Integration of Aquaculture Wastewater Treatment and Chlorella vulgaris Cultivation as a Sustainable Method for Biofuel Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Study Organisation
2.2. Materials
2.2.1. Inoculum of the C. vulgaris Biomass
2.2.2. Characteristics of the Growth Media
2.2.3. Anaerobic Sludge Inoculum
2.3. Experimental Stands and Procedures
2.3.1. Continuous Cultivation of C. vulgaris
2.3.2. Biogas Potential of Microalgae Biomass
2.4. Analytical Methods
2.5. Statistical Analysis
3. Results and Discussion
3.1. Aquaculture Wastewater Treatment
3.2. Growth and Characterisation of the C. vulgaris Biomass
3.3. FAMEs Composition of the Algae Biomass
3.4. Biogas and Methane Potential of the Algae Biomass
4. Microalgae in Wastewater Treatment—Potential and Challenges
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ansari, F.A.; Singh, P.; Guldhe, A.; Bux, F. Microalgal Cultivation Using Aquaculture Wastewater: Integrated Biomass Generation and Nutrient Remediation. Algal Res. 2017, 21, 169–177. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Ahmad, A.; Imron, M.F.; Abdullah, S.R.S.; Othman, A.R.; Hasan, H.A. Achieving a Biocircular Economy in the Aquaculture Sector Through Waste Valorization. Toxics 2025, 13, 131. [Google Scholar] [CrossRef]
- Shitu, A.; Tadda, M.A.; Zhao, J.; Danhassan, U.A.; Ye, Z.; Liu, D.; Chen, W.; Zhu, S. Review of Recent Advances in Utilising Aquaculture Wastewater for Algae Cultivation and Microalgae-Based Bioproduct Recovery. Environ. Geochem. Health 2024, 46, 485. [Google Scholar] [CrossRef]
- Sarker, N.K.; Kaparaju, P. Microalgal Bioeconomy: A Green Economy Approach Towards Achieving Sustainable Development Goals. Sustainability 2024, 16, 11218. [Google Scholar] [CrossRef]
- Simionov, I.A.; Barbu, M.; Vasiliev, I.; Condrachi, L.; Titica, M.; Ifrim, G.; Cristea, D.; Nuță, F.M.; Petrea, Ș.M. Prospective Technical and Technological Insights into Microalgae Production Using Aquaculture Wastewater Effluents. J. Environ. Manag. 2025, 377, 124537. [Google Scholar] [CrossRef]
- Rocha, C.P.; Cabral, H.N.; Marques, J.C.; Gonçalves, A.M.M. A Global Overview of Aquaculture Food Production with a Focus on the Activity’s Development in Transitional Systems—The Case Study of a South European Country (Portugal). J. Mar. Sci. Eng. 2022, 10, 417. [Google Scholar] [CrossRef]
- FAO Report: Global Fisheries and Aquaculture Production Reaches a New Record High. Available online: https://www.fao.org/newsroom/detail/fao-report-global-fisheries-and-aquaculture-production-reaches-a-new-record-high/en (accessed on 13 July 2025).
- Velenturf, A.P.M.; Purnell, P. Principles for a Sustainable Circular Economy. Sustain. Prod. Consum. 2021, 27, 1437–1457. [Google Scholar] [CrossRef]
- Esteves, A.F.; Soares, S.M.; Salgado, E.M.; Boaventura, R.A.R.; Pires, J.C.M. Microalgal Growth in Aquaculture Effluent: Coupling Biomass Valorisation with Nutrients Removal. Appl. Sci. 2022, 12, 12608. [Google Scholar] [CrossRef]
- Viegas, C.; Gouveia, L.; Gonçalves, M. Aquaculture Wastewater Treatment through Microalgal. Biomass Potential Applications on Animal Feed, Agriculture, and Energy. J. Environ. Manag. 2021, 286, 112187. [Google Scholar] [CrossRef]
- Zieliński, M.; Dębowski, M.; Kazimierowicz, J.; Świca, I. Microalgal Carbon Dioxide (CO2) Capture and Utilization from the European Union Perspective. Energies 2023, 16, 1446. [Google Scholar] [CrossRef]
- Gupta, R.; Mishra, N.; Singh, G.; Mishra, S.; Lodhiyal, N.; Biotechnology, B. Microalgae Cultivation and Value-Based Products from Wastewater: Insights and Applications. Blue Biotechnol. 2024, 1, 20. [Google Scholar] [CrossRef]
- Guldhe, A.; Ansari, F.A.; Singh, P.; Bux, F. Heterotrophic Cultivation of Microalgae Using Aquaculture Wastewater: A Biorefinery Concept for Biomass Production and Nutrient Remediation. Ecol. Eng. 2017, 99, 47–53. [Google Scholar] [CrossRef]
- Chen, F.; Leng, Y.; Lu, Q.; Zhou, W. The Application of Microalgae Biomass and Bio-Products as Aquafeed for Aquaculture. Algal Res. 2021, 60, 102541. [Google Scholar] [CrossRef]
