Simulation of Biomass Gasification and Syngas Methanation for Methane Production with H2/CO Ratio Adjustment in Aspen Plus
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstock Characteristics
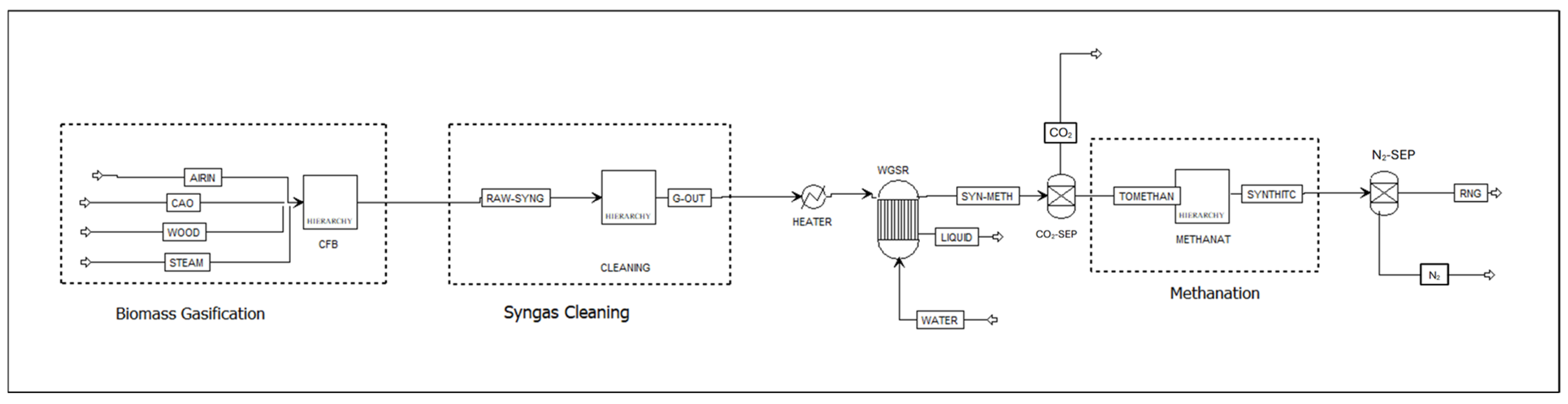
2.2. Aspen Plus Biomass Gasification Model
2.3. Aspen Plus Syngas Cleaning and Conditioning Model
| Impurities in Raw Syngas | Cleaning Technique | Unit Operation in Aspen Plus, Figure 3 |
|---|---|---|
| Particulate matter | Cyclone | Cyclone |
| Tars -In situ tar cracking -Downstream tar removal | CaO catalyst Physical scrubber | ‘CFB’: RPlug reactors ‘TAR-ABS’: Five-stage low-temperature absorber using palm oil as an absorbent [27] |
| NH3 | Chemical scrubber | ‘AMMONIA’: Rstoick reactor [28] |
