Development of Process Configurations and Simulation of Biofuel Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Biobutanol Process Description
2.2. Simulation Development
2.2.1. Compound Selection and Creation in DWSIM
2.2.2. Data Collection
2.2.3. Assumptions
- (i)
- The feedstock composition reported in the literature varies considerably. Lignocellulose composition is presented either in terms of cellulose, hemicellulose and lignin percentage or as carbohydrate mass percentage (e.g., glucan, xylan, arabinan, mannan, galactan). When the lignocellulose composition is presented in the form of cellulose, hemicellulose and lignin, it is assumed that the entire mass of hemicellulose consists of xylan and cellulose is composed of cellobiose.
- (ii)
- Some studies implement simultaneous saccharification and fermentation (SSF). To ensure consistency in the simulation, separate conversion reactors were used for saccharification and fermentation instead of combining them into a single reactor.
- (iii)
- Clostridial species exhibit carbon catabolite repression (CCR) when fermenting mixed sugar. In the fermentation reaction setup, it was assumed that the conversion of xylose into fermentation products was as efficient as that of glucose.
- (iv)
- In instances where data for specific compounds during pretreatment was not provided in the literature, it was assumed that no conversion of these compounds occurred.
- (v)
- Several studies involve physical pretreatment methods such as biomass chipping and milling, which were not integrated into the simulation flowsheet.
2.3. Flowsheet Devlopment for Biomass Converison Modeling
2.3.1. Operating Units
2.3.2. Reaction Sets
3. Results
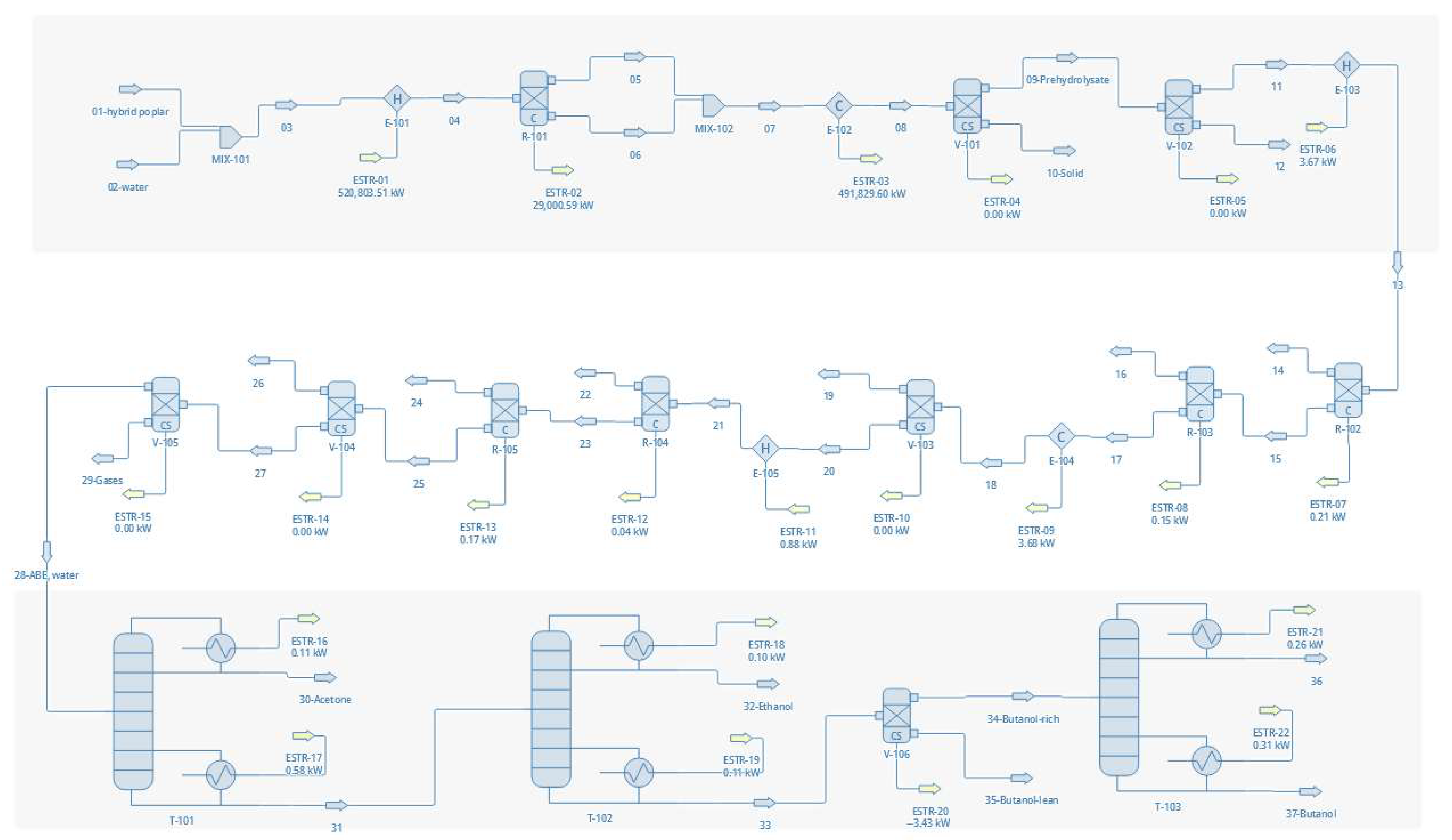
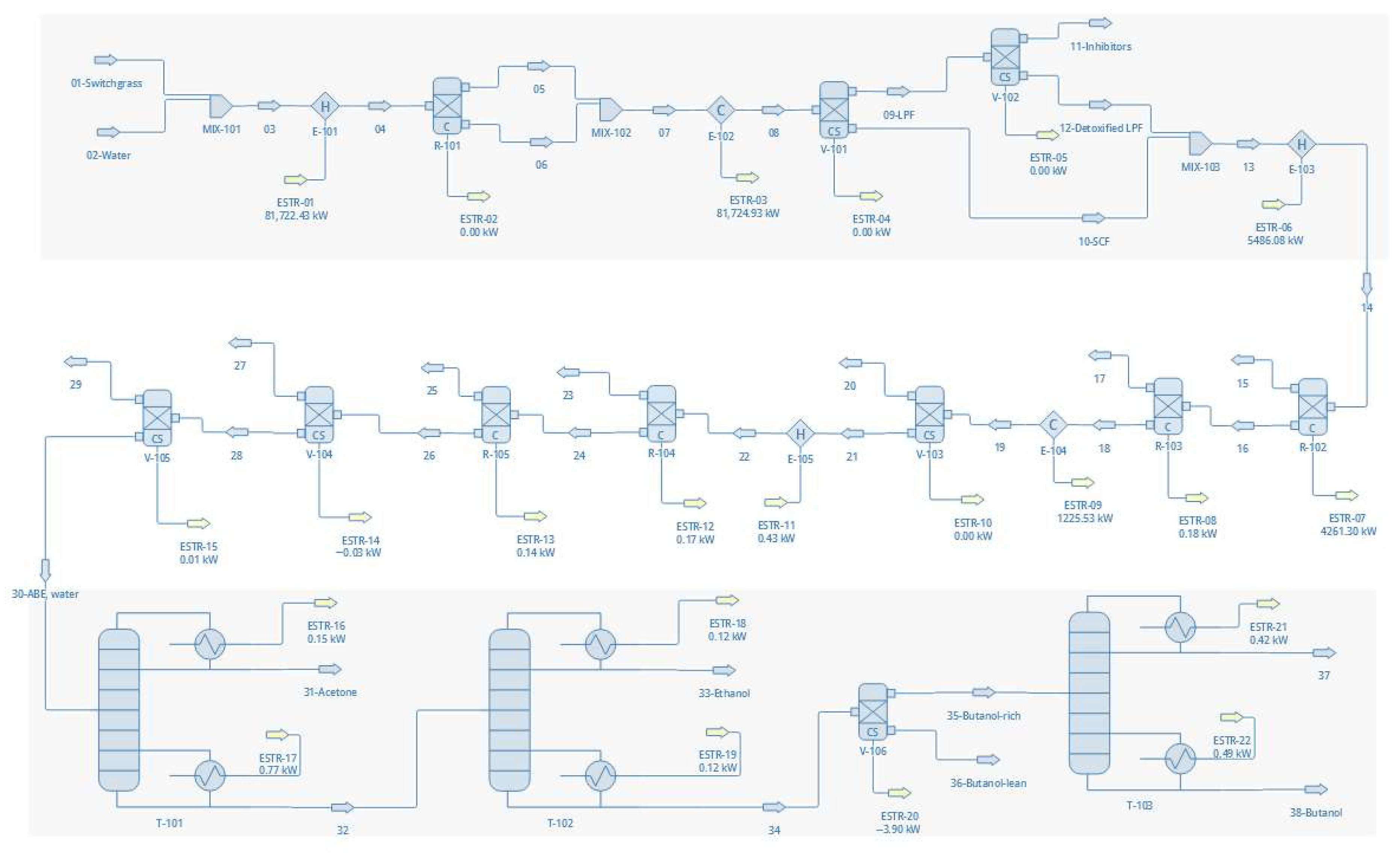
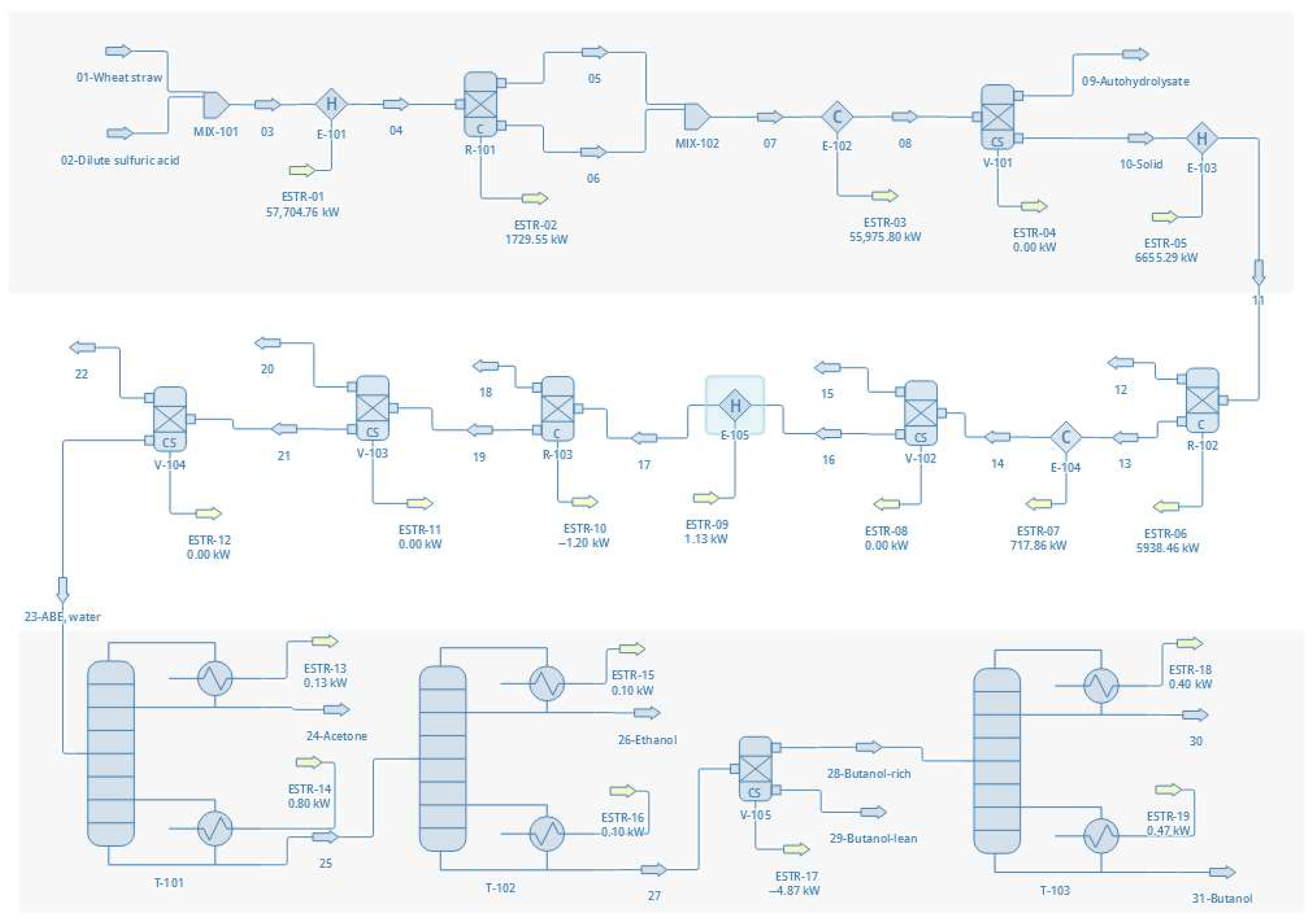
3.1. Flowsheet Development
3.2. Model Validation
3.3. Implementatio of Process Configurations in DWSIM
3.3.1. Feedstock Selection
3.3.2. Flowsheet Configurations
- Configuration 1—Autohydrolysis on hybrid poplar wood
- Configuration 2—Autohydrolysis on elmood
- Configuration 3—Autohydrolysis on switchgrass
- Configuration 4—Dilute sulfuric acid pretreatment on wheat straw
