Abstract
The energy self-sufficiency of wastewater treatment plants has become an essential aspect of sustainable water and energy resource management. On the other hand, due to the expansion of urban conglomerations and agricultural activities, as well as more frequent and erratic meteorological phenomena (e.g., droughts), the majority of EU nations are confronted with water scarcity and the deterioration of water quality. As a consequence, EU member states pledged to implement “tertiary treatment” in all municipal wastewater treatment facilities by the end of 2040. This publication presents an analysis of the efficiency of an energy-efficient gravity cloth disk filter used for treating municipal wastewater in a treatment plant located in a tourist resort in Poland, operating under variable hydraulic loading conditions. Gravity cloth disk filters appear to be the least energy-consuming. The energy consumption of disk filters was 13 Wh/m3 in 2024. The filter ensures the leveling of disturbances in the operation of earlier treatment stages, particularly in terms of retaining total suspended solids (TSSs). The achieved efficiency of TSS removal was 45%. The TSS value in the outflow from the filter did not exceed the limit value from the permit (35 mg/L). When operated correctly, additional filtration and disinfection may become essential components of a wastewater treatment plant, enabling the achievement of wastewater quality that supports water recovery for technological and agricultural purposes, particularly in small, non-industrial areas. They should also consume less energy than other advanced technologies used in the third and fourth stages of wastewater treatment.
1. Introduction
The new Directive (EU) 2024/3019 of the European Parliament and of the Council, dated 27 November 2024, concerning the treatment of municipal wastewater, requires member states to, among other things, tighten the parameters for treating municipal wastewater. Its goal is to improve surface and groundwater quality and reduce the amount of pollutants entering the soil and groundwater. The directive indicates, among other things, that by 2039, all municipal wastewater treatment plants serving agglomerations with a population of 150,000 or more will be required to have increased removal of nitrogen and phosphorus from wastewater at levels of 8.0 and 0.5 mg/L (tertiary treatment). By 2045, a maximum concentration of nutrients in the effluent of 10 mg N/L and 0.7 mg P/L will be required for treatment plants with a population equivalent (p.e.) of 10,000 and above. The directive also introduces the need for additional wastewater treatment that removes a broad spectrum of micropollutants (“stage IV treatment”), which by 2045 will be mandatory for all treatment plants with a p.e. of 150,000 or more, and those with a p.e. of 10,000 or more, based on a risk assessment [1].
At the same time, the directive obliges EU member states to maintain energy self-sufficiency and to systematically promote the reuse of treated wastewater before it is discharged into the environment, particularly in areas affected by water scarcity. When used for irrigation in agriculture, reclaimed water should meet the requirements of Regulation (EU) 2020/741 on water reuse [2,3,4,5]. This regulation establishes the minimum requirements for reusing reclaimed water for agricultural irrigation in a circular economy, depending on the crop type. For example, the highest quality class of reclaimed water, A, is used for the irrigation of crops for consumption in their raw state, the edible part of which is in direct contact with the reclaimed water. The requirements for reclaimed water in class A are as follows: turbidity (TB) ≤ 5 NTU, total suspended solids (TSSs) ≤ 10 mg/L, biochemical oxygen demand (BOD) ≤ 10 mg/L, and E. coli ≤ 10 CFU/100 mL [4].
The adopted changes mean a significant transformation of the infrastructure and technology used in wastewater treatment plants throughout the EU. Increasing emphasis is being placed not only on the quality of treatment and the possibility of reusing water, but also on energy efficiency and the pursuit of energy self-sufficiency for these facilities. Already, in some European countries, such as Germany, Denmark, France, and Italy, recycled water is widely used, and treatment plants are increasingly using renewable energy sources and energy recovery from sewage sludge to minimize external energy consumption [4,6,7,8,9,10].
Today, traditional wastewater treatment plants (WWTPs) operate based on three basic wastewater treatment methods: physical (mechanical), chemical, and biological. Based on these, multi-process wastewater treatment systems are built. These processes are conventionally divided into four stages. Stage I is the so-called pre-treatment, which involves removing contaminants in the form of larger floating bodies (screenings), sand, suspended solids, oils, and fats. Stage II is biological treatment (or chemical treatment, less commonly used for pollutant removal), in which the aim is to reduce the pollutant load, expressed in terms of the value of the biochemical oxygen demand BOD5. Due to the equipment used, the second stage of treatment is one of the most energy-intensive ones in the WWTP. After biological treatment, a leftover surplus activated sewage sludge needs separate treatment [11].
The new guidelines introduce an obligatory stage III of wastewater treatment, utilizing both chemical and biological methods. This stage aims to reduce further pollutants, as determined by the value of BOD, and achieve the highly effective removal of nitrogen and phosphorus compounds, utilizing, among other energy-efficient processes, physical methods such as gravity sedimentation or filtration.
