Hydrogen Energy Storage via Carbon-Based Materials: From Traditional Sorbents to Emerging Architecture Engineering and AI-Driven Optimization
Abstract
1. Introduction
| Material | Precursor | SBET (m2/g) | Pore Size | ΔHads (kJ/mol) | H2 Uptake (77 K) | H2 Uptake (298 K) | Cycling Stability |
|---|---|---|---|---|---|---|---|
| Activated carbon (AC) | Anthracite/coconut shell/biomass | up to ~3220 | ultramicropores (<0.9 nm) | ~7–8 | 6.0 wt.% (77 K, 4 MPa) | 0.6 wt.% (298 K, 5 MPa) | High |
| Carbon nanotubes (CNTs) | Graphitic (CVD-grown) | ~1000 (SWNT) | micro/mesoporous (defects) | ~7 | 2.0 wt.% (77 K, 40 bar) | 0.2 wt.% (298 K, 200 bar) | High |
| Graphene | Graphite (exfoliated) | ~2600 | 2D sheets, stacked micropores | 4–6 | 1.2 wt.% (77 K) | 0.1 wt.% (298 K) | High |
| Ti3C2Tx MXene | Ti3AlC2 (MAX phase) | - | 2D interlayer spacing | - | 10.5 wt.% (77 K, 25 bar) | - | High |
| MOFs (e.g., HKUST-1) | e.g., Cu2(BTC)3, Zn4O(BDC)3 | ~1000–4000 | uniform micropores | ~5 | up to ~9 wt.% (77 K, 50 bar) | ~0.5 wt.% | Moderate |
| Zeolites (e.g., NaX) | Aluminosilicate framework | ~400–700 | micropores (~0.4 nm) | 3–7 | 1.8 wt.% (77 K, 1.5 bar) | <0.1 wt.% | High |
| Porous polymers (HCP/COF) | Crosslinked aromatic monomers | up to ~2000 | micro/mesopores (2–4 nm) | ~5–10 | 5.0 wt.% (77 K, 80 bar) | 0.2 wt.% (298 K, 90 bar) | High |
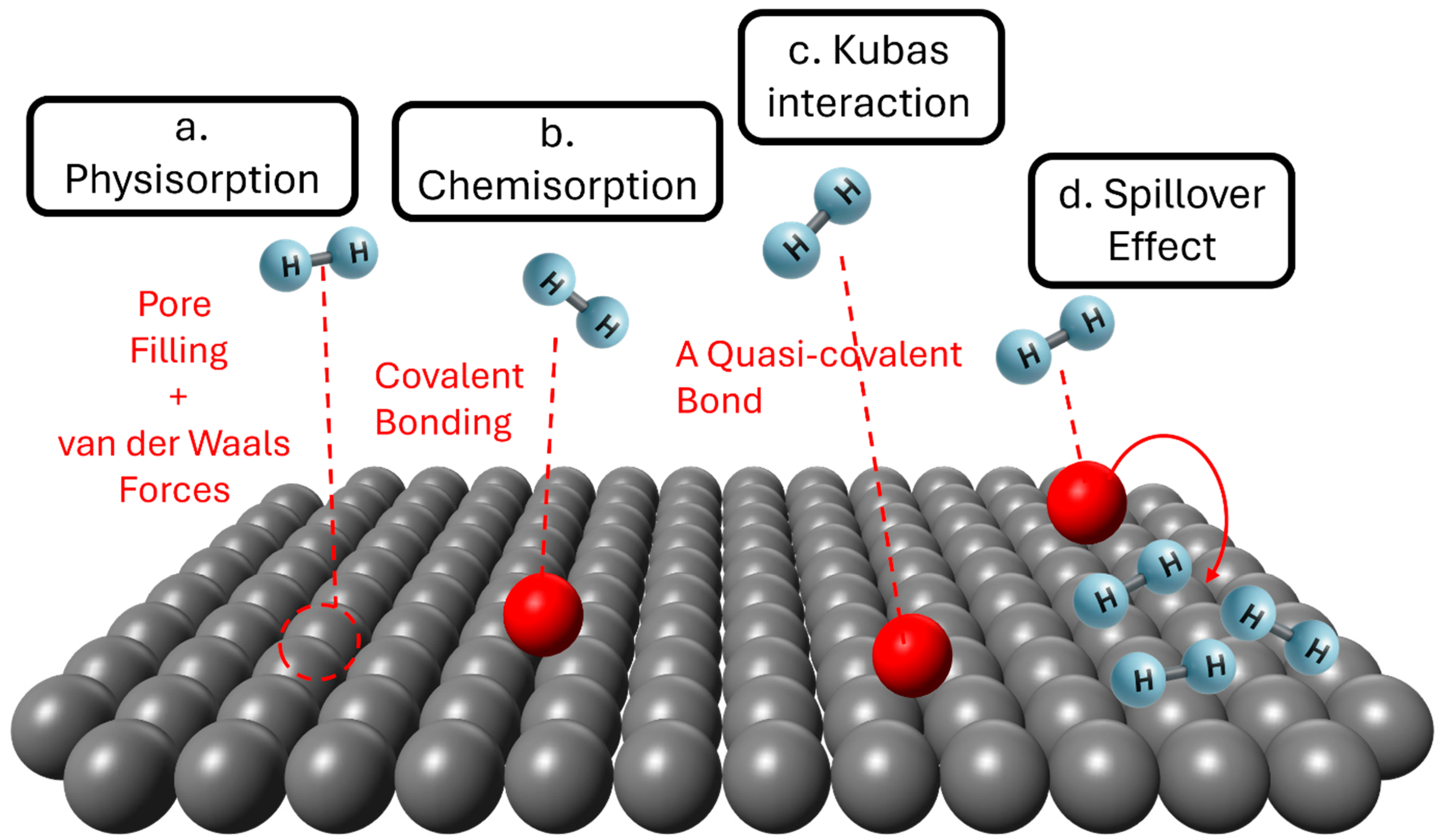
2. Hydrogen Adsorption Mechanisms on Carbon-Based Materials
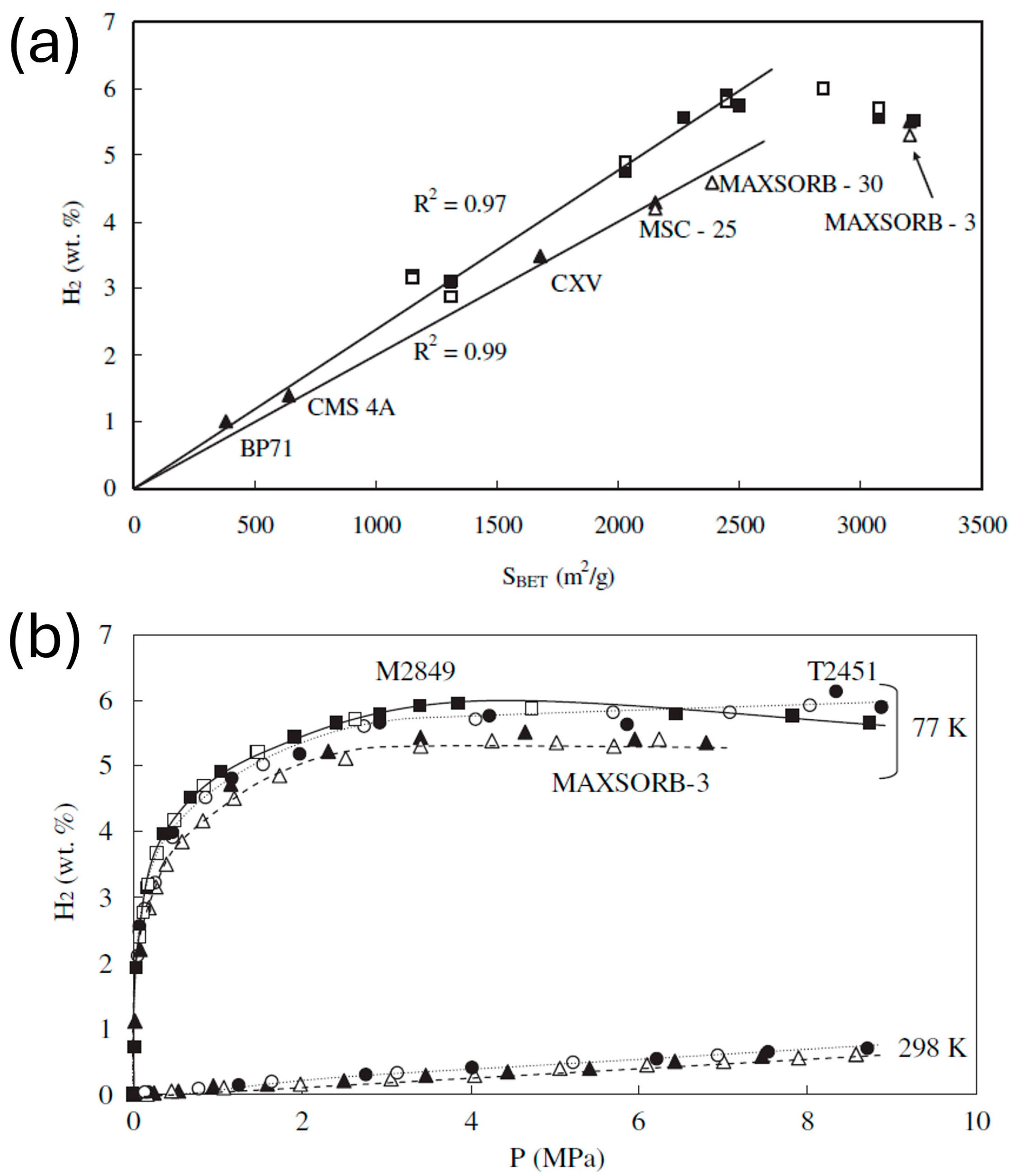
3. Performance Analysis of Carbon-Based Hydrogen Storage Materials
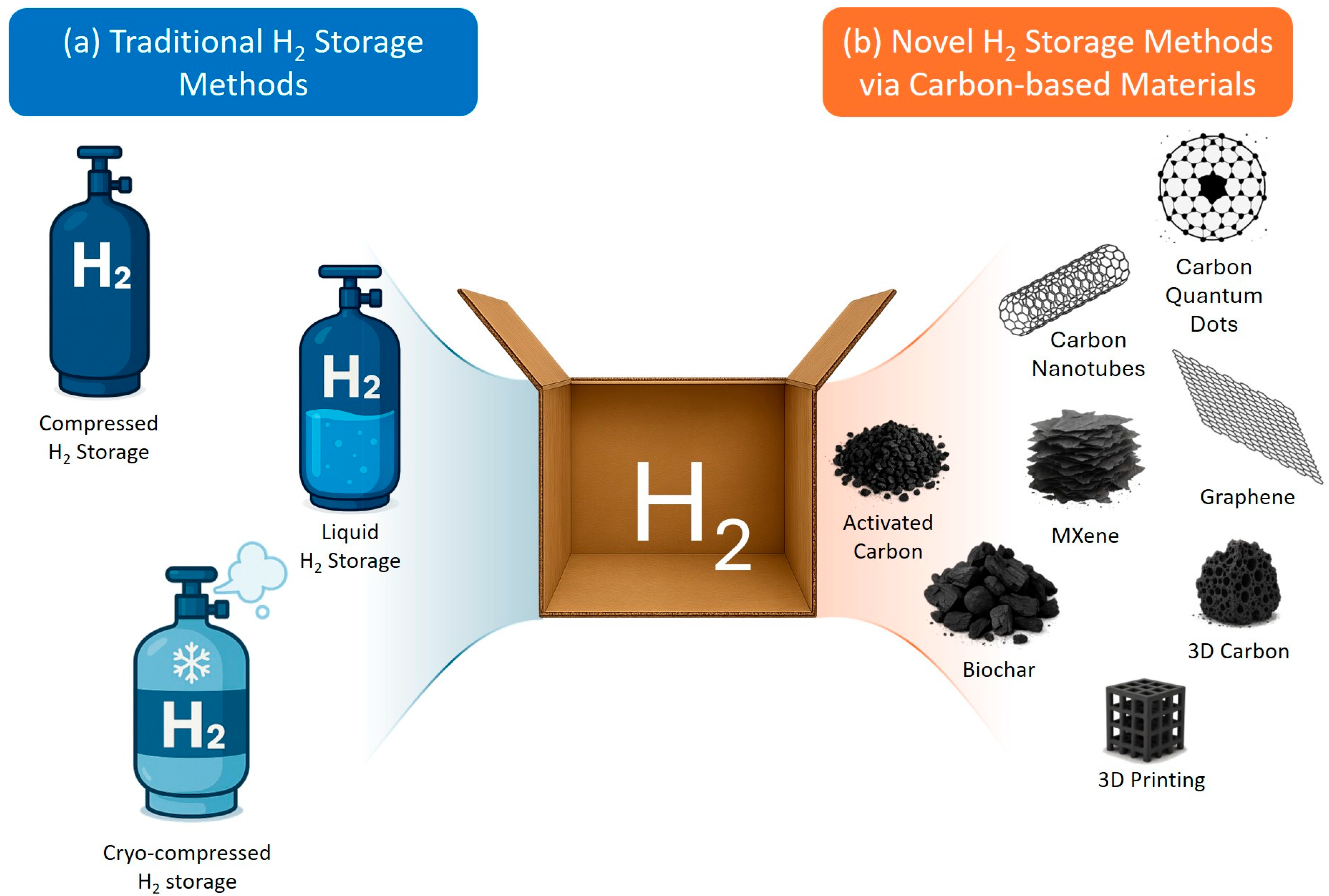
4. Classical Carbon-Based Materials for Hydrogen Storage
5. Emerging Carbon Architectures for Hydrogen Storage
5.1. MXenes and Two-Dimensional Architectures
5.2. Doped and Metal-Decorated Classical Carbons
5.3. Three-Dimensional Architectures: Foams, Aerogels, and Monoliths
5.4. Additive Manufacturing of Architected Carbon Sorbents
6. Hydrogen Storage Conditions and Practical Evaluation Metrics
6.1. Temperature and Pressure Dependencies
6.2. Usable Capacity and Reversibility
6.3. Volumetric Capacity and Packing Density
6.4. Thermal Stability and Cycling Behavior
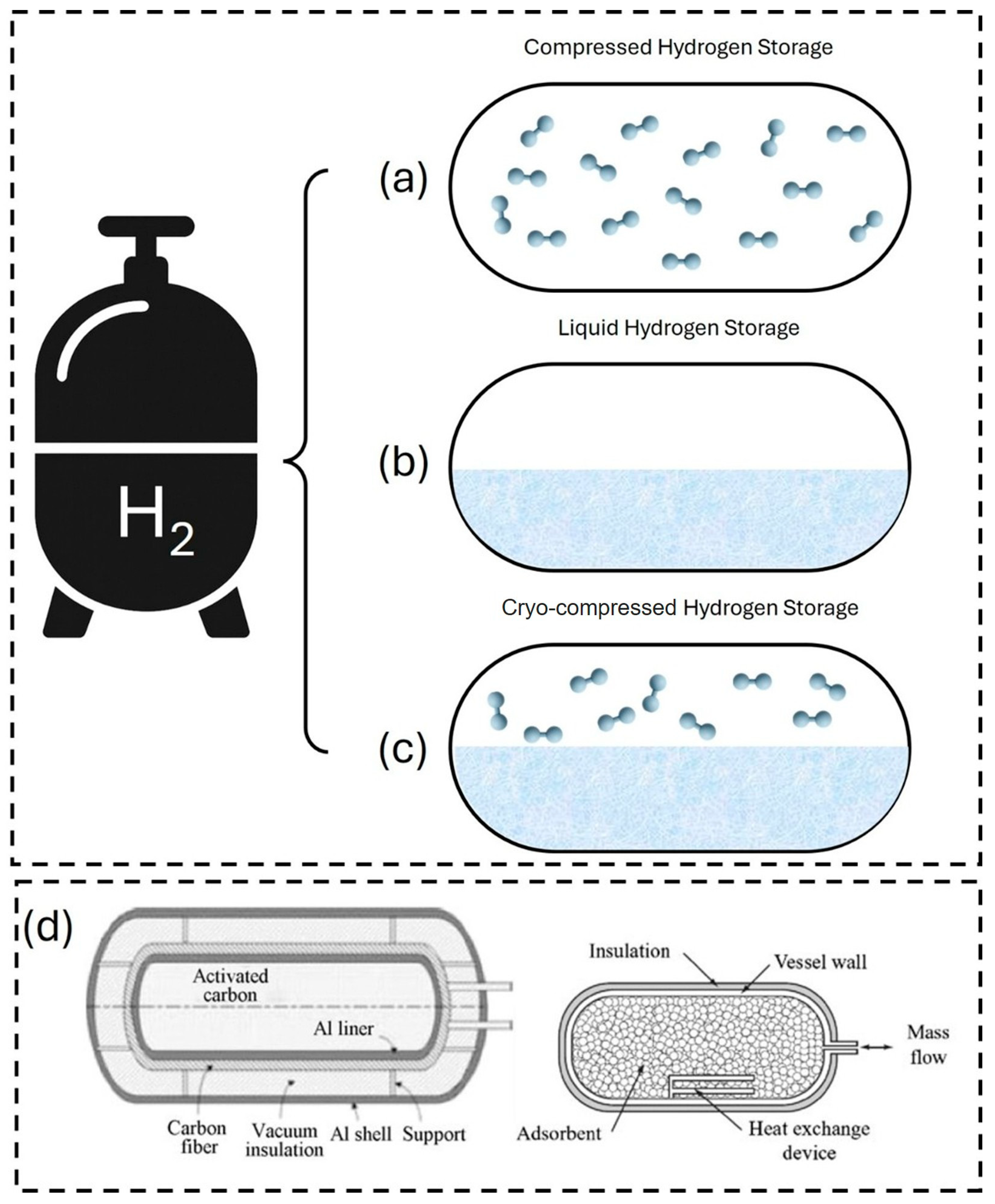
7. Real-World Applications, Commercialization Status, and System-Level Integration
8. AI and Machine Learning in Carbon-Sorbent Design for Hydrogen Storage
8.1. Predictive Modeling of Hydrogen Uptake
8.2. Optimization of Carbon Material Synthesis
8.3. Datasets and Feature Engineering
8.4. Emerging AI Approaches for Carbon Sorbent Discovery
8.5. AI/ML-Driven Workflow for Carbon Sorbent Development and Deployment
8.6. Balancing AI Predictions and Experimental Progress
8.7. Current Limitations of AI and ML
9. Challenges and Future Directions
10. Conclusions
Funding
Conflicts of Interest
References
- Anand, C.; Chandraja, B.; Nithiya, P.; Akshaya, M.; Tamizhdurai, P.; Shoba, G.; Subramani, A.; Kumaran, R.; Yadav, K.K.; Gacem, A.; et al. Green hydrogen for a sustainable future: A review of production methods, innovations, and applications. Int. J. Hydrogen Energy 2025, 111, 319–341. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, C.; Bai, F.; Wang, W.; An, S.; Zhao, K.; Li, Z.; Li, J.; Sun, H. A comprehensive review of the promising clean energy carrier: Hydrogen production, transportation, storage, and utilization (HPTSU) technologies. Fuel 2024, 355, 129455. [Google Scholar] [CrossRef]
- Reda, B.; Elzamar, A.A.; AlFazzani, S.; Ezzat, S.M. Green hydrogen as a source of renewable energy: A step towards sustainability, an overview. Environ. Dev. Sustain. 2024, 1–21. [Google Scholar] [CrossRef]
- Fu, H.; Pan, Z.; Wenderott, J. Engaging with clean power: Integrated, hands-on lessons on photocatalytic hydrogen production for high school students. Discov. Educ. 2025, 4, 105. [Google Scholar] [CrossRef]
- Tang, D.; Tan, G.-L.; Li, G.-W.; Liang, J.-G.; Ahmad, S.M.; Bahadur, A.; Humayun, M.; Ullah, H.; Khan, A.; Bououdina, M. State-of-the-art hydrogen generation techniques and storage methods: A critical review. J. Energy Storage 2023, 64, 107196. [Google Scholar] [CrossRef]
- Osman, A.I.; Nasr, M.; Eltaweil, A.S.; Hosny, M.; Farghali, M.; Al-Fatesh, A.S.; Rooney, D.W.; Abd El-Monaem, E.M. Advances in hydrogen storage materials: Harnessing innovative technology, from machine learning to computational chemistry, for energy storage solutions. Int. J. Hydrogen Energy 2024, 67, 1270–1294. [Google Scholar] [CrossRef]
- Moradi, R.; Groth, K.M. Hydrogen storage and delivery: Review of the state of the art technologies and risk and reliability analysis. Int. J. Hydrogen Energy 2019, 44, 12254–12269. [Google Scholar] [CrossRef]
- Rimza, T.; Saha, S.; Dhand, C.; Dwivedi, N.; Patel, S.S.; Singh, S.; Kumar, P. Carbon-Based Sorbents for Hydrogen Storage: Challenges and Sustainability at Operating Conditions for Renewable Energy. ChemSusChem 2022, 15, e202200281. [Google Scholar] [CrossRef]
- Ouyang, L.; Huang, J.; Wang, H.; Liu, J.; Zhu, M. Progress of hydrogen storage alloys for Ni-MH rechargeable power batteries in electric vehicles: A review. Mater. Chem. Phys. 2017, 200, 164–178. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, X.; Li, Y.; Zhao, Y.; Shu, X.; Zhang, G.; Yang, T.; Liu, Y.; Wu, P.; Ding, Z. Rare-Earth Metal-Based Materials for Hydrogen Storage: Progress, Challenges, and Future Perspectives. Nanomaterials 2024, 14, 1671. [Google Scholar] [CrossRef]
