Instability Mechanisms and Wellbore-Stabilizing Drilling Fluids for Marine Gas Hydrate Reservoirs: A Review
Abstract
1. Introduction
2. Mechanisms of Gas Hydrate Reservoir Destabilization
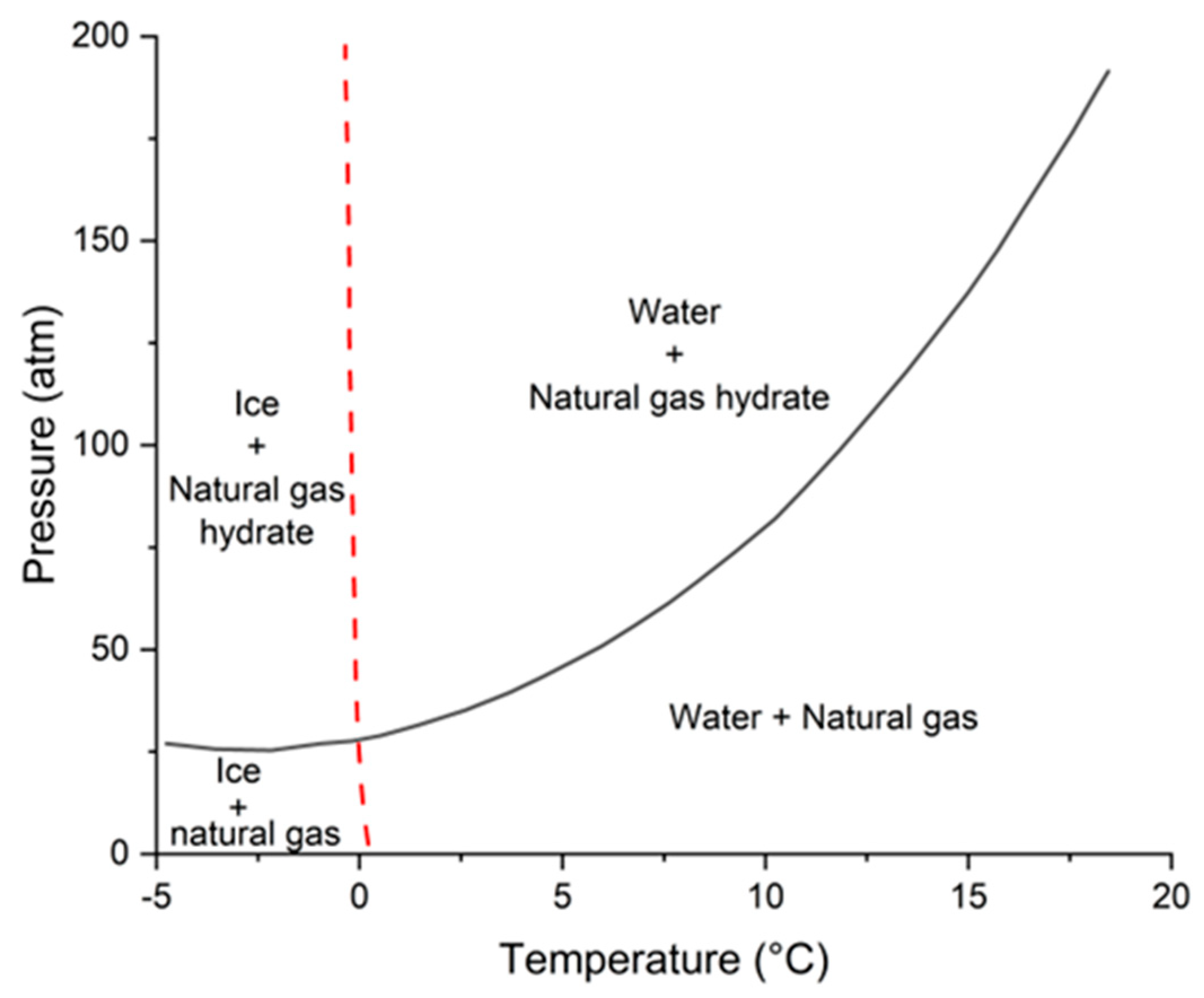
2.1. Thermodynamic Destabilization
- Drilling fluid circulation: Circulating drilling fluid, even after cooling in the marine riser, typically has a temperature higher than that of the seabed formation. Continuous convective and conductive heat transfer from fluid elevates near-wellbore temperatures [50].
- Frictional heating: the rotating drill bit generates substantial localized heat during rock fracturing, causing significant temperature spikes at the bottom of the hole [51].
- Operational fluctuations: Routine operations, such as pump start/stop cycles, or pipe connections, cause transient pressure drops from circulating to static conditions. These fluctuations can momentarily depress the bottom-hole pressure below the hydrate equilibrium pressure, particularly in reservoirs with narrow thermodynamic stability windows [56].
- Rapid pore pressure elevation [57]: Hydrate decomposition produces immense volumes of gas. For instance, 1 cm3 of solid methane hydrate can liberate approximately 164 cm3 of methane gas at standard conditions [58]. The release of this gas into a confined pore volume causes an abrupt and significant increase in pore pressure. This overpressure then propagates rapidly through the newly formed high-permeability network. If this pressure front reaches the wellbore and exceeds the hydrostatic pressure of the drilling fluid, it will trigger a gas influx, which can escalate into a blowout.
- Swab pressures: Rapid tripping of the drill string upward in the wellbore generates a transient low-pressure zone immediately below the drill bit, known as the “swabbing” effect [59]. This phenomenon occurs as the upward movement of the drill string generates a transient reduction in bottom-hole pressure by drawing fluids upward faster than they can be replaced, thereby destabilizing hydrates near the wellbore.
2.2. Kinetic Destabilization
- Catastrophic permeability increase: Hydrate-bearing sediments are typically characterized by low permeability due to hydrate crystals occluding pore throats. Upon hydrate decomposition, this solid phase transforms into mobile fluids, causing permeability to increase by several orders of magnitude (e.g., from 10−3 mD to more than 103 mD) [79,80,81]. This dramatic shift transforms zones that once impermeable barriers into high-flux conduits, which can compromise the integrity of caprocks and overlying formations [82,83,84].
- Rapid gas mobilization: During hydrate decomposition, the gas release rate can far exceed the dissipation capacity of the surrounding formation, particularly when triggered in zones with sharply increased permeability. This rapid mobilization produces high-velocity gas–water flows that can erode fine sediments, enlarge pore channels, and further accelerate decomposition. Such positive feedback between gas liberation and sediment destabilization can rapidly evolve into uncontrolled fluid migration toward the wellbore, amplifying the risk of gas influx and well control challenges [85].
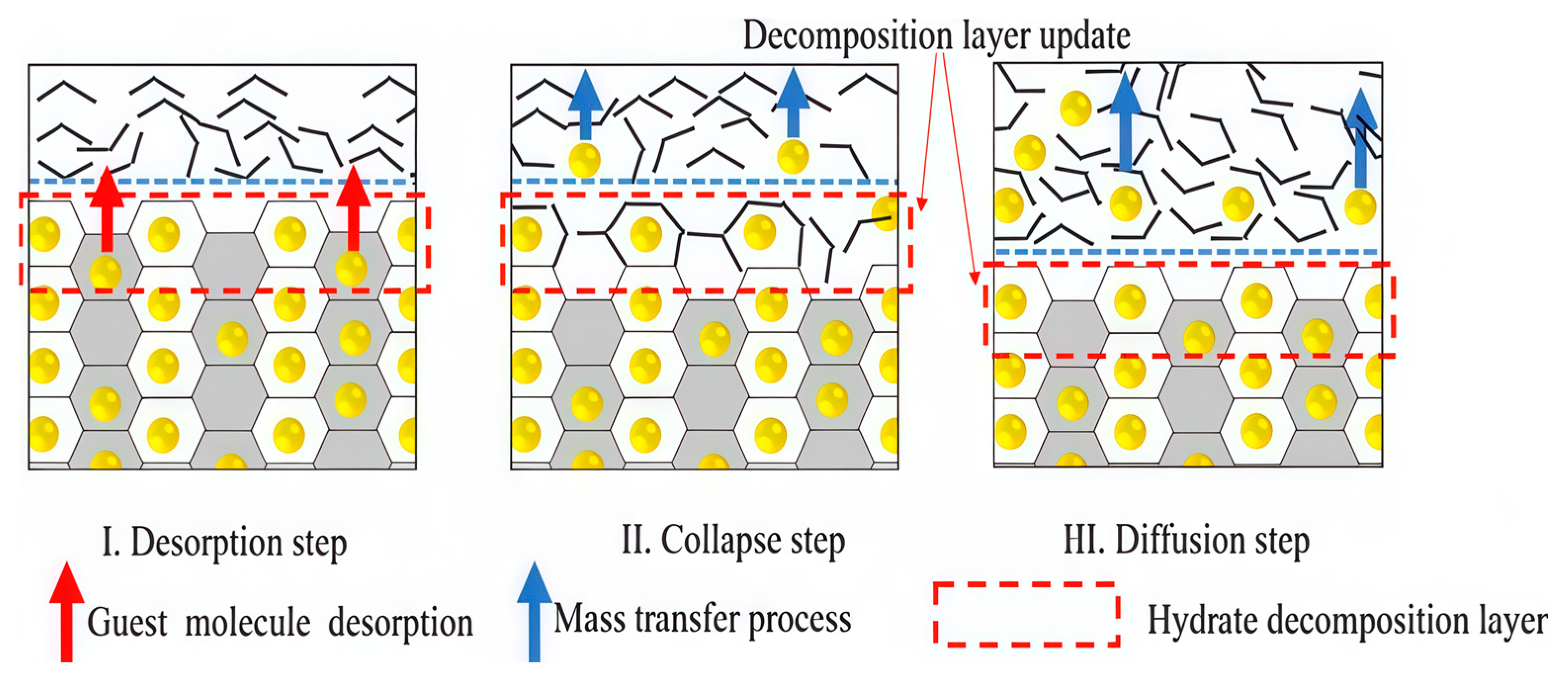
2.3. Mechanical Destabilization
- Loss of cementation and strength degradation: This is the most fundamental failure mechanism. As hydrates decompose, the phase transition converts load-bearing solids into non-load-bearing pore fluids (gas and water). This loss of the primary cementing agent causes a catastrophic reduction in the sediment’s cohesive strength and stiffness. The formation effectively transitions from a weakly cemented rock to a loose, unconsolidated sand, rendering it highly susceptible to shear or tensile failure under ambient stress conditions, which leads to borehole collapse and spalling [45].
- Reduction in effective stress: The stability of the sediment skeleton is governed by effective stress (σ’), defined by Terzaghi’s principle as the total stress (σ) minus the pore pressure (Pp): σ’ = σ − Pp. As established, hydrate decomposition induces an abrupt elevation in Pp. Even if the total stress remains constant, this rise in pore pressure significantly reduces the effective stress acting on the rock framework. When σ’ falls below the sediment’s yield strength, plastic deformation and failure occur. This process, known as poroelastic instability, is a critical pathway for mechanical destabilization in NGH reservoirs [95,96].
- Wellbore stress concentration: The act of drilling creates a cavity that disrupts the original triaxial stress state of the formation. Stress redistributes around the wellbore, creating zones of high stress concentration, typically orientated perpendicular to the maximum horizontal stress direction. For a formation already weakened by hydrate decomposition, even routine stress concentrations can be sufficient to exceed its diminished failure envelope, initiating borehole breakouts with characteristic “V” or “U” shapes [97,98].
