Abstract
We fabricated dye-sensitized solar cells (DSSCs) co-sensitized with the organic dye YD2 and a spiropyran derivative (SPNO2), a photochromic molecule capable of reversible isomerization under light irradiation. Upon UV exposure, SPNO2 converts from its closed spiropyran (SP) form to the open photomerocyanine (PMC) form, which absorbs visible light and changes the optical properties of the photoelectrode. Spectroscopic analysis showed an 18% decrease in transmittance at 540 nm after UV irradiation and a 10% increase following visible light exposure. These changes were accompanied by a 0.5% increase in power conversion efficiency (η) after 5 min of UV irradiation, and a 0.83% decrease after 10 min of visible light. Although direct electron injection from PMC into TiO2 appears inefficient, the enhanced performance is attributed to Förster resonance energy transfer (FRET) from PMC to YD2. This photoresponsive behavior highlights a co-sensitization strategy that combines dynamic optical control and efficient energy transfer. Our findings demonstrate a promising approach to designing smart DSSCs with externally tunable photovoltaic properties using photochromic sensitizers.
1. Introduction
As a representative class of emerging photovoltaic technologies, dye-sensitized solar cells (DSSCs) have attracted considerable attention due to their economical fabrication, tunable optical features, such as coloration and semi-transparency, mechanical compliance, and satisfactory energy conversion capability, making them viable candidates for integration into applications beyond traditional silicon photovoltaics [1,2,3,4,5,6,7,8]. Recent advances have enabled DSSCs to achieve power conversion efficiency (PCE) as high as 15.2% under AM 1.5G standard solar illumination [8]. Among the various organic sensitizers developed for DSSCs, the zinc porphyrin derivative known as YD2 (Figure 1), formally designated as [5-[Bis(4-hexylphenyl)amino]-1,5-[2-(4-carboxyphenyl)ethynyl]-10,20-bis(3,5-di-tert-butylphenyl)porphyrinato]zinc(II), stands out due to its strong and broad absorption across the visible spectrum, high molar absorptivity [1], and favorable alignment for electron injection into TiO2-based photoanodes. Its relatively simple architecture and reduced dependence on rare elements also make YD2 a cost-effective option. Notably, DSSCs incorporating YD2 have demonstrated PCEs reaching up to 11.0% [9]. To further improve device performance, co-sensitization strategies have been explored wherein YD2 is paired with complementary dyes. For instance, dye mixtures combining YD2 and ZnPc3 in a 3:1 ratio have been shown to broaden the light absorption window and enhance charge separation [9,10,11]. The broader importance of co-sensitization strategies in DSSCs has been highlighted in both early and recent studies. A systematic analysis of co-sensitization effects was reported by Zhang et al., demonstrating how complementary dye combinations can significantly influence charge injection and overall device efficiency [12]. More comprehensive reviews have summarized the progress and design principles of co-sensitized DSSCs, emphasizing molecular compatibility, spectral complementarity, and interfacial charge dynamics [13]. Building on these foundations, Keremane et al. recently investigated co-sensitization effects in detail using new dye architectures, providing fresh insights into light-harvesting mechanisms and stability enhancement [14]. Together, these works establish co-sensitization as a robust and versatile approach to improving DSSC performance. Additionally, Förster resonance energy transfer (FRET) has emerged as a promising mechanism to facilitate exciton migration and improve charge generation efficiency in co-sensitized systems [15,16,17,18,19,20]. Beyond chemical optimization, external stimuli such as mechanical compression, magnetic fields, thermal activation, and light irradiation have also been investigated to dynamically modulate DSSC behavior [21,22,23,24]. In this study, we report on DSSCs co-sensitized with YD2 and the photochromic spiropyran derivative SPNO2 (Figure 2), examining their reversible modulation of optical absorption and photovoltaic performance under alternating UV and visible light exposure.
Figure 1.
Molecular structure of YD2.
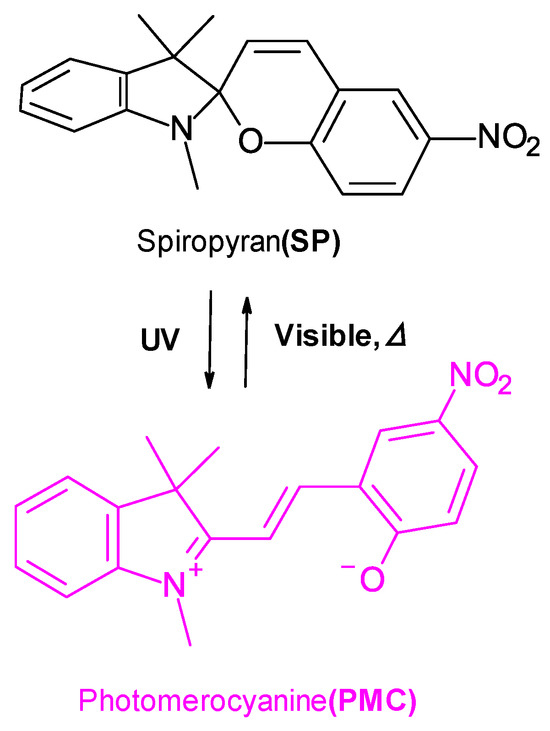
Figure 2.