- Mueller, J.; Pauly, M.; Molkentin, J.; Ostermeyer, U.; van Muilekom, D.R.; Rebl, A.; Goldammer, T.; Lindemeyer, J.; Schultheiß, T.; Seibel, H.; et al. Microalgae as Functional Feed for Atlantic Salmon: Effects on Growth, Health, Immunity, Muscle Fatty Acid and Pigment Deposition. Front. Mar. Sci. 2023, 10, 1273614. [Google Scholar] [CrossRef]
- Siddik, M.A.B.; Sørensen, M.; Islam, S.M.M.; Saha, N.; Rahman, M.A.; Francis, D.S. Expanded Utilisation of Microalgae in Global Aquafeeds. Rev. Aquac. 2024, 16, 6–33. [Google Scholar] [CrossRef]
- Sharma, A.K.; Jaryal, S.; Sharma, S.; Dhyani, A.; Tewari, B.S.; Mahato, N. Biofuels from Microalgae: A Review on Microalgae Cultivation, Biodiesel Production Techniques and Storage Stability. Processes 2025, 13, 488. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Świca, I.; Kazimierowicz, J. Algae Biomass as a Potential Source of Liquid Fuels. Phycology 2021, 1, 105–118. [Google Scholar] [CrossRef]
- Singh, P.K.; Saxena, A.; Tyagi, R.; Sindhu, R.; Binod, P.; Tiwari, A. Biomass Valorization of Agriculture Wastewater Grown Freshwater Diatom Nitzschia Sp. for Metabolites, Antibacterial Activity, and Biofertilizer. Bioresour. Technol. 2023, 377, 128976. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Vdovychenko, A.; Kazimierowicz, J. The Use of the Autotrophic Culture of Arthrospira platensis for CO2 Fixation from Biogas Combustion. Processes 2024, 12, 396. [Google Scholar] [CrossRef]
- Alavianghavanini, A.; Shayesteh, H.; Bahri, P.A.; Vadiveloo, A.; Moheimani, N.R. Microalgae Cultivation for Treating Agricultural Effluent and Producing Value-Added Products. Sci. Total Environ. 2024, 912, 169369. [Google Scholar] [CrossRef]
- Amaral, E.T.; Bender, L.B.Y.C.; Rizzetti, T.M.; Schneider, R.d.C.d.S. Removal of Organic Contaminants in Water Bodies or Wastewater by Microalgae of the Genus Chlorella: A Review. Case Stud. Chem. Environ. Eng. 2023, 8, 100476. [Google Scholar] [CrossRef]
- Pooja, K.; Priyanka, V.; Rao, B.C.S.; Raghavender, V. Cost-Effective Treatment of Sewage Wastewater Using Microalgae Chlorella vulgaris and Its Application as Bio-Fertilizer. Energy Nexus 2022, 7, 100122. [Google Scholar] [CrossRef]
- Ma, R.; Xie, S.; Jia, H.; Pu, L.; Peng, L.; Ge, C. Nutritional Profile and Bioremediation of Tropical Marine Aquaculture with an Integrated Microalgae and Bacteria Symbiosis. Water Air Soil Pollut. 2022, 233, 172. [Google Scholar] [CrossRef]
- Vadiveloo, A.; Matos, A.P.; Chaudry, S.; Bahri, P.A.; Moheimani, N.R. Effect of CO2 Addition on Treating Anaerobically Digested Abattoir Effluent (ADAE) Using Chlorella Sp. (Trebouxiophyceae). J. CO2 Util. 2020, 38, 273–281. [Google Scholar] [CrossRef]
- Markou, G. Fed-Batch Cultivation of Arthrospira and Chlorella in Ammonia-Rich Wastewater: Optimization of Nutrient Removal and Biomass Production. Bioresour. Technol. 2015, 193, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Fernández, F.G.A.; Reis, A.; Wijffels, R.H.; Barbosa, M.; Verdelho, V.; Llamas, B. The Role of Microalgae in the Bioeconomy. New Biotechnol. 2021, 61, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Minaoui, F.; Hakkoum, Z.; Chabili, A.; Douma, M.; Mouhri, K.; Loudiki, M. Biostimulant Effect of Green Soil Microalgae Chlorella vulgaris Suspensions on Germination and Growth of Wheat (Triticum aestivum Var. Achtar) and Soil Fertility. Algal Res. 2024, 82, 103655. [Google Scholar] [CrossRef]
- Lananan, F.; Jusoh, A.; Ali, N.; Lam, S.S.; Endut, A. Effect of Conway Medium and f/2 Medium on the Growth of Six Genera of South China Sea Marine Microalgae. Bioresour. Technol. 2013, 141, 75–82. [Google Scholar] [CrossRef]
- Dudek, M.; Dębowski, M.; Kazimierowicz, J.; Zieliński, M.; Quattrocelli, P.; Nowicka, A. The Cultivation of Biohydrogen-Producing Tetraselmi subcordiformis Microalgae as the Third Stage of Dairy Wastewater Aerobic Treatment System. Sustainability 2022, 14, 12085. [Google Scholar] [CrossRef]
- Zieliński, M.; Barczak, Ł.; Rusanowska, P.; Kazimierowicz, J.; Dębowski, M. Comparison of Biohydrogen Production by Tetraselmis subcordiformis During Cultivation Using Soil-Less Agricultural Wastewater and Effluent from Microbial Fuel Cells. Energies 2024, 17, 5287. [Google Scholar] [CrossRef]