- Klopčič, N.; Grimmer, I.; Winkler, F.; Sartory, M.; Trattner, A. A review on metal hydride materials for hydrogen storage. J. Energy Storage 2023, 72, 108456. [Google Scholar] [CrossRef]
- Scarpati, G.; Frasci, E.; Di Ilio, G.; Jannelli, E. A comprehensive review on metal hydrides-based hydrogen storage systems for mobile applications. J. Energy Storage 2024, 102, 113934. [Google Scholar] [CrossRef]
- Mahmoud, L.A.M.; Rowlandson, J.L.; Fermin, D.J.; Ting, V.P.; Nayak, S. Porous carbons: A class of nanomaterials for efficient adsorption-based hydrogen storage. RSC Appl. Interfaces 2025, 2, 25–55. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, D.; Wu, Q.; Wang, H.; Wang, L.; Liu, B.; Zhou, A.; He, J. MXene: A New Family of Promising Hydrogen Storage Medium. J. Phys. Chem. A 2013, 117, 14253–14260. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, H.; Wu, Q.; Ye, X.; Zhou, A.; Sun, D.; Wang, L.; Liu, B.; He, J. Two-dimensional Sc2C: A reversible and high-capacity hydrogen storage material predicted by first-principles calculations. Int. J. Hydrogen Energy 2014, 39, 10606–10612. [Google Scholar] [CrossRef]
- Butler, C.; Mays, T.J.; Sahadevan, V.; O’Malley, R.; Graham, D.P.; Bowen, C.R. Hydrogen storage capacity of freeze cast microporous monolithic composites. Mater. Adv. 2024, 5, 6864–6872. [Google Scholar] [CrossRef]
- Orangi, J.; Tetik, H.; Parandoush, P.; Kayali, E.; Lin, D.; Beidaghi, M. Conductive and highly compressible MXene aerogels with ordered microstructures as high-capacity electrodes for Li-ion capacitors. Mater. Today Adv. 2021, 9, 100135. [Google Scholar] [CrossRef]
- Usman, M.R. Hydrogen storage methods: Review and current status. Renew. Sustain. Energy Rev. 2022, 167, 112743. [Google Scholar] [CrossRef]
- Ren, J.; Musyoka, N.M.; Langmi, H.W.; Mathe, M.; Liao, S. Current research trends and perspectives on materials-based hydrogen storage solutions: A critical review. Int. J. Hydrogen Energy 2017, 42, 289–311. [Google Scholar] [CrossRef]
- Saeid, M.F.; Abdulkadir, B.A.; Fauzi, M.A.; Setiabudi, H.D. Carbon Materials for Hydrogen Storage: A Bibliometric Analysis on Current Trends and Future Prospects. Environ. Qual. Manag. 2025, 34, e70109. [Google Scholar] [CrossRef]
- Attia, N.F.; Policicchio, A.; Conte, G.; Agostino, R.G.; Alkahlawy, A.; Elashery, S.E.A. Green fabrication of cost-effective and sustainable nanoporous carbons for efficient hydrogen storage and CO2/H2 separation. Int. J. Hydrogen Energy 2024, 92, 1160–1171. [Google Scholar] [CrossRef]
- Antil, B.; Olhan, S.; Vander Wal, R.L. Production of Graphitic Carbon from Renewable Lignocellulosic Biomass Source. Minerals 2025, 15, 262. [Google Scholar] [CrossRef]
- Ahmed, A.; Seth, S.; Purewal, J.; Wong-Foy, A.G.; Veenstra, M.; Matzger, A.J.; Siegel, D.J. Exceptional hydrogen storage achieved by screening nearly half a million metal-organic frameworks. Nat. Commun. 2019, 10, 1568. [Google Scholar] [CrossRef] [PubMed]
- Villajos, J.A. Experimental Volumetric Hydrogen Uptake Determination at 77 K of Commercially Available Metal-Organic Framework Materials. C 2022, 8, 5. [Google Scholar] [CrossRef]
- Chen, Z.; Kirlikovali, K.O.; Idrees, K.B.; Wasson, M.C.; Farha, O.K. Porous materials for hydrogen storage. Chem 2022, 8, 693–716. [Google Scholar] [CrossRef]
- Kudiiarov, V.; Lyu, J.; Semyonov, O.; Lider, A.; Chaemchuen, S.; Verpoort, F. Prospects of hybrid materials composed of MOFs and hydride-forming metal nanoparticles for light-duty vehicle hydrogen storage. Appl. Mater. Today 2021, 25, 101208. [Google Scholar] [CrossRef]
- Cousins, K.; Zhang, R. Highly Porous Organic Polymers for Hydrogen Fuel Storage. Polymers 2019, 11, 690. [Google Scholar] [CrossRef]
- Rodrigues, J.F.; Florea, L.; de Oliveira, M.C.F.; Diamond, D.; Oliveira, O.N. Big data and machine learning for materials science. Discov. Mater. 2021, 1, 12. [Google Scholar] [CrossRef]
- Ayogu, I.I.; Oguzie, K.L.; Oguzie, E.E. 17—Machine learning assisted low carbon technologies for accelerating deployment of hydrogen economy. In Accelerating the Transition to a Hydrogen Economy; Kurniawan, T.A., Vara Prasad, M.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2025; pp. 387–403. [Google Scholar]
- Oh, H.; Tumanov, N.; Ban, V.; Li, X.; Richter, B.; Hudson, M.R.; Brown, C.M.; Iles, G.N.; Wallacher, D.; Jorgensen, S.W.; et al. Small-pore hydridic frameworks store densely packed hydrogen. Nat. Chem. 2024, 16, 809–816. [Google Scholar] [CrossRef]
- Kusdhany, M.I.M.; Lyth, S.M. New insights into hydrogen uptake on porous carbon materials via explainable machine learning. Carbon 2021, 179, 190–201. [Google Scholar] [CrossRef]
- Barghi, S.H.; Tsotsis, T.T.; Sahimi, M. Chemisorption, physisorption and hysteresis during hydrogen storage in carbon nanotubes. Int. J. Hydrogen Energy 2014, 39, 1390–1397. [Google Scholar] [CrossRef]
- Bastos-Neto, M.; Patzschke, C.; Lange, M.; Möllmer, J.; Möller, A.; Fichtner, S.; Schrage, C.; Lässig, D.; Lincke, J.; Staudt, R.; et al. Assessment of hydrogen storage by physisorption in porous materials. Energy Environ. Sci. 2012, 5, 8294–8303. [Google Scholar] [CrossRef]