The filtration process involves the separation of solid and liquid phases. The medium directed to the porous bed is a suspension, i.e., a mixture of solid impurities in the liquid phase. The retention of impurities from the mixture is based on the mechanical action of the filter layer, which involves wedging solid impurities into its pores, i.e., colmatation. Colmatation is a complex phenomenon through which, in addition to the mechanical retention of the solid phase in the filter bed, physical, chemical, and biological colmatation can occur. Physical–chemical colmatation is caused by the adsorption of solid phase particles on the surface of filter layer grains, most often under conditions of the electrostatic polarization of these grains. Chemical colmatation is caused by depositing insoluble mineral substances under the given conditions. Biological colmatation, on the other hand, is caused by the retention of bacteria in the sediment, whose activity can increase the colmatation process [12,13].
A precise description of the filtration process is complicated. Hence, conducting experiments over the broadest possible range of parameter variation is necessary, as the gravitational inflow of liquids into the filter layer can occur under different conditions. For example, two variants of such a process model can be distinguished. The first one has colmatation without the growth of the sediment layer (single-phase wastewater at constant and variable pressure on the bed). The second one would be colmatation with the increase in the sediment layer (a mixture of water and solids at constant and variable pressures in the porous layer). In practice, filtration is implemented using various devices, including gravity filters, vacuum filters, pressure filters, filter presses, and centrifuges [12,13].
Systems for recovering water from treated municipal wastewater in the third stage of treatment use structurally similar modern solutions for continuous filtration in the form of so-called disk filters (Culligan, Krevox, Huber). These types of technologies not only ensure the high quality of reclaimed wastewater for reuse, but also contribute to the idea of increasing energy efficiency and the treatment plant’s pursuit of energy self-sufficiency [14,15,16].
The final IV stage of treatment is known as water renewal, which involves the removal of micropollutants, including chemical contaminants introduced into the wastewater during an earlier stage of treatment. Currently, physical–chemical processes such as adsorption, membrane filtration, advanced oxidation processes (AOPs), and final disinfection via ozonation or UV lamps are most commonly employed [17].
The energy aspect of wastewater treatment is still rarely discussed, as it has always been secondary to the effectiveness of removing pollutants from wastewater. Under the new EU regulations, which require member states to upgrade all wastewater treatment plants with the III and IV stages of treatment, energy efficiency will not be one of the options for improving the energy balance of treatment plants, but will become a criterion for the operation of a facility at socially acceptable treatment costs, e.g., per m3 of wastewater.
This study aimed to evaluate the effectiveness of stage III wastewater treatment using an energy efficient filtration process under seasonal changes in the hydraulic load of a municipal wastewater treatment plant in a selected coastal town.
2. Materials and Methods
2.1. Wastewater Treatment Plant
The study was conducted at a wastewater treatment plant (WWTP) located in a coastal town. The treatment plant operates using a standard system for municipal treatment plants, which involves mechanical and biological treatment in the activated sludge process, with the highly efficient removal of nutrients—Enhanced Biological Nutrient Removal (EBNR). Due to its sensitive location (coastal area), the WWTP must maintain the stability of the treated wastewater parameters regardless of the operating conditions. They change due to the high variability in the hydraulic load, especially during the summer season. Table 1 presents the water permit conditions for wastewater quality parameters applicable to the studied WWTP. In 2023, the WWTP was modernized, achieving a maximum capacity of 6000 m3/d and 53,800 p.e. Since then, the studied WWTP’s technological system has been using elements of stage III (high-efficiency nutrient removal using final filtration, Figure 1 and Figure 2a,b) and stage IV (final disinfection of wastewater with UV light) for municipal wastewater treatment, also allowing for wastewater recycling and water recovery.

Table 1.
Permissible values of parameters in treated municipal wastewater according to the water permit for the studied WWTP.

Figure 1.
View of Mecana AG disk filter: (a) rotating filter disks; (b) filter assembly; and (c) filter segments. Source: [18].
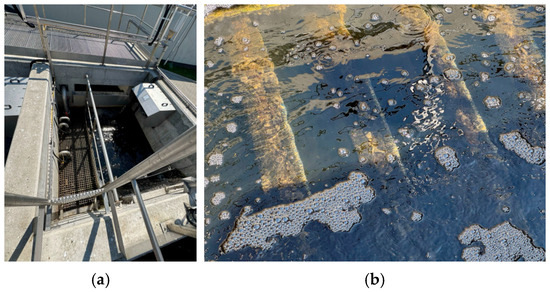
Figure 2.
Elements of the final wastewater treatment of the studied WWTP: (a) gravity filtration chamber; (b) rotary filter disks.
The studied WWTP uses a filter with a maximum capacity of 575 m3/h from Mecana AG [18]—Figure 1. Wastewater is transported from the outlet of the secondary settling tanks to a concrete tank that houses the filter. It consists of 6 disks, each 2.1 m in diameter. The total area is 30 m2 (Figure 1b). Each disk consists of 6 segments individually attached to a rotating shaft that acts as a central filtrate pipe. Each segment is covered with a filter fabric (OptiFiber PES-13, Mecana AG (Reichenburg, Switzerland)) (Figure 1c). The shaft on the outlet side sits on two non-metallic support rollers and one guide roller, and on plain bearings on the inlet side.
