Selective Lithium Plating on Graphite–Silicon Composite Anodes During Fast Charging in Rechargeable Lithium Batteries
Abstract
1. Introduction
2. Experimental Procedures
2.1. Electrode Fabrication and Cell Preparation
2.2. Electrochemical Experiments
2.2.1. Cell Activation
2.2.2. Lithium Plating Test
3. Results and Discussion
4. Conclusions
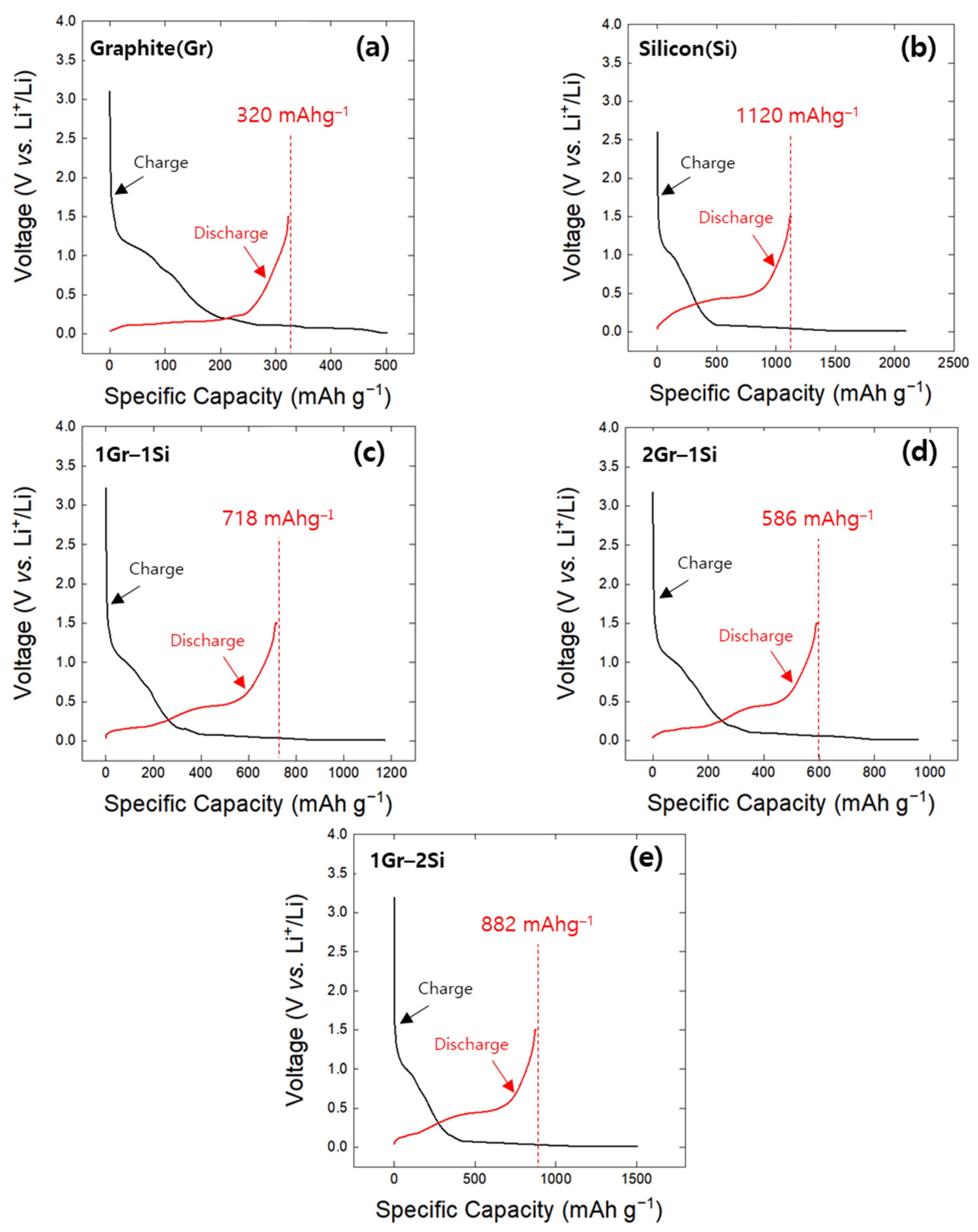
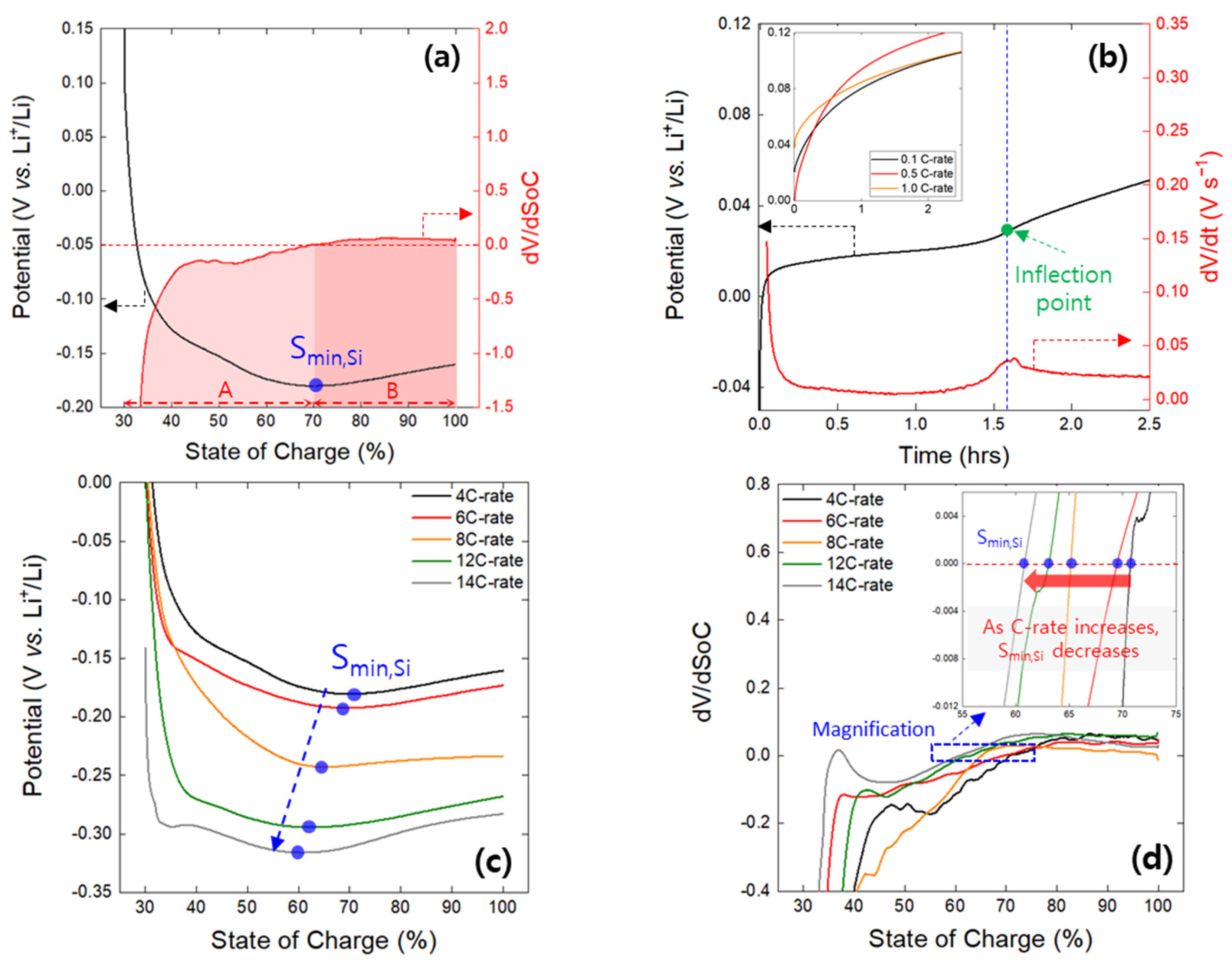
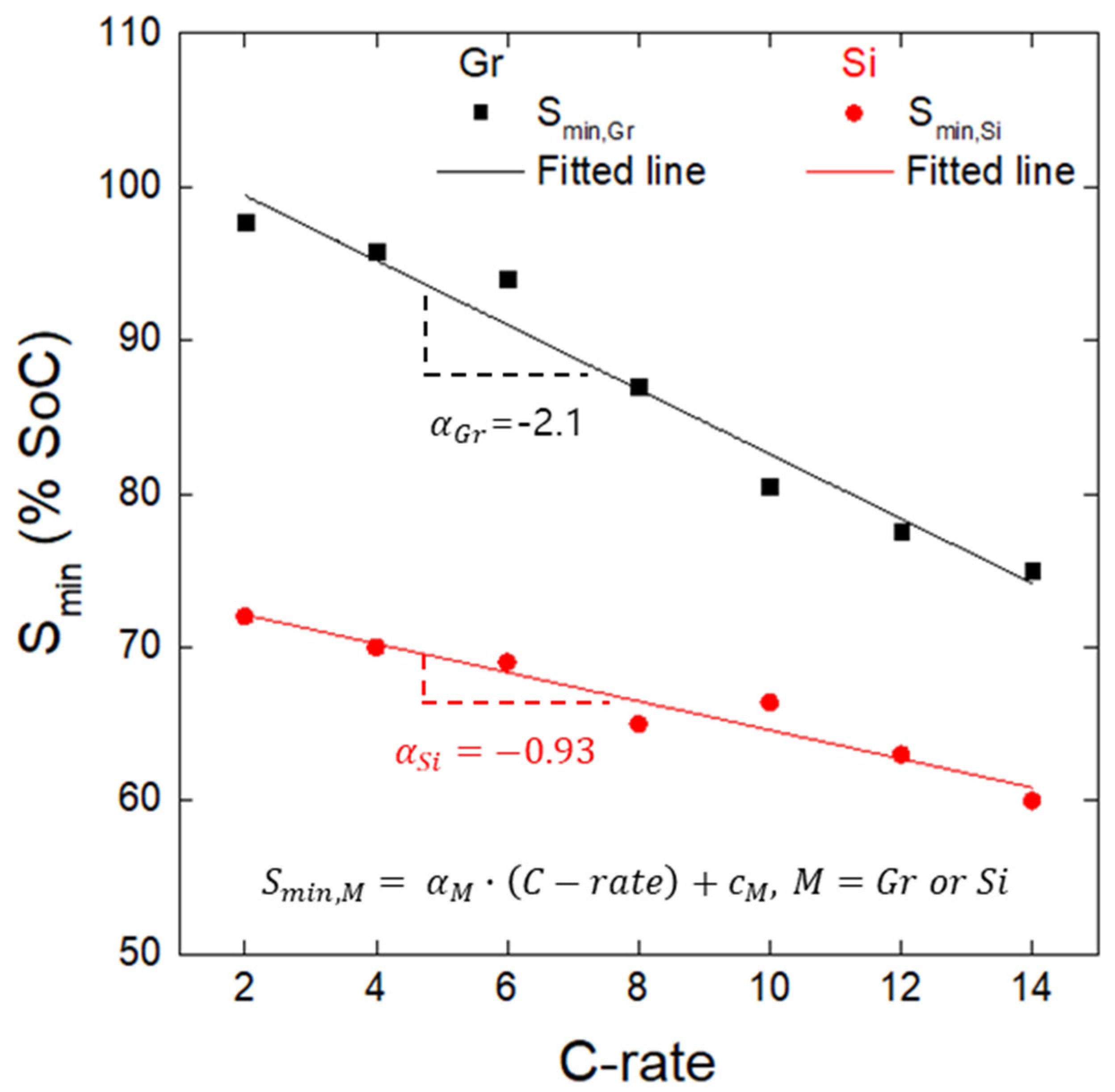
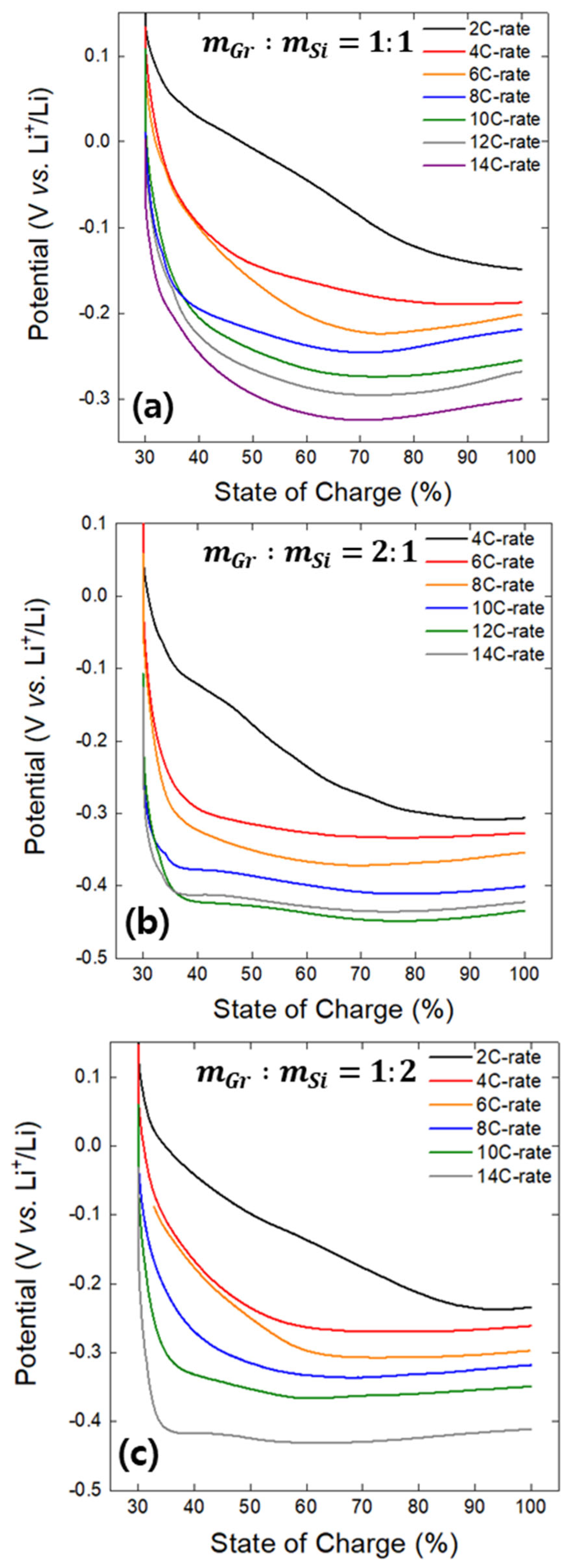
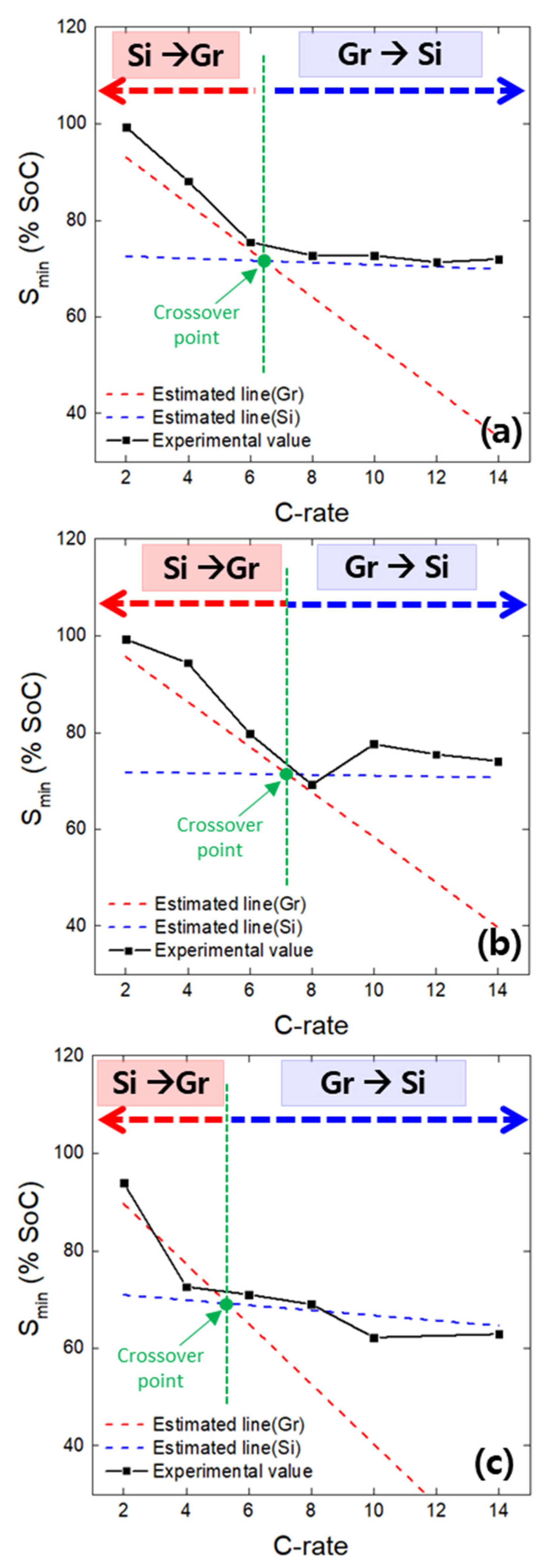
- The minima observed in the charge potential curves of the Gr and Si single electrodes were designated as the onset SoC for lithium plating (Smin). The appearance of inflection points on the open-circuit potential curves of both the Gr and Si electrodes, charged beyond the Smin value, indirectly confirmed the presence of lithium plating on the electrode surfaces. It was confirmed that Smin has a linear relationship with the charging rate (C-rate), and, based on this, a linear empirical equation between Smin and the C-rate was derived.
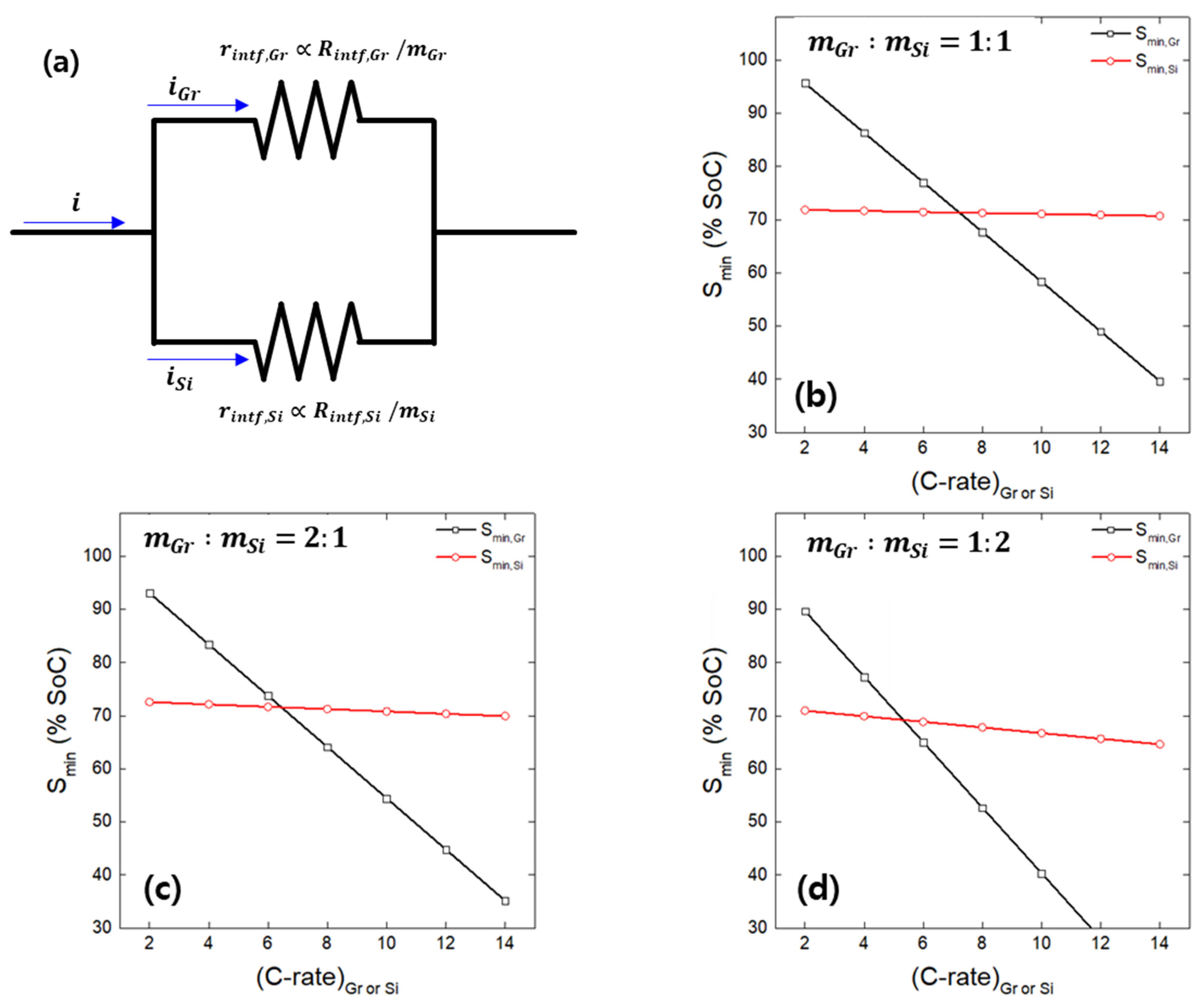
- Based on the interfacial resistances of Gr and Si, the Gr–Si ratio in the composite electrode, and the linear relationship between Smin and the C-rate, the lithium plating behavior on Gr and Si in the hypothetical Gr–Si composite electrode