- Llabrés-Luengo, P.; Mata-Alvarez, J. Kinetic Study of the Anaerobic Digestion of Straw-Pig Manure Mixtures. Biomass 1987, 14, 129–142. [Google Scholar] [CrossRef]
- Van Wychen, S.; Ramirez, K.; Laurens, L.M.L. Determination of Total Lipids as Fatty Acid Methyl Esters (FAME) by In Situ Transesterification: Laboratory Analytical Procedure (LAP); National Renewable Energy Lab.: Golden, CO, USA, 2016. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Barczak, Ł.; Rusanowska, P.; Dudek, M.; Zieliński, M. Characterization of Lipid Production in Chlorella Sp. Cultivated in Different Plant Fertilizers. Energies 2024, 17, 6193. [Google Scholar] [CrossRef]
- Enwereuzoh, U.O.; Harding, K.G.; Low, M.; Harding, K.; Uo, E.; Coutinho, T.; Mokgehle, S. Fish Farm Effluent as a Nutrient Source for Algae Biomass Cultivation. S. Afr. J. Sci. 2021, 117. [Google Scholar] [CrossRef] [PubMed]
- Lugo, L.A.; Thorarinsdottir, R.I.; Bjornsson, S.; Palsson, O.P.; Skulason, H.; Johannsson, S.; Brynjolfsson, S. Remediation of Aquaculture Wastewater Using the Microalga Chlorella sorokiniana. Water 2020, 12, 3144. [Google Scholar] [CrossRef]
- Hawrot-Paw, M.; Koniuszy, A.; Gałczynska, M.; Zajac, G.; Szyszlak-Bargłowicz, J. Production of Microalgal Biomass Using Aquaculture Wastewater as Growth Medium. Water 2019, 12, 106. [Google Scholar] [CrossRef]
- Moreno-Garcia, L.; Gariépy, Y.; Barnabé, S.; Raghavan, G.S.V. Effect of Environmental Factors on the Biomass and Lipid Production of Microalgae Grown in Wastewaters. Algal Res. 2019, 41, 101521. [Google Scholar] [CrossRef]
- Fernandes, T.V.; Suárez-Muñoz, M.; Trebuch, L.M.; Verbraak, P.J.; Van de Waal, D.B. Toward an Ecologically Optimized N:P Recovery from Wastewater by Microalgae. Front. Microbiol. 2017, 8, 263965. [Google Scholar] [CrossRef]
- Yu, H.C.; Lay, C.H.; Abdul, P.M.; Wu, J.Y. Enhancing Lipid Production of Chlorella Sp. by Mixotrophic Cultivation Optimization. Processes 2023, 11, 1892. [Google Scholar] [CrossRef]
- Choi, H.J.; Lee, S.M. Effect of the N/P Ratio on Biomass Productivity and Nutrient Removal from Municipal Wastewater. Bioprocess Biosyst. Eng. 2015, 38, 761–766. [Google Scholar] [CrossRef]
- Carletti, M.; Barbera, E.; Filippini, F.; Sforza, E. Effect of Ammonium/Nitrate Ratio on Microalgae Continuous Cultures: Species-Specificity of Nutrient Uptake and Modelling Perspectives. J. Water Process Eng. 2024, 58, 104762. [Google Scholar] [CrossRef]
- Scherholz, M.L.; Curtis, W.R. Achieving PH Control in Microalgal Cultures through Fed-Batch Addition of Stoichiometrically-Balanced Growth Media. BMC Biotechnol. 2013, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Salbitani, G.; Carfagna, S. Ammonium Utilization in Microalgae: A Sustainable Method for Wastewater Treatment. Sustainability 2021, 13, 956. [Google Scholar] [CrossRef]
- Sutherland, D.L. Improving Microalgal Tolerance to High Ammonia with Simple Organic Carbon Addition for More Effective Wastewater Treatment. J. Water Process Eng. 2022, 47, 102667. [Google Scholar] [CrossRef]
- Sanz-Luque, E.; Chamizo-Ampudia, A.; Llamas, A.; Galvan, A.; Fernandez, E. Understanding Nitrate Assimilation and Its Regulation in Microalgae. Front. Plant Sci. 2015, 6, 163887. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, T.; Yu, X.; Bates, P.D.; Dong, T.; Chen, S. High-Density Fed-Batch Culture of a Thermotolerant Microalga Chlorella sorokiniana for Biofuel Production. Appl. Energy 2013, 108, 281–287. [Google Scholar] [CrossRef]
- Sakarika, M.; Kornaros, M. Effect of PH on Growth and Lipid Accumulation Kinetics of the Microalga Chlorella vulgaris Grown Heterotrophically under Sulfur Limitation. Bioresour. Technol. 2016, 219, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Qin, L.; Feng, S.; Huang, D.; Wang, Z.; Zhu, S. The Joint Effect of Ammonium and PH on the Growth of Chlorella vulgaris and Ammonium Removal in Artificial Liquid Digestate. Bioresour. Technol. 2021, 325, 124690. [Google Scholar] [CrossRef]
