Numerical Study of Self-Heating Maintenance Performance of an Integrated Regenerative Catalytic Reactor
Abstract
1. Introduction
2. Mathematical Modeling and Experimental Data Processing
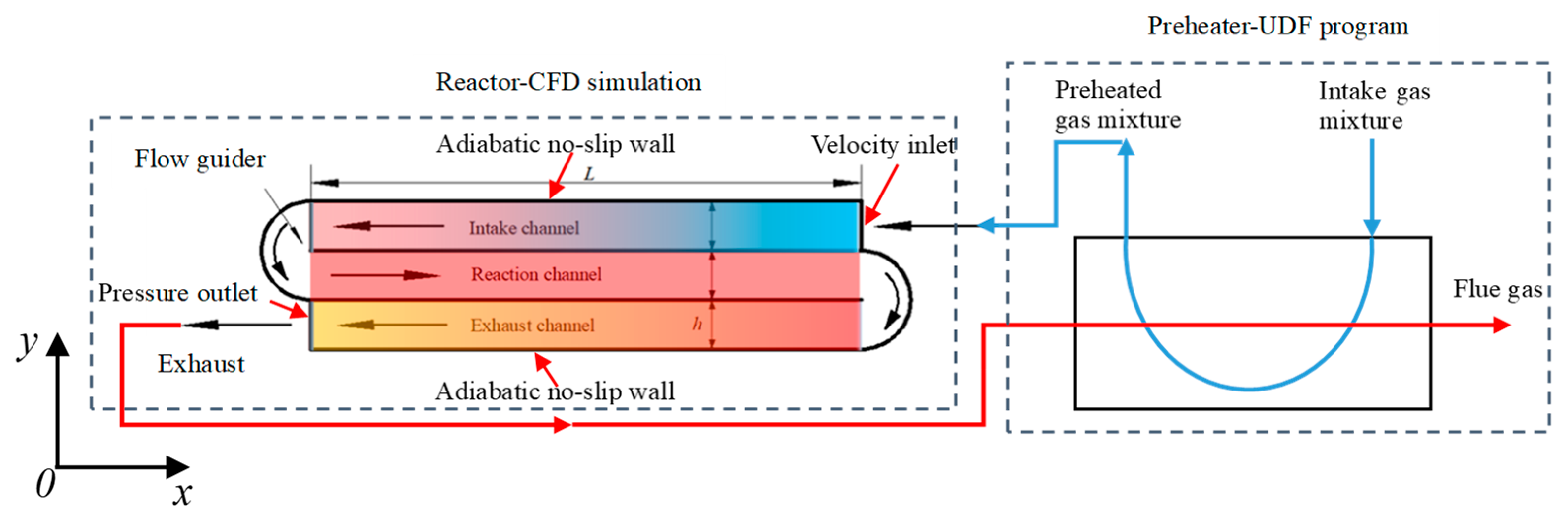
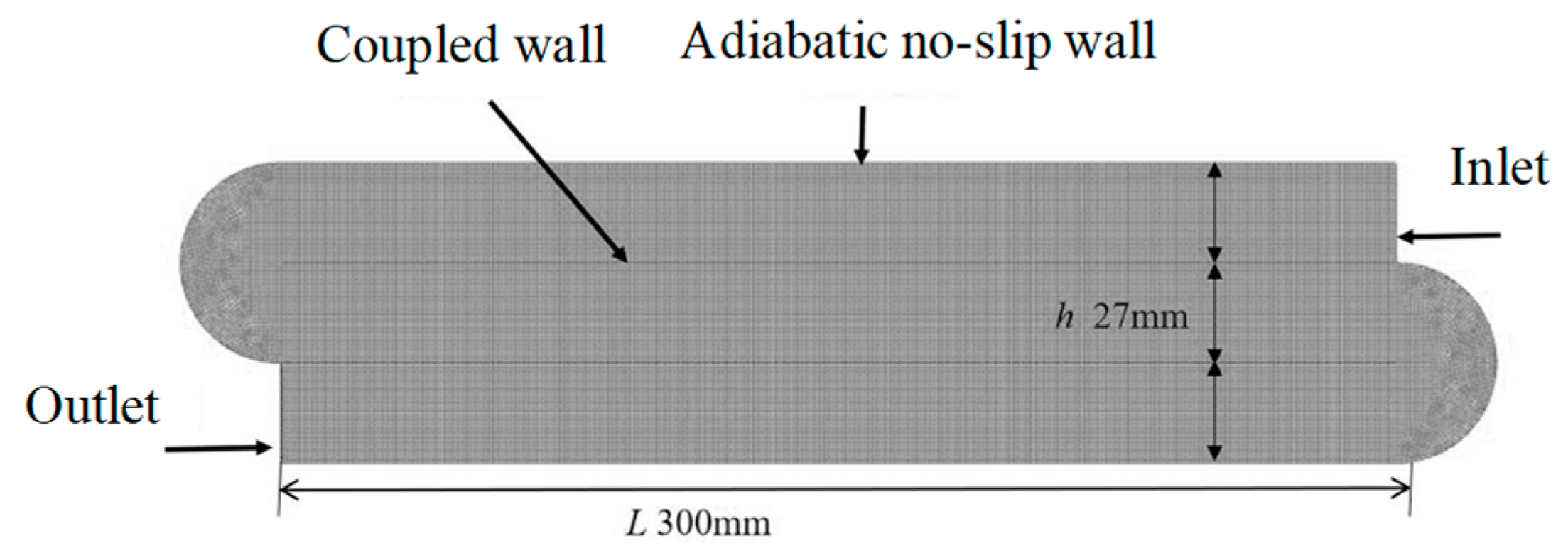
2.1. Geometric Model
2.2. Numerical Model
- (1)
- The methane–air mixture and its combustion products are modeled as an incompressible ideal gas.
