Au–MIL Nanocomposites with Enhanced Borohydride Oxidation Kinetics for Potential Use in Direct Liquid Fuel Cells
Abstract
1. Introduction
| Anode: BH4− + 8OH− → BO2− + 6H2O + 8e− | E0 = −1.24 V vs. SHE | (1) |
| Cathode: O2 + 2H2O + 4e− → 4OH− | E0 = 0.40 V vs. SHE | (2) |
| Overall: BH4− + 2O2 → BO2− + 2H2O | E0 = 1.64 V | (3) |
| Step 1: BH4− + H2O → BH3OH− + H2 | (4) | |
| Step 2: BH3OH− + H2O → BO2− + 3H2 | (5) | |
| Overall: BH4− + 2H2O → BO2− + 4H2 | (6) |
2. Materials and Methods
2.1. Chemicals
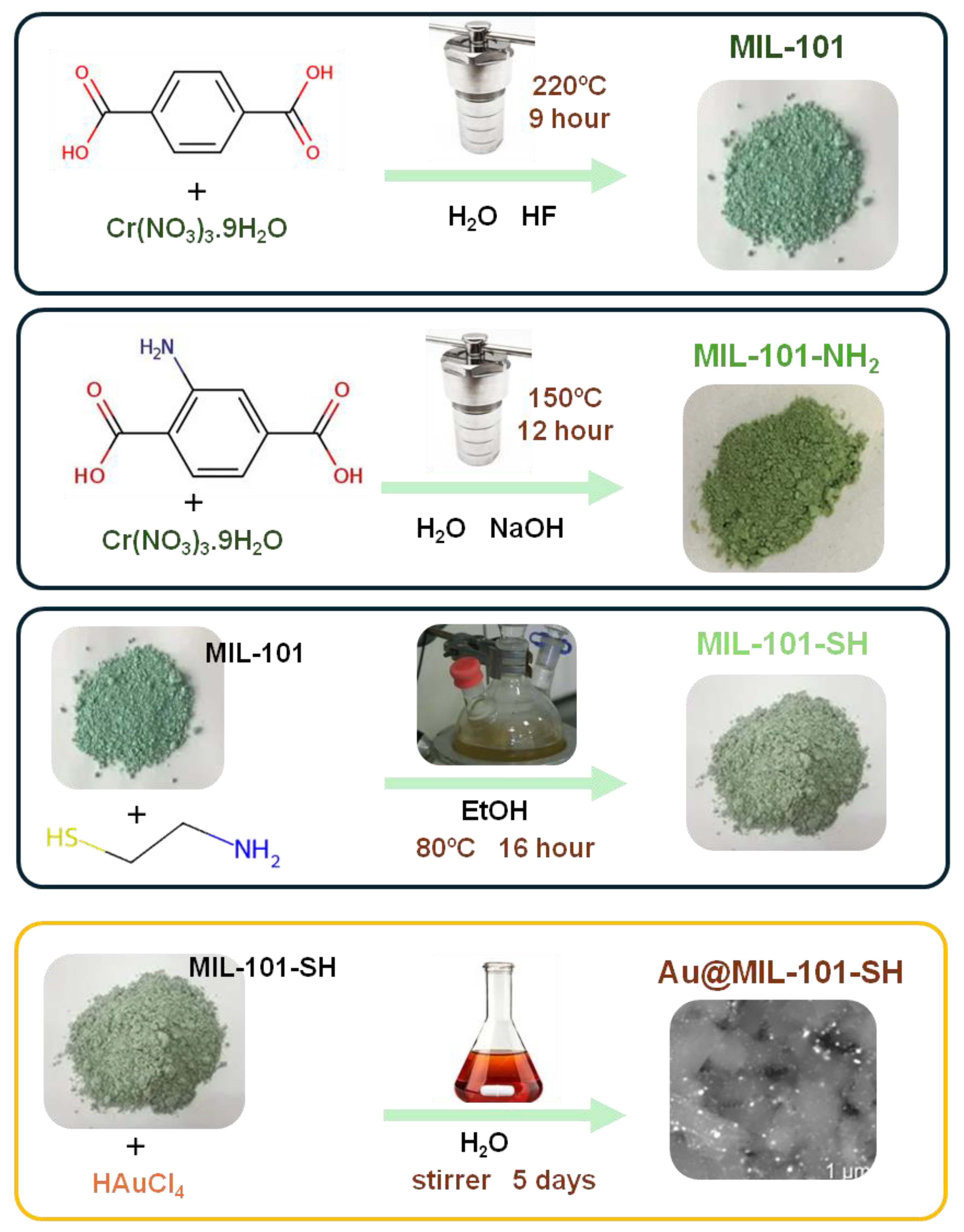
2.2. Synthesis of the Materials
2.3. Physicochemical Characterization
2.4. Electrochemical Characterization
3. Results and Discussion
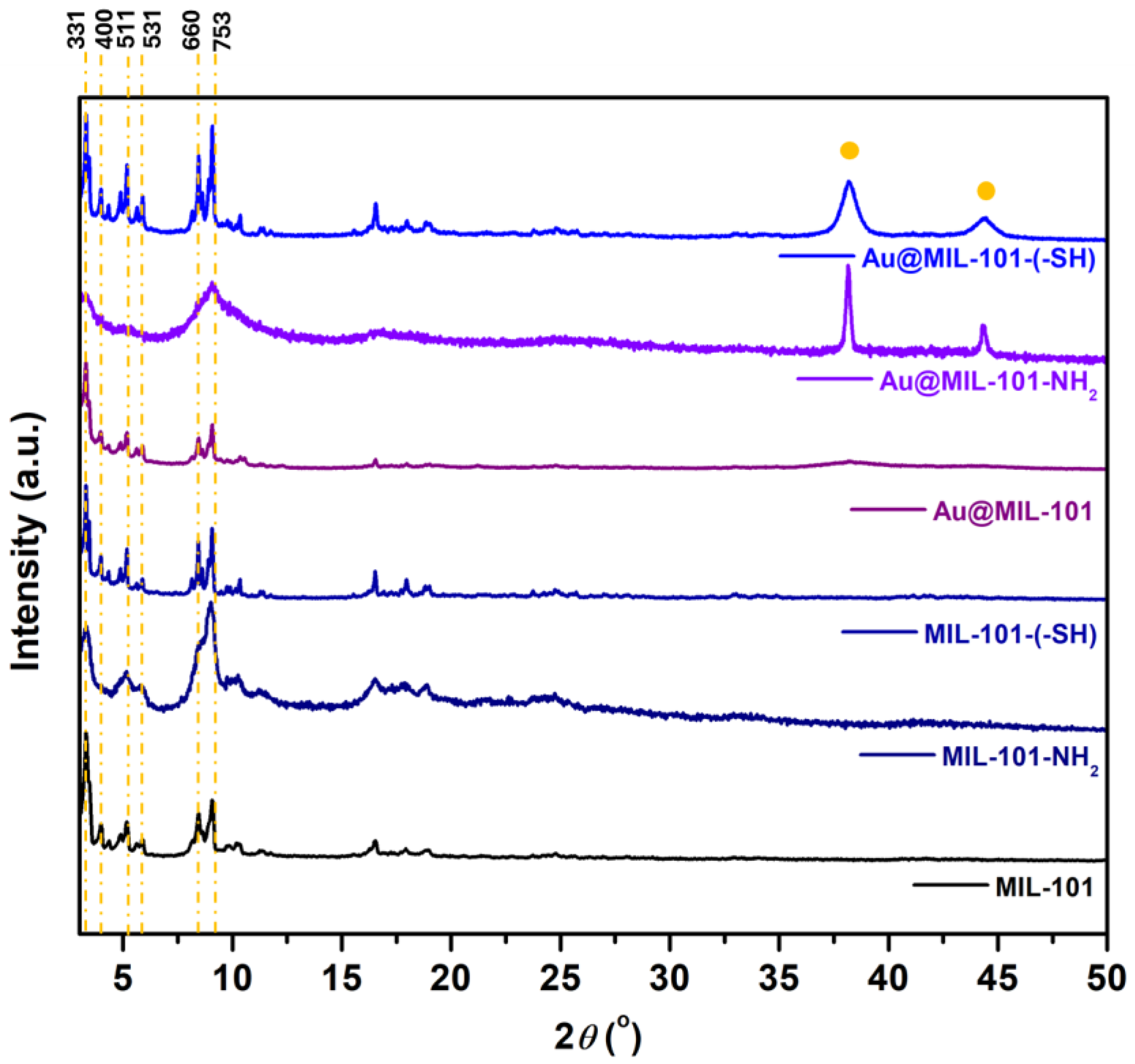
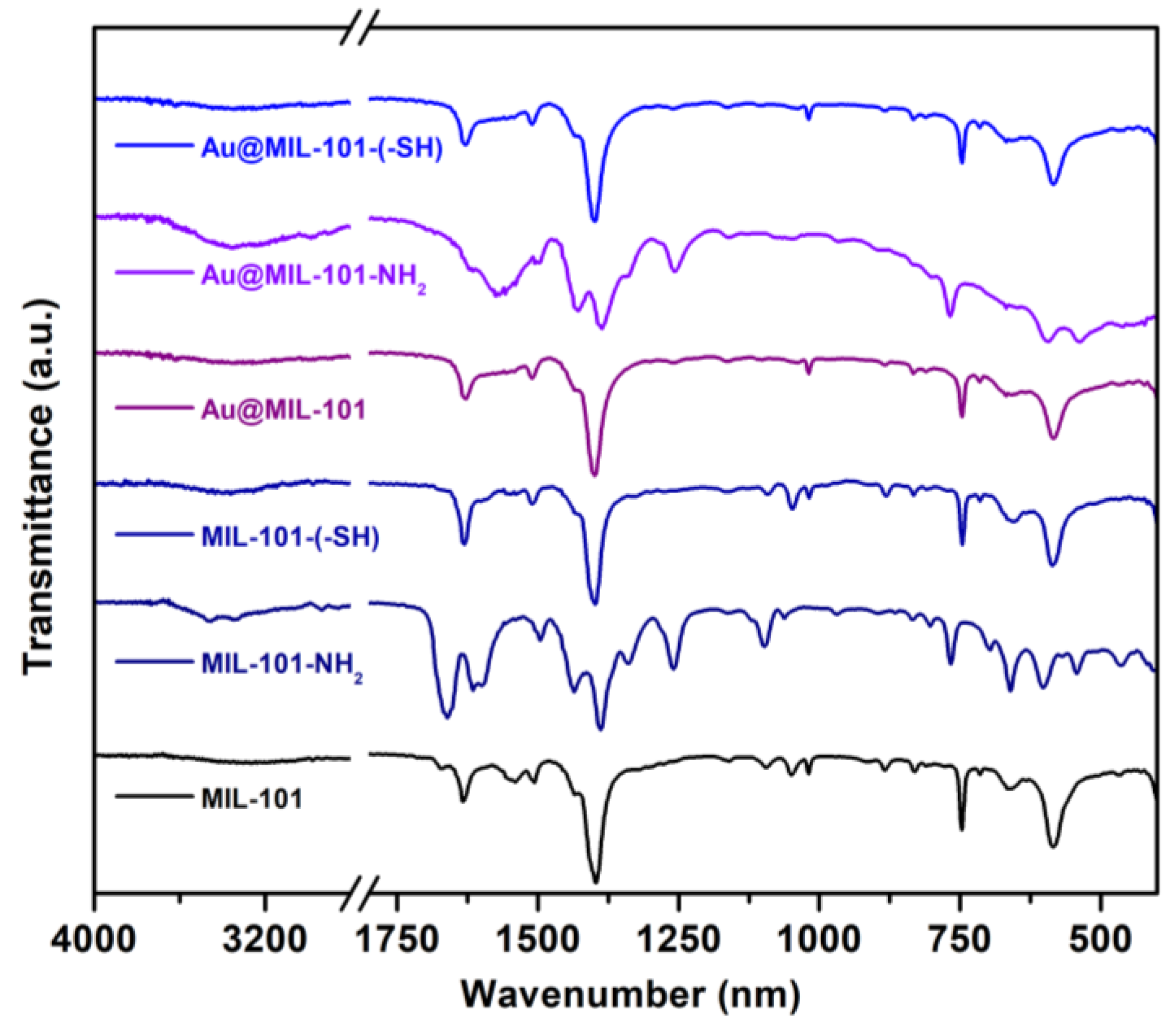
3.1. Characterization of the MIL-Based Materials
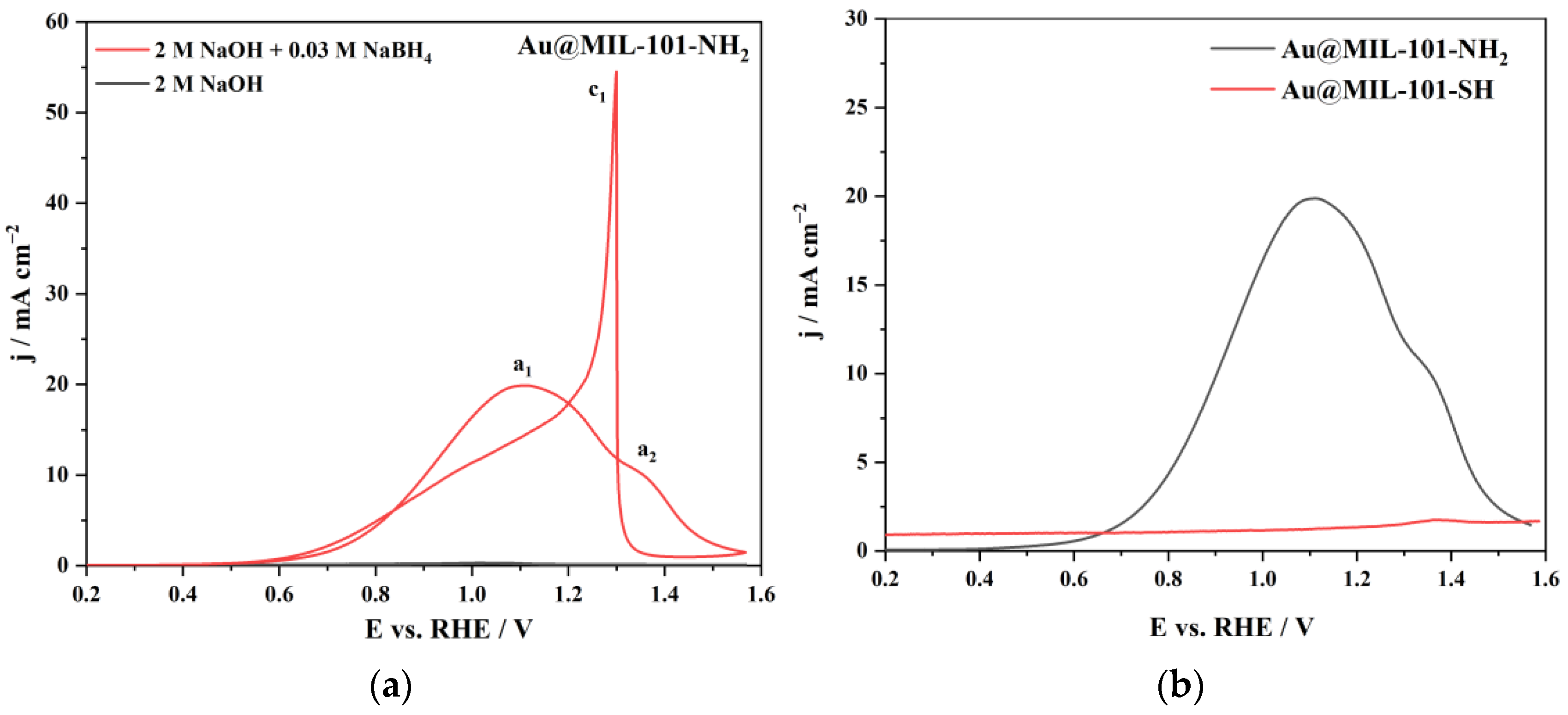
3.2. Cyclic Voltammetry Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qasem, N.A.A.; Abdulrahman, G.A.Q. A Recent Comprehensive Review of Fuel Cells: History, Types, and Applications. Int. J. Energy Res. 2024, 36, 7271748. [Google Scholar] [CrossRef]
- Javeed, A.; Rehman, F.; Draz, U.; Rehman, Z.U.; Farooq, N.; Karami, A.M.; Hussain, S. Structural and Electrochemical Studies of Triple Conducting Nanocomposites for Energy Conversion Devices. Solid State Ion. 2024, 407, 116499. [Google Scholar] [CrossRef]
- Šljukić, B.; Santos, D.M.F. Direct borohydride fuel cells (DBFCs). In Direct Liquid Fuel Cells; Akay, R.G., Bayrakçeken Yurtcan, A., Eds.; Elsevier: Istanbul, Turkey, 2021; pp. 203–232. [Google Scholar]
- Backović, G.; Milikić, J.; De Negri, S.; Saccone, A.; Šljukić, B.; Santos, D.M.F. Enhanced borohydride oxidation kinetics at gold-rare earth alloys. J. Alloys Compd. 2021, 857, 158273. [Google Scholar] [CrossRef]
- Manna, P.; Debgupta, J.; Bose, S.; Das, S.K. A mononuclear CoII coordination complex locked in a confined space and acting as an electrochemical water-oxidation catalyst: A ‘ship-in-a-bottle’ approach. Angew. Chem. Int. Ed. 2016, 55, 2425–2430. [Google Scholar] [CrossRef]
