Electrical–Thermal Aging Performance of PAH-Modified Interfacial Coating Agent for HVDC Cable Accessory
Abstract
1. Introduction
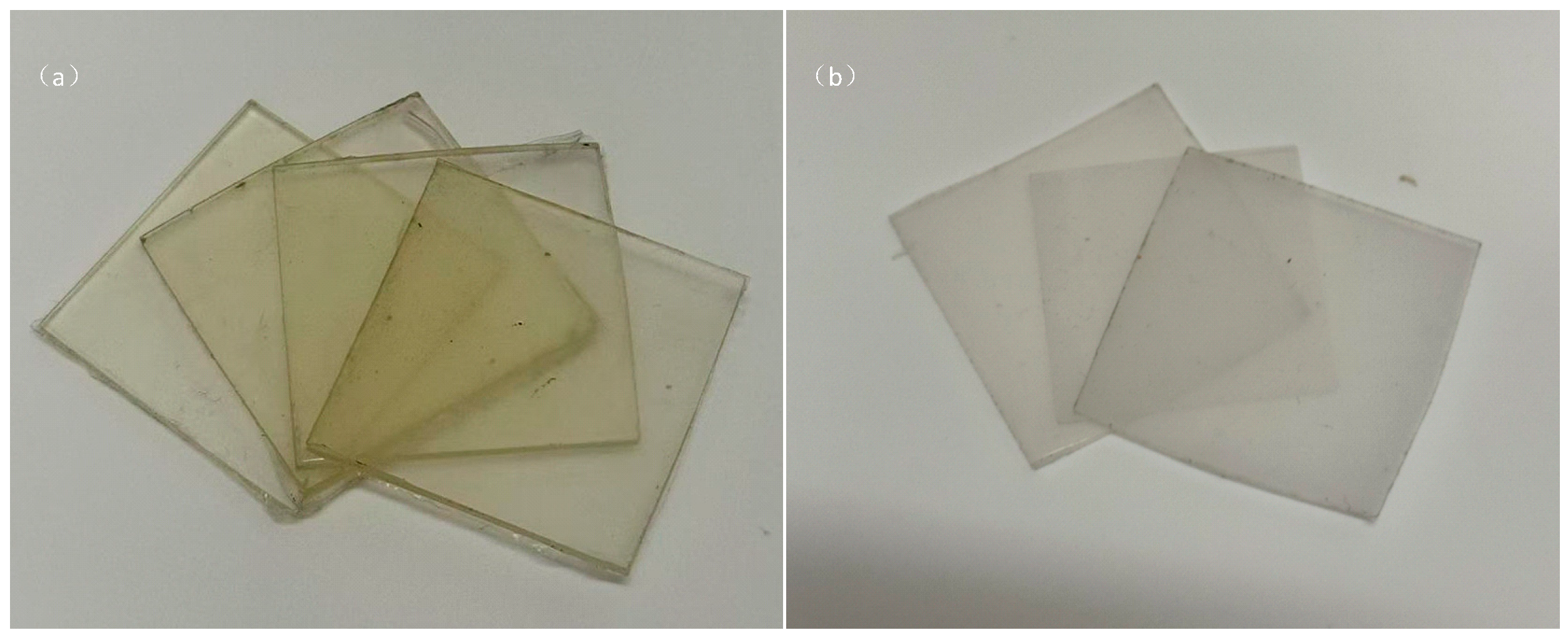
1.1. Sample Preparation
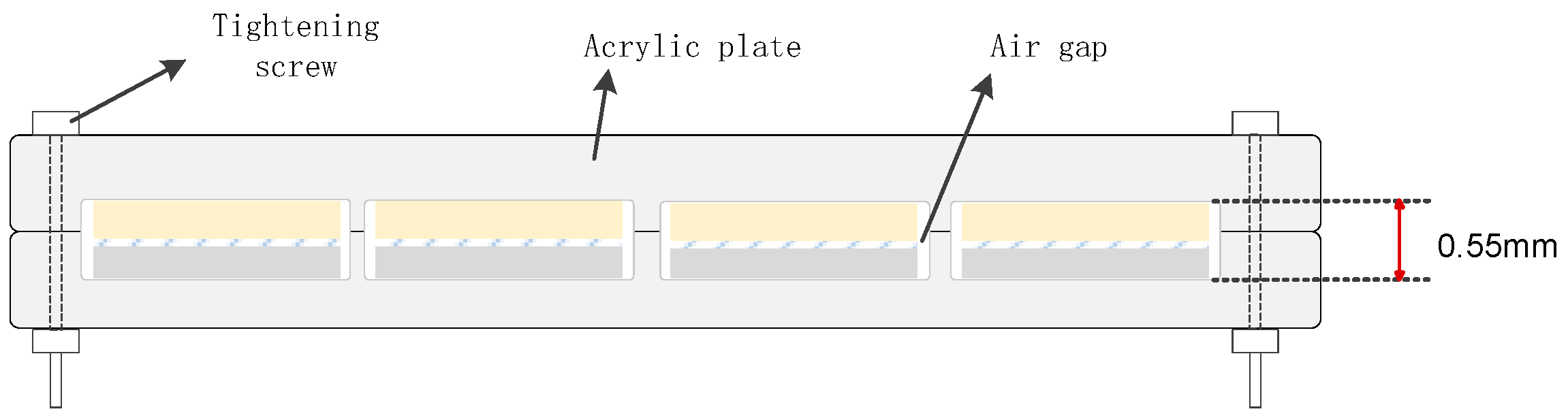
1.1.1. XLPE Sample Preparation
1.1.2. EPDM Sample Preparation