- Configuration 5—Dilute sulfuric acid pretreatment on pulp and paper side-stream
- Configuration 6—Dilute acetic acid pretreatment on switchgrass
4. Discussion
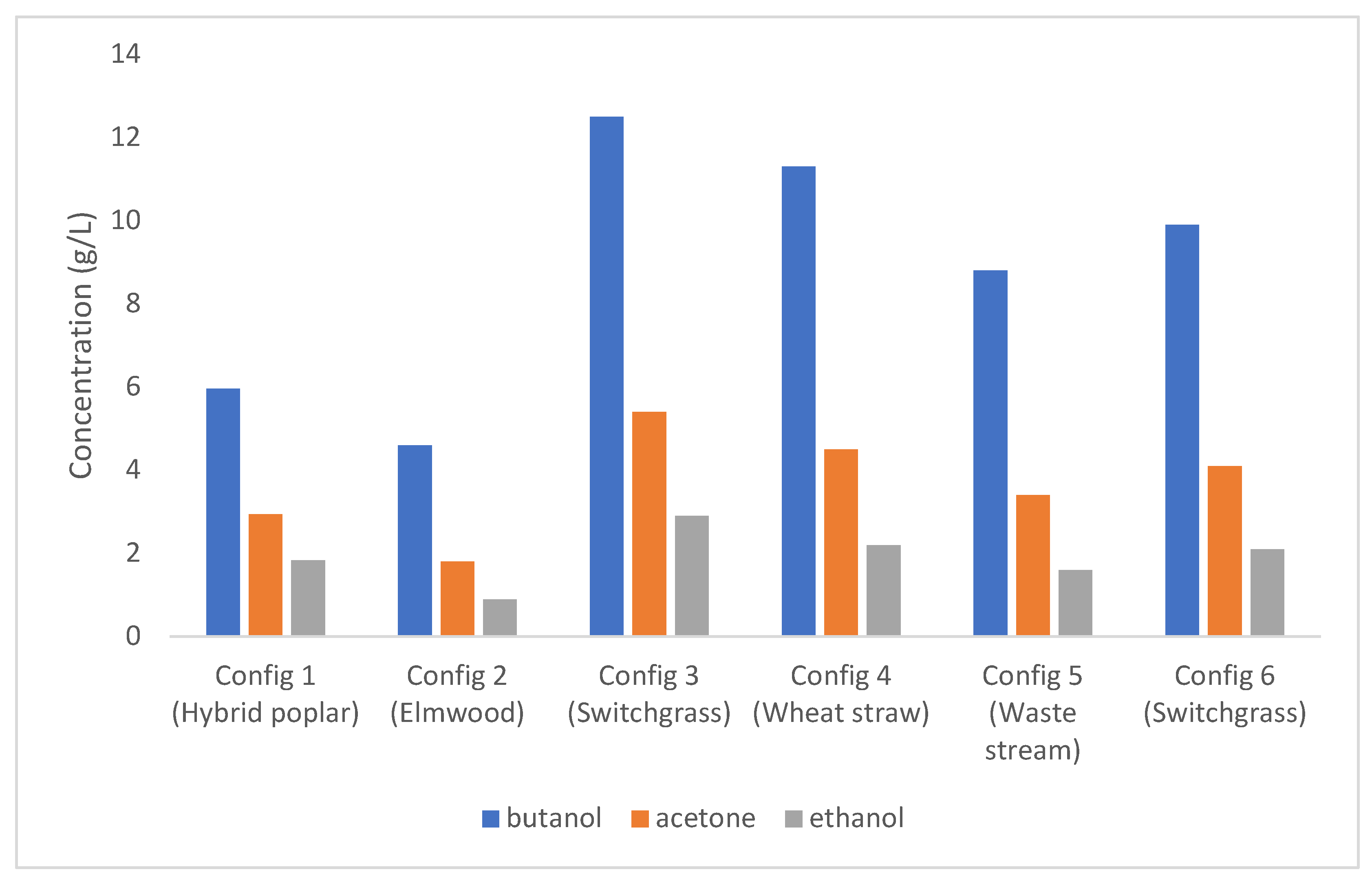
4.1. Fermentation Products
4.2. Software Performance
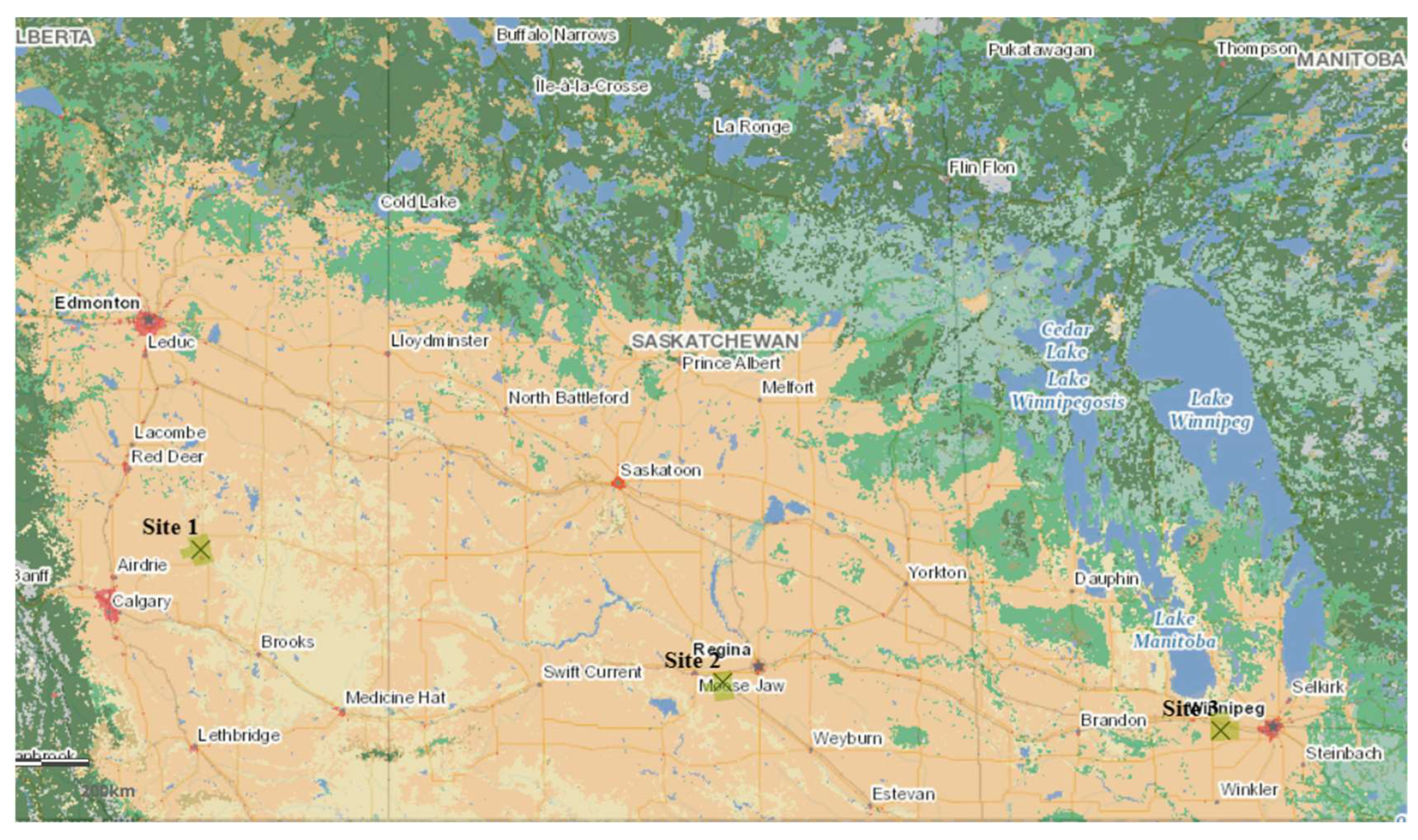
4.3. Potential Biorefinery Locations in Canada
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| GHG | Greenhouse Gas |
| LCB | Lignocellulosic Biomass |
| ABE | Acetone–Butanol–Ethanol |
| CCR | Carbon Catabolite Repression |
| CR | Conversion Reactor |
| CS | Compound Separator |
| SSF | Simultaneous Saccharification and Fermentation |
| SHF | Separate Hydrolysis and Fermentation |
| SLR | Solid Liquid Ratio |
References
- Tilsted, J.P.; Mah, A.; Nielsen, T.D.; Finkill, G.; Bauer, F. Petrochemical transition narratives: Selling fossil fuel solutions in a decarbonizing world. Energy Res. Soc. Sci. 2022, 94, 102880. [Google Scholar] [CrossRef]
- Zhao, S.; Song, Q.; Liu, L.; Li, J.; Zhao, D. Uncovering the lifecycle carbon emissions and its reduction pathways: A case study of petroleum refining enterprise. Energy Convers. Manag. 2024, 301, 118048. [Google Scholar] [CrossRef]
- IEA. Global Energy Crisis. Available online: https://www.iea.org/topics/global-energy-crisis (accessed on 25 May 2025).
- Yuan, X.; Su, C.-W.; Umar, M.; Shao, X.; Lobont, O.-R. The race to zero emissions: Can renewable energy be the path to carbon neutrality? J. Environ. Manag. 2022, 308, 114648. [Google Scholar] [CrossRef]
- Williams, C.L.; Westover, T.L.; Emerson, R.M.; Tumuluru, J.S.; Chenlin, L. Sources of biomass feedstock variability and the potential impact on biofuels production. Bioenergy Res. 2016, 9, 1–14. [Google Scholar] [CrossRef]
- Obergruber, M.; Hönig, V.; Jenčík, J.; Hájek, J.; Schlehöfer, D.; Herink, T. Lignocellulosic Bioethanol and Biobutanol as a Biocomponent for Diesel Fuel. Materials 2021, 14, 5597. [Google Scholar] [CrossRef]
- Liu, W.-J.; Yu, H.-Q. Thermochemical Conversion of Lignocellulosic Biomass into Mass-Producible Fuels: Emerging Technology Progress and Environmental Sustainability Evaluation. ACS Environ. Au 2022, 2, 98–114. [Google Scholar] [CrossRef]