was estimated. Within the charging rate range used in the study (2 to 14 C-rate), it was observed that the Smin values for Gr and Si intersected as the C-rate increased. This suggests that, at a specific C-rate (the crossover point), the active material undergoing preferential lithium plating changes.
- An analysis of the variation in Smin with the C-rate using actual Gr–Si composite electrodes with varying Gr–Si ratios revealed a transition in the lithium plating behavior: Smin followed that of Gr at low rates and Si at high rates, with the crossover point indicating the shift in plating behavior. This unusual behavior observed in the composite electrode is attributed to rate-dependent lithium reaction pathways: at low rates, there is simultaneous lithium plating on Si and lithium insertion into Gr; at high rates, there is simultaneous lithium plating on Gr and lithium alloying with Si. Furthermore, an analysis of the potential curve minima of composite electrodes as a function of the charge C-rate revealed that the onset of lithium plating on Si and Gr can be estimated.
- The observed differences in the lithium plating behavior between Gr and Si are likely influenced by their intrinsic properties. At low C-rates, Gr may tend to favor earlier lithium plating, which could be related to its high electrical conductivity facilitating efficient electron transport during slower lithiation. In contrast, at higher C-rates, Si might become more prone to lithium plating, possibly due to its lower lithium diffusivity, which limits the rate at which lithium ions can diffuse into the material, leading to lithium accumulation and plating on the surface.
- This study provides valuable insights into the C-rate-dependent lithium plating of Gr–Si composite anodes by interpreting it through validated electrochemical signals. However, surface characterization and elemental analysis were not conducted in this work. Incorporating these techniques in future studies could offer a more comprehensive understanding of the electrode’s physicochemical state under fast-charging conditions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, X.; Wang, H.; Wang, X.; Gao, Y.; Allu, S.; Cakmak, E.; Wang, Z. Internal short circuit and failure mechanisms of lithium-ion pouch cells under mechanical indentation abuse conditions: An experimental study. J. Power Sources 2020, 455, 227939. [Google Scholar] [CrossRef]
- Du, J.; Ouyang, D. Progress of Chinese electric vehicles industrialization in 2015: A review. Appl. Energy 2017, 188, 529–546. [Google Scholar] [CrossRef]
- Nykvist, B.; Nilsson, M. Rapidly falling costs of battery packs for electric vehicles. Nat. Clim. Change 2015, 5, 329–332. [Google Scholar] [CrossRef]