- Villajos, J.A.; Zimathies, A.; Prinz, C. A fast procedure for the estimation of the hydrogen storage capacity by cryoadsorption of metal-organic framework materials from their available porous properties. Int. J. Hydrogen Energy 2021, 46, 29323–29331. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Zhang, W.; Zhao, Y.; Wang, J.; Liu, F.; Li, J.; Guo, X.; Li, X. Fabrication of Nano Pt–Co–Cu Sites in the Heterostructured Catalysts for Hydrogen Generation. ACS Appl. Nano Mater. 2024, 7, 22061–22070. [Google Scholar] [CrossRef]
- Qadeer, M.A.; Zhang, X.; Farid, M.A.; Tanveer, M.; Yan, Y.; Du, S.; Huang, Z.-F.; Tahir, M.; Zou, J.-J. A review on fundamentals for designing hydrogen evolution electrocatalyst. J. Power Sources 2024, 613, 234856. [Google Scholar] [CrossRef]
- Thaweelap, N.; Plerdsranoy, P.; Poo-arporn, Y.; Khajondetchairit, P.; Suthirakun, S.; Fongkaew, I.; Hirunsit, P.; Chanlek, N.; Utke, O.; Pangon, A.; et al. Ni-doped activated carbon nanofibers for storing hydrogen at ambient temperature: Experiments and computations. Fuel 2021, 288, 119608. [Google Scholar] [CrossRef]
- Skipper, C.V.J.; Hamaed, A.; Antonelli, D.M.; Kaltsoyannis, N. The Kubas interaction in M(ii) (M = Ti, V, Cr) hydrazine-based hydrogen storage materials: A DFT study. Dalton Trans. 2012, 41, 8515–8523. [Google Scholar] [CrossRef]
- Singh, A.K.; Yakobson, B.I. First principles calculations of H-storage in sorption materials. J. Mater. Sci. 2012, 47, 7356–7366. [Google Scholar] [CrossRef]
- Ghotia, S.; Rimza, T.; Singh, S.; Dwivedi, N.; Srivastava, A.K.; Kumar, P. Hetero-atom doped graphene for marvellous hydrogen storage: Unveiling recent advances and future pathways. J. Mater. Chem. A 2024, 12, 12325–12357. [Google Scholar] [CrossRef]
- Shen, H.; Li, H.; Yang, Z.; Li, C. Magic of hydrogen spillover: Understanding and application. Green Energy Environ. 2022, 7, 1161–1198. [Google Scholar] [CrossRef]
- Yeh, C.-H.; Thang, H.V.; Reyes, Y.I.A.; Coluccini, C.; Chen, H.-Y.T. DFT Insights into Hydrogen Spillover Mechanisms: Effects of Metal Species, Size, and Support. J. Phys. Chem. C 2025, 129, 6185–6195. [Google Scholar] [CrossRef]
- Zhang, W.; Bao, W.; Chen, F.; Li, J.; Yu, L.; Liu, R.; Chi, C.; Yu, J.; Zhao, X.; Zhu, B. Non-noble metal based catalysts with hydrogen spillover mechanism for carbon-based hydrogen storage materials. Int. J. Hydrogen Energy 2024, 88, 945–955. [Google Scholar] [CrossRef]
- Pyle, D.S.; Gray, E.M.; Webb, C.J. Hydrogen storage in carbon nanostructures via spillover. Int. J. Hydrogen Energy 2016, 41, 19098–19113. [Google Scholar] [CrossRef]
- Psofogiannakis, G.M.; Froudakis, G.E. Fundamental studies and perceptions on the spillover mechanism for hydrogen storage. Chem. Commun. 2011, 47, 7933–7943. [Google Scholar] [CrossRef]
- Tian, M.; Lennox, M.J.; O’Malley, A.J.; Porter, A.J.; Krüner, B.; Rudić, S.; Mays, T.J.; Düren, T.; Presser, V.; Terry, L.R.; et al. Effect of pore geometry on ultra-densified hydrogen in microporous carbons. Carbon 2021, 173, 968–979. [Google Scholar] [CrossRef]
- Singh, R.; Wang, L.; Ostrikov, K.; Huang, J. Designing Carbon-Based Porous Materials for Carbon Dioxide Capture. Adv. Mater. Interfaces 2024, 11, 2202290. [Google Scholar] [CrossRef]
- Mananghaya, M.R. Hydrogen saturation limit of Ti-doped BN nanotube with B-N defects: An insight from DFT calculations. Int. J. Hydrogen Energy 2018, 43, 10368–10375. [Google Scholar] [CrossRef]
- Luo, D.; Zhang, X. The effect of oxygen–containing functional groups on the H2 adsorption of graphene–based nanomaterials: Experiment and theory. Int. J. Hydrogen Energy 2018, 43, 5668–5679. [Google Scholar] [CrossRef]
- Chen, J.; Tang, Z.; Zhu, D.; Sheng, L.; Li, Z.; Yang, Y.; Wang, J.; Tang, Y.; He, X.; Xu, H. Three-Dimensional Covalent Organic Framework for Efficient Hydrogen Storage through Polarization-Wall Engineering. Nano Lett. 2025, 25, 6268–6275. [Google Scholar] [CrossRef]
- Jaramillo, D.E.; Jiang, H.Z.H.; Evans, H.A.; Chakraborty, R.; Furukawa, H.; Brown, C.M.; Head-Gordon, M.; Long, J.R. Ambient-Temperature Hydrogen Storage via Vanadium(II)-Dihydrogen Complexation in a Metal–Organic Framework. J. Am. Chem. Soc. 2021, 143, 6248–6256. [Google Scholar] [CrossRef]
- Gao, X.; Zhong, Z.; Huang, L.; Mao, Y.; Wang, H.; Liu, J.; Ouyang, L.; Zhang, L.; Han, M.; Ma, X.; et al. The role of transition metal doping in enhancing hydrogen storage capacity in porous carbon materials. Nano Energy 2023, 118, 109038. [Google Scholar] [CrossRef]
- Fomkin, A.; Pribylov, A.; Men’shchikov, I.; Shkolin, A.; Aksyutin, O.; Ishkov, A.; Romanov, K.; Khozina, E. Adsorption-Based Hydrogen Storage in Activated Carbons and Model Carbon Structures. Reactions 2021, 2, 209–226. [Google Scholar] [CrossRef]
- Cruz-Martínez, H.; García-Hilerio, B.; Montejo-Alvaro, F.; Gazga-Villalobos, A.; Rojas-Chávez, H.; Sánchez-Rodríguez, E.P. Density Functional Theory-Based Approaches to Improving Hydrogen Storage in Graphene-Based Materials. Molecules 2024, 29, 436. [Google Scholar] [CrossRef]
- Wang, H.; Gao, Q.; Hu, J. High Hydrogen Storage Capacity of Porous Carbons Prepared by Using Activated Carbon. J. Am. Chem. Soc. 2009, 131, 7016–7022. [Google Scholar] [CrossRef]
- Gupta, A.; Baron, G.V.; Perreault, P.; Lenaerts, S.; Ciocarlan, R.-G.; Cool, P.; Mileo, P.G.M.; Rogge, S.; Van Speybroeck, V.; Watson, G.; et al. Hydrogen Clathrates: Next Generation Hydrogen Storage Materials. Energy Storage Mater. 2021, 41, 69–107. [Google Scholar] [CrossRef]
- Samantaray, S.S.; Putnam, S.T.; Stadie, N.P. Volumetrics of Hydrogen Storage by Physical Adsorption. Inorganics 2021, 9, 45. [Google Scholar] [CrossRef]