- Huang, H.; Khanna, M.; Önal, H.; Chen, X. Stacking low carbon policies on the renewable fuels standard: Economic and greenhouse gas implications. Energy Policy 2013, 56, 5–15. [Google Scholar] [CrossRef]
- Luo, H.; Liu, Z.; Xie, F.; Bilal, M.; Peng, F. Lignocellulosic biomass to biobutanol: Toxic effects and response mechanism of the combined stress of lignin-derived phenolic acids and phenolic aldehydes to Clostridium acetobutylicum. Ind. Crops Prod. 2021, 170, 113722. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Y.; Guan, M.; Tang, H.; Wang, Z.; Lin, L.; Pang, H. Production of butanol from lignocellulosic biomass: Recent advances, challenges, and prospects. RSC Adv. 2022, 12, 18848–18863. [Google Scholar] [CrossRef]
- Trirahayu, D.A.; Abidin, A.Z.; Putra, R.P.; Hidayat, A.S.; Safitri, E.; Perdana, M.I. Process Simulation and Design Considerations for Biodiesel Production from Rubber Seed Oil. Fuels 2022, 3, 563–579. [Google Scholar] [CrossRef]
- Rosova, A.; Behun, M.; Khouri, S.; Cehlar, M.; Ferencz, V.; Sofranko, M. Case study: The simulation modeling to improve the efficiency and performance of production process. Wireless Netw. 2022, 28, 863–872. [Google Scholar] [CrossRef]
- Pasha, M.K.; Dai, L.; Liu, D.; Guo, M.; Du, W. An overview to process design, simulation and sustainability evaluation of biodiesel production. Biotechnol. Biofuels 2021, 14, 129. [Google Scholar] [CrossRef]
- Lassmann, T.; Kravanja, P.; Friedl, A. Simulation of the downstream processing in the ethanol production from lignocellulosic biomass with ASPEN Plus® and IPSEpro. Energy Sustain. Soc. 2014, 4, 27. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, J.; Bao, J. Cost Evaluation of Cellulase Enzyme for Industrial-Scale Cellulosic Ethanol Production Based on Rigorous Aspen Plus Modeling. Bioprocess Biosyst. Eng. 2016, 39, 133–140. [Google Scholar] [CrossRef]
- Haigh, K.; Petersen, A.M.; Gottumukkala, L.; Mandegari, M.A.; Naleli, K.; Görgens, J. Simulation and comparison of processes for biobutanol production from lignocellulose via ABE fermentation. Biofuels Bioprod. Biorefining 2018, 12, 1023–1036. [Google Scholar] [CrossRef]
- Ashani, P.N.; Shafiei, M.; Karimi, K. Biobutanol production from municipal solid waste: Technical and economic analysis. Bioresour. Technol. 2020, 308, 123267. [Google Scholar] [CrossRef]