Figure 2 presents the filter unit mounted in the examined WWTP. The filter structure is mounted in the tank (Figure 2a) on 4 wall brackets, and the filter disks are always completely submerged in the wastewater (Figure 2b). The minimum wastewater level is set by a baffle installed at the outlet.
As a result of the gravitational flow of wastewater, the suspended solids are retained on the porous filter fabric. The filtrate is directed through the central shaft and ascending chamber to the overflow baffle. The progressing colmatation causes an increase in filtration resistance and, consequently, in the level of wastewater (inflow) in the tank. When the limit level is reached (about 25–35 cm above the minimum wastewater level), the control system starts the cleaning cycle of the filter fabric. The motor rotates disks (1–2 min−1), and the pump, through the suction heads adjacent to the filter fabric, removes the solids accumulated on its surface and transports them back to the previous stage of the treatment process (e.g., the primary settling tank). After the cleaning phase, the resistance of the filter decreases, and the inflowing wastewater level drops. The flow rate again reaches its maximum value at the minimum level of the wastewater in the chamber.
The manufacturer of the gravity filter states that the average energy consumption is low, at 3 Wh/m3 of treated wastewater. In 2024, the WWTP treated 1,232,269 m3 of wastewater. Taking into account the filter manufacturer’s data, the calculated theoretical energy consumption of the filter was 3697 kWh. In 2024, the cost of 1 MWh of energy in Poland was approximately EUR 189, so the estimated annual operating cost of the gravity filter was approximately EUR 697. However, according to the data provided by the WWTP operator, the gravity filter consumed 15,859 kWh in 2024. Therefore, the actual unit energy consumption (12.87 Wh/m3) differs substantially from the manufacturer’s data (3 Wh/m3). The estimated cost of energy used by the filter in 2024 was approximately EUR 2997.
2.2. Technological and Analytical Procedure
The sampling of wastewater was conducted before (S1) and after the filtration chamber (S2) over a wide time range, from 29 April 2024 to 12 December 2024, as shown in Figure 3.
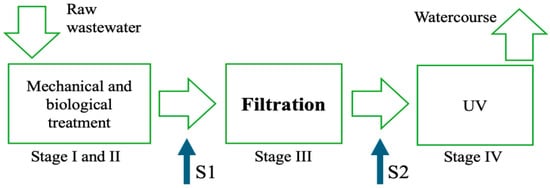
Figure 3.
Sampling points (S1 and S2) on the simplified technological scheme of the studied WWTP.
Samples of wastewater treated via mechanical–biological processes (S1, Figure 3) and treated additionally via filtration (S2, Figure 3) were analyzed on site and in the laboratory of the Department of Water, Wastewater and Waste Technology, Koszalin University of Technology. The outcome variable parameters analyzed on site were temperature T [°C], pH, specific conductivity SC [μS/cm], and turbidity TB [NTU]. Other analyses performed in the laboratory were as follows: total suspended solids, TSSs [mg/L] (direct weighing method), chemical oxygen demand, COD [mg/L] (COD Digestion Vials, Low Range, spectrophotometric measurement, Hach Method 8000, Hach (Loveland, CO, USA)), total organic carbon, TOC [mg/L] (Shimadzu TOC/VCPH analyzer with IR detection, Shimadzu (Kyoto, Japan)), orthophosphate, PO4-P [mg/L], ammonium nitrogen, NH4-N [mg/L], and nitrate nitrogen, NO3-N [mg/L] (Hach DR5000 spectrophotometer, Hach (Loveland, CO, USA)).
As part of the statistical analysis, a hypothesis test was conducted to verify whether the gravitational filtration process using Mecana AG SF6/30 fabric disk filters resulted in a significant reduction in the analyzed output variables. For this purpose, the null hypothesis H0: = 0 was tested against the alternative hypothesis H1: ≠ 0, where denotes the mean difference between influent and filtrate values in the entire population. The analyses were performed at a significance level of α = 0.05, with n − 1 degrees of freedom (where n is the number of measurements). The calculations are presented in tabular form, including variance σ2, standard deviation σ, Student’s t-test statistic, and the critical value from Student’s t-distribution. In addition, the mean values and standard deviations σ of the examined output parameters were calculated. Based on these results, the average efficiency of the Mecana AG SF6/30 fabric disk filters in the third stage of wastewater treatment was computed and graphically illustrated.
3. Results and Discussion
The study revealed that, despite the variability in hydraulic loading observed within the analyzed time range of 74,744 to 166,932 m3/month (Figure 4a), the WWTP operated at a high level of efficiency, which is attributed to the facility’s recent modernization. During sampling, there were no disturbances in the operation of the mechanical–biological system, and the average daily wastewater inflow to the plant was from 2144 m3/d in December to 5228 m3/d in July (Figure 4b).
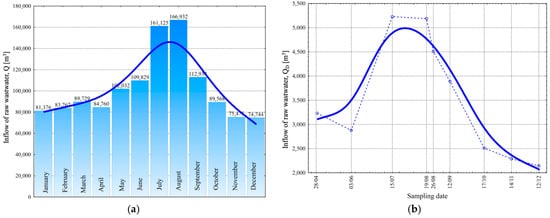
Figure 4.