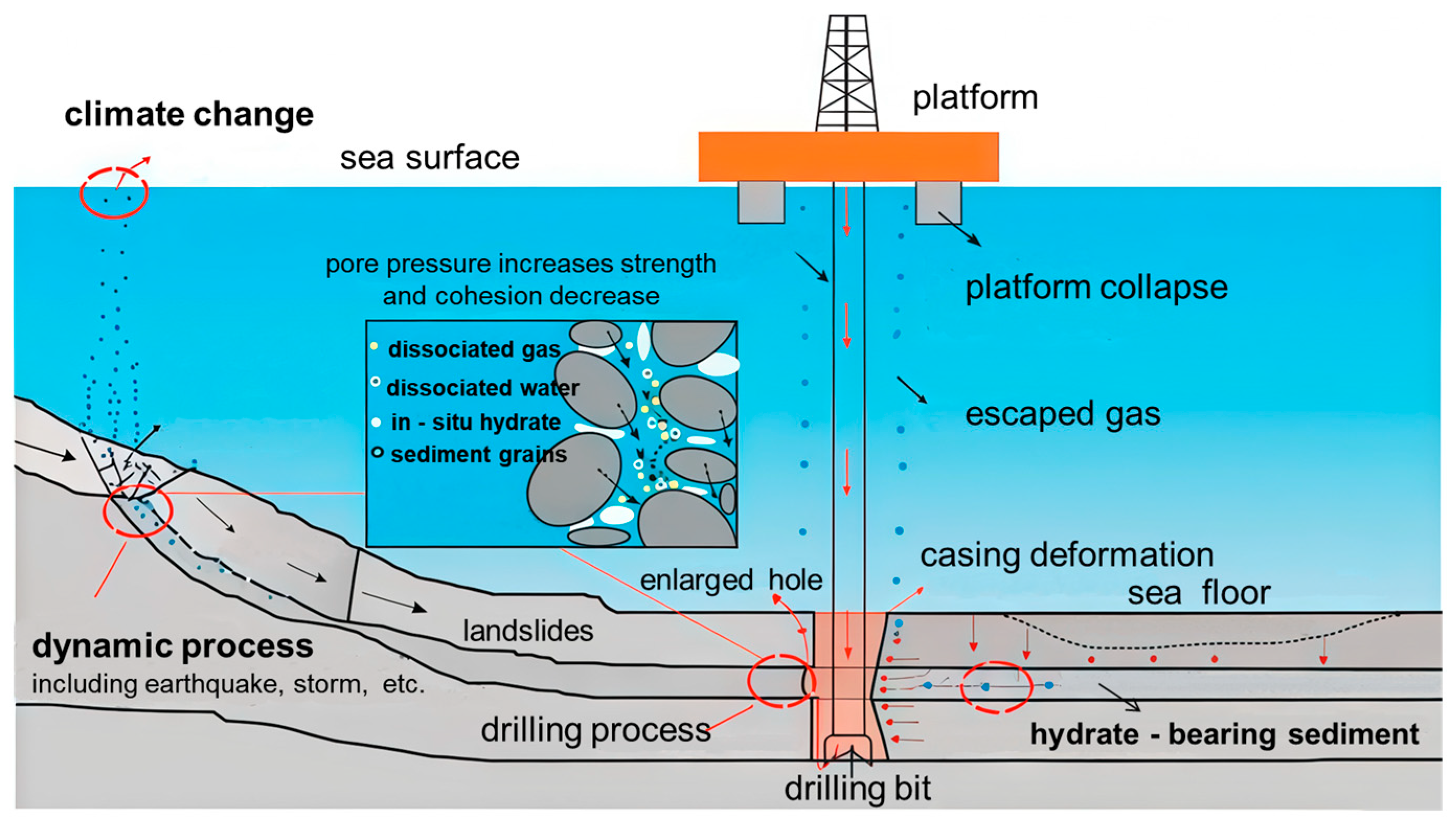
3. Advanced Drilling Fluid for Hydrate Wellbore Stability
3.1. Thermodynamic Inhibitors
3.1.1. Inorganic Salts
3.1.2. Alcohols and Amines
3.2. Kinetic Inhibitors and Anti-Agglomerants

3.3. Wellbore Strengthening and Sealing Technologies
3.4. Integrated Fluid Systems
4. Future Trends and Perspectives
4.1. Advanced Modeling
- Most THMC models rely on constitutive laws for hydrate-bearing sediments that are derived from static, quasi-equilibrium triaxial tests. There is a critical lack of experimental data and corresponding validated models that capture the dynamic, rate-dependent mechanical behavior of sediments during rapid dissociation, where properties like cohesion and friction angle change almost instantaneously. Future research must focus on developing and experimentally validating dynamic constitutive models that accurately reflect this transient weakening process.
- Current models often use simplified relationships to describe how permeability changes with hydrate saturation and porosity. However, the strong coupling between mechanical deformation (e.g., pore collapse, micro-fracturing under stress) and fluid flow pathways is not well captured. We lack robust, experimentally verified models that dynamically link the evolving stress–strain state of the sediment to its anisotropic permeability tensor during decomposition.
- There is a disconnect between pore-scale models that resolve individual grain and hydrate crystal interactions and continuum-scale reservoir models. Upscaling techniques that can accurately translate complex pore-scale physics (like the formation of localized high-permeability “wormholes” or the mechanics of filter cake formation with nanoparticles) into representative parameters (e.g., effective permeability, capillary pressure curves) for larger-scale THMC simulations are urgently needed.
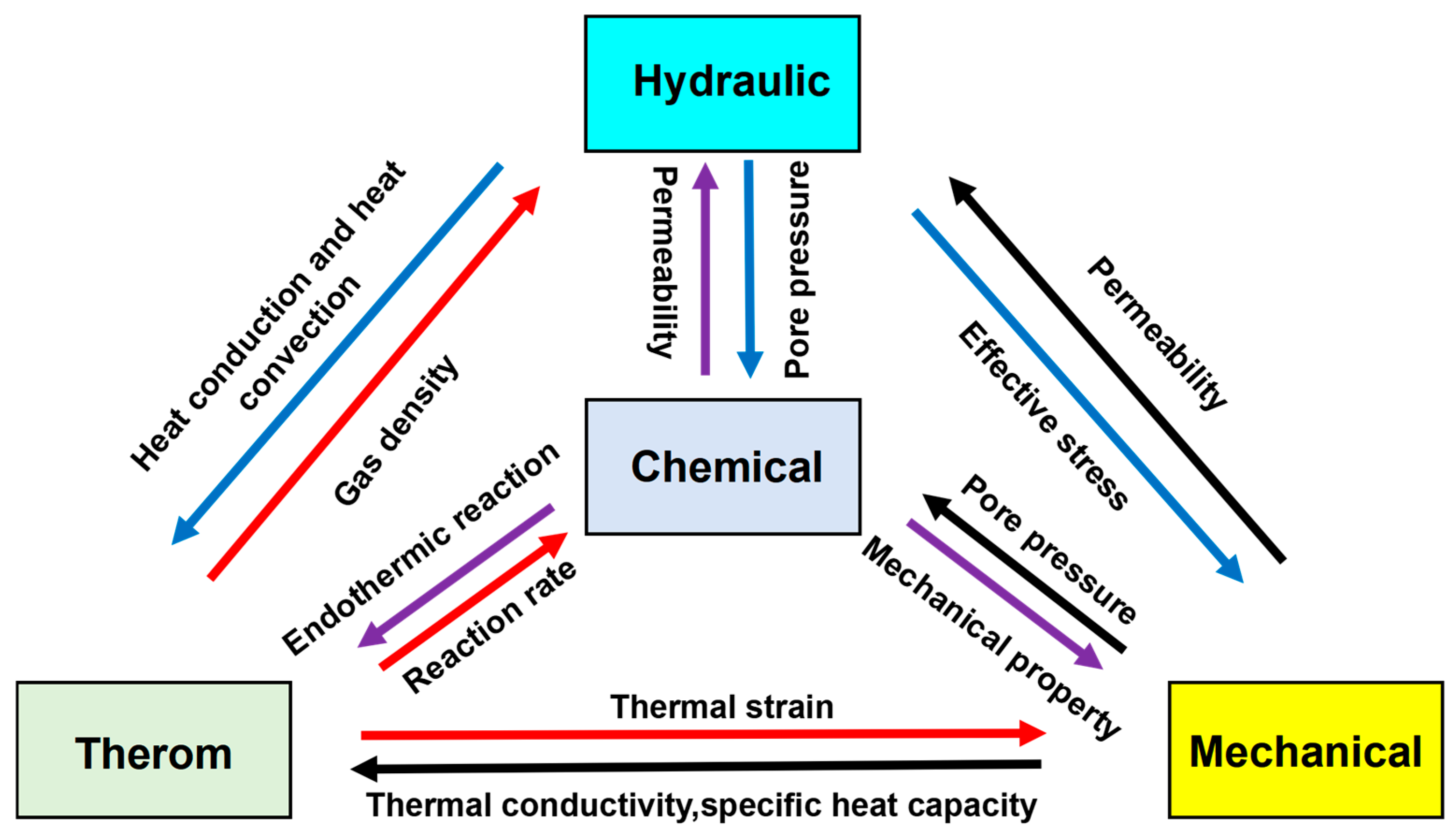
4.2. Intelligent and Eco-Friendly Materials
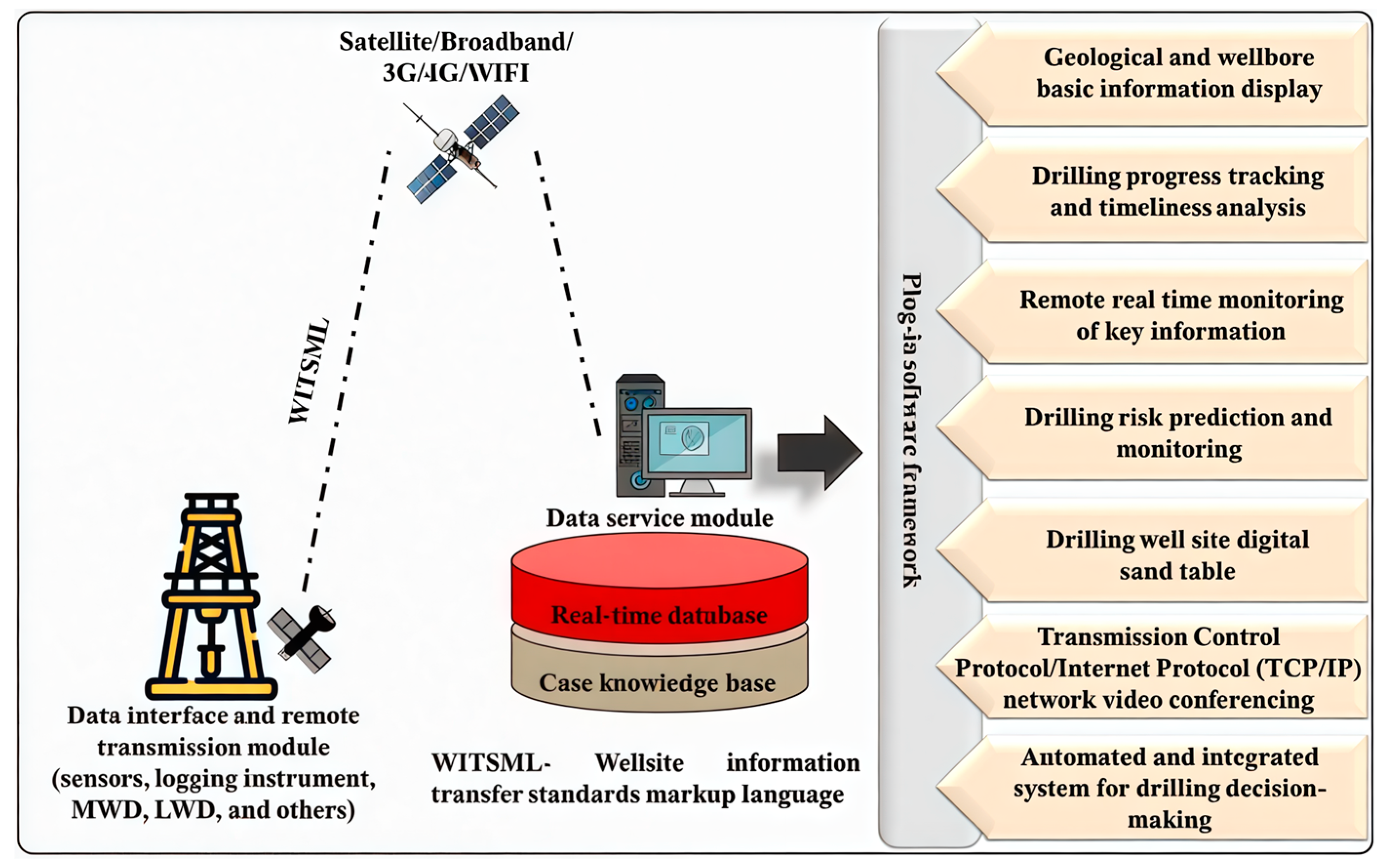
4.3. Intelligent Drilling Systems: Real-Time Diagnosis and Dynamic Control
5. Conclusions and Recommendations
- NGH reservoir destabilization is a multiphysics-coupled process. Instability arises not caused by an isolated factor but by a tightly linked chain reaction involving thermodynamic decomposition, accelerated kinetic-driven fluid release, and mechanical strength degradation. This underscores that any viable stability solutions must holistically address the coupled thermal, hydraulic, mechanical, and chemical challenges. Strategies based on thermodynamic inhibition or mechanical sealing alone are insufficient.
- Current drilling fluids lack true synergistic integration. By synergistic integration, we refer to the holistic design of drilling fluid formulations where the individual additives not only perform their respective functions but also enhance each other’s effectiveness without introducing trade-offs. While existing drilling fluid systems have demonstrated feasibility in specific field trials, they function primarily as a physical superposition of additives rather than as a synergistically engineered system. Potent inhibition often compromises rheological performance or environmental compatibility, and the long-term efficacy of sealing materials within complex chemical environments remains poorly understood.