- Kornienko, N.; Zhao, Y.; Kley, C.S.; Zhu, C.; Kim, D.; Lin, S.; Chang, C.J.; Yaghi, O.M.; Yang, P. Metal–organic frameworks for electrocatalytic reduction of carbon dioxide. J. Am. Chem. Soc. 2015, 137, 14129–14135. [Google Scholar] [CrossRef]
- Backović, G.; Šljukić, B.; Saydan Kanberoğlu, G.; Yurderi, M.; Bulut, A.; Zahmakıran, M.; Santos, D.M.F. Ruthenium(0) nanoparticles stabilized by metal-organic framework as an efficient electrocatalyst for borohydride oxidation reaction. Int. J. Hydrogen Energy 2020, 45, 27056–27066. [Google Scholar] [CrossRef]
- Nagle, L.C.; Rohan, J.F. Nanoporous gold anode catalyst for direct borohydride fuel cell. Int. J. Hydrogen Energy 2011, 36, 10319–10326. [Google Scholar] [CrossRef]
- Ong, B.C.; Kamarudin, S.K.; Basri, S. Direct liquid fuel cells: A review. Int. J. Hydrogen Energy 2017, 42, 10142–10157. [Google Scholar] [CrossRef]
- Wee, J.-H. Which type of fuel cell is more competitive for portable application: Direct methanol fuel cells or direct borohydride fuel cells? J. Power Sources 2006, 161, 1–10. [Google Scholar] [CrossRef]
- Olu, P.-Y.; Job, N.; Chatenet, M. Evaluation of anode (electro)catalytic materials for the direct borohydride fuel cell: Methods and benchmarks. J. Power Sources 2016, 327, 235–257. [Google Scholar] [CrossRef]
- De Leon, C.P.; Walsh, F.C.; Pletcher, D.; Browning, D.J.; Lakeman, J.B. Direct borohydride fuel cells. J. Power Sources 2006, 155, 172–181. [Google Scholar] [CrossRef]
- Ma, J.; Choudhury, N.A.; Sahai, Y. A comprehensive review of direct borohydride fuel cells. Renew. Sustain. Energy Rev. 2010, 14, 183–199. [Google Scholar] [CrossRef]
- Rostamikia, G.; Janik, M.J. Direct Borohydride Oxidation: Mechanism Determination and Design of Alloy Catalysts Guided by Density Functional Theory. Energy Environ. Sci. 2010, 3, 1269–1278. [Google Scholar] [CrossRef]
- Yi, L.; Wei, W.; Zhao, C.; Tian, L.; Liu, J.; Wang, X. Enhanced activity of Au–Fe/C anodic electrocatalyst for direct borohydride-hydrogen peroxide fuel cell. J. Power Sources 2015, 285, 325–333. [Google Scholar] [CrossRef]