Average monthly wastewater inflows in 2024 (a) and average daily inflows on the test day (b) for the WWTP according to the operator’s data.
During the test period, no short-term episodes of qualitative deterioration of the inflow of treated wastewater to the plant were observed, so the full range of possibilities of the final gravity filtration process was not observed. Nevertheless, the results indicate that unfavorable changes in wastewater quality after biological treatment, which may occur within a specific range of parameters, can be offset by filtration, thereby securing the final effect of treatment stability.
The filtration process’s mechanism is primarily based on the retention of solids in wastewater, which has already been biologically treated, on the filter fabric. Therefore, the primary measure of the effectiveness of the gravity filtration process is the analysis of the concentration values of total suspended solids TSSs (Figure 5a) and turbidity TB (Figure 5b).
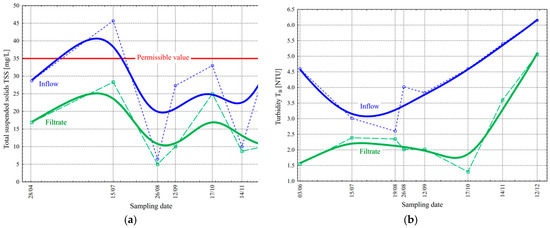
Figure 5.
Change in the values of total suspended solids TSSs [mg/L] (a) and turbidity TB [NTU] (b) before and after the final filtration process in the studied period.
The TSS value in the inflow varied from 6.5 to 45.7 mg/L (mean value = 27.3 mg/L, standard deviation σ = 14.3 mg/L) during the study period. On the other hand, the filtrate changed from 5.0 to 28.3 mg/L ( = 14.9 mg/L and σ = 8.8 mg/L). Wilén et al. achieved a reduction in TSSs to 1 mg/L in their research on cloth filters [19]. The values obtained at the tested WWTP were higher, but still, they were under the limit value resulting from the water permit (Table 1), which is 35 mg/L.
The value of the TB in the inflow varied from 2.6 to 6.15 NTU ( = 4.28 NTU and σ = 1.18 NTU), while in the filtrate, it changed from 1.3 to 5.06 NTU ( = 2.54 NTU and σ = 1.23 NTU). The observed changes in the values of these parameters directly indicate an improvement in wastewater quality after filtration. It is not significant. However, it should be noted that in situations where the TSS value exceeds the water permit (which is visible twice during the test period—Figure 5a), filtration should decrease its value to meet the required permissible value of this parameter. Bourgeous et al. evaluated the cloth media disk filter. In his research, he achieved TB values consistently lower than 2 NTU in the filtrate for inflow values of up to 25 NTU [20].
Biological suspended solids retained during filtration consist mainly of activated sludge flocs lifted from the secondary settling tank during final sedimentation. They contain, among other things, organic substances (cellular components of microorganisms), which are detected in indirect analyses of wastewater (chemical oxygen demand, COD) or directly through the value of total organic carbon, TOC [21,22,23]. The analyses revealed that the retention of suspended solids during the filtration process also directly resulted in decreased values of the COD and TOC in the wastewater (Figure 6). The value of the COD in the inflow varied from 21.0 to 73.5 mg/L ( = 34.2 mg/La and σ = 15.5 mg/L) during the study period. On the other hand, the filtrate changed from 18.0 to 57.5 mg/L ( = 29.1 mg/L and σ = 11.8 mg/L) (Figure 6a). In this case, the limit value in the water permit (Table 1) is 125 mg/L. Sun et al. examined a cloth media filter used for treating the effluent of a municipal wastewater plant, achieving an average removal efficiency of 8% for the COD [24]. In the case of the examined WWTP, the COD removal efficiency was 15%.
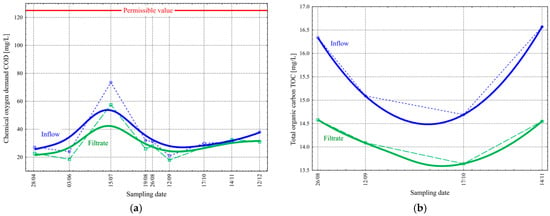
Figure 6.
Change in the values of chemical oxygen demand COD [mg/L] (a) and total organic carbon TOC [mg/L] (b) before and after the final filtration process in the studied period.
The value of the TOC in the inflow varied from 14.7 to 16.6 mg/L ( = 15.7 mg/L and σ = 0.9 mg/L) during the time studied, while in the filtrate, it changed from 13.6 to 14.6 mg/L ( = 14.2 mg/L and σ = 0.4 mg/L) (Figure 6b).
Retention of the solid phase from the activated sludge suspension on the porous surface of the filter, which is not sedimented in the secondary settling tank, can be a source of additional pollution in the treated wastewater, especially in cases of sludge accumulation and infrequent thorough filter cleaning. The retained suspended solids, mainly in the form of microorganisms whose cells are rich in accumulated phosphorus (resulting from high-efficiency nutrient removal), can release phosphorus ions into the treated wastewater (Figure 7a). In the summer, at higher temperatures of 23–24 °C (Figure 7b), the deoxygenation of the activated sludge flocs is more rapid, and nutrients are released [23,25,26,27]. The hydraulic load on the WWTP associated with the holiday season also increased during this period (Figure 4a).