- Bossa, R.; Di Colandrea, M.; Salbitani, G.; Carfagna, S. Phosphorous Utilization in Microalgae: Physiological Aspects and Applied Implications. Plants 2024, 13, 2127. [Google Scholar] [CrossRef]
- Matsui, H.; Shiozaki, K.; Okumura, Y.; Ishikawa, M.; Waqalevu, V.; Hayasaka, O.; Honda, A.; Kotani, T. Effects of Phosphorous Deficiency of a Microalga Nannochloropsis oculata on Its Fatty Acid Profiles and Intracellular Structure and the Effectiveness in Rotifer Nutrition. Algal Res. 2020, 49, 101905. [Google Scholar] [CrossRef]
- Tossavainen, M.; Lahti, K.; Edelmann, M.; Eskola, R.; Lampi, A.M.; Piironen, V.; Korvonen, P.; Ojala, A.; Romantschuk, M. Integrated Utilization of Microalgae Cultured in Aquaculture Wastewater: Wastewater Treatment and Production of Valuable Fatty Acids and Tocopherols. J. Appl. Phycol. 2019, 31, 1753–1763. [Google Scholar] [CrossRef]
- Gao, F.; Li, C.; Yang, Z.H.; Zeng, G.M.; Feng, L.J.; Liu, J.z.; Liu, M.; Cai, H.w. Continuous Microalgae Cultivation in Aquaculture Wastewater by a Membrane Photobioreactor for Biomass Production and Nutrients Removal. Ecol. Eng. 2016, 92, 55–61. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Z.; Xiao, Y.; Yuan, M.; Zhou, C.; Liu, G.; Fang, J.; Yang, B. Biochemical and Morphological Changes Triggered by Nitrogen Stress in the Oleaginous Microalga Chlorella vulgaris. Microorganisms 2022, 10, 566. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.Q.; Park, Y.J.; Winarto, J.; Huynh, P.K.; Moon, J.; Choi, Y.B.; Song, D.G.; Koo, S.Y.; Kim, S.M. Understanding the Impact of Nitrogen Availability: A Limiting Factor for Enhancing Fucoxanthin Productivity in Microalgae Cultivation. Mar. Drugs 2024, 22, 93. [Google Scholar] [CrossRef] [PubMed]
- Tam, N.F.Y.; Wong, Y.S. Effect of Ammonia Concentrations on Growth of Chlorella vulgaris and Nitrogen Removal from Media. Bioresour. Technol. 1996, 57, 45–50. [Google Scholar] [CrossRef]
- Jakhwal, P.; Daneshvar, E.; Skalska, K.; Matsakas, L.; Patel, A.; Park, Y.; Bhatnagar, A. Nutrient Removal and Biomass Production of Marine Microalgae Cultured in Recirculating Aquaculture Systems (RAS) Water with Low Phosphate Concentration. J. Environ. Manag. 2024, 358, 120859. [Google Scholar] [CrossRef] [PubMed]
- Beuckels, A.; Smolders, E.; Muylaert, K. Nitrogen Availability Influences Phosphorus Removal in Microalgae-Based Wastewater Treatment. Water Res. 2015, 77, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Hiltunen, M.; Kannangara, R.; Nakandalage, B.; Söderena, O.; Timilsina, H.; Pirhonen, J.; Pulkkinen, K. Growth and Biomass Composition of Chlorella vulgaris Using Nutrient-Rich Water and CO2 from a Recirculating Aquaculture System. Aquaculture 2026, 610, 742956. [Google Scholar] [CrossRef]
- Peter, A.P.; Chew, K.W.; Koyande, A.K.; Yuk-Heng, S.; Ting, H.Y.; Rajendran, S.; Munawaroh, H.S.H.; Yoo, C.K.; Show, P.L. Cultivation of Chlorella vulgaris on Dairy Waste Using Vision Imaging for Biomass Growth Monitoring. Bioresour. Technol. 2021, 341, 125892. [Google Scholar] [CrossRef]
- Zhai, Q.; Hong, Y.; Wang, X.; Wang, Q.; Zhao, G.; Liu, X.; Zhang, H. Mixing Starch Wastewaters to Balance Nutrients for Improving Nutrient Removal, Microalgae Growth and Accumulation of High Value-Added Products. Water Cycle 2022, 3, 151–159. [Google Scholar] [CrossRef]
- Cai, Y.; Zhai, L.; Fang, X.; Wu, K.; Liu, Y.; Cui, X.; Wang, Y.; Yu, Z.; Ruan, R.; Liu, T.; et al. Effects of C/N Ratio on the Growth and Protein Accumulation of Heterotrophic Chlorella in Broken Rice Hydrolysate. Biotechnol. Biofuels Bioprod. 2022, 15, 102. [Google Scholar] [CrossRef]
- Mao, X.; Zhou, X.; Fan, X.; Jin, W.; Xi, J.; Tu, R.; Naushad, M.; Li, X.; Liu, H.; Wang, Q. Proteomic Analysis Reveals Mechanisms of Mixed Wastewater with Different N/P Ratios Affecting the Growth and Biochemical Characteristics of Chlorella pyrenoidosa. Bioresour. Technol. 2023, 381, 129141. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, Q. Regulatory Mechanisms of Lipid Biosynthesis in Microalgae. Biol. Rev. 2021, 96, 2373–2391. [Google Scholar] [CrossRef]