- Duan, D.; Feng, J.; You, X.; Zhou, X.; Wang, Y.; Chen, L.; Liu, S. Evaluation of Co–Au bimetallic nanoparticles as anode electrocatalyst for direct borohydride-hydrogen peroxide fuel cell. Ionics 2021, 27, 3521–3532. [Google Scholar] [CrossRef]
- Xue, L.; Shi, W.; Song, D.; Huang, X.; Zhang, J.; Zhang, Y.; Yuan, W.; Huang, S.; Sun, K.; Zheng, L.; et al. Constructing a multisite catalyst for achieving efficient and highly selective borohydride oxidation reaction. J. Colloid Interface Sci. 2025, 699, 138253. [Google Scholar] [CrossRef]
- Raul, C.K.; Chatterjee, T.; Halder, M.; Sinha, R.; Dey, S.; Basu, S.; Meikap, A.K. Reverse Micelle Synthesis of AuyNi100−y Nanoparticles Decorated Multiwalled Carbon Nanotubes as High-Performance Anode Electrocatalysts for Direct Borohydride-Hydrogen Peroxide Fuel Cells. ChemNanoMat 2025, 11, e202500005. [Google Scholar] [CrossRef]
- Raul, C.K.; Dey, S.; Halder, M.; Karmakar, R.; Basu, S.; Meikap, A.K. Synthesis of AuxCo100−x/MWCNT Nanoparticles as an Efficient Anode Electrocatalyst for Borohydride Oxidation in Alkaline Medium. J. Appl. Electrochem. 2023, 53, 977–990. [Google Scholar] [CrossRef]
- Xue, L.; Liu, C.; Ye, J.; Zhang, J.; Kang, L.; Zhang, Y.; Shi, W.; Guo, W.; Huang, X.; Yang, X.; et al. Engineering Partially Oxidized Gold via Oleylamine Modifier as a High-Performance Anode Catalyst in a Direct Borohydride Fuel Cell. ACS Appl. Mater. Interfaces 2024, 16, 27145–27154. [Google Scholar] [CrossRef]
- Gaudin, L.F.; Funston, A.M.; Bentley, C.L. Drop-Cast Gold Nanoparticles Are Not Always Electrocatalytically Active for the Borohydride Oxidation Reaction. Chem. Sci. 2024, 15, 5456–5466. [Google Scholar] [CrossRef]
- Yi, L.; Wang, Q.; Peng, X.; Deng, W.; Ma, X.; Li, X.; Yi, W. ZIF-Driven N-Doped Carbon Supported Co, Ni-Codoped Au Nanoparticle Catalyst as Efficient Anode Catalyst for Borohydride Electrooxidation Reaction. Ionics 2023, 29, 1543–1551. [Google Scholar] [CrossRef]