- Madden, D.G.; O’Nolan, D.; Rampal, N.; Babu, R.; Çamur, C.; Al Shakhs, A.N.; Zhang, S.-Y.; Rance, G.A.; Perez, J.; Maria Casati, N.P.; et al. Densified HKUST-1 Monoliths as a Route to High Volumetric and Gravimetric Hydrogen Storage Capacity. J. Am. Chem. Soc. 2022, 144, 13729–13739. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Nazir, G.; Heo, K.; Hussain, S.; Ikram, M.; Mahmood, Q.; Alshahrani, T.; Abd-Rabboh, H.S.M. Single step strategy to prepare highly microporous carbons derived from melamine and terephthalaldehyde for high-performance material-based hydrogen storage. J. Energy Storage 2023, 66, 107468. [Google Scholar] [CrossRef]
- Agboola, O.D.; Benson, N.U. Physisorption and Chemisorption Mechanisms Influencing Micro (Nano) Plastics-Organic Chemical Contaminants Interactions: A Review. Front. Environ. Sci. 2021, 9, 678574. [Google Scholar] [CrossRef]
- Xia, W.; Qiu, B.; Xia, D.; Zou, R. Facile preparation of hierarchically porous carbons from metal-organic gels and their application in energy storage. Sci. Rep. 2013, 3, 1935. [Google Scholar] [CrossRef]
- Cai, L.-F.; Zhan, J.-M.; Liang, J.; Yang, L.; Yin, J. Structural control of a novel hierarchical porous carbon material and its adsorption properties. Sci. Rep. 2022, 12, 3118. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Xu, Y.; Luo, W.; Wang, C.; Ma, L. Conversion of Biomass Wastes into Activated Carbons by Chemical Activation for Hydrogen Storage. ChemistrySelect 2020, 5, 11221–11228. [Google Scholar] [CrossRef]
- Andersson, J.; Grönkvist, S. Large-scale storage of hydrogen. Int. J. Hydrogen Energy 2019, 44, 11901–11919. [Google Scholar] [CrossRef]
- Visaria, M.; Mudawar, I.; Pourpoint, T. Enhanced heat exchanger design for hydrogen storage using high-pressure metal hydride: Part 1. Design methodology and computational results. Int. J. Heat Mass Transf. 2011, 54, 413–423. [Google Scholar] [CrossRef]
- Shi, T.; Xu, H. Integration of hydrogen storage and heat storage in thermochemical reactors enhanced with optimized topological structures: Charging process. Appl. Energy 2022, 327, 120138. [Google Scholar] [CrossRef]
- Eisapour, A.H.; Fung, A.S.; Shafaghat, A.; Khosravi, K. Enhancing hydrogen storage efficiency in metal hydride tanks through conical heat exchangers and phase change material integration. Int. J. Hydrogen Energy 2025, 109, 1090–1107. [Google Scholar] [CrossRef]
- Ruiz Puigdollers, A.; Schlexer, P.; Tosoni, S.; Pacchioni, G. Increasing Oxide Reducibility: The Role of Metal/Oxide Interfaces in the Formation of Oxygen Vacancies. ACS Catal. 2017, 7, 6493–6513. [Google Scholar] [CrossRef]
- Zhao, W.; Fierro, V.; Zlotea, C.; Aylon, E.; Izquierdo, M.T.; Latroche, M.; Celzard, A. Activated carbons with appropriate micropore size distribution for hydrogen adsorption. Int. J. Hydrogen Energy 2011, 36, 5431–5434. [Google Scholar] [CrossRef]
- Jordá-Beneyto, M.; Lozano-Castelló, D.; Suárez-García, F.; Cazorla-Amorós, D.; Linares-Solano, Á. Advanced activated carbon monoliths and activated carbons for hydrogen storage. Microporous Mesoporous Mater. 2008, 112, 235–242. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B.; Mokaya, R. High density hydrogen storage in superactivated carbons from hydrothermally carbonized renewable organic materials. Energy Environ. Sci. 2011, 4, 1400–1410. [Google Scholar] [CrossRef]
- Srinivas, G.; Zhu, Y.; Piner, R.; Skipper, N.; Ellerby, M.; Ruoff, R. Synthesis of graphene-like nanosheets and their hydrogen adsorption capacity. Carbon 2010, 48, 630–635. [Google Scholar] [CrossRef]
- Guo, C.X.; Wang, Y.; Li, C.M. Hierarchical Graphene-Based Material for Over 4.0 Wt % Physisorption Hydrogen Storage Capacity. ACS Sustain. Chem. Eng. 2013, 1, 14–18. [Google Scholar] [CrossRef]
- Baburin, I.A.; Klechikov, A.; Mercier, G.; Talyzin, A.; Seifert, G. Hydrogen adsorption by perforated graphene. Int. J. Hydrogen Energy 2015, 40, 6594–6599. [Google Scholar] [CrossRef]
- Tian, W.; Gao, Q.; Tan, Y.; Zhang, Y.; Xu, J.; Li, Z.; Yang, K.; Zhu, L.; Liu, Z. Three-dimensional functionalized graphenes with systematical control over the interconnected pores and surface functional groups for high energy performance supercapacitors. Carbon 2015, 85, 351–362. [Google Scholar] [CrossRef]
- Chow, D.; Burns, N.; Boateng, E.; van der Zalm, J.; Kycia, S.; Chen, A. Mechanical Exfoliation of Expanded Graphite to Graphene-Based Materials and Modification with Palladium Nanoparticles for Hydrogen Storage. Nanomaterials 2023, 13, 2588. [Google Scholar] [CrossRef] [PubMed]
- El-Gendy, D.M.; Ghany, N.A.A.; El Sherbini, E.E.F.; Allam, N.K. Adenine-functionalized Spongy Graphene for Green and High-Performance Supercapacitors. Sci. Rep. 2017, 7, 43104. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Gray, K.A. Graphene-encapsulated nanocomposites: Synthesis, environmental applications, and future prospects. Sci. Total Environ. 2024, 955, 176753. [Google Scholar] [CrossRef]
- Fu, H.; Huang, J.; Gray, K. Crumpled graphene balls adsorb micropollutants from water selectively and rapidly. Carbon 2021, 183, 958–969. [Google Scholar] [CrossRef]
- Zhou, C.; Szpunar, J.A. Hydrogen Storage Performance in Pd/Graphene Nanocomposites. ACS Appl. Mater. Interfaces 2016, 8, 25933–25940. [Google Scholar] [CrossRef]
- Rashidi, A.M.; Nouralishahi, A.; Khodadadi, A.A.; Mortazavi, Y.; Karimi, A.; Kashefi, K. Modification of single wall carbon nanotubes (SWNT) for hydrogen storage. Int. J. Hydrogen Energy 2010, 35, 9489–9495. [Google Scholar] [CrossRef]