- Ajitha, V.; Sreevidya, C.P.; Sarasan, M.; Park, J.C.; Mohandas, A.; Singh, I.S.B.; Puthumana, J.; Lee, J.S. Effects of Zinc and Mercury on ROS-Mediated Oxidative Stress-Induced Physiological Impairments and Antioxidant Responses in the Microalga Chlorella vulgaris. Environ. Sci. Pollut. Res. 2021, 28, 32475–32492. [Google Scholar] [CrossRef] [PubMed]
- Gora, A.H.; Ambasankar, K.; Sandeep, K.P.; Rehman, S.; Agarwal, D.; Ahmad, I.; Ramachandran, K. Effect of Dietary Supplementation of Crude Microalgal Extracts on Growth Performance, Survival and Disease Resistance of Lates Calcarifer (Bloch, 1790) Larvae. Indian J. Fish. 2019, 66, 64–72. [Google Scholar] [CrossRef]
- Peralta-Sánchez, J.M.; Rabelo-Ruiz, M.; Martín-Platero, A.M.; Vizcaíno, A.J.; Flores-Moreno, S.; Macías-Vidal, J.; Martos-Sitcha, J.A.; Alarcón-López, F.J.; Baños, A.; Valdivia, E.; et al. Microalgae and Phytase Dietary Supplementation Improved Growth and Gut Microbiota in Juvenile European Seabass (Dicentrarchus labrax). BMC Genom. 2024, 25, 838. [Google Scholar] [CrossRef]
- Saber, H.; Galal, H.R.; Abo-Eldahab, M.; Alwaleed, E. Enhancing the Biodiesel Production in the Green Alga Chlorella vulgaris by Heavy Metal Stress and Prediction of Fuel Properties from Fatty Acid Profiles. Environ. Sci. Pollut. Res. 2024, 31, 35952–35968. [Google Scholar] [CrossRef]
- Moradi, P.; Saidi, M. Biodiesel Production from Chlorella vulgaris Microalgal-Derived Oil via Electrochemical and Thermal Processes. Fuel Process. Technol. 2022, 228, 107158. [Google Scholar] [CrossRef]
- Khalaji, M. Evaluation of Fatty Acid Profiles of Chlorella vulgaris Microalgae Grown in Dairy Wastewater for Producing Biofuel. J. Environ. Health Sci. Eng. 2022, 20, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Cha, T.S.; Chee, J.Y.; Loh, S.H.; Jusoh, M. Oil Production and Fatty Acid Composition of Chlorella vulgaris Cultured in Nutrient-Enriched Solid-Agar-Based Medium. Bioresour. Technol. Rep. 2018, 3, 218–223. [Google Scholar] [CrossRef]
- Sadvakasova, A.K.; Kossalbayev, B.D.; Bauenova, M.O.; Balouch, H.; Leong, Y.K.; Zayadan, B.K.; Huang, Z.; Alharby, H.F.; Tomo, T.; Chang, J.S.; et al. Microalgae as a Key Tool in Achieving Carbon Neutrality for Bioproduct Production. Algal Res. 2023, 72, 103096. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Malik, A.; Vijay, V.K. Comparative Evaluation of Biomass Production and Bioenergy Generation Potential of Chlorella Spp. through Anaerobic Digestion. Appl. Energy 2014, 114, 790–797. [Google Scholar] [CrossRef]
- Varol, A.; Ugurlu, A. Biogas Production from Microalgae (Spirulina platensis) in a Two Stage Anaerobic System. Waste Biomass Valorization 2016, 7, 193–200. [Google Scholar] [CrossRef]
- Tossavainen, M.; Edelmann, M.; Lahti-Leikas, K.; Kivimäki, S.; Kymäläinen, M.; Piironen, V.; Lampi, A.M.; Ojala, A.; Romantschuk, M. Chemical Composition and Biomethane Production Potential of Euglena Gracilis Biomass and Extraction Residue from Supercritical CO2 Extraction. Bioresour. Technol. Reports 2022, 19, 101140. [Google Scholar] [CrossRef]
- Hawrot-Paw, M.; Koniuszy, A.; Ratomski, P.; Sąsiadek, M.; Gawlik, A. Biogas Production from Arthrospira platensis Biomass. Energies 2023, 16, 3971. [Google Scholar] [CrossRef]
- Abusweireh, R.S.; Rajamohan, N.; Sonne, C.; Vasseghian, Y. Algae Biogas Production Focusing on Operating Conditions and Conversion Mechanisms—A Review. Heliyon 2023, 9, e17757. [Google Scholar] [CrossRef]
- Montingelli, M.E.; Tedesco, S.; Olabi, A.G. Biogas Production from Algal Biomass: A Review. Renew. Sustain. Energy Rev. 2015, 43, 961–972. [Google Scholar] [CrossRef]
- Sialve, B.; Bernet, N.; Bernard, O. Anaerobic Digestion of Microalgae as a Necessary Step to Make Microalgal Biodiesel Sustainable. Biotechnol. Adv. 2009, 27, 409–416. [Google Scholar] [CrossRef]
- Szaja, A.; Montusiewicz, A.; Lebiocka, M. Variability of Micro- and Macro-Elements in Anaerobic Co-Digestion of Municipal Sewage Sludge and Food Industrial By-Products. Int. J. Environ. Res. Public Health 2023, 20, 5405. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, P.; Yang, X.; Lin, P.; Wang, Y.; Cheng, M.; Ren, L. Process Performance and Microbial Communities in Anaerobic Co-Digestion of Sewage Sludge and Food Waste with a Lower Range of Carbon/Nitrogen Ratio. Bioenergy Res. 2022, 15, 1664–1674. [Google Scholar] [CrossRef]