| Acid gases (CO2 and H2S) | Physical scrubber | A Selexol (DEPG) scrubber for acid gas removal, i.e., H2S and CO2. The Selexol process consists of two columns: absorber ‘ACIDGASR’ and stripper ‘STRIPPER’ [29]. |
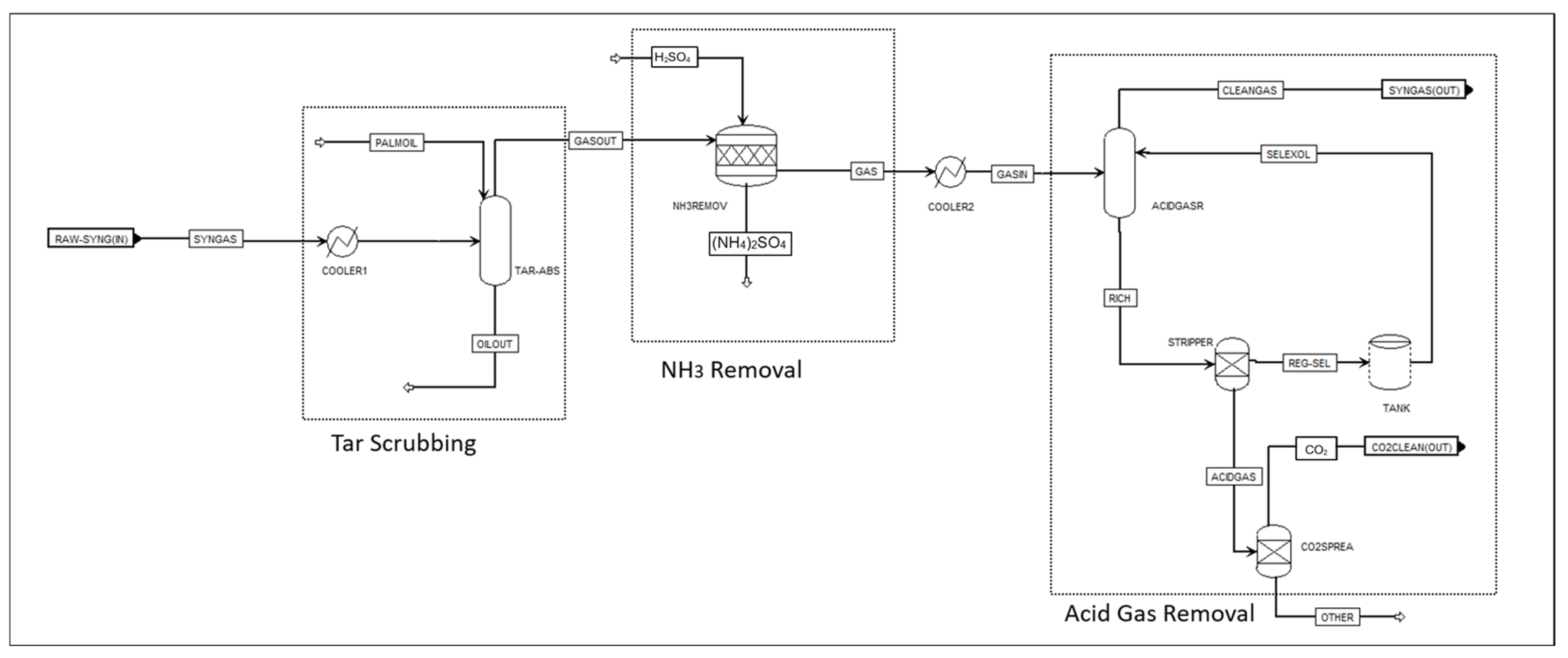
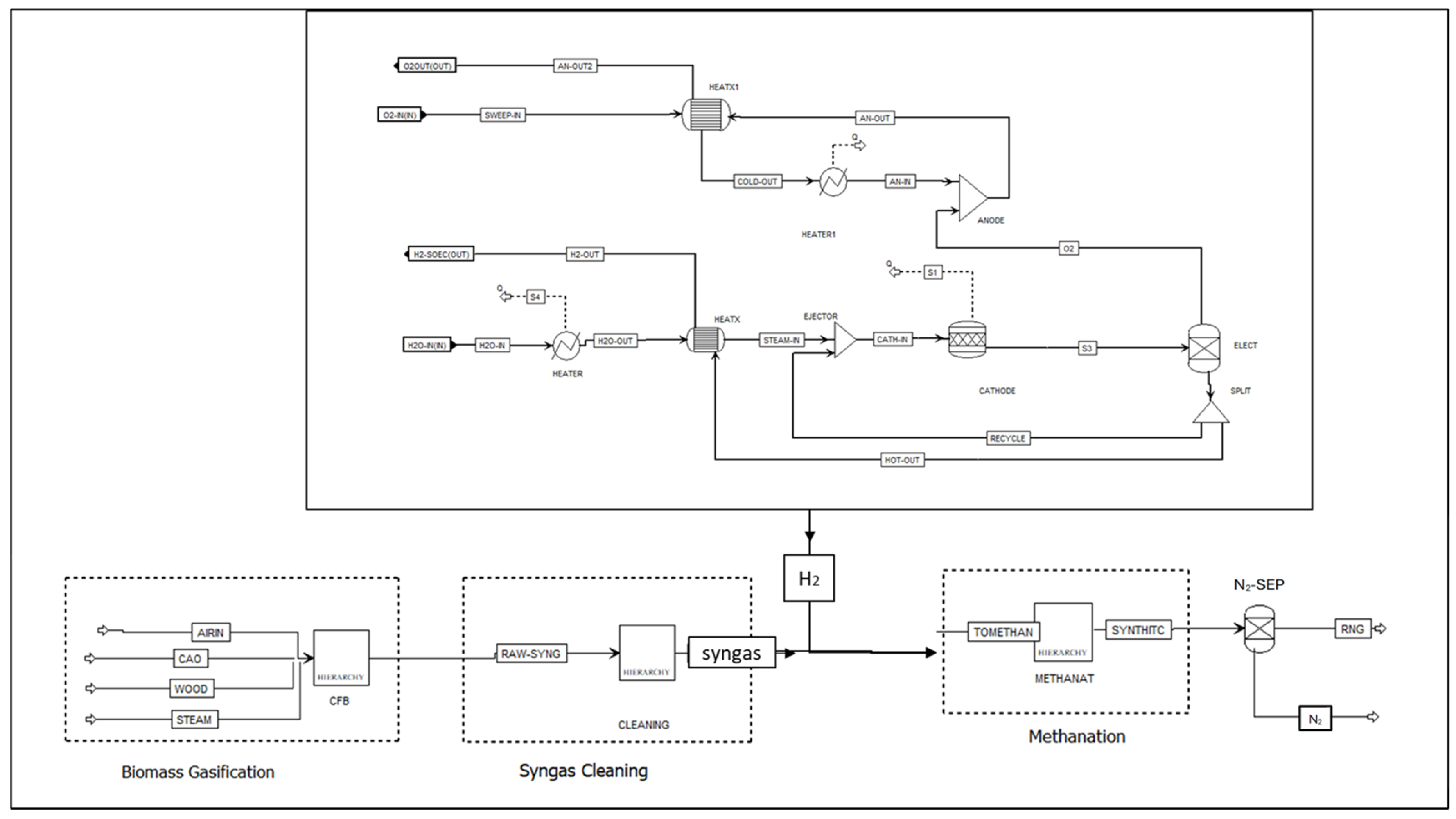
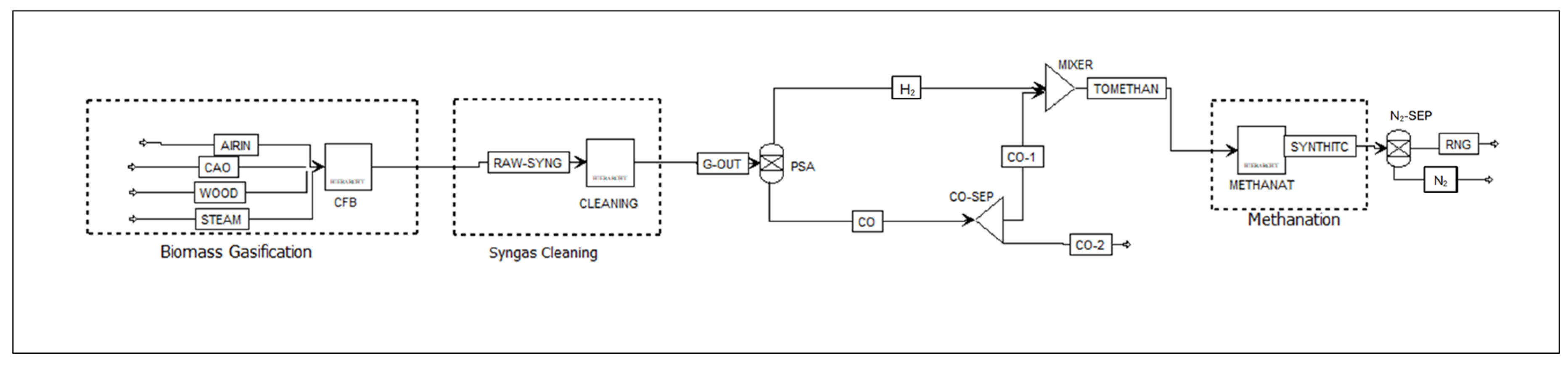
2.4. H2/CO Ratio Adjustment
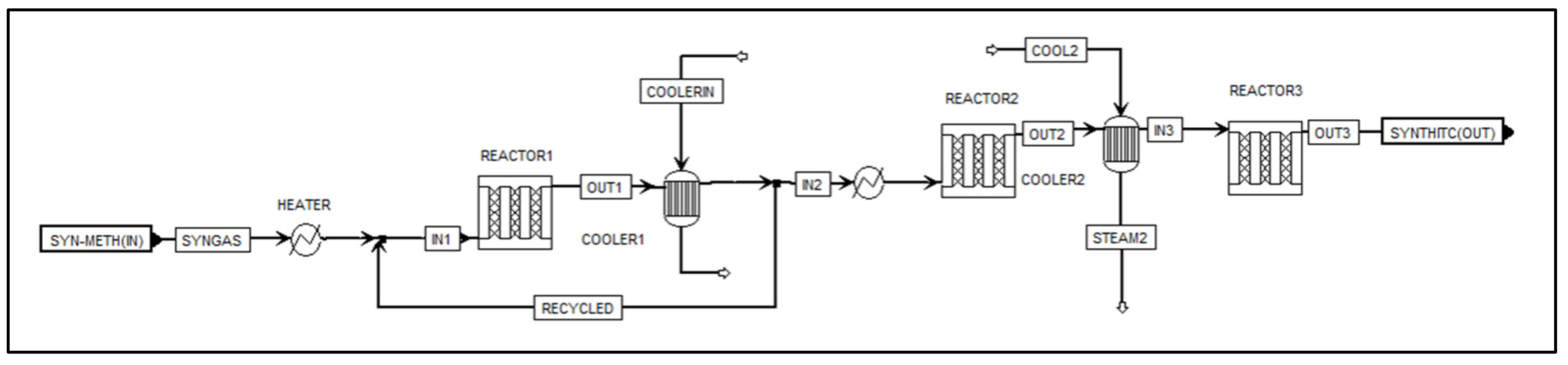
2.5. Aspen Plus Syngas Methanation Model
2.6. Process Evaluation
3. Results
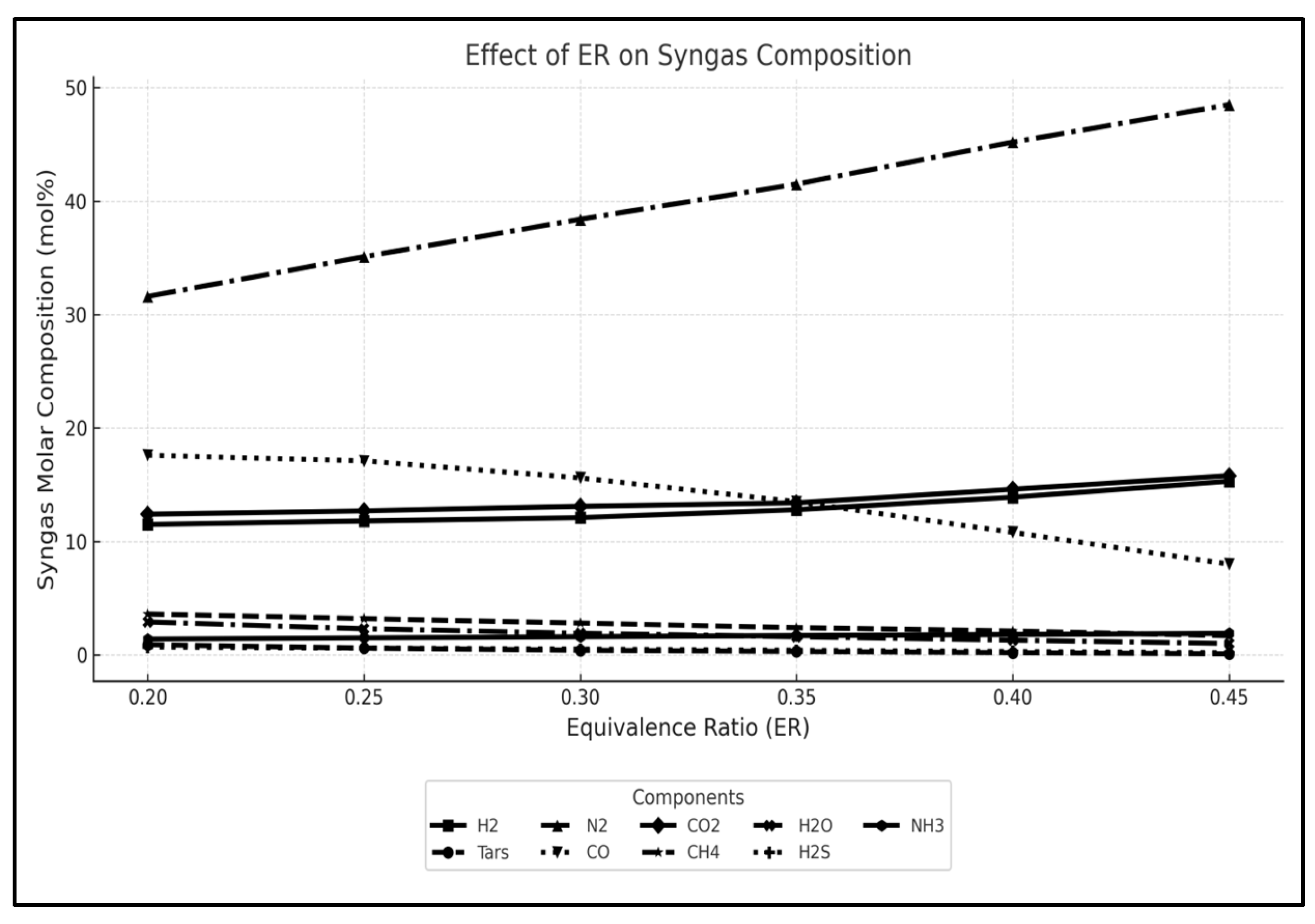
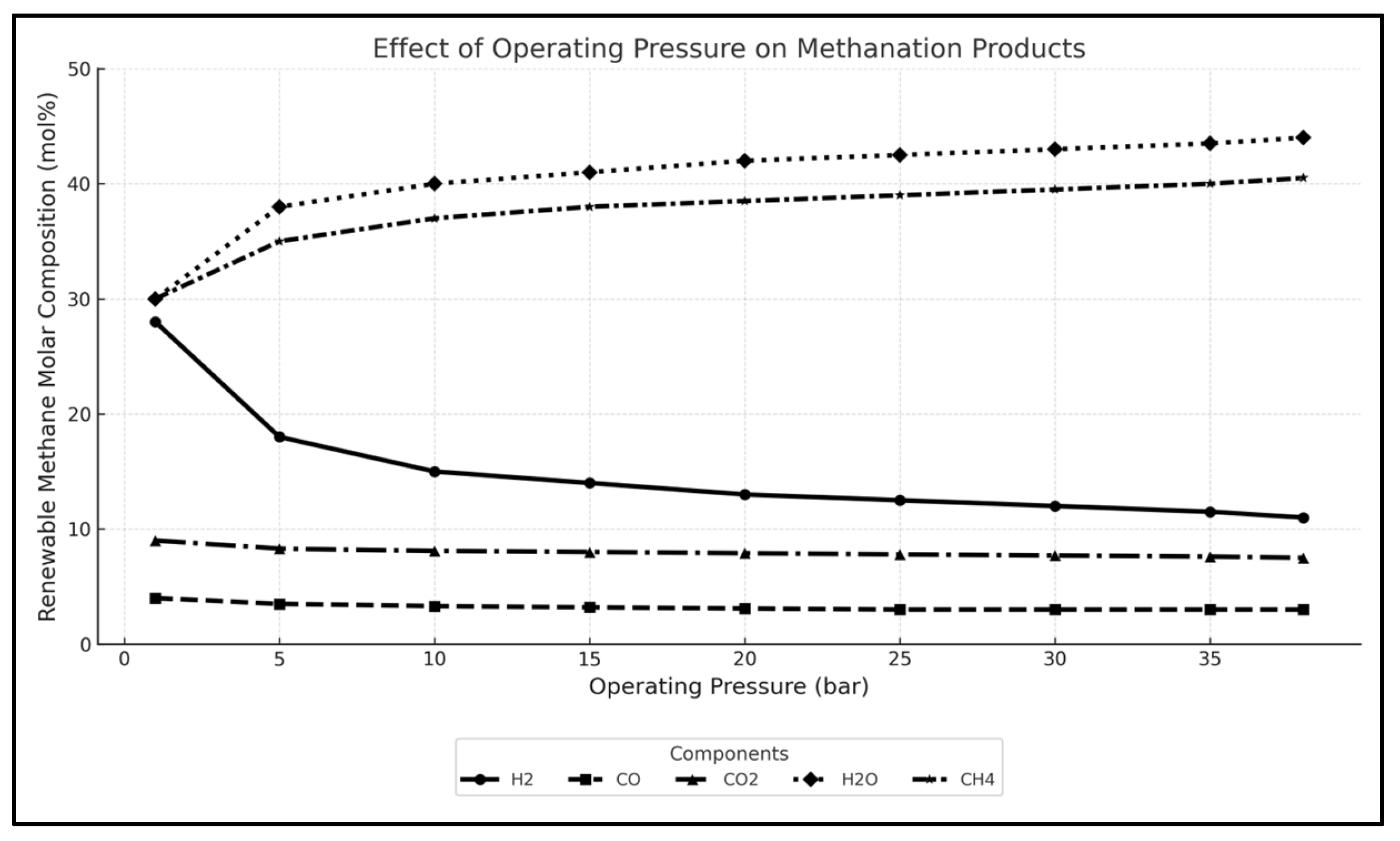
3.1. Sensitivity Analysis
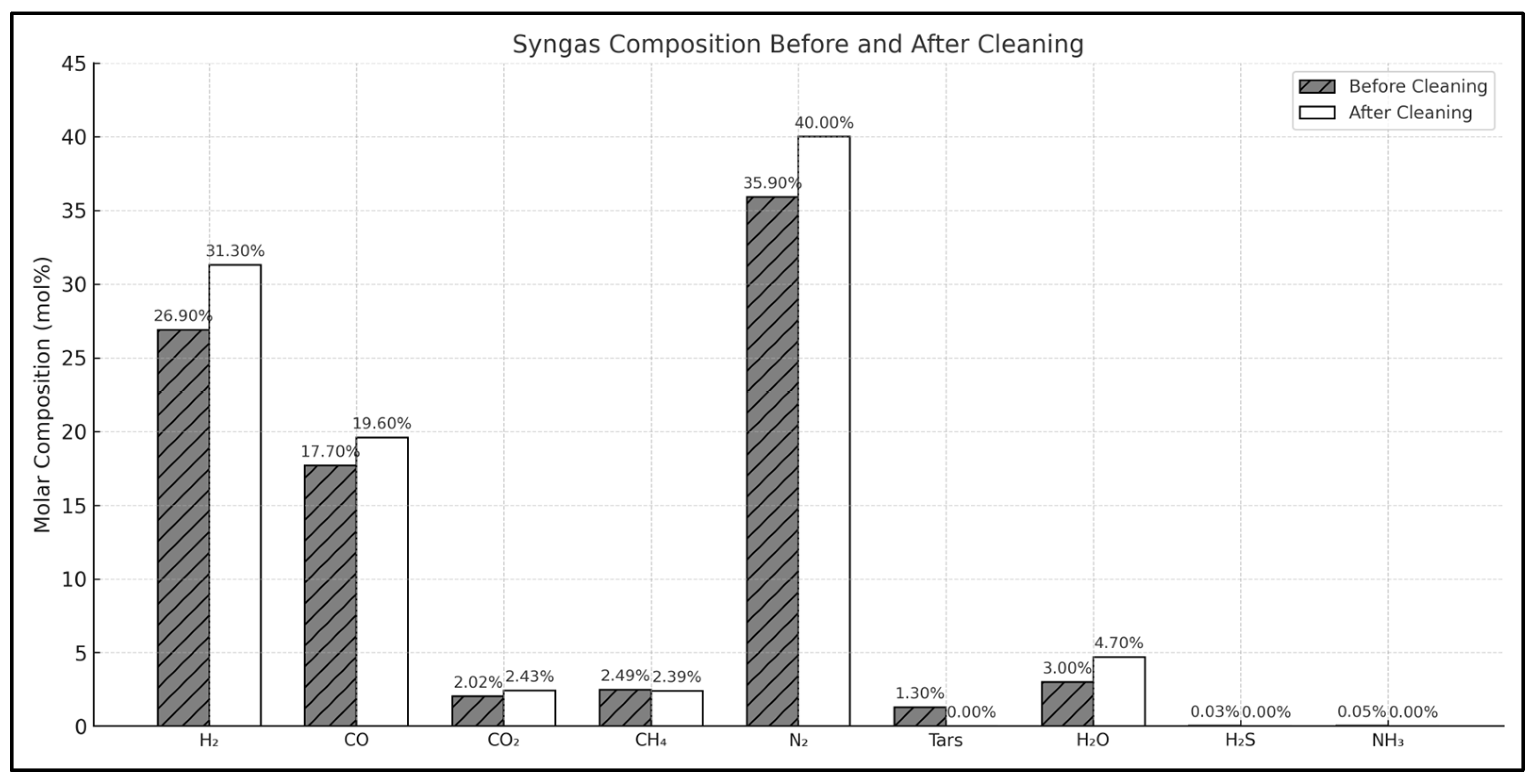
3.2. Syngas Cleaning
3.3. System Level Efficiency
3.4. Model Validation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Biomass Gasification Chemical Reactions Kinetics
| Reaction | Reaction Kinetics | Reference | ||
|---|---|---|---|---|