- Saha, P.; Rahman, M.M.; Hill, C.M. Borohydride Oxidation Electrocatalysis at Individual, Shape-Controlled Au Nanoparticles. Electrochem. Sci. Adv. 2021, 2, 1234–1244. [Google Scholar] [CrossRef]
- Balčiūnaitė, A.; Zabielaitė, A.; Sukackienė, Z.; Kepenienė, V.; Šimkūnaitė, D.; Selskis, A.; Tamašauskaitė-Tamašiūnaitė, L.; Norkus, E. Fabrication of Efficient Gold–Nickel-Supported Titania Nanotube Electrocatalysts for Sodium Borohydride–Hydrogen Peroxide Fuel Cells. Coatings 2022, 12, 850. [Google Scholar] [CrossRef]
- Yi, L.; Peng, X.; Ma, X.; Gao, Y.; Wang, X.; Lu, Y. Investigation of ZIF-Derived Co, N Co-Doped Porous Carbon-Supported Au Nanoparticles as an Effective Catalyst for Borohydride Electrooxidation. New J. Chem. 2021, 45, 21206–21214. [Google Scholar] [CrossRef]
- Yi, L.; Peng, X.; Meng, Y.; Ding, Y.; Wang, X.; Lu, Y. N-Doped Carbon-Coated Co2P-Supported Au Nanocomposite as the Anode Catalyst for Borohydride Electrooxidation. New J. Chem. 2021, 45, 14779–14788. [Google Scholar] [CrossRef]
- Uzundurukan, A.; Akça, E.S.; Budak, Y.; Devrim, Y. Carbon Nanotube–Graphene Supported Bimetallic Electrocatalyst for Direct Borohydride Hydrogen Peroxide Fuel Cells. Renew. Energy 2021, 172, 1351–1364. [Google Scholar] [CrossRef]
- Rao, R.; Ma, S.; Gao, B.; Bi, F.; Chen, Y.; Yang, Y.; Liu, N.; Wu, M.; Zhang, X. Recent advances of metal-organic framework-based and derivative materials in the heterogeneous catalytic removal of volatile organic compounds. J. Colloid Interface Sci. 2023, 636, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, J.; Li, Y.; Lou, Z.; Li, Y. A literature review of MOF derivatives of electromagnetic wave absorbers mainly based on pyrolysis. Int. J. Miner. Metall. Mater. 2023, 30, 446–473. [Google Scholar] [CrossRef]
- Parsaei, S.; Rashid, M.; Ghoorchian, A.; Dashtian, K.; Mowla, D. Bi-metal-organic framework-derived S-scheme InP/CuO-C heterostructure for robust photocatalytic degradation of ciprofloxacin in a microfluidic photoreactor. Chem. Eng. J. 2023, 475, 146448. [Google Scholar] [CrossRef]