- (2)
- The porosity of the porous medium remains constant.
- (3)
- Catalyst coatings are uniformly distributed, with homogeneous surface reactions.
- (4)
- Gas dispersion effects within the porous medium are neglected.
- (5)
- Radiative heat transfer within the porous medium is disregarded.
- (6)
- Gravitational effects on the gas phase are neglected.
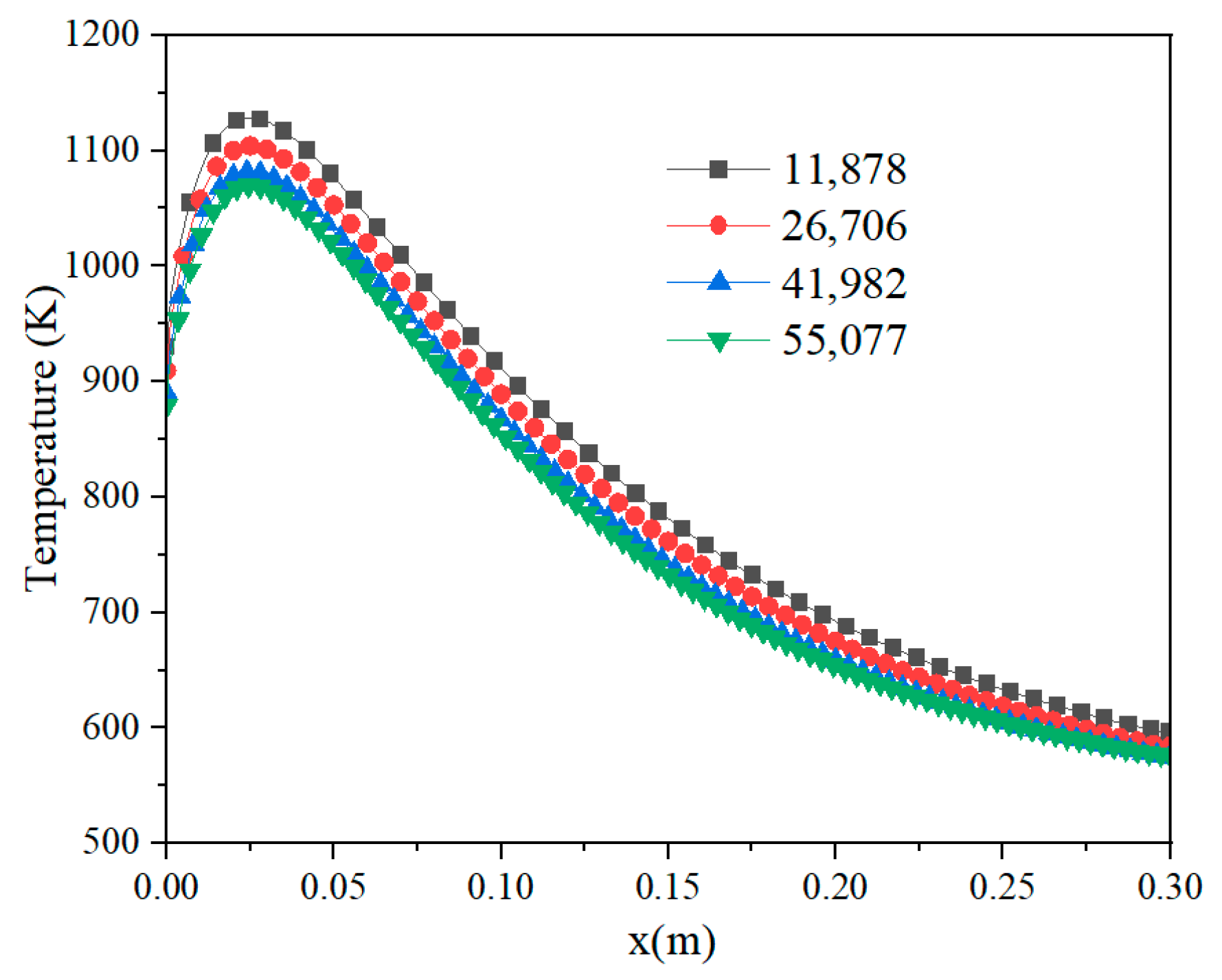