| Dense Zone | Partial oxidation | [43,44,45] | ||
Mc = 12.01 kg/kmol and g/m3 | ||||
| Hydrogen oxidation | [] [O2] | |||
| Dilute Zone | Heterogeneous Water-gas | [H2O] [H2][CO] | [46,47,48] | |
| Boudouard | [CO2] [CO]2 | |||
| Methanation | [H2] []0.5 | |||
| Water-gas shift | [CO]0.5[ [H2]0.5 [] | [49,50] | ||
| Methane-reforming | [] [] [CO] [H2]2 | [46,50,51] |
Appendix A.2. Methanation Chemical Reaction Kinetics
- CO methanation reaction
- Water-Gas-Shift reaction
- CO2 methanation reaction [52]
| CO Methanation Reaction | WGS Reaction | Steam Reforming Reaction |
|---|---|---|
| 7.83 | 1.25 | |
| 8.23 (bar) | ||
| 6.12 (bar) | ||
| 6.65 (bar) | () | |
| 1.77 (bar) | () | |
References
- de Jong, W.; van Ommen, J.R. Biomass as a Sustainable Energy Source for the Future: Fundamentals of Conversion Processes; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Sofer, S.S.; Zaborsky, O.R. Biomass Conversion Processes for Energy and Fuels, 2nd ed.; Plenum Press: New York, NY, USA, 1981. [Google Scholar] [CrossRef][Green Version]
- Kohl, A.L.; Nielsen, R. Gas Purification, 5th ed.; Gulf Pub: Houston, TL, USA, 1997. [Google Scholar][Green Version]
- Mills, G.A.; Steffgen, F.W. Catalytic Methanation. Sci. Eng. 2006, 8, 159–210. [Google Scholar] [CrossRef]
- Hayes, R.E.; Thomas, W.J.; Hayes, K.E. A study of the nickel-catalysed methanation reaction. J. Catal. 1985, 92, 312–326. [Google Scholar] [CrossRef]
- Twigg, M.V. Catalyst Handbook, 2nd ed.; Manson Publishing: London, UK, 1996. [Google Scholar][Green Version]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation—From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Hohlein, M.B.; Niessen, H.; Range, J.; Schiebahn, H.J.R.; Vorwerk, M. Methane from synthesis gas and operation of high-temperature methanation. Nucl. Eng. Des. 1984, 78, 241–250. [Google Scholar] [CrossRef]
- Kernforschungsanlage Jülich GmbH. Projekt Nukleare Fernenergie: Zusammenfassender Bericht zum Projekt Nukleare Fernenergie (NFE); Internal Report; Kernforschungsanlage Jülich GmbH: Jülich, Germany, 1985. [Google Scholar][Green Version]
- Kopyscinski, J. Production of Synthetic Natural Gas in a Fluidised Bed Reactor; Eidgenössische Technische Hochschule ETH Zürich: Zürich, Switzerland, 2010. [Google Scholar] [CrossRef]
- Seemann, M. Methanation of Biosyngas in a Fluidized Bed Reactor. 2006. Available online: https://research.chalmers.se/en/publication/125548 (accessed on 6 April 2023).