- Rashid, M.; Parsaei, S.; Ghoorchian, A.; Dashtian, K.; Mowla, D. A spiral shape microfluidic photoreactor with MOF(NiFe)-derived NiSe-Fe3O4/C heterostructure for photodegradation of tetracycline. J. Ind. Eng. Chem. 2023, 121, 275–286. [Google Scholar] [CrossRef]
- Gumilar, G.; Kaneti, Y.V.; Henzie, J.; Chatterjee, S.; Na, J.; Yuliarto, B.; Nugraha, N.; Patah, A.; Bhaumik, A.; Yamauchi, Y. General synthesis of hierarchical sheet/plate-like M-BDC (M = Cu, Mn, Ni, and Zr) metal–organic frameworks for electrochemical non-enzymatic glucose sensing. Chem. Sci. 2020, 11, 3644–3655. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; García, H. Metal–organic framework (MOF) compounds: Photocatalysts for redox reactions and solar fuel production. Angew. Chem. Int. Ed. 2016, 55, 5414–5445. [Google Scholar] [CrossRef] [PubMed]
- Zorainy, M.Y.; Gar Alalm, M.; Kaliaguine, S.; Boffito, D.C. Revisiting the MIL-101 metal–organic framework: Design, synthesis, modifications, advances, and recent applications. J. Mater. Chem. A 2021, 9, 22159–22217. [Google Scholar] [CrossRef]
- Eom, H.H.; Kim, H.; Lee, J.W. Silver-incorporated NH2-MIL-101(Cr) electrode for highly efficient iodide removal through pseudocapacitive deionization. Sep. Purif. Technol. 2025, 364, 132360. [Google Scholar] [CrossRef]
- Santos, D.M.F.; Sequeira, C.A.C. Cyclic voltammetry investigation of borohydride oxidation at a gold electrode. Electrochim. Acta 2010, 55, 6775–6781. [Google Scholar] [CrossRef]
- Santos, D.M.F.; Sequeira, C.A.C. Chronopotentiometric investigation of borohydride oxidation at a gold electrode. J. Electrochem. Soc. 2010, 157, F16–F21. [Google Scholar] [CrossRef]
- Santos, D.M.F.; Sequeira, C.A.C. Determination of kinetic and diffusional parameters for sodium borohydride oxidation on gold electrodes. J. Electrochem. Soc. 2009, 156, F67–F72. [Google Scholar] [CrossRef]
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surblé, S.; Margiolaki, I. A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef]