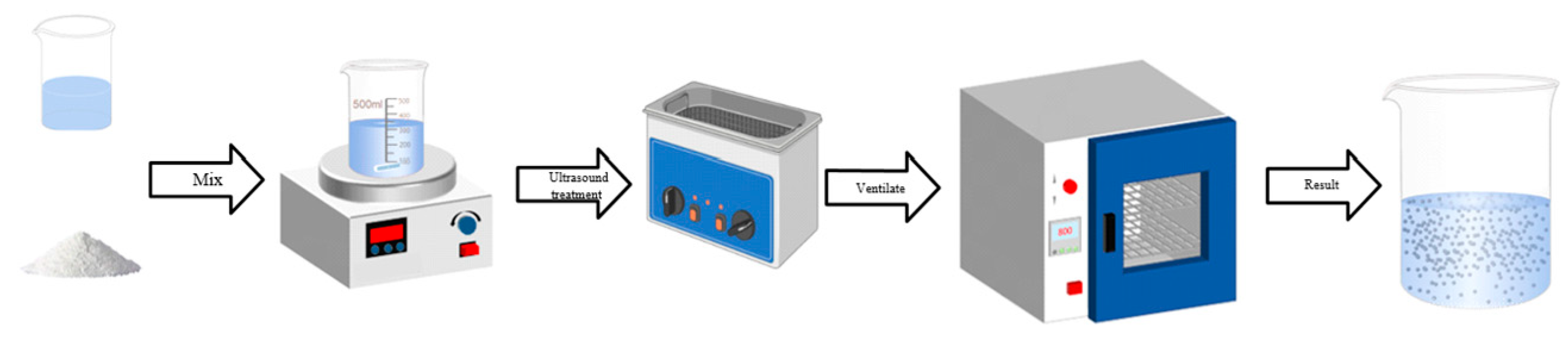
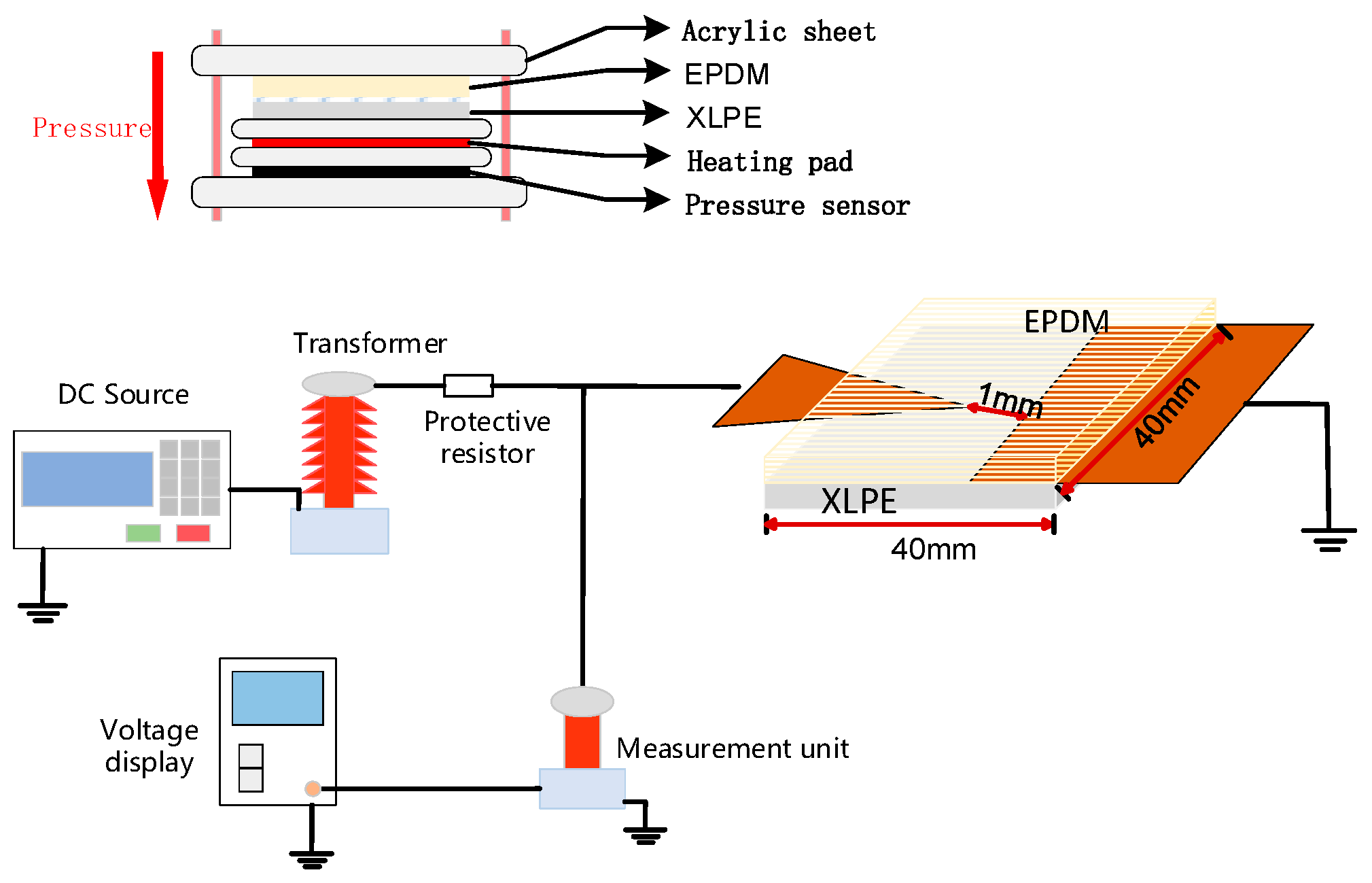
1.1.3. Preparation of Coating Modifying Agent
1.2. Experiment Procedures
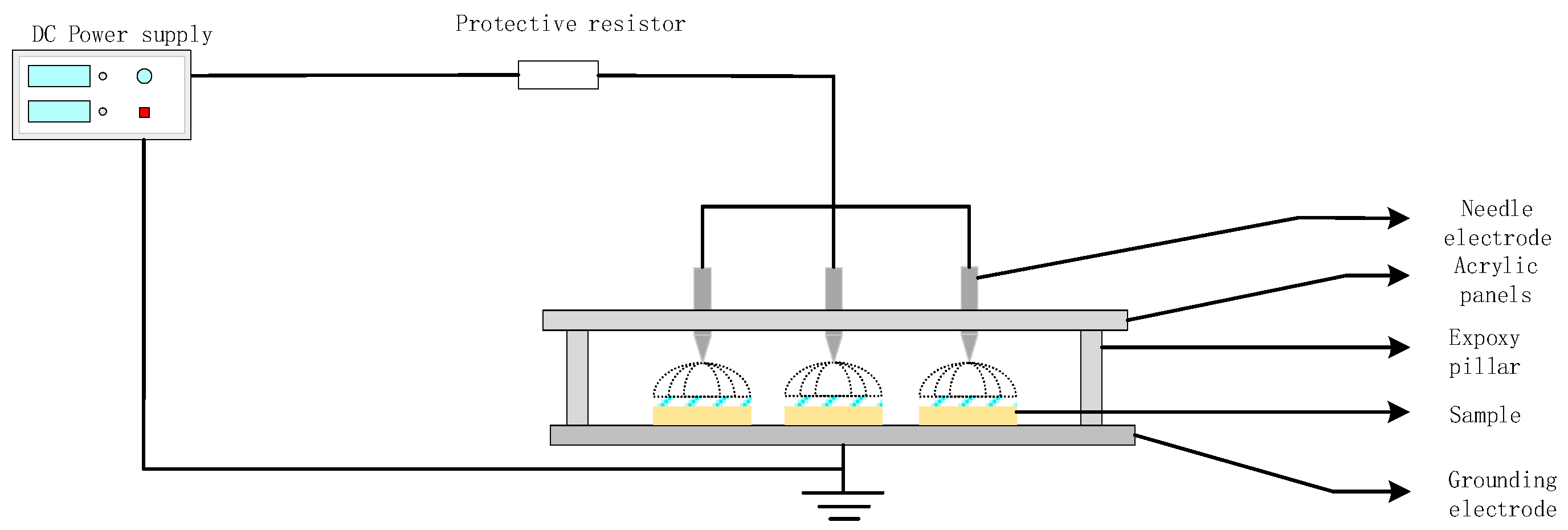
1.2.1. Corona Aging Treatment
1.2.2. Hot and Cold Cycle Aging Treatment