- Swindon Plant. Advanced Biofuel Solutions Ltd. Available online: https://absl.tech/swindon-plant (accessed on 20 June 2024).
- ENGIE Produces Renewable Gas from Solid Non-Recyclable Waste Gasification|Bioenergy International. Bioenergy International [Online]. 2024. Available online: https://bioenergyinternational.com/engie-produces-renewable-gas-from-solid-non-recyclable-waste-gasification/ (accessed on 7 October 2024).
- Begum, S.; Rasul, M.G.; Akbar, D.; Ramzan, N. Performance Analysis of an Integrated Fixed Bed Gasifier Model for Different Biomass Feedstocks. Energies 2013, 6, 6508–6524. [Google Scholar] [CrossRef]
- Suwatthikul, A.; Limprachaya, S.; Kittisupakorn, P.; Mujtaba, I.M. Simulation of Steam Gasification in a Fluidised Bed Reactor with Energy Self-Sufficient Condition. Energies 2017, 10, 314. [Google Scholar] [CrossRef]
- Damartzis, T.; Michailos, S.; Zabaniotou, A. Energetic assessment of a combined heat and power integrated biomass gasification–internal combustion engine system by using Aspen Plus®. Fuel Process. Technol. 2012, 95, 37–44. [Google Scholar] [CrossRef]
- Eikeland, M.S.; Thapa, R.K.; Halvorsen, B.M. Aspen Plus Simulation of Biomass Gasification with known Reaction Kinetic. In Proceedings of the 56th Conference on Simulation and Modelling (SIMS 56), Linköping, Sweden, 7–9 October 2015; Volume 119, pp. 149–156. [Google Scholar] [CrossRef]
- Höhlein, B.; Menzer, R.; Range, J. High temperature methanation in the long-distance nuclear energy transport system. Appl. Catal. 1981, 1, 125–139. [Google Scholar] [CrossRef]
- Topsøe, H. From Solid Fuels to Substitute Natural Gas (SNG) Using TREMPTM Topsøe Recycle Energy-Efficient Methanation Process; Haldor Topsøe: Lyngby, Denmark, 2009. [Google Scholar]
- Bouallou, C. Modeling and simulation of CO methanation process for renewable electricity storage. Energy 2014, 75, 81–88. [Google Scholar] [CrossRef]
- Zhang, J.; Fatah, N.; Capela, S.; Kara, Y.; Guerrini, O.; Khodakov, A.Y. Kinetic investigation of carbon monoxide hydrogenation under realistic conditions of methanation of biomass derived syngas. Fuel 2013, 111, 845–854. [Google Scholar] [CrossRef]
- Santos, J.; Hornung, A.; Ouadi, M. Thermochemical Conversion of Biomass and Upgrading of Bio-Products to Produce Fuels and Chemicals. In Catalysis for Clean Energy and Environmental Sustainability: Biomass Conversion and Green Chemistry; Pant, A.E., Gupta, K.K., Eds.; Springer International Publishing: Cham, Switzerland, 2021; Volume 1, pp. 1–47. [Google Scholar] [CrossRef]
- Sreejith, C.C.; Muraleedharan, C.; Arun, P. Performance prediction of steam gasification of wood using an Aspen Plus thermodynamic equilibrium model. Int. J. Sustain. Energy 2014, 33, 416–434. [Google Scholar] [CrossRef]
- Wang, Y.; Kinoshita, C.M. Kinetic model of biomass gasification. Sol. Energy 1993, 51, 19–25. [Google Scholar] [CrossRef]
- Simell, P.; Kurkela, E.; Ståhlberg, P.; Hepola, J. Catalytic hot gas cleaning of gasification gas. Catal. Today 1996, 27, 55–62. [Google Scholar] [CrossRef]
- Florin, N.H.; Harris, A.T. Hydrogen production from biomass coupled with carbon dioxide capture: The implications of thermodynamic equilibrium. Int. J. Hydrogen Energy 2007, 32, 4119–4134. [Google Scholar] [CrossRef]
- Giakoumis, E.G. Analysis of 22 vegetable oils’ physico-chemical properties and fatty acid composition on a statistical basis, and correlation with the degree of unsaturation. Renew. Energy 2018, 126, 403–419. [Google Scholar] [CrossRef]
- Ammonia in Syngas. [Online]. Available online: https://www.envitechinc.com/air-pollution-control-innovations/bid/20554/Ammonia-in-Syngas (accessed on 6 December 2022).
