Determination of Residual Oil in Biodiesel via Quasi-Isothermal Thermogravimetry (TGA-qISO) and Differential Scanning Calorimetry (DSC)
Abstract
1. Introduction
2. Materials and Methods
2.1. Quasi-Isothermal Thermogravimetry (TGA-qISO)
- Equilibrate the temperature at 25 °C;
- Stop the next segment if the mass variation is greater than 2% min−1;
- Apply a 10 °C min−1 ramp from 25–350 °C;
- Stop the next segment if the sample mass variation is less than 2%;
- Use a 125 min isotherm;
- Apply a 10 °C min−1 ramp to 550 °C.
2.2. Differential Scanning Calorimetry (DSC)
2.3. Determination of the Linear Regression Coefficients
2.4. Analysis of Variance (ANOVA)
3. Results
3.1. Quasi-Isothermal Thermogravimetry (TG-qISO)
3.2. Differential Scanning Calorimetry (DSC)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TG/DTG | Thermogravimetry/Derivative Thermogravimetry |
| TGA-qISO | Quasi-Isothermal Thermogravimetry |
| DSC | Differential Scanning Calorimetry |
| INMETRO | National Institute of Metrology |
References
- Barroso, R.M.; Muñoz, A.E.P.; Cai, J. Social and Economic Performance of Tilapia Farming in Brazil; Fisheries and Aquaculture Circular No. 1181; FAO: Rome, Italy, 2019. [Google Scholar]
- Pires, D.R.; Morais, A.C.N.d.; Costa, J.F.d.; Góes, L.C.D.S.d.A.; Oliveira, G.M.d. Utilization of waste edible fish: Application and feasibility. Rev. Verde 2015, 9, 34–46. Available online: https://www.gvaa.com.br/revista/index.php/RVADS/article/view/2956 (accessed on 21 January 2025).
- Rose Meire, V.; Ivã Guidini, L. Organic waste generated in fish farming. Res. Technol. 2016, 13, 1–6. [Google Scholar]
- Jayasinghe, P.; Hawboldt, K. A review of bio-oils from waste biomass: Focus on fish processing waste. Renew. Sustain. Energy Rev. 2012, 16, 798–821. [Google Scholar] [CrossRef]
- Menegazzo, M.L.; Lucas, B.F.; Alcade, L.B.; Petenucci, M.E.; Fonseca, G.G. Production of biodiesel via methyl and ethyl routes from Nile tilapia and hybrid Sorubim crude oils. J. Environ. Chem. Eng. 2015, 3, 150–154. [Google Scholar] [CrossRef]
- Mota, F.A.S.; Costa Filho, J.T.; Barreto, G.A. The Nile tilapia viscera oil extraction for biodiesel production in Brazil: An economic analysis. Renew. Sustain. Energy Rev. 2019, 108, 1–10. [Google Scholar] [CrossRef]
- Ministry of Mines and Energy. Resolution N° 8, of December 19, 2023. Available online: https://www.gov.br/mme/pt-br/assuntos/conselhos-e-comites/cnpe/resolucoes-do-cnpe/2023/ResoluoCNPE8Publicada.pdf (accessed on 21 January 2025).