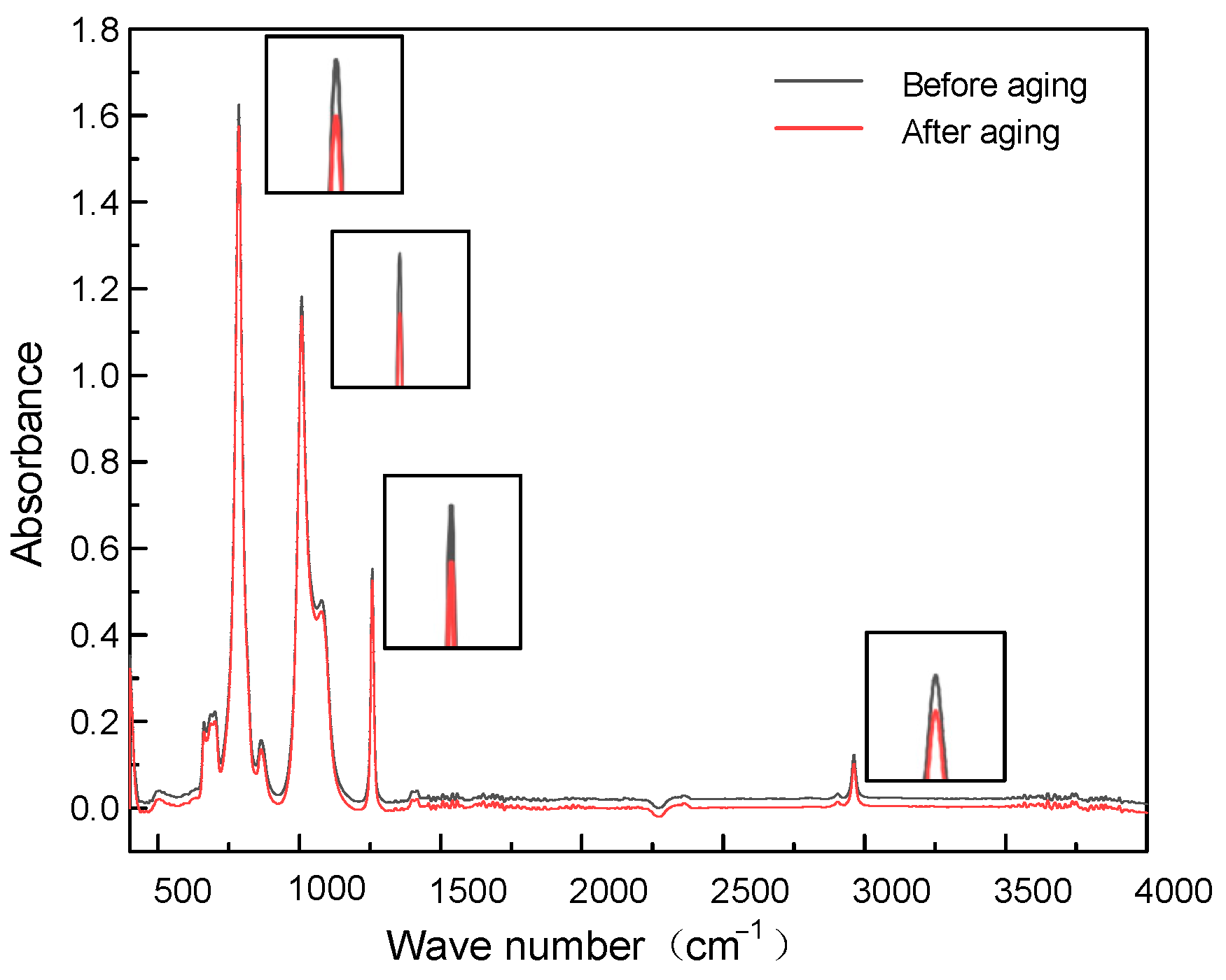
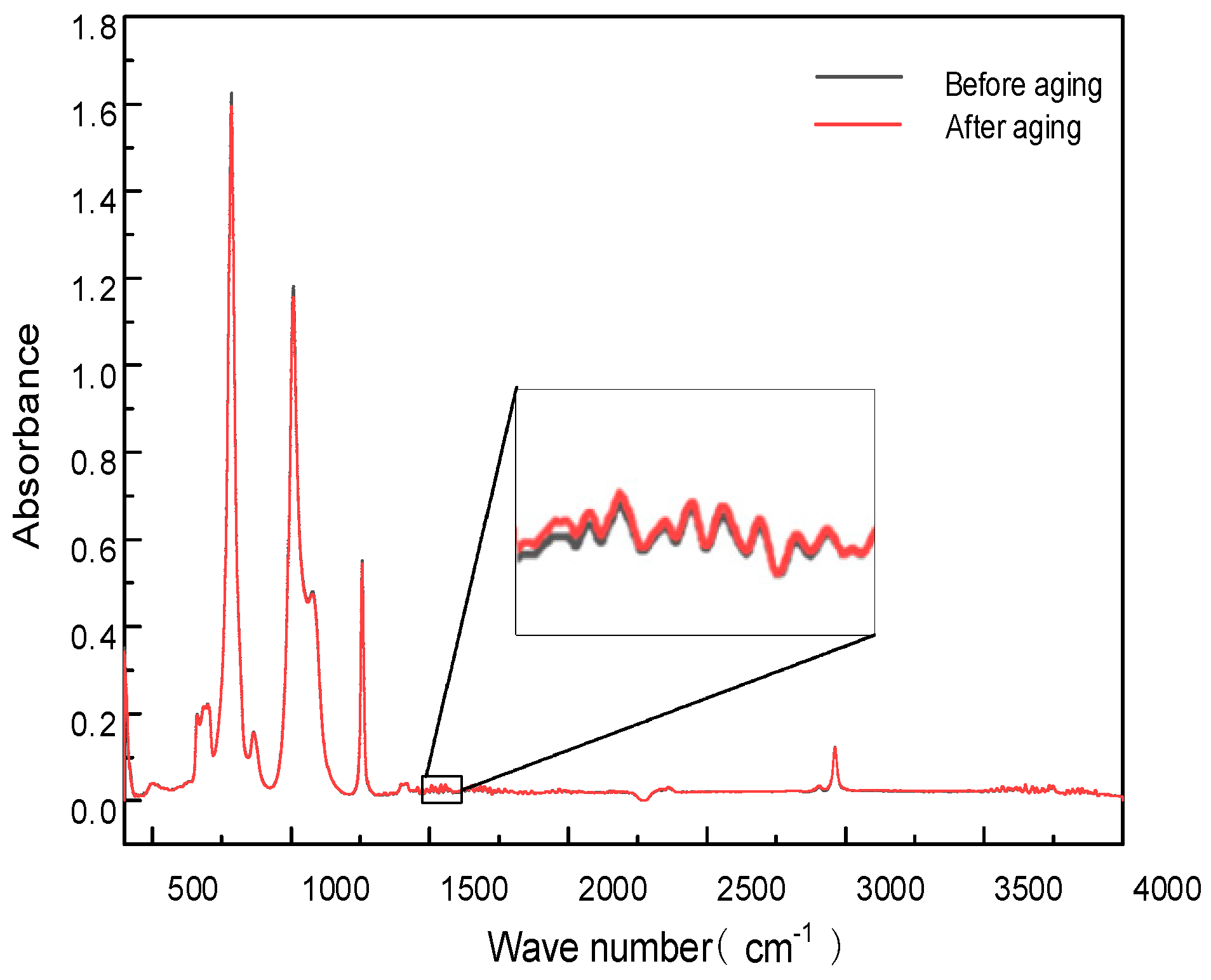
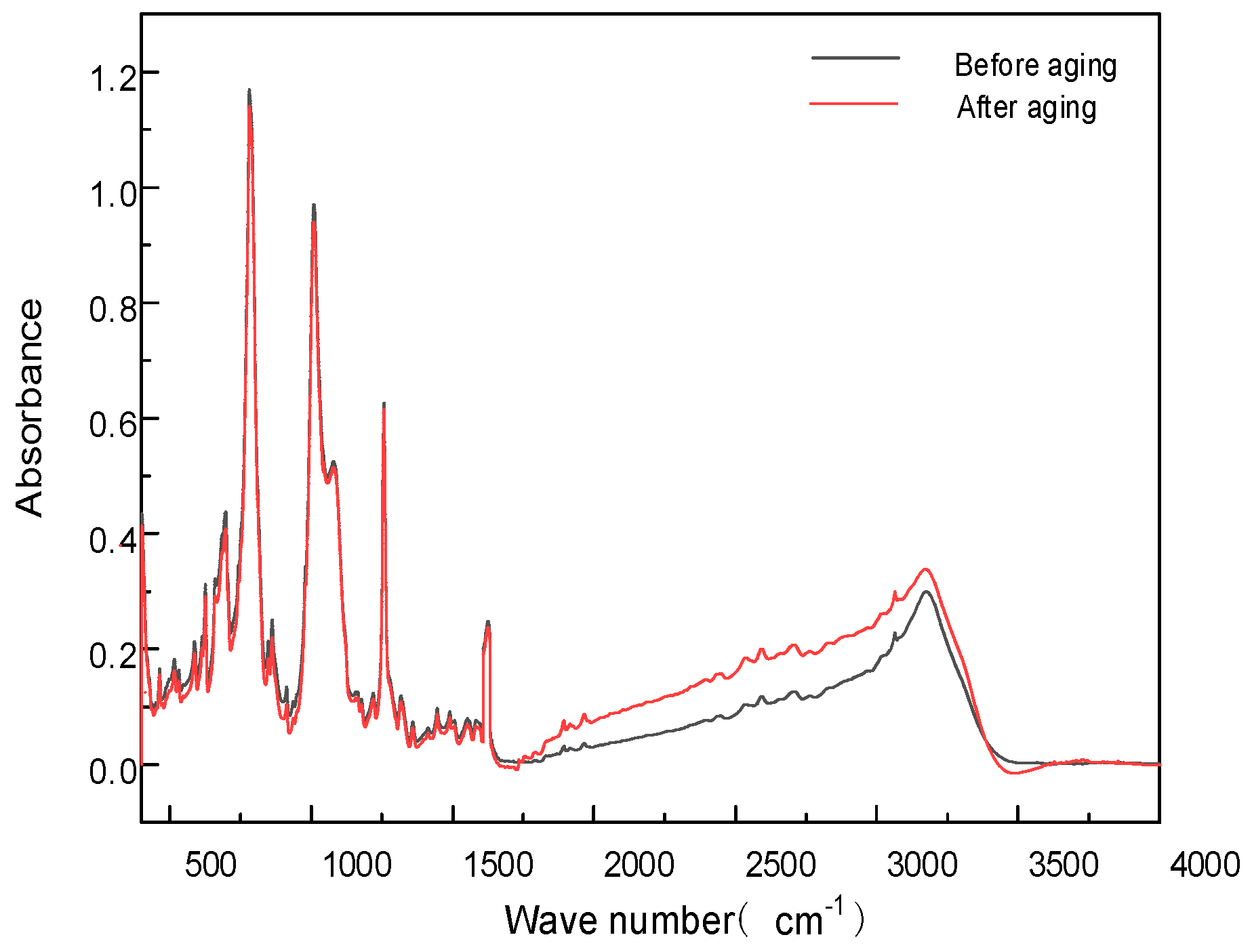
1.2.3. FT-IR Analysis
1.2.4. DC Breakdown Testing
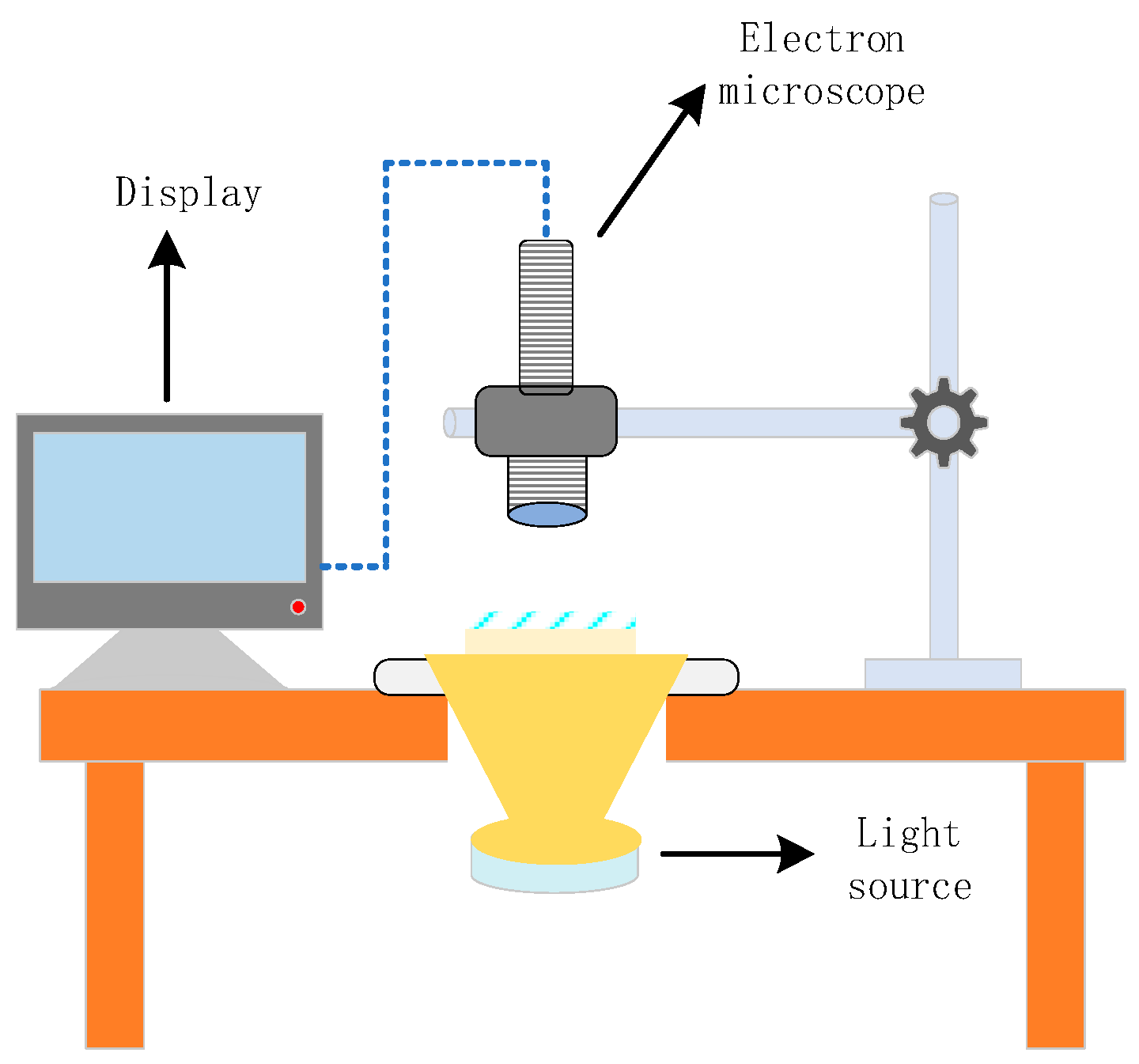
1.2.5. Microstructure Analysis
1.2.6. Quantum Chemical Computing
2. Results
