Technoeconomic Assessment of Biogas Production from Organic Waste via Anaerobic Digestion in Subtropical Central Queensland, Australia
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Preparation and Homogenization
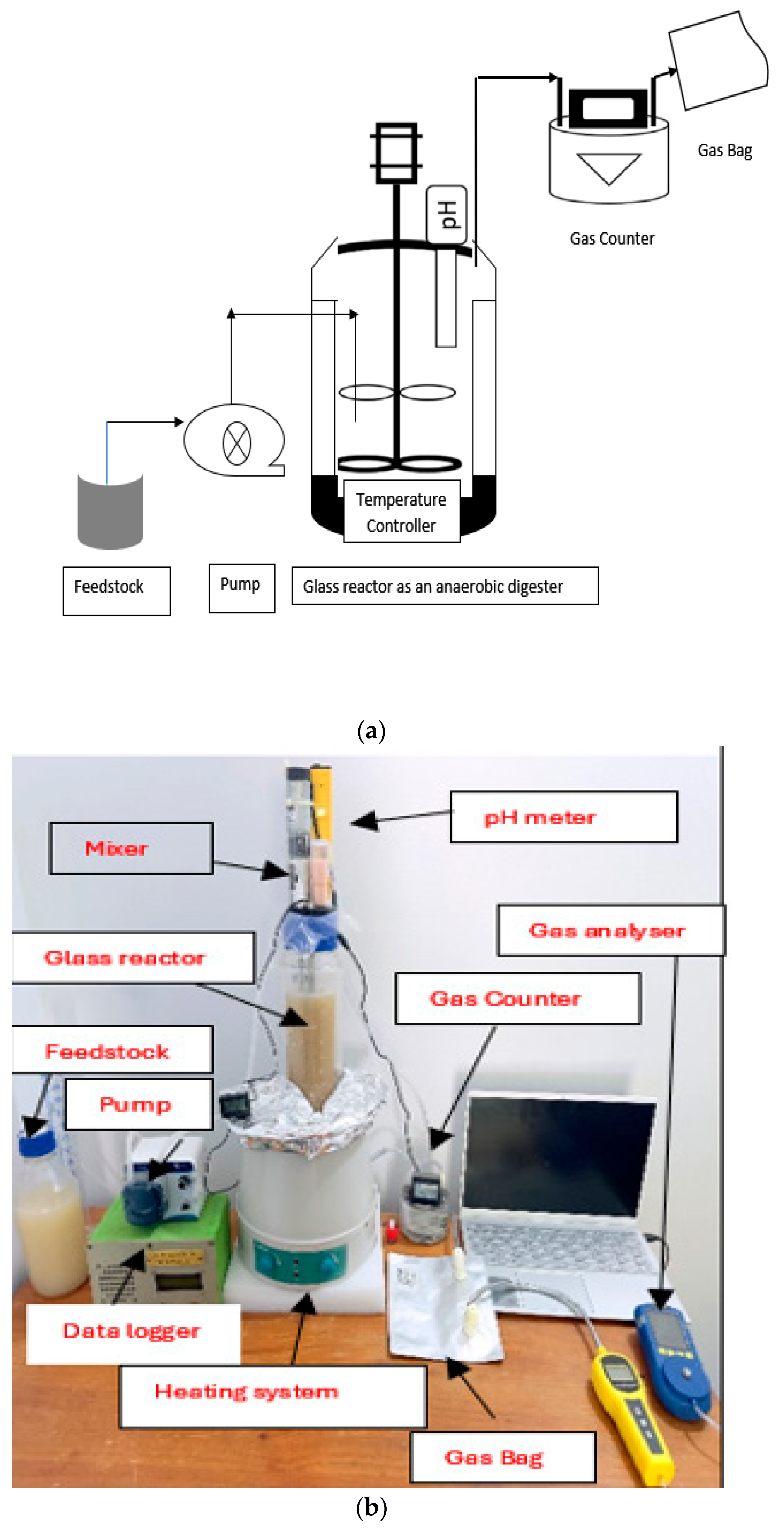
2.3. Experimental Setup
2.4. Analytical Methods
2.5. Technoeconomic Analysis
3. Results and Discussion
3.1. Characterization of Samples
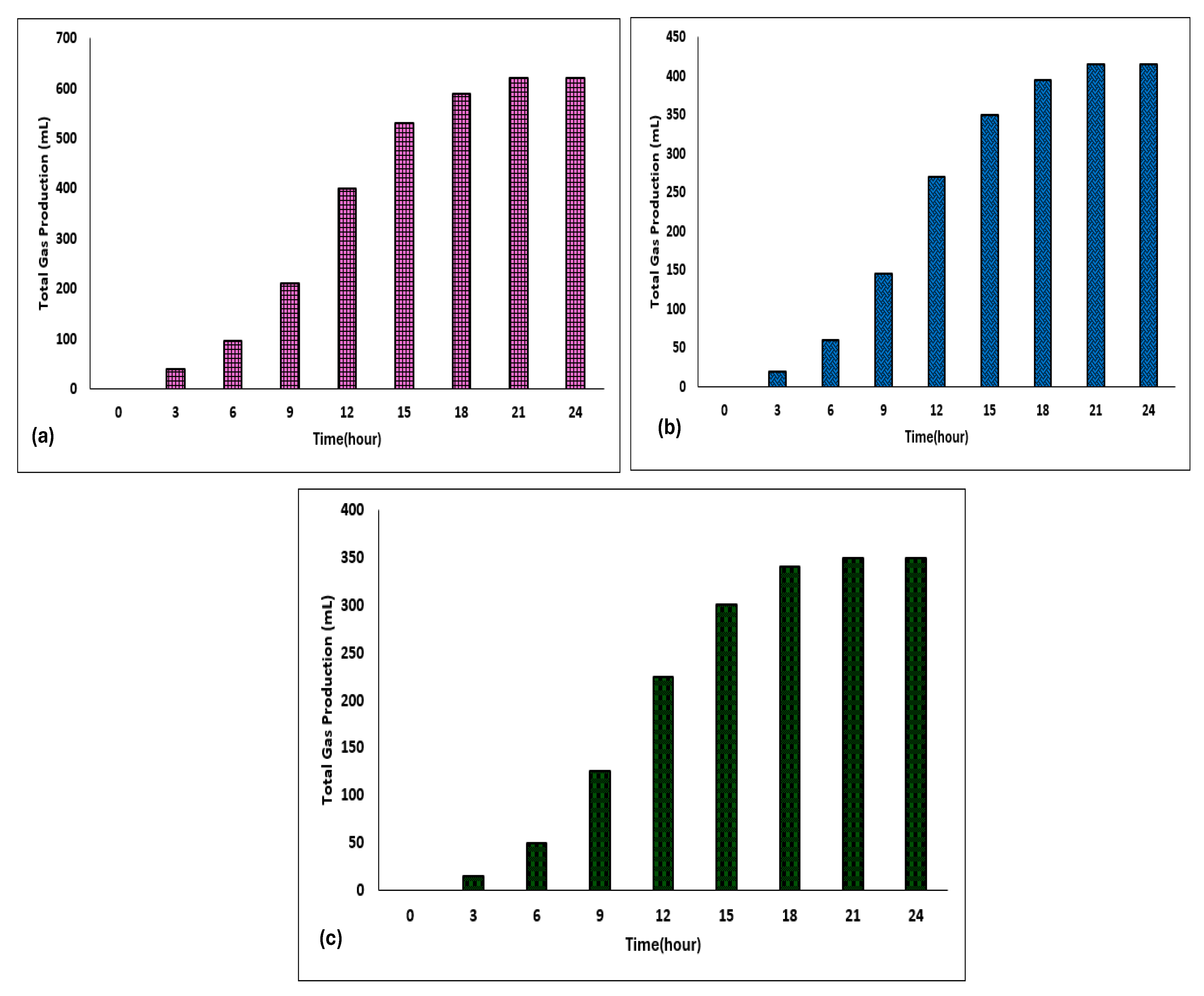
3.2. Temporal Dynamics of Biogas Production Through Anaerobic Digestion (24 h Time Intervals)
3.3. Cumulative Gas Production and Methane Concentration Profile (24 h Time Intervals)
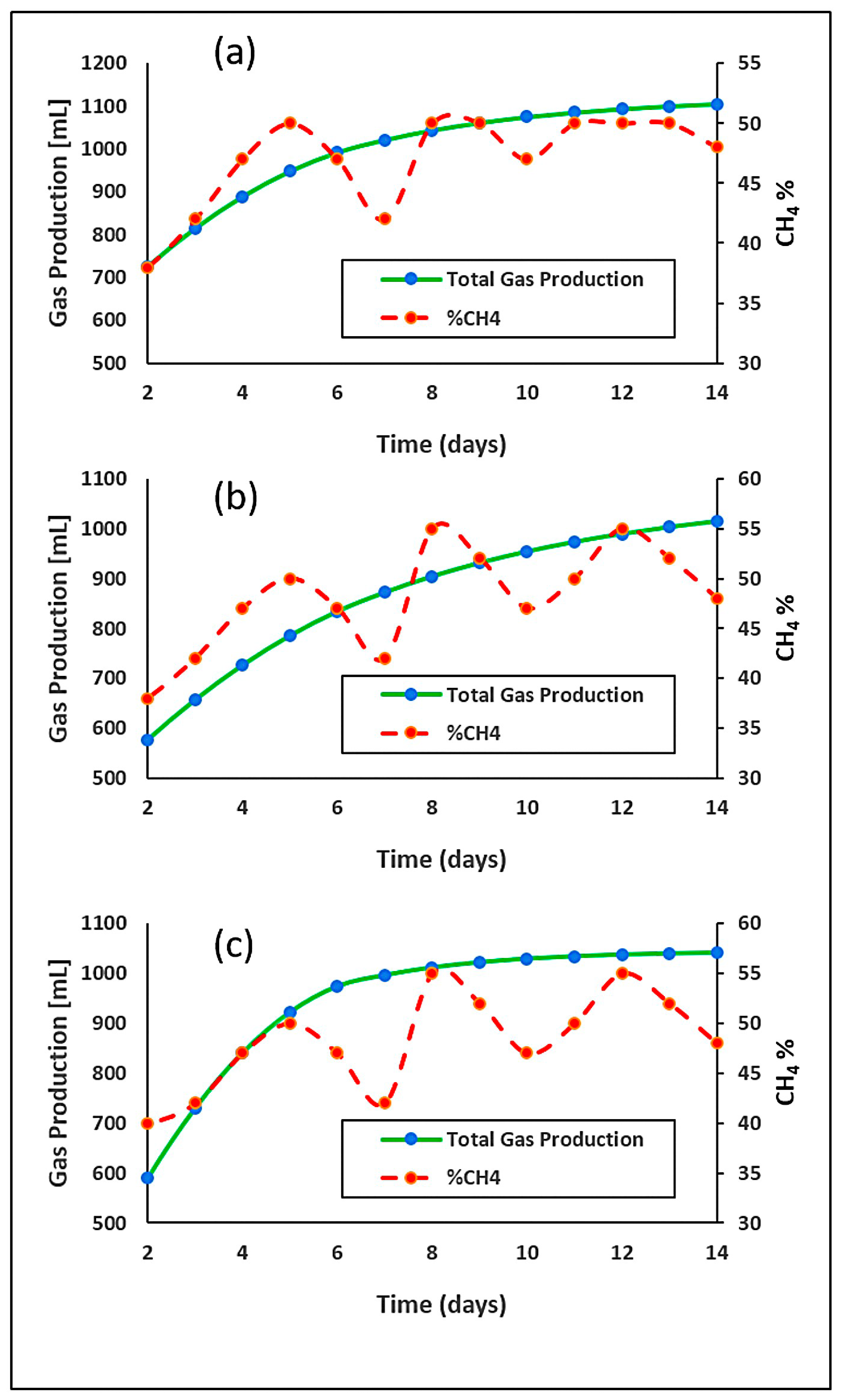
3.4. Cumulative Gas Production and Methane Concentration Profile (14-Day Time Intervals)
4. Technoeconomic Analysis
4.1. Cost Estimation
4.2. Benefit Estimation
4.3. Financial Indicators
4.4. Sensitivity Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Belousov, A.; Lushpeev, V.; Sokolov, A.; Sultanbekov, R.; Tyan, Y.; Ovchinnikov, E.; Shvets, A.; Bushuev, V.; Islamov, S. Experimental Research of the Possibility of Applying the Hartmann–Sprenger Effect to Regulate the Pressure of Natural Gas in Non-Stationary Conditions. Processes 2025, 13, 1189. [Google Scholar] [CrossRef]
- Obaideen, K.; Abdelkareem, M.A.; Wilberforce, T.; Elsaid, K.; Sayed, E.T.; Maghrabie, H.M.; Olabi, A. Biogas role in achievement of the sustainable development goals: Evaluation, Challenges, and Guidelines. J. Taiwan Inst. Chem. Eng. 2022, 131, 104207. [Google Scholar] [CrossRef]
- Cazaudehore, G.; Guyoneaud, R.; Evon, P.; Martin-Closas, L.; Pelacho, A.; Raynaud, C.; Monlau, F. Can anaerobic digestion be a suitable end-of-life scenario for biodegradable plastics? A critical review of the current situation, hurdles, and challenges. Biotechnol. Adv. 2022, 56, 107916. [Google Scholar] [CrossRef] [PubMed]