- Chen, M.-L.; Zhou, S.-Y.; Xu, Z.; Ding, L.; Cheng, Y.-H. Metal-organic frameworks of MIL-100(Fe,Cr) and MIL-101(Cr) for aromatic amines adsorption from aqueous solutions. Molecules 2019, 24, 3718. [Google Scholar] [CrossRef]
- Yallappa, S.; Manjanna, J.; Dhananjaya, B.L. Phytosynthesis of stable Au, Ag and Au–Ag alloy nanoparticles using J. Sambac leaves extract. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 137, 236–243. [Google Scholar] [CrossRef]
- Sheikh Alivand, M.; Tehrani, N.H.M.H.; Shafiei-Alavijeh, M.; Rashidi, A.; Kooti, M.; Pourreza, A.; Fakhraie, S. Synthesis of a modified HF-free MIL-101(Cr) nanoadsorbent with enhanced gas selectivity. J. Environ. Chem. Eng. 2019, 7, 102946. [Google Scholar] [CrossRef]
- Liu, Q.; Ning, L.; Zheng, S.; Tao, M.; Shi, Y.; He, Y. Adsorption of carbon dioxide by MIL-101(Cr): Regeneration conditions and influence of flue gas contaminants. Sci. Rep. 2013, 3, 2916. [Google Scholar] [CrossRef]
- Celik, C.; Boyaci San, F.G.; Sarac, H.I. Effects of operation conditions on direct borohydride fuel cell performance. J. Power Sources 2008, 185, 197–201. [Google Scholar] [CrossRef]
- Ma, J.; Sahai, Y.; Buchheit, R.G. Direct borohydride fuel cell using Ni-based composite anodes. J. Power Sources 2010, 195, 4709–4713. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Rashidi, N.; Mahmoodi, R.; Omer, M. Preparation of Pt/G and PtNi/G nanocatalysts for borohydride oxidation. Mater. Chem. Phys. 2018, 208, 207–219. [Google Scholar] [CrossRef]
- Milikić, J.; Tapia, A.; Stamenović, U.; Vodnik, V.; Otoničar, M.; Škapin, S.; Santos, D.M.F.; Šljukić, B. High-Performance Metal (Au,Cu)–Polypyrrole Nanocomposites for Electrochemical Borohydride Oxidation in Fuel Cell Applications. Int. J. Hydrogen Energy 2022, 47, 36990–37001. [Google Scholar] [CrossRef]
- Yi, L.; Peng, X.; Ma, X.; Gao, Y.; Wang, X.; Lu, Y. Study of ZIF-Derived Iron and Nitrogen Co-Doped Porous Carbon Supported Au Nanoparticles as Electrocatalyst for Borohydride Oxidation Reaction. Ionics 2022, 28, 849–858. [Google Scholar] [CrossRef]
