1. Introduction
Gas-insulated switchgear (GIS) has become essential equipment in modern power systems due to its compact design and high reliability [
1]. As a key functional component of GIS, insulators fulfill dual roles of mechanical support and electrical insulation. Under the combined effects of long-term power frequency voltage and variations in environmental stress, they are susceptible to surface flashover [
2,
3]. This reliability issue has emerged as a significant bottleneck limiting system safety. To tackle this challenge, researchers have conducted extensive studies on GIS insulator surface discharge and flashover characteristics, mainly attributing insulator failures to surface charge accumulation [
4]. Under the influence of electric field forces, these charges migrate and accumulate on the insulator surface, resulting in the distortion of the local electric field and consequently encouraging the development of surface flashover [
5].
In the field of GIS equipment, SF
6 and its gas mixtures have long been employed as the primary insulating medium. However, due to their significant greenhouse effect, their usage and emissions raise serious environmental concerns [
6]. Therefore, there is an urgent need to find relatively environmentally friendly insulating gases to replace SF
6. Perfluoroisobutyronitrile (C
4F
7N) is widely recognized as the most promising SF
6 alternative due to its excellent dielectric strength (approximately twice that of SF
6) and a low global warming potential of 2090 [
7]. However, its relatively high liquefaction temperature (−4.7 °C) necessitates mixing with buffer gases such as carbon dioxide (CO
2) to meet equipment operational requirements [
8].
Existing research demonstrates significant differences in charge accumulation behavior between C
4F
7N gas mixtures and traditional SF
6 mixtures. B. Zhang et al. experimentally observed that even with 25% C
4F
7N content, the surface charge density remains substantially higher than under pure SF
6 conditions, primarily attributed to higher-amplitude discharge pulses generated in C
4F
7N mixtures [
9]. W. Zhou et al. compared power frequency breakdown characteristics between C
4F
7N/CO
2 and C
4F
7N/N
2 mixtures, finding that the former exhibits stronger synergistic effects, with the breakdown strength of 20% C
4F
7N/CO
2 reaching 48.85 kV/mm at 0.5 MPa [
10]. D. Li et al. investigated surface charge accumulation on polymer insulators in air and C
4F
7N/CO
2 mixtures under AC voltage, revealing a three-tier concentric charge distribution influenced by voltage phase and gas properties [
11].
In practical engineering applications, O
2 is typically added as a second buffer gas to suppress carbon deposition caused by decomposition products under discharge [
12,
13]. Although the C
4F
7N/CO
2/O
2 ternary gas mixture shows both environmental friendliness and excellent insulation performance, it significantly alters the charge behavior characteristics at gas–solid interfaces [
14]. However, study with respect to the effect of gas content in such a C
4F
7N/CO
2/O
2 ternary mixture on the surface charge accumulation of epoxy insulator under AC voltage is very limited. The present study is performed mainly in this respect.
This study investigates the influence of varying C4F7N and O2 concentrations on the surface charge distribution of scaled disk-type insulators by establishing a simulated GIS environment chamber and surface charge measurement platform. The charge distribution differences under aluminum metal particle-contaminated and metal particle-free conditions were analyzed, revealing the synergistic mechanisms among gas components. Results demonstrate that increasing C4F7N content significantly suppresses surface charge accumulation, with more pronounced suppression effects observed in the presence of metal particles. In contrast, variations in O2 concentration exhibit a weaker influence on charge accumulation, showing no regular regulatory effect within the 2–14% range.
3. Experimental Results
3.1. Effect of C4F7N Concentrations on Surface Charge Distribution
Figure 4 presents the surface charge distribution patterns on insulators after 5 min of 20 kV AC voltage application, with C
4F
7N concentrations of 2%, 6%, 10%, and 14% in metal particle-free conditions. The charge distribution exhibits relatively stable and low-density characteristics across the insulator surface. This observation suggests minimal charge accumulation during operation under undisturbed conditions, with such low-density distributions demonstrating negligible influence on surface flashover initiation.