- Kang, Y.; Deng, C.; Chen, Y.; Liu, X.; Liang, Z.; Li, T.; Hu, Q.; Zhao, Y. Molecular sieve-modified separator for high-performance lithium-ion batteries. Nanoscale Res. Lett. 2020, 15, 107. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Tian, J.; Ma, X.; Wang, B.; Li, W.; Wang, Q. Adhesive nanocomposites of hypergravity induced Co3O4 nanoparticles and natural gels as Li-ion battery anode materials with high capacitance and low resistance. RSC Adv. 2017, 7, 21061–21067. [Google Scholar] [CrossRef]
- Prior, A. Past, Present and Future; Oxford University Press: Oxford, UK, 1967. [Google Scholar]
- Liu, Z.; Yu, Q.; Zhao, Y.; He, R.; Xu, M.; Feng, S.; Li, S.; Zhou, L. Silicon oxides: A promising family of anode materials for lithium-ion batteries. Chem. Soc. Rev. 2019, 48, 285–309. [Google Scholar] [CrossRef]
- Kim, M.; Robertson, D.C.; Dees, D.W.; Yao, K.P.; Lu, W.; Trask, S.E.; Kirner, J.T.; Bloom, I. Estimating the diffusion coefficient of lithium in graphite: Extremely fast charging and a comparison of data analysis techniques. J. Electrochem. Soc. 2021, 168, 070506. [Google Scholar] [CrossRef]
- Son, S.B.; Trask, S.; Tsai, Y.; Lopykinski, S.; Kim, M.; Bloom, I. Across-Depth Heterogeneity and Irreversibility of Fast-Charge-Driven Lithium Plating. J. Electrochem. Soc. 2022, 169, 060506. [Google Scholar] [CrossRef]