- Milikić, J.; Oliveira, R.C.P.; Tapia, A.; Santos, D.M.F.; Zdolšek, N.; Trtić-Petrović, T.; Vraneš, M.; Šljukić, B. Ionic Liquid-Derived Carbon-Supported Metal Electrocatalysts as Anodes in Direct Borohydride-Peroxide Fuel Cells. Catalysts 2021, 11, 632. [Google Scholar] [CrossRef]
- Aziz, D.M.; Hassan, S.A. Acid-free synthesis of MIL-101(Cr) for enhanced photocatalytic reduction of CO2. Inorg. Chem. Commun. 2025, 178, 114551. [Google Scholar] [CrossRef]
- Shi, K.; Li, X.; Tian, Z.; Luo, Y.; Ding, R.; Zhu, Y.; Yao, H. Synergistic and efficient photocatalytic degradation of rhodamine B and tetracycline in wastewater based on novel S-scheme heterojunction phosphotungstic Acid@MIL-101(Cr). J. Environ. Manag. 2025, 373, 123716. [Google Scholar] [CrossRef]
- Moeini, Z.; Azhdarpoor, A.; Yousefinejad, S.; Hashemi, H. Removal of atrazine from water using titanium dioxide encapsulated in NH2MIL-101 (Cr). J. Clean. Prod. 2019, 224, 238–245. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, X.; Wei, C.; Hu, Y. Spatial directional separation and synergetic treatment of Cr(VI) and Rhodamine B. Sci. Total Environ. 2022, 842, 156836. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, D.; Xu, Z.; Liu, R. MIL-101(Fe)-derived nickel–iron quasi-metal organic framework as efficient catalyst. J. Colloid Interface Sci. 2025, 691, 137429. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yao, L.; He, J.; Li, J.; Xu, S.; Li, N.; Zhu, Y.; Chen, X.; Zhu, R. Enhanced degradation of tetracycline under natural sunlight. J. Hazard. Mater. 2023, 449, 131024. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Peng, L.; Yang, H.; Waterhouse, G.I.N.; Shang, L.; Zhang, T. MIL-101-derived mesoporous carbon supporting highly exposed Fe single-atom sites. Adv. Mater. 2021, 33, 2101038. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, D.; Zhao, Y.; Sun, J. Hydrogen bond donor functionalized poly(ionic liquids)@MIL-101 for CO2 capture. J. Colloid Interface Sci. 2022, 618, 22–33. [Google Scholar] [CrossRef]


| Au@MIL-101-NH2 | Au@MIL-101-SH | |
|---|---|---|
| bAu (μmolAu gMIL−1) | 77.8 | 330 |
| cAu (mgAu gMIL−1) | 15.3 | 65 |