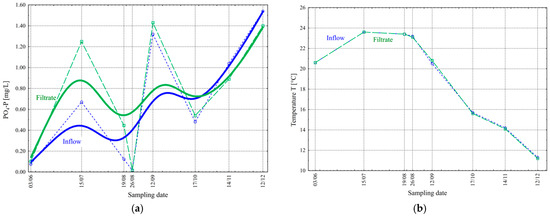
Figure 7.
Change in the values of PO4-P concentration [mg/L] (a) and temperature T [°C] (b) before and after the final filtration process in the studied period.
Analysis of the values of orthophosphate concentration in the inflow during the study period showed changes ranging from 0.016 to 1.54 mg/L ( = 0.65 mg/L and σ = 0.59 mg/L), while in the filtrate, from 0.016 to 1.43 mg/L ( = 0.76 mg/L and σ = 0.57 mg/L) (Figure 7a). Wilén et al. achieved total phosphorus concentrations below 0.2 mg/L in filtrate from a cloth filter [19]. On the other hand, Sun et al. achieved an average removal efficiency of 28.76% for total phosphorus [24]. Both authors claim to have achieved the removal of phosphorus on cloth filters, which was not the case in our studies conducted in the summer.
The wastewater temperature during the studied period ranged from 11 °C in winter to 24 °C in summer (Figure 7b).
During filtration, physical and biological processes can occur on the filter material [21,22,25,26,28,29]. Biological processes may be the cause of changes in pH and specific conductivity values. The pH value in the inflow ranged from 6.88 to 8.08 ( = 7.64 mg/L and σ = 0.39 mg/L) during the study period, while in the filtrate it changed from 6.84 to 7.71 ( = 7.33 mg/L and σ = 0.27 mg/L) (Figure 8a). The values fell within the permitted range (Table 1). On the other hand, the value of the conductivity in the inflow varied from 942 to 1038 μS/cm ( = 975 μS/cm and σ = 35 μS/cm) during the study period, while in the filtrate, the range was from 945 to 1118 μS/cm ( = 994 μS/cm and σ = 54 μS/cm) (Figure 7b).
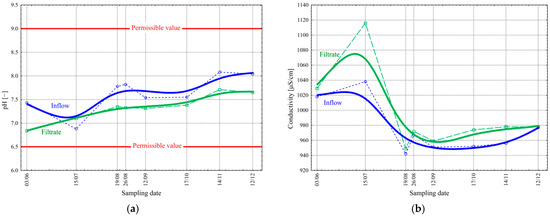
Figure 8.
Change in the pH values (a) and specific conductivity SC [μS/cm] (b) before and after the final filtration process in the studied period.
It can be seen that the filtration process stabilized the pH value, lowering it to a small extent, probably due to the biochemical processes caused by microorganisms colmatating on the filter surface. Ions formed during biological processes (including ammonium and nitrate) can lower the pH. It is noticeable that practically, over the entire period of the tests, the value of specific conductivity in the filtrate is higher than in the inflow. The most significant difference was observed in July, which is attributed to the increase in temperature and the amount of inflowing wastewater. Also, the ongoing mineralization of organic substrates retained on the filter, internal respiration processes, and, as mentioned earlier, the release of orthophosphates, may contribute to higher values of specific conductivity due to the release of inorganic ions (including ammonium and nitrate). Despite the minimal nutrient content in the wastewater, the filter material exposed to sunlight also provides a suitable substrate for the growth and development of various types of organisms, i.e., phytoplankton and algae. Such organisms absorb the elements present in the wastewater and excrete metabolic products, thereby affecting the quality of the treated wastewater [21,22,25,26,28]. The filtration process is not a complete remedy for the problems of biological wastewater treatment. It provides only partial protection against the increased suspended solids (containing, of course, the full range of organic and mineral compounds) in the effluent, with little effect on the pollutants present in the wastewater in dissolved form.
The filtration effect on nitrogen compounds in the wastewater was analyzed for ammonia nitrogen (NH4-N) and nitrate (NO3-N)—Figure 9.
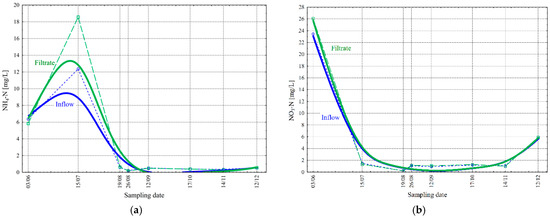
Figure 9.
Change in the concentration values of ammonia nitrogen NH4-N [mg/L] (a) and nitrate nitrogen NO3-N [mg/L] (b) before and after the final filtration process in the studied period.