2.3. Grid Independence Validation
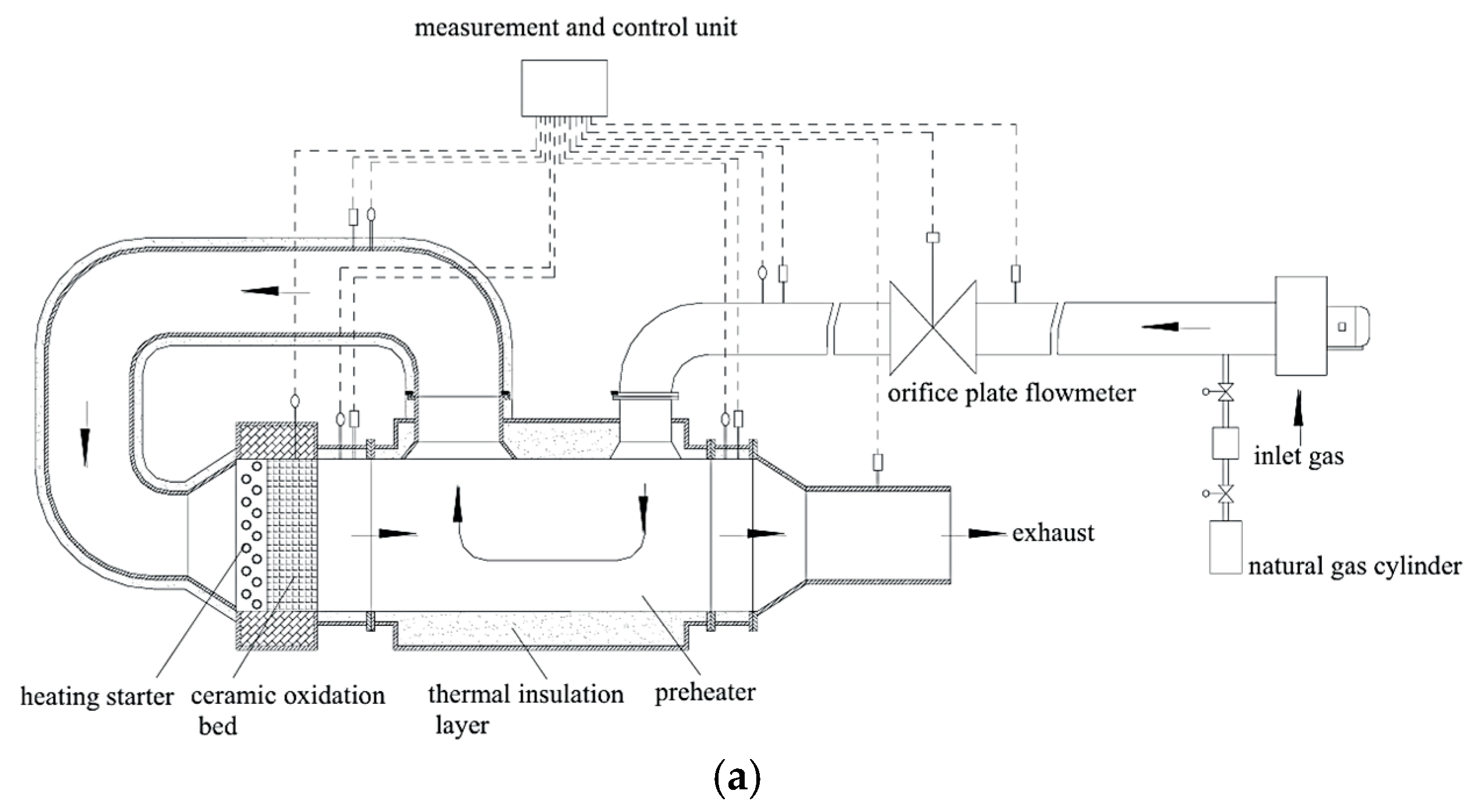
2.4. The Preheated Catalytic Oxidation Reactor Experiment Platform
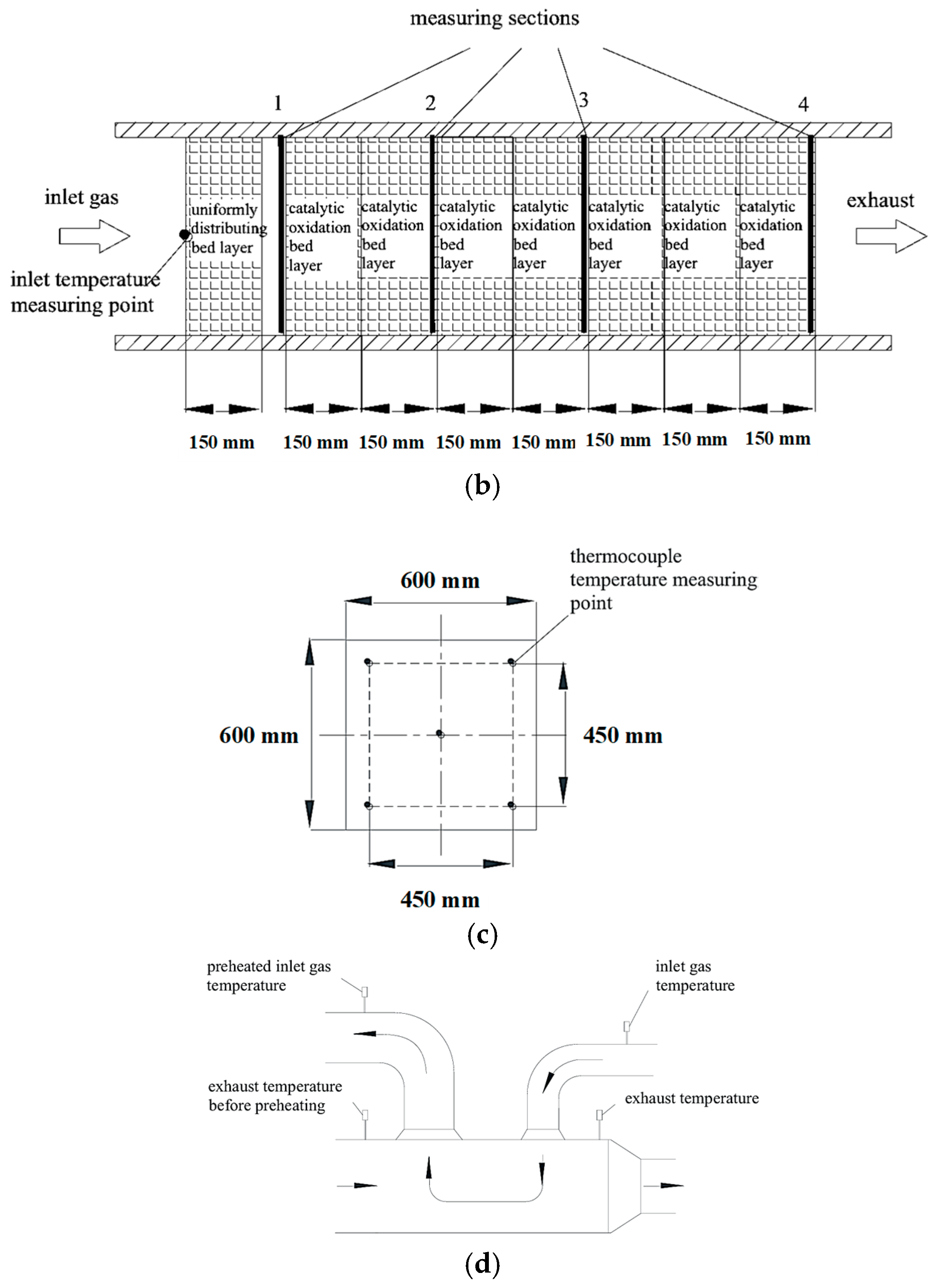
2.4.1. Structure and Measurement
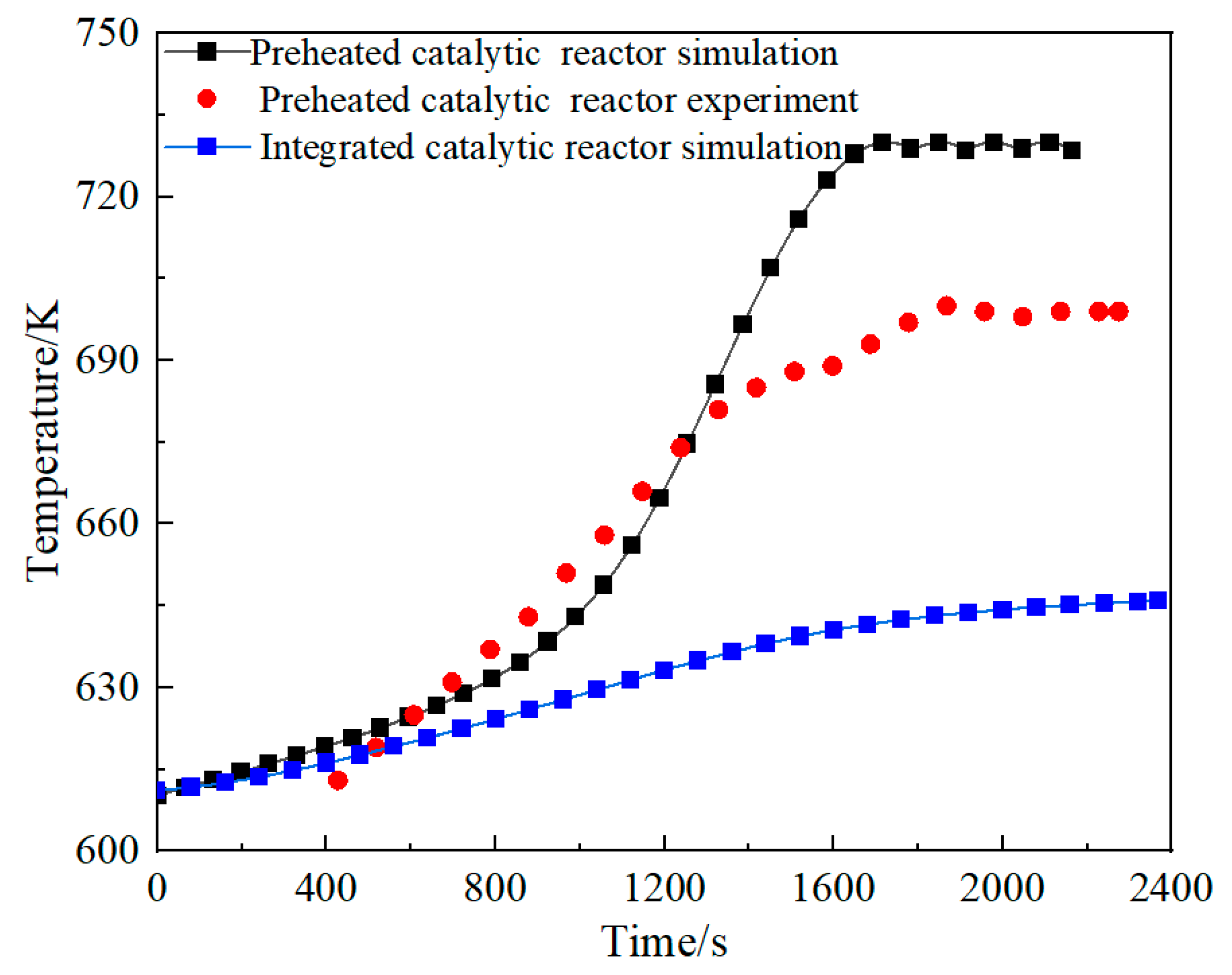
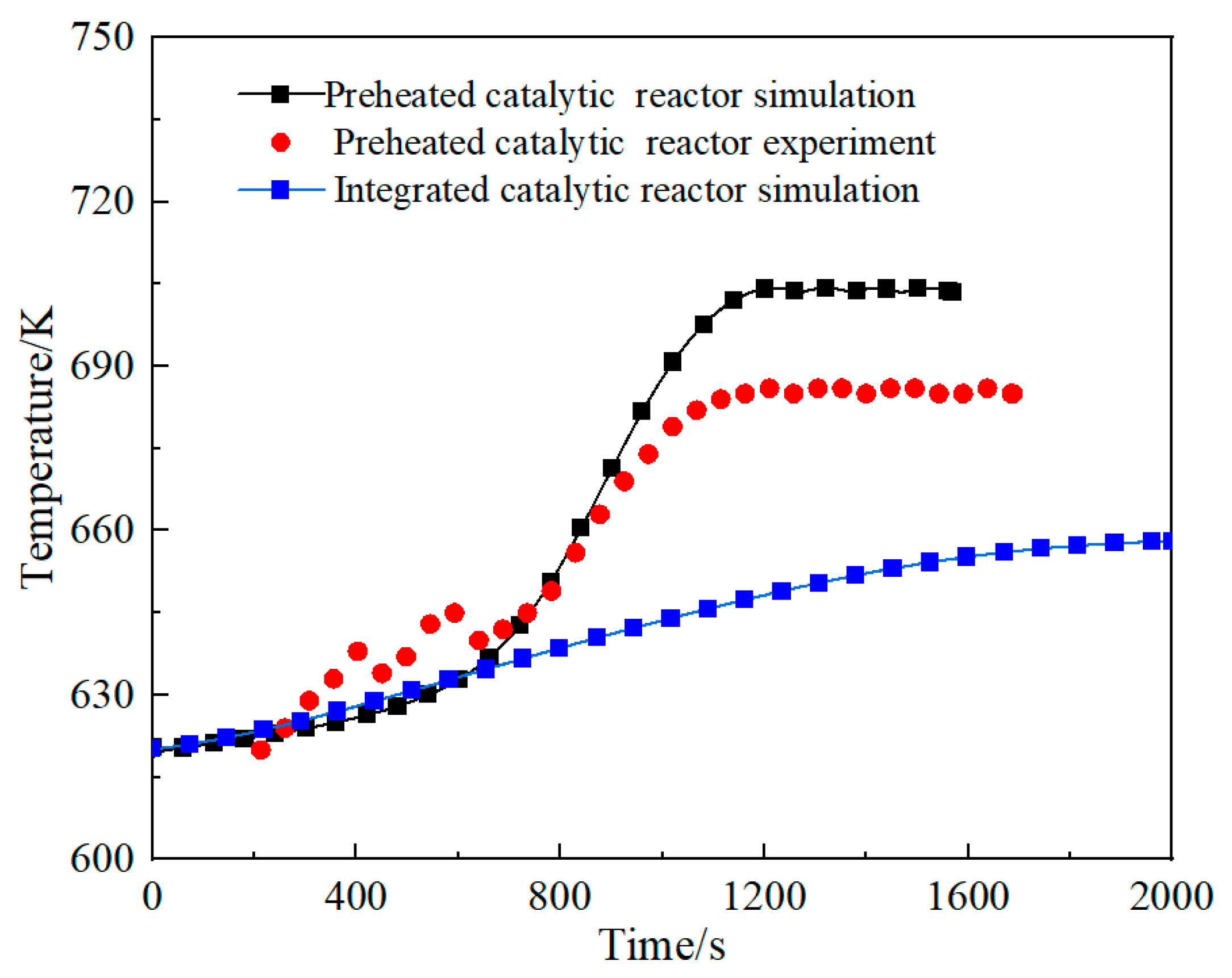
2.4.2. Processing of Preheater Experimental Data
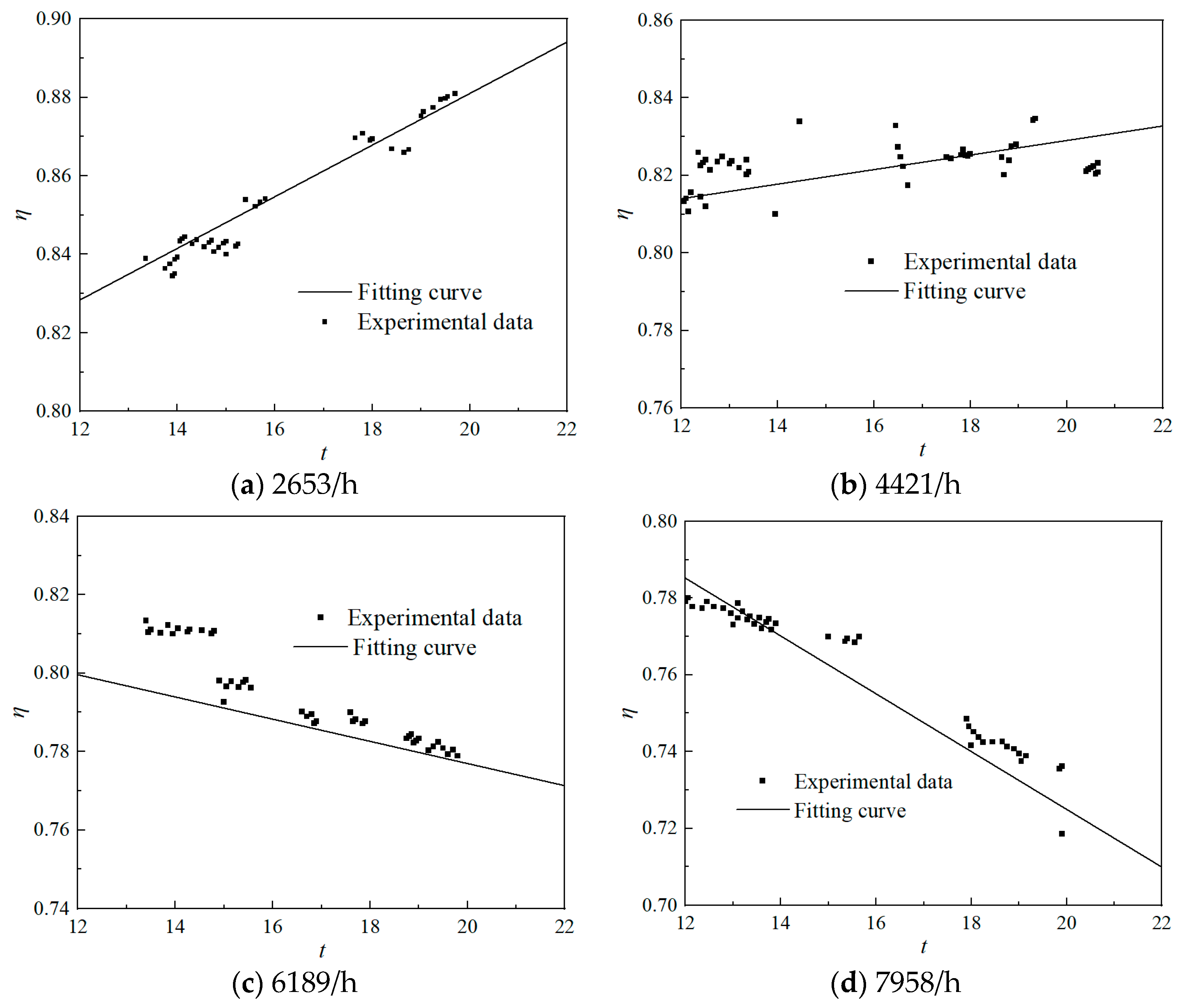