- Kim, M.; Yang, Z.; Bloom, I. The lithiation/delithiation behavior of Si-based electrodes: A connection between electrochemistry and mechanics. J. Electrochem. Soc. 2021, 168, 010523. [Google Scholar] [CrossRef]
- Kim, M.; Yang, Z.; Son, S.B.; Trask, S.E.; Jansen, A.; Bloom, I. Effect of cathode on crosstalk in Si-based lithium-ion cells. J. Mater. Chem. A 2021, 9, 26904–26916. [Google Scholar] [CrossRef]
- Kim, M.; Yang, Z.; Trask, S.E.; Bloom, I. Understanding the effect of cathode composition on the interface and crosstalk in NMC/Si full cells. ACS Appl. Mater. Interfaces 2022, 14, 15103–15111. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhu, B.; Lu, Z.; Liu, N.; Zhu, J. Challenges and recent progress in the development of Si anodes for lithium-ion battery. Adv. Energy Mater. 2017, 7, 1700715. [Google Scholar] [CrossRef]
- He, Y.; Jiang, L.; Chen, T.; Xu, Y.; Jia, H.; Yi, R.; Xue, D.; Song, M.; Genc, A.; Bouchet-Marquis, C.; et al. Progressive growth of the solid–electrolyte interphase towards the Si anode interior causes capacity fading. Nat. Nanotechnol. 2021, 16, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Kim, N.; Lee, T.; Lee, Y.; Ma, J.; Chae, S.; Sung, J.; Cha, H.; Yoo, Y.; Cho, J. Calendering-compatible macroporous architecture for silicon–graphite composite toward high-energy lithium-ion batteries. Adv. Mater. 2020, 32, 2003286. [Google Scholar] [CrossRef]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef]