Reversible photoinduced isomerization of SPNO2 between the closed spiropyran (SP) and open photomerocyanine (PMC) forms under UV and visible light irradiation.
Photochromic molecules, particularly spiropyran derivatives, have been studied as functional additives in dye-sensitized solar cells (DSSCs) due to their ability to reversibly alter both optical appearance and photovoltaic performance in response to external light stimuli [23,25,26]. For example, Dryza and co-workers examined the photochemical behavior of 1,3,3-trimethylindolino-6′-nitrobenzopyrylospiran (SPNO2) embedded within γ-cyclodextrin cavities on metal oxide nanoparticle substrates. Under ultraviolet (UV) irradiation, SPNO2 undergoes a reversible transformation from the non-absorbing spiro (SP) form to the colored photomerocyanine (PMC) form (Figure 2), which changes the absorption characteristics and may interact with neighboring sensitizers such as YD2. In particular, Förster resonance energy transfer (FRET) to SQ2—a squaraine-based dye recognized for its strong absorption in the visible spectrum and efficient electron injection—has been suggested [27]. In our earlier work, we provided detailed characterization of SPNO2’s isomerization and optical switching within SQ2-based DSSCs [28]. Departing from that context, this study focuses on YD2-based cells to investigate the role of SPNO2 as a co-sensitizer with tunable optical and electronic behavior. We previously demonstrated that combining SPNO2 with SQ2 allowed control over both coloration and power output through light irradiation [28]. Extending that concept, we now apply a similar strategy to YD2-only systems, capitalizing on YD2’s strong absorption and high photovoltaic capability. Accordingly, DSSCs incorporating both YD2 and SPNO2 were fabricated and evaluated for light-responsive modulation. The SPNO2 component serves as a photoresponsive sensitizer, undergoing reversible interconversion between the SP and PMC forms when exposed to UV or visible light. We propose that FRET from PMC to YD2 facilitates tunable absorption behavior and enhances photoinduced charge separation. To validate this, we conducted spectral and electrical measurements under alternating illumination and assessed FRET efficiency through fluorescence lifetime analysis using photoanodes sensitized with PMC, either alone or combined with YD2.
2. Materials and Methods
All chemicals and solvents utilized in this study were either analytical- or spectroscopic-grade and were used without additional purification. The TiO2 paste (Ti-nanoxide T/SP), iodide-based electrolyte solution (Iodolyte Z-50), and the porphyrin dye YD2 were obtained from Solaronix (Aubonne, Switzerland). The photochromic compound 1,3,3-trimethylindolino-6′-nitrobenzopyrylo-spiran (SPNO2) was purchased from Tokyo Kasei (Tokyo, Japan). Fluorine-doped tin oxide (FTO) glass substrates (sheet resistance: 9.3 Ω/□) were provided by Asahi Glass Co., Ltd. (Tokyo, Japan) Spectroscopy-grade benzene and ethanol were sourced from Wako Pure Chemical Industries (Odawara, Japan).
Photoanode fabrication involved depositing TiO2 paste onto FTO glass using the doctor-blade method. After coating, the substrates were gradually heated in air to 450 °C over 50 min and sintered at that temperature for 1 h. The resulting mesoporous TiO2 films had a circular photoactive area approximately 0.6 cm in diameter.
Two types of photoanodes were prepared: one sensitized only with YD2 and the other co-sensitized with both YD2 and SPNO2. For the former, TiO2 electrodes were immersed in an ethanolic YD2 solution (3.0 × 10−4 M) at room temperature for 24 h. For co-sensitization, these YD2-loaded electrodes were additionally soaked in a 0.16 M benzene solution of SPNO2 for 30 min.
Counter electrodes were prepared by depositing a platinum layer onto clean FTO glass via sputtering in an argon environment. The final DSSCs were assembled in a sandwich-like configuration using an 80 μm thick thermoplastic film (Roland DGS-305-BK, Roland DG Corporation, Shizuoka, Japan) as a spacer. Electrolyte infiltration between electrodes was achieved through capillary action.
The optical absorption spectra of photoanodes were recorded with a UV-Vis spectrophotometer (Hitachi U-3310, Hitachi High-Tech Corporation, Tokyo, Japan). Emission spectra and fluorescence lifetimes (τ) were measured using a fluorescence spectrometer (PerkinElmer LS55, PerkinElmer, Inc., Shelton, CT, USA) and a time-resolved fluorescence spectrometer (Hamamatsu C11367, Hamamatsu Photonics K.K., Hamamatsu City, Japan), respectively.