- Fahim, M.A.; Alsahhaf, T.A.; Elkilani, A. Acid Gas Processing and Mercaptans Removal. In Fundamentals of Petroleum Refining, 1st ed.; Elsevier: Oxford, UK, 2010; Volume 15, pp. 377–402. [Google Scholar] [CrossRef]
- Aziz, M.; Darmawan, A.; Juangsa, F.B. Hydrogen production from biomasses and wastes: A technological review. Int. J. Hydrogen Energy 2021, 46, 33756–33781. [Google Scholar] [CrossRef]
- Sazali, N. Emerging technologies by hydrogen: A review. Int. J. Hydrogen Energy 2020, 45, 18753–18771. [Google Scholar] [CrossRef]
- Wulf, C.; Linssen, J.; Zapp, P. Power-to-Gas—Concepts, Demonstration, and Prospects. In Hydrogen Supply Chain: Design, Deployment and Operation; Academic Press: London, UK, 2018; Volume 9, pp. 309–345. [Google Scholar] [CrossRef]
- Cai, Q.; Luna-Ortiz, E.; Adjiman, C.S.; Brandon, N.P. The Effects of Operating Conditions on the Performance of a Solid Oxide Steam Electrolyser: A Model-Based Study. Fuel Cells 2010, 10, 1114–1128. [Google Scholar] [CrossRef]
- Defner, B.; Krenn, U.-P.H. Modeling and Simulation of System Concepts for High-Temperature, Solid Oxide Electrolysis. Master’s Thesis, University of Graz, Graz, Austria, 2016. [Google Scholar]
- Ni, M.; Leung, M.K.; Leung, D.Y. Parametric study of solid oxide fuel cell performance. Energy Convers. Manag. 2007, 48, 1525–1535. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Ping, Y.; Hu, D.; Xu, G.; Gu, F.; Su, F. A thermodynamic analysis of methanation reactions of carbon oxides for the production of synthetic natural gas. RSC Adv. 2012, 2, 2358. [Google Scholar] [CrossRef]
- Wan, C.; Yu, F.; Zhang, Y.; Li, Q.; Wooten, J. Material Balance and Energy Balance Analysis for Syngas Generation by a Pilot-Plant Scale Downdraft Gasifier. J. Biobased Mater. Bioenergy 2013, 7, 690–695. [Google Scholar] [CrossRef]
- Chein, R.-Y.; Yu, C.-T.; Wang, C.-C. Numerical simulation on the effect of operating conditions and syngas compositions for synthetic natural gas production via methanation reaction. Fuel 2016, 185, 394–409. [Google Scholar] [CrossRef]
- Wu, J.; Xu, B.; Lou, Z.; Zhou, X. Performance analysis of a biomass circulating fluidized bed gasifier. Biomass-Bioenergy 1992, 3, 105–110. [Google Scholar] [CrossRef]
- Jens Rostrup-Nielsen, L.; Christiansen, J. Concepts in Syngas Manufacture; Imperial College Press: London, UK, 2011. [Google Scholar]
- Samipour, S.; Manshadi, M.D.; Setoodeh, P. CO2 Removal from Biogas and Syngas. In Advances in Carbon Capture; Elsevier: Amsterdam, The Netherlands, 2020; pp. 455–477. [Google Scholar] [CrossRef]
- You, Y.-W.; Lee, D.-G.; Yoon, K.-Y.; Moon, D.-K.; Kim, S.M.; Lee, C.-H. H2 PSA purifier for CO removal from hydrogen mixtures. Int. J. Hydrogen Energy 2012, 37, 18175–18186. [Google Scholar] [CrossRef]
- Petersen, I.; Werther, J. Experimental investigation and modeling of gasification of sewage sludge in the circulating fluidized bed. Chem. Eng. Process.-Process. Intensif. 2005, 44, 717–736. [Google Scholar] [CrossRef]
- Westbrook, C.K.; Dryer, F.L. Chemical kinetic modeling of hydrocarbon combustion. Prog. Energy Combust. Sci. 1984, 10, 1–57. [Google Scholar] [CrossRef]
- Champion, W.M.; Cooper, C.D.; Mackie, K.R.; Cairney, P. Development of a chemical kinetic model for a biosolids fluidized-bed gasifier and the effects of operating parameters on syngas quality. J. Air Waste Manag. Assoc. 2013, 64, 160–174. [Google Scholar] [CrossRef]
- Syamlal, M.; Bissett, L.A. METC Gasifier Advanced Simulation (MGAS) Model; Technical Report No. DOE/MC/24146–3117; U.S. Department of Energy, Morgantown Energy Technology Center: Morgantown, WV, USA, 1992.
- Solli, K.-A.; Thapa, R.K.; Moldestad, B.M.E. Screening of Kinetic Rate Equations for Gasification Simulation Models. In Proceedings of the 9th EUROSIM 2016 & SIMS 2016, Oulu, Finland, 12–16 September 2016. [Google Scholar] [CrossRef]
- de Souza-Santos, M. Comprehensive modelling and simulation of fluidized bed boilers and gasifiers. Fuel 1989, 68, 1507–1521. [Google Scholar] [CrossRef]
- Snider, D.M.; Clark, S.M.; O’ROurke, P.J. Eulerian–Lagrangian method for three-dimensional thermal reacting flow with application to coal gasifiers. Chem. Eng. Sci. 2011, 66, 1285–1295. [Google Scholar] [CrossRef]
- Bustamante, F.; Enick, R.M.; Cugini, A.; Killmeyer, R.P.; Howard, B.H.; Rothenberger, K.S.; Ciocco, M.V.; Morreale, B.D.; Chattopadhyay, S.; Shi, S. High-temperature kinetics of the homogeneous reverse water–gas shift reaction. AIChE J. 2004, 50, 1028–1041. [Google Scholar] [CrossRef]
- Gómez-Barea, A.; Leckner, B. Modeling of biomass gasification in fluidized bed. Prog. Energy Combust. Sci. 2010, 36, 444–509. [Google Scholar] [CrossRef]