The NH4-N concentration in the inflow changed from 0.21 to 12.36 mg/L ( = 2.67 mg/L and σ = 4.43 mg/L). In the filtrate, on the other hand, the values ranged from 0.18 to 18.57 mg/L ( = 3.37 mg/L and σ = 6.43 mg/L) (Figure 9a). The value of the NO3-N concentration in the inflow varied from 0.17 to 23.44 mg/L ( = 4.39 mg/L and σ = 8.35 mg/L), while in the filtrate, the change in value was from 0.21 to 26.11 mg/L ( = 4.76 mg/L and σ = 8.80 mg/L) (Figure 9b). Higher values of NO3-N and NH4-N concentrations after the filter may be caused by the biological decomposition of organic substances via microorganisms retained on the filter cloth, releasing inorganic ions, including ammonium and nitrate. The effective retention of suspended solids on the filter surface influenced the release of NH4-N and PO4-P (Figure 7a) from the biological structure of the activated sludge suspension. It should be noted that an increase in temperature (Figure 7b), a crucial parameter in biological processes, enhances the dynamics of decomposition processes, resulting in greater differences in NH4-N and PO4-P concentrations before and after the filter.
Figure 10 presents the average removal efficiency obtained during research. The highest efficiency of removal was achieved for TSSs and TB, at 45% and 41%, respectively.
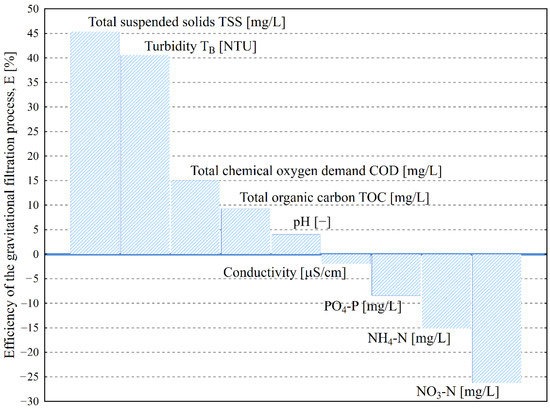
Figure 10.
Average efficiency of cloth disk filters SF6/30 using Mecana AG in the third stage of wastewater treatment with regard to examined parameters.
Table 2 presents the results of the statistical analysis of the mean differences in the inflow and filtrate data, focusing on selected indicators of municipal wastewater pollution. Verification was performed at a significance level of α = 0.05.

Table 2.
Results of statistical analysis of the filtration process of municipal wastewater.
The statistical analysis in Table 2 confirms the previously described changes in the values of selected outcome variable parameters in relation to the independent variable parameters for the inflow and filtrate of municipal wastewater filtration. The following outcome variables—TSSs, TB, COD, TOC, and pH—are impacted by the filtration process. On the other hand, the process does not affect SC, PO4-P, NH4-N, or NO3-N.
Results from other researchers show that disk cloth filters also remove microplastics (10–5000 μm), achieving an efficiency of up to 99.99% [30]. Some also observed the removal of metals [19]. However, the filtrate after disk filters requires further disinfection due to the poor removal efficiency of pathogens [19,31].
4. Conclusions
An important aspect of sustainable water and energy management is the energy self-sufficiency of wastewater treatment plants. The new Directive of the European Parliament and of the Council of the EU obliges companies to meet their energy needs exclusively from their sources, without the need to import energy from outside. Most of the process equipment used in this industry is energy-intensive, which is why energy-efficient solutions are being sought. The introduction of advanced third and fourth stages of wastewater treatment using physical and chemical processes (filtration, adsorption, membrane separation, deep oxidation techniques, photo-oxidation, catalytic oxidation, electro-oxidation, and final disinfection using ozonation or UV radiation) allows for the recovery of water that can be reused for industrial, agricultural, or municipal purposes. There are two main disadvantages of those processes. Intermediate decomposition products formed during these processes may be more toxic than the primary compounds. Gravity cloth disk filters appear to be less energy-consuming and require fewer resources. In 2024, the unit energy consumption of the examined cloth filter was 12.87 Wh/m3, which corresponds to 1.04 kWh/kg of TSSs removed. The integration of the filtration system with energy recovery infrastructure, such as biogas plants that process sewage sludge, enables the effective combination of wastewater treatment and energy production processes. Sludge generated during filtration and other wastewater treatment stages can be directed to anaerobic digestion, where biogas is produced—a renewable energy source used to generate heat and electricity for the treatment plant’s own needs. Additionally, heat recovered from cogeneration can be utilized for heat fermentation chambers or other technological processes. Such an optimized sludge management system not only reduces the amount of waste to be disposed of, but also increases the treatment plant’s energy self-sufficiency, thereby reducing operating costs and greenhouse gas emissions. Modern WWTPs utilizing these solutions will produce reclaimed water and energy, enhance their autonomy, and mitigate their ecological footprint [32,33].
Studies conducted on the operation of an additional technological node, i.e., filtration after biological treatment at a selected coastal WWTP, showed that it is an essential element of the facility’s operation. It ensures the leveling of disturbances in the operation of earlier treatment stages, particularly in terms of the suspended solid retention of activated sludge. The highest efficiency of removal was achieved for TSSs and TB, at 45% and 40%, respectively.