Comparative analysis reveals a consistent reduction in surface charge accumulation with increasing C4F7N content. At 14% C4F7N concentration, the peak positive and negative charge densities show reductions of 46.58% and 22.22%, respectively, compared to the 2% concentration case. Notably, higher C4F7N concentrations promote predominant negative charge accumulation, with density values substantially exceeding those of positive charges. These findings indicate that C4F7N not only enhances insulation performance by suppressing ionization and reducing charge accumulation but also modulates charge polarity distribution.
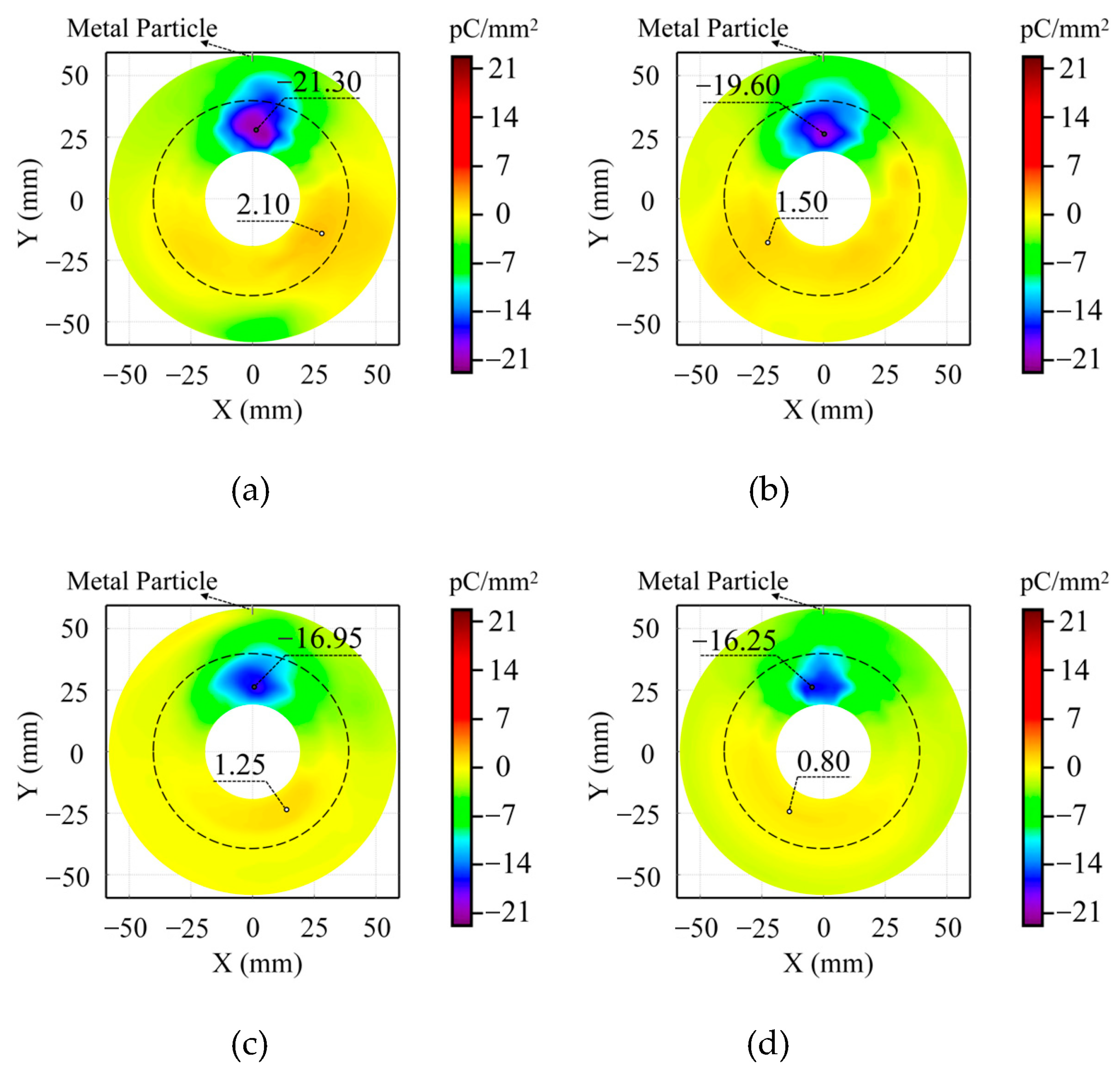
Figure 5 illustrates the surface charge distribution characteristics of the insulator after applying 20 kV AC voltage for 5 min with metal particles positioned on the ground electrode and C
4F
7N mixing ratios of 2%, 6%, 10%, and 14%. The experimental results reveal particularly pronounced charge accumulation in non-planar regions of the insulator, with a negative charge speckle observed near the metal particles. In contrast, planar regions exhibited relatively minimal charge accumulation without other distinct charge concentration areas.