- Rowan, M.; Umenweke, G.C.; Epelle, E.I.; Afolabi, I.C.; Okoye, P.U.; Gunes, B.; Okolie, J.A. Anaerobic co-digestion of food waste and agricultural residues: An overview of feedstock properties and the impact of biochar addition. Digit. Chem. Eng. 2022, 4, 100046. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Kulupa, T.; Kubiak, A.; Wolna-Maruwka, A.; Pilarski, K.; Niewiadomska, A. Anaerobic Digestion of Food Waste—A Short Review. Energies 2023, 16, 5742. [Google Scholar] [CrossRef]
- Singh, K.; Senapati, K.; Sarma, K. Synthesis of superparamagnetic Fe3O4 nanoparticles coated with green tea polyphenols and their use for removal of dye pollutant from aqueous solution. J. Environ. Chem. Eng. 2017, 5, 2214–2221. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Quan, X.; Chen, S. Enhanced anaerobic digestion of waste activated sludge digestion by the addition of zero valent iron. Water Res. 2014, 52, 242–250. [Google Scholar] [CrossRef]
- Bridgewater, L.L. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- García, A.-R.F.; Guez, J.-S.; Dussap, C.G.; Fontanille, P.; Christophe, G. Effect of redox potential on biohydrogen production during dark fermentation of food wastes in bioreactor. Int. J. Hydrogen Energy 2025, 141, 1199–1210. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; Zhao, Y.; Yuan, X.; Cui, Z. Effect of Temperature on the Inocula Preservation, Mesophilic Anaerobic Digestion Start-Up, and Microbial Community Dynamics. Agronomy 2024, 14, 2991. [Google Scholar] [CrossRef]
- Panigrahi, S.; Sharma, H.B.; Dubey, B.K. Anaerobic co-digestion of food waste with pretreated yard waste: A comparative study of methane production, kinetic modeling and energy balance. J. Clean. Prod. 2020, 243, 118480. [Google Scholar] [CrossRef]
- Feng, R.; Li, J.; Dong, T.; Li, X. Performance of a novel household solar heating thermostatic biogas system. Appl. Therm. Eng. 2016, 96, 519–526. [Google Scholar] [CrossRef]
- Moreno, L.; González, A.; Cuadros-Salcedo, F.; Cuadros-Blázquez, F. Feasibility of a novel use for agroindustrial biogas. J. Clean. Prod. 2017, 144, 48–56. [Google Scholar] [CrossRef]