- Greses, S.; Jimenez, J.; González-Fernández, C.; Steyer, J.P. Modelling of Anaerobic Digestion of Microalgae Biomass: Effect of Overloading Perturbation. Bioresour. Technol. 2024, 399, 130625. [Google Scholar] [CrossRef]
- Kalra, R.; Gaur, S.; Goel, M. Microalgae Bioremediation: A Perspective towards Wastewater Treatment along with Industrial Carotenoids Production. J. Water Process Eng. 2021, 40, 101794. [Google Scholar] [CrossRef]
- Gurreri, L.; Rindina, M.C.; Luciano, A.; Falqui, L.; Fino, D.; Mancini, G. Microalgae production in an industrial scale photobioreactor plant: A comprehensive life cycle assessment. Sustain. Chem. Pharm. 2024, 139, 101598. [Google Scholar] [CrossRef]
- Kisielewska, M.; Zielinski, M.; Debowski, M.; Kazimierowicz, J.; Romanowska-Duda, Z.; Dudek, M. Effectiveness of Scenedesmus Sp. Biomass Grow and Nutrients Removal from Liquid Phase of Digestates. Energies 2020, 13, 1432. [Google Scholar] [CrossRef]
- Zieliński, M.; Dębowski, M.; Kazimierowicz, J. Outflow from a Biogas Plant as a Medium for Microalgae Biomass Cultivation—Pilot Scale Study and Technical Concept of a Large-Scale Installation. Energies 2022, 15, 2912. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Kisielewska, M.; Kazimierowicz, J.; Dudek, M.; Świca, I.; Rudnicka, A. The Cultivation of Lipid-Rich Microalgae Biomass as Anaerobic Digestate Valorization Technology—A Pilot-Scale Study. Processes 2020, 8, 517. [Google Scholar] [CrossRef]
- Vishwakarma, R.; Dhaka, V.; Ariyadasa, T.U.; Malik, A. Exploring Algal Technologies for a Circular Bio-Based Economy in Rural Sector. J. Clean. Prod. 2022, 354, 131653. [Google Scholar] [CrossRef]
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current Status of the Algae Production Industry in Europe: An Emerging Sector of the Blue Bioeconomy. Front. Mar. Sci. 2021, 7, 1247. [Google Scholar] [CrossRef]
- Hasselström, L.; Gröndahl, F. Payments for Nutrient Uptake in the Blue Bioeconomy—When to Be Careful and When to Go for It. Mar. Pollut. Bull. 2021, 167, 112321. [Google Scholar] [CrossRef] [PubMed]
- Abomohra, A.E.F.; Almutairi, A.W. A Close-Loop Integrated Approach for Microalgae Cultivation and Efficient Utilization of Agar-Free Seaweed Residues for Enhanced Biofuel Recovery. Bioresour. Technol. 2020, 317, 124027. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Ummalyma, S.B. Bioremediation and Biomass Production of Microalgae Cultivation in River Water Contaminated with Pharmaceutical Effluent. Bioresour. Technol. 2020, 307, 123233. [Google Scholar] [CrossRef]
- Culaba, A.B.; Ubando, A.T.; Ching, P.M.L.; Chen, W.H.; Chang, J.S. Biofuel from Microalgae: Sustainable Pathways. Sustainability 2020, 12, 8009. [Google Scholar] [CrossRef]
- Ebhodaghe, S.O.; Imanah, O.E.; Ndibe, H. Biofuels from Microalgae Biomass: A Review of Conversion Processes and Procedures. Arab. J. Chem. 2022, 15, 103591. [Google Scholar] [CrossRef]
- Ganesan, R.; Manigandan, S.; Samuel, M.S.; Shanmuganathan, R.; Brindhadevi, K.; Lan Chi, N.T.; Duc, P.A.; Pugazhendhi, A. A Review on Prospective Production of Biofuel from Microalgae. Biotechnol. Rep. 2020, 27, e00509. [Google Scholar] [CrossRef]
- Goswami, R.K.; Agrawal, K.; Upadhyaya, H.M.; Gupta, V.K.; Verma, P. Microalgae Conversion to Alternative Energy, Operating Environment and Economic Footprint: An Influential Approach towards Energy Conversion, and Management. Energy Convers. Manag. 2022, 269, 116118. [Google Scholar] [CrossRef]
- Su, B.; Lin, Y.; Wang, J.; Quan, X.; Chang, Z.; Rui, C. Sewage Treatment System for Improving Energy Efficiency Based on Particle Swarm Optimization Algorithm. Energy Rep. 2022, 8, 8701–8708. [Google Scholar] [CrossRef]
- Sun, H.; Huang, K.; Zhang, X.; Ren, H.; Ye, L. Stable Isotope Probing Reveals Specific Assimilating Bacteria of Refractory Organic Compounds in Activated Sludge. Water Res. 2022, 212, 118105. [Google Scholar] [CrossRef]
