Influence of Overcharge Abuse on the Thermal-Electrochemical Performance of Sodium Ion Cells
Abstract
1. Introduction
2. Experiment
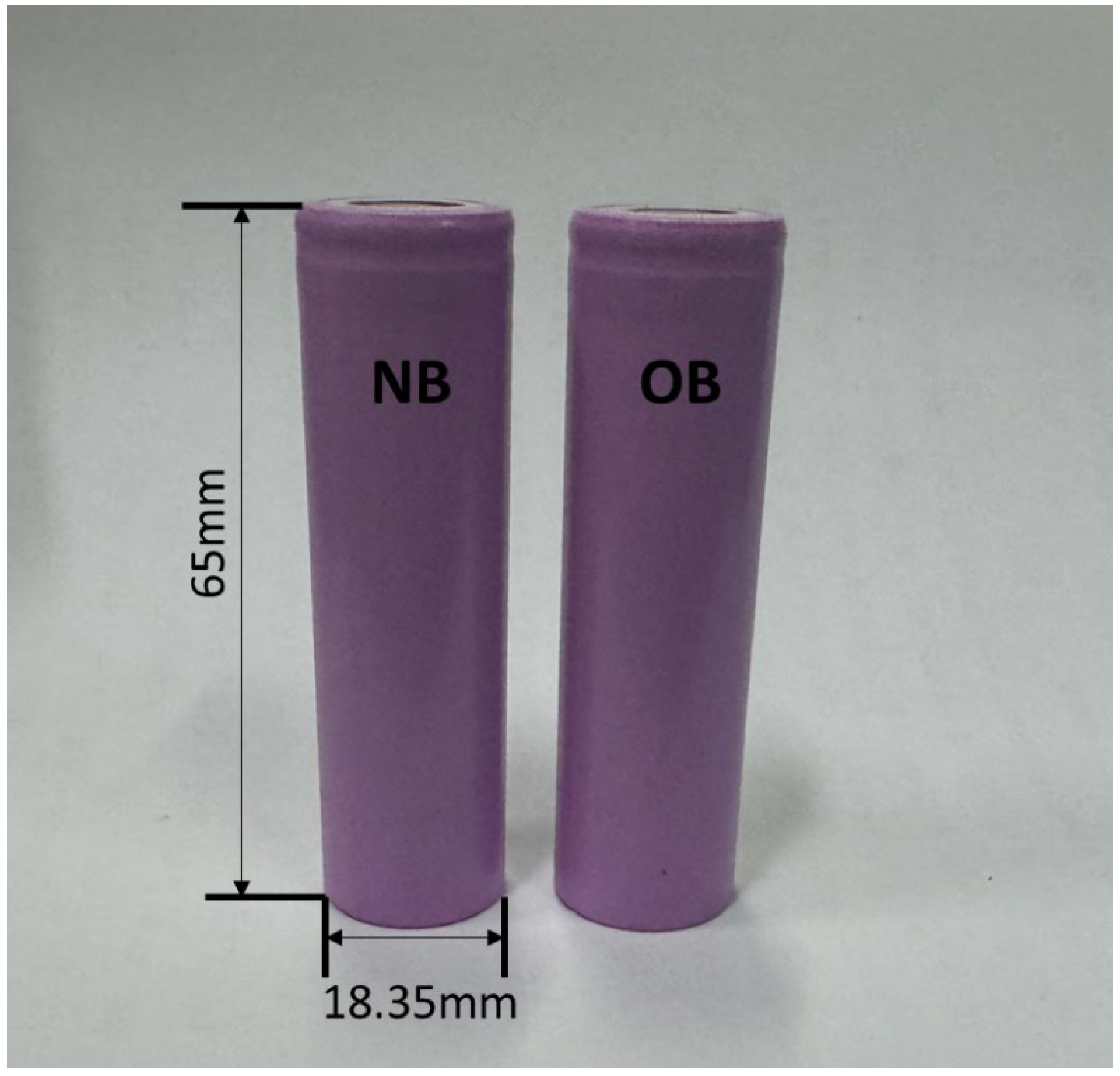
2.1. Sodium-Ion Battery Cells for Experimental Testing
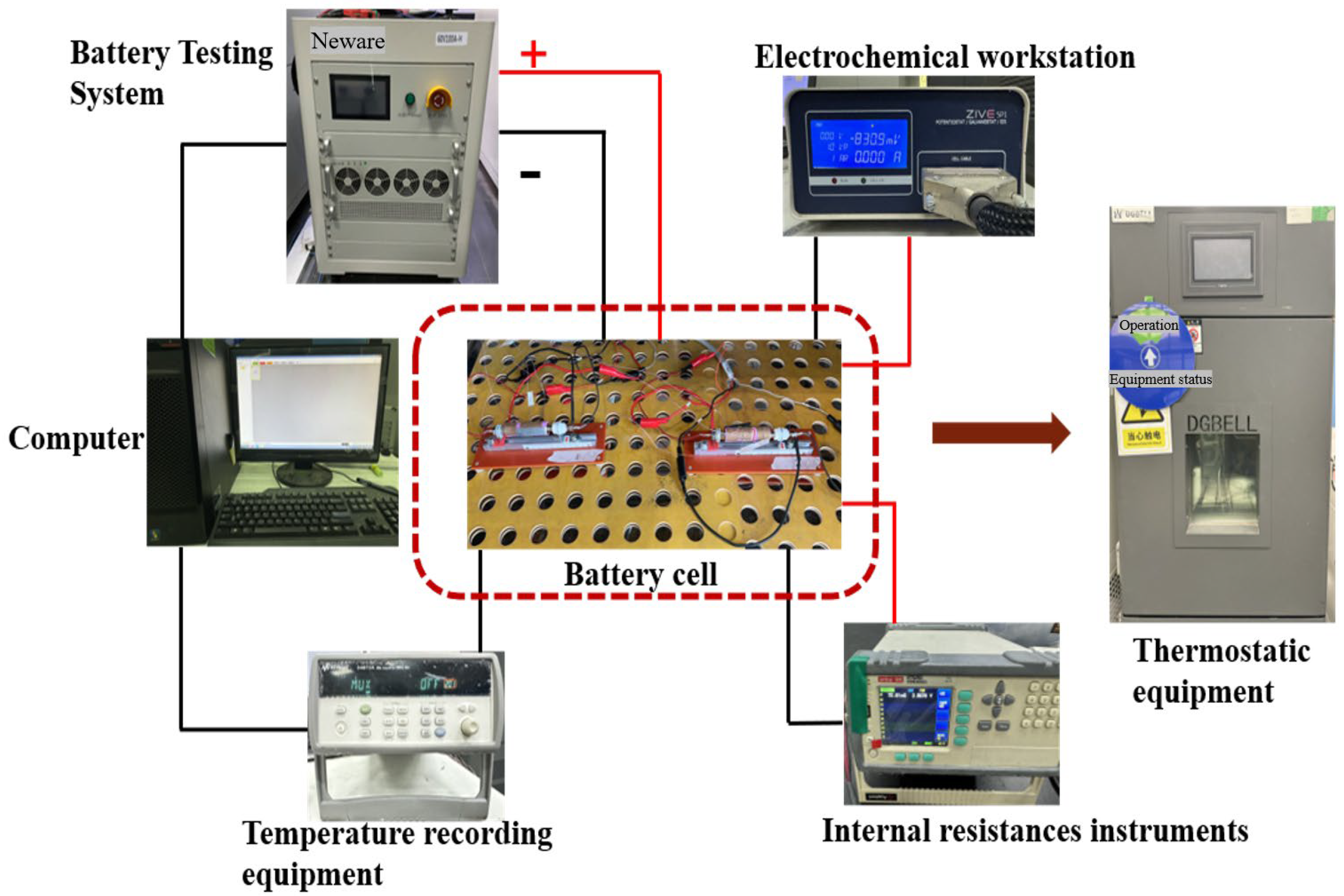
2.2. Establishment of the Experimental Testing System
2.3. Electrochemical Performance Testing
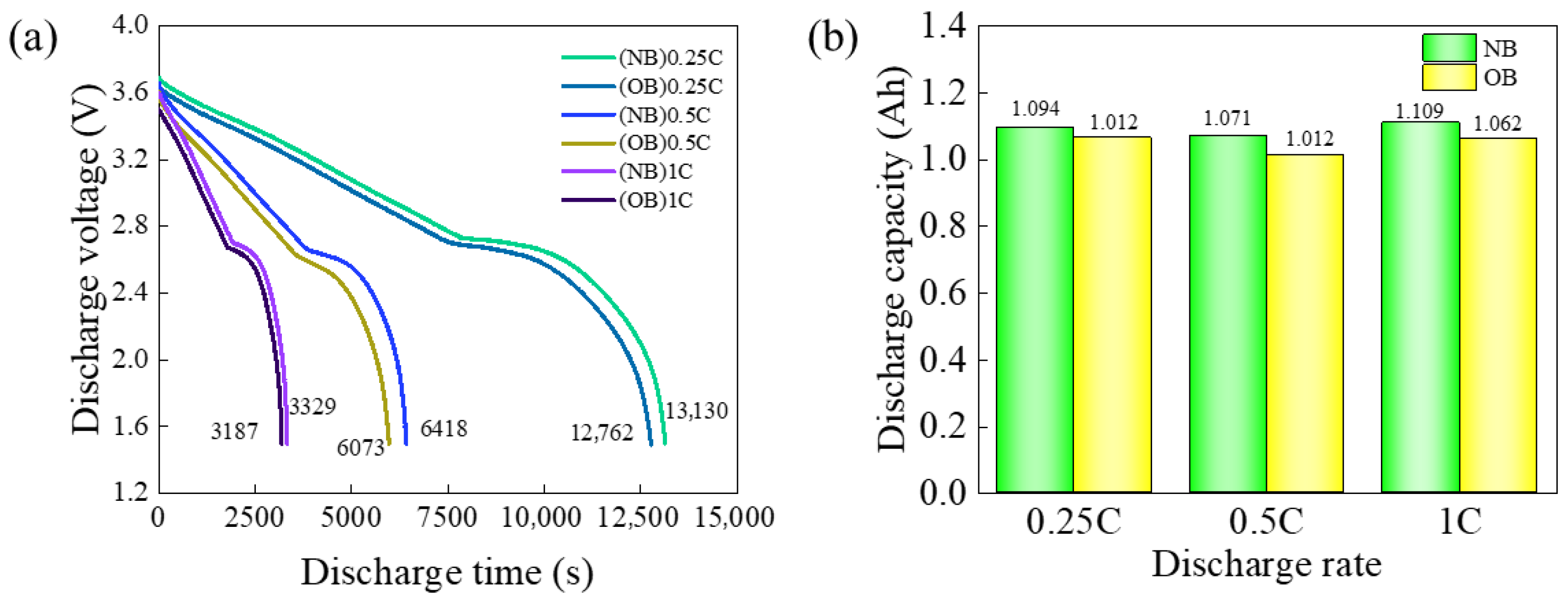
2.3.1. Variation in the Discharge Voltage Plateau
2.3.2. Discharge Capacity
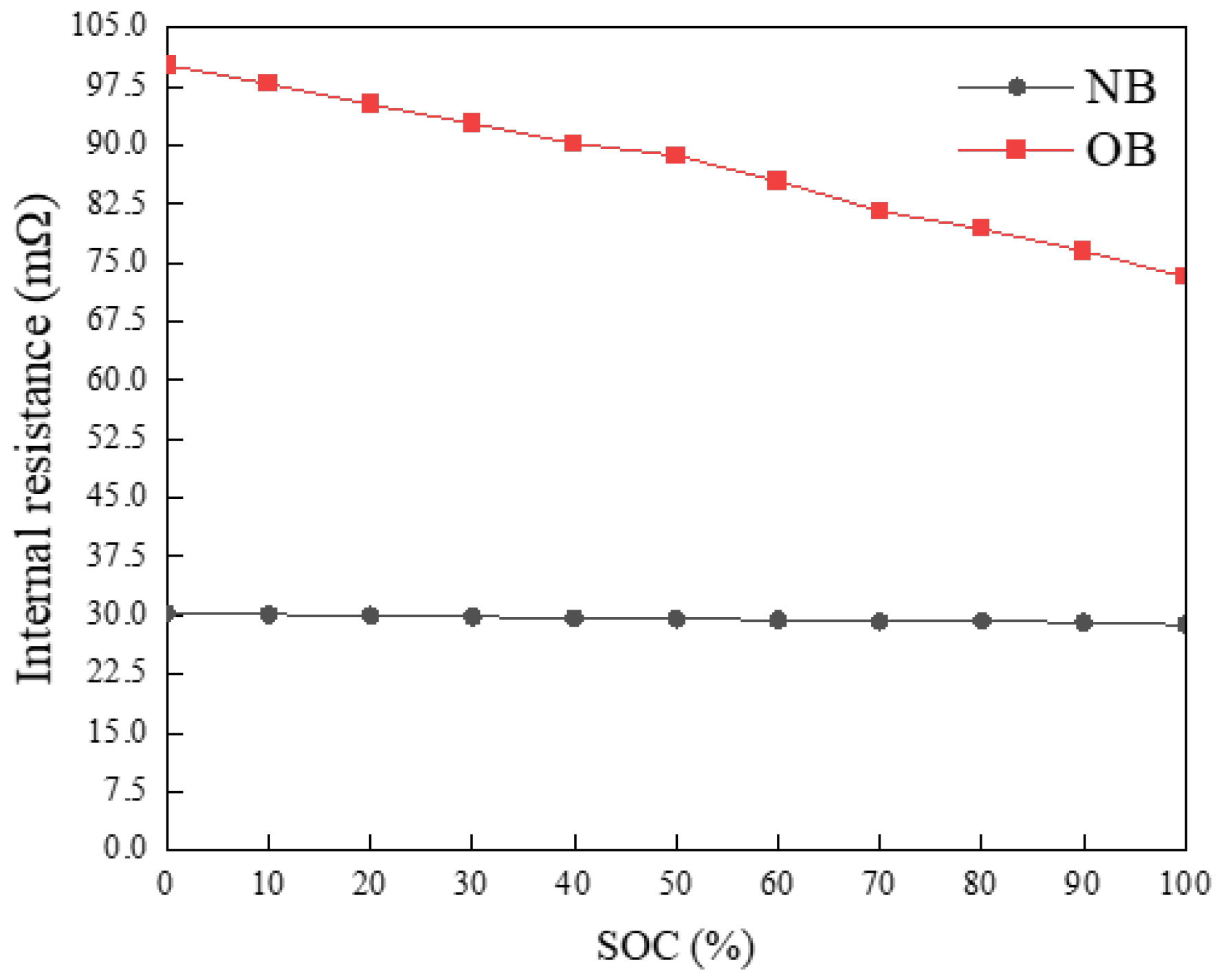
2.3.3. Internal Resistance Test
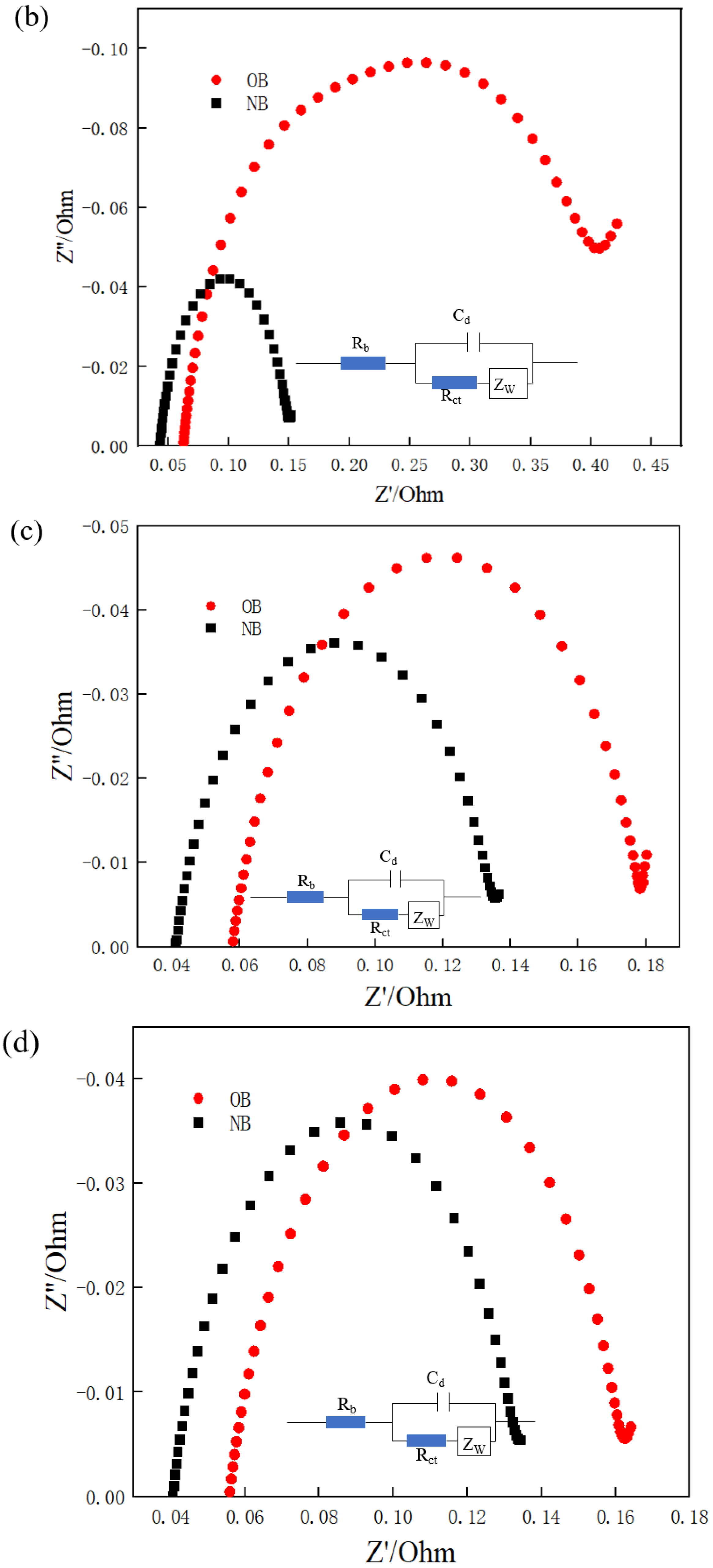
2.3.4. Comparison of EIS
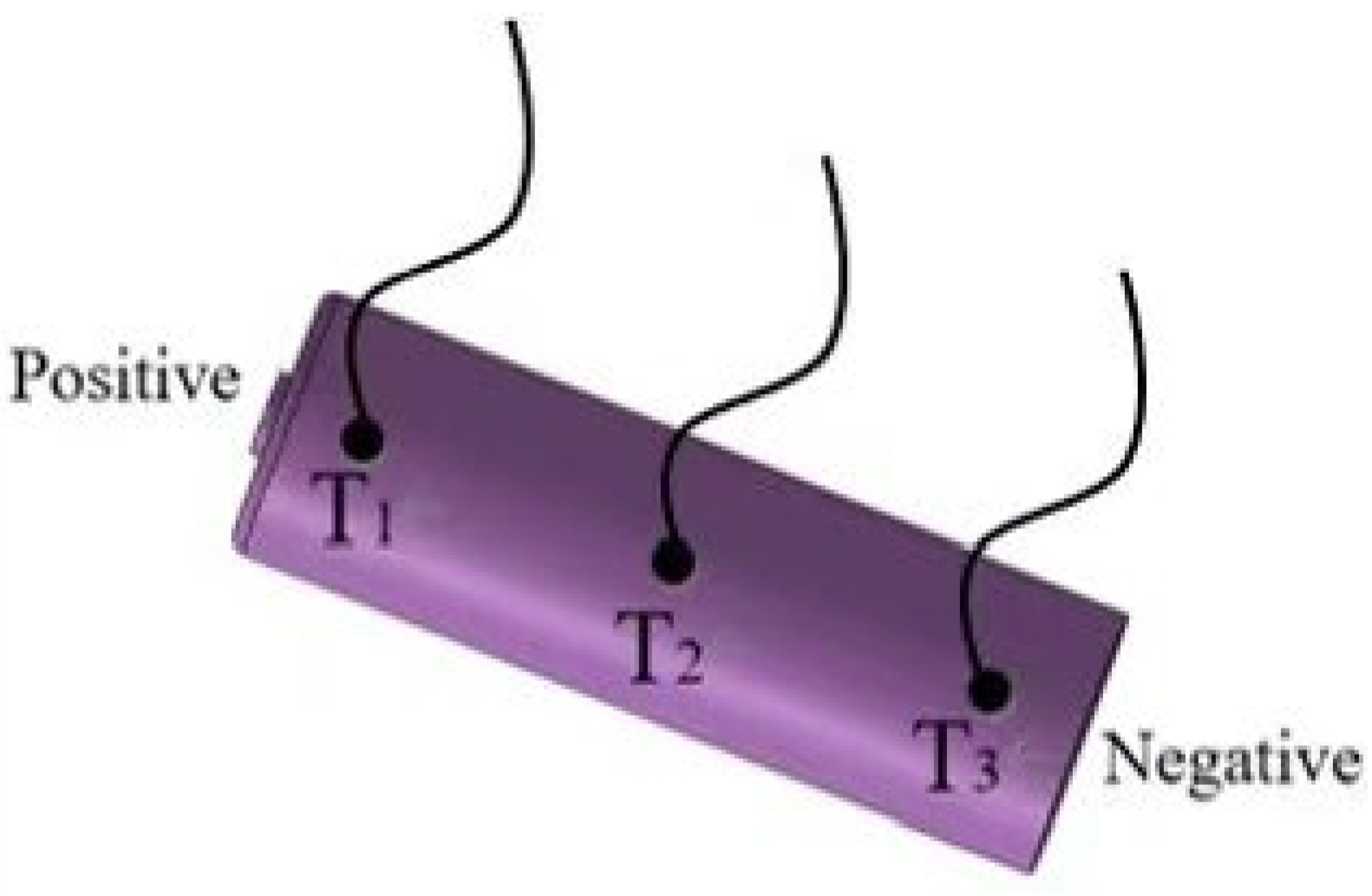
2.4. Analysis of Temperature Variations
2.5. Microscopic Morphology and Crystal Structure of Internal Electrode Materials in NB and OB Cells
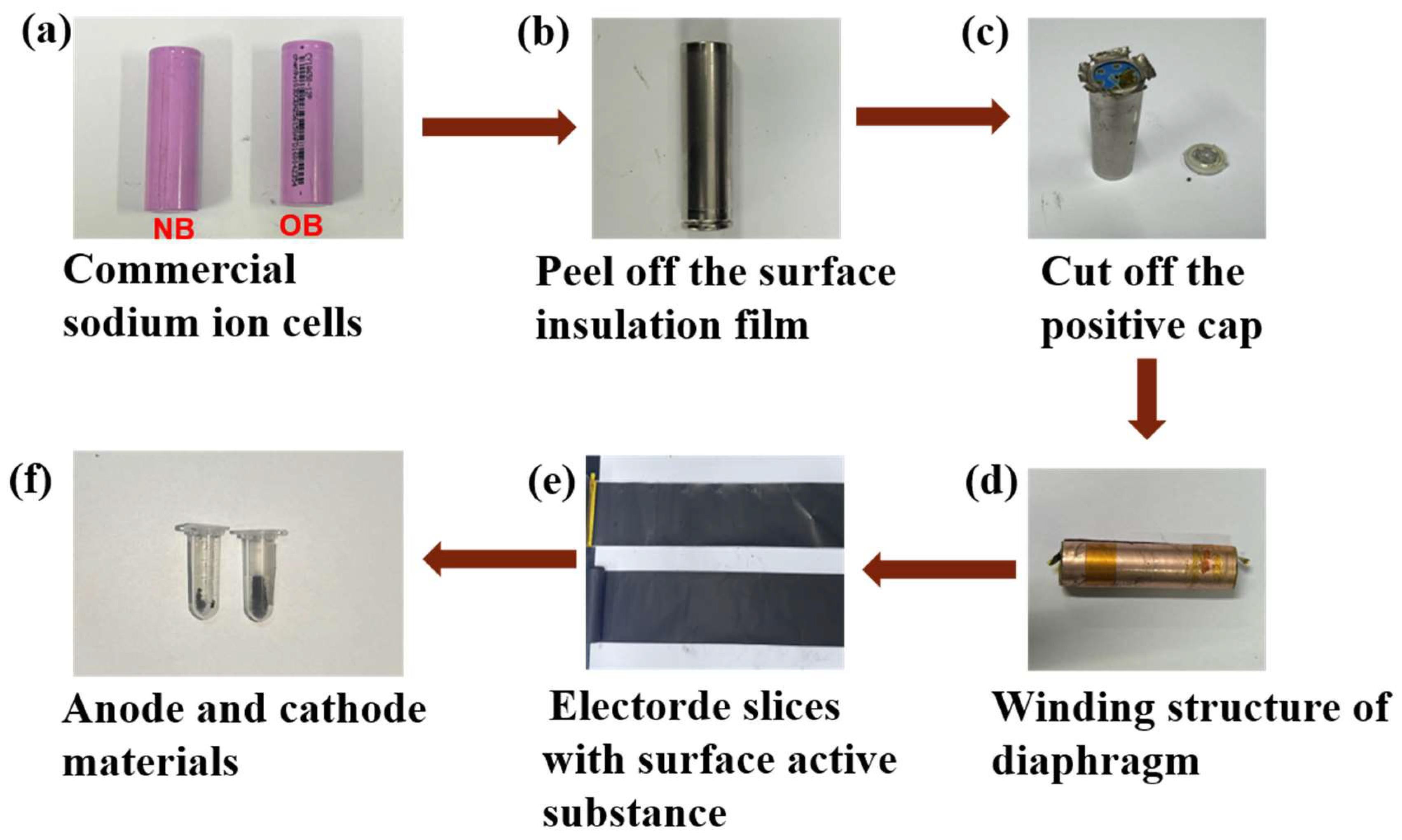