- Chen, Y.J.; Haung, T.M. Planar-oriented ripple based greedy search algorithm for vector quantization. In Proceedings of the 2012 Computing, Communications and Applications Conference, Dindigul, India, 11–13 January 2012; pp. 227–230. [Google Scholar]
- Xianrong, H.; Fengju, S. Evaluation on the composite benefit of household biogas digesters. Renew. Energy 2006, 2, 4–6. [Google Scholar]
- Kurchania, A.K.; Panwar, N.L.; Pagar, S.D. Development of domestic biogas stove. Biomass Convers. Biorefinery 2011, 1, 99–103. [Google Scholar] [CrossRef]
- He, K.; Liu, Y.; Tian, L.; He, W.; Cheng, Q. Review in anaerobic digestion of food waste. Heliyon 2024, 10, e28200. [Google Scholar] [CrossRef]
- Brown, D.; Li, Y. Solid state anaerobic co-digestion of yard waste and food waste for biogas production. Bioresour. Technol. 2013, 127, 275–280. [Google Scholar] [CrossRef]
- Dhull, P.; Kumar, S.; Yadav, N.; Lohchab, R.K. A comprehensive review on anaerobic digestion with focus on potential feedstocks, limitations associated and recent advances for biogas production. Environ. Sci. Pollut. Res. 2024, 31, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Ait Oubba, Z.; Ounini, K.; Cherroud, S.; Ainane, A.; Ainane, T. From computational thinking to computational action: Case of waste treatment processes by composting. J. Anal. Sci. Appl. Biotechnol. 2024, 6, 92–99. [Google Scholar]
- Ainane, T.; Abdoul-Latif, F.M.; Ainane, A.; Kornaros, M.; Abbi, R.; Achira, M.; Brulé, M. Optimization by design of experiments of the preparation of biochar from olive pomace and its physicochemical characterizations. J. Anal. Sci. Appl. Biotechnol. 2023, 5, 39156. [Google Scholar]
- Lee, W.; Kim, Y.; Kim, H.; Kim, M. Comparison of Anaerobic Co-Digestion of Food Waste and Livestock Manure at Various Mixing Ratios under Mesophilic and Thermophilic Temperatures. Sustainability 2024, 16, 7653. [Google Scholar] [CrossRef]