2.4.3. Fitting of Preheater Formulas for Different Space Velocities
2.4.4. Self-Heating Maintenance Iterative Calculation Method
3. Results and Discussion
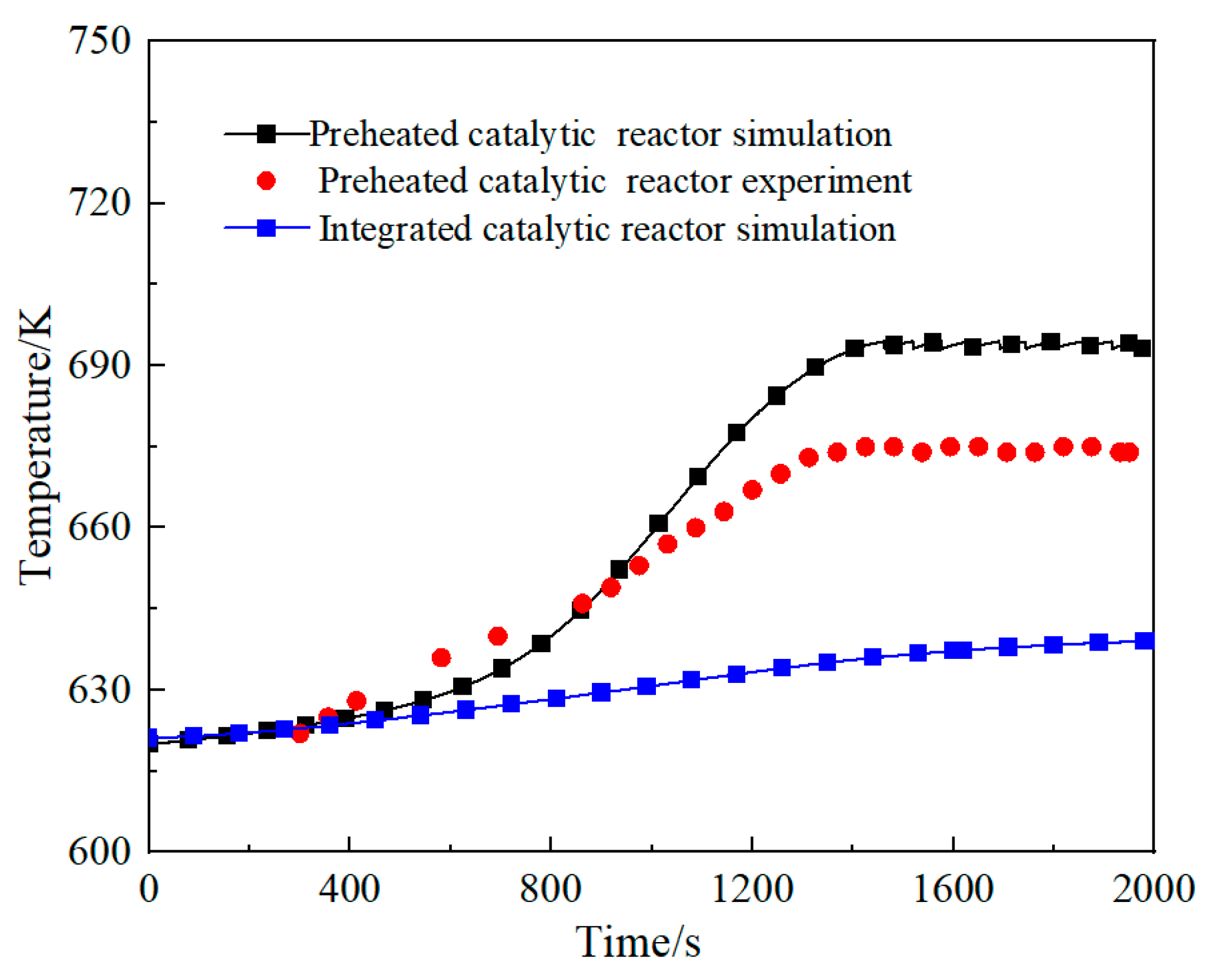
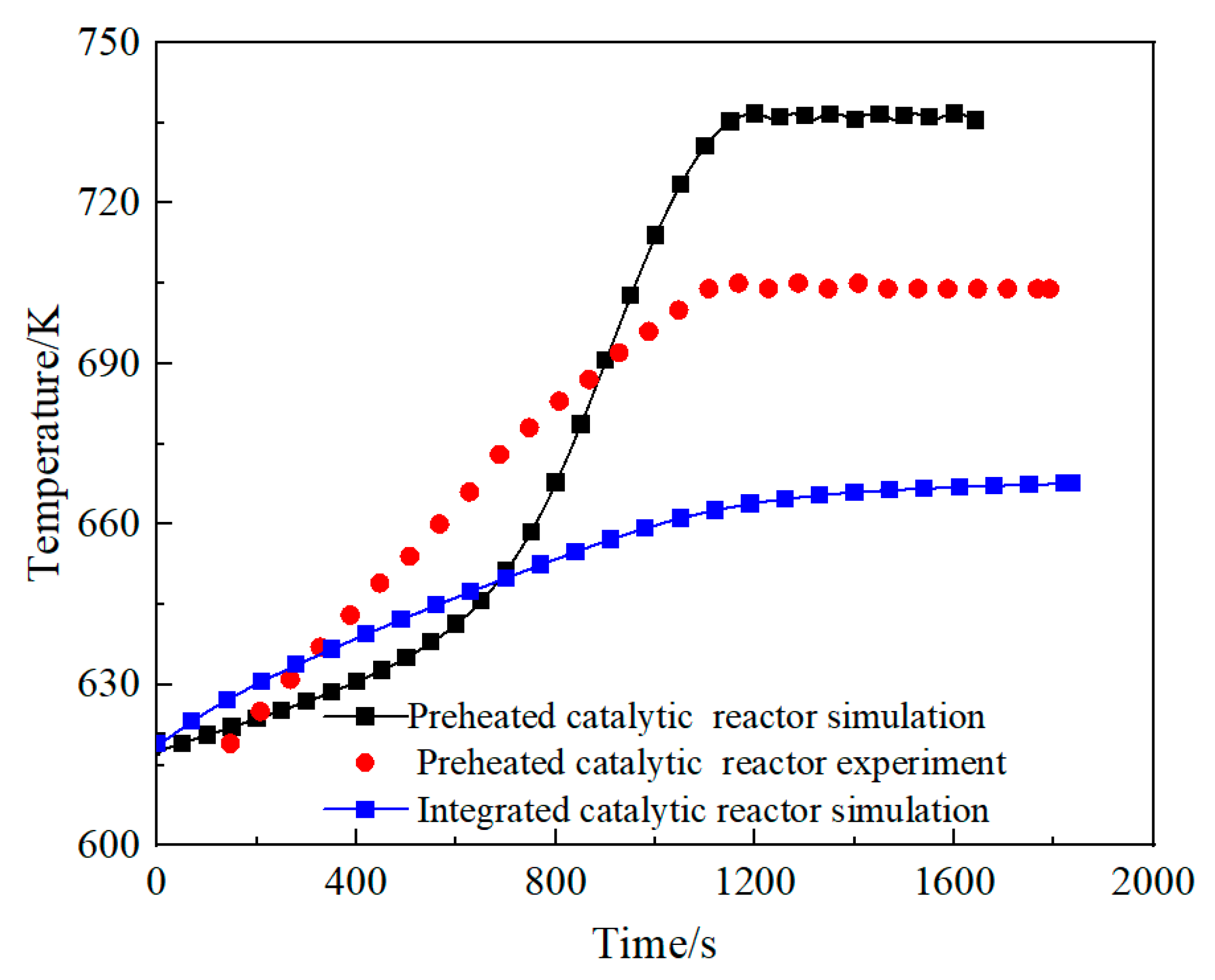
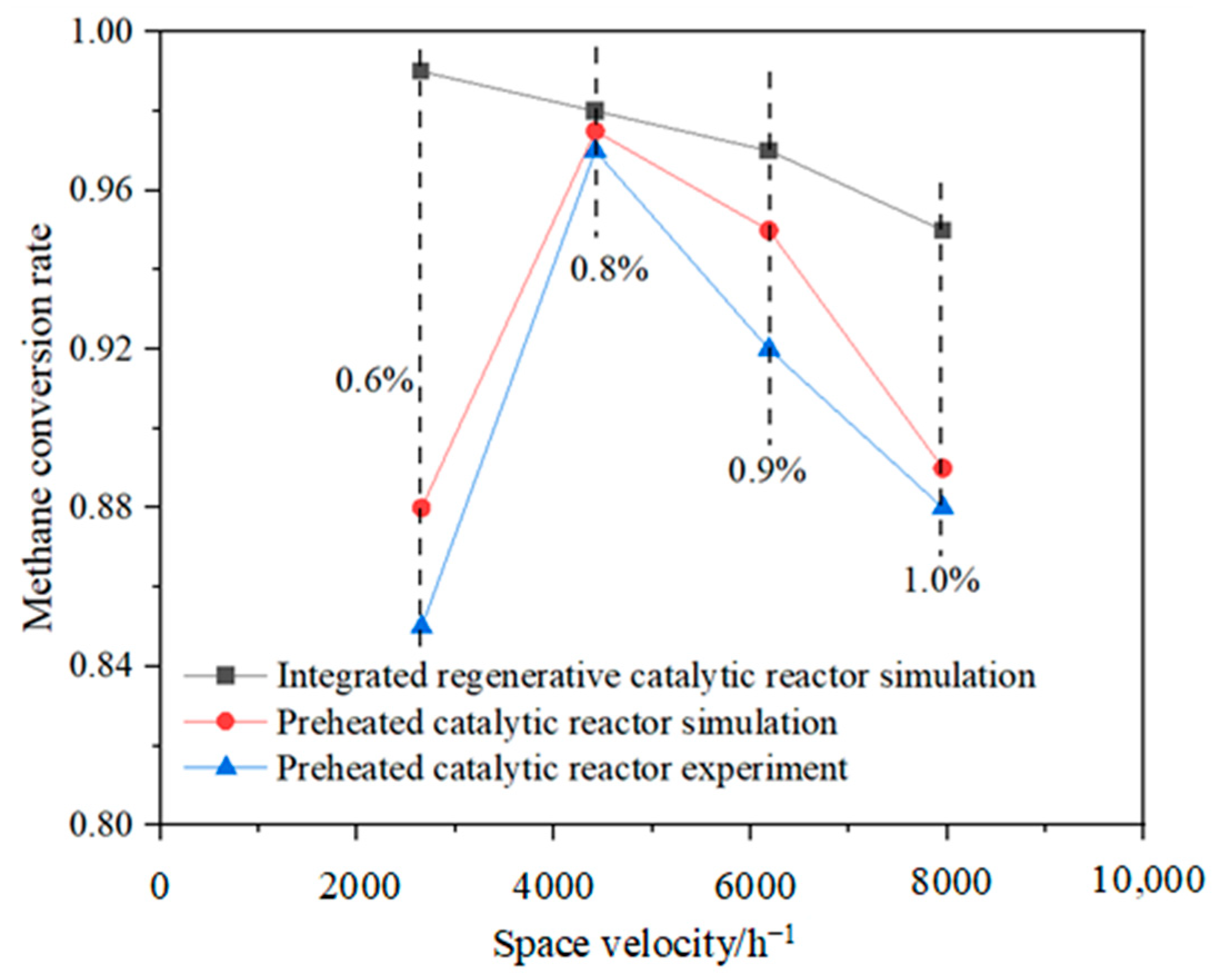
3.1. Comparison of the Performance of the Integrated Regenerative Catalytic Reactor
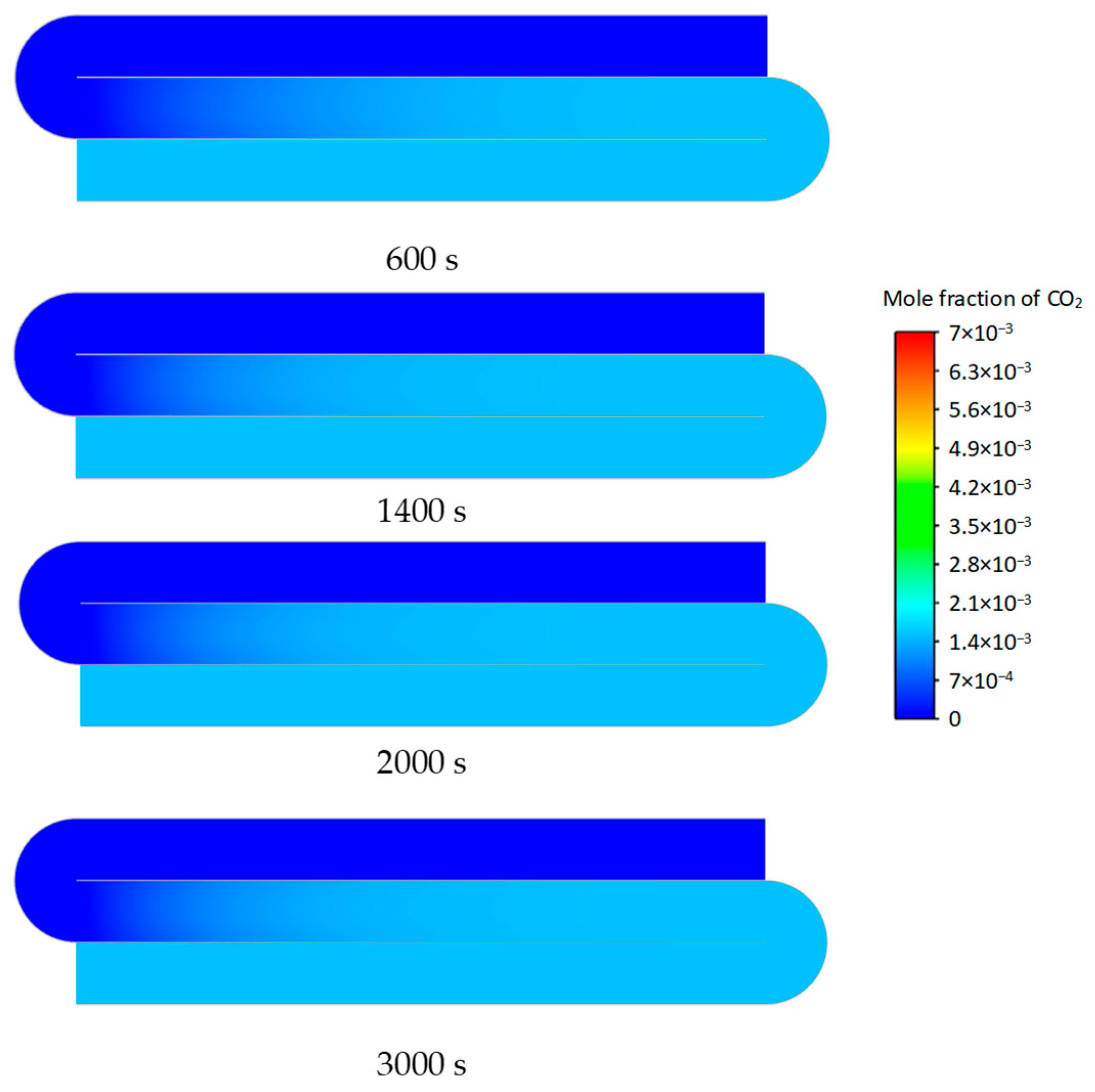