- Sitoe, B.V.; Costa, L.G.D.; Santos, B.A.D.; Santos, D.Q.; Borges Neto, W. Monitoring of adulterants in crambe methyl biodiesel in mixtures with diesel, using ft-mir and multivariate control charts based on net analyte signal. Química Nova 2020, 43, 1–8. [Google Scholar] [CrossRef]
- Chand, P.; Reddy, C.V.; Verkade, J.G.; Wang, T.; Grewell, D. Thermogravimetric quantification of biodiesel produced via alkali catalyzed transesterification of soybean oil. Energy Fuels 2009, 23, 989–992. [Google Scholar] [CrossRef]
- Sousa, F.P.; Luciano, M.A.; Pasa, V.M.D. Thermogravimetry and viscometry for assessing the ester content (FAME and FAEE). Fuel Process. Technol. 2013, 109, 133–140. [Google Scholar] [CrossRef]
- Gaglieri, C.; Alarcon, R.T.; de Moura, A.; Mendes, R.A.; Caires, F.J. Is Thermogravimetry an efficient alternative to gas chromatography in degree of biodiesel conversion? J. Therm. Anal. Calorim. 2019, 135, 2591–2597. [Google Scholar] [CrossRef]
- Ionashiro, M.; Caires, F.J.; Gomes, D.J.C. Giolite: Fundamentals of Thermogravimetry and Differential Thermal Analysis/Differential Scanning Calorimetry, 2nd ed.; Giz Editorial: São Paulo, Brazil, 2014; pp. 1–192. [Google Scholar]