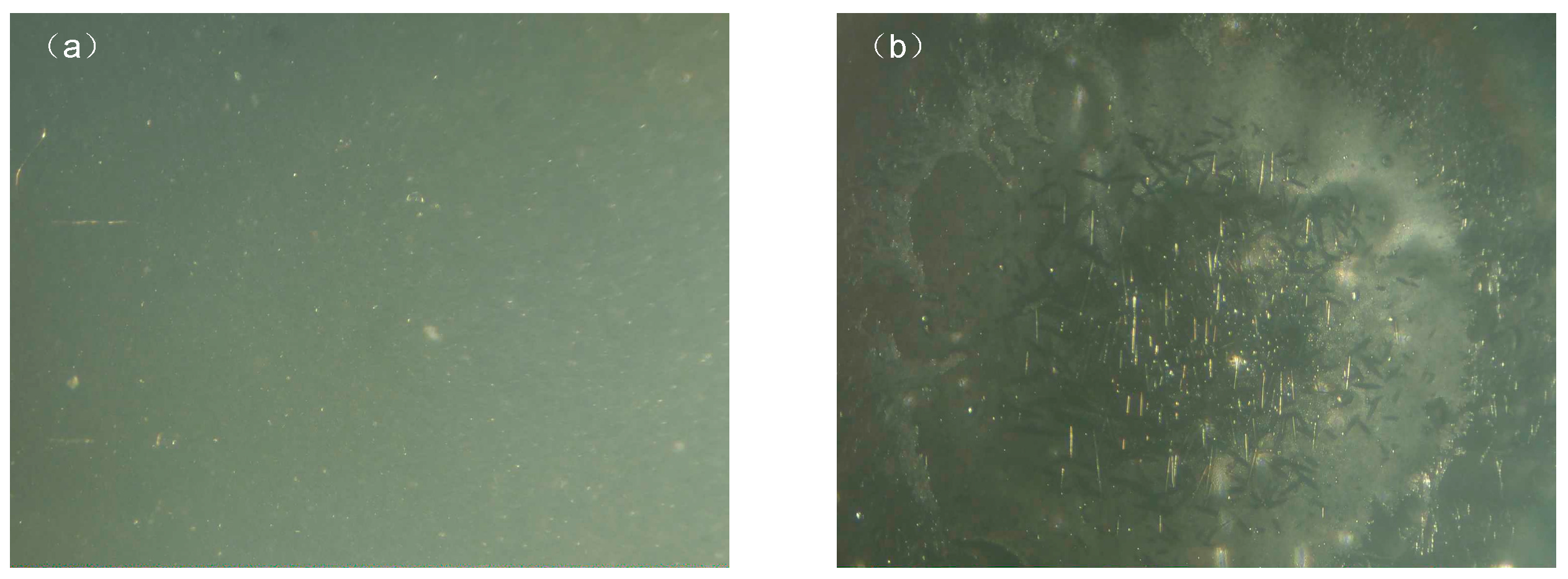
2.1. Effect of Corona Aging on Insulation Performance
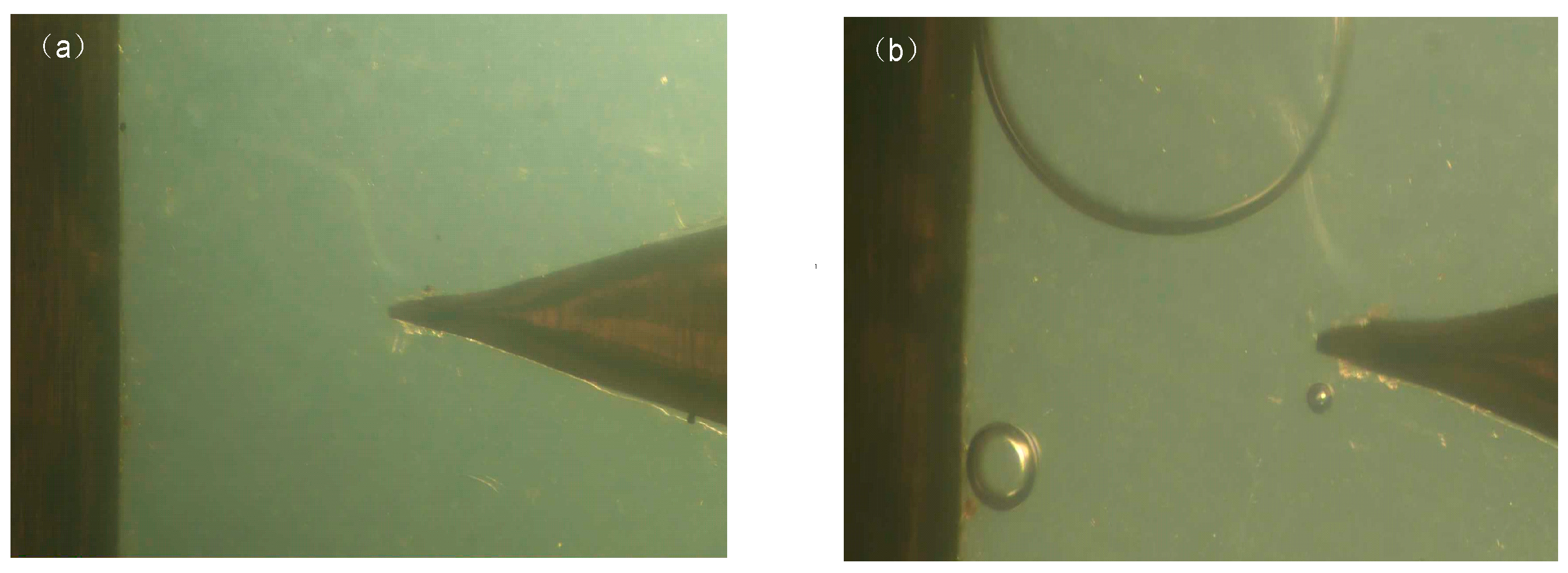
Microstructure Observation
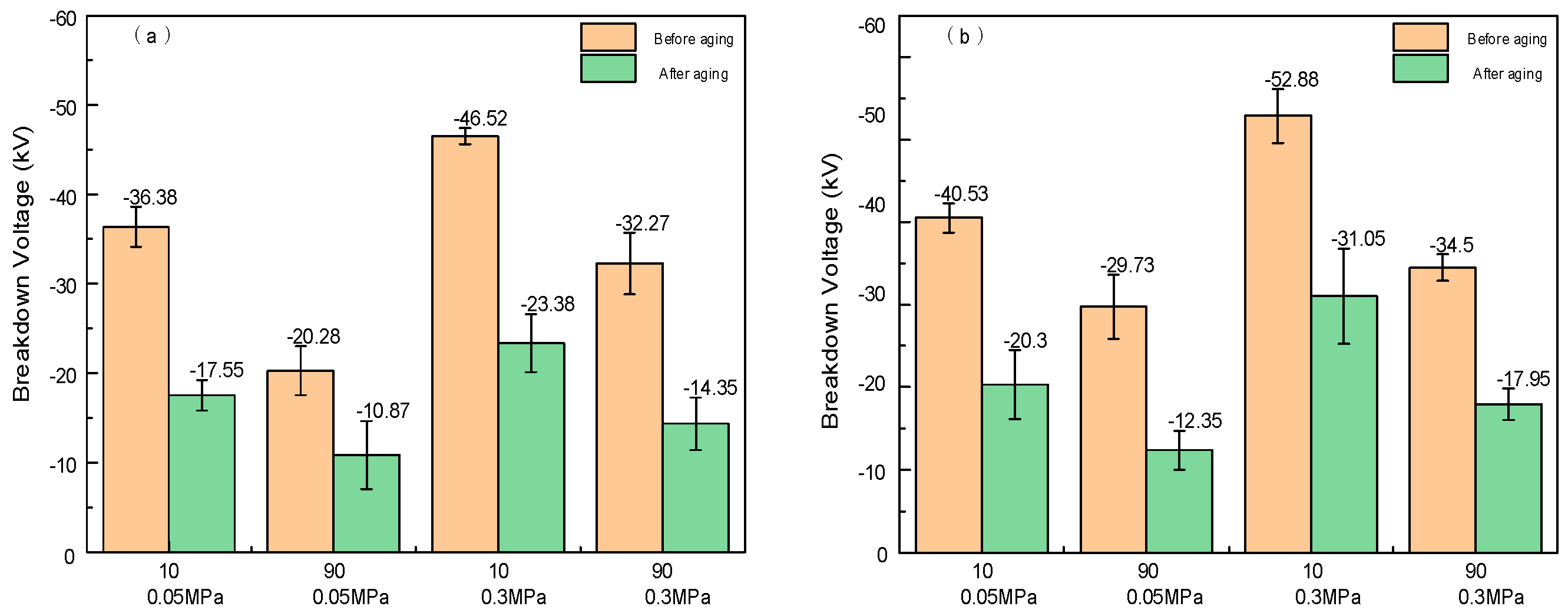
2.2. DC Breakdown Characteristics
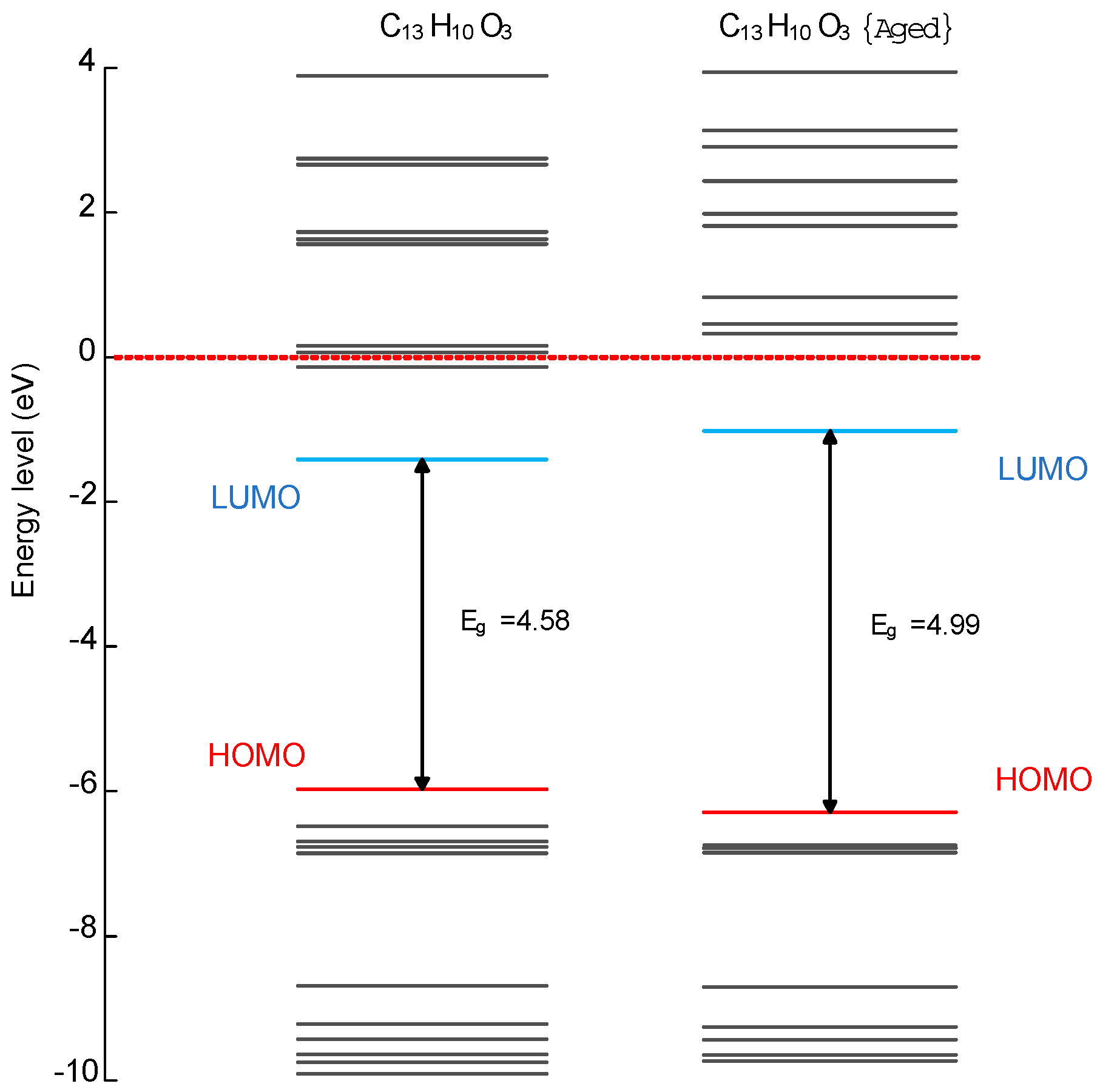
2.3. Aging Mechanism Analysis
- The molecular chains of modified silicone oil undergo decomposition due to the impact of electrons from the needle electrode during corona aging.
- New hydroxyl groups that form on the benzene rings are altering the chemical properties of the composition.