Photovoltaic performance was evaluated under AM1.5 simulated sunlight using a 500 W xenon lamp (Ushio SX-UI502XQ, Ushio Inc., Tokyo, Japan), with light intensity calibrated via a Melles Griot 13PEM001 power meter (Melles Griot, Boulder, CO, USA). J–V curves were obtained with a source-measure unit (Advantest R6243, Advantest Corporation, Yokohama-shi, Japan), while monochromatic J–V testing was performed using a Keysight B2901A (Keysight Technologies Inc., Santa Rosa, CA, USA) source and MT10-T monochromator (Bunkoukeiki, Tokyo, Japan). Illumination intensity was validated with Thorlabs PM400 (Thorlabs, Inc., Newton, NJ, USA) and Melles Griot power meters (Melles Griot, Boulder, CO, USA).
External light-responsive tests involved exposure to UV and visible light from an Alfa Mirage MR-UV system, delivering doses of 6 J (UV) and 90 J (Vis), respectively. All measurements were carried out at room temperature. During solar performance evaluations, a 0.16 cm2 aperture mask defined the active cell area. Device metrics including short-circuit current density (Jsc), open-circuit voltage (Voc), fill factor (FF), overall power conversion efficiency (η), and incident photon-to-current efficiency (IPCE) were assessed. Data analysis and visualization were conducted using Origin® 2023b (OriginLab, Northampton, MA, USA).
3. Results and Discussion
Upon immersing the TiO2 photoelectrode in a benzene solution containing YD2-only, the electrode surface quickly shifted from colorless to green. As illustrated in Figure 3, this change was accompanied by a transmittance spectrum featuring an absorption band spanning 300–750 nm. This spectral feature aligns with the characteristic absorption of YD2-only in benzene, implying that YD2-only molecules were successfully adsorbed onto the TiO2 surface. Subsequent immersion of the YD2-only coated electrode in a benzene solution containing SPNO2 resulted in further spectral changes, with a noticeable reduction in transmittance observed around 500–650 nm (red trace in Figure 3). In addition, the electrode’s appearance changed from green to a reddish-green hue, suggesting the adsorption of SPNO2 onto the YD2-only anchored surface. SPNO2 exhibits absorption in the visible region, where its isomers—SP and PMC—have peak absorptions at approximately 350 nm and 540 nm, respectively. Therefore, the observed decline in transmittance between 540–560 nm can be specifically attributed to the formation of the PMC isomer upon UV irradiation, confirming its photoconversion on the electrode surface.
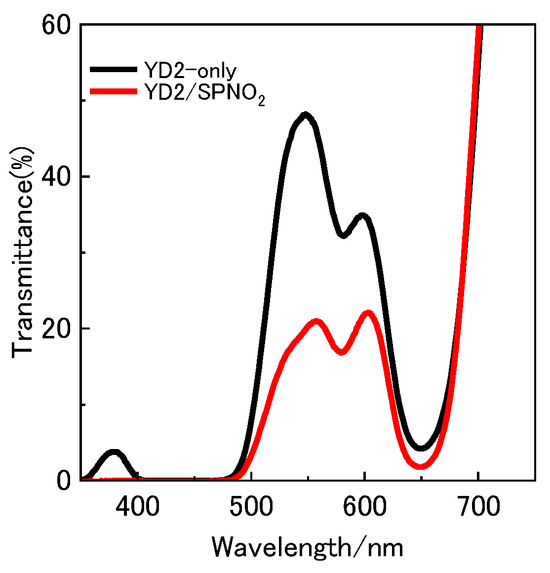
Figure 3.
Ultraviolet–visible (UV–Vis) transmittance spectra of TiO2-based photoelectrodes sensitized with YD2-only (black curve) and co-sensitized with YD2 and SPNO2 (red curve). The spectra highlight the reduced transmittance in the visible region (500–650 nm) due to SPNO2 adsorption, particularly in its photoinduced PMC form.
The current–voltage (J–V) characteristics of DSSCs incorporating YD2-only alone and co-sensitized with YD2/SPNO2 were measured under AM1.5 illumination, as shown by the black and red curves in Figure 4A. The data confirm that electron injection from YD2-only into the conduction band of TiO2 nanoparticles was effectively promoted via its photosensitization. The energy conversion efficiencies (η) for the devices containing only YD2-only and those co-sensitized with YD2/SPNO2 were found to be 1.24% and 0.71%, respectively. This decrease in efficiency is likely due to the reduced light absorption by YD2-only, which may be caused by surface adsorption of SPNO2 (both SP and PMC forms) on the electrode.
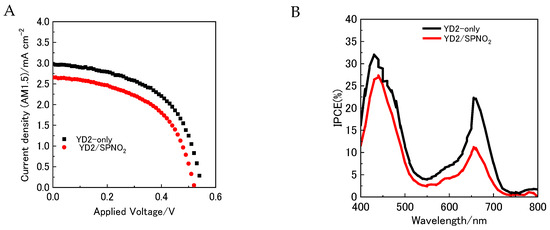
Figure 4.