- Cortes, M.R.; de Queiroz, J.F.; dos Santos, T.M.; Cavalheiro, L.F.; Misutsu, M.; Ravaglia, L.M.; Alcantara, G.B.; Vian, L.H.; Nazário, C.E.; Simionatto, E.; et al. Applying quasi-isothermal thermogravimetry to determine degrees of oil conversion into biodiesel. J. Therm. Anal. Calorim. 2022, 147, 4397–4402. [Google Scholar] [CrossRef]
- National Institute of Metrology, Quality and Technology—INMETRO. DOQ-CGCRE-008: Guidance on Validation of Analytical Methods. 2020. Available online: https://app.sogi.com.br/Manager/texto/arquivo/exibir/arquivo?eyJ0eXAiOiJKV1QiLCJhbGciOiJIUzI1NiJ9AFFIjAvMTM4ODM3NS9TR19SZXF1aXNpdG9fTGVnYWxfVGV4dG8vMC8wL0RPUS1DZ2NyZS04XzA5LnBkZi8wLzAiAFFBcMYdNmecpDn0m0Dj4vzJmvMJZMAYtW6mtkIlj0C7fk (accessed on 21 January 2025).
- Ribani, M.; Bottoli, C.B.G.; Collins, C.H.; Jardim, I.C.S.F.; Melo, L.F.C. Validation for chromatographic and electrophoretic methods. Química Nova 2004, 27, 771–780. [Google Scholar] [CrossRef]