- Lee, J.H.; Rhee, K.Y.; Park, S.J. Effects of cryomilling on the structures and hydrogen storage characteristics of multi-walled carbon nanotubes. Int. J. Hydrogen Energy 2010, 35, 7850–7857. [Google Scholar] [CrossRef]
- Salaheldeen, M.; M. Abu-Dief, A.; El-Dabea, T. Functionalization of Nanomaterials for Energy Storage and Hydrogen Production Applications. Materials 2025, 18, 768. [Google Scholar] [CrossRef]
- Kawasaki, K.; Harada, I.; Akaike, K.; Wei, Q.; Koshiba, Y.; Horike, S.; Ishida, K. Complex chemistry of carbon nanotubes toward efficient and stable p-type doping. Commun. Mater. 2024, 5, 21. [Google Scholar] [CrossRef]
- Pinjari, S.; Bera, T.; Kapur, G.S.; Kjeang, E. The mechanism and sorption kinetic analysis of hydrogen storage at room temperature using acid functionalized carbon nanotubes. Int. J. Hydrogen Energy 2023, 48, 1930–1942. [Google Scholar] [CrossRef]
- Chronopoulos, D.D.; Saini, H.; Tantis, I.; Zbořil, R.; Jayaramulu, K.; Otyepka, M. Carbon Nanotube Based Metal–Organic Framework Hybrids From Fundamentals Toward Applications. Small 2022, 18, 2104628. [Google Scholar] [CrossRef]
- Ghiyasiyan-Arani, M.; Salavati-Niasari, M. Decoration of green synthesized S, N-GQDs and CoFe2O4 on halloysite nanoclay as natural substrate for electrochemical hydrogen storage application. Sci. Rep. 2022, 12, 8103. [Google Scholar] [CrossRef]
- Annamalai, A.; Sangaraju, S.; Elumalai, S. Insights into carbon dots and conventional semiconductors hybrids: A trendsetter in photocatalytic hydrogen generation. Coord. Chem. Rev. 2025, 535, 216646. [Google Scholar] [CrossRef]
- Meng, X.; Shen, F.; Wang, D.; Zhang, S.; Hou, J.; Ding, L.; Sun, J. Carbon dots-based hybrid materials: Synthesis, properties and applications in environmental pollution control. Chem. Eng. J. 2025, 503, 158278. [Google Scholar] [CrossRef]
- Kesarwani, R.; Bhatnagar, A.; Verma, S.K.; Hudson, M.S.L.; Shaz, M.A. Enhancement in hydrogen sorption behaviour of MgH2 catalyzed by graphene quantum dots. Int. J. Hydrogen Energy 2024, 67, 1026–1032. [Google Scholar] [CrossRef]
- Blankenship, L.S.; Mokaya, R. Cigarette butt-derived carbons have ultra-high surface area and unprecedented hydrogen storage capacity. Energy Environ. Sci. 2017, 10, 2552–2562. [Google Scholar] [CrossRef]
- Cheng, F.; Liang, J.; Zhao, J.; Tao, Z.; Chen, J. Biomass Waste-Derived Microporous Carbons with Controlled Texture and Enhanced Hydrogen Uptake. Chem. Mater. 2008, 20, 1889–1895. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, Y.; Ding, N.; Liang, L.; Zhao, S.; Yin, D.; Cheng, Y.; Wang, C.; Wang, L. Preparation and hydrogen storage performance of poplar sawdust biochar with high specific surface area. Ind. Crops Prod. 2023, 200, 116788. [Google Scholar] [CrossRef]
- Deng, L.; Zhao, Y.; Sun, S.; Feng, D.; Zhang, W. Thermochemical method for controlling pore structure to enhance hydrogen storage capacity of biochar. Int. J. Hydrogen Energy 2023, 48, 21799–21813. [Google Scholar] [CrossRef]
- Sultana, A.I.; Chambers, C.; Ahmed, M.M.N.; Pathirathna, P.; Reza, T. Multifunctional Loblolly Pine-Derived Superactivated Hydrochar: Effect of Hydrothermal Carbonization on Hydrogen and Electron Storage with Carbon Dioxide and Dye Removal. Nanomaterials 2022, 12, 3575. [Google Scholar] [CrossRef]
- Sultana, A.I.; Saha, N.; Reza, M.T. Upcycling simulated food wastes into superactivated hydrochar for remarkable hydrogen storage. J. Anal. Appl. Pyrolysis 2021, 159, 105322. [Google Scholar] [CrossRef]
- Mopoung, S.; Singse, W. Cotton-derived biochar fibers modified by doping Al2O3 and MgSO4 for application to hydrogen storage. Int. J. Renew. Energy Dev. 2025, 14, 10. [Google Scholar] [CrossRef]
- Zhang, W.; Xi, R.; Li, Y.; Zhang, Y.; Wang, P.; Hu, D. Recent development of transition metal doped carbon materials derived from biomass for hydrogen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 32436–32454. [Google Scholar] [CrossRef]
- Yang, X.; Kong, J.; Lu, X.; Su, J.; Hou, Q.; Li, W. Hydrogen storage properties of metal borohydrides and their improvements: Research progress and trends. Int. J. Hydrogen Energy 2024, 60, 308–323. [Google Scholar] [CrossRef]
- Rawat, S.; Wang, C.-T.; Lay, C.-H.; Hotha, S.; Bhaskar, T. Sustainable biochar for advanced electrochemical/energy storage applications. J. Energy Storage 2023, 63, 107115. [Google Scholar] [CrossRef]
- Li, Z.; Fan, G.; Guo, Q.; Li, Z.; Su, Y.; Zhang, D. Synergistic strengthening effect of graphene-carbon nanotube hybrid structure in aluminum matrix composites. Carbon 2015, 95, 419–427. [Google Scholar] [CrossRef]
- Bhadane, P.; Chakraborty, S. Cross-material synergies of carbon nanomaterials, MOFs, and COFs: Innovative approaches for sustainable environmental remediation and resource recovery. Coord. Chem. Rev. 2025, 535, 216669. [Google Scholar] [CrossRef]
- Soni, K.; Panwar, N.L.; Lanjekar, P.R. Emergence of carbonaceous material for hydrogen storage: An overview. Clean Energy 2024, 8, 147–168. [Google Scholar] [CrossRef]
- Ghotia, S.; Kumar, A.; Sudarsan, V.; Dwivedi, N.; Singh, S.; Kumar, P. Multilayered Ti3C2Tx MXenes: A prominent materials for hydrogen storage. Int. J. Hydrogen Energy 2024, 52, 100–107. [Google Scholar] [CrossRef]