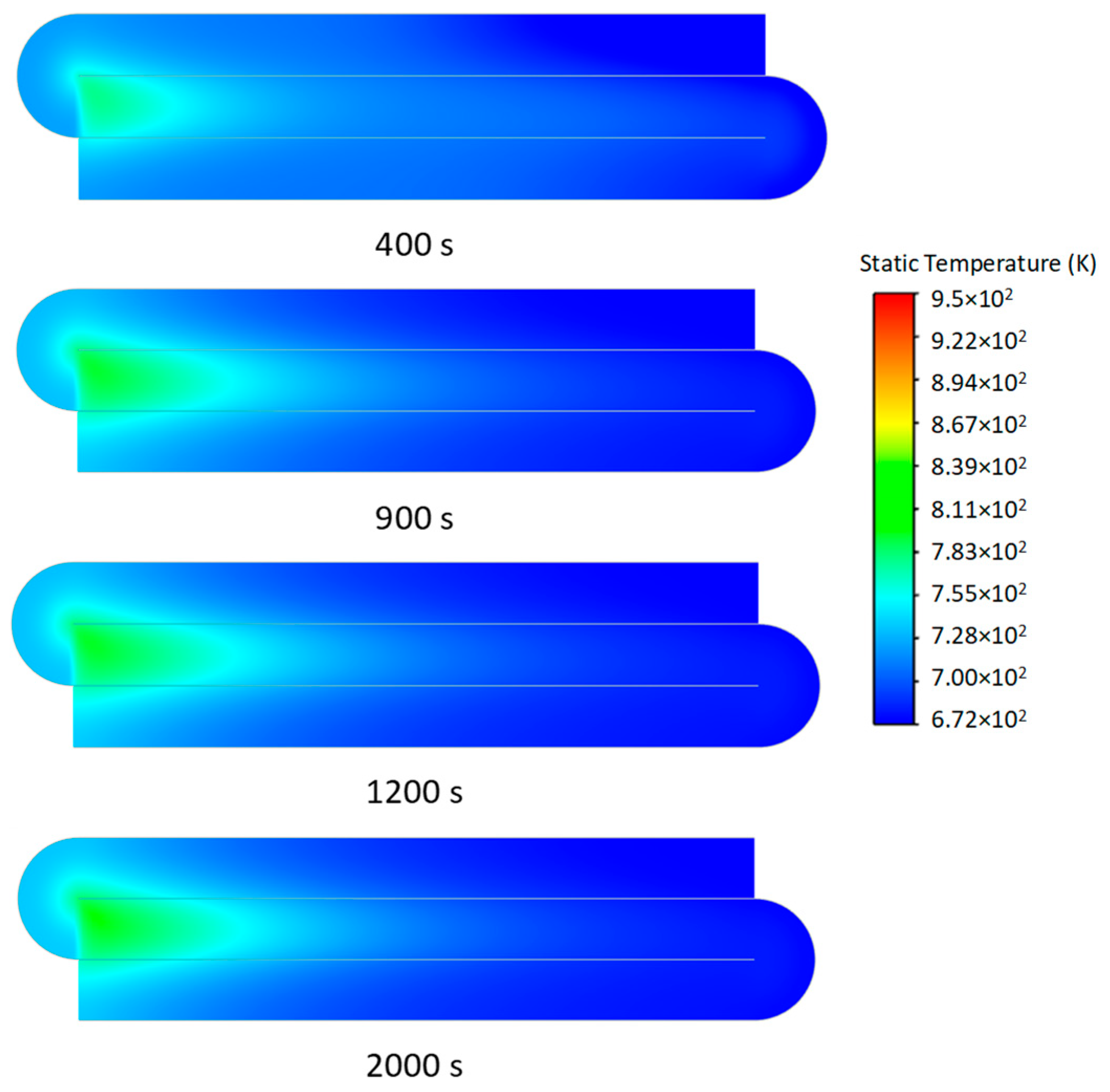
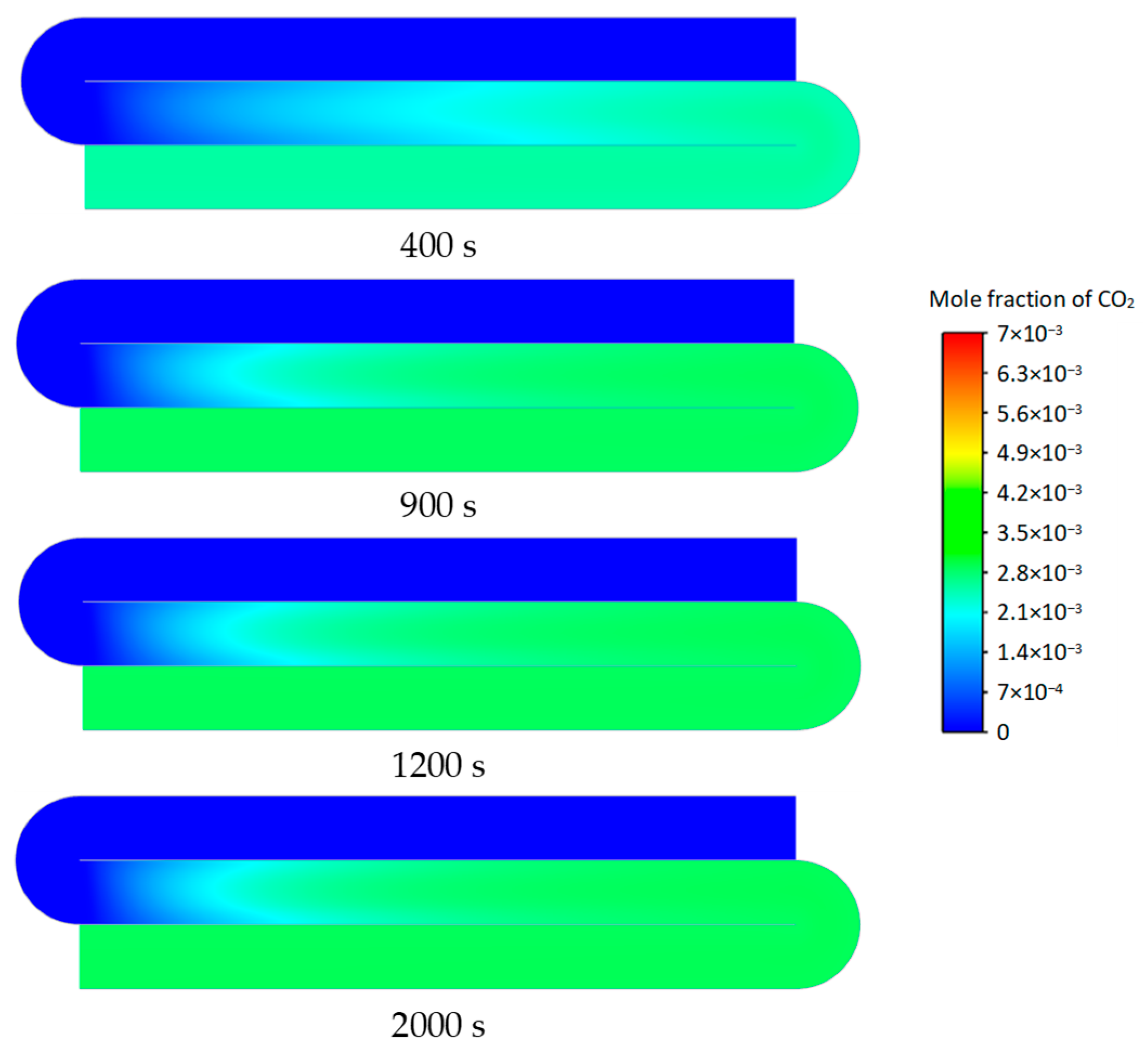
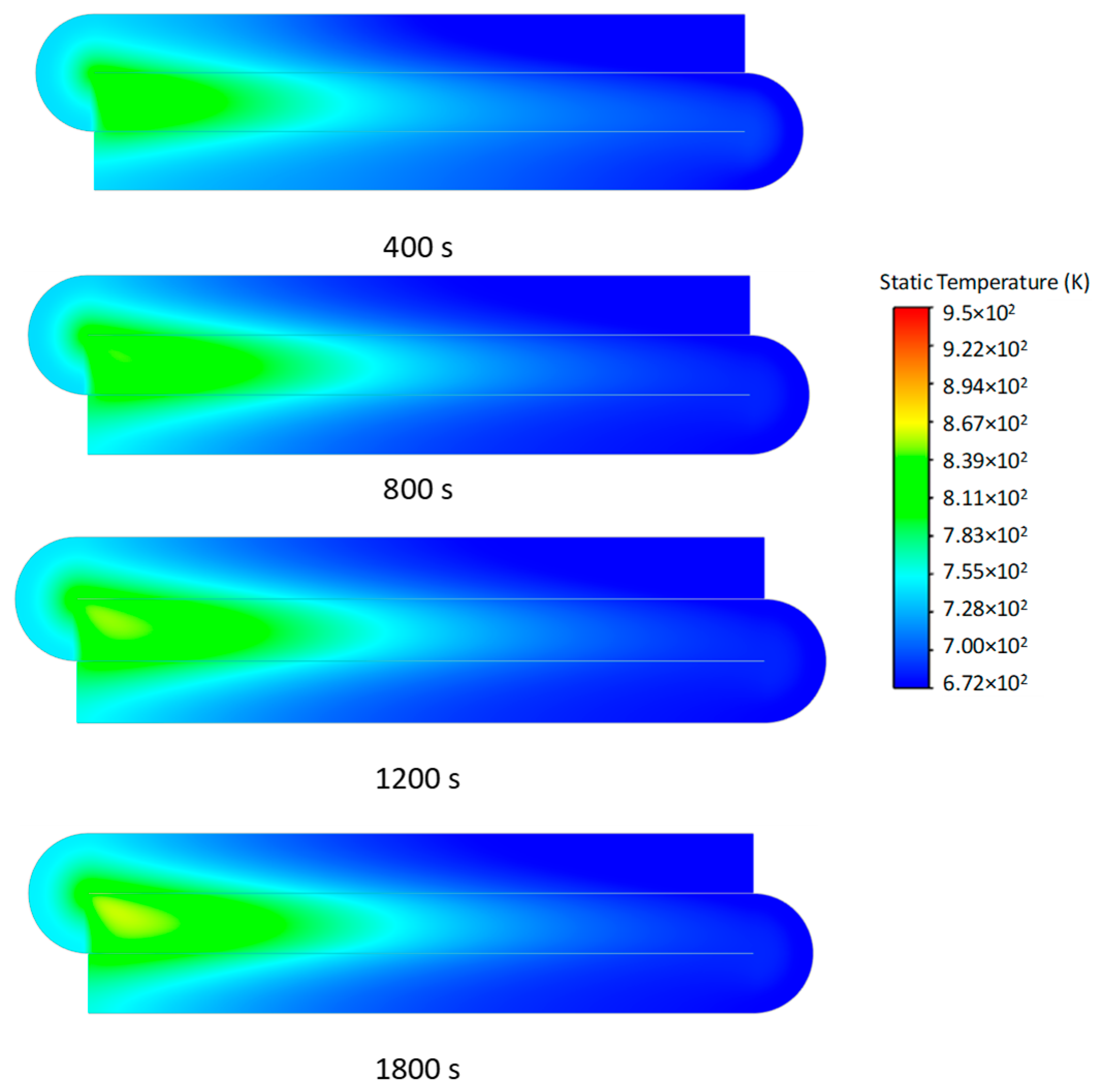
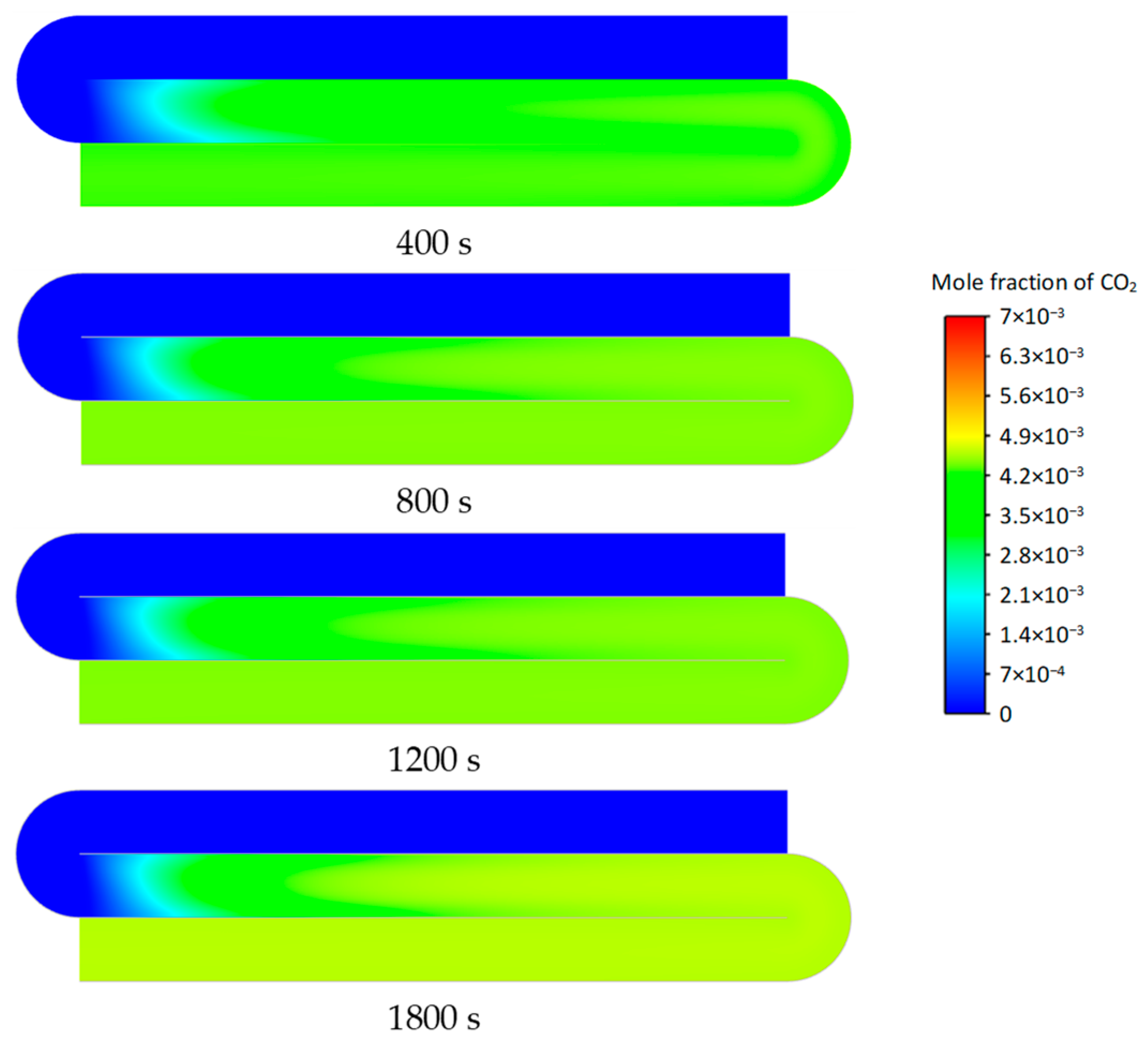
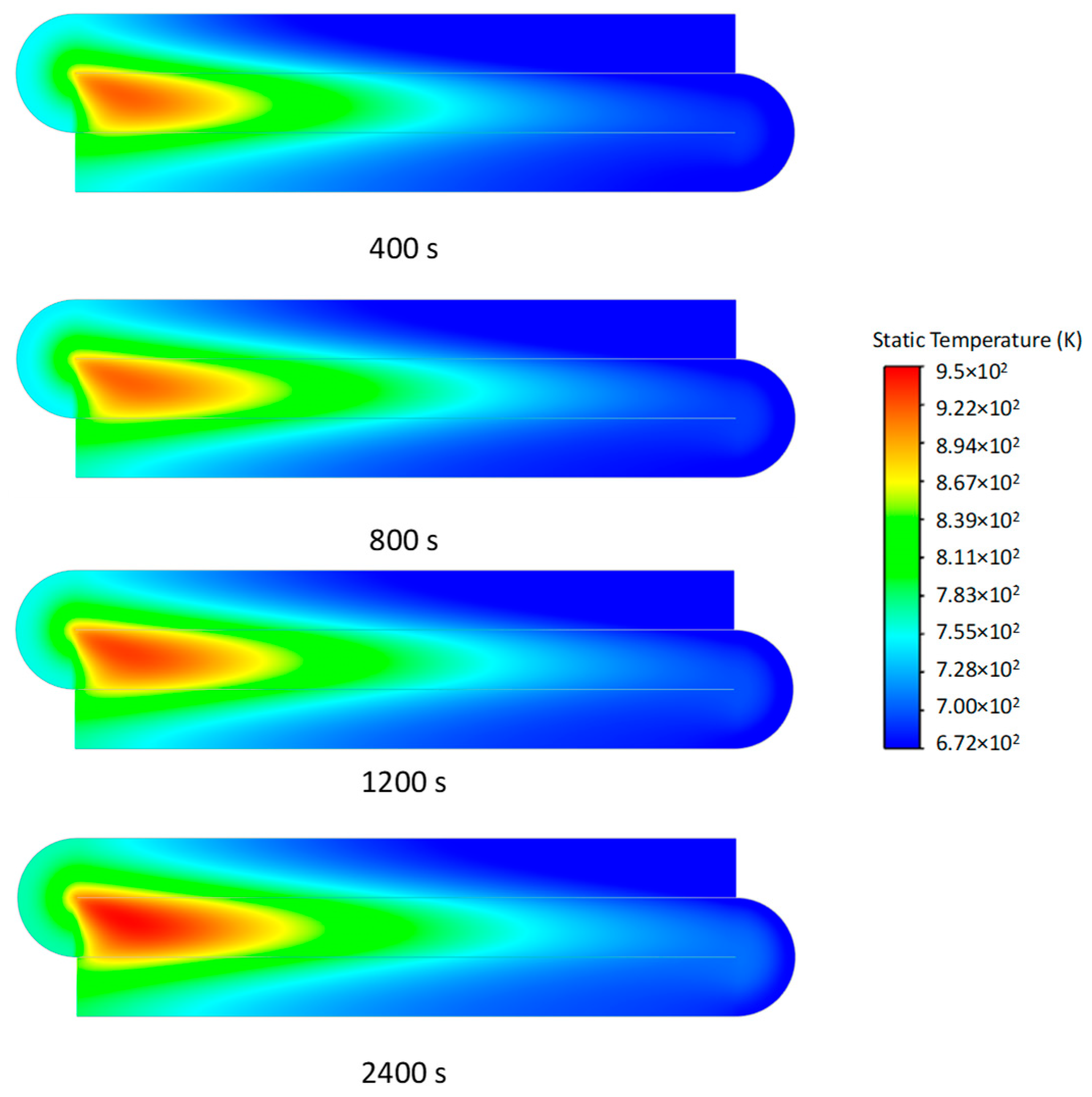
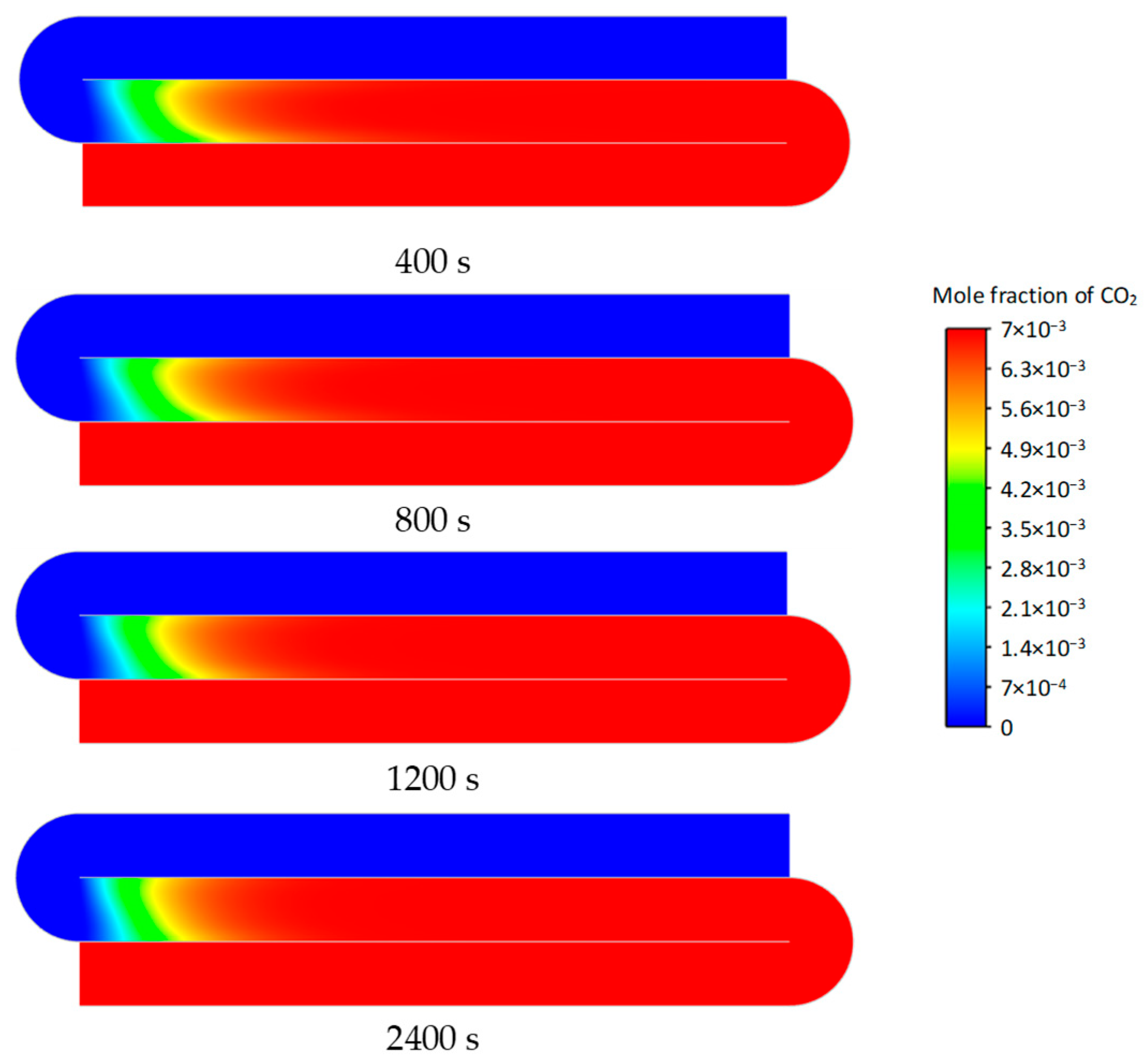
3.2. Transient Characteristics of the Self-Heating Maintenance State
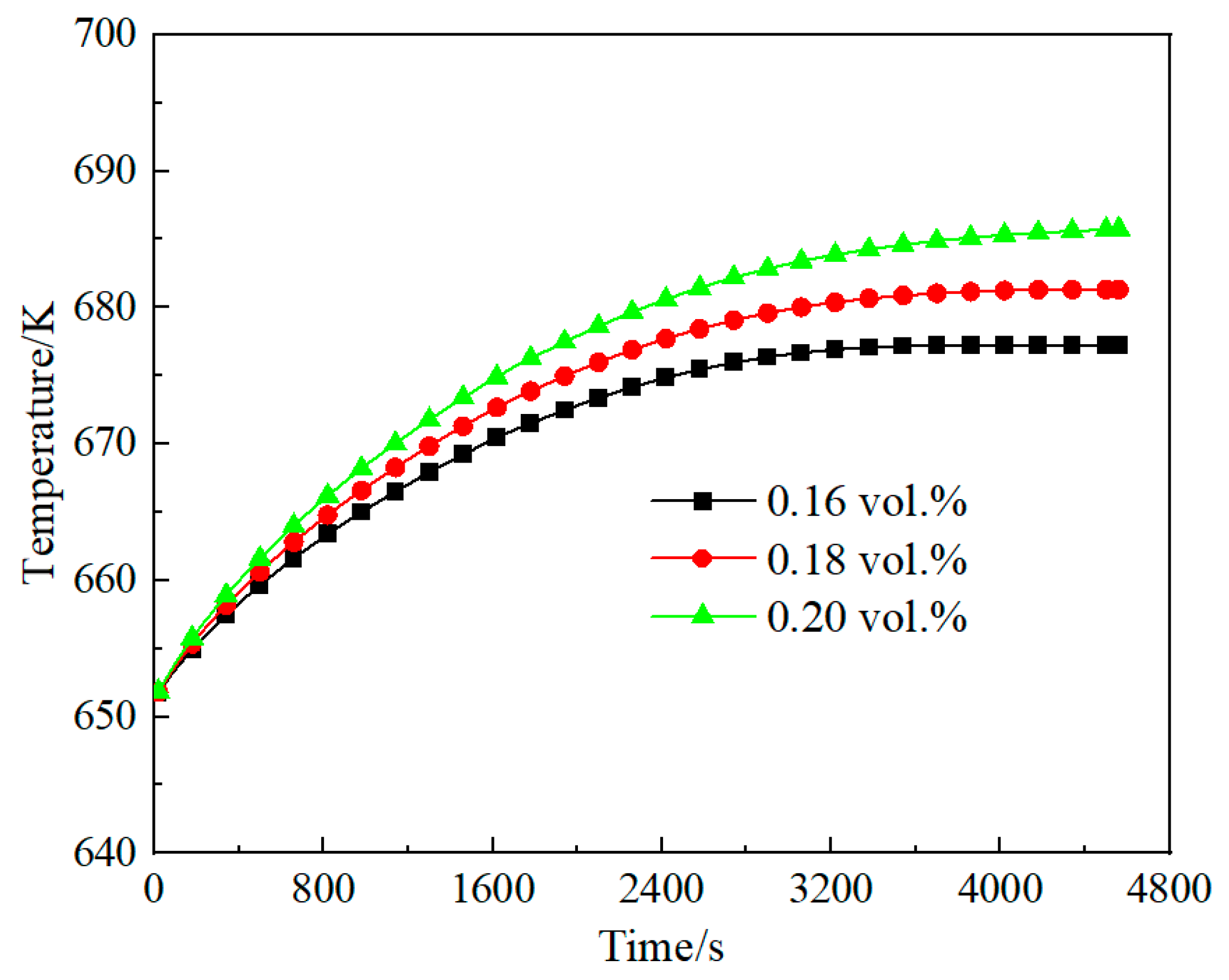
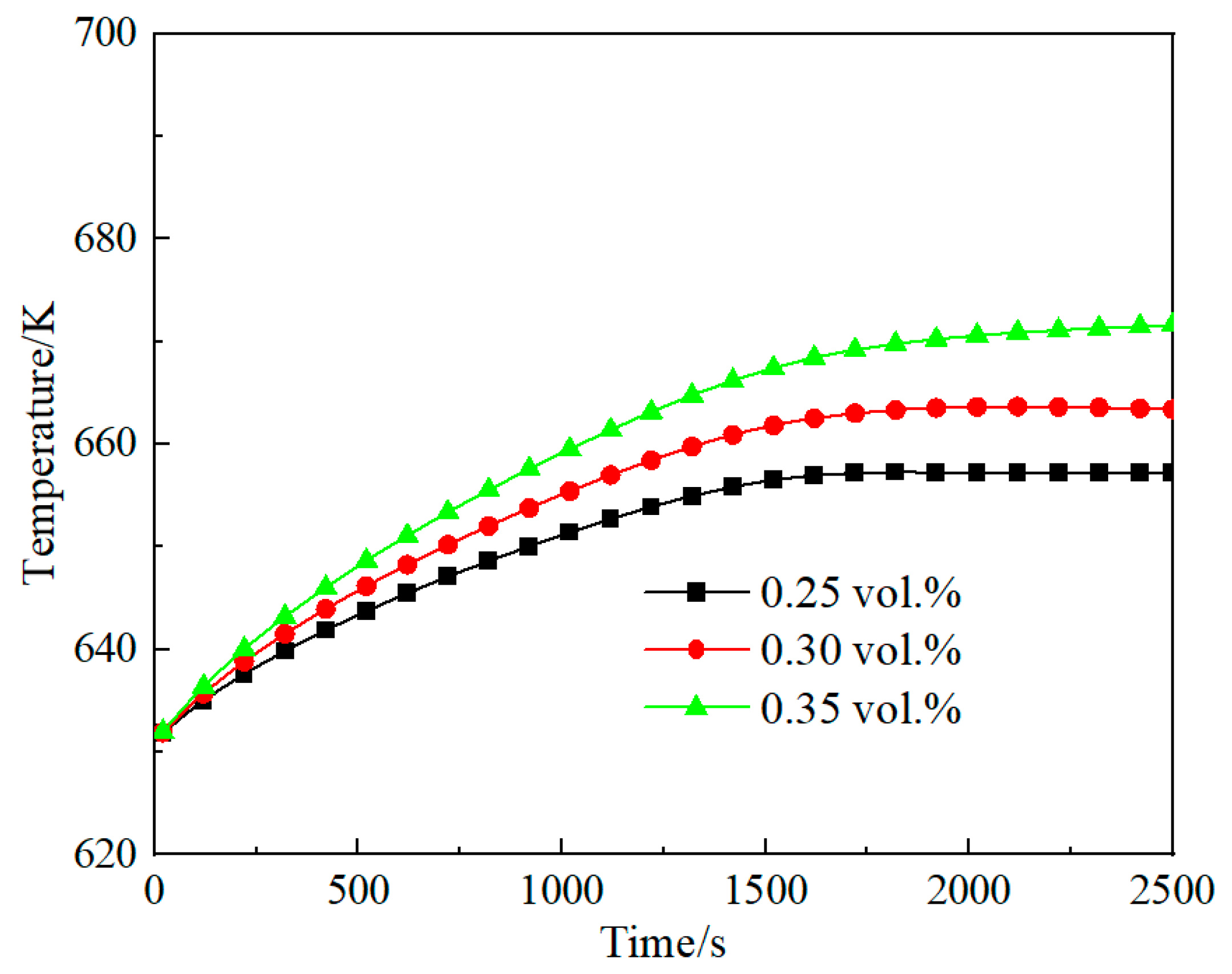