During the operation of a filter, a colmatation process occurs on its surface, consisting of a reduction in the porosity of the filter material. This results in an unfavorable increase in filtration resistance, and thus, a decrease in the device’s operational efficiency. However, special attention should be paid to the phenomenon associated with the colmatation of the filtration layer—possible biological processes carried out by retained activated sludge microorganisms or inhabiting phytoplankton, whose metabolites and products of organic decomposition can affect the final quality of treated wastewater increasing concentrations of PO4-P (8%), NH4-N (15%), and NO3-N (26%) in the filtrate, compared to the inflow.
The effectiveness of filter cleaning, especially in situations of an increased suspended solid concentration, is crucial during periods of higher temperatures and the concomitant increase in hydraulic load, which is essential for the correct operation of the device.
When operated correctly, additional filtration and disinfection may become essential components of the WWTP system, enabling the achievement of wastewater quality suitable for various uses, including technological, agricultural, and other purposes. The results of the analyses indicate that filtration with UV disinfection (the effectiveness of which needs to be confirmed) offers the possibility of using the recovered water for agricultural irrigation, with a quality class of B–D.
Author Contributions
Conceptualization, K.S. and J.P.; literature review, K.S., J.P., T.D., K.P., R.Ś.-D. and K.I.; methodology, J.P., T.D., K.P. and R.Ś.-D.; investigation J.P., T.D., K.P. and R.Ś.-D.; formal analysis, J.P., T.D., K.P. and R.Ś.-D.; writing, J.P., T.D., K.P., R.Ś.-D. and K.I.; conclusions and discussion, K.S., J.P. and K.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
This research has been supported by Koszalin University of Technology within the framework of project No. 524.01.17 and by Bialystok University of Technology within the framework of project No. WZ/WB–IIŚ/2/2024.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Directive (EU) 2024/3019 of the European Parliament and of the Council of 27 November 2024 Concerning Urban Wastewater Treatment. 2024. Available online: https://eur-lex.europa.eu/eli/dir/2024/3019/oj/eng (accessed on 24 April 2025).
- Regulation (EU) 2020/741 of the European Parliament and of the Council of 25 May 2020 on Minimum Requirements for Water Reuse. Available online: https://eur-lex.europa.eu/eli/reg/2020/741/oj/eng (accessed on 24 April 2025).
- Preisner, M.; Smol, M.; Horttanainen, M.; Deviatkin, I.; Havukainen, J.; Klavins, M.; Ozola-Davidane, R.; Kruopienė, J.; Szatkowska, B.; Appels, L.; et al. Indicators for Resource Recovery Monitoring within the Circular Economy Model Implementation in the Wastewater Sector. J. Environ. Manag. 2022, 304, 114261. [Google Scholar] [CrossRef]
- Ramm, K.; Smol, M. Analysis of the Possibility of Using Reclaimed Water Depending on the Quality Class in the European Countries. Sustainability 2023, 15, 12781. [Google Scholar] [CrossRef]
- Ossowicz-Rupniewska, P.E.; Kucharska, E.; Klebeko, J.; Kopciuch, E.; Bilska, K.; Janus, E. Effect of the Type of Amino Acid on the Biodegradation of Ibuprofen Derivatives. Arch. Environ. Prot. 2023, 49, 46–69. [Google Scholar] [CrossRef]
- Ramm, K.; Smol, M. The Potential for Water Recovery from Urban Waste Water—The Perspective of Urban Waste Water Treatment Plant Operators in Poland. J. Environ. Manag. 2024, 358, 120890. [Google Scholar] [CrossRef] [PubMed]
- D’Adamo, I.; Gastaldi, M.; Morone, P.; Rosa, P.; Sassanelli, C.; Settembre-Blundo, D.; Shen, Y. Bioeconomy of Sustainability: Drivers, Opportunities and Policy Implications. Sustainability 2021, 14, 200. [Google Scholar] [CrossRef]
- ReNutriWater. Available online: https://interreg-baltic.eu/project/renutriwater/ (accessed on 24 April 2025).
- WaterMan. Available online: https://interreg-baltic.eu/project/waterman/ (accessed on 24 April 2025).
- Dona, C.G.W.; Fukushi, K.; Mohan, G. Reusing Treated Wastewater and Biosolids in Urban Agriculture: Social Feasibility of a Decentralized Approach for Underserved Settlements. Environ. Chall. 2025, 19, 101159. [Google Scholar] [CrossRef]
- Fernandes, J.; Ramísio, P.J.; Puga, H. A Comprehensive Review on Various Phases of Wastewater Technologies: Trends and Future Perspectives. Eng 2024, 5, 2633–2661. [Google Scholar] [CrossRef]
- Piekarski, J.; Dąbrowski, T. Investigations on Colmatation during Filtration Process on the Porous Deposit. Pol. J. Environ. Stud. 2009, 10, 51–56. [Google Scholar]
- Piekarski, J. Computation of Filtration Bed Porosity Based on Selected Filtration Coefficient Equations by Application of Numerical Methods. Rocz. Ochr. Sr. 2020, 22, 1084–1096. [Google Scholar]
- Culligan Water. Available online: https://www.culligan.com/commercial (accessed on 24 April 2025).