- Cortes-Peña, Y.; Kumar, D.; Singh, V.; Guest, J.S. BioSTEAM: A Fast and Flexible Platform for the Design, Simulation, and Techno-Economic Analysis of Biorefineries under Uncertainty. ACS Sustain. Chem. Eng. 2020, 8, 3302–3310. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, A.; Abánades, A. Comparative Analysis of Energy and Exergy Performance of Hydrogen Production Methods. Entropy 2020, 22, 1286. [Google Scholar] [CrossRef]
- Silva, S.S.O.; Nascimento, M.R.; Lima, R.J.P.; Luna, F.M.T.; Cavalcante Júnior, C.L. Experimental and Simulation Studies for Purification and Etherification of Glycerol from the Biodiesel Industry. AppliedChem 2023, 3, 492–508. [Google Scholar] [CrossRef]
- Ullah, K.; Asaad, S.M.; Inayat, A. Process modelling and optimization of hydrogen production from biogas by integrating DWSIM with response surface methodology. Digit. Chem. Eng. 2025, 14, 100205. [Google Scholar] [CrossRef]
- Tangsriwong, K.; Lapchit, P.; Kittijungjit, T.; Klamrassamee, T.; Sukjai, Y.; Laoonual, Y. Modeling of chemical processes using commercial and open-source software: A comparison between Aspen Plus and DWSIM. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Bangkok, Thailand, 11–14 December 2019. [Google Scholar]
- Nayak, P.; Dalve, P.; Sai, R.A.; Jain, R.; Moudgalya, K.M.; Naren, P.R.; Fritzson, P.; Wagner, D. Chemical Process Simulation Using OpenModelica. Ind. Eng. Chem. Res. 2019, 58, 11164–11174. [Google Scholar] [CrossRef]
- Medeiros, D. DWSIM-Open-Source Chemical Process Simulator. Available online: http://sourceforge.net/projects/dwsim (accessed on 25 May 2025).
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 28 May 2025).
- Parameters of the Modified UNIFAC (Dortmund) Model. Available online: https://www.ddbst.com/PublishedParametersUNIFACDO.html (accessed on 28 May 2025).
- Akhtar, N.; Gupta, K.; Goyal, D.; Goyal, A. Recent advances in pretreatment technologies for efficient hydrolysis of lignocellulosic biomass. Environ. Prog. Sustain. Energy 2016, 35, 489–511. [Google Scholar] [CrossRef]
- du Pasquier, J.; Paës, G.; Perré, P. Principal factors affecting the yield of dilute acid pretreatment of lignocellulosic biomass: A critical review. Bioresour. Technol. 2023, 369, 128439. [Google Scholar] [CrossRef]