The findings demonstrate a significant reduction in surface charge accumulation as C4F7N content increases, indicating enhanced insulation performance at higher concentrations. Specifically, at the C4F7N concentration of 14%, the maximum positive and negative charge densities decreased by 61.90% and 23.71%, respectively, compared to the 2% concentration case. This suggests that as C4F7N concentration rises from trace levels, its inhibitory effect on surface charge accumulation becomes markedly stronger, with particularly pronounced suppression of positive charges.
This phenomenon arises from the strong electronegativity of C4F7N, as its molecular outer orbitals exhibit high electron affinity. When critical concentration thresholds are reached, C4F7N effectively captures free electrons generated during gas-phase ionization, forming stable negative ions. The C4F7N molecule, while polar, forms stable negative ions primarily through electron capture by its cyanide group (-CN), where the combination of electron-withdrawing character and molecular orbital structure facilitates negative ion stabilization. Furthermore, the substantial molecular mass of C4F7N facilitates the deposition of these negative ions onto the insulator surface under electric fields, promoting negative charge accumulation. Concurrently, C4F7N significantly suppresses the ionization process, reducing positive ion generation and consequently decreasing positive charge accumulation—a mechanism consistent with the observed predominance of negative charge accumulation. These results confirm that even under conditions where metal particles serve as external discharge sources, higher C4F7N concentrations maintain their inhibitory effect on charge distribution characteristics.
Figure 6 illustrates the characteristics of electric field distribution along insulator surfaces under varying C
4F
7N mixing ratios, resulting from surface charge accumulation along the radial direction of metal particles after the removal of AC voltage. The peak surface electric field consistently appears in non-planar regions, regardless of variations in C
4F
7N concentration. At the C
4F
7N concentration of 2%, the maximum field strength of 0.56 kV/mm is found at a radial position of 27.34 mm, due to significant negative charge accumulation in this region, which greatly enhances the field magnitude. In contrast, the planar region shows relatively lower field magnitudes because of diminished charge accumulation.
When increasing C4F7N concentration to 6%, the maximum field strength shifts toward the high-voltage electrode, appearing at a 24.34 mm radial position with a value of 0.55 kV/mm, representing a 1.79% reduction compared to the 2% case. Concurrently, planar regions show decreasing field strength as charge density further diminishes relative to the 2% condition. At higher concentrations of 10% and 14% C4F7N, non-planar regions demonstrate markedly reduced field distributions compared to lower concentrations, with maximum field strengths of 0.41 kV/mm and 0.44 kV/mm, respectively, corresponding to reductions of 26.79% and 21.43% relative to the 2% case. Furthermore, planar regions maintain consistently low field magnitudes comparable to the 6% condition, exhibiting only minor fluctuations.