- Xu, J.; Froment, G.F. Methane steam reforming, methanation and water-gas shift: I. Intrinsic kinetics. AIChE J. 1989, 35, 88–96. [Google Scholar] [CrossRef]
- Elnashaie, S.S.E.H.; Elshishini, S.S. Modelling, Simulation, and Optimization of Industrial Fixed Bed Catalytic Reactors; Gordon and Breach Science: London, UK, 1993. [Google Scholar]

| Ultimate Analysis (wt%) | Proximate Analysis | ||
|---|---|---|---|
| C | 46.4 | Moisture | 9 wt% |
| H | 6.1 | Fixed Carbon | 20.3 wt% |
| N | <0.10 | Volatiles | 70.9 wt% |
| S | <0.10 | HHV | 18.6 MJ/kg |
| O | 46.6 | ||
| Ash | 0.7 | ||
| S1 | S2 | S3 | |
|---|---|---|---|
| Biomass gasification | 78% | 78% | 78% |
| Syngas cleaning | 85% | 85% | 85% |
| WGS | 90.6% | - | - |
| CO2 capture post-WGS | 80% | - | - |
| SOE | - | 85% | - |
| PSA for H2-CO splitting | - | - | 76.5% |
| Methanation | 82.4% | 81.6% | 81.2% |
| overall efficiency | 59% | 62% | 44.36% |
| Scenario | Key Features | Overall Efficiency | Strengths | Weaknesses |
|---|---|---|---|---|
| S1 | Adjust H2/CO via WGS, CO2 capture | 59% | High methane yield, CO2 capture | Energy loss due to CO2 capture, moderate efficiency |
| S2 | H2 added via SOE | 62% | Potential for renewable integration | Low SOE efficiency, the lowest overall efficiency |
| S3 | H2/CO separation and recombination for a 3:1 ratio | 44.36% | Higher efficiency, more straightforward process | No CO2 capture, efficiency loss during separation |
| Gasification Model | Experiment [39] | Relative Error | |
|---|---|---|---|
| H2 | 21.4% | 16.3% | 5.0% |
| CO | 15.3% | 16.7% | 1.4% |
| CO2 | 19.0% | 15.6% | 3.4% |
| CH4 | 6.3% | 6.8% | 0.4% |
| N2 | 38.0% | 43.3% | 5.3% |
| Carbon conversion (%) | 84.8% | 94.9% | 10.1% |
| HHV (MJ/m3) | 6.84 | 6.87 | 3.2% |
| Cold gas efficiency (CGE) | 82.4% | 75.2% | 7.2% |
| Feed | Recycled Stream | Rin1 | Rout1 | Rout2 | Rout3 | ||
|---|---|---|---|---|---|---|---|
| Aspen Plus Methanation Model | Gas flow (m3(STP)/h) | 492.5 | 562.54 | 1061.99 | 907.22 | 308.64 | 305.91 |
| Pressure (bar) | 27 | ||||||
| Temperature °C | 17 | 233 | 239 | 631 | 405 | 348 | |
| H2 | 67.60% | 20.67% | 42.39% | 20.67% | 5.43% | 4.80% | |
| H2 (mol%) relative error | (5.03%) | (2.51%) | (5.03%) | (4.54%) | (2.17%) | ||
| CO | 10.38% | 0.24% | 4.94% | 0.24% | 1.41% | 1.18% | |
| CO (mol%) relative error | (1.57%) | (0.80%) | (1.57%) | (1.37%) | (1.18%) | ||
| CO2 | 8.93% | 4.72% | 6.67% | 4.72% | 0.01% | 0.02% | |
| CO2 (mol%) relative error | (0.07%) | (0.02%) | (0.07%) | (2.46%) | (0.69%) | ||
| CH4 | 9.83% | 37.81% | 24.86% | 37.81% | 44.71% | 45.14% | |
| CH4 (mol%) relative error | (3.13%) | (1.57%) | (3.13%) | (1.56%) | (1.65%) | ||
| H2O | 0.07% | 31.88% | 17.15% | 31.88% | 43.38% | 43.79% | |
| H2O (mol%) relative error | (3.18%) | (1.57%) | (3.18%) | (3.81%) | (1.17%) | ||
| N2 | 3.19% | 4.68% | 3.99% | 4.68% | 5.05% | 5.07% | |
| N2 (mol%) relative error | (3.18%) | (1.57%) | (3.18%) | (3.81%) | (1.17%) | ||
| ADAM I Project | Gas flow (m3(STP)/h) | 441 | 521 | 962 | 832 | 282 | 271 |
| Pressure (bar) | 27 | 33.4 | 26.85 | 26.7 | 26.6 | 26.5 | |
| Temperature °C | 17 | 193/239 | 306 | 651 | 485 | 343 | |
| H2 | 67.59% | 25.70% | 44.90% | 25.70% | 9.97% | 2.63% | |
| CO | 10.38% | 1.81% | 5.74% | 1.81% | 0.04% | 0% | |
| CO2 | 8.93% | 4.79% | 6.69% | 4.79% | 2.47% | 0.71% | |
| CH4 | 9.83% | 34.68% | 23.29% | 34.68% | 43.15% | 46.79% | |
| H2O | 0.07% | 28.70% | 15.58% | 28.70% | 39.57% | 44.96% | |
| N2 | 3.19% | 4.32% | 3.80% | 4.32% | 4.80% | 4.90% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Zakwani, S.; Ouadi, M.; Mohammed, K.; Steinberger-Wilckens, R. Simulation of Biomass Gasification and Syngas Methanation for Methane Production with H2/CO Ratio Adjustment in Aspen Plus. Energies 2025, 18, 4319. https://doi.org/10.3390/en18164319
Al Zakwani S, Ouadi M, Mohammed K, Steinberger-Wilckens R. Simulation of Biomass Gasification and Syngas Methanation for Methane Production with H2/CO Ratio Adjustment in Aspen Plus. Energies. 2025; 18(16):4319. https://doi.org/10.3390/en18164319
Chicago/Turabian StyleAl Zakwani, Suaad, Miloud Ouadi, Kazeem Mohammed, and Robert Steinberger-Wilckens. 2025. "Simulation of Biomass Gasification and Syngas Methanation for Methane Production with H2/CO Ratio Adjustment in Aspen Plus" Energies 18, no. 16: 4319. https://doi.org/10.3390/en18164319
APA StyleAl Zakwani, S., Ouadi, M., Mohammed, K., & Steinberger-Wilckens, R. (2025). Simulation of Biomass Gasification and Syngas Methanation for Methane Production with H2/CO Ratio Adjustment in Aspen Plus. Energies, 18(16), 4319. https://doi.org/10.3390/en18164319