- Guan, W.; Xu, G.; Duan, J.; Shi, S. Acetone–Butanol–Ethanol Production from Fermentation of Hot-Water-Extracted Hemicellulose Hydrolysate of Pulping Woods. Ind. Eng. Chem. Res. 2018, 57, 775–783. [Google Scholar] [CrossRef]
- Amiri, H.; Karimi, K. Autohydrolysis: A promising pretreatment for the improvement of acetone, butanol, and ethanol production from woody materials. Chem. Eng. Sci. 2015, 137, 722–729. [Google Scholar] [CrossRef]
- Wang, P.; Chen, Y.M.; Wang, Y.; Lee, Y.Y.; Zong, W.; Taylor, S.; McDonald, T.; Wang, Y. Towards comprehensive lignocellulosic biomass utilization for bioenergy production: Efficient biobutanol production from acetic acid pretreated switchgrass with Clostridium saccharoperbutylacetonicum N1-4. Appl. Energy 2019, 236, 551–559. [Google Scholar] [CrossRef]
- Rajan, K.; Carrier, D.J. Effect of dilute acid pretreatment conditions and washing on the production of inhibitors and on recovery of sugars during wheat straw enzymatic hydrolysis. Biomass Bioenergy 2014, 62, 222–227. [Google Scholar] [CrossRef]
- Sandar, S.; Yang, M.; Turunen, O.; Vepsäläinen, J.; Pappinen, A.; Kuittinen, S. Acetone-butanol-ethanol fermentation from different pulp and paper manufacturing process side-streams. BioResources 2020, 15, 9265–9290. [Google Scholar] [CrossRef]
- Meramo-Hurtado, S.; González-Delgado, A.; Rehmann, L.; Quiñones-Bolaños, E.; Mehrvar, M. Comparison of Biobutanol Production Pathways via Acetone–Butanol–Ethanol Fermentation Using a Sustainability Exergy-Based Metric. ACS Omega 2020, 5, 18710–18730. [Google Scholar] [CrossRef]
- Luyben, W.L. Control of the heterogeneous azeotropic n-butanol/water distillation system. Energy Fuels 2008, 22, 4249–4258. [Google Scholar] [CrossRef]
- Kujawska, A.; Kujawski, J.; Bryjak, M.; Kujawski, W. ABE fermentation products recovery methods—A review. Renew. Sustain. Energy Rev. 2015, 48, 648–661. [Google Scholar] [CrossRef]
- Lima, M.A.; Lavorente, G.B.; da Silva, H.K.P.; Bragatto, J.; Rezende, C.A.; Bernardinelli, O.D.; de Azevedo, E.R.; Gomez, L.D.; McQueen-Mason, S.J.; Labate, C.A.; et al. Effects of pretreatment on morphology, chemical composition and enzymatic digestibility of eucalyptus bark: A potentially valuable source of fermentable sugars for biofuel production—Part 1. Biotechnol. Biofuels 2013, 6, 75. [Google Scholar] [CrossRef]