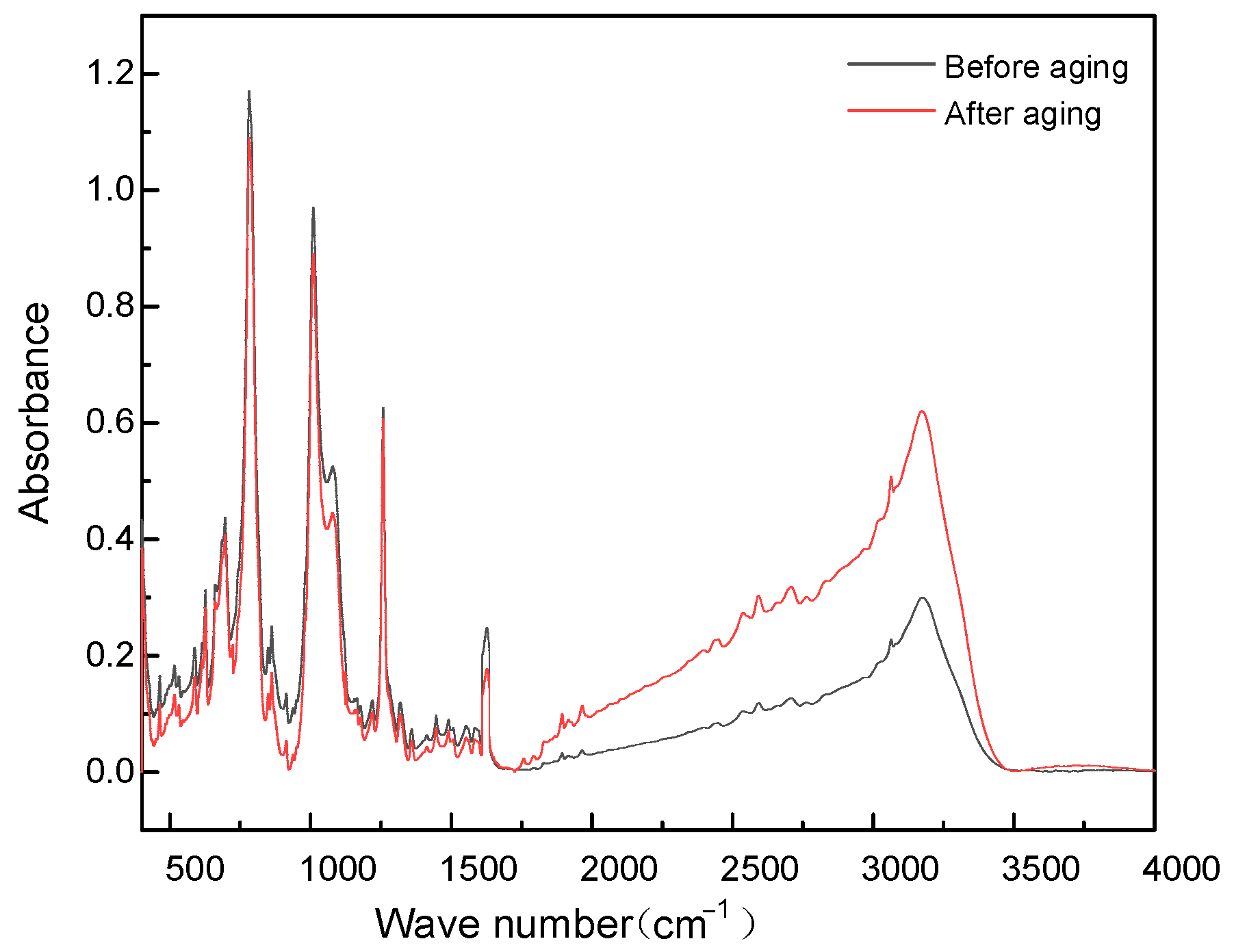
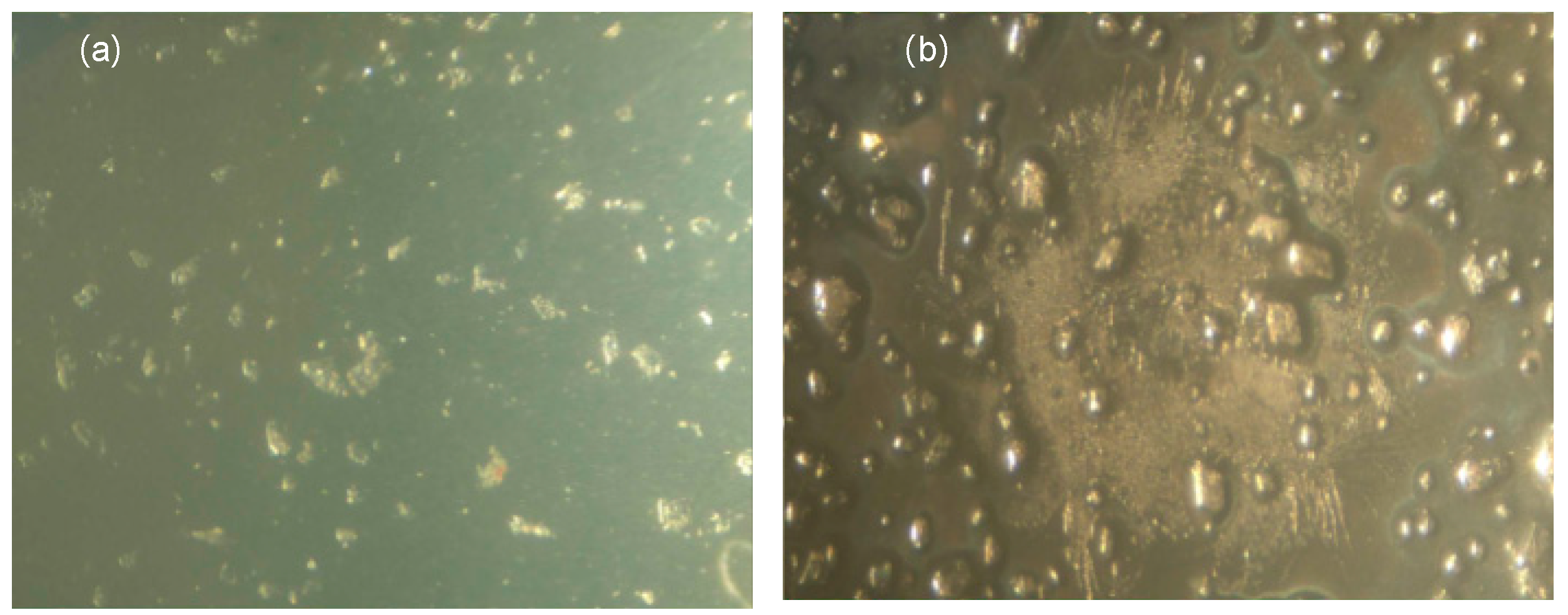
2.4. Effect of Thermal Cycling Aging on Insulation Performance
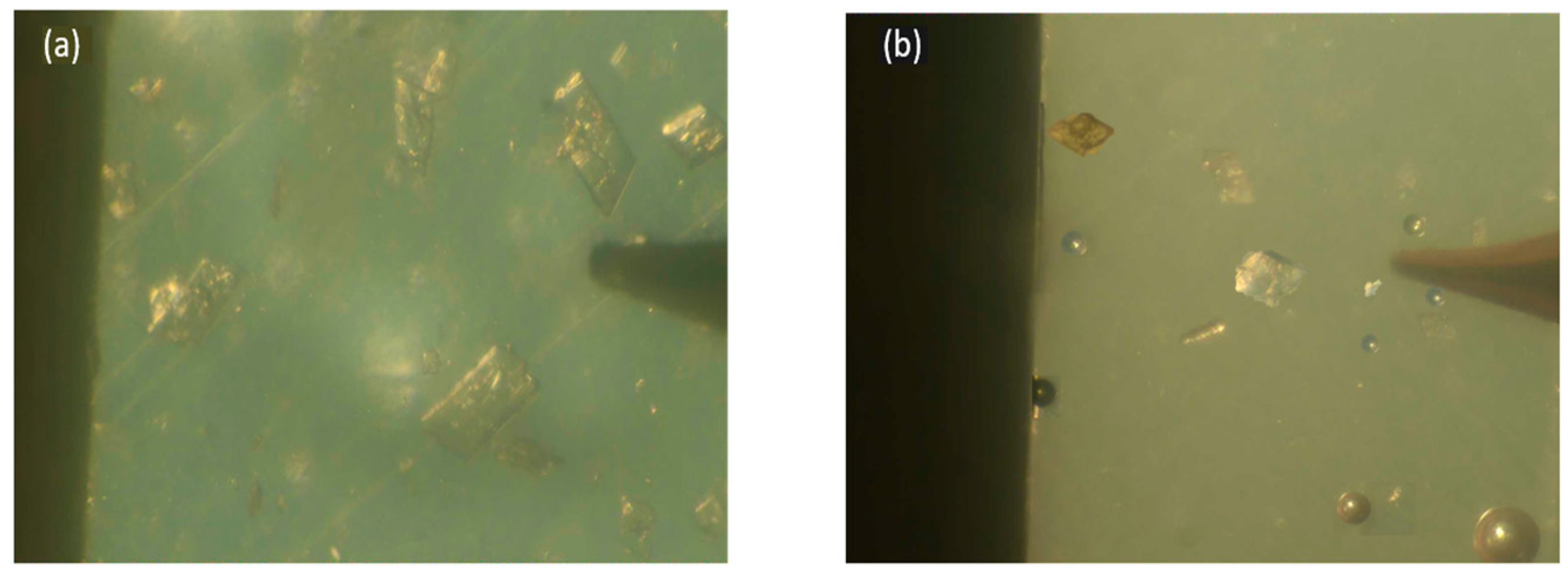
Microstructure Observation
2.5. DC Breakdown Characteristics
2.6. Aging Mechanism Analysis
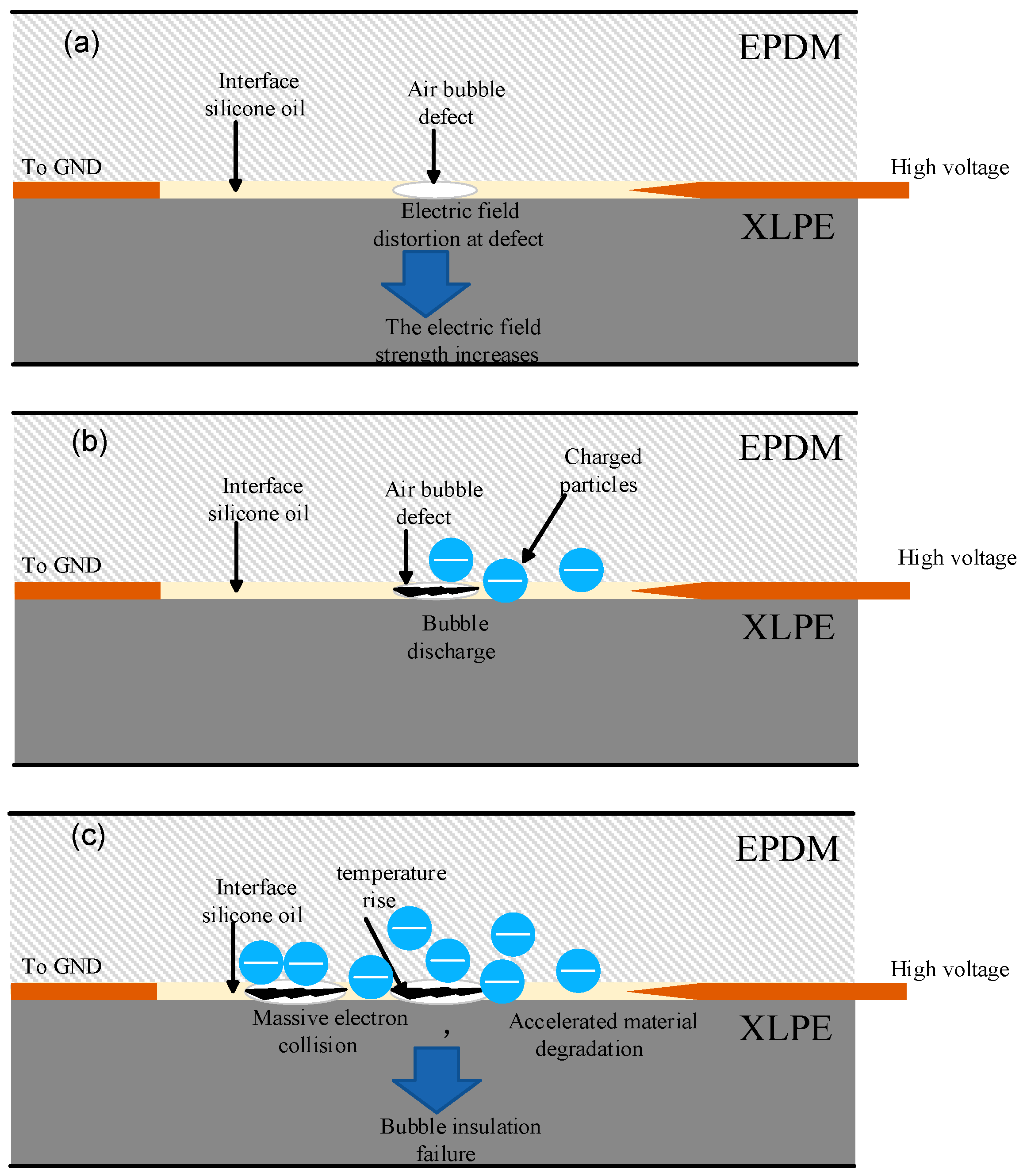
3. Discussion
Mechanisms of Bubble Formation and Interface Breakdown Development
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sonerud, B.; Mäkelä, T.; Josefsson, S. Design Evaluation of HVDC Flexible Joints using Numerical Simulations and Conductivity Measurements. In Annual Report—Conference on Electrical Insulation and Dielectric Phenomena, CEIDP; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2020; pp. 447–450. [Google Scholar] [CrossRef]
- Fernandez, J.; Guffond, R. Breakdown Prediction Modelling of XLPE Insulation under HVDC Thermoelectrical Stress. In Annual Report—Conference on Electrical Insulation and Dielectric Phenomena, CEIDP; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2022; pp. 459–462. [Google Scholar] [CrossRef]
- Fard, M.A.; Farrag, M.E.; McMeekin, S.; Reid, A. Electrical treeing in cable insulation under different HVDC operational conditions. Energies 2018, 11, 2406. [Google Scholar] [CrossRef]
- Fournier, D.; Lamarre, L. Effect of Pressure and Temperature on Interfacial Breakdown between Two Dielectric Surfaces. In Proceedings of the 1992 Annual Report Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Victoria, BC, Canada, 18–21 October 1992; pp. 229–235. [Google Scholar] [CrossRef]
- Tian, X.; Li, Y. Research on Electrical Characteristics of Insulating Silicone Oil Based on Polarization-Depolarization Current Method. In Proceedings of the 2020 IEEE 1st China International Youth Conference on Electrical Engineering (CIYCEE), Wuhan, China, 1–4 November 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1–6. [Google Scholar] [CrossRef]
- Chen, Z.; Tang, J.; Luo, C.; Zhang, J. A Study on Aging Characteristics of Silicone Oil in HV Oil-filled Cable Termination Based on Infrared Thermal Imaging Test. MATEC Web Conf. 2015, 22, 05026. [Google Scholar] [CrossRef]