- Anuchitsakol, S.; Dilokekunakul, W.; Khongtor, N.; Chaemchuen, S.; Klomkliang, N. Combined experimental and simulation study on H2 storage in oxygen and nitrogen co-doped activated carbon derived from biomass waste: Superior pore size and surface chemistry development. RSC Adv. 2023, 13, 36009–36022. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.P.; Bhatnagar, A.; Shukla, V.; Soni, P.K.; Singh, S.; Verma, S.K.; Shaneeth, M.; Sekkar, V.; Srivastava, O.N. Hydrogen storage properties of carbon aerogel synthesized by ambient pressure drying using new catalyst triethylamine. Int. J. Hydrogen Energy 2020, 45, 30818–30827. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, Y.; Jing, Z.; Liu, X.; Liu, R.; Tao, R.; Cai, T.; Li, Y.; Zhou, Y.; Shuai, M. Three-dimensional layered porous graphene aerogel hydrogen getters. Int. J. Hydrogen Energy 2022, 47, 15296–15307. [Google Scholar] [CrossRef]
- Konno, H.; Onishi, H.; Yoshizawa, N.; Azumi, K. MgO-templated nitrogen-containing carbons derived from different organic compounds for capacitor electrodes. J. Power Sources 2010, 195, 667–673. [Google Scholar] [CrossRef]
- Jung, H.; Park, K.T.; Gueye, M.N.; So, S.H.; Park, C.R. Bio-inspired graphene foam decorated with Pt nanoparticles for hydrogen storage at room temperature. Int. J. Hydrogen Energy 2016, 41, 5019–5027. [Google Scholar] [CrossRef]
- Blanco-Rey, M.; Juaristi, J.I.; Alducin, M.; López, M.J.; Alonso, J.A. Is Spillover Relevant for Hydrogen Adsorption and Storage in Porous Carbons Doped with Palladium Nanoparticles? J. Phys. Chem. C 2016, 120, 17357–17364. [Google Scholar] [CrossRef]
- Lyu, J.; Kudiiarov, V.; Lider, A. An Overview of the Recent Progress in Modifications of Carbon Nanotubes for Hydrogen Adsorption. Nanomaterials 2020, 10, 255. [Google Scholar] [CrossRef]
- Vanmathi, S.; Awasthi, H.; Pal, A.; Goel, S. IoT enabled carbon cloth-based 3D printed hydrogen fuel cell integrated with supercapacitor for low-power microelectronic devices. Sci. Rep. 2024, 14, 16953. [Google Scholar] [CrossRef]
- Kreider, M.C.; Sefa, M.; Fedchak, J.A.; Scherschligt, J.; Bible, M.; Natarajan, B.; Klimov, N.N.; Miller, A.E.; Ahmed, Z.; Hartings, M.R. Toward 3D printed hydrogen storage materials made with ABS-MOF composites. Polym. Adv. Technol. 2018, 29, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Free, Z.; Hernandez, M.; Mashal, M.; Mondal, K. A Review on Advanced Manufacturing for Hydrogen Storage Applications. Energies 2021, 14, 8513. [Google Scholar] [CrossRef]
- Wu, R.; Wang, C.; Xu, G.; Fan, M.; Huang, Z.; Zeng, T.; Wang, X. Preparation and Characteristics of Porous Mullite Ceramics by 3D Printing and In-Situ Synthesis. Materials 2025, 18, 956. [Google Scholar] [CrossRef]
- Compton, B.G.; Lewis, J.A. 3D-Printing of Lightweight Cellular Composites. Adv. Mater. 2014, 26, 5930–5935. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, R.; Sun, Z.; Liu, Q.; He, X.; Li, H.; Ye, H.; Yang, X.; Wei, X.; Li, Z.; et al. Centrifugal multimaterial 3D printing of multifunctional heterogeneous objects. Nat. Commun. 2022, 13, 7931. [Google Scholar] [CrossRef]
- Fierro, V.; Szczurek, A.; Zlotea, C.; Marêché, J.F.; Izquierdo, M.T.; Albiniak, A.; Latroche, M.; Furdin, G.; Celzard, A. Experimental evidence of an upper limit for hydrogen storage at 77K on activated carbons. Carbon 2010, 48, 1902–1911. [Google Scholar] [CrossRef]
- DOE. DOE Technical Targets for Hydrogen Storage Systems for Material Handling Equipment. Available online: https://www.energy.gov/eere/fuelcells/doe-technical-targets-hydrogen-storage-systems-material-handling-equipment#:~:text=Table_title:%20DOE%20Technical%20Targets%20for%20Hydrogen%20Storage,Units:%20bar%20%28abs%29%20|%202020:%203%20| (accessed on 1 June 2025).
- Chen, M.; Masum, S.A.; Sadasivam, S.; Thomas, H.R.; Mitchell, A.C. Modeling Gas Adsorption–Desorption Hysteresis in Energetically Heterogeneous Coal and Shale. Energy Fuels 2023, 37, 2149–2163. [Google Scholar] [CrossRef]
- Bliznakov, S.; Lefterova, E.; Dimitrov, N. Electrochemical PCT isotherm study of hydrogen absorption/desorption in AB5 type intermetallic compounds. Int. J. Hydrogen Energy 2008, 33, 5789–5794. [Google Scholar] [CrossRef]
- Hossain Bhuiyan, M.M.; Siddique, Z. Hydrogen as an alternative fuel: A comprehensive review of challenges and opportunities in production, storage, and transportation. Int. J. Hydrogen Energy 2025, 102, 1026–1044. [Google Scholar] [CrossRef]
- O’Malley, K.; Ordaz, G.; Adams, J.; Randolph, K.; Ahn, C.C.; Stetson, N.T. Applied hydrogen storage research and development: A perspective from the U.S. Department of Energy. J. Alloys Compd. 2015, 645, S419–S422. [Google Scholar] [CrossRef]
- Sdanghi, G.; Nicolas, V.; Mozet, K.; Schaefer, S.; Maranzana, G.; Celzard, A.; Fierro, V. A 70 MPa hydrogen thermally driven compressor based on cyclic adsorption-desorption on activated carbon. Carbon 2020, 161, 466–478. [Google Scholar] [CrossRef]
- Elyasi, S.; Saha, S.; Hameed, N.; Mahon, P.J.; Juodkazis, S.; Salim, N. Emerging trends in biomass-derived porous carbon materials for hydrogen storage. Int. J. Hydrogen Energy 2024, 62, 272–306. [Google Scholar] [CrossRef]
- Yu, Z.; Deschamps, J.; Hamon, L.; Karikkethu Prabhakaran, P.; Pré, P. Hydrogen adsorption and kinetics in MIL-101(Cr) and hybrid activated carbon-MIL-101(Cr) materials. Int. J. Hydrogen Energy 2017, 42, 8021–8031. [Google Scholar] [CrossRef]