- Future breakthroughs hinge on cross-scale mechanistic understanding and precision material design. The primary limitation of current technology is an insufficient understanding of the microscopic interactions that govern macroscopic phenomena. Transformative advances will therefore emerge from foundational science—specifically, from cross-scale theoretical and experimental research that enables a predictive understanding of stabilization processes and facilitates the “design-on-demand” of new functional materials.
- Investigate coupled mechanisms across scales. This requires a multi-pronged approach: (i) at the molecular scale, using simulations to elucidate inhibitor-hydrate interactions to guide rational inhibitor design; (ii) at the pore scale, using microfluidics and advanced imaging to model multiphase flow and sealing mechanisms within dynamic pore networks; and (iii) at the reservoir scale, developing fully coupled THMC numerical models that integrate these microscopic insights to accurately predict wellbore behavior under realistic drilling conditions.
- Develop function-oriented intelligent and sustainable materials. Research should shift from passive defense to active response by designing “smart” materials (e.g., stimuli-responsive polymers) that can sense downhole triggers like temperature changes and autonomously activate sealing or inhibitive functions. Concurrently, a strong focus must be placed on eco-friendly materials, such as high-performance, biodegradable additives derived from renewable biomass, to ensure environmental compatibility.
- Establish feedback control methodologies based on real-time monitoring. This involves three key steps: (i) developing AI-driven diagnostic algorithms that can identify subtle precursors to instability from real-time LWD/MWD data; (ii) building dynamic models that quantitatively link drilling fluid properties to wellbore stability status; and (iii) integrating these elements into closed-loop control systems that can automatically adjust fluid properties in real-time to proactively manage wellbore stability.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Koh, C.A. Towards a fundamental understanding of natural gas hydrates. Chem. Soc. Rev. 2002, 31, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Sloan, E.D., Jr. Fundamental principles and applications of natural gas hydrates. Nature 2003, 426, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Seo, Y.-T.; Lee, J.-W.; Moudrakovski, I.; Ripmeester, J.A.; Chapman, N.R.; Coffin, R.B.; Gardner, G.; Pohlman, J. Complex gas hydrate from the Cascadia margin. Nature 2007, 445, 303–306. [Google Scholar] [CrossRef]
- Kvenvolden, K.A. Methane hydrate—A major reservoir of carbon in the shallow geosphere? Chem. Geol. 1988, 71, 41–51. [Google Scholar] [CrossRef]
- Sloan, E.D., Jr.; Koh, C.A. Clathrate Hydrates of Natural Gases; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Hassanpouryouzband, A.; Joonaki, E.; Farahani, M.V.; Takeya, S.; Ruppel, C.; Yang, J.; English, N.J.; Schicks, J.M.; Edlmann, K.; Mehrabian, H. Gas hydrates in sustainable chemistry. Chem. Soc. Rev. 2020, 49, 5225–5309. [Google Scholar] [CrossRef]
- Demirbas, A.; Rehan, M.; Al-Sasi, B.O.; Nizami, A.-S. Evaluation of natural gas hydrates as a future methane source. Pet. Sci. Technol. 2016, 34, 1204–1210. [Google Scholar] [CrossRef]
- Milkov, A.V. Global estimates of hydrate-bound gas in marine sediments: How much is really out there? Earth-Sci. Rev. 2004, 66, 183–197. [Google Scholar] [CrossRef]
- Boswell, R.; Collett, T.S. Current perspectives on gas hydrate resources. Energy Environ. Sci. 2011, 4, 1206–1215. [Google Scholar] [CrossRef]
- Wallmann, K.; Pinero, E.; Burwicz, E.; Haeckel, M.; Hensen, C.; Dale, A.; Ruepke, L. The global inventory of methane hydrate in marine sediments: A theoretical approach. Energies 2012, 5, 2449–2498. [Google Scholar] [CrossRef]
- Collett, T.; Bahk, J.-J.; Baker, R.; Boswell, R.; Divins, D.; Frye, M.; Goldberg, D.; Husebø, J.; Koh, C.; Malone, M. Methane Hydrates in Nature Current Knowledge and Challenges. J. Chem. Eng. Data 2015, 60, 319–329. [Google Scholar] [CrossRef]
- Ruppel, C. Permafrost-associated gas hydrate: Is it really approximately 1% of the global system? J. Chem. Eng. Data 2015, 60, 429–436. [Google Scholar] [CrossRef]
- Boswell, R.; Hancock, S.; Yamamoto, K.; Collett, T.; Pratap, M.; Lee, S.-R. Natural gas hydrates: Status of potential as an energy resource. Future Energy 2020, 111–131. [Google Scholar] [CrossRef]
- Kastner, M.; Myers, M.; Koh, C.A.; Moridis, G.; Johnson, J.E.; Thurmond, J. Energy Transition and Climate Mitigation Require Increased Effort on Methane Hydrate Research; ACS Publications: Washington, DC, USA, 2022. [Google Scholar]
- Moridis, G.J.; Collett, T.S.; Boswell, R.; Kurihara, M.; Reagan, M.T.; Koh, C.; Sloan, E.D. Toward production from gas hydrates: Current status, assessment of resources, and simulation-based evaluation of technology and potential. SPE Reserv. Eval. Eng. 2009, 12, 745–771. [Google Scholar] [CrossRef]
- Sloan, E.D. Natural Gas Hydrates in Flow Assurance; Gulf Professional Publishing: Oxford, UK, 2010. [Google Scholar]
- Moridis, G.J.; Collett, T.S.; Pooladi-Darvish, M.; Hancock, S.; Santamarina, C.; Boswell, R.; Kneafsey, T.; Rutqvist, J.; Kowalsky, M.B.; Reagan, M.T. Challenges, uncertainties, and issues facing gas production from gas-hydrate deposits. SPE Reserv. Eval. Eng. 2011, 14, 76–112. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; You, Z.; Wu, P.; Qu, Y.; Zhang, A.; Sun, X.; Song, Y. A particle-scale investigation of mechanical behavior of cemented hydrate-bearing sediment using Discrete Element Method. Geomech. Energy Environ. 2023, 33, 100436. [Google Scholar] [CrossRef]
- Ghaedi, M.; Rahimpour, M.R. Modeling and simulation of exploration and exploitation natural gas hydrate. In Advances Natural Gas: Formation, Processing, and Applications. Volume 8: Natural Gas Process Modelling and Simulation; Elsevier: Amsterdam, The Netherlands, 2024; pp. 609–628. [Google Scholar]
- McConnell, D.R.; Zhang, Z.; Boswell, R. Review of progress in evaluating gas hydrate drilling hazards. Mar. Pet. Geol. 2012, 34, 209–223. [Google Scholar] [CrossRef]
- He, Y.; Song, B.; Li, Q. Coupling submarine slope stability and wellbore stability analysis with natural gas hydrate drilling and production in submarine slope strata in the South China Sea. J. Mar. Sci. Eng. 2023, 11, 2069. [Google Scholar] [CrossRef]
- Zhou, R.; Bai, B.; Chen, L.; Zong, Y.; Wu, N. A granular thermodynamic constitutive model considering THMC coupling effect for hydrate-bearing sediment. Ocean Eng. 2024, 310, 118689. [Google Scholar] [CrossRef]
- Ebeltoft, H.; Majeed, Y.; Sœrgärd, E. Hydrate control during deepwater drilling: Overview and new drilling-fluids formulations. SPE Drill. Complet. 2001, 16, 19–26. [Google Scholar] [CrossRef]
- Elhenawy, S.; Khraisheh, M.; Almomani, F.; Al-Ghouti, M.A.; Hassan, M.K.; Al-Muhtaseb, A.A. Towards gas hydrate-free pipelines: A comprehensive review of gas hydrate inhibition techniques. Energies 2022, 15, 8551. [Google Scholar] [CrossRef]
- Majid, A.A.A.; Worley, J.; Koh, C.A. Thermodynamic and kinetic promoters for gas hydrate technological applications. Energy Fuels 2021, 35, 19288–19301. [Google Scholar] [CrossRef]
- Lederhos, J.; Long, J.; Sum, A.; Christiansen, R.; Sloan, E., Jr. Effective kinetic inhibitors for natural gas hydrates. Chem. Eng. Sci. 1996, 51, 1221–1229. [Google Scholar] [CrossRef]
- Lachance, J.W.; Sloan, E.D.; Koh, C.A. Determining gas hydrate kinetic inhibitor effectiveness using emulsions. Chem. Eng. Sci. 2009, 64, 180–184. [Google Scholar] [CrossRef]
- Alcázar-Vara, L.A.; Cortés-Monroy, I.R. Drilling Fluids for Deepwater Fields: An Overview; IntechOpen: London, UK, 2017. [Google Scholar]
- Gao, Y.; Wang, Y.; Xin, G.; Wang, X.; Yue, C.; Chen, L. Experimental Study on the Effect of Combination of Thermodynamic Inhibitors’ and Kinetic Inhibitors’ Hydrate Inhibition. Front. Energy Res. 2021, 9, 718673. [Google Scholar] [CrossRef]
- Rong, L.; Santra, A.; Ross, G.; Advincula, R.C. Polymer grafted graphene via atom transfer radical polymerization (ATRP): A rheology improver in oil-based drilling fluids. MRS Commun. 2023, 13, 445–450. [Google Scholar] [CrossRef]
- Noor, A.A.; Khan, M.A.; Zhang, Y.; Lv, K.; Sun, J.; Liu, C.; Li, M.-C. Modified natural polymers as additives in high-temperature drilling fluids: A review. Int. J. Biol. Macromol. 2025, 287, 138556. [Google Scholar] [CrossRef]
- Phrampus, B.J.; Hornbach, M.J. Recent changes to the Gulf Stream causing widespread gas hydrate destabilization. Nature 2012, 490, 527–530. [Google Scholar] [CrossRef]
- Burton, Z.F.; Kroeger, K.F.; Hosford Scheirer, A.; Seol, Y.; Burgreen-Chan, B.; Graham, S.A. Tectonic uplift destabilizes subsea gas hydrate: A model example from Hikurangi margin, New Zealand. Geophys. Res. Lett. 2020, 47, e2020GL087150. [Google Scholar] [CrossRef]
- Weldeab, S.; Schneider, R.R.; Yu, J.; Kylander-Clark, A. Evidence for massive methane hydrate destabilization during the penultimate interglacial warming. Proc. Natl. Acad. Sci. USA 2022, 119, e2201871119. [Google Scholar] [CrossRef]
- Burton, Z.F.; Dafov, L.N. Salt diapir-driven recycling of gas hydrate. Geochem. Geophys. Geosyst. 2023, 24, e2022GC010704. [Google Scholar] [CrossRef]
- Dafov, L.N.; Burton, Z.F.; Haines, S.S.; Scheirer, A.H.; Masurek, N.; Boswell, R.; Frye, M.; Seol, Y.; Graham, S.A. Terrebonne Basin, Gulf of Mexico gas hydrate resource evaluation and 3-D modeling of basin-scale sedimentation, salt tectonics, and hydrate system evolution since the early Miocene. Mar. Pet. Geol. 2025, 176, 107330. [Google Scholar] [CrossRef]
- Kwon, T.-H.; Song, K.-I.; Cho, G.-C. Destabilization of marine gas hydrate-bearing sediments induced by a hot wellbore: A numerical approach. Energy Fuels 2010, 24, 5493–5507. [Google Scholar] [CrossRef]
- Popenoe, P.; Schmuck, E.; Dillon, W.; Schwab, W.; Lee, H.; Twichell, D. The Cape Fear Landslide: Slope Failure Associated with Salt Diapirism and Gas Hydrate Decomposition. In Submarine Landslides: Selected Studies in the US Exclusive Economic Zone; Schwab, W.C., Lee, H.J., Twichell, D.C., Eds.; US Geological Survey: Washington, DC, USA, 1993; pp. 40–53. [Google Scholar]