| mAu (μgAu) | 1.74 | 7.38 |
| jp (mA cm−2) | 19.9 | 1.76 |
| ip (A μgAu−1) | 11.4 | 2.38 × 10−4 |
| Electrocatalyst | n | α | β | Eaapp/kJ mol−1 | Source |
|---|---|---|---|---|---|
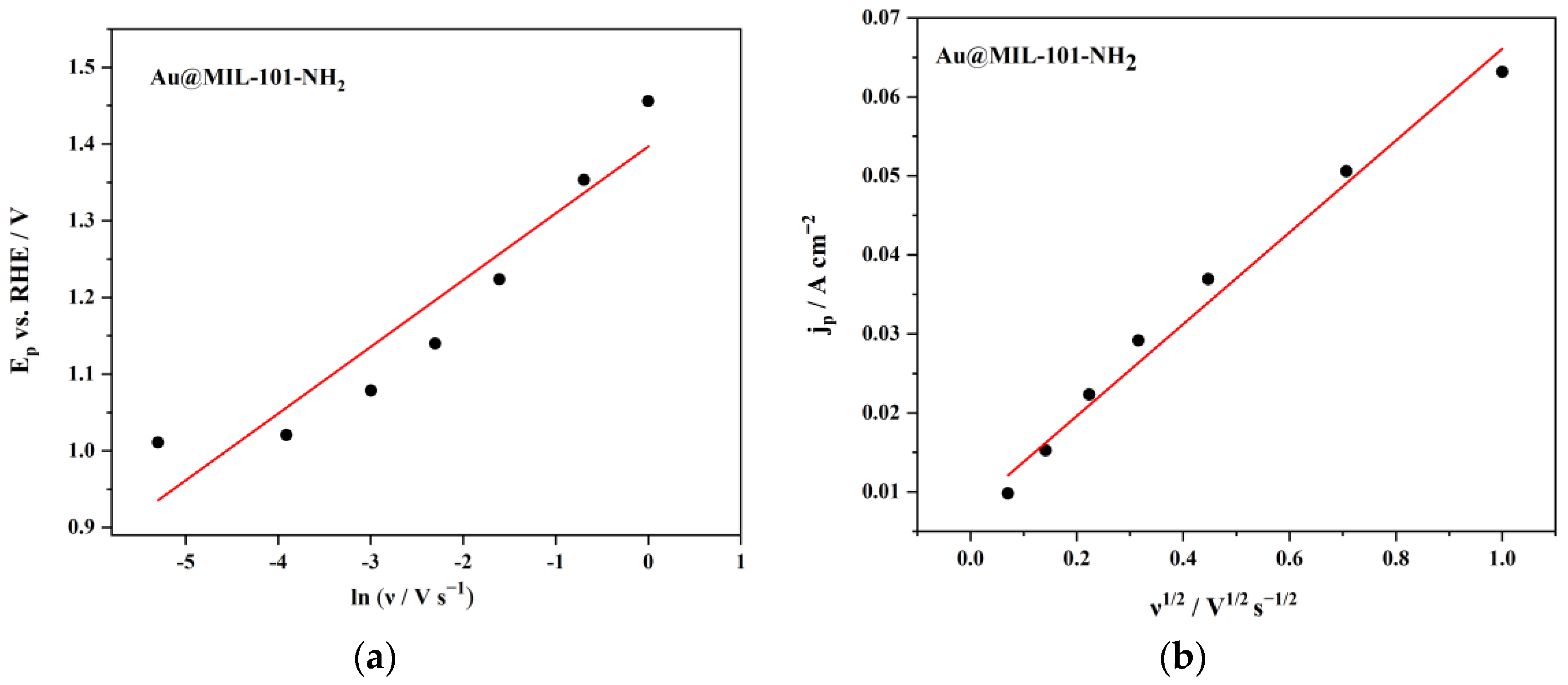
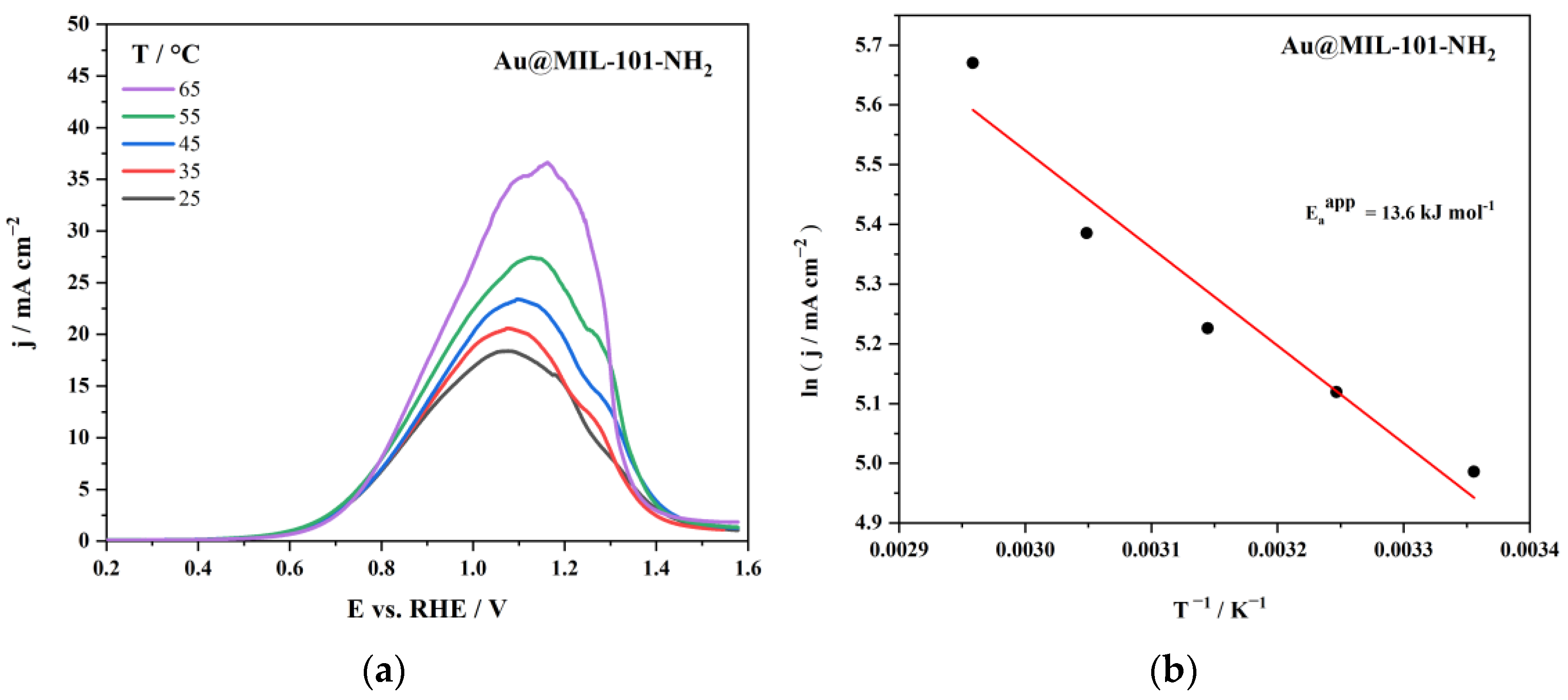
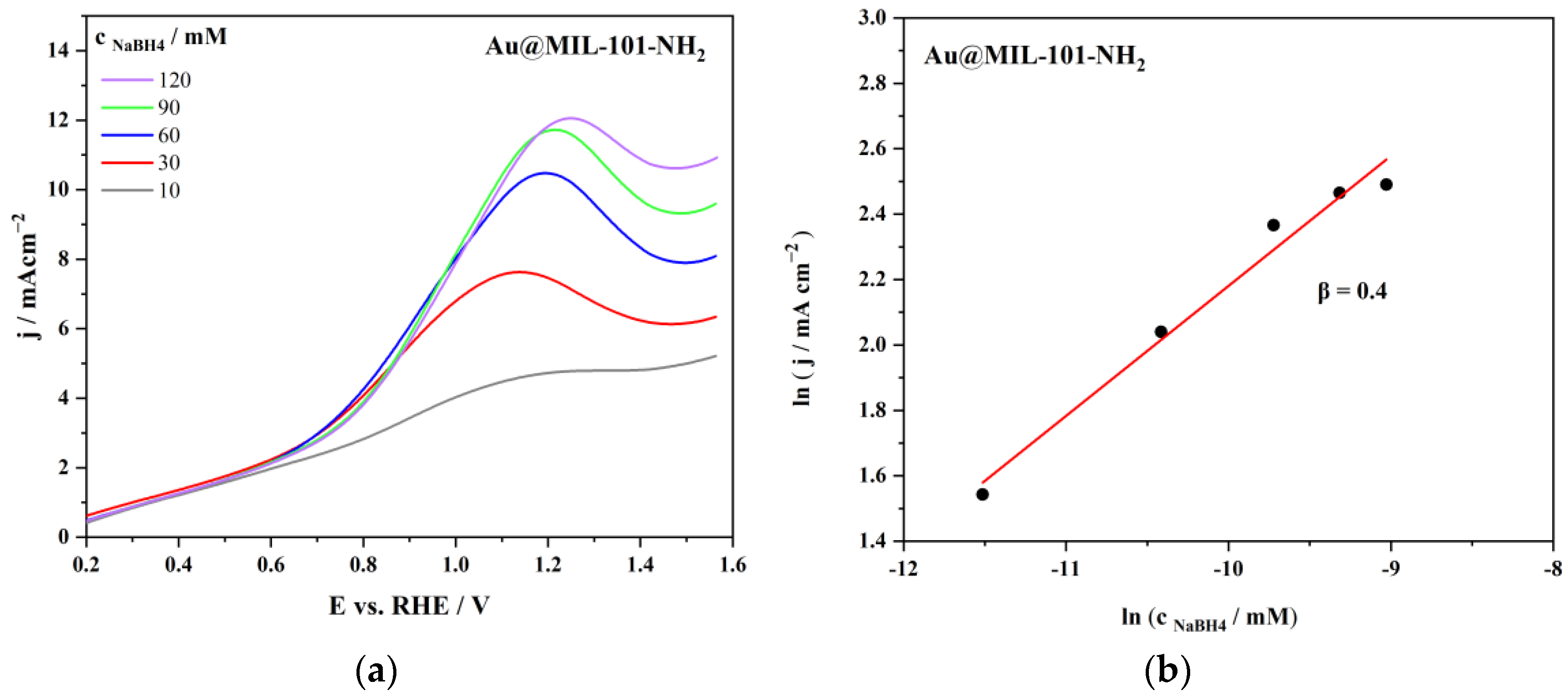
| Au@MIL-101-NH2 | 7.97 | 0.85 | 0.4 | 13.6 | This work |
| CoFe&AuC | 5.4 | - | - | - | [17] |
| Au50Ni50/MWCNT | 5.8 | - | - | 7.4 | [18] |
| Au74Co26/MWCNT | 4.7 | - | - | 8.2 | [19] |
| AuPPy | 4.4 | - | - | - | [47] |
| Au/FeNPC | 7 | - | - | - | [48] |
| Au/CoNPC | 7.5 | - | - | - | [25] |
| Au/c-IL | 2.4 | - | 1.0 | 13.8 | [49] |
| Au–RE alloys (RE = Sm, Dy, Ho, Y) | 2.4–4.4 | 0.60–0.83 | 1.0 | 16.4–20.2 | [4] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belhaj, I.; Becker, A.; Viana, A.M.; Gusmão, F.M.B.; Chaves, M.; Šljukić, B.; Balula, S.S.; Cunha-Silva, L.; Santos, D.M.F. Au–MIL Nanocomposites with Enhanced Borohydride Oxidation Kinetics for Potential Use in Direct Liquid Fuel Cells. Energies 2025, 18, 4503. https://doi.org/10.3390/en18174503
Belhaj I, Becker A, Viana AM, Gusmão FMB, Chaves M, Šljukić B, Balula SS, Cunha-Silva L, Santos DMF. Au–MIL Nanocomposites with Enhanced Borohydride Oxidation Kinetics for Potential Use in Direct Liquid Fuel Cells. Energies. 2025; 18(17):4503. https://doi.org/10.3390/en18174503
Chicago/Turabian StyleBelhaj, Ines, Alexander Becker, Alexandre M. Viana, Filipe M. B. Gusmão, Miguel Chaves, Biljana Šljukić, Salete S. Balula, Luís Cunha-Silva, and Diogo M. F. Santos. 2025. "Au–MIL Nanocomposites with Enhanced Borohydride Oxidation Kinetics for Potential Use in Direct Liquid Fuel Cells" Energies 18, no. 17: 4503. https://doi.org/10.3390/en18174503
APA StyleBelhaj, I., Becker, A., Viana, A. M., Gusmão, F. M. B., Chaves, M., Šljukić, B., Balula, S. S., Cunha-Silva, L., & Santos, D. M. F. (2025). Au–MIL Nanocomposites with Enhanced Borohydride Oxidation Kinetics for Potential Use in Direct Liquid Fuel Cells. Energies, 18(17), 4503. https://doi.org/10.3390/en18174503