(A) Current density–voltage (J–V) characteristics of dye-sensitized solar cells (DSSCs) fabricated with YD2-only (black curve) and YD2/SPNO2 co-sensitized (red curve) photoelectrodes under simulated AM1.5 solar illumination (100 mW cm−2). A circular aperture mask (0.16 cm2) was employed to define the active area. (B) Incident photon-to-current conversion efficiency (IPCE) spectra of the same DSSCs measured under monochromatic illumination in the 400–800 nm wavelength range.
The incident photon-to-current efficiency (IPCE) spectra of DSSCs incorporating YD2-only alone and those co-sensitized with YD2/SPNO2 were recorded under illumination in the 400–800 nm wavelength range (Figure 4B, black and red curves). A prominent peak appears at approximately 440 nm, and the overall spectral profile resembles the transmittance behavior of YD2-only adsorbed on TiO2.
The surface color of the photoelectrode containing both YD2/SPNO2 exhibited dynamic changes upon alternating light irradiation, as shown in the inset in Figure 5. Following 5 min of UV exposure, the initially green electrode turned red; subsequent visible light irradiation for 10 min restored the green coloration, which again shifted to red after an additional 5-min UV treatment. The electrode appeared green at the outset due to the presence of green-colored YD2-only and the nearly transparent SP form of SPNO2 adsorbed on TiO2 [22,26]. Upon UV irradiation, the formation of the red-colored isomer PMC from SPNO2 accounted for the reddish hue, while YD2-only retained its original green appearance [22,26].
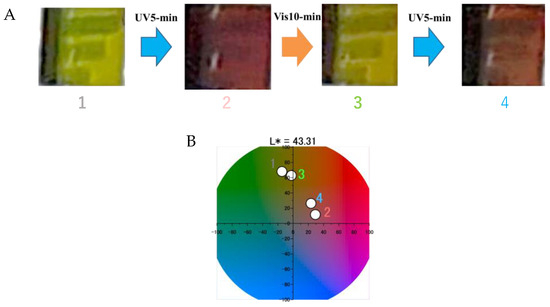
Figure 5.
Photographs showing the photoisomerization behavior of the YD2/SPNO2-sensitized TiO2 electrode under alternating ultraviolet (UV) and visible (Vis) light irradiation. (A) Sequential images labeled 1–4: (1) natural state; (2) after 5 min UV irradiation; (3) after 5 min UV followed by 10 min Vis irradiation; (4) after 5 min UV + 10 min Vis + additional 5 min UV irradiation. (B) Corresponding CIE Lab chromaticity diagram indicating the color changes induced by the isomerization of SPNO2 on the TiO2 surface, with numbers 1–4 matching the conditions shown in (A).
Upon immersing the YD2-only coated TiO2 photoelectrode in a benzene solution containing SPNO2, its surface color rapidly shifted from green to a reddish-green hue. The corresponding transmittance spectrum (black line in Figure 6) displayed near-zero transmittance in the 400–500 nm region. As also seen in Figure 3, this spectral pattern closely corresponds to the absorption characteristics of YD2-only in benzene, indicating a physical interaction—likely physisorption—between SPNO2 and the pre-adsorbed YD2-only layer. Figure 6A presents the transmittance spectra of the YD2/SPNO2-modified photoelectrode following sequential light exposures: 5 min of UV (red), 10 min of visible light (green), and an additional 5 min of UV (blue). Figure 6B highlights the variation in transmittance at 540 nm for each of these conditions. The PMC isomer, which absorbs at this wavelength, exhibited an 18% decrease in transmittance after the first UV irradiation, followed by a 10% recovery upon visible light exposure, and then a further 7.5% reduction after the final UV irradiation. These fluctuations at 540 nm support the reversible formation of the PMC isomer on the TiO2 surface in response to alternating light stimuli.
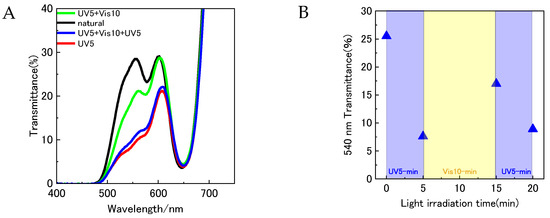
Figure 6.
(A) UV–Vis transmittance spectra of YD2/SPNO2-sensitized TiO2 photoelectrodes under sequential light irradiation conditions: natural light (black line), UV irradiation for 5 min (red line), UV for 5 min followed by visible light for 10 min (green line), and UV for 5 min + Vis for 10 min + UV for 5 min (blue line). (B) Evolution of transmittance intensity at 540 nm (red circles) corresponding to the first irradiation (UV, 5 min), second irradiation (Vis, 10 min), and third irradiation (UV, 5 min), highlighting the reversible modulation behavior.