- Choi, J.W.; Cui, Y.; Nix, W.D. Size-dependent fracture of Si nanowire battery anodes. J. Mech. Phys. Solids 2011, 59, 1717–1730. [Google Scholar]
- Ruffo, R.; Hong, S.S.; Chan, C.K.; Huggins, R.A.; Cui, Y. Impedance analysis of silicon nanowire lithium ion battery anodes. J. Phys. Chem. C 2009, 113, 11390–11398. [Google Scholar] [CrossRef]
- Song, T.; Xia, J.; Lee, J.-H.; Lee, D.H.; Kwon, M.-S.; Choi, J.-M.; Wu, J.; Doo, S.K.; Chang, H.; Park, W.I.; et al. Arrays of sealed silicon nanotubes as anodes for lithium ion batteries. Nano Lett. 2010, 10, 1710–1716. [Google Scholar] [CrossRef]
- Li, X.; Gu, M.; Hu, S.; Kennard, R.; Yan, P.; Chen, X.; Wang, C.; Sailor, M.J.; Zhang, J.-G.; Liu, J. Mesoporous silicon sponge as an anti-pulverization structure for high-performance lithium-ion battery anodes. Nat. Commun. 2014, 5, 4105. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, W.; Kang, W.; Ye, Y.; Yuan, Y.; Qiu, Z.; Wang, C.; Zhang, X.; Ke, Y. Silicon-nanoparticle-based composites for advanced lithium-ion battery anodes. Nanoscale 2020, 12, 7461–7484. [Google Scholar] [CrossRef]
- Gao, P.; Huang, X.; Zhao, Y.; Hu, X.; Cen, D.; Gao, G.; Bao, Z.; Mei, Y.; Di, Z.; Wu, G. Formation of Si hollow structures as promising anode materials through reduction of silica in AlCl3–NaCl molten salt. ACS Nano 2018, 12, 11481–11490. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; McDowell, M.T.; Ryu, I.; Wu, H.; Liu, N.; Hu, L.; Nix, W.D.; Cui, Y. Interconnected silicon hollow nanospheres for lithium-ion battery anodes with long cycle life. Nano Lett. 2011, 11, 2949–2954. [Google Scholar] [CrossRef]
- Shi, J.; Gao, H.; Hu, G.; Zhang, Q. Core-shell structured Si@C nanocomposite for high-performance Li-ion batteries with a highly viscous gel as precursor. J. Power Sources 2019, 438, 227001. [Google Scholar] [CrossRef]
- Dimov, N.; Kugino, S.; Yoshio, M. Carbon-coated silicon as anode material for lithium ion batteries: Advantages and limitations. Electrochim. Acta 2003, 48, 1579–1587. [Google Scholar] [CrossRef]
- Chen, D.; Mei, X.; Ji, G.; Lu, M.; Xie, J.; Lu, J.; Lee, J.Y. Reversible lithium-ion storage in silver-treated nanoscale hollow porous silicon particles. Angew. Chem. Int. Ed. 2012, 51, 2409–2413. [Google Scholar] [CrossRef]
- Murugesan, S.; Harris, J.T.; Korgel, B.A.; Stevenson, K.J. Copper-coated amorphous silicon particles as an anode material for lithium-ion batteries. Chem. Mater. 2012, 24, 1306–1315. [Google Scholar] [CrossRef]
- Kulova, T.L.; Skundin, A.M.; Pleskov, Y.V.; Terukov, E.I.; Kon’kov, O.I. Lithium insertion into amorphous silicon thin-film electrodes. J. Electroanal. Chem. 2007, 600, 217–225. [Google Scholar] [CrossRef]