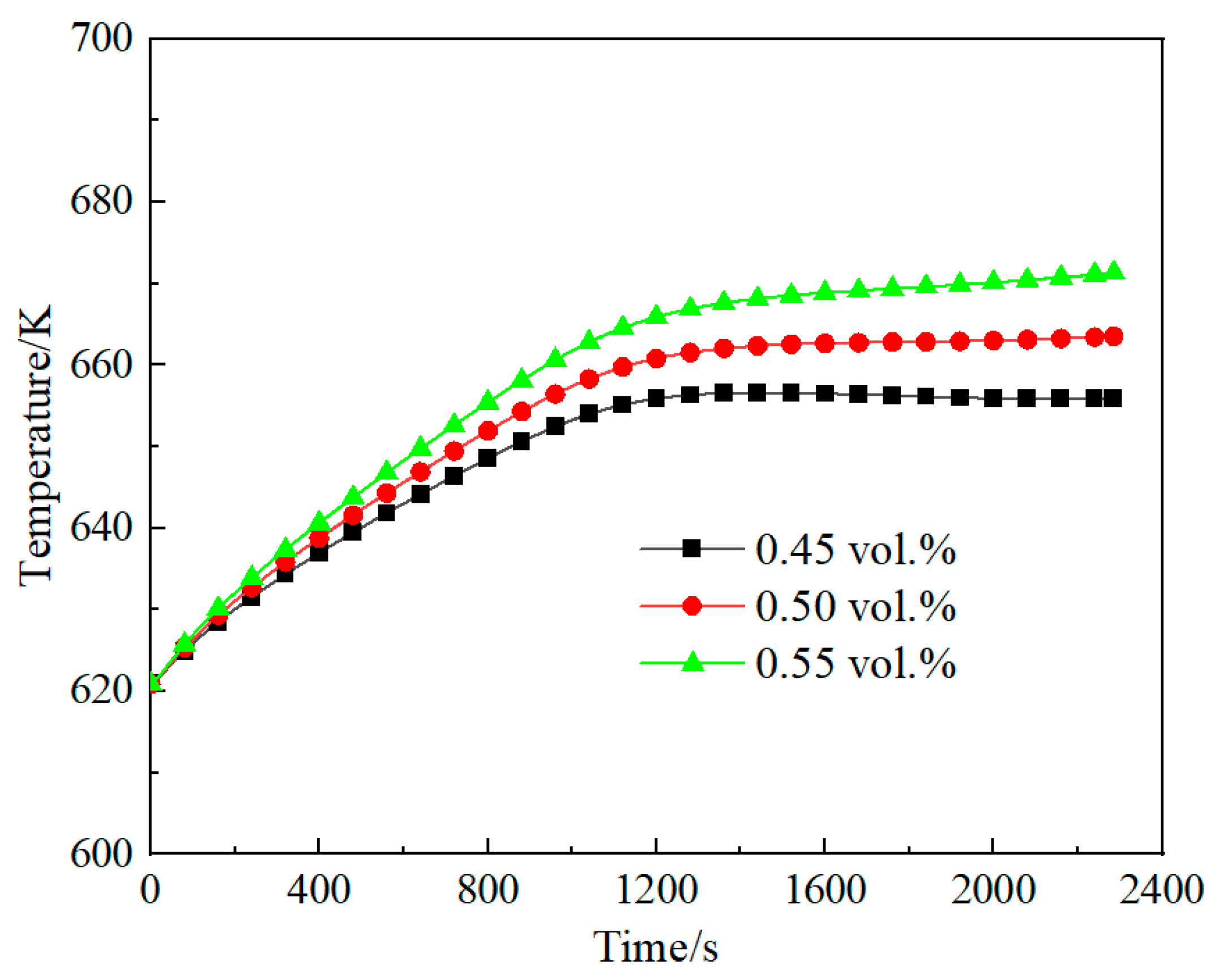
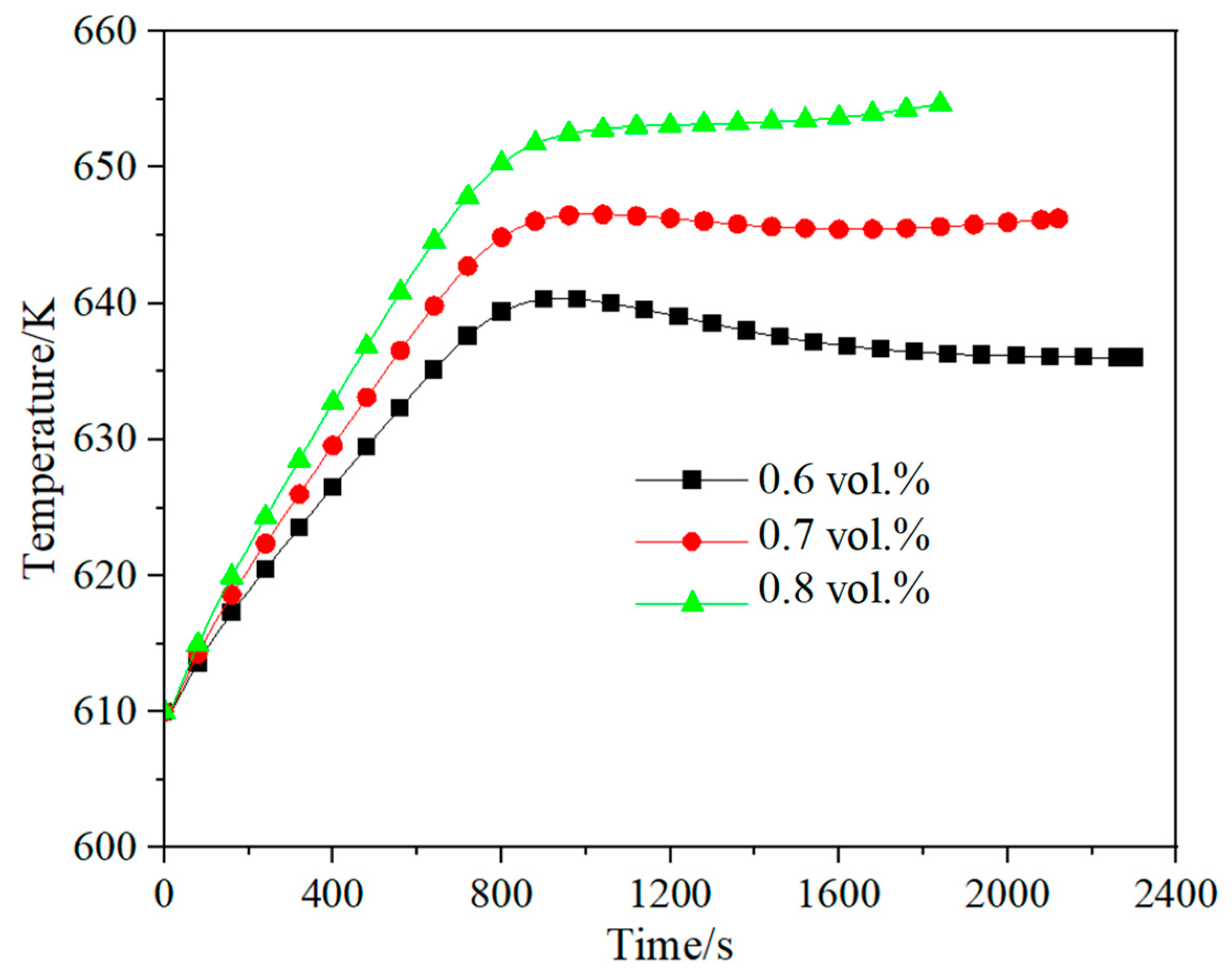
3.3. Effect of Methane Concentration on Self-Heating Maintenance Characteristics
4. Conclusions
- (1)
- Comparative analysis shows that under the same inlet conditions, the self-heating maintenance performance of integrated regenerative catalytic reactors is superior to traditional preheated catalytic reactors. Numerical simulations show that the integrated regenerative catalytic reactor achieves self-heating maintenance operation at significantly lower inlet temperatures. Compared with the preheated catalytic reactor, the integrated regenerative catalytic reactor showed a decrease in self-heating-maintained inlet temperatures of 54 K, 70 K, 84 K, and 40 K at space velocities of 2653 h−1, 4421 h−1, 6189 h−1, and 7958 h−1, respectively.