Figure 7A (black markers) shows the current–voltage (J–V) characteristics of DSSCs incorporating both YD2/SPNO2 under AM1.5 simulated sunlight. The measurements indicate that YD2-only facilitated electron injection into the conduction band of TiO2 nanoparticles through its photosensitizing function. The device’s photovoltaic behavior was also evaluated under different external light conditions: UV irradiation for 5 min, visible light for 10 min, and a subsequent 5-min UV exposure (red, green, and blue markers in Figure 7A, respectively). The corresponding energy conversion efficiencies (η), derived from Figure 7B, changed in sequence from 0.70% to 1.21%, then dropped to 0.38%, and slightly increased to 0.45% with alternating light exposure. These findings suggest that the PMC isomer absorbs light and contributes to photocurrent generation, thereby functioning as a photosensitizer. The error bars in Figure 7B represent the standard deviation calculated from three independently fabricated DSSC devices per condition. Furthermore, the short-circuit current density (Jsc), open-circuit voltage (Voc), fill factor (FF), and overall efficiency (η) were extracted from the J–V data and are summarized in Table 1.
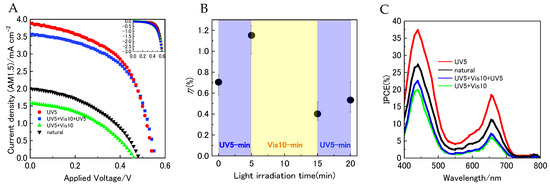
Figure 7.
(A) Current density–voltage (J–V) characteristics of YD2/SPNO2-based dye-sensitized solar cells (DSSCs) measured under AM1.5 illumination (100 mW cm−2), with external ultraviolet (UV) and visible (Vis) light irradiation. The inset displays the dark current density behavior of the same devices. (B) Power conversion efficiency (η) of YD2/SPNO2-based DSSCs under alternating UV and Vis irradiation cycles, illustrating reversible photovoltaic modulation. (C) Incident photon-to-current efficiency (IPCE) spectra of YD2/SPNO2-based DSSCs recorded under 400–800 nm monochromatic illumination with external UV and Vis light exposure.

Table 1.
Photovoltaic performance of YD2/SPNO2-containing DSSCs under different external light irradiation conditions.
The YD2/SPNO2-based dye-sensitized solar cell (DSSC) demonstrated photovoltaic parameters of Jsc = 2.2 mA cm−2, Voc = 0.54 V, FF = 0.51, and an energy conversion efficiency (η) of 0.70% under standard conditions. To examine the device’s response to external optical stimuli, it was sequentially exposed to ultraviolet and visible light. The evolution of η followed the trend 0.70% (natural) → 1.21% (UV for 5 min) → 0.38% (UV 5 + Vis 10 min) → 0.45% (UV 5 + Vis 10 + UV 5 min). Similarly, the short-circuit current density (Jsc) varied under the same series of light exposures as follows: 2.2 → 3.4 → 1.6 → 1.9 mA cm−2. These results indicate that both η and Jsc increased upon UV irradiation and decreased following visible light exposure. In contrast, Voc and FF remained nearly constant throughout the irradiation cycles. This suggests that the modulation of η primarily stems from changes in Jsc. Interestingly, although Jsc increased by approximately 54.5% after UV exposure, the corresponding transmittance dropped by 18.5%, implying that the relationship between light absorption and photocurrent generation is not strictly linear. Taken together, these findings support the hypothesis that PMC contributes to photocurrent generation by functioning as a light-absorbing sensitizer.
To evaluate the photoelectric conversion performance of DSSCs co-sensitized with YD2/SPNO2, incident photon-to-current efficiency (IPCE) spectra were recorded over the 400–800 nm wavelength range, as shown in Figure 7C. The maximum IPCE value reached approximately 27% at 440 nm. Figure 7C (red, green, and blue markers) also illustrates the spectral changes under successive external light exposures: UV for 5 min, visible light for 10 min, and a second UV irradiation for 5 min. Following UV irradiation, the IPCE values increased across the 400–700 nm range, while subsequent exposure to visible light led to a decline in this region. These observations imply that the concentration of the PMC isomer increases after UV exposure and diminishes after visible light irradiation. This reversible behavior suggests that PMC contributes to the modulation of photovoltaic efficiency in the co-sensitized device.
The YD2-only dye anchors to the TiO2 surface through ester linkages formed between its carboxyl functional groups and surface hydroxyls on the TiO2 nanoparticles [25,26]. This molecular interaction facilitates efficient electron transfer from YD2-only to the conduction band of TiO2, resulting in a photovoltaic conversion efficiency of approximately 1.24% in DSSCs, utilizing YD2-only as the primary sensitizer.