- Paull, C.K.; Ussler, W., III; Dillon, W.P. Potential role of gas hydrate decomposition in generating submarine slope failures. Nat. Gas Hydrate Ocean. Permafr. Environ. 2000, 5, 149–156. [Google Scholar]
- Maslin, M.; Owen, M.; Betts, R.; Day, S.; Dunkley Jones, T.; Ridgwell, A. Gas hydrates: Past and future geohazard? Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 2369–2393. [Google Scholar] [CrossRef]
- Handwerger, A.L.; Rempel, A.W.; Skarbek, R.M. Submarine landslides triggered by destabilization of high-saturation hydrate anomalies. Geochem. Geophys. Geosyst. 2017, 18, 2429–2445. [Google Scholar] [CrossRef]
- Song, B.; Cheng, Y.; Yan, C.; Han, Z.; Ding, J.; Li, Y.; Wei, J. Influences of hydrate decomposition on submarine landslide. Landslides 2019, 16, 2127–2150. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Liao, C.; Jiang, M.; Peng, M. A two-stage probabilistic approach for the risk assessment of submarine landslides induced by gas hydrate exploitation. Appl. Ocean Res. 2020, 99, 102158. [Google Scholar] [CrossRef]
- Clennell, M.B.; Hovland, M.; Booth, J.S.; Henry, P.; Winters, W.J. Formation of natural gas hydrates in marine sediments: 1. Conceptual model of gas hydrate growth conditioned by host sediment properties. J. Geophys. Res. Solid Earth 1999, 104, 22985–23003. [Google Scholar] [CrossRef]
- Freij-Ayoub, R.; Tan, C.; Clennell, B.; Tohidi, B.; Yang, J. A wellbore stability model for hydrate bearing sediments. J. Pet. Sci. Eng. 2007, 57, 209–220. [Google Scholar] [CrossRef]
- Henry, P.; Thomas, M.; Clennell, M.B. Formation of natural gas hydrates in marine sediments: 2. Thermodynamic calculations of stability conditions in porous sediments. J. Geophys. Res. Solid Earth 1999, 104, 23005–23022. [Google Scholar] [CrossRef]
- Xu, W.; Ruppel, C. Predicting the occurrence, distribution, and evolution of methane gas hydrate in porous marine sediments. J. Geophys. Res. Solid Earth 1999, 104, 5081–5095. [Google Scholar] [CrossRef]
- Tang, L.G.; Xiao, R.; Huang, C.; Feng, Z.; Fan, S.S. Experimental investigation of production behavior of gas hydrate under thermal stimulation in unconsolidated sediment. Energy Fuels 2005, 19, 2402–2407. [Google Scholar] [CrossRef]
- Yang, M.; Fu, Z.; Zhao, Y.; Jiang, L.; Zhao, J.; Song, Y. Effect of depressurization pressure on methane recovery from hydrate–gas–water bearing sediments. Fuel 2016, 166, 419–426. [Google Scholar] [CrossRef]
- Chen, H.; Luo, M.; Jiang, D.; Wu, Y.; Ma, C.; Yu, X.; Wang, M.; Yang, Y.; Liu, H.; Zhang, Y. Research on the Formation and Plugging Risk of Gas Hydrate in a Deepwater Drilling Wellbore: A Case Study. Processes 2023, 11, 488. [Google Scholar] [CrossRef]
- Chandrasekhar, S.V. Annular Couette–Poiseuille flow and heat transfer of a power-law fluid–analytical solutions. J. Non-Newton. Fluid Mech. 2020, 286, 104402. [Google Scholar] [CrossRef]
- Chang, X.; Zhou, J.; Guo, Y.; He, S.; Wang, L.; Chen, Y.; Tang, M.; Jian, R. Heat transfer behaviors in horizontal wells considering the effects of drill pipe rotation, and hydraulic and mechanical frictions during drilling procedures. Energies 2018, 11, 2414. [Google Scholar] [CrossRef]
- Wu, W.-T.; Massoudi, M. Heat transfer and dissipation effects in the flow of a drilling fluid. Fluids 2016, 1, 4. [Google Scholar] [CrossRef]
- Yin, B.; Chen, C.; Feng, K.; Liu, S.; Ren, M.; Wang, Z.; Sun, B. Multiphase Transient Flow in Wellbore during Shallow Hydrate Reservoir Drilling in Deep Water. ACS Omega 2025, 10, 13701–13714. [Google Scholar] [CrossRef]
- Li, G.; Moridis, G.J.; Zhang, K.; Li, X.-S. Evaluation of gas production potential from marine gas hydrate deposits in Shenhu area of South China Sea. Energy Fuels 2010, 24, 6018–6033. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kanno, T.; Wang, X.-X.; Tamaki, M.; Fujii, T.; Chee, S.-S.; Wang, X.-W.; Pimenov, V.; Shako, V. Thermal responses of a gas hydrate-bearing sediment to a depressurization operation. RSC Adv. 2017, 7, 5554–5577. [Google Scholar] [CrossRef]
- Xu, W.; Germanovich, L.N. Excess pore pressure resulting from methane hydrate dissociation in marine sediments: A theoretical approach. J. Geophys. Res. Solid Earth 2006, 111, B1. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, X.; Li, Q. Formation of layered fracture and outburst during gas hydrate dissociation. J. Pet. Sci. Eng. 2011, 76, 212–216. [Google Scholar] [CrossRef]
- Mohammad, A.; Davidrajuh, R. Modeling of swab and surge pressures: A survey. Appl. Sci. 2022, 12, 3526. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, H.; Jiang, M. Elastoplastic analytical investigation of wellbore stability for drilling in methane hydrate-bearing sediments. J. Nat. Gas Sci. Eng. 2020, 79, 103344. [Google Scholar] [CrossRef]
- Hu, T.; Wang, H.; Jiang, M. Analytical approach for the fast estimation of time-dependent wellbore stability during drilling in methane hydrate-bearing sediment. J. Nat. Gas Sci. Eng. 2022, 99, 104422. [Google Scholar] [CrossRef]
- Wang, H.; Chen, X.; Jiang, M.; Guo, Z. Analytical investigation of wellbore stability during drilling in marine methane hydrate-bearing sediments. J. Nat. Gas Sci. Eng. 2019, 68, 102885. [Google Scholar] [CrossRef]
- Sun, W.; Pei, J.; Wei, N.; Zhao, J.; Xue, J.; Zhou, S.; Zhang, L.; Kvamme, B.; Li, Q.; Li, H. Sensitivity analysis of reservoir risk in marine gas hydrate drilling. Petroleum 2021, 7, 427–438. [Google Scholar] [CrossRef]
- Liu, W.; Sun, Z.; Hu, J.; Chen, S.; Wu, K.; Sun, Y.; Luo, J.; Li, X. Prediction of hydrate formation risk based on temperature–pressure field coupling in the deepwater gas well cleanup process. Energy Fuels 2021, 35, 2024–2032. [Google Scholar] [CrossRef]
- Sun, W.; Wei, N.; Zhao, J.; Zhou, S.; Zhang, L.; Li, Q.; Jiang, L.; Zhang, Y.; Li, H.; Xu, H. Wellbore temperature and pressure field in deep-water drilling and the applications in prediction of hydrate formation region. Front. Energy Res. 2021, 9, 696392. [Google Scholar] [CrossRef]
- Ye, H.; Wu, X.; Li, D. Numerical Simulation of Natural Gas Hydrate Exploitation in Complex Structure Wells: Productivity Improvement Analysis. Mathematics 2021, 9, 2184. [Google Scholar] [CrossRef]
- Kim, H.; Bishnoi, P.R.; Heidemann, R.A.; Rizvi, S.S. Kinetics of methane hydrate decomposition. Chem. Eng. Sci. 1987, 42, 1645–1653. [Google Scholar] [CrossRef]
- Alavi, S.; Ripmeester, J. Nonequilibrium adiabatic molecular dynamics simulations of methane clathrate hydrate decomposition. J. Chem. Phys. 2010, 132, 144703. [Google Scholar] [CrossRef]
- Windmeier, C.; Oellrich, L.R. Theoretical Study of Gas Hydrate Decomposition Kinetics—Model Development. J. Phys. Chem. A 2013, 117, 10151–10161. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Helmig, R.; Wohlmuth, B. Non-isothermal, multi-phase, multi-component flows through deformable methane hydrate reservoirs. Comput. Geosci. 2015, 19, 1063–1088. [Google Scholar] [CrossRef]
- Fu, J.; Su, Y.; Jiang, W.; Xiang, X.; Li, B. Multiphase flow behavior in deep water drilling: The influence of gas hydrate. Energy Sci. Eng. 2020, 8, 1386–1403. [Google Scholar] [CrossRef]
- Sun, X.; Xiao, P.; Shi, Q.; Wang, L.; Xu, Z.; Bu, Y.; Wang, X.; Sun, Y.; Sun, C.; Chen, G. Rate-limiting factors in hydrate decomposition through depressurization across various scales: A mini-review. Chin. J. Chem. Eng. 2024, 67, 206–219. [Google Scholar] [CrossRef]
- Wang, B.; Fan, Z.; Wang, P.; Liu, Y.; Zhao, J.; Song, Y. Analysis of depressurization mode on gas recovery from methane hydrate deposits and the concomitant ice generation. Appl. Energy 2018, 227, 624–633. [Google Scholar] [CrossRef]
- Rueff, R.M.; Dendy Sloan, E.; Yesavage, V.F. Heat capacity and heat of dissociation of methane hydrates. AIChE J. 1988, 34, 1468–1476. [Google Scholar] [CrossRef]
- Circone, S.; Kirby, S.H.; Stern, L.A. Thermal regulation of methane hydrate dissociation: Implications for gas production models. Energy Fuels 2005, 19, 2357–2363. [Google Scholar] [CrossRef]
- Falenty, A.; Kuhs, W.F. “Self-Preservation” of CO2 Gas Hydrates Surface Microstructure and Ice Perfection. J. Phys. Chem. B 2009, 113, 15975–15988. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, N.; He, C.; Sun, Z.; Zhang, Z.; Hao, X.; Chen, Q.; Bu, Q.; Liu, C.; Sun, J. Nucleation probability and memory effect of methane-propane mixed gas hydrate. Fuel 2021, 291, 120103. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, C.; Yang, M.; Liu, W.; Xu, K.; Liu, Y.; Song, Y. Existence of a memory effect between hydrates with different structures (I, II, and H). J. Nat. Gas Sci. Eng. 2015, 26, 330–335. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, X.; Zhang, L.; Wang, Z.; Wang, D.; Dai, S. An analytical model for the permeability in hydrate-bearing sediments considering the dynamic evolution of hydrate saturation and pore morphology. Geophys. Res. Lett. 2021, 48, e2021GL093397. [Google Scholar] [CrossRef]
- Li, Z.-D.; Tian, X.; Li, Z.; Xu, J.-Z.; Zhang, H.-X.; Wang, D.-J. Experimental study on growth characteristics of pore-scale methane hydrate. Energy Rep. 2020, 6, 933–943. [Google Scholar] [CrossRef]
- Ren, X.; Guo, Z.; Ning, F.; Ma, S. Permeability of hydrate-bearing sediments. Earth-Sci. Rev. 2020, 202, 103100. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Komai, T.; Kawabe, Y.; Tenma, N.; Yamaguchi, T. Formation and Dissociation Behavior of Methane Hydrate in Porous Media-Estimation of Permeability in Methane Hydrate Reservoir, Part 1. Shigen-Sozai 2004, 120, 85–90. [Google Scholar] [CrossRef]
- Zhang, Q.; Qin, X.; Zhang, H.; Dong, Y.; Lu, C.; Li, S.; Xiao, L.; Ma, C.; Bian, H. Experimental Insights Into the In Situ Formation and Dissociation of Gas Hydrate in Sediments of Shenhu, South China Sea. Front. Earth Sci. 2022, 10, 882701. [Google Scholar] [CrossRef]
- Yan, J.; Yan, K.; Huang, T.; Mao, M.; Li, X.; Chen, Z.; Pang, W.; Qin, R.; Ruan, X. Research Progress on Characteristics of Marine Natural Gas Hydrate Reservoirs. Energies 2024, 17, 4431. [Google Scholar] [CrossRef]
