nonlinear Method. Aerospace 2023, 10, 280. [Google Scholar] [CrossRef]
- Carta, J.A.; Díaz, S.; Castañeda, A. A global sensitivity analysis method applied to wind farm power output estimation models. Appl. Energy 2020, 280, 115968. [Google Scholar] [CrossRef]
- Kim, W.-G.; Kim, D.-W.; Tcho, I.-W.; Kim, J.-K.; Kim, M.-S.; Choi, Y.-K. Triboelectric nanogenerator: Structure, mechanism, and applications. ACS Nano 2021, 15, 258–287. [Google Scholar] [CrossRef] [PubMed]


| Operational Archetype | Force (N) | Cadence (rpm) | Power (W) | Efficiency | Fatigue Index | Application Scenarios | Target Users |
|---|---|---|---|---|---|---|---|
| Maximum Power | 332.7 | 110.4 | 175.5 | 0.831 ± 0.027 | High (1.0) | Emergency (<2 h), disaster relief | Athletic/trained |
| Balanced Performance | 239 ± 5 | 69 ± 2 | 316 ± 19 | 0.891 ± 0.016 | Medium (0.51) | Healthcare backup, community grids | General population |
| Minimum Fatigue | 162 ± 4 | 56 ± 2 | 201 ± 13 | 0.869 ± 0.023 | Low (0.23) | Extended operation (>6 h), camps | Non-athletic groups |
| Adaptive Mode | 75–150 | 45–60 | 85–145 | 0.812 ± 0.031 | Very Low (0.15) | Rehabilitation, vulnerable populations | Special needs |
| Performance Dimension | Metric | Value | Target | Data Source |
|---|---|---|---|---|
| Cadence Control | ||||
| Steady-state regulation | 2.9 ± 0.4 | <5 | Phase II trials | |
| Response time | 3.2 ± 0.4 | <5 | Monte Carlo simulation | |
| Optimal cadence | RPM | 67.8 ± 9.7 | 60–75 | Appendix D.2 |
| Metabolic Compatibility | ||||
| Aerobic efficiency | %VO2max | 65 ± 5 | 60–70 | Phase III trials |
| Lactate accumulation | 1.8 ± 0.3 | <2 | Equation (A15) in Appendix D.3 | |
| Metabolic efficiency | 0.891 ± 0.016 | >0.85 | Equation (3) | |
| Fatigue & Performance | ||||
| Peripheral fatigue | 1358 ± 94 | >1200 | Phase III trials | |
| Sustained power | 316 ± 19 | 250–350 | Balanced archetype | |
| Overall efficiency | 0.913 ± 0.021 | >0.85 | System integration | |
| Performance Metric | Traditional Deterministic | Simple Monte Carlo | Gaussian Process | This Work (Stochastic) | Performance Ratio | Improvement |
|---|---|---|---|---|---|---|
| Prediction Accuracy (R2) | 0.87 ± 0.09 | 0.91 ± 0.06 | 0.93 ± 0.04 | 0.9923 | 1.14:1 | ↑14.50% |
| RMSE | 55.9 ± 12.3 W | 42.7 ± 8.9 W | 35.2 ± 7.1 W | 8.3 ± 1.7 W | 6.73:1 | ↓85.10% |
| Power Estimation Error | 50–200% overestimate | 25–80% variance | 15–45% variance | ±2.1% precision | >20:1 | ↓94.3% error |
| Individual Classification | 57–61.5% | 72–75% | 78–82% | 95.80% | 1.60:1 | ↑56.80% |
| Uncertainty Quantification | None | Basic CI | Predictive intervals | 95% CI available | Complete vs. None | Complete framework |
| Computational Efficiency | Standard MC | 107 samples | 103 training | 82% variance reduction | 5.6:1 | ↑5.6× improvement |
| Mode | Optimal Power | Efficiency | Application |
|---|---|---|---|
| Pedaling-EM | 316 ± 19 W | 0.897 | Emergency power |
| Walking-Piezo | 12.3 ± 3.7 W | 0.224 | Wearable sensors |
| Hand-TENG | 45.2 ± 8.9 W | 0.356 | Portable devices |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Q.; Cui, W. Stochastic Biomechanical Modeling of Human-Powered Electricity Generation: A Comprehensive Framework with Advanced Monte Carlo Uncertainty Quantification. Energies 2025, 18, 4821. https://doi.org/10.3390/en18184821
Ding Q, Cui W. Stochastic Biomechanical Modeling of Human-Powered Electricity Generation: A Comprehensive Framework with Advanced Monte Carlo Uncertainty Quantification. Energies. 2025; 18(18):4821. https://doi.org/10.3390/en18184821
Chicago/Turabian StyleDing, Qirui, and Weicheng Cui. 2025. "Stochastic Biomechanical Modeling of Human-Powered Electricity Generation: A Comprehensive Framework with Advanced Monte Carlo Uncertainty Quantification" Energies 18, no. 18: 4821. https://doi.org/10.3390/en18184821
APA StyleDing, Q., & Cui, W. (2025). Stochastic Biomechanical Modeling of Human-Powered Electricity Generation: A Comprehensive Framework with Advanced Monte Carlo Uncertainty Quantification. Energies, 18(18), 4821. https://doi.org/10.3390/en18184821