Upon ultraviolet (UV) irradiation, SPNO2 undergoes a structural transformation from its closed spiropyran (SP) form to the ring-opened photomerocyanine (PMC) form. This photoisomerization induces a visible color change in the TiO2-based photoelectrode, shifting from green to reddish tones, which is accompanied by a decrease in transmittance near 540 nm due to the absorption characteristics of PMC. The resulting dye-sensitized solar cells (DSSCs), fabricated with UV-treated electrodes, exhibited an improvement in power conversion efficiency (η). Subsequent exposure to visible light caused PMC to revert to the original SP form, leading to a recovery of the electrode’s green appearance and a corresponding decline in η. These observations suggest that the optical and photovoltaic properties of the device are closely linked to the isomeric state of SPNO2. Additionally, no significant Förster energy transfer from SPNO2 to YD2-only was detected, indicating that the effect of PMC on device performance may arise through indirect modulation (Figure 8). While Takeshita et al. [23] have previously explored the photovoltaic behavior of PMC, their results indicate that direct electron injection from PMC into the conduction band of TiO2 is energetically unfavorable. Thus, we infer that neither SP nor PMC serves as an active electron donor in the charge injection process within the TiO2 network.
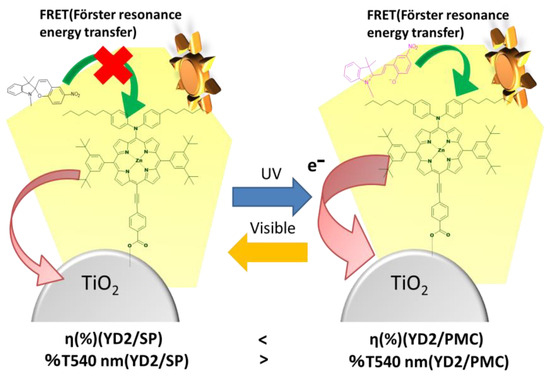
Figure 8.
Schematic illustration of the proposed mechanism underlying the photovoltaic behavior of YD2/SPNO2-based dye-sensitized solar cells (DSSCs). The diagram highlights the role of photoinduced isomerization of SPNO2 and the subsequent Förster resonance energy transfer (FRET) from the photomerocyanine (PMC) form to the YD2 dye, contributing to enhanced light absorption and charge generation under ultraviolet (UV) irradiation.
Although definitive experimental evidence for Förster resonance energy transfer (FRET) from the PMC isomer to YD2-only remains elusive, time-resolved fluorescence data suggest a possible energy transfer pathway. Specifically, we observed a reduction in fluorescence lifetime from 0.90 ns (PMC-TiO2) to 0.41 ns (PMC–YD2-only–TiO2), which may imply donor quenching due to proximity-based energy transfer. This observation aligns with previous findings [26] in which SPNO2 acted as a FRET donor to another dye (SQ2), although that study did not include YD2.
To further probe this phenomenon, the FRET efficiency (ΦFRET) was estimated using average fluorescence lifetimes according to the relation ΦFRET = 1 − (τDA/τD), where τD and τDA are the lifetimes of PMC in the absence and presence of YD2, respectively. This analysis yielded a ΦFRET of approximately 0.54, supporting the possibility of energy transfer within our co-sensitized architecture.
We propose that UV irradiation promotes SPNO2 isomerization from the non-planar SP form to the conjugated PMC form, which acts as a FRET donor to YD2-only, enhancing light-harvesting and improving photocurrent generation. Conversely, exposure to visible light induces reverse isomerization (PMC → SP), diminishing the energy transfer process and consequently reducing efficiency (η). These results collectively suggest a dynamic FRET-mediated mechanism modulating device performance under light stimuli, although further spectroscopic studies are warranted to conclusively validate this model. Additionally, based on energy level estimations reported by Dryza et al., the lowest unoccupied molecular orbital (LUMO) of PMC (~−4.0 eV) lies below the conduction band of TiO2, making direct electron injection energetically unfavorable. This reinforces the hypothesis that PMC serves primarily as an energy donor rather than an electron injector in our DSSC system [27].
Beyond the mechanistic insights, the demonstration of a reversible, light-tunable photovoltaic response provides a conceptual basis for developing adaptive energy devices. Compared with conventional co-sensitization approaches that primarily broaden spectral coverage, the YD2/SPNO2 system offers an additional dimension of dynamic control. Such functionality could be particularly advantageous for semi-transparent DSSCs in building-integrated photovoltaics (BIPVs), where color tuning and light management are as important as efficiency. Furthermore, the present results suggest a broader design principle in which photochromic sensitizers can be rationally combined with porphyrin dyes to achieve multifunctional, responsive solar cells.