3.2. Effect of O2 Concentrations on Surface Charge Distribution
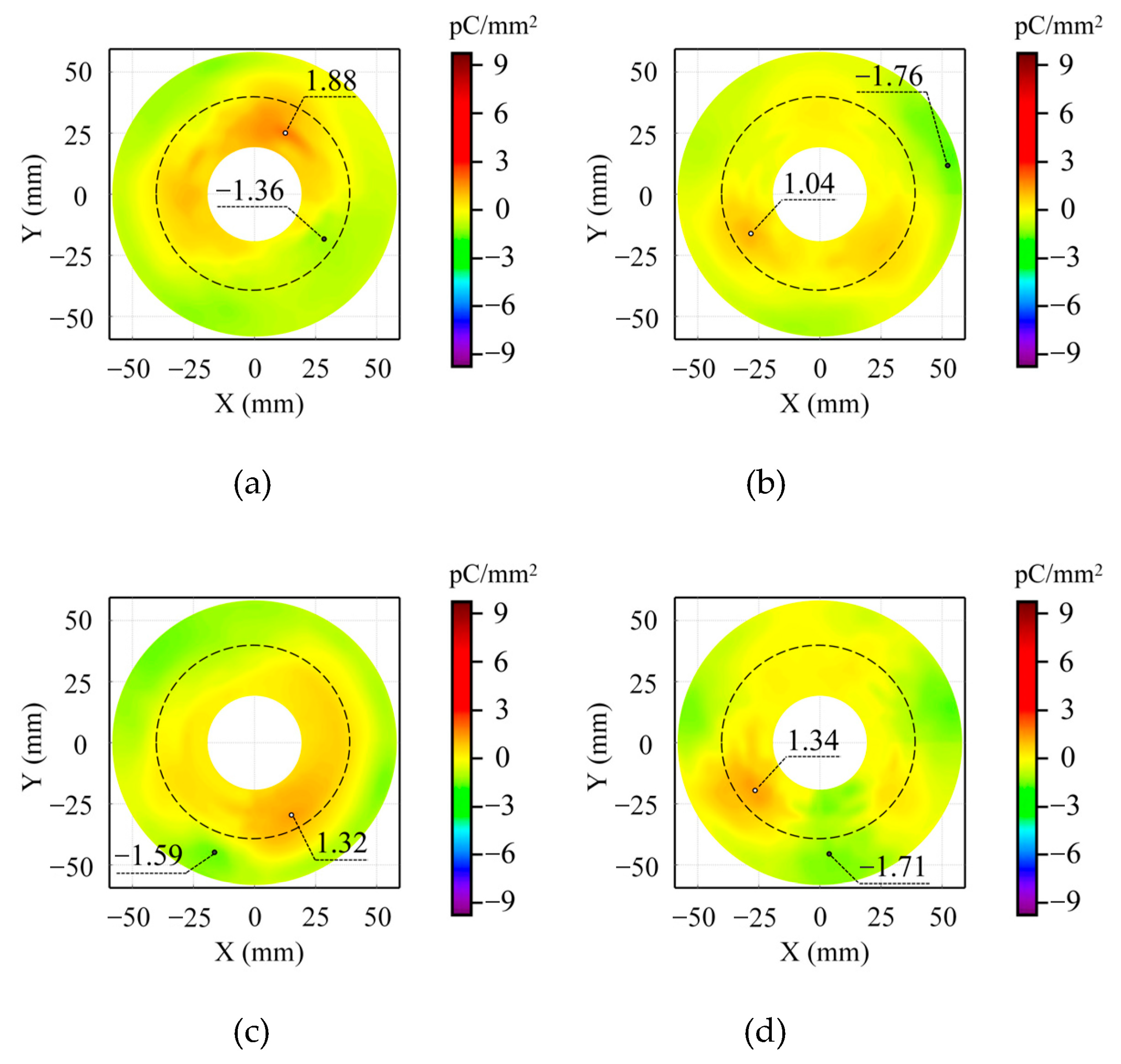
Figure 7 displays the surface charge distribution characteristics of insulators under O
2 mixing ratios of 2%, 6%, 10%, and 14% in the absence of metal particle contamination. Experimental results indicate predominant accumulation of positive charges in non-planar regions, while negative charges dominate in planar areas. Furthermore, the absence of significant gas-phase ionization prevents the generation of substantial charged particles, resulting in minimal charge accumulation on insulator surfaces. Analysis reveals that increasing O
2 content exerts some influence on charge distribution patterns, though without demonstrating clear systematic trends. This observation suggests that, within the experimental conditions examined, variations in O
2 concentration do not significantly affect the dynamic behavior of surface charges on insulators.