- Czekała, W.; Nowak, M.; Bojarski, W. Characteristics of substrates used for biogas production in terms of water content. Fermentation 2023, 9, 449. [Google Scholar] [CrossRef]
- Czekała, W.; Jasiński, T.; Grzelak, M.; Witaszek, K.; Dach, J. Biogas plant operation: Digestate as the valuable product. Energies 2022, 15, 8275. [Google Scholar] [CrossRef]
- Møller, H.B.; Sommer, S.G.; Ahring, B.K. Methane productivity of manure, straw and solid fractions of manure. Biomass Bioenergy 2004, 26, 485–495. [Google Scholar] [CrossRef]
- Angelidaki, I.; Ellegaard, L. Codigestion of manure and organic wastes in centralized biogas plants: Status and future trends. Appl. Biochem. Biotechnol. 2003, 109, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Karakashev, D.; Batstone, D.J.; Angelidaki, I. Influence of environmental conditions on methanogenic compositions in anaerobic biogas reactors. Appl. Environ. Microbiol. 2005, 71, 331–338. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Feedstock 1 (FW) | Feedstock 2 (FW+CD) | Feedstock 3 (FW+CD+GW) | Unit | Test Method |
|---|---|---|---|---|---|
| PH | 5.70 | 4.87 | 6.60 | - | pH meter |
| COD | 89,000 | 98,500 | 40,000 | mg/L | CENLAB/WI/CHEM-TM/006 (In House Method based on APHA 5220 D) |
| BOD | 2020 | 1710 | 1070 | mg/L | CENLAB/WI/CHEM-TM/005 (In-House method based on APHA 5210B) |
| TOC | 45,400 | 32,700 | 24,100 | mg/L | In-House Method based on APHA 5310 |
| TS | 147,410 | 99,880 | 107,634 | mg/L | APHA 2540 |
| Carbon | 4.67 | 1.42 | 2.99 | % | In-House Method using CHNS Analyzer |
| Hydrogen | 10.60 | 12.33 | 11.28 | % | In-House Method using CHNS Analyzer |
| Nitrogen | 0.33 | 0.17 | 0.26 | % | In-House Method using CHNS Analyzer |
| Sulphur | 0.79 | 0.08 | 0.06 | % | In-House Method using CHNS Analyzer |
| Cost Component | Amount (AUD) | Remarks |
|---|---|---|
| Digester Tank (500 L) | 800 | Durable material, fabricated locally |
| Piping and Valves | 400 | High-quality PVC materials |