4. Conclusions
In this study, we constructed dye-sensitized solar cells incorporating YD2-only and the photochromic dye SPNO2 and examined how their photovoltaic performance responds to external light stimuli. The devices exhibited reversible modulation of both light absorption and photoelectric conversion efficiency (η), increasing after UV irradiation and decreasing upon exposure to visible light. Spectral analysis confirmed that SPNO2 underwent photoinduced isomerization from the closed (SP) to the open (PMC) form under UV light. These findings indicate that the enhancement in photovoltaic response is likely attributed to Förster resonance energy transfer (FRET) from the photoactivated PMC to the YD2-only dye, contributing to co-sensitization and improved light-harvesting efficiency.
While the absolute efficiencies remain modest, this study demonstrates a novel mechanism for real-time tunability in DSSCs, which could inform future strategies in smart photovoltaic applications and responsive coatings. Furthermore, as the devices are based on a semi-transparent DSSC configuration, they are particularly suitable for integration into building-integrated photovoltaics (BIPVs) or aesthetic applications requiring see-through functionality.
Author Contributions
Conceptualization, M.H.; methodology, M.H. and R.E.; software, M.H.; validation, M.H. and R.E.; formal analysis, R.E.; investigation, K.O.; resources, M.H. and K.O.; data curation, K.O.; writing—original draft preparation, M.H. and K.O.; writing—review and editing, M.H., K.O., and R.E.; visualization, K.O.; supervision, M.H.; project administration, M.H.; funding acquisition, M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, M.H., upon reasonable request.
Acknowledgments
Members of the Fukui University of Technology are gratefully acknowledged for their financial support. Tomokazu Tanaka and Tatsuya Takeshita are acknowledged for valuable discussions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yadagiri, B.; Kumar Kaliamurthy, A.; Yoo, K.; Cheol Kang, H.; Ryu, J.; Kwaku Asiam, F.; Lee, J.J. Molecular Engineering of Photosensitizers for Solid-State Dye-Sensitized Solar Cells: Recent Developments and Perspectives. ChemistryOpen 2023, 12, e202300170. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B.F.E.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, M.K.; Graetzel, M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef]
- Raut, P.; Kishnani, V.; Mondal, K.; Gupta, A.; Jana, S.C. A Review on Gel Polymer Electrolytes for Dye-Sensitized Solar Cells. Micromachines 2022, 13, 680. [Google Scholar] [CrossRef]
- Kokkonen, M.; Talebi, P.; Zhou, J.; Asgari, S.; Soomro, S.A.; Elsehrawy, F.; Halme, J.; Ahmad, S.; Hagfeldt, A.; Hashmi, S.G. Advanced research trends in dye-sensitized solar cells. J. Mater. Chem. A Mater. 2021, 9, 10527–10545. [Google Scholar] [CrossRef]
- Gratzel, M. Dye-sensitized solar cells. J. Photochem. Photobiol. C Photochem. Rev. 2003, 4, 145–153. [Google Scholar] [CrossRef]
- Shen, H.; Li, J.; Zhao, L.; Zhang, S.; Wang, W.; Oron, D.; Lin, H. Synergistic recombination suppression by an inorganic layer and organic dye molecules in highly photostable quantum dot sensitized solar cells. Phys. Chem. Chem. Phys. PCCP 2014, 16, 6250–6256. [Google Scholar] [CrossRef]
- Imahori, H.; Umeyama, T.; Ito, S. Large pi-aromatic molecules as potential sensitizers for highly efficient dye-sensitized solar cells. Acc. Chem. Res. 2009, 42, 1809–1818. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, D.; Suo, J.; Cao, Y.; Eickemeyer, F.T.; Vlachopoulos, N.; Zakeeruddin, S.M.; Hagfeldt, A.; Grätzel, M. Hydroxamic acid pre-adsorption raises the efficiency of cosensitized solar cells. Nature 2023, 613, 60–65. [Google Scholar] [CrossRef]
- Vallejo, W.; Lerma, M.; Diaz-Uribe, C. Dye sensitized solar cells: Meta-analysis of effect sensitizer-type on photovoltaic efficiency. Heliyon 2025, 11, e41092. [Google Scholar] [CrossRef]
- Suerkan, S.N.; Arslan, N.; Gokceoren, A.T.; Cakar, S.; Sevim, A.M.; Gul, A.; Ozacar, M. A3B type Zn(II) phthalocyanines and porphyrin cocktail dye sensitizers for highly efficient DSSCs. J. Photochem. Photobiol. A Chem. 2025, 464, 116333. [Google Scholar] [CrossRef]