- Krevox. Available online: https://www.krevox.com/ (accessed on 24 April 2025).
- HUBER Technology. Available online: https://www.huber-se.com/products/detail/huber-pile-cloth-media-filter-rotafilt/ (accessed on 24 April 2025).
- Belete, B.; Desye, B.; Ambelu, A.; Yenew, C. Micropollutant Removal Efficiency of Advanced Wastewater Treatment Plants: A Systematic Review. Environ. Health Insights 2023, 17, 1–11. [Google Scholar] [CrossRef]
- Mecana AG. Available online: https://www.mecana.ch/en/technologies/pile-cloth-media-filtration/disk-filter (accessed on 24 April 2025).
- Wilén, B.-M.; Johansen, A.; Mattsson, A. Assessment of Sludge Particle Removal from Wastewater by Disc Filtration. Water Pract. Technol. 2012, 7, wpt2012037. [Google Scholar] [CrossRef]
- Bourgeous, K.N.; Riess, J.; Tchobanoglous, G.; Darby, J.L. Performance Evaluation of a Cloth-Media Disk Filter for Wastewater Reclamation. Water Environ. Res. 2003, 75, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Leão, I.; Antunes, J.; Baptista, I.; Jorge, R.; Marinheiro, L.; Löblich, S.; Vaz-Moreira, I.; Manaia, C.M. Treating Domestic Wastewater towards Freshwater Quality: Bacterial Community and Antibiotic Resistance Profiles Highlight Critical Steps and Improvement Opportunities. J. Environ. Chem. Eng. 2025, 13, 116172. [Google Scholar] [CrossRef]
- Christianson, L.E.; Lepine, C.; Sharrer, K.L.; Summerfelt, S.T. Denitrifying Bioreactor Clogging Potential during Wastewater Treatment. Water Res. 2016, 105, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, Y.; Zhai, Y.; Xu, Z. Enhanced Phosphorus Release from Waste Activated Sludge Triggered by Ferrate/Sulfite Oxidation Process: The Overlooked Role of High Valent Iron. J. Environ. Chem. Eng. 2024, 12, 111617. [Google Scholar] [CrossRef]
- Sun, S.Q.; Bi, L.J.; Xu, P.P.; Jiang, C.B.; Tan, W.C.; Wu, F.T.; Nie, X.B. Pilot Studies on Cloth Media Filter Applied in WWTP for the Treatment of Secondary Effluent. AMR 2011, 183–185, 683–689. [Google Scholar] [CrossRef]
- Velten, H.; Krahe, D.; Hasport, N.; Fundneider, T.; Grabbe, U.; Knorr, L.; Theilen, U. Pile Cloth Media Filtration for Harvesting Microalgae Used for Wastewater Treatment. Fermentation 2024, 10, 325. [Google Scholar] [CrossRef]
- Gutierrez, M. Municipal Tertiary Treatment: Disc Filters Enhanced by Pleated Configuration. Filtr. Sep. 2009, 46, 22–24. [Google Scholar] [CrossRef]
- Cheng, X.; Huang, Y.; Li, R.; Pu, X.; Huang, W.; Yuan, X. Impacts of Water Temperature on Phosphorus Release of Sediments under Flowing Overlying Water. J. Contam. Hydrol. 2020, 235, 103717. [Google Scholar] [CrossRef]
- Waqas, S.; Harun, N.Y.; Lock, S.S.M.; Alsaadi, A.S. Energy-Efficient Single-Stage Membrane Rotating Biological Contactor for Wastewater Treatment. Bioresour. Technol. Rep. 2024, 25, 101776. [Google Scholar] [CrossRef]
- Ignatowicz, K. Use of Natural Waste Materials as Sorbents for Limiting the Migration of Pesticides from Graveyards. Przem. Chem. 2008, 87, 464–466. [Google Scholar]
- Bitter, H.; Krause, L.; Kirchen, F.; Fundneider, T.; Lackner, S. Semi-Crystalline Microplastics in Wastewater Plant Effluents and Removal Efficiencies of Post-Treatment Filtration Systems. Water Res. X 2022, 17, 100156. [Google Scholar] [CrossRef]
- Levine, A.D.; Harwood, V.J.; Farrah, S.R.; Scott, T.M.; Rose, J.B. Pathogen and Indicator Organism Reduction Through Secondary Effluent Filtration: Implications for Reclaimed Water Production. Water Environ. Res. 2008, 80, 596–608. [Google Scholar] [CrossRef]
- Dąbrowski, W.; Żyłka, R.; Rynkiewicz, M. EVALUATION OF ENERGY CONSUMPTION IN AGRO-INDUSTRIAL WASTEWATER TREATMENT PLANT. J. Ecol. Eng. 2016, 17, 73–78. [Google Scholar] [CrossRef]
- Żyłka, R.; Dąbrowski, W.; Malinowski, P.; Karolinczak, B. Modeling of Electric Energy Consumption during Dairy Wastewater Treatment Plant Operation. Energies 2020, 13, 3769. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).