- Li, J.; Han, C.; Du, B.; Takada, T. Deep trap sites suppressing space charge injection in polycyclic aromatic compounds doped XLPE composite. IET Nanodielectrics 2020, 3, 10–13. [Google Scholar] [CrossRef]
- Zhang, C.C.; Li, Y.F.; Hu, M.Y.; Ma, F.L.; Zhao, H.; Han, B.Z. Conductivity Properties of XLPE Insulation Used for HVDC Cable After Accelerated Thermal Ageing; IEEE: Piscataway, NJ, USA, 2018. [Google Scholar]
- Isabella, N.; Tim, B.; Marvin, B.; Bastian, S. Effect of Ageing on the Dielectric properties of EPDM for HVDC Cable Joints. In Proceedings of the VDE High Voltage Technology 2020; ETG-Symposium. [VDE Verlag], Online, 9–11 November 2020. [Google Scholar]
- Xing, Y.; Guo, X.; Kong, F.; Pathiraja, K.; Wang, Y.; Li, J. Interfacial breakdown characteristics of XLPE/EPDM double-layer for DC cable terminations with different treatment conditions. J. Phys. D Appl. Phys. 2025, 58, 115503. [Google Scholar] [CrossRef]
- Evangelou, C.; Zaky, A.A.; Megahed, I.Y. The effect of organic additives on the breakdown strength of transformer oil. J. Phys. D Appl. Phys. 1973, 6, 103. [Google Scholar] [CrossRef]
- Angerer, L. Effect of organic additives on electrical breakdown in transformer oil and liquid paraffin. Proc. Inst. Electr. Eng. 1965, 112, 1025. [Google Scholar] [CrossRef]
- Brian, S. Fourier Transform Infrared Spectroscopy Fundamentals of Second Edition; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2011. [Google Scholar]
- Gong, Y.; Chen, X.; Wu, W. Application of fourier transform infrared (FTIR) spectroscopy in sample preparation: Material characterization and mechanism investigation. Adv. Sample Prep. 2024, 11, 100122. [Google Scholar] [CrossRef]
- Arkles, B.; Launer, P.J. Infrared Analysis of Organsilicon Compounds: Spectra-Structure Correlations. Available online: https://www.researchgate.net/publication/273439829_Silicon_Compounds_Silanes_Silicones (accessed on 14 April 2025).