- Moraes, P.S.; Igansi, A.V.; Cadaval, T.R., Jr.; Pinto, L.A. Biodiesel produced from crude, degummed, neutralized and bleached oils of Nile tilapia waste: Production efficiency, physical-chemical quality and economic viability. Renew. Energy 2020, 161, 110–119. [Google Scholar] [CrossRef]
- de Lima Ribeiro, F.A.; Ferreira, M.M.C.; Morano, S.C.; da Silva, L.R.; Schneider, R.P. Validation spreadsheet: A new tool for estimating figures of merit in the validation of univariate analytical methods. Química Nova 2008, 31, 164–171. [Google Scholar] [CrossRef]
- Skoog, D.A.; West, M.D.; Holler, F.J.; Crouch, S.R. Fundamentos de Química Analítica, 8th ed.; Pioneira Thomson Learning: São Paulo, Brazil, 2006. [Google Scholar]
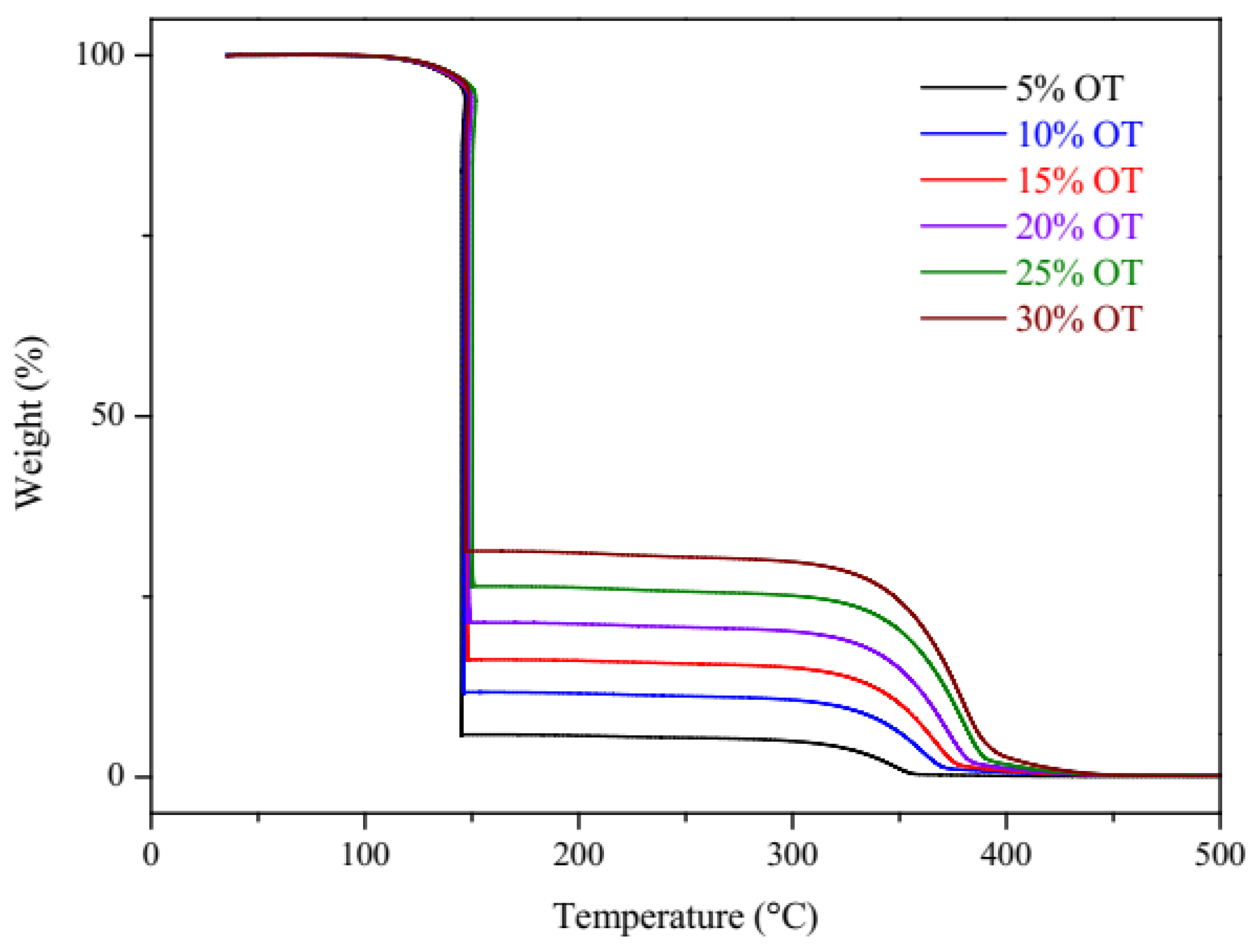

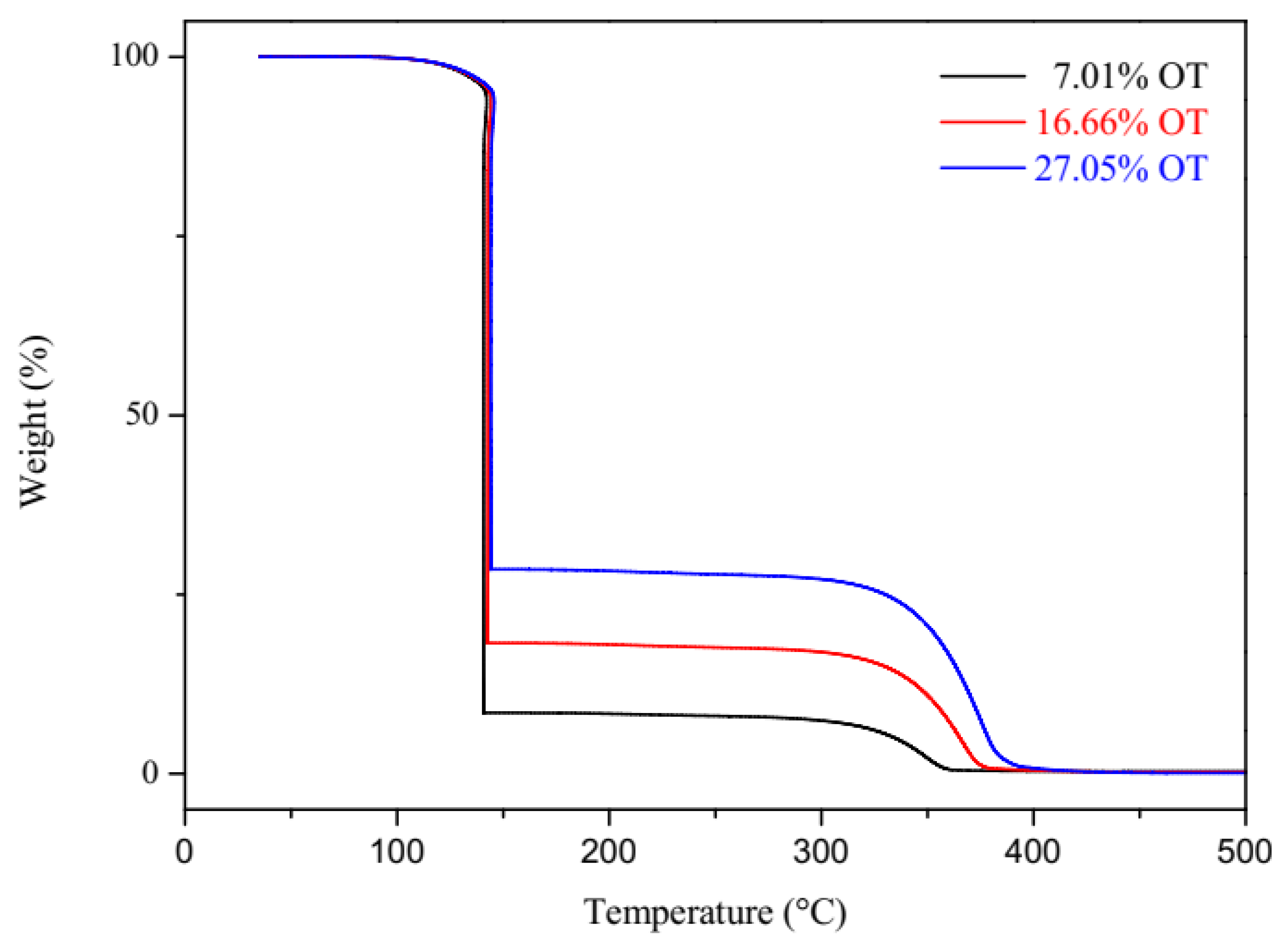
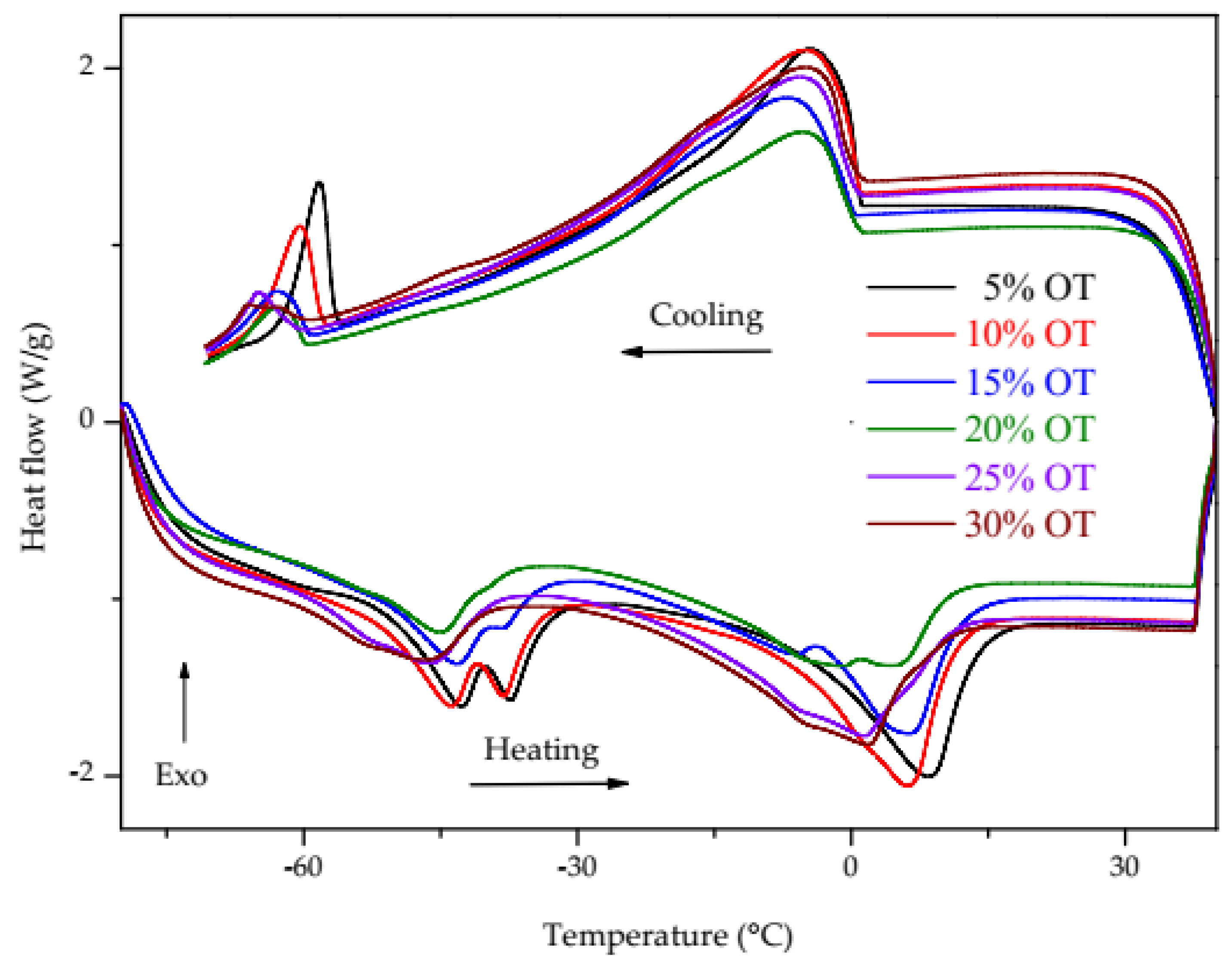
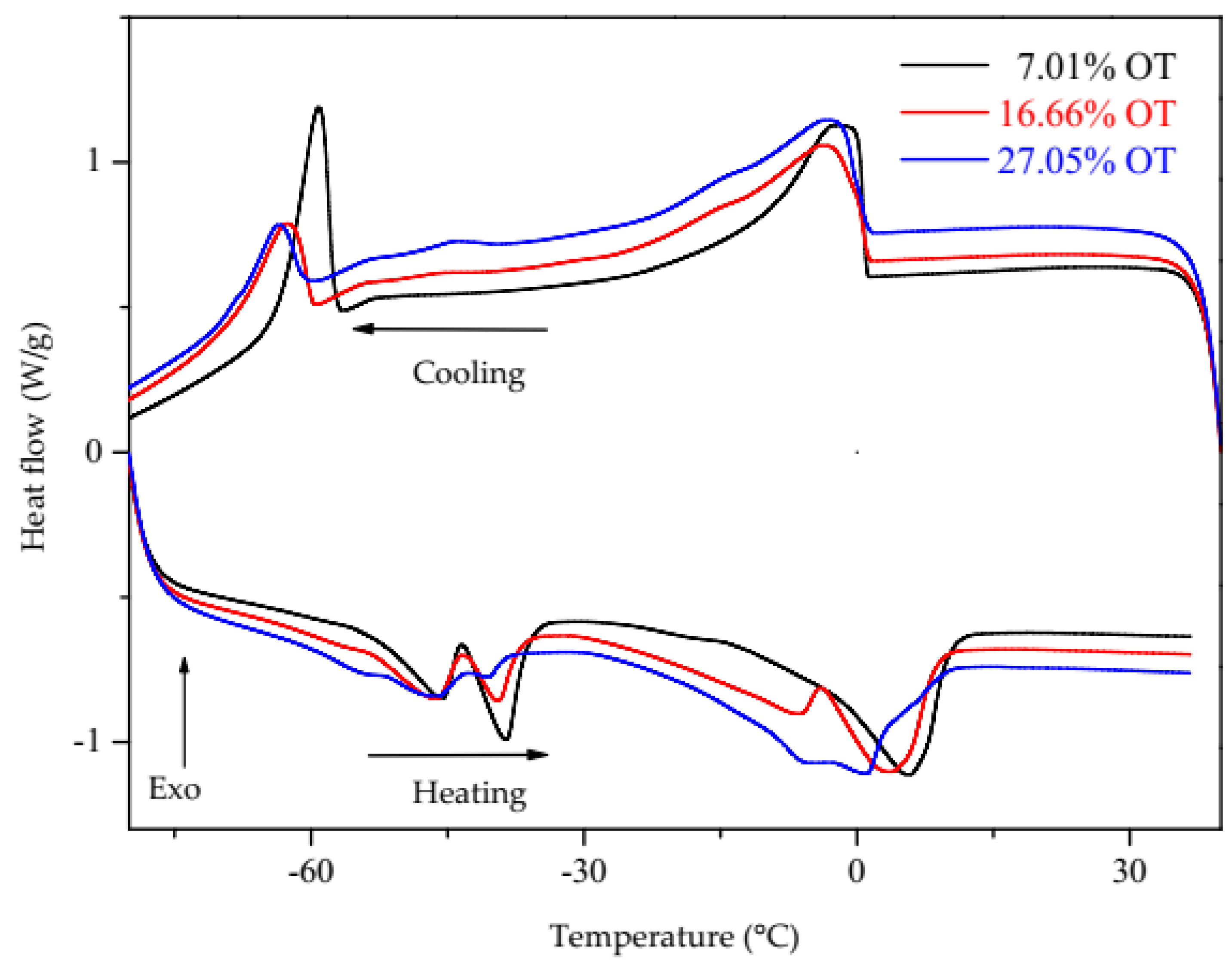
| Oil/Biodiesel Blends | Oil Content (% m/m) | ∆H (J/g) (1.93 to −24 °C) P1 | ∆H (J/g) (−55 to −64 °C) P2 | ∆H (J/g) (−56 to −30 °C) P3 | ∆H (J/g) (−7.38 to 19.84) P3 |
|---|---|---|---|---|---|