- Rossi, E.; Pecorini, I.; Iannelli, R. Multilinear Regression Model for Biogas Production Prediction from Dry Anaerobic Digestion of OFMSW. Sustainability 2022, 14, 4393. [Google Scholar] [CrossRef]
- Burch, T.R.; Firnstahl, A.D.; Spencer, S.K.; Larson, R.A.; Borchardt, M.A. Fate and Seasonality of Antimicrobial Resistance Genes during Full-Scale Anaerobic Digestion of Cattle Manure across Seven Livestock Production Facilities. J. Environ. Qual. 2022, 51, 352–363. [Google Scholar] [CrossRef]
- Iglesias, R.; Muñoz, R.; Polanco, M.; Díaz, I.; Susmozas, A.; Moreno, A.D.; Guirado, M.; Carreras, N.; Ballesteros, M. Biogas from Anaerobic Digestion as an Energy Vector: Current Upgrading Development. Energies 2021, 14, 2742. [Google Scholar] [CrossRef]
- Liyanaarachchi, V.C.; Premaratne, M.; Ariyadasa, T.U.; Nimarshana, P.H.V.; Malik, A. Two-Stage Cultivation of Microalgae for Production of High-Value Compounds and Biofuels: A Review. Algal Res. 2021, 57, 102353. [Google Scholar] [CrossRef]
- Belwal, T.; Chemat, F.; Venskutonis, P.R.; Cravotto, G.; Jaiswal, D.K.; Bhatt, I.D.; Devkota, H.P.; Luo, Z. Recent Advances in Scaling-up of Non-Conventional Extraction Techniques: Learning from Successes and Failures. TrAC Trends Anal. Chem. 2020, 127, 115895. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Conrad, M.; Sun, S.N.; Sanchez, A.; Rocha, G.J.M.; Romaní, A.; Castro, E.; Torres, A.; Rodríguez-Jasso, R.M.; Andrade, L.P.; et al. Engineering Aspects of Hydrothermal Pretreatment: From Batch to Continuous Operation, Scale-up and Pilot Reactor under Biorefinery Concept. Bioresour. Technol. 2020, 299, 122685. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Prinsen, P.; Raheem, A.; Luque, R.; Zhao, M. Sustainability Analysis of Microalgae Production Systems: A Review on Resource with Unexploited High-Value Reserves. Environ. Sci. Technol. 2018, 52, 14031–14049. [Google Scholar] [CrossRef]
- Li, G.; Hao, Y.; Yang, T.; Xiao, W.; Pan, M.; Huo, S.; Lyu, T. Enhancing Bioenergy Production from the Raw and Defatted Microalgal Biomass Using Wastewater as the Cultivation Medium. Bioengineering 2022, 9, 637. [Google Scholar] [CrossRef] [PubMed]
- Ubando, A.T.; Anderson, S.; Ng, E.; Chen, W.H.; Culaba, A.B.; Kwon, E.E. Life Cycle Assessment of Microalgal Biorefinery: A State-of-the-Art Review. Bioresour. Technol. 2022, 360, 127615. [Google Scholar] [CrossRef]
- Chamkalani, A.; Zendehboudi, S.; Rezaei, N.; Hawboldt, K. A Critical Review on Life Cycle Analysis of Algae Biodiesel: Current Challenges and Future Prospects. Renew. Sustain. Energy Rev. 2020, 134, 110143. [Google Scholar] [CrossRef]
- Jasni, J.; Yasin, M.; Haiza, N.; Ruslan, N.; Nurul, C.; Che, A.; Arisht, S.N.; Bajunaid Hariz, H.; Sobri Takriff, M.; Sajab, M.S. Strategies of Utilising Agriculture Wastewater for Microalgae Cultivation and Its Possible Applications: A Review Microalgal Biomass Harvesting for Subsequence Production of Valuable Products View Project MISC SMAFEG Research Project View Project Strategi. ASM Sci. J. 2021, 16, 2021. [Google Scholar] [CrossRef]
- Wicker, R.J.; Kwon, E.; Khan, E.; Kumar, V.; Bhatnagar, A. The Potential of Mixed-Species Biofilms to Address Remaining Challenges for Economically-Feasible Microalgal Biorefineries: A Review. Chem. Eng. J. 2023, 451, 138481. [Google Scholar] [CrossRef]
- Song, Y.; Wang, L.; Qiang, X.; Gu, W.; Ma, Z.; Wang, G. The Promising Way to Treat Wastewater by Microalgae: Approaches, Mechanisms, Applications and Challenges. J. Water Process Eng. 2022, 49, 103012. [Google Scholar] [CrossRef]
- Chan, S.S.; Khoo, K.S.; Chew, K.W.; Ling, T.C.; Show, P.L. Recent Advances Biodegradation and Biosorption of Organic Compounds from Wastewater: Microalgae-Bacteria Consortium—A Review. Bioresour. Technol. 2022, 344, 126159. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Varjani, S.; Jeevanantham, S.; Yaashikaa, P.R.; Thamarai, P.; Abirami, B.; George, C.S. A Review on Algal-Bacterial Symbiotic System for Effective Treatment of Wastewater. Chemosphere 2021, 271, 129540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, W.; Guo, Y.; Zhang, Z.; Shi, W.; Cui, F.; Lens, P.N.L.; Tay, J.H. Microalgal-Bacterial Consortia: From Interspecies Interactions to Biotechnological Applications. Renew. Sustain. Energy Rev. 2020, 118, 109563. [Google Scholar] [CrossRef]