- Zhang, P.; Gao, W.; Ding, Y.; Liu, B. Mesoscale numerical investigation of fines detachment, migration, and erosion mechanisms in gas hydrate extraction. Acta Geotech. 2025, 1–18. [Google Scholar] [CrossRef]
- Li, X.; Lu, H.; Zhang, P.; Yu, L.; Xiao, C.; Li, Y. Numerical Simulation of Secondary Hydrate Formation Characteristics and Effectiveness of Prevention Methods. Energies 2024, 17, 5045. [Google Scholar] [CrossRef]
- Kou, X.; Zhang, H.; Li, X.-S.; Chen, Z.-Y.; Wang, Y. Interfacial Heat and Mass Transfer Effects on Secondary Hydrate Formation under Different Dissociation Conditions. Langmuir 2024, 40, 3617–3627. [Google Scholar] [CrossRef]
- Yan, Y.; Yamada, Y.; Liang, Y. Enhancing the efficacy of kinetic hydrate inhibitors through synergistic effects: A review. Gas Sci. Eng. 2025, 138, 205611. [Google Scholar] [CrossRef]
- Ning, F.; Guo, D.; Din, S.U.; Zhang, H.; Ou, W.; Fang, B.; Liang, Y.; Zhang, L.; Lee, K.; Koh, C.A. The kinetic effects of hydrate anti-agglomerants/surfactants. Fuel 2022, 318, 123566. [Google Scholar] [CrossRef]
- Song, S.; Shi, B.; Yu, W.; Ding, L.; Chen, Y.; Yu, Y.; Ruan, C.; Liu, Y.; Wang, W.; Gong, J. A new methane hydrate decomposition model considering intrinsic kinetics and mass transfer. Chem. Eng. J. 2019, 361, 1264–1284. [Google Scholar] [CrossRef]
- Kimoto, S.; Oka, F.; Fushita, T. A chemo–thermo–mechanically coupled analysis of ground deformation induced by gas hydrate dissociation. Int. J. Mech. Sci. 2010, 52, 365–376. [Google Scholar] [CrossRef]
- Li, Q.; Liu, J.; Wang, S.; Guo, Y.; Han, X.; Li, Q.; Cheng, Y.; Dong, Z.; Li, X.; Zhang, X. Numerical insights into factors affecting collapse behavior of horizontal wellbore in clayey silt hydrate-bearing sediments and the accompanying control strategy. Ocean Eng. 2024, 297, 117029. [Google Scholar] [CrossRef]
- Wang, T.; Ding, Y.; Wang, R.; Qian, A.; Lu, H.; Zhou, B. Discrete element modeling of the effect of hydrate distribution heterogeneity on the mechanical behavior of cemented hydrate-bearing sediments. J. Mar. Sci. Eng. 2023, 11, 831. [Google Scholar] [CrossRef]
- Li, Y.; Wu, P.; Sun, X.; Liu, W.; Song, Y. Mechanical behaviors of hydrate-bearing sediment with different cementation spatial distributions at microscales. Iscience 2021, 24, 102448. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, L.; Ning, F.; Lei, H.; Liu, T.; Hu, G.; Lu, H.; Lu, J.; Liu, C.; Jiang, G. Production potential and stability of hydrate-bearing sediments at the site GMGS3-W19 in the South China Sea: A preliminary feasibility study. Mar. Pet. Geol. 2017, 86, 447–473. [Google Scholar] [CrossRef]
- Zhou, S.; Li, Q.; Zhu, J.; Gu, Q.; Wang, L.; Wu, Z. Experimental Study on Permeability of Methane Hydrate Clayey Interbedded Sediments Considering Effective Stress and Hydrate Dissociation. Energy Fuels 2023, 37, 11113–11124. [Google Scholar] [CrossRef]
- Sun, T.; Wen, Z.; Yang, J. Research on Wellbore Stability in Deepwater Hydrate-Bearing Formations during Drilling. Energies 2024, 17, 823. [Google Scholar] [CrossRef]
- Yan, C.; Li, Y.; Yan, X.; Cheng, Y.; Han, Z.; Tian, W.; Ren, X. Wellbore shrinkage during drilling in methane hydrate reservoirs. Energy Sci. Eng. 2019, 7, 930–942. [Google Scholar] [CrossRef]
- Lu, J.; Xiong, Y.; Li, D.; Shen, X.; Wu, Q.; Liang, D. Experimental investigation of characteristics of sand production in wellbore during hydrate exploitation by the depressurization method. Energies 2018, 11, 1673. [Google Scholar] [CrossRef]
- Song, J.; Fu, J.; Xiong, Y.; Pang, W.; He, Y.; Liu, L.; Huang, T.; Liu, C.; Li, Y.; Li, J. State-of-the-art brief review on sanding problem of offshore natural gas hydrates sediments. Energy Sci. Eng. 2022, 10, 253–273. [Google Scholar] [CrossRef]
- Wu, N.; Li, Y.; Chen, Q.; Liu, C.; Jin, Y.; Tan, M.; Dong, L.; Hu, G. Sand production management during marine natural gas hydrate exploitation: Review and an innovative solution. Energy Fuels 2021, 35, 4617–4632. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, L.; Zhang, R.; Ren, S. Assessment of natural gas production from hydrate-bearing sediments with unconsolidated argillaceous siltstones via a controlled sandout method. Energy 2018, 160, 654–667. [Google Scholar] [CrossRef]
- Mishra, S.; Ojha, K. Chemical Sand Consolidation: An Overview. J. Pet. Eng. Technol. 2015, 5, 21–34. [Google Scholar]
- Yamamoto, K.; Terao, Y.; Fujii, T.; Ikawa, T.; Seki, M.; Matsuzawa, M.; Kanno, T. Operational overview of the first offshore production test of methane hydrates in the Eastern Nankai Trough. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 5–8 May 2014; p. 25243. [Google Scholar]
- Qiu, K.; Yamamoto, K.; Birchwood, R.; Chen, Y. Well-integrity evaluation for methane-hydrate production in the deepwater Nankai Trough. SPE Drill. Complet. 2015, 30, 52–67. [Google Scholar] [CrossRef]
- Contreras Puerto, O. Wellbore Strengthening by Means of Nanoparticle-Based Drilling Fluids. Ph.D. Thesis, University of Calgary, Calgary, AB, Canada, 2014. [Google Scholar]
- Li, Z.-H.; Yu, J.-X.; Wang, Y.; Li, X.-S. Experimental Investigation on Deformation of Natural Gas Hydrate in the Process of Decompressing. Energy Procedia 2019, 158, 5510–5516. [Google Scholar] [CrossRef]
- Wang, J.; He, Z.; Yan, Y.; Liu, L.; Yan, J.; Liao, B.; Zhao, K.; Li, Y.; Chen, L. Development of a dual-functional inhibitor for natural gas hydrates and construction of drilling fluid system. Gas Sci. Eng. 2024, 122, 205218. [Google Scholar] [CrossRef]
- Lal, B.; Bavoh, C.B.; Ofei, T.N. Hydrates Drilling Muds Rheological Properties. In Hydrate Control in Drilling Mud; Springer: Cham, Switzerland, 2022; pp. 91–114. [Google Scholar]
- Kelland, M.A. History of the development of low dosage hydrate inhibitors. Energy Fuels 2006, 20, 825–847. [Google Scholar] [CrossRef]
- Koh, C.; Westacott, R.E.; Zhang, W.; Hirachand, K.; Creek, J.; Soper, A. Mechanisms of gas hydrate formation and inhibition. Fluid Phase Equilibria 2002, 194, 143–151. [Google Scholar] [CrossRef]
- Kumar, A.; Palodkar, A.V.; Gautam, R.; Choudhary, N.; Veluswamy, H.P.; Kumar, S. Role of salinity in clathrate hydrate based processes. J. Nat. Gas Sci. Eng. 2022, 108, 104811. [Google Scholar] [CrossRef]
- Sa, J.-H.; Kwak, G.-H.; Han, K.; Ahn, D.; Lee, K.-H. Gas hydrate inhibition by perturbation of liquid water structure. Sci. Rep. 2015, 5, 11526. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Chen, M.; Pang, W.; Xu, Z.; Liu, Z.; Wen, H.; Lei, X. Experimental and Modeling Study on Methane Hydrate Equilibrium Conditions in the Presence of Inorganic Salts. Molecules 2024, 29, 3702. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Lv, X.; Ge, K.; Jiang, Z.; Li, Y. Experimental measurement and thermodynamic modeling of methane hydrate phase equilibria in the presence of chloride salts. Chem. Eng. J. 2020, 395, 125126. [Google Scholar] [CrossRef]
- Kim, H.; Veluswamy, H.P.; Seo, Y.; Linga, P. Morphology Study on the Effect of Thermodynamic Inhibitors during Methane Hydrate Formation in the Presence of NaCl. Cryst. Growth Des. 2018, 18, 6984–6994. [Google Scholar] [CrossRef]
- Davoodi, S.; Al-Shargabi, M.; Wood, D.A.; Rukavishnikov, V.S.; Minaev, K.M. Thermally stable and salt-resistant synthetic polymers as drilling fluid additives for deployment in harsh sub-surface conditions: A review. J. Mol. Liq. 2023, 371, 121117. [Google Scholar] [CrossRef]
- Du, C.Y.; Guerra, A.; McElligott, A.; Maric, M.; Rey, A.; Servio, P. Effects of Poly (styrene/Pentafluorostyrene-block-vinylpyrrolidone) Amphiphilic Kinetic Hydrate Inhibitors on the Dynamic Viscosity of Methane Hydrate Systems at High-Pressure Driving Forces. Ind. Eng. Chem. Res. 2023, 62, 11795–11804. [Google Scholar] [CrossRef]
- Aminnaji, M.; Tohidi, B.; Burgass, R.; Atilhan, M. Effect of injected chemical density on hydrate blockage removal in vertical pipes: Use of MEG/MeOH mixture to remove hydrate blockage. J. Nat. Gas Sci. Eng. 2017, 45, 840–847. [Google Scholar] [CrossRef]
- Keshavarz, L.; Ghaani, M.R.; English, N.J. Thermodynamic evaluation of inhibitors for methane-hydrate formation. Fuel 2022, 324, 124672. [Google Scholar] [CrossRef]
- Nasir, Q.; Suleman, H.; Elsheikh, Y.A. A review on the role and impact of various additives as promoters/inhibitors for gas hydrate formation. J. Nat. Gas Sci. Eng. 2020, 76, 103211. [Google Scholar] [CrossRef]
- Tabaaza, G.A.; Haq, I.U.; Zain, D.B.; Lal, B. Toxicological issues of conventional gas hydrate inhibitors. Process Saf. Prog. 2022, 41, S135–S140. [Google Scholar] [CrossRef]
- Shahnazar, S.; Bagheri, S.; Termeh Yousefi, A.; Mehrmashhadi, J.; Karim, M.; Kadri, N.A. Structure, mechanism, and performance evaluation of natural gas hydrate kinetic inhibitors. Rev. Inorg. Chem. 2018, 38, 1–19. [Google Scholar] [CrossRef]
- Aminnaji, M.; Anderson, R.; Hase, A.; Tohidi, B. Can kinetic hydrate inhibitors inhibit the growth of pre-formed gas hydrates? Gas Sci. Eng. 2023, 109, 104831. [Google Scholar] [CrossRef]
- Yang, C.; Zi, M.; Wu, G.; Zou, X.; Liu, K.; Chen, D. Concentration effect of kinetic hydrate inhibitor on hydrate formation and inhibition. Fuel 2022, 323, 124448. [Google Scholar] [CrossRef]
- Zhang, Q.; Cai, W.; Li, Z.; Lu, H. Insights into behaviors of guest and host molecules in methane hydrate formation process in the presence of kinetic inhibitors via in-situ micro-Raman spectroscopy. Fuel 2024, 358, 130195. [Google Scholar] [CrossRef]
- Kelland, M.A. A review of kinetic hydrate inhibitors from an environmental perspective. Energy Fuels 2018, 32, 12001–12012. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, W.; Chen, L.; Li, K.; Ge, Y.; Li, J. Investigation on the Adhesion Force between Tetrabutylammonium Bromide Hydrate Particles Using Atomic Force Microscopy. Langmuir 2024, 40, 20848–20858. [Google Scholar] [CrossRef]
- Kang, S.-P.; Lee, D.; Lee, J.-W. Anti-Agglomeration Effects of Biodegradable Surfactants from Natural Sources on Natural Gas Hydrate Formation. Energies 2020, 13, 1107. [Google Scholar] [CrossRef]
- Liu, K.; Zi, M.; Zou, X.; Yao, Y.; Wang, Y.; Yang, C.; Chen, D. Determining the critical time points for hydrate formation in the presence of kinetic hydrate inhibitors: A rheological and kinetic study. Fuel 2024, 376, 132702. [Google Scholar] [CrossRef]