- Ayalew, M.E. DFT Studies on Molecular Structure, Thermodynamics Parameters, HOMO-LUMO and Spectral Analysis of Pharmaceutical Compound Quinoline (Benzo[b]Pyridine). J. Biophys. Chem. 2022, 13, 29–42. [Google Scholar] [CrossRef]
- Takada, T.; Miyake, H.; Tanaka, Y.; Yoshida, M. Quantum Chemical Calculation Studies on Interface Charge Transfer between Electrode and Polyethylene under Electrical Stress. In Proceedings of the 2014 International Symposium on Electrical Insulating Materials, Niigata, Japan, 1–5 June 2014. [Google Scholar]
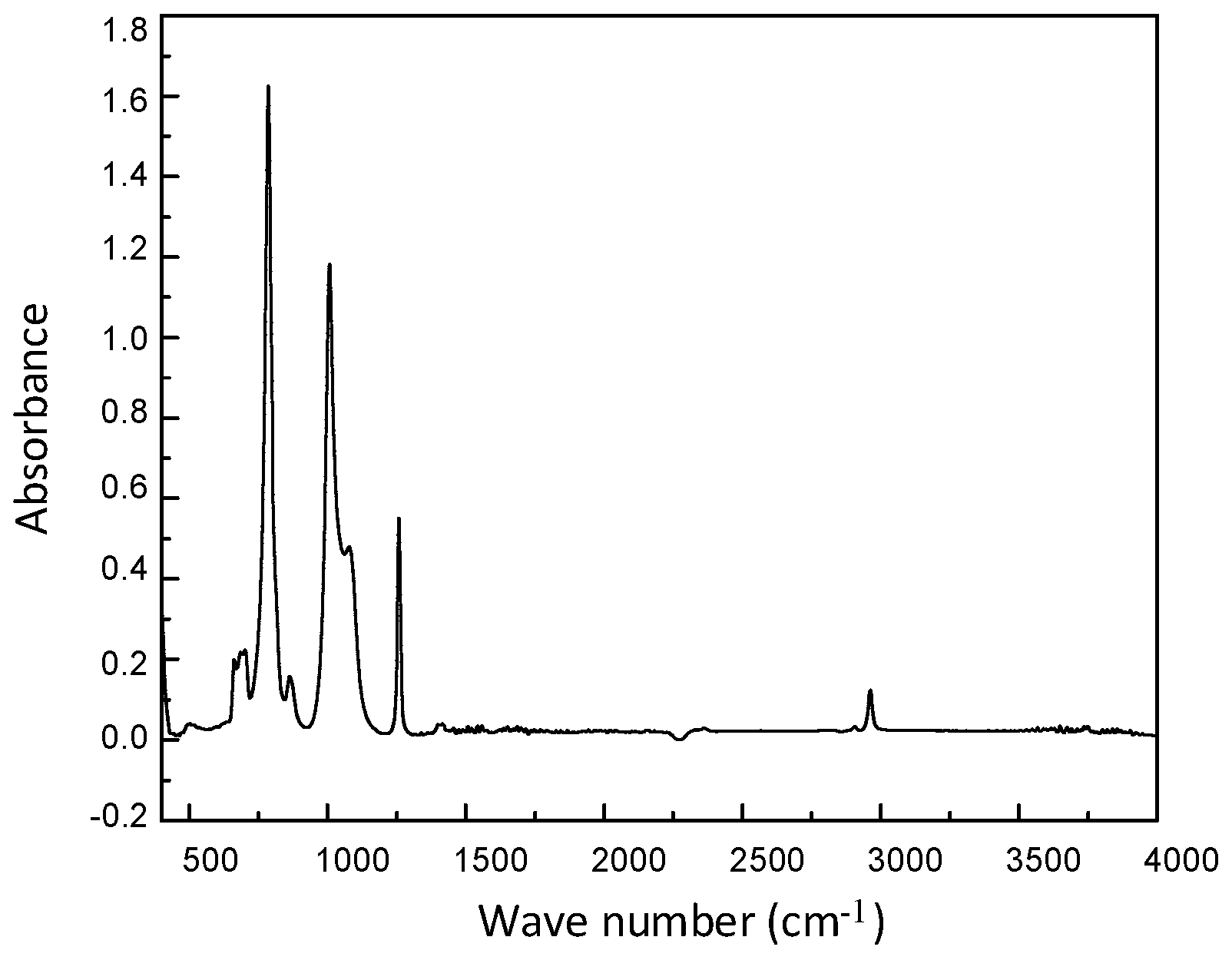
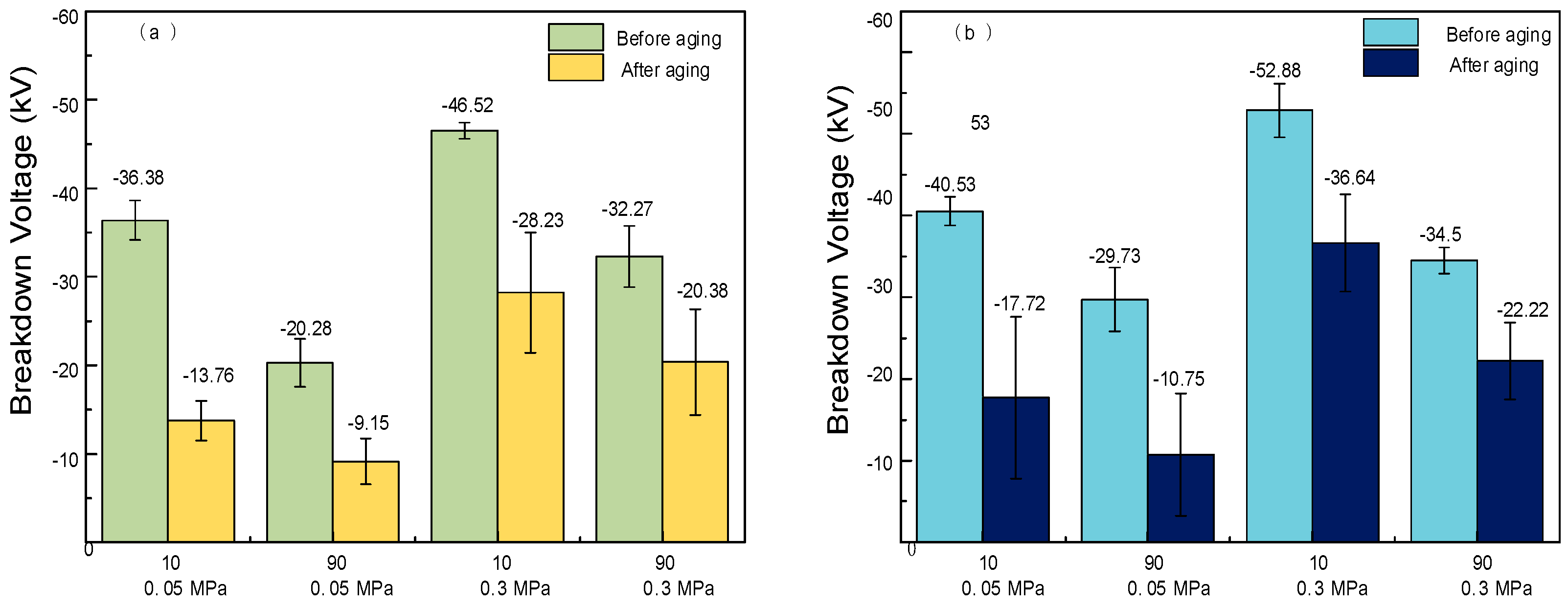
| Assignment | Wave Number (cm−1) |
|---|---|
| Si(CH3)3 | 700 |
| Si(CH3)2 | 780–840 |
| C-H in Si(CH3)3 | 2960 |
| Si-CH3 | 1240–1280 |
| Si-O-Si | 1000–1130 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Pathiraja, K.; Guo, X.; Hui, B.; Fu, M.; Zhao, L.; Wang, Y.; Li, J. Electrical–Thermal Aging Performance of PAH-Modified Interfacial Coating Agent for HVDC Cable Accessory. Energies 2025, 18, 3767. https://doi.org/10.3390/en18143767
Zhu W, Pathiraja K, Guo X, Hui B, Fu M, Zhao L, Wang Y, Li J. Electrical–Thermal Aging Performance of PAH-Modified Interfacial Coating Agent for HVDC Cable Accessory. Energies. 2025; 18(14):3767. https://doi.org/10.3390/en18143767
Chicago/Turabian StyleZhu, Wenbo, Kaulya Pathiraja, Xu Guo, Baojun Hui, Mingli Fu, Linjie Zhao, Yuhuai Wang, and Jin Li. 2025. "Electrical–Thermal Aging Performance of PAH-Modified Interfacial Coating Agent for HVDC Cable Accessory" Energies 18, no. 14: 3767. https://doi.org/10.3390/en18143767
APA StyleZhu, W., Pathiraja, K., Guo, X., Hui, B., Fu, M., Zhao, L., Wang, Y., & Li, J. (2025). Electrical–Thermal Aging Performance of PAH-Modified Interfacial Coating Agent for HVDC Cable Accessory. Energies, 18(14), 3767. https://doi.org/10.3390/en18143767