2.5.1. Disassembly of Full NB and OB Cells
2.5.2. Scanning Electron Microscopy (SEM)
2.5.3. X-Ray Diffraction Analysis (XRD)
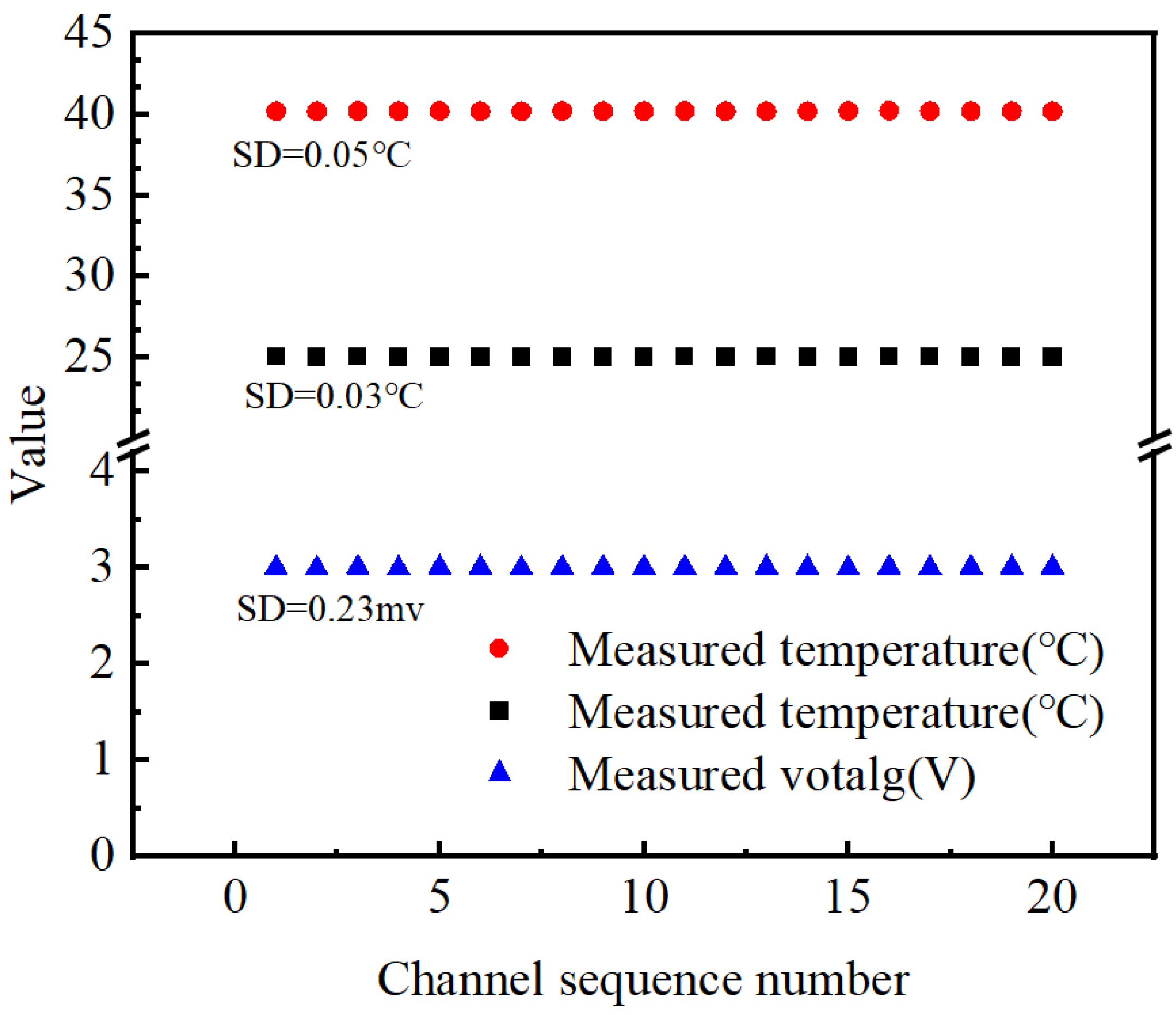
2.6. Experimental Uncertainty Analysis
3. Results and Discussion
3.1. Comparative Analysis of the Discharge Voltage Plateau and Discharge Capacity Between NB and OB Cells
3.2. Internal Resistance Variations Post-Overcharge
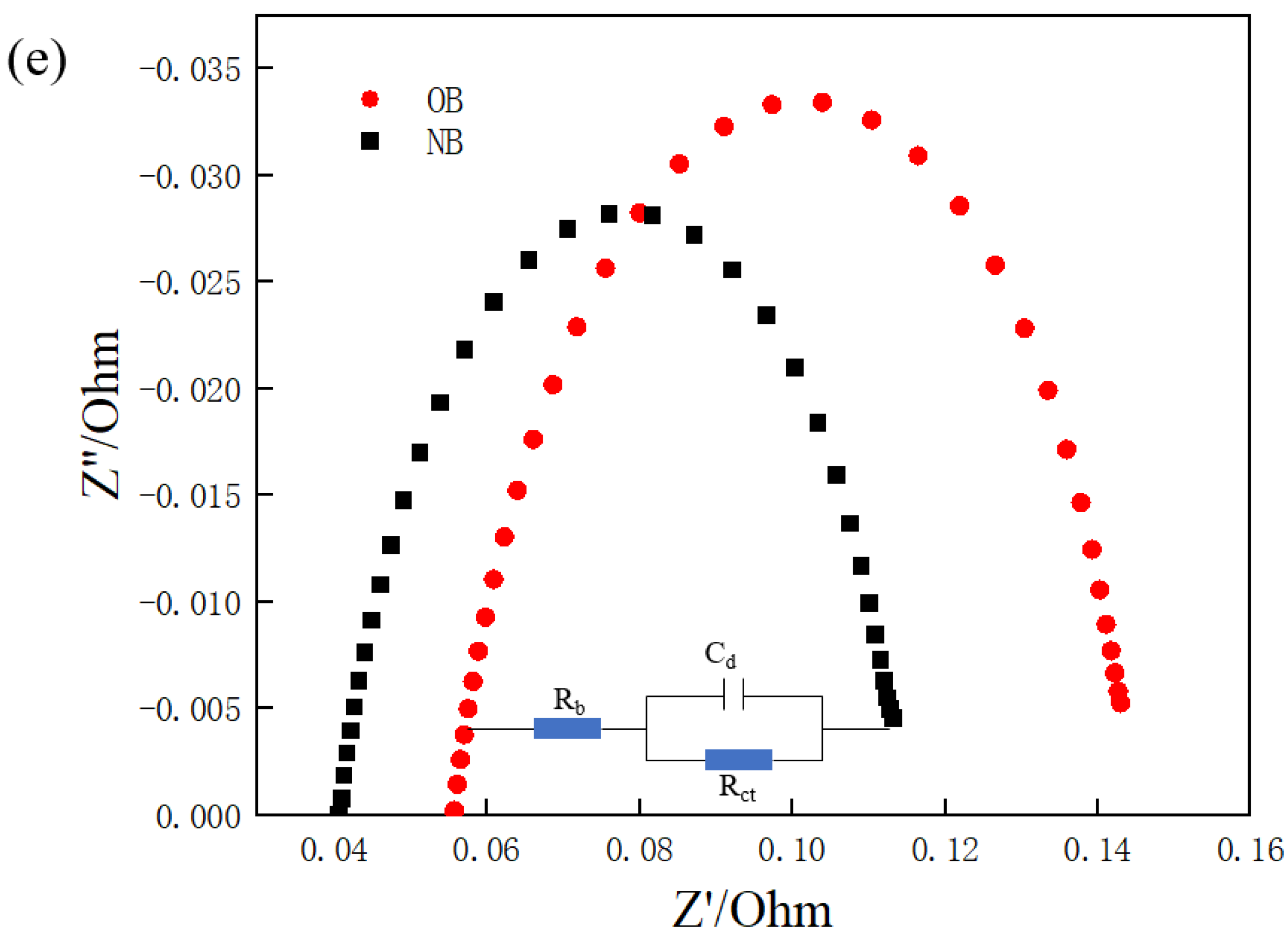
3.3. EIS Analysis
3.4. Analysis of Heat Generation Behavior
3.5. Appearance and Morphology of Electrode Materials
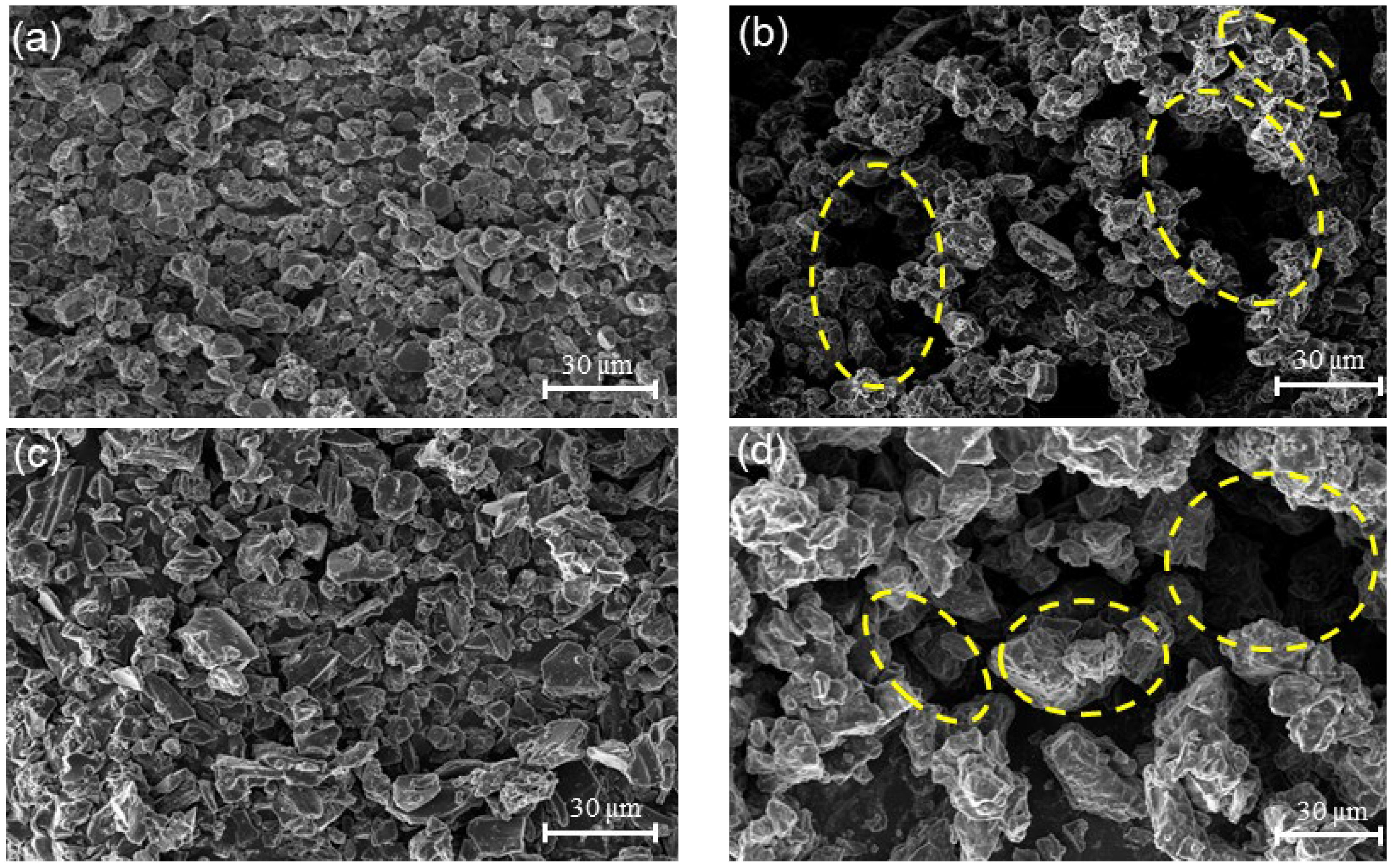
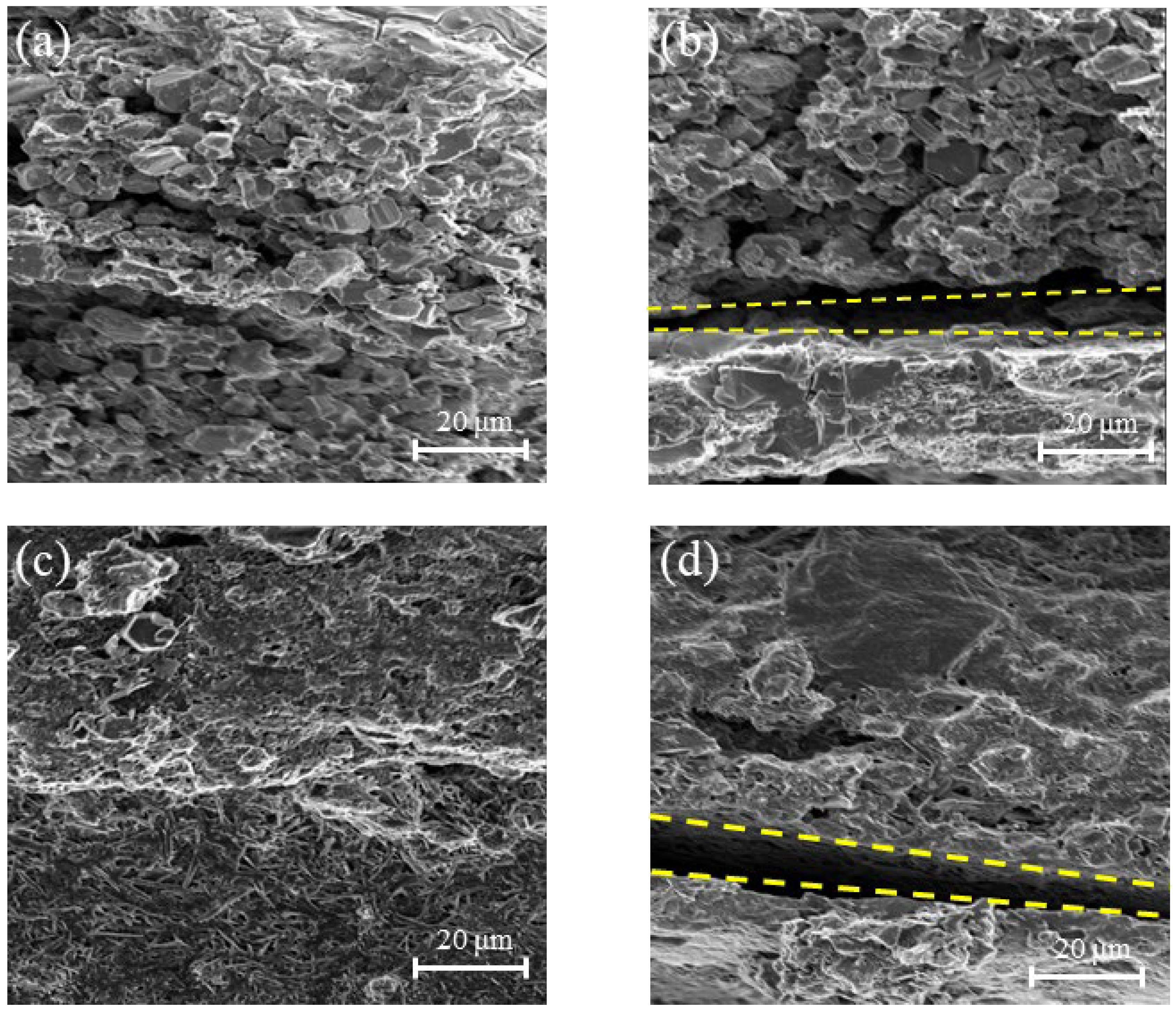
3.6. Microscopic Morphology of Electrode Materials
3.7. Microscopic Morphology of Electrode Sheet Cross-Sections
3.8. XRD Patterns of Electrode Materials
4. Conclusions and Outlooks
- (1)