| 5% OT | 4.98 | 34.35 ± 1.38 | 26.02 ± 1.08 | 24.09 ± 1.14 | 41.32 ± 0.72 |
| 10% OT | 10.15 | 33.80 ± 3.22 | 20.84 ± 1.12 | 20.90 ± 1.37 | 40.45 ± 1.59 |
| 15% OT | 15.07 | 33.37 ± 1.96 | 14.06 ± 1.00 | 17.86 ± 1.62 | 42.16 ± 1.71 |
| 20% OT | 20.16 | 25.73 ± 1.76 | 10.99 ± 1.28 | 12.85 ± 1.86 | 37.91 ± 2.90 |
| 25% OT | 25.04 | 27.96 ± 1.58 | 9.58 ± 1.10 | 12.56 ± 0.65 | 42.75 ± 1.35 |
| 30% OT | 30.04 | 25.03 ± 5.70 | 5.90 ± 1.54 | 8.46 ± 1.62 | 35.23 ± 6.57 |
| Events | Line Equation | R |
|---|---|---|
| P1 (1.93 to −24 °C) | y = 38.09 − 0.4156 x | −0.9027 |
| P2 (−55 to −64 °C) | y = 28.56 − 0.8015 x | −0.9723 |
| P3 (−56 to −30 °C) | y = 26.02 − 0.5451 x | −0.9903 |
| P4 (−7.38 to 19.84 °C) | y = 41.02 − 0.08693 x | −0.3473 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortes, M.R.; de Queiroz, J.F.; Amorim, M.J.R.; Machate, D.J.; Simionatto, E.; Nazário, C.E.D.; de Oliveira, L.C.S. Determination of Residual Oil in Biodiesel via Quasi-Isothermal Thermogravimetry (TGA-qISO) and Differential Scanning Calorimetry (DSC). Energies 2025, 18, 3518. https://doi.org/10.3390/en18133518