- Vasiliev, L.L.; Kanonchik, L.E.; Babenko, V.A. Thermal management of the adsorption-based vessel for hydrogeneous gas storage. J. Eng. Phys. Thermophys. 2012, 85, 987–996. [Google Scholar] [CrossRef]
- Shahzad, S.; Alsenani, T.R.; Alrumayh, O.; Altamimi, A.; Kilic, H. Adaptive hydrogen buffering for enhanced flexibility in constrained transmission grids with integrated renewable energy system. Int. J. Hydrogen Energy 2025, 144, 637–651. [Google Scholar] [CrossRef]
- Singla, M.K.; Gupta, J.; Safaraliev, M.; Nijhawan, P.; Oberoi, A.S. Hydrogen storage in activated carbon for fuel cell-powered vehicles: A cost-effective and sustainable approach. Int. J. Hydrogen Energy 2024, 58, 446–458. [Google Scholar] [CrossRef]
- Manoharan, Y.; Hosseini, S.E.; Butler, B.; Alzhahrani, H.; Senior, B.T.; Ashuri, T.; Krohn, J. Hydrogen Fuel Cell Vehicles; Current Status and Future Prospect. Appl. Sci. 2019, 9, 2296. [Google Scholar] [CrossRef]
- Liu, L.; Ilyushechkin, A.; Liang, D.; Cousins, A.; Tian, W.; Chen, C.; Yin, J.; Schoeman, L. Metal Hydride Composite Structures for Improved Heat Transfer and Stability for Hydrogen Storage and Compression Applications. Inorganics 2023, 11, 181. [Google Scholar] [CrossRef]
- Marocco, P.; Ferrero, D.; Lanzini, A.; Santarelli, M. The role of hydrogen in the optimal design of off-grid hybrid renewable energy systems. J. Energy Storage 2022, 46, 103893. [Google Scholar] [CrossRef]
- Samir De, B.; Singh, A.; Ji Dixit, R.; Khare, N.; Elias, A.; Basu, S. Hydrogen generation in additively manufactured membraneless microfluidic electrolysis cell: Performance evaluation and accelerated stress testing. Chem. Eng. J. 2023, 452, 139433. [Google Scholar] [CrossRef]
- Fu, H.; Pan, Z.; Lai, Y.-J.S.; Ananpattarachai, J.; Serpa, M.; Shapiro, N.; Zhao, Z.; Westerhoff, P. Green hydrogen production via a photocatalyst-enabled optical fiber system: A promising route to net-zero emissions. Energy Clim. Change 2025, 6, 100175. [Google Scholar] [CrossRef]
- Fu, H.; Wang, T.-H.; Doong, R.-a.; Lai, Y.-J.S.; Garcia-Segura, S.; Zhao, Z.; Westerhoff, P. Boosting Hydrogen Production via Water Splitting: An ITO Plus g-C3N4 Nanomaterial Enabled Polymer Optical Fiber Design. ACS Mater. Lett. 2024, 6, 2267–2275. [Google Scholar] [CrossRef]
- Kayikci, Y.; Ali, M.R.; Khan, S.A.; Ikpehai, A. Examining dynamics of hydrogen supply chains. Technol. Forecast. Soc. Change 2025, 215, 124101. [Google Scholar] [CrossRef]
- Wang, Y.; Shahbeik, H.; Moradi, A.; Rafiee, S.; Shafizadeh, A.; Khoshnevisan, B.; Ghafarian Nia, S.A.; Nadian, M.H.; Li, M.; Pan, J.; et al. Predictive modeling for hydrogen storage in functionalized carbonaceous nanomaterials using machine learning. J. Energy Storage 2024, 97, 112914. [Google Scholar] [CrossRef]
- Davoodi, S.; Thanh, H.V.; Wood, D.A.; Mehrad, M.; Al-Shargabi, M.; Rukavishnikov, V.S. Machine-learning models to predict hydrogen uptake of porous carbon materials from influential variables. Sep. Purif. Technol. 2023, 316, 123807. [Google Scholar] [CrossRef]
- Heravi, M.J.T.; Yasari, E.; Farhadian, N. Data-driven modelling and optimization of hydrogen adsorption on carbon nanostructures. Int. J. Hydrogen Energy 2022, 47, 25704–25723. [Google Scholar] [CrossRef]
- Ibrahim, A.F.; Hussein, M.A. Leveraging machine learning for prediction and optimization of texture properties of sustainable activated carbon derived from waste materials. Sci. Rep. 2025, 15, 11313. [Google Scholar] [CrossRef]
- Thanh, H.V.; Ebrahimnia Taremsari, S.; Ranjbar, B.; Mashhadimoslem, H.; Rahimi, E.; Rahimi, M.; Elkamel, A. Hydrogen Storage on Porous Carbon Adsorbents: Rediscovery by Nature-Derived Algorithms in Random Forest Machine Learning Model. Energies 2023, 16, 2348. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, L.; Ye, X.; Wu, J.; Ying, H.; Zhu, W.; Yu, Z.; Wu, X. State-of-the-art review on various applications of machine learning techniques in materials science and engineering. Chem. Eng. Sci. 2025, 306, 121147. [Google Scholar] [CrossRef]
- Wang, T.; Pan, R.; Martins, M.L.; Cui, J.; Huang, Z.; Thapaliya, B.P.; Do-Thanh, C.-L.; Zhou, M.; Fan, J.; Yang, Z.; et al. Machine-learning-assisted material discovery of oxygen-rich highly porous carbon active materials for aqueous supercapacitors. Nat. Commun. 2023, 14, 4607. [Google Scholar] [CrossRef]
- Dave, A.; Mitchell, J.; Kandasamy, K.; Wang, H.; Burke, S.; Paria, B.; Póczos, B.; Whitacre, J.; Viswanathan, V. Autonomous Discovery of Battery Electrolytes with Robotic Experimentation and Machine Learning. Cell Rep. Phys. Sci. 2020, 1, 100264. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, C.; Deng, H.; Zheng, B.; Su, J.-W.; Shutt, K.; Lin, J. Accelerate Synthesis of Metal–Organic Frameworks by a Robotic Platform and Bayesian Optimization. ACS Appl. Mater. Interfaces 2021, 13, 53485–53491. [Google Scholar] [CrossRef]
- Park, H.; Yan, X.; Zhu, R.; Huerta, E.A.; Chaudhuri, S.; Cooper, D.; Foster, I.; Tajkhorshid, E. A generative artificial intelligence framework based on a molecular diffusion model for the design of metal-organic frameworks for carbon capture. Commun. Chem. 2024, 7, 21. [Google Scholar] [CrossRef]

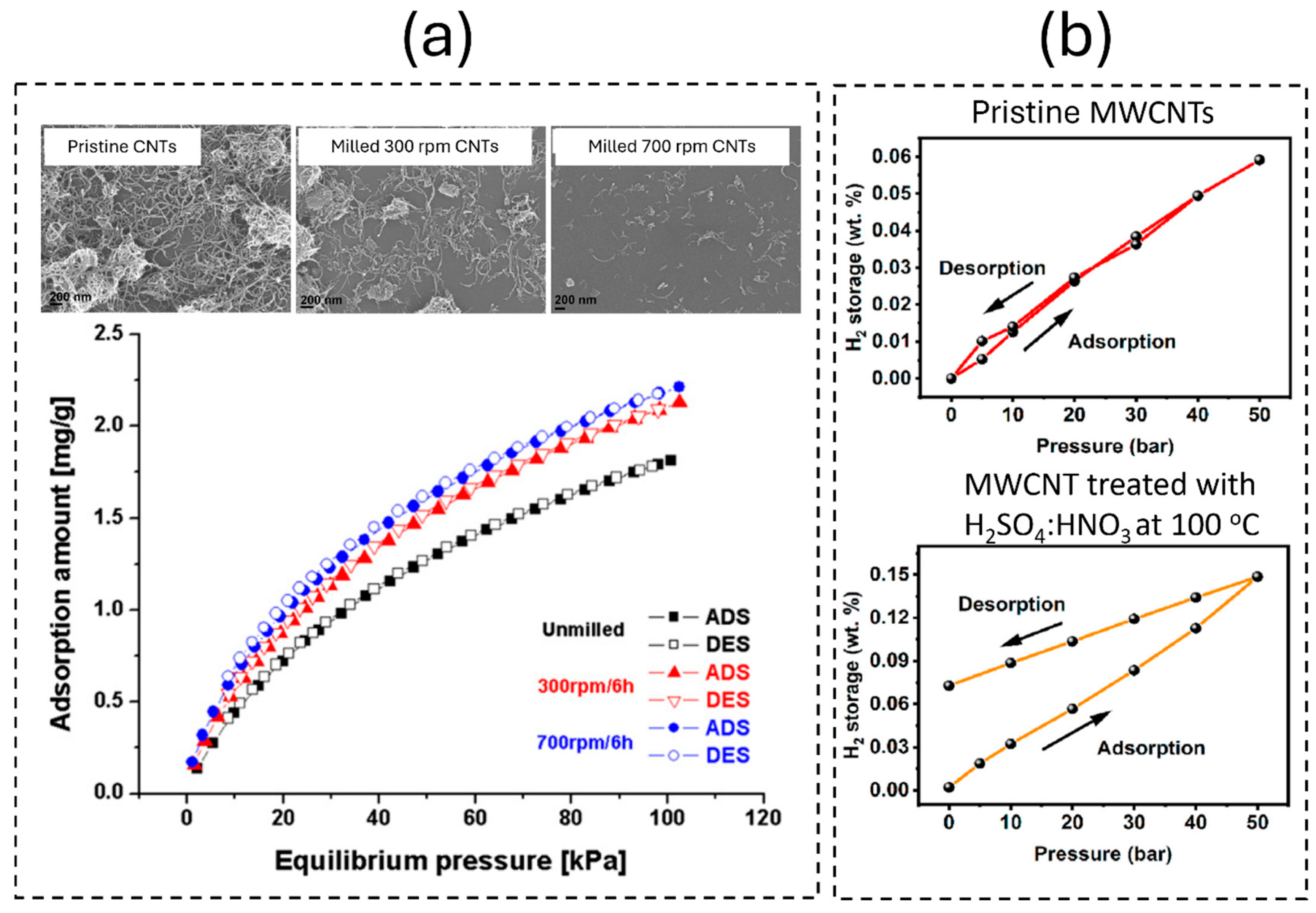
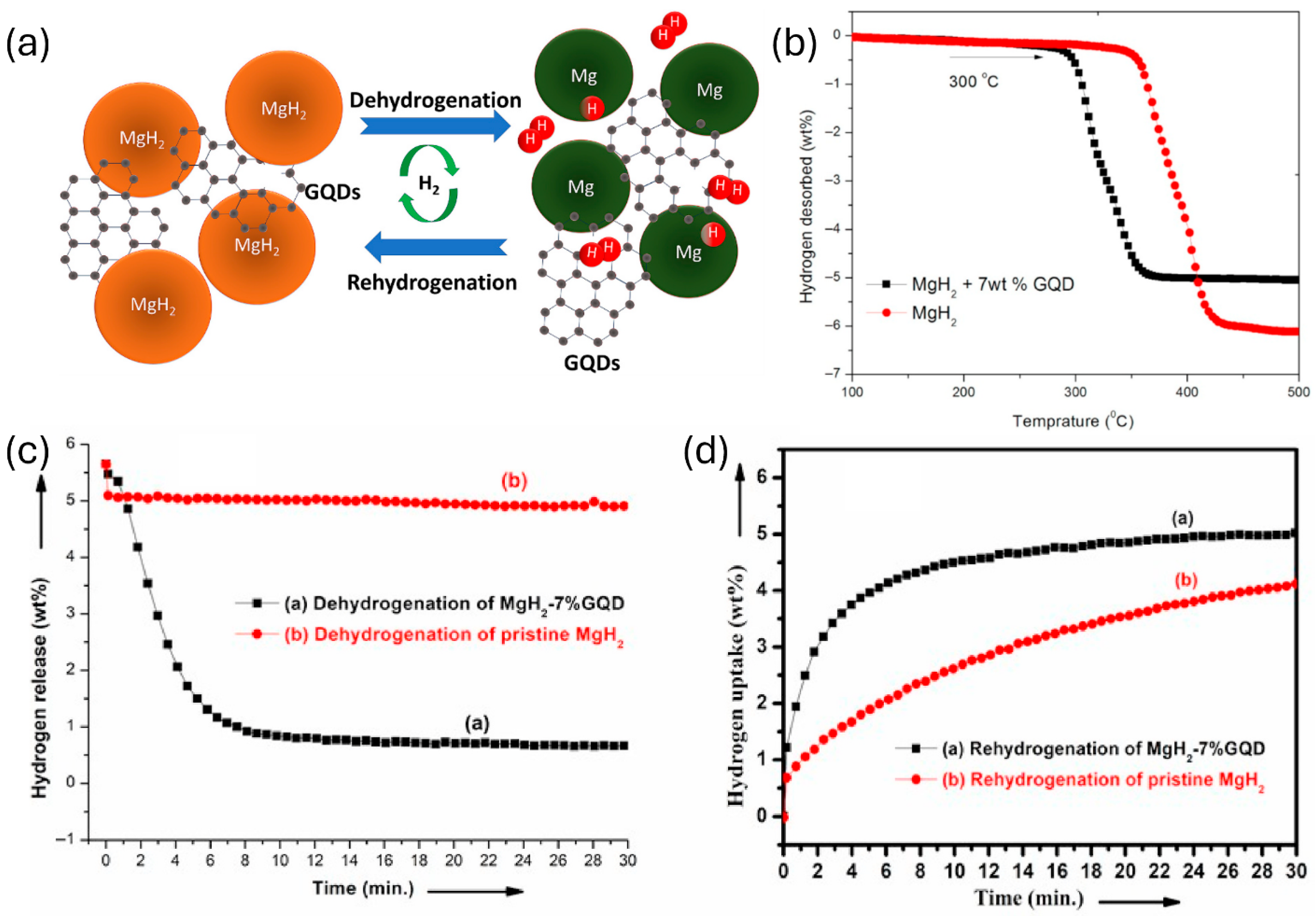
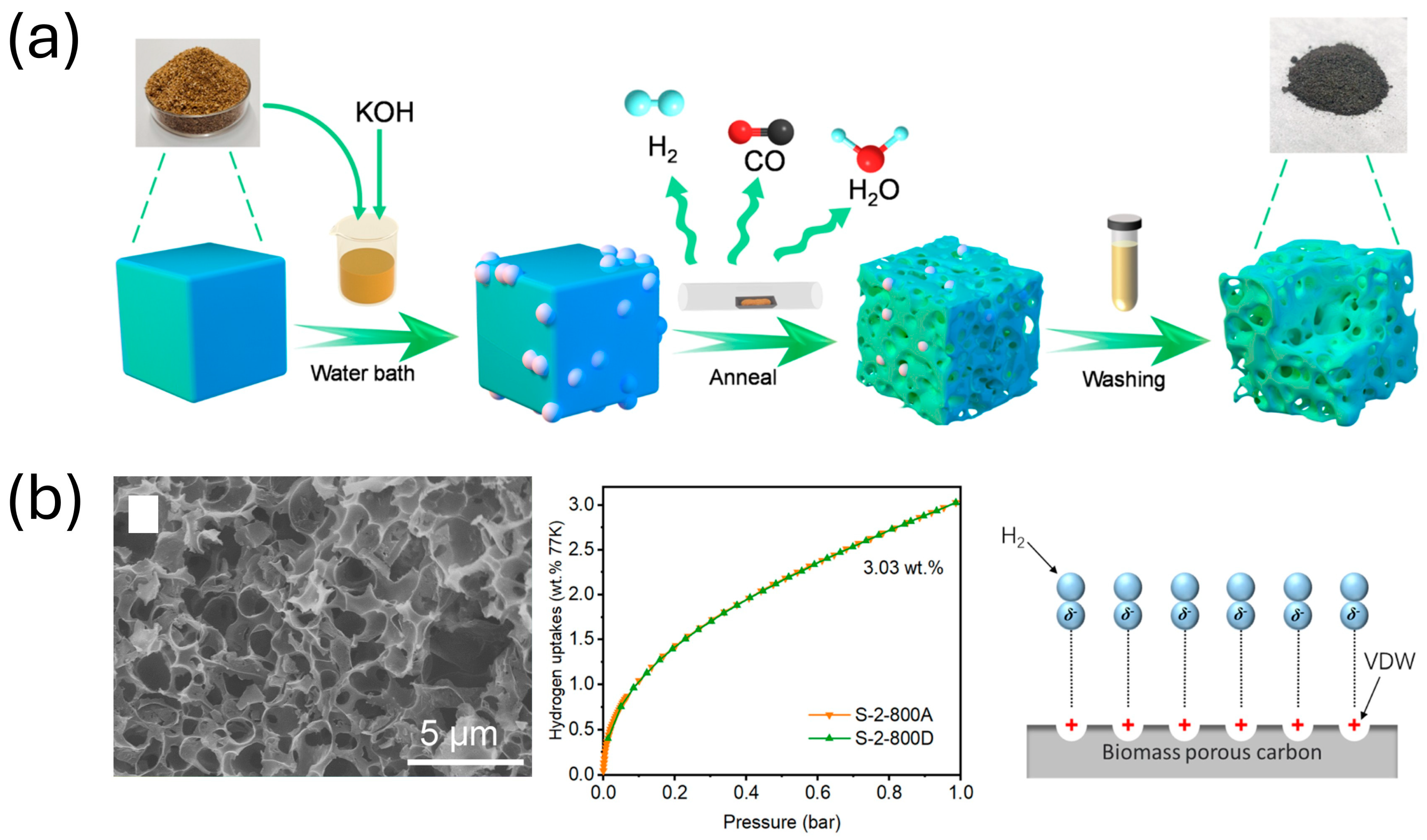
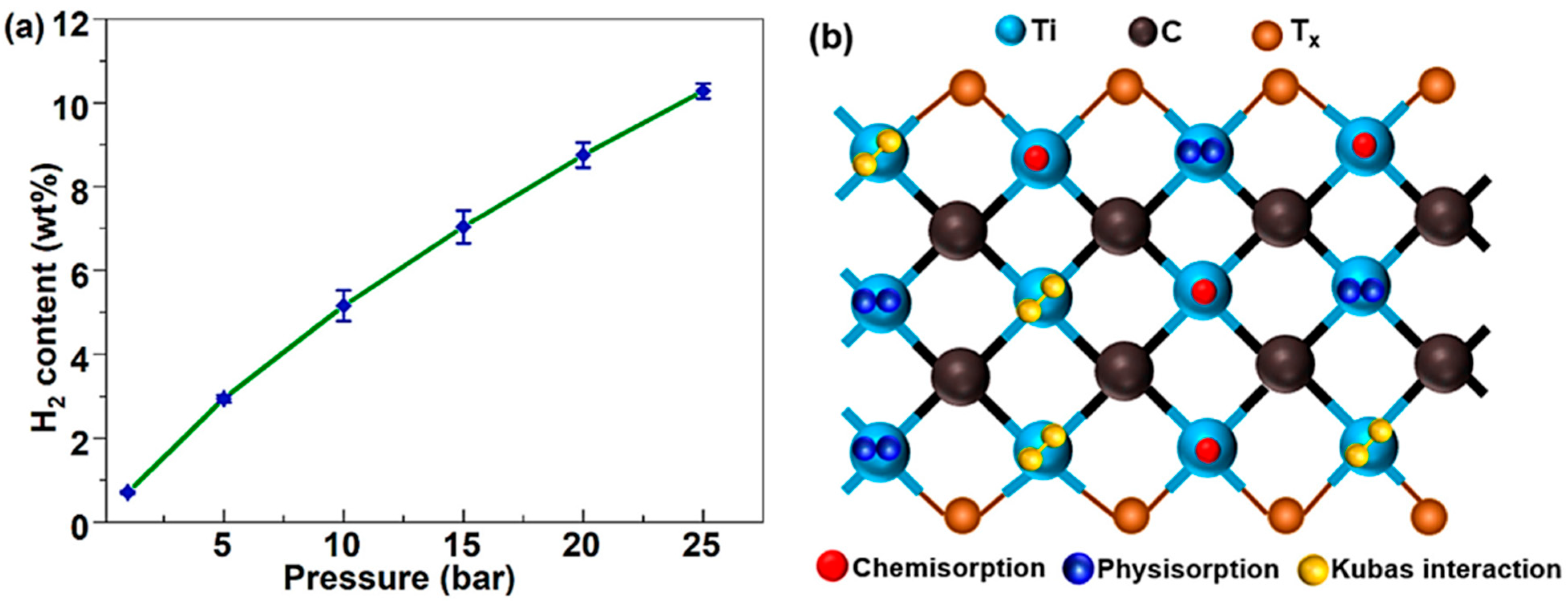
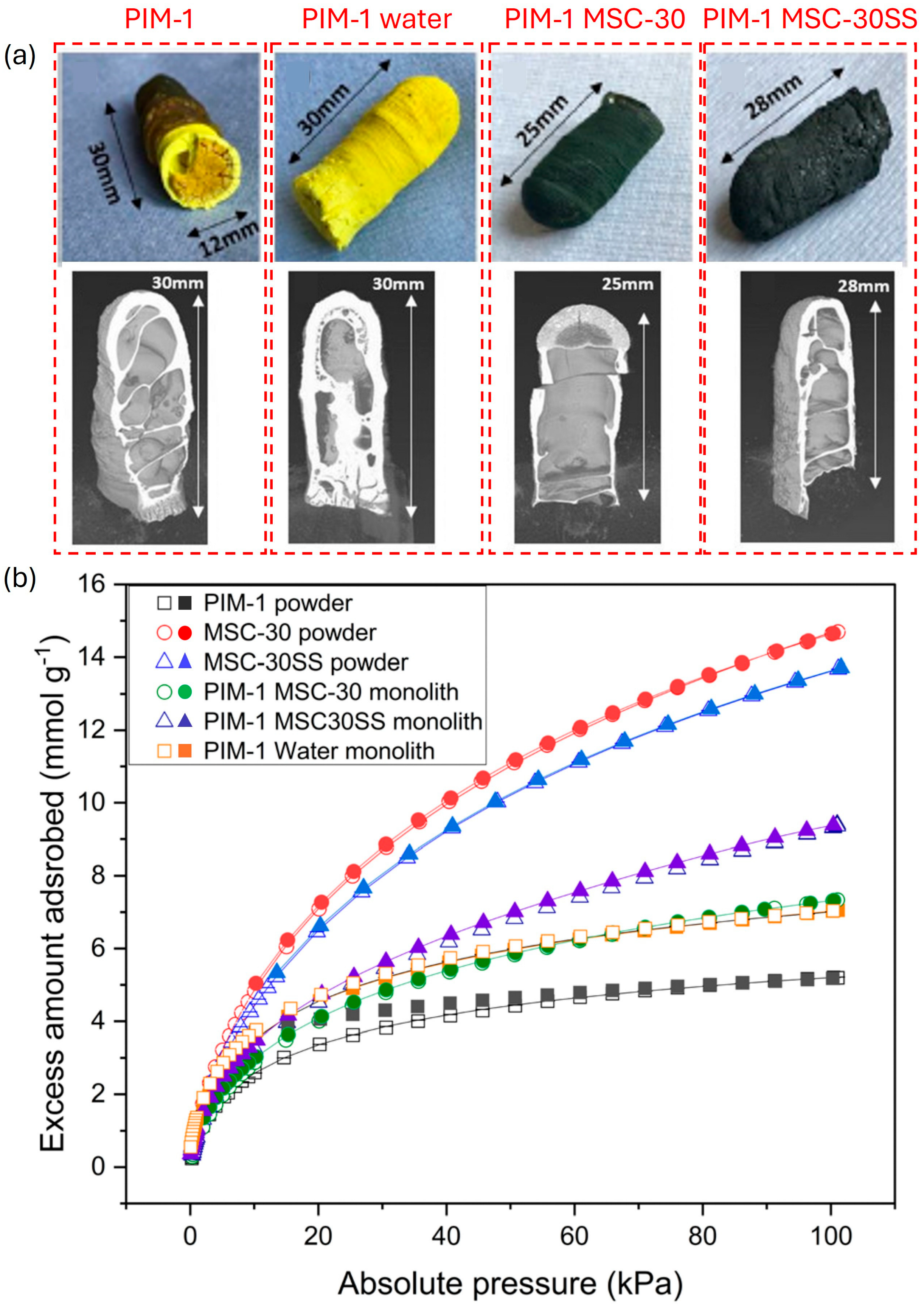
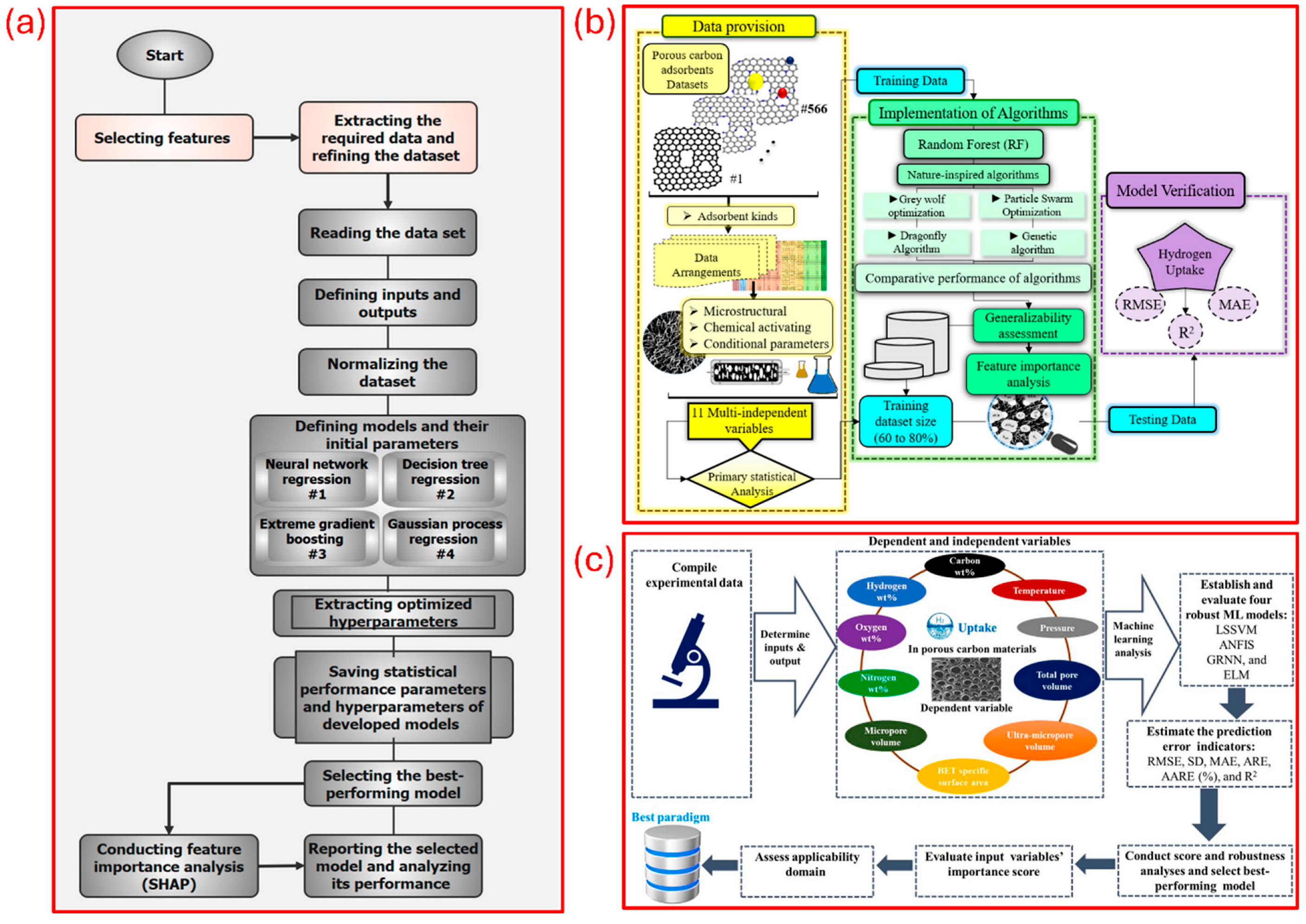
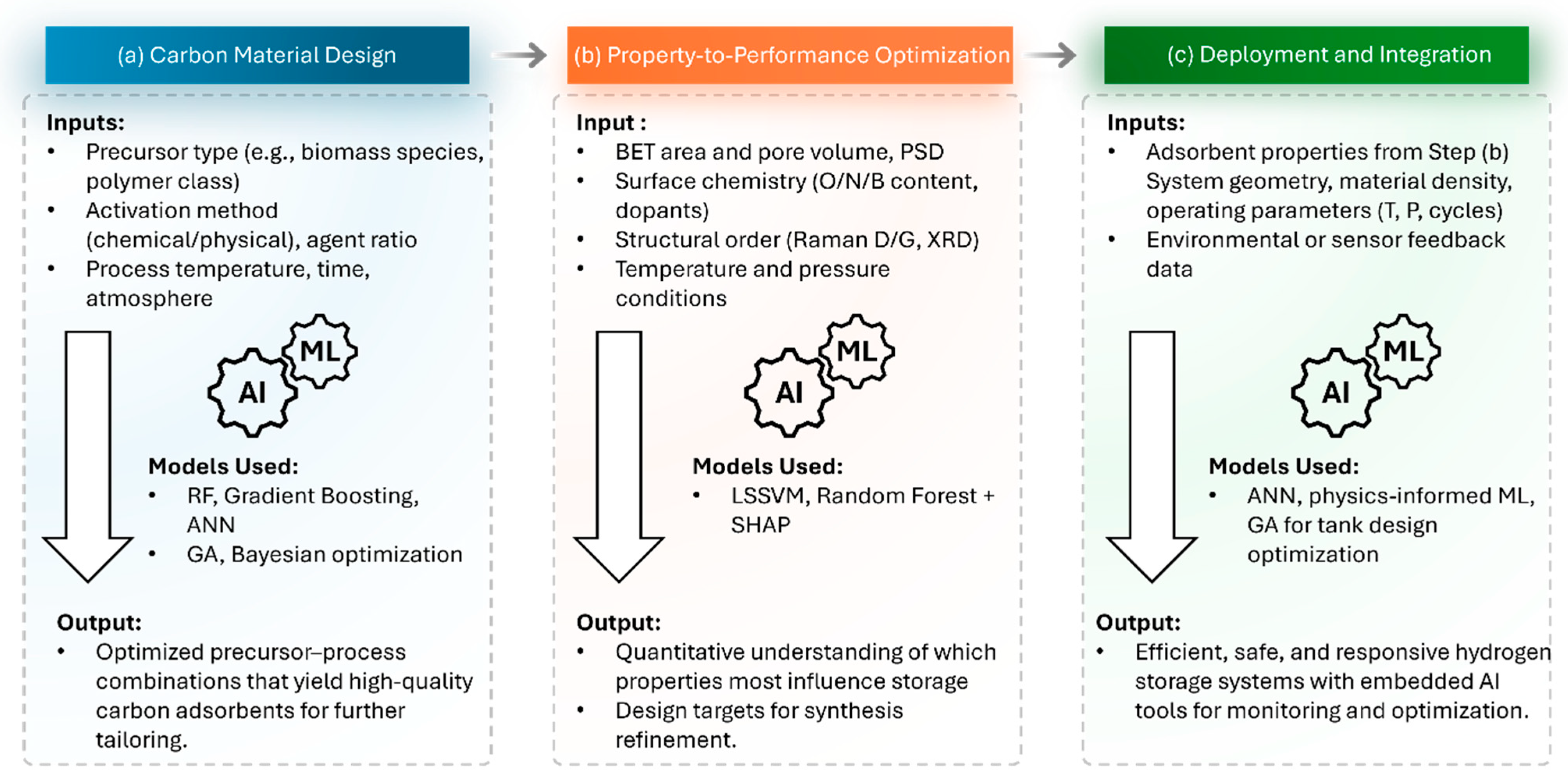