- At 25 °C, OB cells exhibited a 2.81% reduction in discharge time compared to NB cells at a discharge rate of 0.25 C, with a corresponding discharge capacity of 1.064 Ah. At a discharge rate of 0.5 C, the discharge time of OB decreased by 5.38%, accompanied by a discharge capacity of 1.012 Ah. Furthermore, at a discharge rate of 1 C, the discharge time of OB decreased by 4.26%, with a discharge capacity of 1.062 Ah. When the temperature was elevated to 40 °C, the OB discharge time decreased by 3.17% at a discharge rate of 0.25 C, with a discharge capacity of 1.154 Ah. At a discharge rate of 0.5 C, the OB discharge time decreased by 3.27%, with a discharge capacity of 1.131 Ah. Lastly, at a discharge rate of 1 C, the discharge time of OB was decreased by 5.47%, with a discharge capacity of 1.082 Ah. After the same charging procedure, the internal resistance of OB increased by 46.72 Ω before CC discharge (SOC = 100%) and by 65.99 Ω after discharge (SOC = 0%), compared to NB. The macroscopic thermal-electrical characteristic data of NB and OB cells indicated that overcharging significantly diminished both battery charge–discharge time and discharge capacity. Higher discharge rates exacerbated these reductions, resulting in more pronounced declines. Additionally, overcharging elevated battery internal resistance and AC impedance, further deteriorating the charge–discharge capacity and shortening the cell’s service life.
- (2)
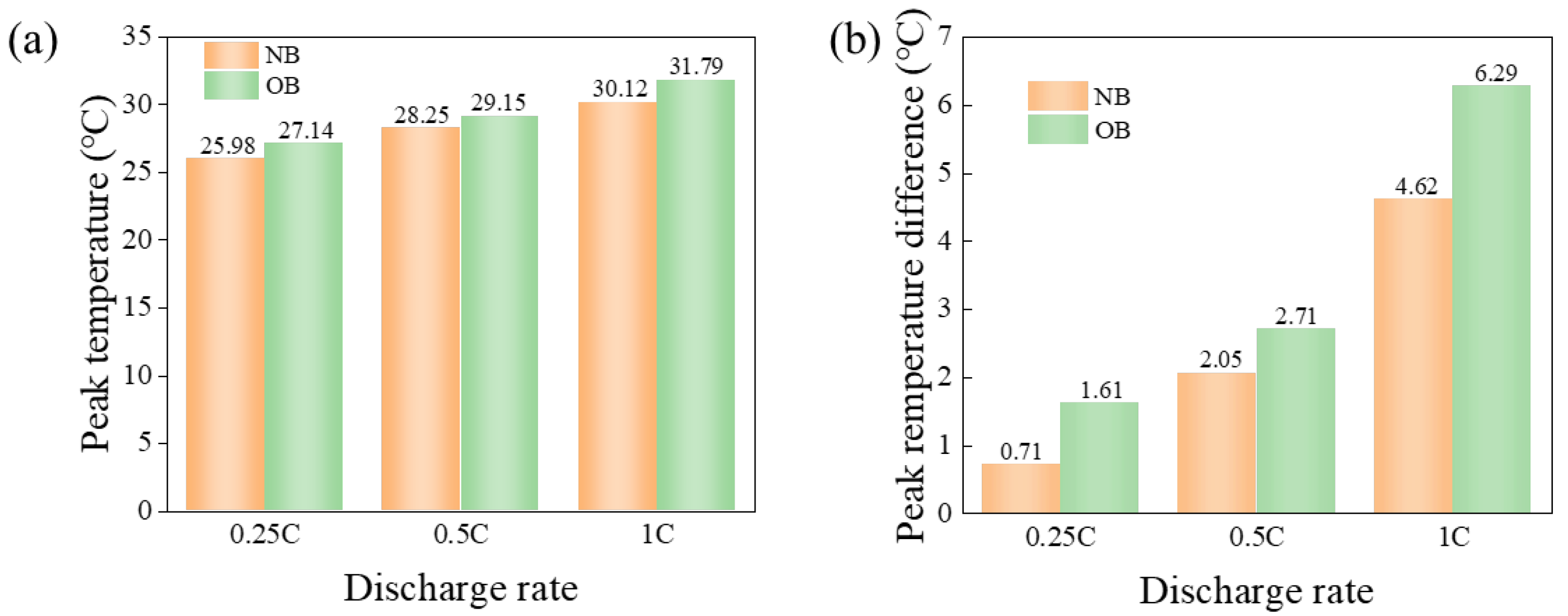
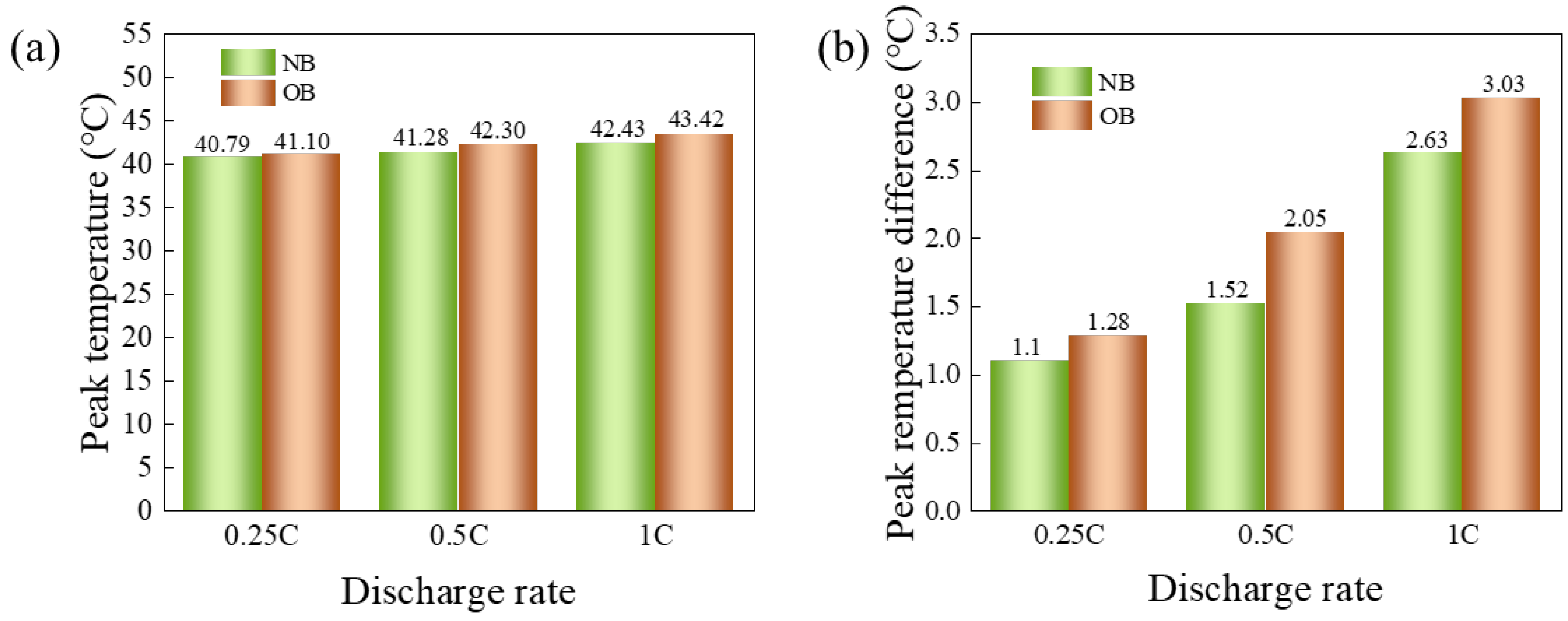
- At an ambient temperature of 25 °C, the OB exhibited peak temperature differences of 1.61 °C, 2.71 °C, and 6.29 °C at discharge rates of 0.25 C, 0.5 C, and 1 C, respectively. These values represented increases of 0.9 °C, 0.66 °C, and 1.67 °C compared to the NB under the same conditions. At the maximum discharge rate of 1 C, the OB peak temperature reached 31.79 °C, which was 5.6% higher than that of the NB. When the ambient temperature rose to 40 °C and the discharge rate was maintained at 1 C, the peak temperature and temperature difference of OB were 43.42 °C and 3.03 °C, respectively, exceeding those of the NB by 0.99 °C and 0.4 °C. These results suggested that overcharging exacerbated heat generation and temperature variability within the power battery cell, leading to a significant deterioration in electrochemical performance. Overcharge abuse promoted heat generation and accelerated temperature rise within the battery cell, increasing the risk of thermal runaway and potential safety hazards.