Figure 8 presents the surface charge distribution characteristics on insulators after applying 20 kV AC voltage for 5 min with metal particles positioned on the ground electrode. Experimental results reveal substantial accumulation of negative charges near metal particles in non-planar regions, forming a distinct negative charge speckle. Comparative analysis across different O
2 concentrations indicates no systematic influence of O
2 on surface charge distribution patterns. When increasing O
2 content from 2% to 14% in the gas mixture, charge distribution characteristics remain predominantly governed by local electric field distortion induced by metal particles. A persistent negative charge speckle appears near the metal particle in non-planar regions, with maximum charge density values fluctuating between −19.40 pC/mm
2 and −19.95 pC/mm
2, demonstrating minimal overall variation. Although elevated O
2 concentrations may cause slight local charge density modifications, the basic charge distribution pattern shows no significant alteration. These findings suggest that while the electronegativity of O
2 and its electron capture capability may influence charge accumulation to some degree, variations in O
2 concentration exert limited effects on surface charge dynamic behavior under these experimental conditions, failing to exhibit measurable systematic trends.
Figure 9 illustrates the electric field distribution along insulator surfaces derived from accumulated surface charges along the radial direction of metal particles after power interruption, under varying O
2 mixing ratios. The observed field distribution characteristics remain predominantly governed by local electric field distortion induced by metal particles, with O
2 concentration demonstrating minimal influence as it increases from 2% to 14% in the gas mixture. At 2% O
2 concentration, the maximum surface electric field strength of 0.54 kV/mm occurs at the 24.23 mm radial position. In planar regions, field magnitude exhibits a gradual enhancement toward the ground electrode, potentially attributable to higher negative charge density accumulation near the ground electrode combined with weaker counteracting effects from surrounding charge-induced fields. When increasing O
2 concentration to 6%, 10%, and 14%, the position of maximum field strength remains unchanged from the 2% case, with only minor amplitude variations observed. Planar regions maintain consistent field magnitudes around 0.3 kV/mm across all concentrations, showing negligible variation between different mixing ratios.
4. Discussion
Systematic comparison of experimental results under varying C
4F
7N and O
2 concentrations demonstrates the dominant role of C
4F
7N in regulating surface charge accumulation, primarily due to the strong electronegative properties inherent in its molecular structure. The fluorine atoms and cyanide groups (-CN) within C
4F
7N molecules confer exceptionally high electron affinity, enabling efficient capture of free electrons to form stable negative ions [
17]. Increasing C
4F
7N content in the gas mixture enhances this electronegative effect through two distinct suppression mechanisms: (1) significant inhibition of initial electron avalanche development during gas-phase ionization, reducing charged particle generation rates; (2) markedly decreased migration velocities of the formed negative ions under electric fields due to their larger mass. The combined effect of these two factors leads to a significant decrease in the charge density accumulated on the surface of the insulator. The metal particles, as intense field emission sources, amplify the electronegativity advantage of C
4F
7N, resulting in 15–20% greater charge suppression at 6–14% concentrations compared to particle-free conditions.
Conversely, the indirect effects of O
2 become even less pronounced under such strong interference, O
2 exhibits distinctly auxiliary characteristics within the ternary system. Its mechanism influences insulation performance indirectly through chemical reactions with C
4F
7N decomposition products rather than direct participation in charge transport processes. Under partial discharge or arcing conditions, C
4F
7N molecules undergo cleavage to produce reactive radicals such as CFx and CN, which readily polymerize into conductive carbon particles [
18]. The addition of O
2 converts these carbon particles into gaseous CO/CO
2 through oxidation reactions, effectively minimizing the accumulation of solid carbon deposits on insulator surfaces. Notably, within the experimental parameter range (2–14% O
2 content), this indirect influence shows no significant concentration dependence, suggesting that O
2 oxidation effects may reach saturation at relatively low concentrations.