- (2)
- Transient temperature and CO2 concentration fields in the integrated regenerative catalytic reactor are analyzed. Temperature contours reveal gradual thermal gradient formation between channels, with initial asymmetric high-temperature zones (e.g., downward-shifted hotspots at higher velocities). CO2 distribution evolves from partial to complete methane conversion, showing progressive shortening of reaction zones until stabilization. At 7958 h−1, CO2 generation accelerates, achieving full conversion by 800 s. Spatial analysis highlights wall-proximity reaction delays, particularly near the lower walls.
- (3)
- Space velocity significantly impacts methane concentration requirements for self-heat maintenance. At lower space velocity (2653 h−1), the minimum methane concentration for self-sustaining operation is 0.16 vol.%, while higher velocity (7958 h−1) requires 0.6 vol.% methane concentration. For any given space velocity, increased methane concentration necessitates higher reactor inlet temperatures to achieve thermal equilibrium. Increased space velocity narrows operable methane ranges and demands higher concentrations for thermal equilibrium due to reduced preheater heat exchange efficiency from shorter flue gas residence times.
- (4)
- Based on its capability to achieve self-sustained combustion at ultra-low methane concentrations (0.16 vol.%) with high heat recovery efficiency and 99% methane conversion, the integrated regenerative catalytic reactor significantly reduces preheating energy consumption (>50%) and enables annual CO2e mitigation of 1200 tons per unit (1000 Nm3/h scale). This design supports global methane abatement goals under the “Dual Carbon” strategy by converting dilute emissions into CO2/H2O without auxiliary fuel.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Nie, B.; Li, G.; Liu, X.; Yin, F.; Wang, Z.; He, H.; Bao, S.; Fu, H.; Huang, J. Experimental study and correlation analysis on thermal storage oxidation of ventilation air methane mixed with pulverized coal. Case Stud. Therm. Eng. 2025, 68, 105885. [Google Scholar] [CrossRef]
- Gosiewski, K.; Pawlaczyk, A. Catalytic or thermal reversed flow combustion of coal mine ventilation air methane: What is better choice and when? Chem. Eng. J. 2014, 238, 78–85. [Google Scholar] [CrossRef]
- Tang, A.; Yang, J.; Shao, S.; Cai, T.; Zhang, Z.; Xiao, L. Experimental investigations on the thermal destabilization and performance enhancement of ventilation air methane in a reversed flow oxidation reactor. Chem. Eng. Process. 2024, 204, 109958. [Google Scholar] [CrossRef]
- He, X.; Lei, L. Optimizing methane recovery: Techno-economic feasibility analysis of N2-selective membranes for the enrichment of ventilation air methane. Sep. Purif. Technol. 2021, 259, 118180. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Q.; Liu, X.; Lin, B.; Li, D. Investigations on the mitigation of ventilation air methane and energy recovery in site trial thermal flow-reversal reactor. Chem. Eng. Process. 2022, 170, 108703. [Google Scholar] [CrossRef]
- Ajrash AL-Zuraiji, M.J.; Zanganeh, J.; Moghtaderi, B. Application of flame arrester in mitigation of explosion and flame deflagration of ventilation air methane. Fuel 2019, 257, 115985. [Google Scholar] [CrossRef]
- Ursueguía, D.; Marín, P.; Díaz, E.; Ordóñez, S. A new strategy for upgrading ventilation air methane emissions combining adsorption and combustion in a lean-gas turbine. J. Nat. Gas Sci. Eng. 2021, 88, 103808. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, T.; Liu, S.; Nie, B.; Yuan, B.; Han, Y. Insight into ventilation air methane combustion of ultralow sub-nanometer palladium catalyst within the MFI zeolite. J. Environ. Sci. 2025, 155, 1–12. [Google Scholar] [CrossRef]
- Lyu, X.; Qiao, Y.; Yuan, D.; Zhang, Z.; Zuo, W.; Hua, J.; Wang, Y.; Zhang, L. Investigation and CFD simulation of coal dust explosion accident in confined space: A case study of Gaohe Coal Mine Ventilation Air Methane oxidation power plant. Fire Saf. J. 2024, 149, 104237. [Google Scholar] [CrossRef]
- Moncada Quintero, C.W.; Ercolino, G.; Specchia, S. Combined silicon carbide and zirconia open cell foams for the process intensification of catalytic methane combustion in lean conditions: Impact on heat and mass transfer. Chem. Eng. J. 2022, 429, 132448. [Google Scholar] [CrossRef]
- Akbari, E.; Alavi, S.M.; Rezaei, M.; Larimi, A. CeO2-promoted BaO-MnOx catalyst for lean methane catalytic combustion at low temperatures: Improved catalytic efficiency and light-off temperature. Int. J. Hydrogen Energy 2022, 47, 13004–13021. [Google Scholar] [CrossRef]
- Huang, Q.; Yin, C.; Lei, Y.; Fang, Y.; Wang, H.; Wu, H.; Luo, H.; Xu, Y.; Wang, Y.; Miao, Y.; et al. Potassium-promoted high-entropy Mg-Al spinel with active lattice oxygen and improved hydrothermal stability for catalytic combustion of lean methane. Sep. Purif. Technol. 2025, 359, 130672. [Google Scholar] [CrossRef]
- Ramos, H.S.F.; Mmbaga, J.P.; Hayes, R.E. Catalyst optimization in a catalytic flow reversal reactor for lean methane combustion. Catal. Today 2023, 407, 182–193. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, J.; Zhou, C.; Xue, Z.; Zheng, Y.; Tang, H.; Liu, Z. Catalytic combustion of lean methane over different Co3O4 nanoparticle catalysts. Heliyon 2023, 9, e21994. [Google Scholar] [CrossRef]
- Varbar, M.; Alavi, S.M.; Rezaei, M.; Akbari, E. Lean methane catalytic combustion over the mesoporous MnOx-Ni/MgAl2O4 catalysts: Effects of Mn loading. Int. J. Hydrogen Energy 2022, 47, 39829–39840. [Google Scholar] [CrossRef]
- Tang, S.; Li, W.; Jin, J.; Zhang, X.; Shen, Z.; Gui, Y. Lean methane catalytic combustion using Pd/ZSM-5 catalysts prepared by ammonia evaporation method. Mol. Catal. 2025, 579, 115092. [Google Scholar] [CrossRef]
- Shen, Z.; Nabavi, S.A.; Clough, P.T. Design and performance testing of a monolithic nickel-based SiC catalyst for steam methane reforming. Appl. Catal. A Gen. 2024, 670, 119529. [Google Scholar] [CrossRef]
- Pandit, D.M.; Bollini, P.; Balakotaiah, V. Optimization of monolith reactor design for autothermal oxidative dehydrogenation of ethane over a MoVTeNbOx catalyst. Chem. Eng. J. 2025, 509, 16266. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, Y.; Zhang, Y.; Li, T.; Li, J.; Chen, Z.; Norinaga, K. Regulation of temperature distribution in fixed bed reactor for CO2 methanation through “CHESS” monolith structure catalyst. Appl. Therm. Eng. 2024, 236, 121826. [Google Scholar] [CrossRef]
- Sarkar, B.; Ratnakar, R.R.; Balakotaiah, V. Bifurcation analysis of catalytically assisted hydrogen combustion in monolith reactors. Chem. Eng. J. 2021, 425, 130318. [Google Scholar] [CrossRef]
- Sandu, V.C.; Cormos, A.M.; Dumbrava, I.D.; Imre-Lucaci, A.; Cormos, C.C.; de Boer, R.; Boon, J.; Sluijter, S. Assessment of CO2 capture efffciency in packed bed versus 3D-printed monolith reactors for SEWGS using CFD modeling. Int. J. Greenh. Gas Control 2021, 111, 103447. [Google Scholar] [CrossRef]
- Liu, X.; Zeng, Y.; Chen, J.; Zhong, Z.; Xing, W. Research progress on the monolithic catalyst for hydrogenation of CO2 to methane. Chin. J. Chem. Eng. 2025, 80, 184–197. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, H.; Yang, L.; Wu, D.; He, S.; Yang, H.; Xiong, H. Methane-selective oxidation to methanol and ammonia selective catalytic reduction of NOx over monolithic Cu/SSZ-13 catalysts: Are hydrothermal stability and active sites same? Fuel 2022, 309, 122178. [Google Scholar] [CrossRef]
- Chen, J.; Yan, L.; Song, W.; Xu, D. Effect of heat recirculation on the combustion stability of methane-air mixtures in catalytic micro-combustors. Appl. Therm. Eng. 2017, 115, 702–714. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, Z.; Wang, B.; Wang, Z.; Yin, Z.; Zhang, Z. Optimization design and performance analysis of methanol reforming reactor for exhaust waste heat recovery of marine engine. Int. J. Hydrogen Energy 2024, 49, 1593–1604. [Google Scholar] [CrossRef]
- He, G.; Zhang, H.; Zhou, W.; Zeng, H.; Shen, Z.; Liu, H. Reaction kinetic and application of methane reforming with CO2/H2O in flue gas for heat recovery and carbon conversion. Chem. Eng. J. 2025, 513, 162996. [Google Scholar] [CrossRef]
- Miranda, A.F.; Rodríguez, M.L.; Borio, D.O. Open-loop dynamic analysis of the catalytic oxidation of VOCs with heat recovery. Chem. Eng. Res. Des. 2023, 193, 432–446. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, Y.; Yao, N.; Zhang, J.; Huang, Y.; Han, D.; Pan, W. Recent advancements in ammonia catalytic cracking and waste heat reforming technologies: A review. Energy Convers. Manag. 2025, 326, 119530. [Google Scholar] [CrossRef]
- Chen, Q.; Mao, M.; Gao, M.; Liu, Y.; Shi, J.; Li, J. Design and Performance Investigation of a Compact Catalytic Reactor Integrated with Heat Recuperator. Energies 2022, 15, 447. [Google Scholar] [CrossRef]
- Mao, M.; Liu, Y.; Liu, R.; Zheng, B.; Meng, J. Experimental Investigations of Cold Flow Mass Distribution and Pressure Drop Characteristics in a Structured Honeycomb Monolith Bed. Open Mech. Eng. J. 2015, 9, 302–311. [Google Scholar]
- Moallemi, F.; Batley, G.; Dupont, V.; Foster, T.J.; Pourkashanian, M.; Williams, A. Chemical modelling and measurements of the catalytic combustion of CH4/air mixtures on platinum and palladium catalysts. Catal. Today 1999, 47, 235–244. [Google Scholar] [CrossRef]
- Zheng, B.; Liu, Y.; Liu, R.; Meng, J. Catalytic oxidation of coal mine ventilation air methane in a preheat catalytic reaction reactor. Int. J. Hydrogen Energy 2015, 40, 3381–3387. [Google Scholar] [CrossRef]
| CH4 | O2 | N2 | CO2 |
|---|---|---|---|
| 0.16–1% | 20.8% | 78.3% | 0.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, F.; Mao, M.; Wang, Y.; Chen, Q. Numerical Study of Self-Heating Maintenance Performance of an Integrated Regenerative Catalytic Reactor. Energies 2025, 18, 4654. https://doi.org/10.3390/en18174654
Zhu F, Mao M, Wang Y, Chen Q. Numerical Study of Self-Heating Maintenance Performance of an Integrated Regenerative Catalytic Reactor. Energies. 2025; 18(17):4654. https://doi.org/10.3390/en18174654
Chicago/Turabian StyleZhu, Fangdong, Mingming Mao, Youtang Wang, and Qiang Chen. 2025. "Numerical Study of Self-Heating Maintenance Performance of an Integrated Regenerative Catalytic Reactor" Energies 18, no. 17: 4654. https://doi.org/10.3390/en18174654
APA StyleZhu, F., Mao, M., Wang, Y., & Chen, Q. (2025). Numerical Study of Self-Heating Maintenance Performance of an Integrated Regenerative Catalytic Reactor. Energies, 18(17), 4654. https://doi.org/10.3390/en18174654