- (3)
- Microscopic examination of the NB and OB revealed that the primary cause of the electrochemical performance decline following overcharging was the degradation of the electrode material structure. This structural damage impeded sodium ion migration between the positive and negative electrodes, further reducing discharge capacity. Additionally, overcharging disrupted the normal electrochemical reactions within the battery, promoting side reactions. These side reactions generated substantial heat and cause abnormal temperature elevations, posing significant safety risks in practical applications.
- (4)
- Consequently, in the actual application of sodium-ion batteries, we should try to avoid subjecting the battery to a variety of abuse conditions (including electrical abuse, thermal abuse and mechanical abuse). Electric abuse includes overcharge, overdischarge, internal short circuit, and so on. In particular, overcharging is the most common electrical abuse. It is necessary to conduct real-time monitoring and monitoring of battery operating state parameters (current, voltage, temperature), that is, the normal and effective operation of the battery management system.
- (1)
- This study examines the electrochemical characteristics and changes in the internal material structure of sodium-ion battery cells subjected to overcharging within the electrical abuse scale. Future studies will include the development of testing platforms for mechanical and thermal abuse to evaluate sodium-ion batteries from various abuse perspectives.
- (2)
- Large format batteries are becoming the mainstream trend in the energy storage field and demonstrate totally different characteristics compared with small size cells. Future research will extend to large-scale sodium-ion battery cells.
- (3)
- This research analyzes sodium-ion battery cells using experimental testing methods supported by relevant theories. The parameter thresholds identified herein serve as preliminary references for BMS design. Their robustness must be rigorously tested in future module prototypes accounting for cell-to-cell variations and aging effects.
- (4)
- This paper focuses on commercial 18650-type sodium-ion battery cells. Future research will expand to include sodium-ion batteries of various geometries and large-scale battery modules. Comprehensive testing at the module level will be conducted, and a range of battery thermal management solutions will be developed to improve safety, particularly in temperature uniformity and control, for OB modules.
- (5)
- Future research will focus on applying machine learning and neural network models to predict sodium-ion battery life, surface temperature, SOC, and state of health. Future research will integrate image statistics and pore analysis to establish a quantitative model between structural parameters and performance degradation. These advancements will play a crucial role in developing battery management systems.
- (6)
- For future studies on 18,650 modules, gas composition analysis using gas chromatography–mass spectrometry (GC–MS) will be implemented. Built-in sensors will measure the rising gas pressure during overcharging to quantify correlations between SOC overages (>110%) and gas species (e.g., CO2, H2).
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| AC | Alternating current |
| CC | Constant current |
| CV | Constant voltage |
| C | The ratio of the rate at which a battery discharges per unit time to its rated capacity |
| Cd | Double layer capacitance |
| Cn | Nominal capacity |
| CSEI | Solid electrolyte interphase capacitance |
| DC | Direct current |
| Dn | Discharge capacity |
| EIS | Electrochemical impedance spectroscopy |
| I | Current |
| LFP | LiFePO4 |
| NBs | Normal batteries |
| NTM | NaxTMO2 |
| NTP | NaTi2(PO4)3 |
| NCM | Li(Ni0.5Co0.2Mn0.3)O2 |
| NC | Nitrogen-doped carbon |
| OBs | Overcharged batteries |
| PDMS | Polydimethylsiloxane copolymer |
| PVDF-HFP | Polyvinylidene fluoride-co-hexafluoropropylene |
| RSEI | Solid electrolyte interphase resistance |
| R | Resistance |
| Rb | Solution resistance |
| Rct | Charge transfer resistance |
| SEM | Scanning electron microscope |
| SD | Standard deviation |
| SEI | Solid electrolyte interface |
| SOC | State of charge |
| XRD | X-ray diffraction analysis |
| Zw | Warburg impedance |
References
- Appiah-Otoo, I.; Chen, X.D.; Ampah, J.D. Does financial structure affect renewable energy consumption? Evidence from G20 countries. Energy 2023, 272, 127130. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, D.D.; Wang, H.; Guo, L. Smart electrochemical energy storage devices with self-protection and self-adaptation abilities. Adv. Mater. 2017, 29, 1703040. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.M.; Wang, T.Y.; Liao, X.B.; Qie, B.Y.; Yang, P.F.; Chen, M.J.; Wng, X.; Srinivasan, A.; Cheng, Q.; Ye, Q.; et al. Accordion-like stretchable Li-ion batteries with high energy density. Energy Storage Mater. 2019, 17, 136–142. [Google Scholar] [CrossRef]
- Usiskin, R.; Lu, Y.X.; Popovic, J.; Law, M.; Balaya, P.; Hu, Y.S.; Maier, J. Fundamentals, status and promise of sodium-based batteries. Nat. Rev. Mater. 2021, 6, 1020–1035. [Google Scholar] [CrossRef]
- Palomares, V.; Serras, P.; Villaluenga, I.; Hueso, K.B.; Carretero-González, J.; Rojo, T. Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ. Sci. 2012, 5, 5884–5901. [Google Scholar] [CrossRef]
- Ramasubramanian, B.; Ling, J.; Jose, R.; Ramakrishna, S. Ten major challenges for sustainable lithium-ion batteries. Cell Rep. Phys. Sci. 2024, 5, 102032. [Google Scholar] [CrossRef]
- Sirengo, K.; Babu, A.; Brennan, B.; Pillai, S.C. Ionic liquid electrolytes for sodium-ion batteries to control thermal runaway. J. Energy Chem. 2023, 81, 321–338. [Google Scholar] [CrossRef]
- Zhao, Q.; Lu, Y.; Chen, J. Advanced organic electrode materials for rechargeable sodium-ion batteries. Adv. Energy Mater. 2017, 7, 1601792. [Google Scholar] [CrossRef]
- Wu, H.; Hao, J.N.; Jiang, Y.L.; Jiao, Y.R.; Liu, J.H.; Xu, X.; Davey, K.; Wang, C.S.; Qiao, S.Z. Alkaline-based aqueous sodium-ion batteries for large-scale energy storage. Nat. Commun. 2024, 15, 575. [Google Scholar] [CrossRef]
- Cai, X.; Yue, Y.; Yi, Z.; Liu, J.; Sheng, Y.; Lu, Y. Challenges and industrial perspectives on the development of sodium ion batteries. Nano Energy 2024, 129, 110052. [Google Scholar] [CrossRef]
- Tarascon, J.M. Na-ion versus li-ion batteries: Complementarity rather than competitiveness. Joule 2020, 4, 1616–1620. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kumar, H.; Kumar, G.; Sharma, S.; Aneja, R.; Sharma, A.K.; Kumar, R.; Kumar, P. Progress and challenges in electrochemical energy storage devices: Fabrication, electrode material, and economic aspects. Chem. Eng. J. 2023, 468, 143706. [Google Scholar] [CrossRef]
- Digital Energy Storage Network. “Terra-Gen Investigates Cause of Fire...”. September 2023. Available online: https://desn.com.cn/news/show-2023875.html (accessed on 7 August 2024). (In Chinese).
- International Energy Network. “Year-End Review: Top 10...-Idaho Power Substation Fire”. December 2023. Available online: https://www.in-en.com/article/html/energy-2329962.shtml (accessed on 8 August 2024). (In Chinese).
- Solarbe Network. “Top 10 International...-Gothenburg Fire Incident”. January 2024. Available online: https://cn.solarbe.com/news/20240103/84105.html (accessed on 2 September 2024). (In Chinese).
- Wang, J.; Ruan, L. Cell-to-cell inconsistency analysis and structure optimization for a liquid-cooled cylindrical battery module. Appl. Therm. Eng. 2023, 224, 120021. [Google Scholar] [CrossRef]
- Zarrabeitia, M.; Salazar, I.; Acebedo, B.; Muñoz-Márquez, M.Á. Stabilization of p2 layered oxide electrodes in sodium-ion batteries through sodium evaporation. Commun. Mater. 2024, 5, 130. [Google Scholar] [CrossRef]
- Velumani, D.; Bansal, A. Thermal behavior of lithium- and sodium-ion batteries: A review on heat generation, battery degradation, thermal runway-perspective and future directions. Energy Fuels 2022, 36, 14000–14029. [Google Scholar] [CrossRef]
- Bordes, A.; Marlair, G.; Zantman, A.; Chesnaye, A.; Lore, P.-A.L.; Lecocq, A. Safety evaluation of a sodium-ion cell: Assessment of vent gas emissions under thermal runaway. ACS Energy Lett. 2022, 7, 3386–3391. [Google Scholar] [CrossRef]
- Wang, Y.; Ou, R.; Yang, J.; Xin, Y.; Singh, P.; Wu, F.; Qian, Y.; Gao, H. The safety aspect of sodium ion batteries for practical applications. J. Energy Chem. 2024, 95, 407–427. [Google Scholar] [CrossRef]
- Wei, T.; Xian, X.L.; Dou, S.X.; Chen, W.; Chou, S.L. Comprehensive analysis and mitigation strategies for safety issues of sodium-ion batteries. Rare Met. 2024, 43, 1343–1349. [Google Scholar] [CrossRef]
- Jin, Y.; Le, P.M.L.; Gao, P.; Xu, Y.; Xiao, B.; Engelhard, M.H.; Cao, X.; Vo, T.D.; Hu, J.; Zhong, L.; et al. Low-solvation electrolytes for high-voltage sodium-ion batteries. Nat. Energy 2022, 7, 718–725. [Google Scholar] [CrossRef]
- Zhang, L.; Tsolakidou, C.; Mariyappan, S.; Tarascon, J.-M.; Trabesinger, S. Unraveling gas evolution in sodium batteries by online electrochemical mass spectrometry. Energy Storage Mater. 2021, 42, 12–21. [Google Scholar] [CrossRef]
- Lu, Z.; Yang, H.; Guo, Y.; He, P.; Wu, S.; Yang, Q.-H.; Zhou, H. Electrolyte sieving chemistry in suppressing gas evolution of sodium-metal batteries. Angew. Chem. Int. Ed. Engl. 2022, 61, e202206340. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, G.Q.; Zhang, J.T.; Zhang, J.Y.; Wu, H.W.; Jiang, L.Q.; Jiang, W.Z.; Liu, J.; Peng, K. Comparative analysis of electrochemical properties and thermal behaviors of sodium ion and lithium ion batteries. Int. J. Electrochem. Sci. 2025, 20, 101027. [Google Scholar] [CrossRef]
- Cao, J.; Ling, Z.; Lin, S.; He, Y.; Fang, X.; Zhang, Z. Thermochemical heat storage system for preventing battery thermal runaway propagation using sodium acetate trihydrate/expanded graphite. Chem. Eng. J. 2022, 433, 133536. [Google Scholar] [CrossRef]
- Xia, X.; Dahn, J.R. Study of the reactivity of na/hard carbon with different solvents and electrolytes. J. Electrochem. Soc. 2012, 159, A515–A519. [Google Scholar] [CrossRef]
- Chayambuka, K.; Mulder, G.; Danilov, D.L.; Notten, P.H.L. From li-ion batteries toward na-ion chemistries: Challenges and opportunities. Adv. Energy Mater. 2020, 10, 2001310. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.-S.; Qi, X.; Rong, X.; Li, H.; Huang, X.; Chen, L. Advanced sodium-ion batteries using superior low cost pyrolyzed anthracite anode: Towards practical applications. Energy Storage Mater. 2016, 5, 191–197. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, G.-L.; Che, H.; Wang, H.; Yang, K.; Yang, X.; Guo, F.; Ren, Y.; Chen, Z.; Amine, K.; et al. Probing thermal and chemical stability of naxni1/3fe1/3mn1/3o2 cathode material toward safe sodium-ion batteries. Chem. Mater. 2018, 30, 4909–4918. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, L.; Chihara, K.; Okada, S.; Yamaki, J.-I.; Matsumoto, S.; Kuze, S.; Nakane, K. Electrochemical and thermal properties of hard carbon-type anodes for na-ion batteries. J. Power Sources 2013, 244, 752–757. [Google Scholar] [CrossRef]
- Barpanda, P.; Liu, G.; Ling, C.D.; Tamaru, M.; Avdeev, M.; Chung, S.-C.; Yamada, Y.; Yamada, A. Na2FeP2O7: A safe cathode for rechargeable sodium-ion batteries. Chem. Mater. 2013, 25, 3480–3487. [Google Scholar] [CrossRef]
- Yue, Y.; Jia, Z.; Li, Y.; Wen, Y.; Lei, Q.; Duan, Q.; Sun, J.; Wang, Q. Thermal runaway hazards comparison between sodium-ion and lithium-ion batteries using accelerating rate calorimetry. Process Saf. Environ. Prot. 2024, 189, 61–70. [Google Scholar] [CrossRef]
- Zhang, L.C.; Zhou, Y.; Li, Y.Q.; Ma, W.L.; Wu, P.; Zhu, X.S.; Wei, S.H.; Zhou, Y.M. Achieving in-situ hybridization of NaTi2(PO4)3 and n-doped carbon through a one-pot solid state reaction for high performance sodium-ion batteries. J. Solid State Chem. 2022, 310, 123036. [Google Scholar] [CrossRef]
- Kanematsu, S.; Matsumoto, K.; Hagiwara, R. Electrochemically stable fluorohydrogenate ionic liquids based on quaternary phosphonium cations. Electrochem. Commun. 2009, 11, 1312–1315. [Google Scholar] [CrossRef]
- Wang, Y.S.; Yu, X.Q.; Xu, S.Y.; Bai, J.M.; Xiao, R.J.; Hu, Y.S.; Li, H.; Yang, X.Q.; Chen, L.Q.; Huang, X.J. Erratum: A zero-strain layered metal oxide as the negative electrode for long-life sodium-ion batteries. Nat. Commun. 2013, 4, 2858. [Google Scholar] [CrossRef]
- DeBlock, R.H.; Wei, Q.L.; Ashby, D.S.; Butts, D.M.; Whang, G.J.; Choi, C.S.; Dunn, B.S. Siloxane-modified, silica-based lonogel as a pseudosolid electrolyte for sodium-ion batteries. ACS Appl. Energy Mater. 2021, 4, 154–163. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, G.Z.; Xu, X.T.; Liao, M.; Li, Y.Y.; Angell, M.; Gu, M.; Zhu, Y.M.; Hung, W.H.; Li, J.C.; et al. A safe and non-flammable sodium metal battery based on an ionic liquid electrolyte. Nat. Commun. 2019, 10, 3302. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, G.T.; Jeong, S.; Dou, X.W.; Geng, C.X.; Kim, Y.; Passerini, S. Large-scale stationary energy storage: Seawater batteries with high rate and reversible performance. Energy Storage Mater. 2019, 16, 56–64. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, Y.Q.; Tang, F.; Li, K.X.; Rong, X.H.; Lu, Y.X.; Chen, L.Q.; Hu, Y.S. Thermal stability of high power 26650-type cylindrical na-ion batteries. Chin. Phys. Lett. 2021, 38, 076501. [Google Scholar] [CrossRef]
- Feng, J.K.; Ci, L.; Xiong, S.L. Biphenyl as overcharge protection additive for nonaqueous sodium batteries. RSC Adv. 2015, 5, 96649–96652. [Google Scholar] [CrossRef]
- Ji, W.X.; Huang, H.; Zhang, X.X.; Zheng, D.; Ding, T.Y.; Lambert, T.H.; Qu, D.Y. A redox-active organic salt for safer na-ion batteries. Nano Energy 2020, 72, 104705. [Google Scholar] [CrossRef]
- Deng, Y.Y.; Jin, T.; Li, C.; Zhang, T.; Zhang, W.J.Y.; Cui, S.J.; Shen, C.; Jiao, L.F.; Huang, H.T.; Xie, K.Y. Lattice strengthening enables reversible anionic redox chemistry in sodium-ion batteries. Energy Storage Mater. 2025, 74, 103935. [Google Scholar] [CrossRef]
- Kolachi, U.A.; Solangi, N.H.; Sun, Y.Z.; Andavar, R.; Shahid, I.; Zhao, J.J.; Pan, J.Q. Precisely controlling the nanostructure ofbimetallic Mg/Zn MOFs to construct a highperformance material for supercapacitors and sodium-ion batteries. Energy Storage Mater. J. Mater. Chem. A 2025. [Google Scholar] [CrossRef]
- Byun, J.; Chang, J.H.; Hwang, C.; Lee, C.R.; Kim, M.; Song, J.H.; Heo, B.; Choi, S.; Han, J.H.; Jeon, H.-J.; et al. Transformative Effect of Li Salt for Proactively Mitigating Interfacial Side Reactions in Sodium-Ion Batteries. Nano-Micro Lett. 2025, 17, 226. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xin, S.; Mai, L.; You, Y. Materials design for high-safety sodium-ion battery. Adv. Energy Mater. 2021, 11, 2000974. [Google Scholar] [CrossRef]
- Ji, C.W.; Zhang, S.Q.; Wang, B.; Sun, J.J.; Zhang, Z.Z.; Liu, Y.Y. Study on thermal safety of the overcharged lithium-ion battery. Fire Technol. 2023, 59, 1089–1114. [Google Scholar] [CrossRef]
- Lv, Y.F.; Geng, X.W.; Luo, W.M.; Chu, T.Y.; Li, H.N.; Liu, D.F.; Cheng, H.; Chen, J.; He, X.; Li, C.C. Review on influence factors and prevention control technologies of lithium-ion battery energy storage safety. J. Energy Storage 2023, 72, 108389. [Google Scholar] [CrossRef]
- Song, Y.H.; Lyu, N.; Shi, S.; Jiang, X.; Jin, Y. Safety warning for lithium-ion batteries by module-space air-pressure variation under thermal runaway conditions. J. Energy Storage 2022, 56, 105911. [Google Scholar] [CrossRef]
- Gui, Q.H.; Xu, B.; Yu, K.; Wang, X.Y.; Li, J.Z. Comparison of NaNi1/3Fe1/3Mn1/3O2 and Na4Fe3(PO4)2(P2O7) cathode sodium-ion battery behavior under overcharging induced thermal runaway. Chem. Eng. J. 2024, 497, 154732. [Google Scholar] [CrossRef]
- Xu, B.; Li, J.Z.; Xie, Y.G.; Gui, Q.H.; Wu, Q.; Liu, W.L.; Mao, L. Capacity degradation study of NaNi1/3Fe1/3Mn1/3O2 cathode sodium-ion batteries induced by overcharge. J. Power Sources 2024, 623, 235448. [Google Scholar] [CrossRef]
- Zakharchenko, T.K.; Nikiforov, D.I.; Serdyukov, G.D.; Komissarov, P.V.; Shkuratov, M.O.; Dzuban, A.V.; Lakienko, G.P.; Gordienko, Y.A.; Yashina, L.V.; Itkis, D.M. cathode materials for sodium-ion batteries Na3V2O2 (PO4) 2F cathodes. Batter. Supercaps 2024, 8, 2566–6223. [Google Scholar] [CrossRef]
- Kurzweil, P.; Scheuerpflug, W.; Schell, C.; Schottenbauer, J. Useful quantities and diagram types for diagnosis and monitoring of electrochemical energy converters using impedance spectroscopy: State of the art, review and outlook. Batteries 2024, 10, 177. [Google Scholar] [CrossRef]
- He, M.L.; Mejdoubi, A.E.L.; Morcrette, M.; Troendle, P.; Castiglioni, R.; Chartouni, D. High power NVPF/HC-based sodium-ion batteries. J. Power Sources 2023, 588, 233741. [Google Scholar] [CrossRef]
- Chen, M.M.; Zhang, W.J.; Chen, C.; Zhang, T.; Zhao, C.; Li, Y.; Wang, H.; Wang, K.H.; Yang, S.C.; Gao, Y.; et al. Breaking boundaries in O3-type NaNi1/3Fe1/3Mn1/3O2 Lattice strengthening enables reversible anionic: An industrially scalable reheating strategy for superior electrochemical performance. J. Energy Chem. 2025, 102, 107–119. [Google Scholar] [CrossRef]
- Wang, X.Y.; Gui, Q.H.; Liu, Z.Y.; Li, J.Z.; Xie, Y.G.; Liu, P.; Mao, L. Evaluation of multiparameter warning capability of sodium-ion battery. IEEE Trans. Instrum. Meas. 2024, 73, 3541308. [Google Scholar] [CrossRef]
- Lin, S.S.; Yang, Z.; Chen, J.; Qiao, Y.; Li, L.; Chou, S.L. Functional electrolyte additives for sodium-ion and sodium-metal batteries: Progress and perspectives. Adv. Funct. Mater. 2024, 34, 2400731. [Google Scholar] [CrossRef]
- Gui, Q.H.; Jin, B.W.; Liu, P.; Yu, K.; Zhang, J.R.; Mao, L. Revealing the Hazard of Mild Electrical Abuse on the SafetyCharacteristics of NaNi1/3Fe1/3Mn1/3O2 Cathode Sodium-Ion Battery. Adv. Sci. 2025, 2501649. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Li, X.X.; Zhang, G.Q.; Guo, J.W.; Huang, Q.Q.; Wang, C.H.; Zhou, D.Q.; Wei, D.Y. Experimental investigation on the essential cause of the degrading performances for an overcharging ternary battery. Int. J. Energy Res. 2020, 44, 3134–3147. [Google Scholar] [CrossRef]
- Zhong, K.W.; Wang, C.H.; Luo, Q.Y.; Zhang, Z.H.; Zheng, J.X. Experimental study of a novel guided sequential immersion cooling system for battery thermal management. Appl. Therm. Eng. 2024, 257, 124337. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, C.; Zhang, J.S.; Shi, Q.L.; Li, K.X.; Yu, B.X.; Xu, C.; Ju, X. The electro-thermal equalization behaviors of battery modules with immersion cooling. Appl. Energy 2023, 351, 121826. [Google Scholar] [CrossRef]
- Mei, W.X.; Liu, Z.; Wang, C.D.; Wu, C.; Liu, Y.B.; Liu, P.J.; Xia, X.D.; Xue, X.B.; Han, X.L.; Sun, J.H.; et al. Operando monitoring of thermal runaway in commerciallithiumion cells via advancd lab-on-fiber technologies. Nat. Commun. 2024, 14, 5251. [Google Scholar] [CrossRef]
- Sun, L.; Sun, W.; You, F.Q. Core temperature modelling and monitoring of lithium-ion battery in the presence of sensor bias. Appl. Energy 2020, 271, 115243. [Google Scholar] [CrossRef]
- Itagaki, M.; Suzuki, S.; Shitanda, I.; Watanabe, K. Electrochemical impedance and complex capacitance to interpret electrochemical capacitor. Electrochemistry 2007, 75, 649–655. [Google Scholar] [CrossRef]
- Lalinde, I.; Berrueta, A.; Arza, J.; Sanchis, P.; Ursua, A. On the characterization of lithium-ion batteries under overtemperature and overcharge conditions: Identification of abuse areas and experimental validation. Appl. Energy 2024, 354, 122205. [Google Scholar] [CrossRef]
- Guo, M.Y.; Zhang, H.; Huang, Z.; Li, W.B.; Zhang, D.Y.; Gao, C.Q.; Gao, F.; He, P.; Wang, J.G.; Chen, W.H.; et al. Liquid template assisted activation for “egg puff”-like hard carbontoward high sodium storage performance. Small 2023, 19, 39. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yamaguchi, T.; Nohira, T.; Hagiwara, R.; Fukunaga, A.; Sakai, S.; Nitta, K. Structural and electrochemical properties of hard carbon negative electrodes for sodium secondary batteries using the Na[FSA]-[C3C1pyrr][FSA] ionic liquid electrolyte. J. Electrochem. 2017, 85, 391–396. [Google Scholar] [CrossRef]




| Items | Technical Parameters |
|---|---|
| Cathode and anode materials | Na(Ni0.4Fe0.2Mn0.4)O2/HC |
| Nominal voltage, V | 3 |
| Nominal capacity, mAh | 1200 (25 ± 2 °C) |
| Charging cut-off voltage, V | 3.8 ± 0.05 |
| Maximum charging current, A | 1.2 |
| Maximum discharge current, A | 1.8 |
| Discharge cut-off voltage, V | 1.5 ± 0.05 |
| Operating temperature interval, °C | Charging: 0–45 Discharging: –20 to 60 |
| Storage temperature interval, °C | –20 to 60 |
| Cell weight, g | 37 ± 1.0 |
| Cell size, mm | Height: 65.0 ± 0.3 Diameter: 18.35 ± 0.2 |
| Characteristics | Mild Overcharge (100–120% SOC) | Severe Overcharge (>140% SOC) |
|---|---|---|
| Voltage range | Slightly exceeds cut-off voltage (this study: 4.2 V vs. 3.8 V) | Far exceeds cut-off voltage (>150% nominal voltage) |
| Capacity fade | Reversible fade (<5%) | Irreversible fade (>20%) |
| Structural damage | SEI thickening | Cathode phase transition/collapse, anode dendrite growth |
| Thermal behavior | Temperature rise ~5 °C | Local hotspots > 10 °C, high thermal runaway risk |
| Safety risk | Low (BMS interruptible) | High (potential thermal runaway) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhang, R.; Duan, F.; Niu, M.; Zhang, G.; Huang, T.; Wang, X.; Wen, Y.; Xu, N.; Liu, X. Influence of Overcharge Abuse on the Thermal-Electrochemical Performance of Sodium Ion Cells. Energies 2025, 18, 3580. https://doi.org/10.3390/en18133580
Zhang J, Zhang R, Duan F, Niu M, Zhang G, Huang T, Wang X, Wen Y, Xu N, Liu X. Influence of Overcharge Abuse on the Thermal-Electrochemical Performance of Sodium Ion Cells. Energies. 2025; 18(13):3580. https://doi.org/10.3390/en18133580
Chicago/Turabian StyleZhang, Jiangyun, Ruli Zhang, Fei Duan, Mingli Niu, Guoqing Zhang, Ting Huang, Xiaoyong Wang, Yuliang Wen, Ning Xu, and Xin Liu. 2025. "Influence of Overcharge Abuse on the Thermal-Electrochemical Performance of Sodium Ion Cells" Energies 18, no. 13: 3580. https://doi.org/10.3390/en18133580
APA StyleZhang, J., Zhang, R., Duan, F., Niu, M., Zhang, G., Huang, T., Wang, X., Wen, Y., Xu, N., & Liu, X. (2025). Influence of Overcharge Abuse on the Thermal-Electrochemical Performance of Sodium Ion Cells. Energies, 18(13), 3580. https://doi.org/10.3390/en18133580