- Li, L.; Fang, C.; Wei, W.; Zhang, L.; Ye, Z.; He, G.; Huang, Y. Nano-ordered structure regulation in delithiated Si anode triggered by homogeneous and stable Li-ion diffusion at the interface. Nano Energy 2020, 72, 104651. [Google Scholar] [CrossRef]
- Wang, G.; Xu, B.; Shi, J.; Wu, M.; Su, H.; Ouyang, C. New insights into Li diffusion in Li–Si alloys for Si anode materials: Role of Si microstructures. Nanoscale 2019, 11, 14042–14049. [Google Scholar] [CrossRef]
- Wu, J.; Cao, Y.; Zhao, H.; Mao, J.; Guo, Z. The critical role of carbon in marrying silicon and graphite anodes for high-energy lithium-ion batteries. Carbon Energy 2019, 1, 57–76. [Google Scholar] [CrossRef]
- Anothumakkool, B.; Holtstiege, F.; Wiemers-Meyer, S.; Nowak, S.; Schappacher, F. Electropolymerization triggered in situ surface modification of electrode interphases: Alleviating first-cycle lithium loss in silicon anode lithium-ion batteries. ACS Sustain. Chem. Eng. 2020, 8, 12788–12798. [Google Scholar] [CrossRef]
- Müller, J.; Abdollahifar, M.; Vinograd, A.; Nöske, M.; Nowak, C.; Chang, S.-J.; Placke, T.; Haselrieder, W.; Winter, M.; Kwade, A.; et al. Si-on-Graphite fabricated by fluidized bed process for high-capacity anodes of Li-ion batteries. Eng. J. 2020, 407, 126603. [Google Scholar] [CrossRef]
- Beaulieu, L.Y.; Hatchard, T.D.; Bonakdarpour, A.; Fleischauer, M.D.; Dahn, J.R. