Research on the Thermal Decomposition Characteristics of PE Outer Sheath of High-Voltage Cables Under Different Humidity Levels
Abstract
1. Introduction
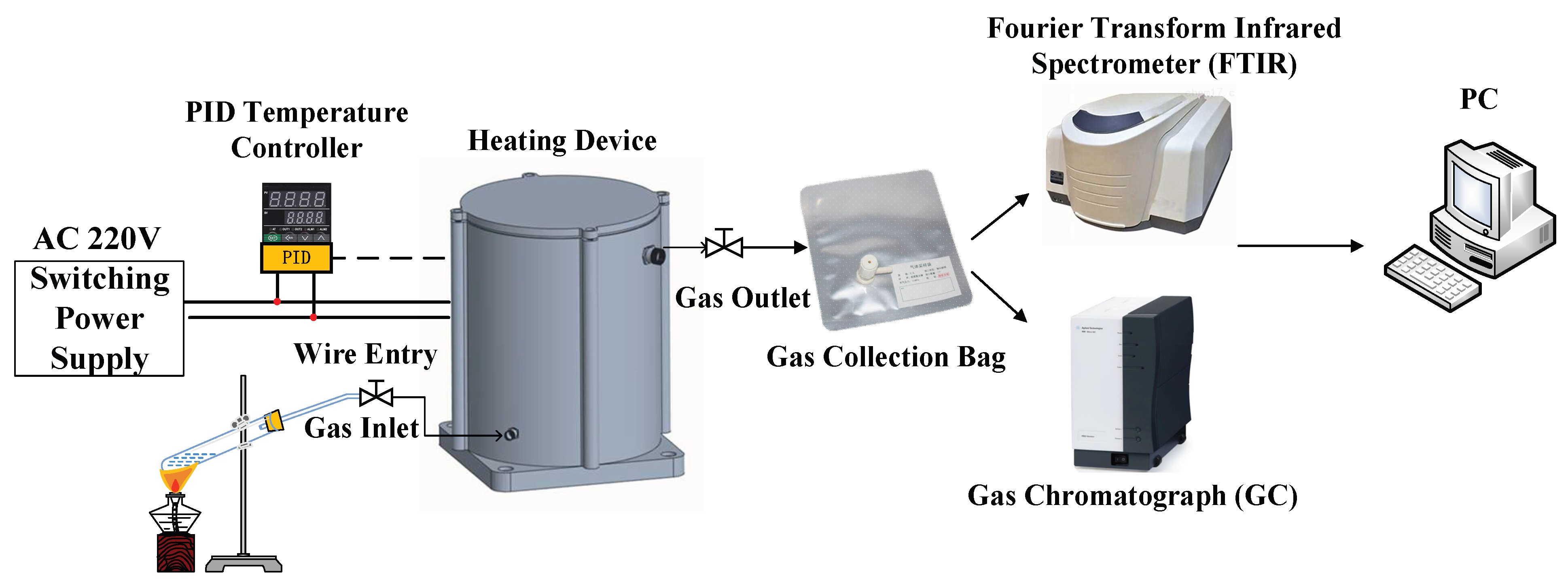
2. Thermal Decomposition Test Method
3. Results and Discussion
3.1. Infrared Spectroscopy Test Results and Analysis
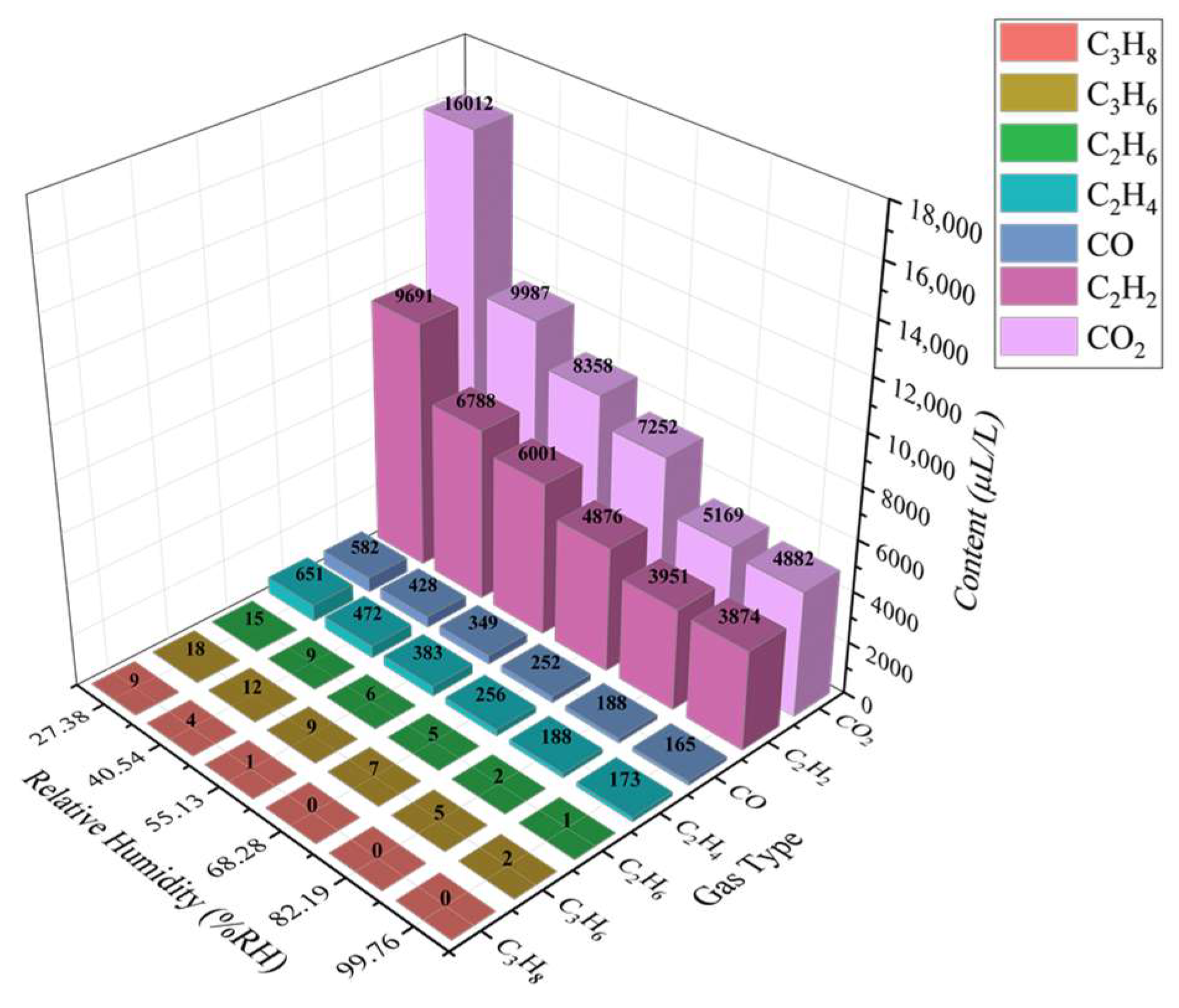
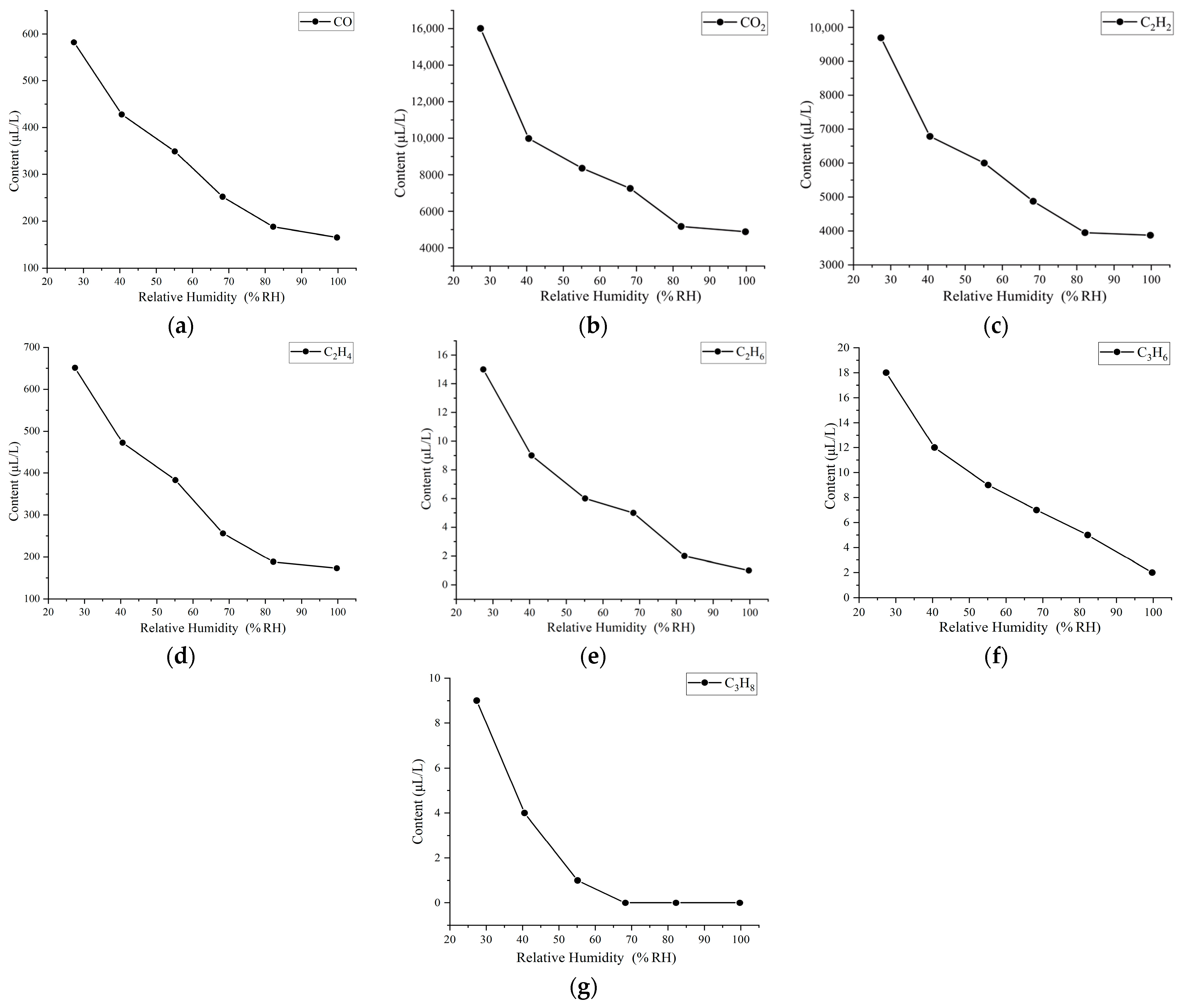
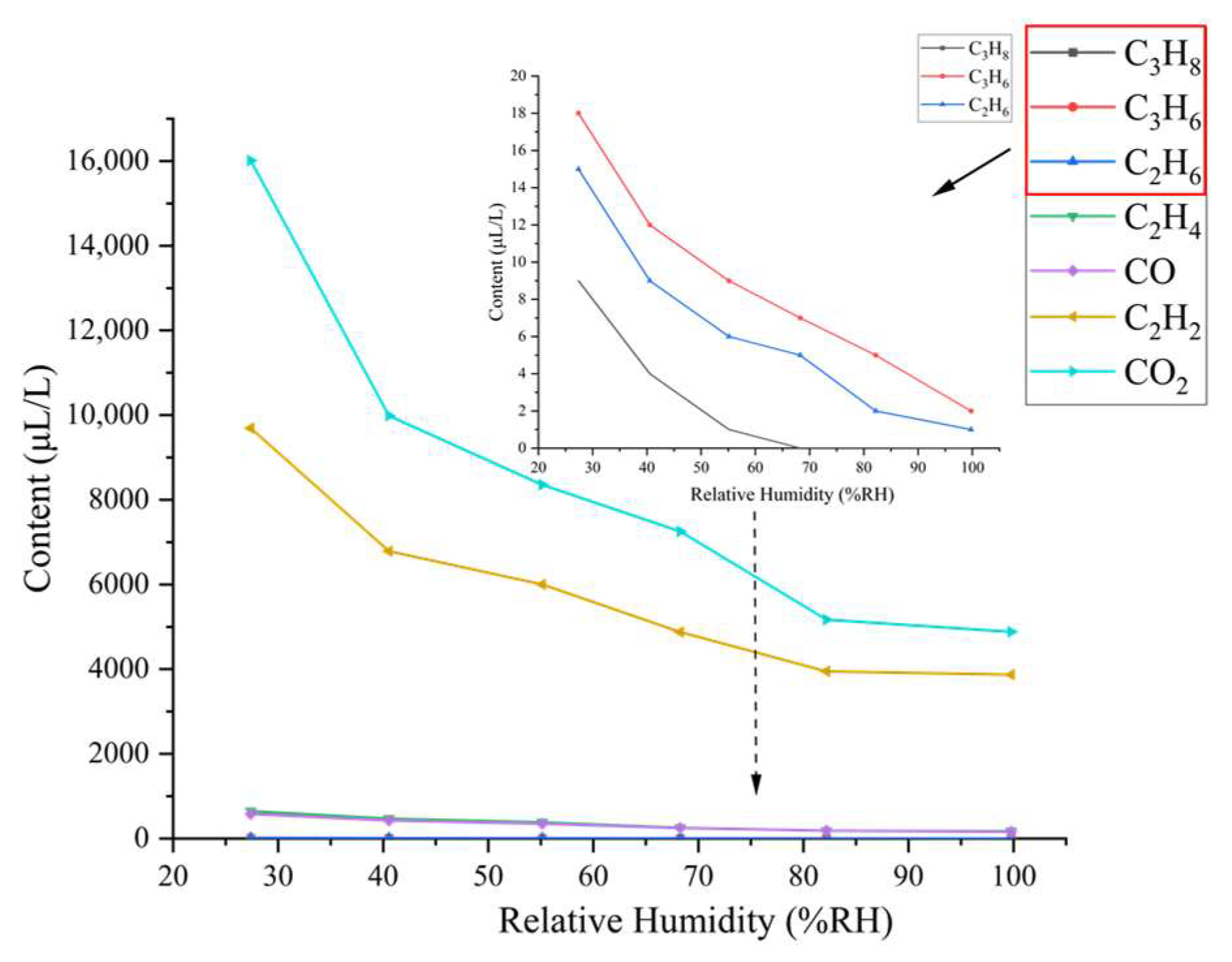
3.2. Gas Chromatography Test Results and Analysis
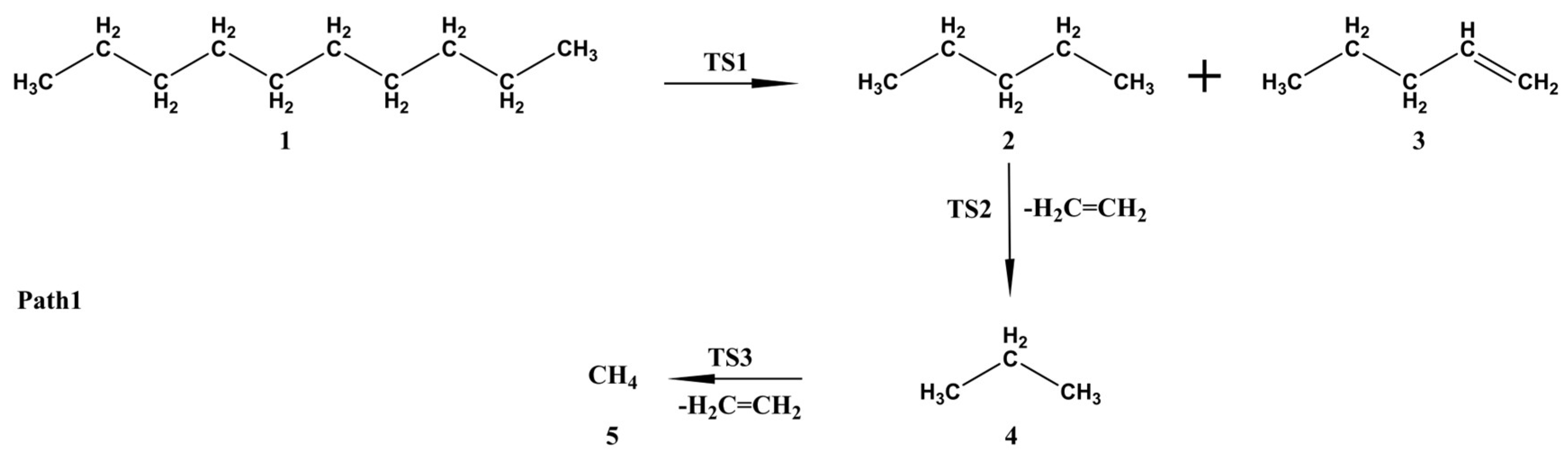
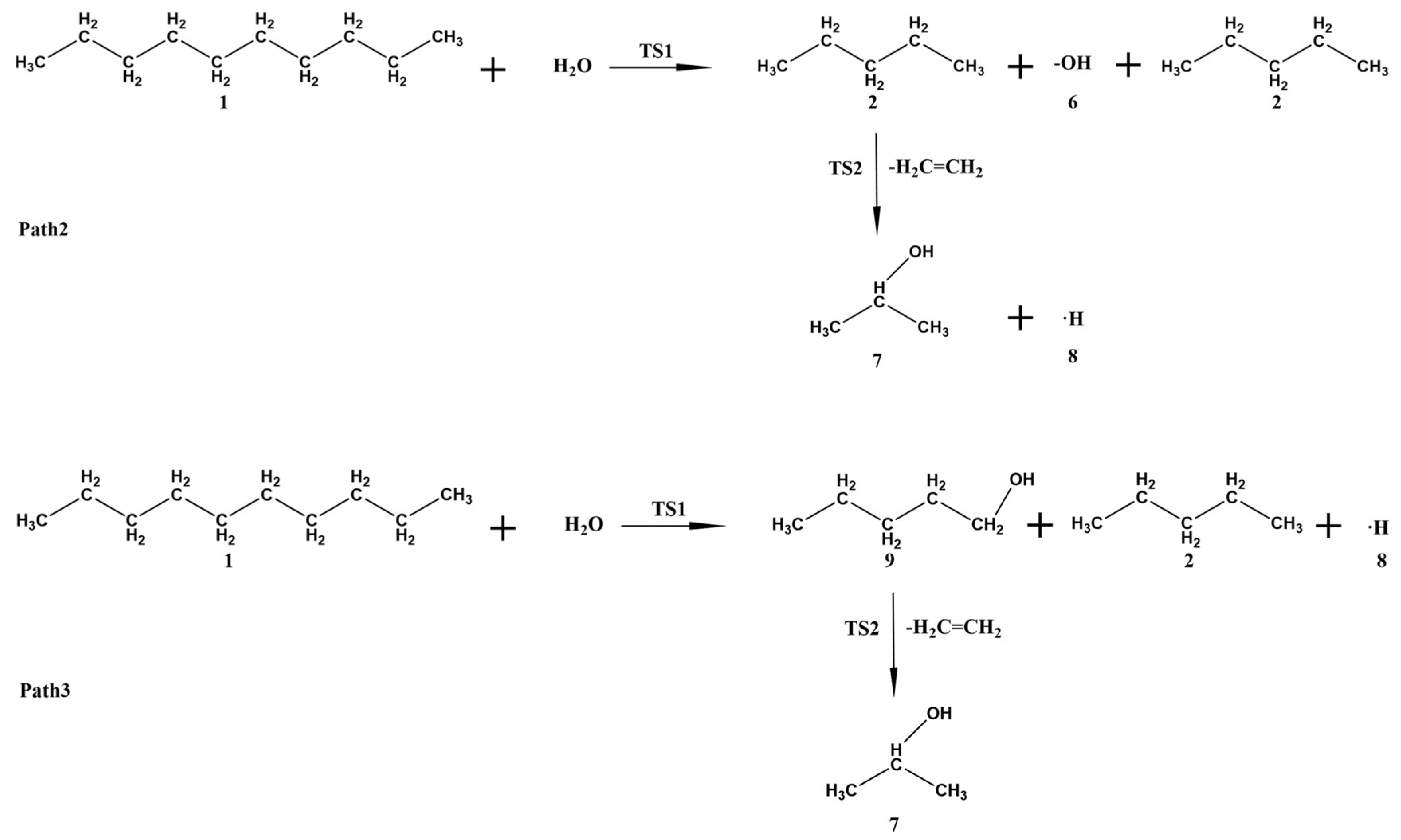
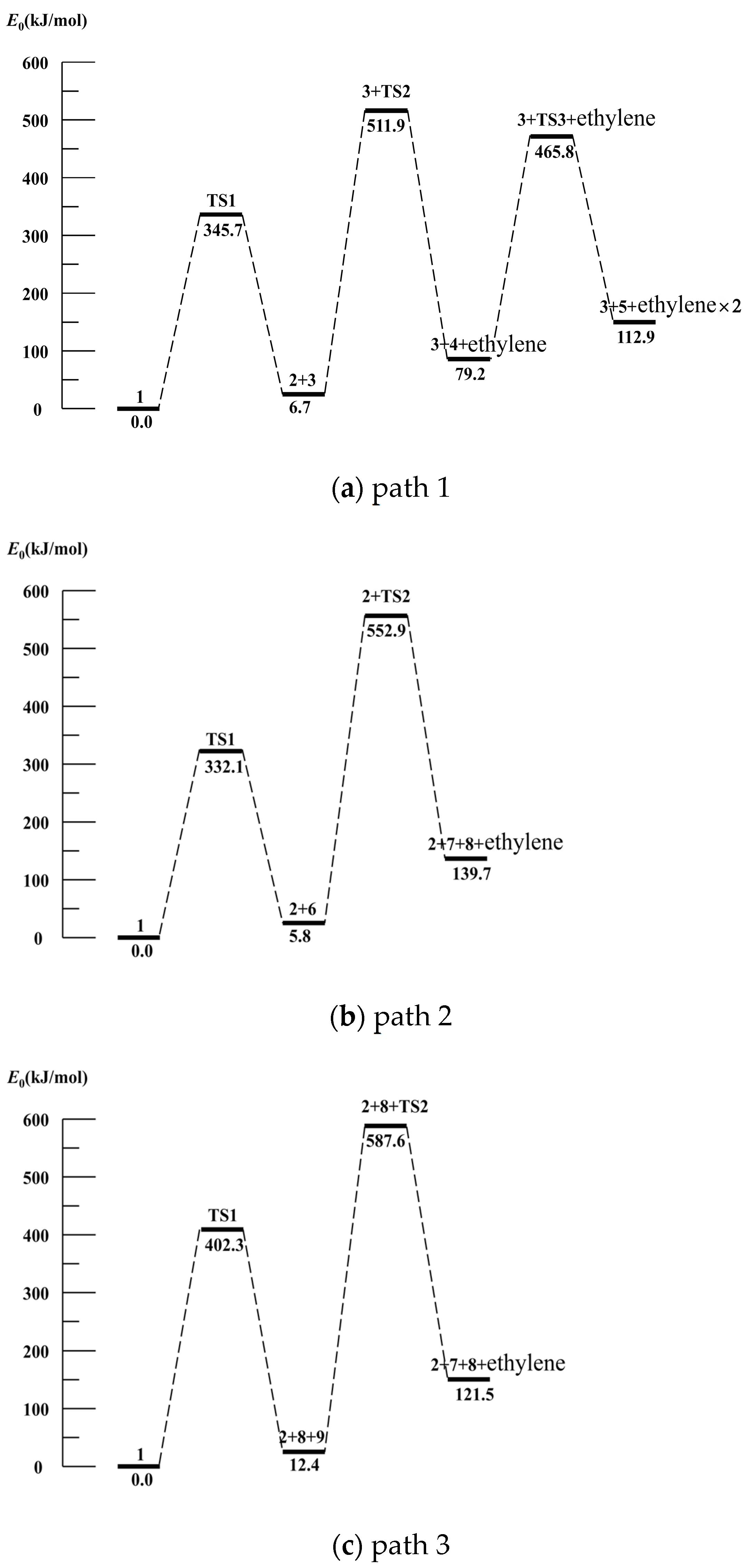
3.3. Gaussian Simulation Calculation Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yuan, J.; Ouyang, B.; Xia, R.; Wang, G.; Liu, S. Research on the effectiveness evaluation method of on-line partial discharge monitoring device for high-voltage cables and field application. High Volt. Eng. 2023, 49, 17–23. [Google Scholar] [CrossRef]
- Wang, T.; Tan, L.; Xie, S.; Ma, B. Development and applications of common utility tunnels in China. Tunn. Undergr. Space Technol. 2018, 76, 92–106. [Google Scholar] [CrossRef]
- Pan, R.; Zhu, G.; Xu, G.; Liu, X. Experimental analysis on burning rate and temperature profile produced by pool fire in a curved tunnel as a function of fire location. Process Saf. Environ. Prot. 2021, 152, 549–567. [Google Scholar] [CrossRef]
- National Fire and Rescue Administration. China Fire Yearbook 2018 Edition; Yunnan People’s Publishing House: Kunming, China, 2018.
- Guo, W.; Pu, Z.; Ren, Z.; Zhou, S.; Quan, L.; Men, Y.; Pan, Z. Analysis of abnormal detection data of fire accident in power cable tunnel and field test study on characteristic parameters of tunnel fire. Front. Energy Res. 2022, 10, 860707. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, N.; Lin, J.; Lua, S.; Liew, K.M. On the pyrolysis characteristic parameters of four flame-retardant classes of PVC sheathless cable insulation materials. J. Anal. Appl. Pyrolysis 2023, 170, 105901. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, G.; Wang, S.; Zhang, H.; Xu, F. TG-FTIR and Py-GC/MS study of the pyrolysis mechanism and composition of volatiles from flash pyrolysis of PVC. J. Energy Inst. 2020, 93, 2362–2370. [Google Scholar] [CrossRef]
- Courty, L.; Garo, J.P. External heating of electrical cables and auto-ignition investigation. J. Hazard. Mater. 2017, 321, 528–536. [Google Scholar] [CrossRef]
- Liu, J.; Ouyang, B.; Xia, R.; Fei, W. Assessment of the insulation aging state of high voltage XLPE cables. Electr. Power Eng. Technol. 2021, 40, 141–149. [Google Scholar] [CrossRef]
- Duan, Y.; Han, M.; Lan, R.; Li, G.; Wang, Z. Insulation Characteristics and Failure Mechanism of High-Voltage Cables under Different Thermal Aging Temperatures. Trans. China Electrotech. Soc. 2024, 39, 45–54. [Google Scholar] [CrossRef]
- Lee, H.G.; Jung, J.S.; Kim, J.G. Measurement of activation energy and accelerated degradation time by thermal analysis methods for polymeric insulating materials. J. Electr. Eng. Technol. 2021, 16, 515–524. [Google Scholar] [CrossRef]
- Xu, D.; Li, Y.; Yang, X.; Zhong, H.; Li, J.; Li, J.; Huang, Y. Enhancing resilience in urban utility tunnels power transmission systems: Analysing temperature distribution in near-wall cable fires for risk mitigation. Tunn. Undergr. Space Technol. 2024, 152, 105911. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Qiu, X.; Zhu, T. A review on fire research of electric power grids of China: State-of-the-art and new insights. Fire Technol. 2024, 60, 1027–1076. [Google Scholar] [CrossRef]
- Huo, Y.; Xu, X.; Li, Y.; Li, R.; Yu, Z.; Wang, W. Experimental and theoretical research on the temperature evolution law of overcurrent fault wires. Fire Mater. 2024, 48, 632–641. [Google Scholar] [CrossRef]
- Liu, Y.; Geyik, U.; Kobald, A.; Yang, A.; Wang, X.; Weimar, U.; Rong, M.; Bârsan, N. Overheat diagnosis of power cable based on gas sensors: Device/material exploration. Sens. Actuators B Chem. 2022, 350, 130837. [Google Scholar] [CrossRef]
- Lei, F.; Chu, J.; Liu, Y.; Yang, A.; Yuan, H.; Wang, X.; Rong, M. Fault diagnosis of cable overheating based on semiconductor gas sensing array. Trans. China Electrotech. Soc. 2023, 38, 3651–3664. [Google Scholar] [CrossRef]
- Yi, J.; Chen, W.; Han, J.; Chen, D. Sensitive and selective detection of plasticizer vapors with modified-SnO2 hollow nanofibers for electrical fire warning. Sens. Actuators B Chem. 2019, 287, 364–370. [Google Scholar] [CrossRef]
- Kannan, P.; Ibrahim, S.; Reddy, K.S.K.; Al Shoaibi, A.; Srinivasakannan, C. A comparative analysis of the kinetic experiments in polyethylene pyrolysis. J. Energy Resour. Technol. 2014, 136, 024001. [Google Scholar] [CrossRef]
- Jing, R.; Wei, G.; Du, K.; Cao, Z.; Yan, H. Experimental Study on Thermal Decomposition of Polyvinyl Chloride Outer Sheath of 110 kV High Voltage Cable. J. Electr. Eng. Technol. 2025, 20, 861–872. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, Y.; Zhang, D.; Fang, R.; Zhang, X. Revealing the pyrolysis mechanism of polypropylene cable insulation: A combined study of TG-GC experiments and RMD/DFT simulations. J. Appl. Polym. Sci. 2024, 141, e55245. [Google Scholar] [CrossRef]
- Kajda-Szcześniak, M.; Czop, M. Comparison of Pyrolysis and Combustion Processes of Vinyl Floor Panels Using Thermogravimetric Analysis (TG-FTIR) in Terms of the Circular Economy. Energies 2022, 15, 1516. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.; Man, P. Kinetic Analysis of Thermal Decomposition of Polyvinyl Chloride at Various Oxygen Concentrations. Fire 2023, 6, 404. [Google Scholar] [CrossRef]
- Decimus, A.; Sonnier, R.; Zavaleta, P.; Suard, S.; Ferry, L. Study of gases released under incomplete combustion using PCFC–FTIR. J. Therm. Anal. Calorim. 2019, 138, 753–763. [Google Scholar] [CrossRef]
- Zheng, X.; Cai, G.; Guo, J.; Gao, W.; Huang, Y.; Tong, X. Combustion characteristics and thermal decomposition mechanism of the flame-retardant cable in urban utility tunnel. Case Stud. Therm. Eng. 2023, 44, 102887. [Google Scholar] [CrossRef]
- Jabłońska, B.; Poznańska, G.; Jabłoński, P.; Zwolińska, J. Thermochemical Valorization of Plastic Waste Containing Low Density Polyethylene, Polyvinyl Chloride and Polyvinyl Butyral into Thermal and Fuel Energy. Energies 2024, 17, 3458. [Google Scholar] [CrossRef]
- Wu, J.; Chen, T.; Luo, X.; Han, D.; Wang, Z.; Wu, J. TG/FTIR analysis on co-pyrolysis behavior of PE, PVC and PS. Waste Manag. 2014, 34, 676–682. [Google Scholar] [CrossRef]
- Honus, S.; Kumagai, S.; Fedorko, G.; Molnár, V.; Yoshioka, T. Pyrolysis gases produced from individual and mixed PE, PP, PS, PVC, and PET-Part I: Production and physical properties. Fuel 2018, 221, 346–360. [Google Scholar] [CrossRef]
- Lv, L.; Duan, C.; Ji, S.; Rao, Z.; Xu, M.; Lin, Z. Overview of ignition mechanism and combustion characteristic of wire and cable. High Volt. Eng. 2022, 48, 612–625. [Google Scholar] [CrossRef]
- GB/T 11017.1-2024; Power Cables with Cross-Linked Polyethylene Insulation and Their Accessories for Rated Voltage of 66 kV(Um = 72.5 kV)and 110 kV(Um = 126 kV)—Part 1: Test Methods and Requirements. Standards Press of China: Beijing, China, 2024.
- Wang, S. Research on the influence of air humidity on coal spontaneous combustion characteristics and oxidation kinetics parameters. Safe. Coal Min. 2024, 55, 98–105. [Google Scholar] [CrossRef]
- Lai, Y.; Liu, X.; Davies, M.; Fisk, C.; Holliday, M.; King, D.; Zhang, Y.; Willmott, J. Characterisation of wood combustion and emission under varying moisture contents using multiple imaging techniques. Fuel 2024, 373, 132397. [Google Scholar] [CrossRef]
- Zhang, H.; Yoshino, H. Analysis of indoor humidity environment in Chinese residential buildings. Build. Environ. 2010, 45, 2132–2140. [Google Scholar] [CrossRef]
- IEC 60229:2007; Electric Cables—Tests on Extruded Oversheaths with a Special Protective Function. International Electrotech-nical Commission: Geneva, Switzerland, 2007.
- GB/T 11017.2-2014; Power Cables with Cross-Linked Polyethylene Insulation and Their Accessories for Rated Voltage of 110 kV (Um = 126 kV)—Part 2: Power Cables. Standards Press of China: Beijing, China, 2024.
- GB/T 13610-2020; Analysis of Natural Gas Composition—Gas Chromatography. Standards Press of China: Beijing, China, 2020.
- Jameel, A.G.A.; Han, Y.; Brignoli, O.; Telalović, S.; Elbaz, A.M.; Im, H.G.; Roberts, W.L.; Sarathy, S.M. Heavy fuel oil pyrolysis and combustion: Kinetics and evolved gases investigated by TGA-FTIR. J. Anal. Appl. Pyrolysis 2017, 127, 183–195. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Yang, X.; Jin, Y.; Lv, Z.; Lan, S.; Zhu, D.; Dang, L. Self-emulsification synthesis of epoxy phosphate ester and its flame-retardant mechanism in flexible poly (vinyl chloride)/magnesium hydroxide composites. J. Appl. Polym. Sci. 2024, 141, e55354. [Google Scholar] [CrossRef]
- Yang, J.; Chen, H.; Zhao, W.; Zhou, J. TG–FTIR-MS study of pyrolysis products evolving from peat. J. Anal. Appl. Pyrolysis 2016, 117, 296–309. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, W.; Yu, Q.; Ji, W. Pyrolysis and carbonization of polyvinyl chloride under electric field: A computational study. Chem. Phys. Lett. 2021, 770, 138450. [Google Scholar] [CrossRef]
- Bockhorn, H.; Hornung, A.; Hornung, U. Mechanisms and kinetics of thermal decomposition of plastics from isothermal and dynamic measurements. J. Anal. Appl. Pyrolysis 1999, 50, 77–101. [Google Scholar] [CrossRef]
- Luo, H.; Zhao, Y.; Li, Y.; Xiang, Y.; He, D.; Pan, X. Aging of microplastics affects their surface properties, thermal decomposition, additives leaching and interactions in simulated fluids. Sci. Total Environ. 2020, 714, 136862. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Wang, Q.; Huang, H.; Li, Y.; Kuang, Y.; Xiang, H.; Liu, J.; Cao, Z. Research on the Thermal Decomposition Characteristics of PE Outer Sheath of High-Voltage Cables Under Different Humidity Levels. Energies 2025, 18, 3537. https://doi.org/10.3390/en18133537
Wu Z, Wang Q, Huang H, Li Y, Kuang Y, Xiang H, Liu J, Cao Z. Research on the Thermal Decomposition Characteristics of PE Outer Sheath of High-Voltage Cables Under Different Humidity Levels. Energies. 2025; 18(13):3537. https://doi.org/10.3390/en18133537
Chicago/Turabian StyleWu, Zhaoguo, Qian Wang, Huixian Huang, Yong Li, Yulai Kuang, Hong Xiang, Junwei Liu, and Zhengqin Cao. 2025. "Research on the Thermal Decomposition Characteristics of PE Outer Sheath of High-Voltage Cables Under Different Humidity Levels" Energies 18, no. 13: 3537. https://doi.org/10.3390/en18133537
APA StyleWu, Z., Wang, Q., Huang, H., Li, Y., Kuang, Y., Xiang, H., Liu, J., & Cao, Z. (2025). Research on the Thermal Decomposition Characteristics of PE Outer Sheath of High-Voltage Cables Under Different Humidity Levels. Energies, 18(13), 3537. https://doi.org/10.3390/en18133537