- Kodji, N.; Souop Tala Foadin, C.; Mohammadou, S.; Tchangnwa Nya, F.; Ejuh, G.W. Implementation of bridged copolymerisation to optimise the optical properties of porphyrin: Applications in dye-sensitized solar cells. Mol. Phys. 2025, 123, e2345727. [Google Scholar] [CrossRef]
- Lee, C.-L.; Lee, W.-H.; Yang, C.-H. The effects of co-sensitization in dye-sensitized solar cells. J. Mater. Sci. 2013, 48, 3448–3453. [Google Scholar] [CrossRef]
- Cole, J.M.; Pepe, G.; Al Bahri, O.K.; Cooper, C.B. Cosensitization in Dye-Sensitized Solar Cells. Chem. Rev. 2019, 119, 7279–7327. [Google Scholar] [CrossRef]
- Keremane, K.S.; Eletmany, M.R.; Abdellah, I.M.; Naik, P.; Adhikari, A.V. New carbazole-based symmetric double D–A type chromophores for DSSC application: Impact of di-anchoring nature on photoelectrochemical processes. J. Photochem. Photobiol. A Chem. 2025, 466, 116368. [Google Scholar] [CrossRef]
- Patwari, J.; Sardar, S.; Liu, B.; Lemmens, P.; Pal, S.K. Three-in-one approach towards efficient organic dye-sensitized solar cells: Aggregation suppression, panchromatic absorption and resonance energy transfer. Beilstein J. Nanotechnol. 2017, 8, 1705–1713. [Google Scholar] [CrossRef]
- Ananthakumar, S.; Balaji, D.; Ram Kumar, J.; Moorthy Babu, S. Role of co-sensitization in dye-sensitized and quantum dot-sensitized solar cells. SN Appl. Sci. 2019, 1, 186. [Google Scholar] [CrossRef]
- Malzner, F.J.; Willgert, M.; Constable, E.C.; Housecroft, C.E. The way to panchromatic copper(I)-based dye-sensitized solar cells: Co-sensitization with the organic dye SQ2. J. Mater. Chem. A 2017, 5, 13717–13729. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.; Kim, D.Y.; Seo, Y. Co-sensitization of metal free organic dyes in flexible dye sensitized solar cells. Org. Electron. 2018, 52, 103–109. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Yeh, M.-H.; Lee, C.-P.; Chang, J.; Baheti, A.; Vittal, R.; Justin Thomas, K.R.; Ho, K.-C. Insights into the co-sensitizer adsorption kinetics for complementary organic dye-sensitized solar cells. J. Power Sources 2014, 247, 906–914. [Google Scholar] [CrossRef]
- Demirci, Y.C.; Çakar, S.; Sevim, A.M.; Gül, A.; Özacar, M. DSSCs based on unsymmetrical A3B type Zn(II) and TiO(IV) naphthalenephthalocyanine/porphyrin cocktail dyes: A potential alternative for ruthenium based sensitizers. J. Photochem. Photobiol. A Chem. 2023, 440, 114642. [Google Scholar] [CrossRef]
- Zhu, L.; Li, P.; Sun, H.; Han, X.; Xu, Y.; Wang, X.; Liu, B.; Ozaki, Y.; Zhao, B. An investigation of the effect of high-pressure on charge transfer in dye-sensitized solar cells based on surface-enhanced Raman spectroscopy. Nanoscale 2022, 14, 373–381. [Google Scholar] [CrossRef]
- Klein, M.; Pankiewicz, R.; Zalas, M.; Stampor, W. Magnetic field effects in dye-sensitized solar cells controlled by different cell architecture. Sci. Rep. 2016, 6, 30077. [Google Scholar] [CrossRef] [PubMed]
- Huaulmé, Q.; Mwalukuku, V.M.; Joly, D.; Liotier, J.; Kervella, Y.; Maldivi, P.; Narbey, S.; Oswald, F.; Riquelme, A.J.; Anta, J.A.; et al. Photochromic dye-sensitized solar cells with light-driven adjustable optical transmission and power conversion efficiency. Nat. Energy 2020, 5, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Fathabadi, H. Comparative study on the effect of magnetic field on the photocurrent density of organic, dye-sensitized and silicon solar cells. J. Mater. Sci. Mater. Electron. 2019, 30, 17314–17321. [Google Scholar] [CrossRef]
- Ma, S.; Ting, H.; Ma, Y.; Zheng, L.; Zhang, M.; Xiao, L.; Chen, Z. Smart photovoltaics based on dye-sensitized solar cells using photochromic spiropyran derivatives as photosensitizers. AIP Adv. 2015, 5, 057154. [Google Scholar] [CrossRef]
- Takeshita, T.; Umeda, T.; Hara, M. Fabrication of a dye-sensitized solar cell containing a noncarboxylated spiropyran-derived photomerocyanine with cyclodextrin. J. Photochem. Photobiol. A Chem. 2017, 333, 87–91. [Google Scholar] [CrossRef]
- Dryza, V.; Bieske, E.J. Electron Injection and Energy-Transfer Properties of Spiropyrana-Cyclodextrin Complexes Coated onto Metal Oxide Nanoparticles: Toward Photochromic Light Harvesting. J. Phys. Chem. C 2015, 119, 14076–14084. [Google Scholar] [CrossRef]
- Hara, M.; Ejima, R. Fabrication and Characterization of Co-Sensitized Dye Solar Cells Using Energy Transfer from Spiropyran Derivatives to SQ2 Dye. Molecules 2024, 29, 4896. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).