Cortes MR, de Queiroz JF, Amorim MJR, Machate DJ, Simionatto E, Nazário CED, de Oliveira LCS. Determination of Residual Oil in Biodiesel via Quasi-Isothermal Thermogravimetry (TGA-qISO) and Differential Scanning Calorimetry (DSC). Energies. 2025; 18(13):3518. https://doi.org/10.3390/en18133518
Chicago/Turabian StyleCortes, Mário Rodrigues, Joice Ferreira de Queiroz, Marcio José Rodrigues Amorim, David Johane Machate, Euclésio Simionatto, Carlos Eduardo Domingues Nazário, and Lincoln Carlos Silva de Oliveira. 2025. "Determination of Residual Oil in Biodiesel via Quasi-Isothermal Thermogravimetry (TGA-qISO) and Differential Scanning Calorimetry (DSC)" Energies 18, no. 13: 3518. https://doi.org/10.3390/en18133518
APA StyleCortes, M. R., de Queiroz, J. F., Amorim, M. J. R., Machate, D. J., Simionatto, E., Nazário, C. E. D., & de Oliveira, L. C. S. (2025). Determination of Residual Oil in Biodiesel via Quasi-Isothermal Thermogravimetry (TGA-qISO) and Differential Scanning Calorimetry (DSC). Energies, 18(13), 3518. https://doi.org/10.3390/en18133518