- Pu, Y.; Treasure, T.; Gonzalez, R.; Venditti, R.; Jameel, H. Autohydrolysis pretreatment of mixed hardwoods to extract value prior to combustion. BioResources 2011, 6, 4856–4870. [Google Scholar] [CrossRef]
- Li, M.; Guo, C.; Luo, B. Comparing impacts of physicochemical properties and hydrolytic inhibitors on enzymatic hydrolysis of sugarcane bagasse. Bioprocess Biosyst. Eng. 2020, 43, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Nitsos, C.K.; Choli-Papadopoulou, T.; Matis, K.A.; Triantafyllidis, K.S. Optimization of Hydrothermal Pretreatment of Hardwood and Softwood Lignocellulosic Residues for Selective Hemicellulose Recovery and Improved Cellulose Enzymatic Hydrolysis. ACS Sustain. Chem. Eng. 2016, 4, 4529–4544. [Google Scholar] [CrossRef]
- Adesanya, Y.; Atiyeh, H.K.; Olorunsogbon, T.; Khanal, A.; Okonkwo, C.C.; Ujor, V.C.; Shah, A.; Ezeji, T.C. Viable strategies for enhancing acetone-butanol-ethanol production from non-detoxified switchgrass hydrolysates. Bioresour. Technol. 2022, 44, 126167. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, C.; Yao, R.; Xu, S.; Zhong, J.; Luo, L.; Wang, Y. Butanol fermentation by Clostridium saccharobutylicum based on poplar wood. Bioresources 2015, 10, 5395–5406. [Google Scholar] [CrossRef]
- Agriculture and Agri-Food Canada, Biomass Inventory Mapping and Analysis. Available online: https://open.canada.ca/data/en/dataset/1a759d95-3008-4078-87af-5bb1bdf657b3 (accessed on 20 August 2025).





| Compound | Molecular Formula | Available in DWSIM |
|---|---|---|
| Cellobiose | C12H22O11 | ✕ |
| Xylan | C10H18O9 | ✕ |
| Lignin | C10H12O3 | ✕ |
| Acetate | CH3COO | ✕ |
| Glucolig | C12H22O11 | ✕ |
| Xylolig | C10H18O9 | ✕ |
| Soluble lignin | C10H12O3 | ✕ |
| Glucose | C6H12O6 | √ |
| Xylose | C5H10O5 | √ |
| Furfural | OC4H3CHO | √ |
| 1-butanol | CH3(CH2)3OH | √ |
| Acetone | CH3COCH3 | √ |
| N-butyric acid | CH3CH2CH2COOH | √ |
| Acetic acid | CH3COOH | √ |
| Water | HOH | √ |
| Carbon dioxide | CO2 | √ |
| Hydrogen | H2 | √ |