- Khabibullin, T.; Falcone, G.; Teodoriu, C. Drilling through gas-hydrate sediments: Managing wellbore-stability risks. SPE Drill. Complet. 2011, 26, 287–294. [Google Scholar] [CrossRef]
- Rutqvist, J.; Moridis, G.; Grover, T.; Silpngarmlert, S.; Collett, T.; Holdich, S. Coupled multiphase fluid flow and wellbore stability analysis associated with gas production from oceanic hydrate-bearing sediments. J. Pet. Sci. Eng. 2012, 92, 65–81. [Google Scholar] [CrossRef]
- Atta, A.M.; Ghiaty, E.A.; Shafek, S.H.; El-Segaey, A.A.; Gaffer, A.K. Application of new tetra-cationic imidazolium ionic liquids for capture and conversion of CO2 to amphiphilic calcium carbonate nanoparticles as a green additive in water based drilling fluids. J. Environ. Sci. 2025, 150, 159–176. [Google Scholar] [CrossRef]
- Bai, X.; Yong, X.; Koutsos, V.; Deng, L.; Li, K.; Zhou, Y.; Luo, Y. Dispersive and filter loss performance of calcium carbonate nanoparticles in water for drilling fluid applications. Nanotechnology 2021, 32, 485704. [Google Scholar] [CrossRef]
- Xia, P.; Pan, Y. Effects of nanosilica on the properties of brine-base drilling fluid. Sci. Rep. 2023, 13, 20462. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhou, X.; Shi, L.; Long, Z.; Lu, J.; Liang, D. Study on the hydrate inhibition effect of nano-silica in drilling fluids. J. Nat. Gas Sci. Eng. 2022, 105, 104688. [Google Scholar] [CrossRef]
- An, Y.; Jiang, G.; Qi, Y.; Huang, X.; Shi, H. High-performance shale plugging agent based on chemically modified graphene. J. Nat. Gas Sci. Eng. 2016, 32, 347–355. [Google Scholar] [CrossRef]
- Abdullah, A.H.; Ridha, S.; Mohshim, D.F.; Yusuf, M.; Kamyab, H.; Krishna, S.; Maoinser, M.A. A comprehensive review of nanoparticles: Effect on water-based drilling fluids and wellbore stability. Chemosphere 2022, 308, 136274. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Li, D.; Su, N.; Xie, G.; Wu, L.; Gu, D.; Zhai, Y.; Dai, F.; Luo, P. Study on the preparation and mechanism of two-dimensional nanomaterials as plugging agents for oil-based drilling fluids. Colloids Surf. A Physicochem. Eng. Asp. 2024, 702, 134676. [Google Scholar] [CrossRef]
- Yang, L.; Ou, Z.; Jiang, G. Research progress of elastomer materials and application of elastomers in drilling fluid. Polymers 2023, 15, 918. [Google Scholar] [CrossRef]
- Houton, K.A.; Burslem, G.M.; Wilson, A.J. Development of solvent-free synthesis of hydrogen-bonded supramolecular polyurethanes. Chem. Sci. 2015, 6, 2382–2388. [Google Scholar] [CrossRef]
- Huang, X.; Sun, J.; Lv, K.; Liu, J.; Shen, H.; Zhang, F. Application of core-shell structural acrylic resin/nano-SiO2 composite in water based drilling fluid to plug shale pores. J. Nat. Gas Sci. Eng. 2018, 55, 418–425. [Google Scholar] [CrossRef]
- Meng, X.; Huang, X.; Sun, J.; Lv, K.; Li, H.; Wang, Z.; Yuan, Z. Ultra-high temperature resistant polystyrene/acrylic resin microspheres for enhanced wellbore stability of oil-based drilling fluids. Colloids Surf. A Physicochem. Eng. Asp. 2025, 725, 137637. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, J.; Wang, R.; Liu, F.; Cheng, R.; Qu, Y.; Hao, H.; Bai, Y.; Li, Y.; Geng, Y. Shape memory resin with high temperature resistance for plugging fracture formations drilled with oil-based drilling fluid. Geoenergy Sci. Eng. 2024, 243, 213355. [Google Scholar] [CrossRef]
- Li, X.; Pu, C.; Wei, H.; Huang, F.; Bai, Y.; Zhang, C. Enhanced oil recovery in fractured low-permeability reservoirs by a novel gel system prepared by sustained-release crosslinker and water-soluble thixotropic polymer. Geoenergy Sci. Eng. 2023, 222, 211424. [Google Scholar] [CrossRef]
- Qiao, L.; Liu, C.; Liu, C.; Hu, F.; Li, X.; Wang, C.; Jian, X. Study on new polymer crosslinking agents and their functional hydrogels. Eur. Polym. J. 2020, 134, 109835. [Google Scholar] [CrossRef]
- Merey, Ş. Evaluation of drilling parameters in gas hydrate exploration wells. J. Pet. Sci. Eng. 2019, 172, 855–877. [Google Scholar] [CrossRef]
- Villada, Y.; Giraldo, L.J.; Cardona, C.; Estenoz, D.; Rosero, G.; Lerner, B.; Pérez, M.S.; Riazi, M.; Franco, C.A.; Córtes, F.B. Synergistic effect of nanoparticles and viscoelastic surfactants to improve properties of drilling fluids. Pet. Sci. 2024, 21, 4391–4404. [Google Scholar] [CrossRef]
- Zhao, X.; Qiu, Z.; Zhou, G.; Huang, W. Synergism of thermodynamic hydrate inhibitors on the performance of poly (vinyl pyrrolidone) in deepwater drilling fluid. J. Nat. Gas Sci. Eng. 2015, 23, 47–54. [Google Scholar] [CrossRef]
- Liu, K.; Qu, Y.; Zhang, D.; Liang, B.; Yan, L. The potential of the synergistic use of SiO2@Silane-PEG with molybdenum dialkyldithiocarbamate in water-based drilling fluid. Colloids Surf. A Physicochem. Eng. Asp. 2025, 709, 136101. [Google Scholar] [CrossRef]
- Wang, J.; Yao, Z.; Gong, R.; Guo, P.; Jiang, H.; Wang, Y. Comprehensive Study of Synergistic-Dual-Functional Mechanism of Kinetic and Thermodynamic Inhibitors in a Hydrate Drilling Fluid System. Energy Fuels 2023, 37, 15784–15794. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, R.; Sun, J.; Sun, J.; Lu, C.; Lv, K.; Wang, J.; Wang, J.; Yang, J.; Qu, Y. Effect of Drilling Fluid Invasion on Natural Gas Hydrate Near-Well Reservoirs Drilling in a Horizontal Well. Energies 2021, 14, 7075. [Google Scholar] [CrossRef]
- Morenov, V.; Leusheva, E.; Liu, T. Development of a weighted barite-free formate drilling mud for well construction under complicated conditions. Polymers 2021, 13, 4457. [Google Scholar] [CrossRef]
- Sun, W.-J.; Tian, G.-Q.; Huang, H.-J.; Lu, G.-M.; Ke, C.-Y.; Hui, J.-F.; Zhang, X.-L. Synthesis and characterisation of a multifunctional oil-based drilling fluid additive. Environ. Earth Sci. 2018, 77, 793. [Google Scholar] [CrossRef]
- Di, W.; Zhao, C.; Geng, T.; Sun, Q.; Xu, Z.; Sun, D. Probing the state of water in oil-based drilling fluids. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129770. [Google Scholar] [CrossRef]
- Peixoto, R.D.; Bicudo, T.C.; de A. Moura, H.O.M.; Sousa, A.S.; de Carvalho, L.S. Synthesis of decyl methyl carbonate and a comparative assessment of its performance as the continuous phase of synthetic-based drilling fluids. J. Pet. Sci. Eng. 2021, 199, 108301. [Google Scholar] [CrossRef]
- Ye, J.-L.; Qin, X.-W.; Xie, W.-W.; Lu, H.-L.; Ma, B.-J.; Qiu, H.-J.; Liang, J.-Q.; Lu, J.-A.; Kuang, Z.-G.; Lu, C. The second natural gas hydrate production test in the South China Sea. China Geol. 2020, 3, 197–209. [Google Scholar] [CrossRef]
- Research Consortium for Methane Hydrate Resources in Japan. Japan’s Methane Hydrate R & D Program: Phase 1: Comprehensive Report of Research Results [R/OL]. 2019. Available online: https://www.mh21japan.gr.jp/mh21wp/wp-content/uploads/phase1_200808_english.pdf (accessed on 15 July 2025).
- Echt, T.; Plank, J. An improved test protocol for high temperature carrying capacity of drilling fluids exemplified on a sepiolite mud. J. Nat. Gas Sci. Eng. 2019, 70, 102964. [Google Scholar] [CrossRef]
- Kim, J.-H.; Torres, M.E.; Hong, W.-L.; Choi, J.; Riedel, M.; Bahk, J.-J.; Kim, S.-H. Pore fluid chemistry from the Second Gas Hydrate Drilling Expedition in the Ulleung Basin (UBGH2): Source, mechanisms and consequences of fluid freshening in the central part of the Ulleung Basin, East Sea. Mar. Pet. Geol. 2013, 47, 99–112. [Google Scholar] [CrossRef]
- Li, P.; Fan, Z.; Li, M.; Zhao, L.; Wang, D. Long-term performance and security of gas production for horizontal-well depressurization exploitation: Insights from a coupled thermo-hydro-mechanical-chemical model for the Shenhu hydrate reservoir. Energy Fuels 2023, 37, 14824–14835. [Google Scholar] [CrossRef]
- Uchida, S.; Klar, A.; Yamamoto, K. Sand production modeling of the 2013 Nankai offshore gas production test. In Proceedings of the International Conference on Energy Geotechnics, Kiel, Germany, 29–31 August 2016. [Google Scholar]
- Koji, Y. Gas Hydrate Drilling in the Nankai Trough, Japan. In World Atlas of Submarine Gas Hydrates in Continental Margins; Springer: Cham, Switzerland, 2022; pp. 183–194. [Google Scholar]
- Naeiji, P.; Varaminian, F.; Rahmati, M. The kinetic modeling of methane hydrate growth by using molecular dynamic simulations. Int. J. Heat Mass Transf. 2019, 142, 118356. [Google Scholar] [CrossRef]
- Castillo-Borja, F.; Bravo-Sánchez, U.I. Molecular Dynamics simulation study of the performance of different inhibitors for methane hydrate growth. J. Mol. Liq. 2021, 337, 116510. [Google Scholar] [CrossRef]
- Shao, Z.; Wang, J.; Lv, K.; Liao, B.; Wang, Z.; Bai, Y.; Wang, R.; Sun, J. Experimental and molecular dynamics studies of zwitterionic inhibitors of methane hydrate dissociation. Fuel 2022, 318, 123059. [Google Scholar] [CrossRef]
- Du, Q.-S.; Liu, P.-J.; Deng, J. Empirical Correction to Molecular Interaction Energies in Density Functional Theory (DFT) for Methane Hydrate Simulation. J. Chem. Theory Comput. 2007, 3, 1665–1672. [Google Scholar] [CrossRef]
- Guo, P.; Qiu, Y.-L.; Li, L.-L.; Luo, Q.; Zhao, J.-F.; Pan, Y.-K. Density functional theory study of structural stability for gas hydrate. Chin. Phys. B 2018, 27, 043103. [Google Scholar] [CrossRef]
- Uchida, T.; Yamazaki, K.; Gohara, K. Gas nanobubbles as nucleation acceleration in the gas-hydrate memory effect. J. Phys. Chem. C 2016, 120, 26620–26629. [Google Scholar] [CrossRef]
- Tanaka, H.; Nakatsuka, T.; Koga, K. On the thermodynamic stability of clathrate hydrates IV: Double occupancy of cages. J. Chem. Phys. 2004, 121, 5488–5493. [Google Scholar] [CrossRef]
- Koyama, Y.; Tanaka, H.; Koga, K. On the thermodynamic stability and structural transition of clathrate hydrates. J. Chem. Phys. 2005, 122, 074503. [Google Scholar] [CrossRef]
- Katsumasa, K.; Koga, K.; Tanaka, H. On the thermodynamic stability of hydrogen clathrate hydrates. J. Chem. Phys. 2007, 127, 044509. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Koga, K.; Tanaka, H. Augmented stability of hydrogen clathrate hydrates by weakly polar molecules. J. Chem. Phys. 2009, 131, 214506. [Google Scholar] [CrossRef]
- English, N.J.; Clarke, E.T. Molecular dynamics study of CO2 hydrate dissociation: Fluctuation-dissipation and non-equilibrium analysis. J. Chem. Phys. 2013, 139, 094701. [Google Scholar] [CrossRef] [PubMed]
- Sarupria, S.; Debenedetti, P.G. Molecular dynamics study of carbon dioxide hydrate dissociation. J. Phys. Chem. A 2011, 115, 6102–6111. [Google Scholar] [CrossRef]
- Kondori, J.; Zendehboudi, S.; James, L. New insights into methane hydrate dissociation: Utilization of molecular dynamics strategy. Fuel 2019, 249, 264–276. [Google Scholar] [CrossRef]
- Yan, K.-F.; Zhao, J.-Y.; Chen, H.; Li, X.-S.; Xu, C.-G.; Chen, Z.-Y.; Zhang, Y.; Wang, Y.; Feng, J.-C.; Yu, Y.-S. Exploring hydration mechanism of salt ions on the methane hydrate formation: Insights from experiments, QM calculations and MD simulations. Chem. Eng. Sci. 2023, 276, 118829. [Google Scholar] [CrossRef]
- Cheng, L.; Cui, J.; Li, Z.; Liu, B.; Ban, S.; Chen, G. Molecular dynamics simulation of the formation of methane hydrates in the presence of KHIs. Chem. Eng. Sci. 2021, 236, 116508. [Google Scholar] [CrossRef]
- Sasaki, K.; Ono, S.; Sugai, Y.; Ebinuma, T.; Narita, H.; Yamaguchi, T. Gas production system from methane hydrate layers by hot water injection using dual horizontal wells. J. Can. Pet. Technol. 2009, 48, 21–26. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Komai, T.; Kawamura, T.; Minagawa, H.; Tenma, N.; Yamaguchi, T. Laboratory-scale experiment of methane hydrate dissociation by hot-water injection and numerical analysis for permeability estimation in reservoir: Part 1-Numerical study for estimation of permeability in methane hydrate reservoir. Int. J. Offshore Pol. Eng. 2007, 17, 47–56. [Google Scholar]
- Raymond, L. Temperature distribution in a circulating drilling fluid. J. Pet. Technol. 1969, 21, 333–341. [Google Scholar] [CrossRef]
- Rutqvist, J.; Moridis, G.J. Numerical Studies on the Geomechanical Stability of Hydrate-Bearing Sediments. SPE J. 2009, 14, 267–282. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, C.; Jing, L. Modeling coupled THM processes of geological porous media with multiphase flow: Theory and validation against laboratory and field scale experiments. Comput. Geotech. 2009, 36, 1308–1329. [Google Scholar] [CrossRef]
- Wang, W.; Kosakowski, G.; Kolditz, O. A parallel finite element scheme for thermo-hydro-mechanical (THM) coupled problems in porous media. Comput. Geosci. 2009, 35, 1631–1641. [Google Scholar] [CrossRef]
- Liu, X.; Qu, Z.; Guo, T.; Sun, Y.; Rabiei, M.; Liao, H. A coupled thermo-hydrologic-mechanical (THM) model to study the impact of hydrate phase transition on reservoir damage. Energy 2021, 216, 119222. [Google Scholar] [CrossRef]
- Huang, J.; Jiang, M.; Song, S.; Wang, H. A semi-analytical model considering two-phase flow and full thermal–hydraulic–mechanical (THM) coupling for the mechanical response of formation in hydrate production. Geoenergy Sci. Eng. 2025, 252, 213943. [Google Scholar] [CrossRef]
- Yuan, Y.; Gong, Y.; Xu, T.; Zhu, H. Multiphase flow and geomechanical responses of interbedded hydrate reservoirs during depressurization gas production for deepwater environment. Energy 2023, 262, 125603. [Google Scholar] [CrossRef]
- Dhakal, S.; Gupta, I. Gas Hydrates Reserve Characterization Using Thermo-Hydro-Mechanical Numerical Simulation: A Case Study of Green Canyon 955, Gulf of Mexico. Energies 2023, 16, 3275. [Google Scholar] [CrossRef]
- Zhang, N.; Luo, Z.; Chen, Z.; Liu, F.; Liu, P.; Chen, W.; Wu, L.; Zhao, L. Thermal–hydraulic–mechanical–chemical coupled processes and their numerical simulation: A comprehensive review. Acta Geotech. 2023, 18, 6253–6274. [Google Scholar] [CrossRef]
- He, Y.; Huang, C.; Yan, W.; Zhang, S. A fully coupled multi-physics THCM model for the marine hydrate-bearing sediment. Eng. Comput. 2025. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, F.; Xu, C.; Han, Y. Stability analysis of near-wellbore reservoirs considering the damage of hydrate-bearing sediments. J. Mar. Sci. Eng. 2019, 7, 102. [Google Scholar] [CrossRef]
- Li, P.; Fan, Z.; Zhao, L.; Li, M.; Yang, C.; He, K.; Wang, D. Mechanistic Understanding of Sand Production in Marine Hydrate Exploitation: Insights from Field Tests and THMC Simulations. Energy Fuels 2024, 38, 12730–12740. [Google Scholar] [CrossRef]
- Paull, C.; Reeburgh, W.S.; Dallimore, S.R.; Enciso, G.; Koh, C.A.; Kvenvolden, K.A.; Mankin, C.; Riedel, M. Realizing the Energy Potential of Methane Hydrate for the United States; Committee on Assessment of the Department of Energy’s Methane Hydrate Research and Development Program: Evaluating Methane Hydrate as a Future Energy Resource; National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- Uchida, T.; Kvamme, B.; Coffin, R.B.; Tenma, N.; Oyama, A.; Masutani, S.M. Review of fundamental properties of gas hydrates: Breakout sessions of the international workshop on methane hydrate research and development. Energies 2017, 10, 747. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, B.; Li, Q.; Hao, Q.; Zhang, H.; Nie, Y. A fully coupled thermal–hydro–mechanical–chemical model for simulating gas hydrate dissociation. Appl. Math. Model. 2024, 129, 88–111. [Google Scholar] [CrossRef]
- Tsimpanogiannis, I.N.; Economou, I.G. Monte Carlo simulation studies of clathrate hydrates: A review. J. Supercrit. Fluids 2018, 134, 51–60. [Google Scholar] [CrossRef]
- Nakajima, C.; Ouchi, H.; Tamaki, M.; Akamine, K.; Sato, M.; Ohtsuki, S.; Naiki, M. Sensitivity and Uncertainty Analysis for Natural Gas Hydrate Production Tests in Alaska. Energy Fuels 2022, 36, 7434–7455. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, X.; Wang, Z.; Liang, W.; Zhao, T. THMC Fully Coupled Model of Natural Gas Hydrate under Damage Effect and Parameter Sensitivity Analysis. J. Mar. Sci. Eng. 2023, 11, 612. [Google Scholar] [CrossRef]
- Zhao, X.; Geng, Q.; Zhang, Z.; Qiu, Z.; Fang, Q.; Wang, Z.; Yan, C.; Ma, Y.; Li, Y. Phase change material microcapsules for smart temperature regulation of drilling fluids for gas hydrate reservoirs. Energy 2023, 263, 125715. [Google Scholar] [CrossRef]
- Bae, Y.W.; Kim, J.; Ju, S.H.; Shin, K.; Cheong, I.W. Highly Efficient Recovery of Water-Soluble Polymers in Synergistic Kinetic Inhibition of Gas Hydrate Formation. ACS Appl. Polym. Mater. 2019, 1, 130–135. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, J.; Huang, X.; Lv, K.; Geng, Y. A temperature-sensitive polymer with thinner effect as a rheology modifier in deepwater water-based drilling fluids. J. Mol. Liq. 2024, 393, 123536. [Google Scholar] [CrossRef]
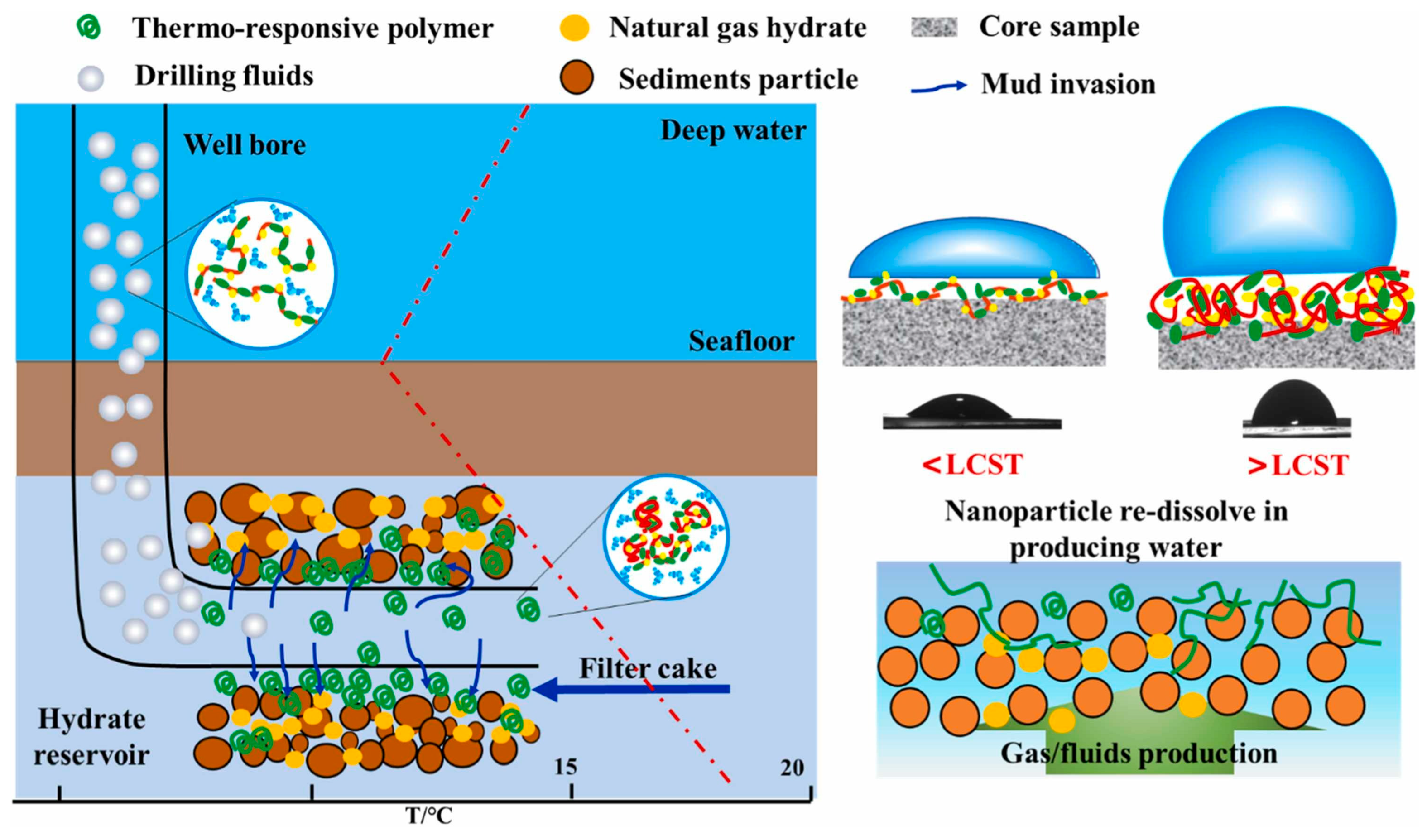
- Li, X.; Huang, W.; Zhen, Z.; Sun, J.; Wang, Z.; Maeda, N. Preparation of thermo-responsive polymer and its application for plugging in hydrate-bearing sediments. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132210. [Google Scholar] [CrossRef]
- Zamora-Ledezma, C.; Narváez-Muñoz, C.; Almeida-Arellano, F.A.; Voiry, D.; Salameh, C.; Medina, E.; Carrión-Matamoros, L.M.; Navas-León, D.G.; Vizuete, K.; Debut, A. Vermiculite and graphene oxide 2D layered nanoparticle for improving rheology and filtration in water-based drilling fluids formulations. Results Eng. 2025, 26, 104796. [Google Scholar] [CrossRef]
- Tian, Y.; Altalbawy, F.M.; Rachchh, N.; Ramachandran, T.; Shankhyan, A.; Karthikeyan, A.; Thatoi, D.N.; Gupta, D.; Norberdiyeva, M.; Al-Badkubi, M.R. Leveraging a novel nanocomposite for enhanced drilling fluid efficiency. Sci. Rep. 2025, 15, 28304. [Google Scholar] [CrossRef]
- Chang, I.; Lee, M.; Tran, A.T.P.; Lee, S.; Kwon, Y.-M.; Im, J.; Cho, G.-C. Review on biopolymer-based soil treatment (BPST) technology in geotechnical engineering practices. Transp. Geotech. 2020, 24, 100385. [Google Scholar] [CrossRef]
- Li, X.; Jiang, G.; He, Y.; Chen, G. Novel Starch Composite Fluid Loss Additives and Their Applications in Environmentally Friendly Water-Based Drilling Fluids. Energy Fuels 2021, 35, 2506–2513. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, Z.; Wang, A.; Wang, C.; Qu, J.; Chen, B.; Wei, B.; Kapu, N.S.; Wen, Y. Cellulose nanofibril-polymer hybrids for protecting drilling fluid at high salinity and high temperature. Carbohydr. Polym. 2020, 229, 115465. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Sun, J.; Xu, Z.; Lv, K.; Dai, Z.; Zhang, F.; Huang, X.; Liu, J. Synthesis of a novel environment-friendly filtration reducer and its application in water-based drilling fluids. Colloids Surf. A Physicochem. Eng. Asp. 2019, 568, 284–293. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Z.; Wang, Z.; Dai, X.; Chen, M.; Zhang, J. Novel lignosulfonate/N, N-dimethylacrylamide/γ-methacryloxypropyl trimethoxy silane graft copolymer as a filtration reducer for water-based drilling fluids. J. Appl. Polym. Sci. 2020, 137, 48274. [Google Scholar] [CrossRef]
- Zhong, H.; Shen, G.; Yang, P.; Qiu, Z.; Jin, J.; Xing, X. Mitigation of lost circulation in oil-based drilling fluids using oil absorbent polymers. Materials 2018, 11, 2020. [Google Scholar] [CrossRef]
- Nazemi, R.; Zargar, G.; Nooripoor, V. Experimental investigation of deformable additives as loss circulation control agent during drilling and well construction. Sci. Rep. 2024, 14, 30423. [Google Scholar] [CrossRef]
- Elkatatny, S.; Ahmed, A.; Abughaban, M.; Patil, S. Deep illustration for loss of circulation while drilling. Arab. J. Sci. Eng. 2020, 45, 483–499. [Google Scholar] [CrossRef]
- Saumya, S.; Narasimhan, B.; Singh, J.; Yamamoto, H.; Vij, J.; Sakiyama, N.; Kumar, P. Acquisition of Logging-While-Drilling (LWD) Multipole Acoustic log Data during the India National Gas Hydrate Program (NGHP) Expedition 02. Mar. Pet. Geol. 2019, 108, 562–569. [Google Scholar] [CrossRef]
- Longo, J.P.N.; Galvão, J.R.; Antes, T.; dos Santos, E.N.; da Silva, J.C.C.; Martelli, C.; Sum, A.K.; Morales, R.E.M.; da Silva, M.J. Sensing Hydrates in Pipes by a Combined Electrical and Optical Fiber Sensor. IEEE Sens. J. 2020, 20, 5012–5018. [Google Scholar] [CrossRef]
- Yadav, U.S.; Shukla, K.M.; Ojha, M.; Kumar, P.; Shankar, U. Assessment of gas hydrate accumulations using velocities derived from vertical seismic profiles and acoustic log data in Krishna-Godavari Basin, India. Mar. Pet. Geol. 2019, 108, 551–561. [Google Scholar] [CrossRef]