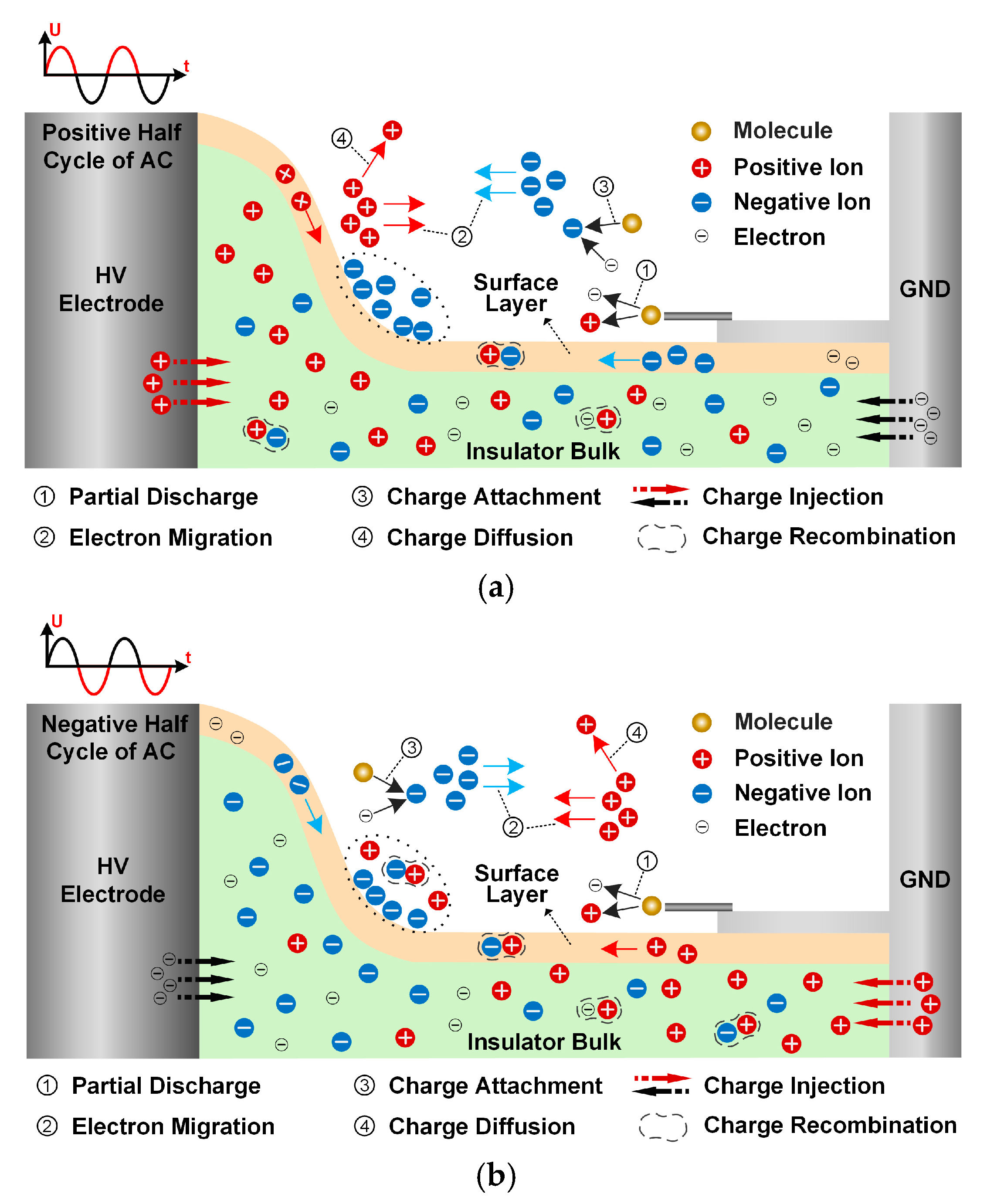
When the metal particle was placed near the grounded electrode as the gas side discharge source, the physical process of surface charge accumulation on the insulator is shown in
Figure 10. Due to the significant enhancement of the electric field at the tip of the metal particles, local discharge is easily induced (marked as ①), which leads to gas ionization and the generation of a large number of charged particles [
19]. Under the action of the electric field force, the charged particles undergo directional migration (marked as ②). During the charge transport process, the generated positive ions and electrons migrate towards the insulator surface and the ground electrode, respectively. Additionally, due to its strong electronegativity, C
4F
7N gas can effectively capture free electrons and attach them to its outer electron orbit to form negative ions, a process marked as ③. Some of the charged particles recombine to form neutral gas molecules, while others diffuse along non-electric field lines (marked as ④). The remaining charged particles deposit on the insulator and electrode surfaces to form surface charges. Notably, the difference in polarity of high-voltage AC leads to significant differences in the direction of charge injection [
20]. This section mainly studies the mechanism of charge injection from the electrode to the insulator body.