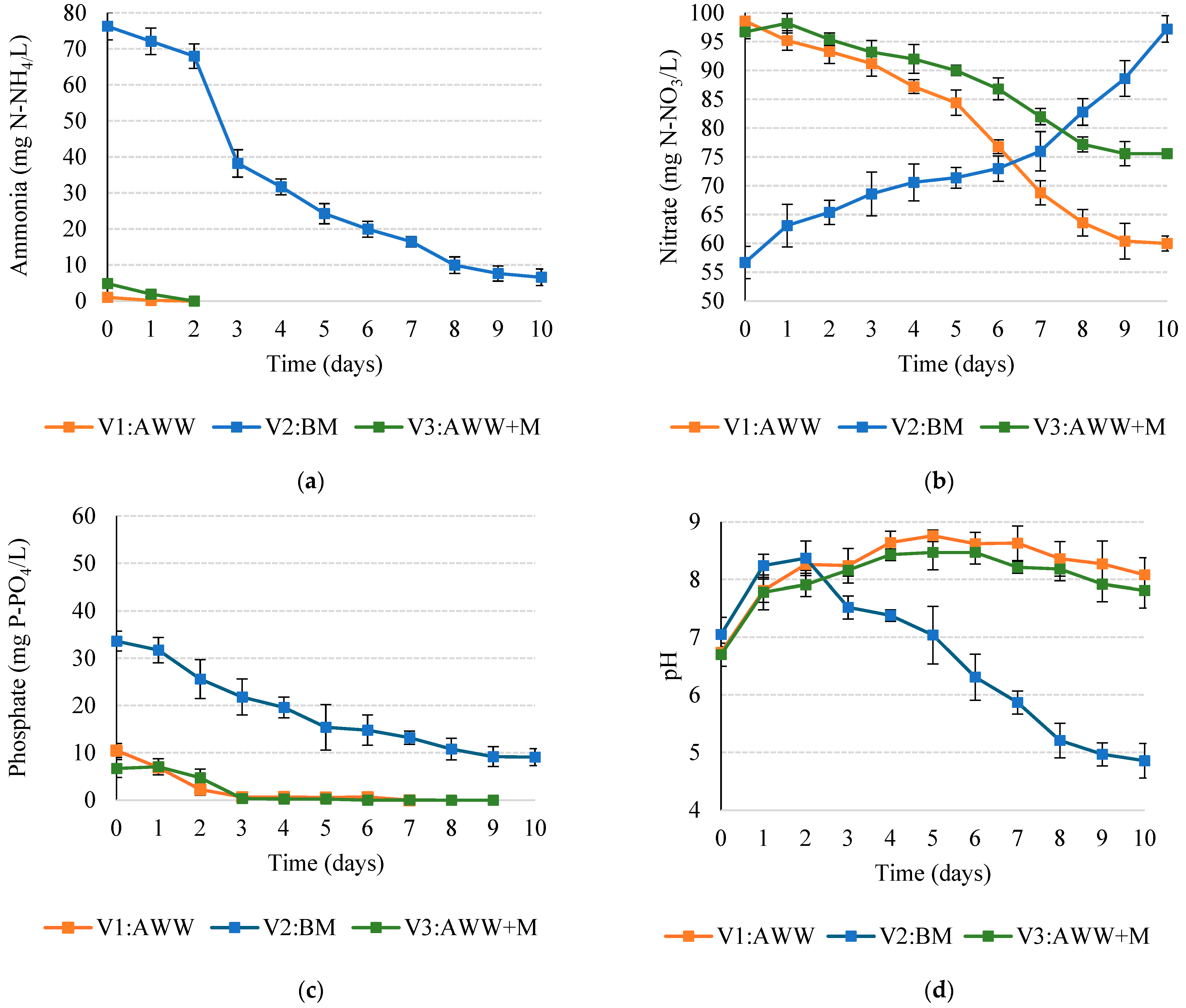

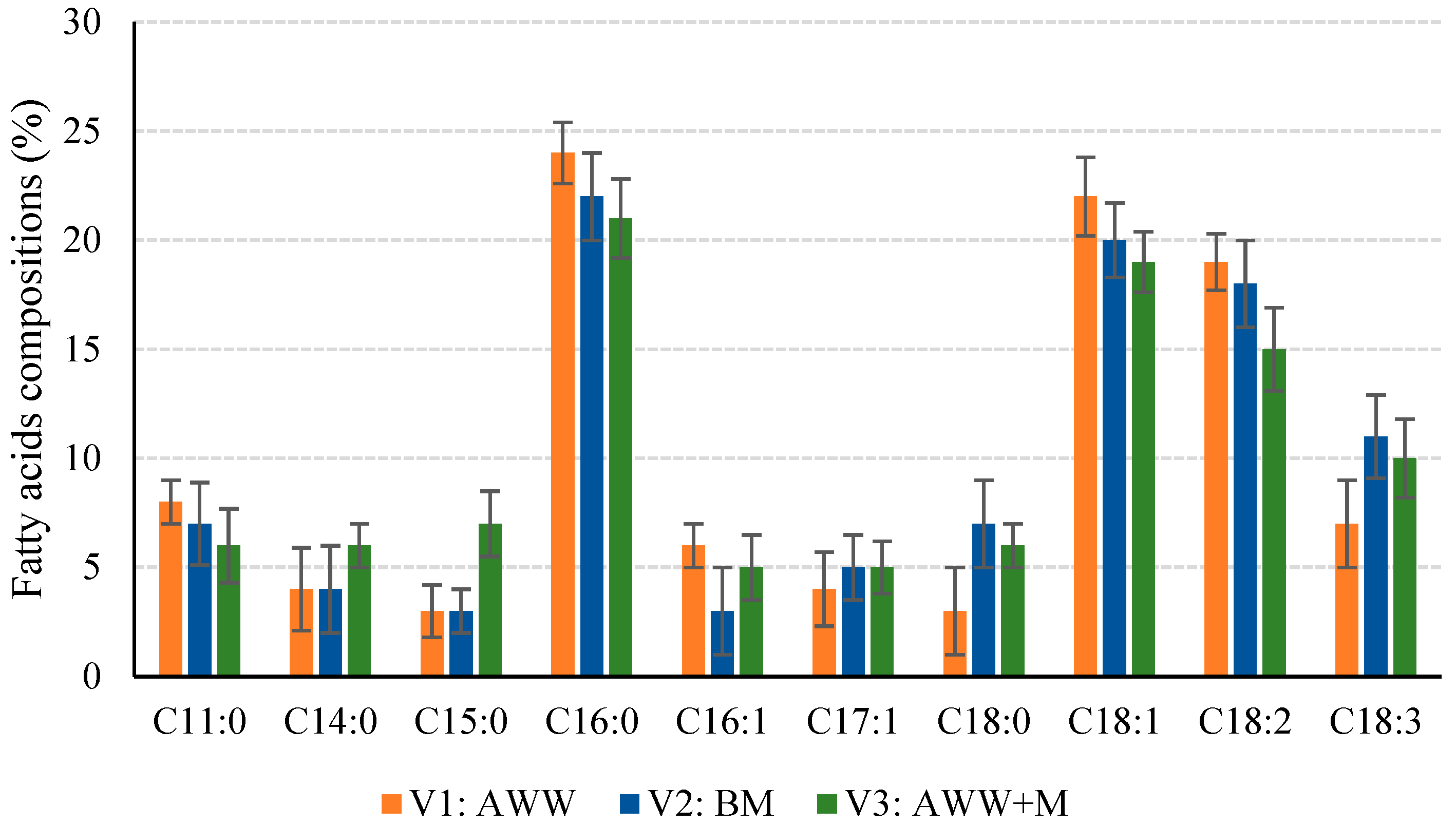

| Parameter | Unit | V1:AWW | V2:BM | V3:AWW+M |
|---|---|---|---|---|
| Ammonia | mg N-NH4/L | 1.03 ± 0.09 | 76.30 ± 2.80 | 4.85 ± 0.25 |
| Nitrate | mg N-NO3/L | 98.60 ± 4.10 | 56.70 ± 2.10 | 96.70 ± 3.60 |
| Phosphate | mg P-PO4/L | 10.50 ± 0.80 | 33.60 ± 1.40 | 6.69 ± 0.50 |
| pH | – | 6.74 ± 0.05 | 7.05 ± 0.04 | 6.70 ± 0.06 |
| Iron | mg Fe/L | 0.094 ± 0.008 | 3.270 ± 0.120 | 2.440 ± 0.095 |
| Potassium | mg K/L | 47.6 ± 2.3 | 190.0 ± 6.2 | 53.8 ± 2.1 |
| Parameter | Unit | Value ± SD |
|---|---|---|
| Total solids (TS) | [% FM *] | 3.9 ± 0.3 |
| Volatile solids (VS) | [% TS] | 60.2 ± 3.5 |
| Mineral solids (MS) | [% TS] | 39.8 ± 3.5 |
| Total carbon (TC) | [mg/g TS] | 372 ± 35 |
| Total organic carbon (TOC) | [mg/g TS] | 322 ± 17 |
| Total nitrogen (TN) | [mg/g TS] | 30.8 ± 2.5 |
| C/N ratio | [–] | 10.2 ± 0.4 |
| Total phosphorus (TP) | [mg/g TS] | 2.4 ± 0.2 |
| pH | [–] | 7.26 ± 0.10 |
| Protein | [% TS] | 20.5 ± 1.5 |
| Lipids | [% TS] | 2.3 ± 0.3 |
| Carbohydrates | [% TS] | 3.1 ± 0.8 |
| Parameters | Unit | V1:AWW | V2:BM | V3:AWW+M |
|---|---|---|---|---|
| Biomass concentration | g VS/L | 2.4 ± 0.04 | 2.2 ± 0.08 | 2.1 ± 0.04 |
| Chlorophyll a | mg/L | 67.6 ± 1.8 | 62.5 ± 2.2 | 59.2 ± 2.8 |
| Volatile solids (VS) | % TS | 88.7 ± 1.3 | 86.9 ± 0.9 | 86.2 ± 1.4 |
| Mineral solids (MS) | % TS | 11.3 ± 1.1 | 13.1 ± 1.0 | 13.8 ± 1.1 |
| Total carbon (TC) | mg/g TS | 477 ± 14 | 471 ± 26 | 442 ± 31 |
| Total organic carbon (TOC) | mg/g TS | 431 ± 9 | 412 ± 21 | 409 ± 16 |
| Total nitrogen (TN) | mg/g TS | 32.5 ± 1.9 | 36.3 ± 1.3 | 37.3 ± 2.7 |
| C/N | 13.3 ± 0.5 | 11.3 ± 1.1 | 10.9 ± 0.9 | |
| Total phosphorus (TP) | mg/g TS | 16.3 ± 2.1 | 16.0 ± 1.7 | 15.2 ± 1.8 |
| pH | mg/g TS | 7.81 ± 0.09 | 8.01 ± 0.12 | 7.94 ± 0.07 |
| Protein | mg/g TS | 203 ± 19 | 227 ± 14 | 233 ± 11 |
| Saccharides | mg/g TS | 212 ± 21 | 197 ± 13 | 216 ± 19 |
| Lipids | mg/g TS | 83.2 ± 3.7 | 81.4 ± 4.3 | 75.6 ± 6.7 |
| Parameter | Unit | Variant | |||
|---|---|---|---|---|---|
| V0—Endogenous Production | V1:AWW | V2:BM | V3:AWW+M | ||
| Biogas | L/kgVS | 83.5 ± 21 | 358 ± 11 | 319 ± 13 | 300 ± 12 |
| k | 1/day | 0.16 | 0.16 | 0.13 | 0.13 |
| r | mL/day | 13.4 | 57.4 | 41.5 | 39.1 |
| CH4 | % | 41.2 ± 0.5 | 60.2 ± 0.7 | 57.1 ± 0.9 | 57.4 ± 0.5 |
| CH4 | L/kgVS | 34.0 ± 9 | 216 ± 7 | 182 ± 7 | 173 ± 6 |
| k | 1/day | 0.15 | 0.15 | 0.14 | 0.15 |
| r | mL/day | 5.1 | 32.4 | 27.3 | 25.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zieliński, M.; Kisielewska, M.; Talpalaru, A.; Rusanowska, P.; Kazimierowicz, J.; Dębowski, M. Integration of Aquaculture Wastewater Treatment and Chlorella vulgaris Cultivation as a Sustainable Method for Biofuel Production. Energies 2025, 18, 4352. https://doi.org/10.3390/en18164352
Zieliński M, Kisielewska M, Talpalaru A, Rusanowska P, Kazimierowicz J, Dębowski M. Integration of Aquaculture Wastewater Treatment and Chlorella vulgaris Cultivation as a Sustainable Method for Biofuel Production. Energies. 2025; 18(16):4352. https://doi.org/10.3390/en18164352
Chicago/Turabian StyleZieliński, Marcin, Marta Kisielewska, Annamaria Talpalaru, Paulina Rusanowska, Joanna Kazimierowicz, and Marcin Dębowski. 2025. "Integration of Aquaculture Wastewater Treatment and Chlorella vulgaris Cultivation as a Sustainable Method for Biofuel Production" Energies 18, no. 16: 4352. https://doi.org/10.3390/en18164352
APA StyleZieliński, M., Kisielewska, M., Talpalaru, A., Rusanowska, P., Kazimierowicz, J., & Dębowski, M. (2025). Integration of Aquaculture Wastewater Treatment and Chlorella vulgaris Cultivation as a Sustainable Method for Biofuel Production. Energies, 18(16), 4352. https://doi.org/10.3390/en18164352