- Pandey, L.; Sain, K. Joint inversion of resistivity and sonic logs using gradient descent method for gas hydrate saturation in the Krishna Godavari offshore basin, India. Mar. Geophys. Res. 2022, 43, 29. [Google Scholar] [CrossRef]
- Gooneratne, C.P.; Li, B.; Moellendick, T.E. Downhole Applications of Magnetic Sensors. Sensors 2017, 17, 2384. [Google Scholar] [CrossRef]
- Kang, D.; Lu, J.A.; Zhang, Z.; Liang, J.; Kuang, Z.; Lu, C.; Kou, B.; Lu, Q.; Wang, J. Fine-grained gas hydrate reservoir properties estimated from well logs and lab measurements at the Shenhu gas hydrate production test site, the northern slope of the South China sea. Mar. Pet. Geol. 2020, 122, 104676. [Google Scholar] [CrossRef]
- Yang, M.; Chong, Z.R.; Zheng, J.; Song, Y.; Linga, P. Advances in nuclear magnetic resonance (NMR) techniques for the investigation of clathrate hydrates. Renew. Sustain. Energy Rev. 2017, 74, 1346–1360. [Google Scholar] [CrossRef]
- Tian, L.; Li, Z.; Cao, Y.; Liu, S.; Song, Y. In Situ Stress Distribution and Variation Monitored by Microseismic Tracking on a Fractured Horizontal Well: A Case Study from the Qinshui Basin. ACS Omega 2022, 7, 14363–14370. [Google Scholar] [CrossRef]
- Martyushev, D.A.; Yang, Y.; Kazemzadeh, Y.; Wang, D.; Li, Y. Understanding the Mechanism of Hydraulic Fracturing in Naturally Fractured Carbonate Reservoirs: Microseismic Monitoring and Well Testing. Arab. J. Sci. Eng. 2024, 49, 8573–8586. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, X.; Ji, Y.; Wang, X. Acoustic Wave Propagation in a Borehole with a Gas Hydrate-Bearing Sediment. J. Mar. Sci. Eng. 2022, 10, 235. [Google Scholar] [CrossRef]
- Lee, H.; Lee, H.P. Formation lithology predictions based on measurement while drilling (MWD) using gradient boosting algorithms. Geoenergy Sci. Eng. 2023, 227, 211917. [Google Scholar] [CrossRef]
- Rashidi, B.; Hareland, G.; Tahmeen, M.; Anisimov, M.; Abdorazakov, S. Real-time bit wear optimization using the intelligent drilling advisory system. In Proceedings of the SPE Russian Petroleum Technology Conference, Moscow, Russia, 26–28 October 2010; p. SPE-136006. [Google Scholar]
- Eyvazi Farab, A.; Shahbazi, K.; Hashemi, A.; Shahbazi, A. Real time determination of casing resistance and wear in an Iranian oilfield based on MWD/LWD data. Pet. Sci. Technol. 2022, 40, 2193–2212. [Google Scholar] [CrossRef]
- Peñaranda, J.; Manchola, J.; Casado, G.; Kovarskiy, E.; Solar, G.; Campos, N.; Diaz, Y.; Hernandez, I.; Gamba, I.; Gutierrez, A. Novel Drilling Engineering Methodology to Enhance Wellbore Positioning and Performance Using Disruptive MWD/LWD Technology. In Proceedings of the SPE Latin America and Caribbean Petroleum Engineering Conference, Port of Spain, Trinidad and Tobago, 14–15 June 2023; p. SPE-213158. [Google Scholar]
- Wu, J.; Liu, W.; Lin, H.; Liu, H.; Peng, C. Application of Artificial Intelligence in Prediction of Wellbore Stability Using Well Logging and Drilling Data. In Proceedings of the 57th U.S. Rock Mechanics/Geomechanics Symposium, Atlanta, GA, USA, 25–28 June 2023; p. 0598. [Google Scholar]
- Gurina, E.; Klyuchnikov, N.; Antipova, K.; Koroteev, D. Forecasting the abnormal events at well drilling with machine learning. Appl. Intell. 2022, 52, 9980–9995. [Google Scholar] [CrossRef]
- Okoro, E.E.; Alaba, A.O.; Sanni, S.E.; Ekeinde, E.B.; Dosunmu, A. Development of an automated drilling fluid selection tool using integral geometric parameters for effective drilling operations. Heliyon 2019, 5, e01713. [Google Scholar] [CrossRef]
- Omomo, K.O.; Esiri, A.E.; Olisakwe, H.C. Hydraulic modeling and real-time optimization of drilling fluids: A future perspective. Glob. J. Res. Eng. Technol. 2024, 2, 030–038. [Google Scholar] [CrossRef]
- Xu, K.; Liu, Z.; Chen, Q.; Zhang, Q.; Ling, X.; Cai, X.; He, Q.; Yang, M. Application of machine learning in wellbore stability prediction: A review. Geoenergy Sci. Eng. 2024, 232, 212409. [Google Scholar] [CrossRef]
- Omomo, K.; Esiri, A.; Olisakwe, H. Towards an integrated model for predictive well control using real-time drilling fluid data. Glob. J. Res. Eng. Technol. 2024, 2, 001–010. [Google Scholar] [CrossRef]
- Davoodi, S.; Al-Shargabi, M.; Wood, D.A.; Mehrad, M. Advancement of artificial intelligence applications in hydrocarbon well drilling technology: A review. Appl. Soft Comput. 2025, 176, 113129. [Google Scholar] [CrossRef]

| Additive Category | Examples | Primary Function and Performance | Relative Cost | Environmental Impact | Challenges |
|---|---|---|---|---|---|
| Thermodynamic Inhibitors (THIs)–Inorganic Salts [113,114,115,116,117] | NaCl, KCl, CaCl2 | Strong inhibition: significantly shifts hydrate phase boundary. Performance is concentration-dependent. | Low | High: high-salinity brine is difficult to treat, corrosive, and can harm marine life upon discharge. | Highly corrosive to equipment; degrades polymer performance (salting-out); high concentration required. |
| Thermodynamic Inhibitors (THIs)–Alcohols/Glycols [118,119,120,121,122] | Methanol, MEG, glycerol | Strong inhibition: Effective water activity reduction. MEG is recyclable. | Moderate to high | Moderate to high: Methanol is toxic and volatile. Glycols have high BOD/COD, requiring costly wastewater treatment. | High dosage required (10–50 wt%); can affect fluid rheology; methanol poses safety risks. |
| Kinetic Hydrate Inhibitors (KHIs) [123,124,125,126] | PVP, PVCap | Delayed nucleation/growth: provides a “time window” of protection at low dosages (0.1–2 wt%). | High | Low to moderate: generally better biodegradability and lower toxicity than THIs. | Effective only under moderate subcooling (<12 °C); less effective against decomposition than formation; performance can be unpredictable. |
| Anti-Agglomerants (AAs) [129,130] | Quaternary ammonium salts (e.g., TBAB) | Prevents blockage: allows hydrate formation but keeps particles dispersed as a flowable slurry. | High | Moderate: some are surfactants with potential aquatic toxicity; requires careful selection. | Compatibility issues with anionic polymers in WBDFs; long-term stability under downhole conditions is uncertain; primarily used in pipelines. |
| Bridging/Sealing Agents (Conventional) [131,132] | CaCO3, sulfonated asphalt | Pore/fracture sealing: forms a filter cake to reduce permeability and fluid loss. | Low to moderate | Moderate: some materials like asphalt have environmental concerns. | Less effective in nano-pores; can cause formation damage if particle size is not matched; filter cake can be erosive. |
| Nanomaterials [133,134,135,136,137] | Nano-SiO2, nano-CaCO3, graphene | Deep sealing and strengthening: penetrates nano-pores for an ultra-low permeability seal; reinforces filter cake structure. | Very high | Uncertain/emerging concern: potential for bioaccumulation and long-term ecotoxicity is still under investigation. | High cost of production; difficult to disperse and maintain stability in high-salinity brines; potential health and environmental risks. |
| Project/Location | Year(s) | Drilling Fluid System | Key Functional Components | Performance | Challenges |
|---|---|---|---|---|---|
| Shenhu Area, South China Sea, China [157] | 2017, 2020 | Low-temperature, composite salt + glycol WBDF with active cooling | NaCl/KCl + glycol (thermodynamic inhibition); multi-modal particles (sealing); low-temperature polymers (rheology). | Successfully maintained wellbore stability for >60 days of continuous production testing; minimal hole enlargement observed. Proved the viability of the “active cooling” strategy. | High energy consumption and complex logistics associated with the surface cooling systems. |
| Nankai Trough, Japan [158] | 2013, 2017 | KCl/polymer WBDF | KCl (thermodynamic inhibition); Partially Hydrolyzed Polyacrylamide (PHPA) for shale stability; sepiolite for rheology control. | Enabled successful drilling, coring, and casing operations in hydrate-bearing intervals. | Encountered severe sand production during the depressurization production phase, leading to premature test termination. This highlights that drilling stability does not guarantee production stability. |
| Offshore India, National Gas Hydrate Program (NGHP) Expedition 02 [159] | 2015 | Seawater-based KCl/polymer WBDF | KCl (inhibition); glycols; polymers (PHPA, PAC); sized CaCO3 (bridging). | Successfully drilled and cored numerous sites in the Krishna-Godavari Basin, recovering high-quality pressure cores. Demonstrated effective hole cleaning and stability for scientific drilling. | The fluid was designed for short-term coring operations, its suitability for long-term production drilling was not tested. |
| Ulleung Basin, East Sea (Japan Sea), South Korea (UBGH2) [160] | 2010 | KCl/glycol/polymer WBDF | KCl + glycol (inhibition); polymers for rheology and filtration control. | Successfully completed logging-while-drilling (LWD) and coring operations in multiple wells, confirming hydrate presence. Maintained wellbore stability for scientific objectives. | Similar to other scientific expeditions, the system’s robustness for commercial-scale, long-duration drilling was not the primary focus. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Xiao, B.; Zhuang, G.; Li, Y.; Li, Q. Instability Mechanisms and Wellbore-Stabilizing Drilling Fluids for Marine Gas Hydrate Reservoirs: A Review. Energies 2025, 18, 4392. https://doi.org/10.3390/en18164392
Liu Q, Xiao B, Zhuang G, Li Y, Li Q. Instability Mechanisms and Wellbore-Stabilizing Drilling Fluids for Marine Gas Hydrate Reservoirs: A Review. Energies. 2025; 18(16):4392. https://doi.org/10.3390/en18164392
Chicago/Turabian StyleLiu, Qian, Bin Xiao, Guanzheng Zhuang, Yun Li, and Qiang Li. 2025. "Instability Mechanisms and Wellbore-Stabilizing Drilling Fluids for Marine Gas Hydrate Reservoirs: A Review" Energies 18, no. 16: 4392. https://doi.org/10.3390/en18164392
APA StyleLiu, Q., Xiao, B., Zhuang, G., Li, Y., & Li, Q. (2025). Instability Mechanisms and Wellbore-Stabilizing Drilling Fluids for Marine Gas Hydrate Reservoirs: A Review. Energies, 18(16), 4392. https://doi.org/10.3390/en18164392