| Installation and Labor | 300 | Skilled labor for setup |
| Solar Heating Integration | 300 | Reduces thermal energy costs |
| Total Initial Investment | 1800 | |
| Annual Operational Costs | 180 | Includes maintenance and utility expenses |
| Benefit Component | Annual Amount (AUD) | Remarks |
|---|---|---|
| Biogas Savings | 657 | Based on LPG replacement at 1.8 m3/day |
| Thermal Energy Savings | 250 | Solar water heating integration |
| Fertilizer Savings | 240 | Digestate at 400 kg/year |
| Total Annual Benefits | 1147 |
| Metric | Value | Interpretation |
|---|---|---|
| Net Present Value (NPV) | AUD 2834 | Positive, indicating profitability |
| Internal Rate of Return (IRR) | 13.5% | Higher than average small-scale investment returns |
| Dynamic Payback Period (DPP) | 3.2 years | Short recovery time for initial investment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmudul, H.M.; Rasul, M.G.; Narayanan, R.; Akbar, D.; Hasan, M.M. Technoeconomic Assessment of Biogas Production from Organic Waste via Anaerobic Digestion in Subtropical Central Queensland, Australia. Energies 2025, 18, 4505. https://doi.org/10.3390/en18174505
Mahmudul HM, Rasul MG, Narayanan R, Akbar D, Hasan MM. Technoeconomic Assessment of Biogas Production from Organic Waste via Anaerobic Digestion in Subtropical Central Queensland, Australia. Energies. 2025; 18(17):4505. https://doi.org/10.3390/en18174505
Chicago/Turabian StyleMahmudul, H. M., M. G. Rasul, R. Narayanan, D. Akbar, and M. M. Hasan. 2025. "Technoeconomic Assessment of Biogas Production from Organic Waste via Anaerobic Digestion in Subtropical Central Queensland, Australia" Energies 18, no. 17: 4505. https://doi.org/10.3390/en18174505
APA StyleMahmudul, H. M., Rasul, M. G., Narayanan, R., Akbar, D., & Hasan, M. M. (2025). Technoeconomic Assessment of Biogas Production from Organic Waste via Anaerobic Digestion in Subtropical Central Queensland, Australia. Energies, 18(17), 4505. https://doi.org/10.3390/en18174505