| Config 1 [29] | Config 2 [30] | Config 3 [31] | Config 4 [32] | Config 5 [33] | Config 6 [31] | |
|---|---|---|---|---|---|---|
| Pretreatment Reactions | ||||||
| (Cellulose)n + mH2O → mGlucolig | 4.07% | 19.2% | 11.0% | n/a * | 0% | 11.8% |
| (Cellulose)n + nH2O → nGlucose | 1.50% | 7.30% | 2.80% | 3.00% | 0% | 4.49% |
| (Xylan)n + mH2O → mXylolig | 32.9% | 1.76% | 37.0% | n/a | 0% | 49.5% |
| (Xylan)n + nH2O → nXylose | 6.50% | 12.3% | 3.10% | 45.1% | 0% | 4.17% |
| (Xylan)n → 2H2O + nFurfural | 1.90% | n/a | 3.65% | n/a | 1.30% | 4.17% |
| Acetate → Acetic acid | 10.2% | n/a | 12.5% | n/a | 0.190% | 27.9% |
| (Lignin)n → nSoluble lignin | 2.26% | n/a | 27.4% | n/a | n/a | 25.5% |
| Enzymatic Hydrolysis Reactions | ||||||
| (Cellulose)n + nH2O → nGlucose | - | 38.4% | 77.7% | 89.3% | 47.0% | 71.0% |
| (Xylan)n + nH2O → nXylose | - | 10.7% | 78.0% | 47.2% | 30.0% | 78.0% |
| Glucolig + H2O → Glucose | 77.7% | - | 77.7% | - | - | 71.0% |
| Xylolig + H2O → Xylose | 38.0% | - | 78.0% | - | - | 78.0% |
| Fermentation Reactions | Conversion (%) |
|---|---|
| Glucose → Butanol + 2CO2 + H2O | 57.0 |
| Glucose + H2O → Acetone + 3CO2 + 4H2 | 27.4 |
| Glucose → 2Ethanol + 2CO2 | 7.40 |
| Glucose → Butyric acid + 2CO2 + 2H2 | 4.30 |
| Glucose → 3Acetic acid | 3.20 |
| 6Xylose → 5Butanol + 10CO2 + 5H2O | 50.0 |
| Xylose → Acetone + 2CO2 + 2H2 | 30.0 |
| 3Xylose → 5Ethanol + 5CO2 | 15.0 |
| 2Xylose → 5Acetic acid | 1.00 |
| Compound | Hybrid Poplar (Config 1) | Elmwood (Config 2) | Switchgrass (Config 3 and 6) | Wheat Straw (Config 4) | Side Stream (Config 5) |
|---|---|---|---|---|---|
| Cellulose | 45.4% | 51.8% | 35.6% | 38.4% | 61.3% |
| Xylan | 19.2% | 22.7% | 19.2% | 19.7% | 7.50% |
| Lignin | 27.5% | 25.1% | 22.6% | 16.9% | 19.0% |
| Acetate | 7.90% | 0.40% | 22.6% | 25.0% | 12.2% |
| Parameter | Config. 1 | Config. 2 | Config. 3 | Config. 4 | Config. 5 | Config. 6 |
|---|---|---|---|---|---|---|
| Feedstock | Hybrid poplar | Elmwood | Switchgrass | Wheat straw | Pulp and paper side stream | Switchgrass |
| Pretreatment method | Autohydrolysis | Autohydrolysis | Autohydrolysis | Dilute sulfuric acid | Dilute sulfuric acid | Dilute acetic acid |