| Parameter | Unit | 2025 Target | Ultimate Target |
|---|---|---|---|
| Gravimetric Energy Density | kWh/kg-system | 1.8 | 2.2 |
| Gravimetric Capacity (system-based) | kg-H2/kg-system | 0.055 | 0.065 |
| Volumetric Energy Density | kWh/L-system | 1.3 | 1.7 |
| Volumetric Capacity (system-based) | kg-H2/L-system | 0.04 | 0.05 |
| Storage System Cost | $/kWh (and $/kg-H2) | 9 ($300) | 8 ($266) |
| Operating Ambient Temperature | °C (with solar load) | −40 to +60 | −40 to +60 |
| Delivery Temperature Range | °C | −40 to +85 | −40 to +85 |
| Delivery Pressure Range | bar | 5 to 12 | 5 to 12 |
| Cycle Life | full charge/discharge cycles | 1500 | 1500 |
| System Fill Time | min (for 4–10 kg H2) | 3 to 5 | 3 to 5 |
| Fuel Purity | % H2 | 99.97% | 99.97% |
| Dormancy Time (cryogenic systems) | days (no boil-off loss) | 10 | 14 |
| H2 Loss after 30 Days (cryo systems) | % lost | ≤10% | ≤5% |
| System Weight | kg (for 5.6 kg usable H2) | ~100–125 kg target | <90 kg (aspirational) |
| System Volume | L (for 5.6 kg usable H2) | ~125–140 L target | <115 L (aspirational) |
| Model/Technique | Application in Carbon Sorbents | Recent Breakthroughs | Advantages | Limitations | Reference |
|---|---|---|---|---|---|
| Random Forest (RF) | Predicting H2 uptake; feature importance analysis | RF + PSO/GWO achieved R2 > 0.91 for H2 uptake prediction using porous carbon dataset (hydrogen storage). | Handles non-linearities; interpretable with SHAP | Can overfit; less interpretable without SHAP | [111] |
| Gaussian Process Regression (GPR) | High-accuracy prediction of sorption behavior | GPR predicted H2 adsorption in functionalized carbon nanomaterials with R2 > 0.955 (hydrogen storage). | High accuracy; quantifies uncertainty | Computationally expensive | [137] |
| Least-Squares Support Vector Machine (LSSVM) | Best performer among multiple ML models for H2 uptake | LSSVM delivered lowest RMSE (~0.24 wt.%) for H2 uptake across 2000+ porous carbon samples (hydrogen storage). | Strong generalization and low RMSE | Sensitive to parameter tuning | [138] |
| Artificial Neural Networks (ANN) | Modeling synthesis–performance relationships | ANN predicted capacitance of porous carbons; SHAP confirmed surface area as key factor (supercapacitor design). | Captures complex nonlinear relationships | Black-box nature; training requires large data. | [139] |
| Polynomial Regression | Simplified modeling of synthesis relationships | Polynomial regression modeled thermal conductivity and phase behavior in carbon-enhanced PCM (thermal energy storage). | Simple and interpretable | Low predictive power for complex patterns | [139] |
| ALAMO | Interpretable algebraic expressions for optimization | ALAMO derived equations for H2 uptake; GA optimized pore structure and conditions (hydrogen storage). | Combines interpretability and ML accuracy. | Limited to polynomial functions | [139] |
| Genetic Algorithm (GA) | Optimization of synthesis parameters | GA + ML optimized biomass activation parameters for porous carbon synthesis (hydrogen storage). | Effective for global search and tuning | Stochastic; may require many evaluations | [140] |
| Bayesian Optimization | Optimization of experiments and synthesis | BO discovered lightweight carbon nanolattices with record-specific strength (structural carbon materials). | Reduces trial and error in expensive experiments | Depends on accurate surrogate models | [140] |
| SHAP (SHapley Additive exPlanations) | Model interpretation and feature impact ranking | SHAP revealed optimal oxygen content (8–12 wt.%) enhances H2 uptake in doped porous carbons (hydrogen storage). | Explains feature influence clearly | Post hoc analysis; not predictive itself | [111] |
| Variational Autoencoders (VAE) | Generating new porous structures with desired features | SMVAE generated novel MOF structures for gas adsorption and separation (gas storage and CO2 capture). | Can explore unseen structure–property space | Requires high-quality training data | [141] |
| Generative Adversarial Networks (GAN) | Morphology generation from images; exploratory design | GAN generated realistic 3D morphologies of porous carbon electrodes (supercapacitor microstructure modeling). | Useful for morphology design and data augmentation | Training instability; hard to validate results | [141] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, H.; Mojiri, A.; Wang, J.; Zhao, Z. Hydrogen Energy Storage via Carbon-Based Materials: From Traditional Sorbents to Emerging Architecture Engineering and AI-Driven Optimization. Energies 2025, 18, 3958. https://doi.org/10.3390/en18153958
Fu H, Mojiri A, Wang J, Zhao Z. Hydrogen Energy Storage via Carbon-Based Materials: From Traditional Sorbents to Emerging Architecture Engineering and AI-Driven Optimization. Energies. 2025; 18(15):3958. https://doi.org/10.3390/en18153958
Chicago/Turabian StyleFu, Han, Amin Mojiri, Junli Wang, and Zhe Zhao. 2025. "Hydrogen Energy Storage via Carbon-Based Materials: From Traditional Sorbents to Emerging Architecture Engineering and AI-Driven Optimization" Energies 18, no. 15: 3958. https://doi.org/10.3390/en18153958
APA StyleFu, H., Mojiri, A., Wang, J., & Zhao, Z. (2025). Hydrogen Energy Storage via Carbon-Based Materials: From Traditional Sorbents to Emerging Architecture Engineering and AI-Driven Optimization. Energies, 18(15), 3958. https://doi.org/10.3390/en18153958