During the positive half-cycle of the AC voltage, negatively charged particles in the gas side and positive ions in the solid side migrate towards the non-planar regions of the insulator surface under the action of the electric field. The surface charge accumulation process is predominantly governed by negatively charged particles conducted from the gas phase, owing to the intrinsically low carrier density characteristic of insulating materials and the limited positive charge injection capacity of high-voltage electrodes. The electrons generated by local discharge, due to their small mass, are more likely to gain kinetic energy and accelerate towards the insulator under the action of the electric field. Most of these electrons are captured by C
4F
7N molecules, forming negative ions and depositing on the insulator surface or remaining in the mixed gas. During the negative half-cycle, although the positive ions generated by local discharge in the gas domain migrate towards the insulator surface, their migration rate is significantly lower than that of electrons due to their larger mass, resulting in most of the positive ions undergoing recombination reactions with negative ions in the gas domain. Before the voltage polarity reverses, only a small number of positive ions can reach the insulator surface and participate in the neutralization reaction. After multiple voltage cycle repetitions, significant surface charge accumulation occurs in the non-planar regions due to the continuous accumulation of negative charges. In contrast, in the planar regions, due to the weak normal component of the surface electric field, almost no charge accumulation from the gas side is observed [
21].
The introduction of metal particles dramatically amplifies these differential effects between gas components. Functioning as intense field emission sources, metal particles trigger vigorous partial discharges that intensify gas ionization processes by several orders of magnitude. Under such extreme conditions, the advantages of C4F7N electronegativity become more pronounced, demonstrating approximately 15–20% greater charge accumulation suppression compared to particle-free conditions, principally because the high-density discharge environment provides increased availability of free electrons for capture. Concurrently, the indirect effects of O2 become further diminished under such strong interference, indicating that anticipated insulation improvements through O2 content adjustment may prove substantially less effective when severe particle contamination exists in the insulation system.
The C
4F
7N/CO
2/O
2 ternary gas mixture exhibits a well-defined synergy: C
4F
7N serves as the functional component, directly regulating charge behavior through electronegative mechanisms; O
2 acts as a stabilizer, maintaining medium purity via chemical pathways; while CO
2 principally fulfils the basic function of adjusting critical parameters of the gas mixture. This mechanism achieves optimal balance between environmental compatibility and insulation reliability, providing crucial theoretical foundations for designing next-generation GIS equipment. It has to be mentioned that, in our previous research, we have compared the charge accumulation behavior in SF
6/N
2 with that in C
4F
7N/CO
2, and we have concluded that in C
4F
7N/CO
2 mixture, charge accumulation is more pronounced since the dielectric strength of C
4F
7N is lower than that of SF
6 [
22]. In other words, gaseous ionization and surface charge accumulation become more serious problems in C
4F
7N-based mixtures than in SF
6. That is the reason why we focus on the content of such C
4F
7N-based ternary mixture on the charge accumulation feature.