| Pretreatment conditions | SLR of 1:5 at 170 °C for 60 min | SLR of 1:10 at 180 °C for 60 min | SLR of 1:10 at 170 °C for 10 min | SLR of 1:10 at 140 °C for 30 min with 0.01% sulfuric acid | SLR of 1:10 at 180 °C for 10 min with 0.2% sulfuric acid | SLR of 1:10 at 170 °C for 10 min with 3 g/L acetic acid |
| Solid/liquid separation | Vacuum filtration | Cheesecloth | Vacuum filtration | Centrifugation | Vacuum filtration | Vacuum filtration |
| Enzymatic hydrolysis | Liquid prehydrolysate | Treated solid | Liquid prehydrolysate and solid | Treated solid | Treated solid | Liquid prehydrolysate and solid |
| Hydrolysis temperature | 36 °C | 45 °C | 30 °C | 50 °C | 30 °C | 30 °C |
| Hydrolysis method | SSF | SHF | SSF | Saccharification only | SSF | SSF |
| Detoxification metod | Charcoal adsorption | Not specified | Activated carbon adsorption | Water wash | Not specified | Activated carbon adsorption |
| Fermentaionmicrobe | C. acetobutylicum ATCC 824 | C. acetobutylicum NRRL B-591 | C. saccharoperbutylacetonicum | Not specified | C. acetobutylicum DSM 1731 | C. saccharoperbutylacetonicum |
| Reference | [29] | [30] | [31] | [32] | [33] | [31] |
| Hardwood biomass | |||
| Site 1 | Site 2 | ||
| Latitude and Longitude | +46.698931 −70.876572 | +45.481278 −72.073673 | |
| Area requirement (km2) | 400 | 700 | |
| Wheat straw biomass | |||
| Site 1 | Site 2 | Site 3 | |
| Latitude and Longitude | +51.520299 −112.729126 | +50.301202 −105.126587 | +49.849958 −97.875611 |
| Area requirement (km2) | 700 | 700 | 800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasprzak, J.; Marinova, M. Development of Process Configurations and Simulation of Biofuel Production. Energies 2025, 18, 4713. https://doi.org/10.3390/en18174713
Kasprzak J, Marinova M. Development of Process Configurations and Simulation of Biofuel Production. Energies. 2025; 18(17):4713. https://doi.org/10.3390/en18174713
Chicago/Turabian StyleKasprzak, Joanna, and Mariya Marinova. 2025. "Development of Process Configurations and Simulation of Biofuel Production" Energies 18, no. 17: 4713. https://doi.org/10.3390/en18174713
APA StyleKasprzak, J., & Marinova, M. (2025). Development of Process Configurations and Simulation of Biofuel Production. Energies, 18(17), 4713. https://doi.org/10.3390/en18174713








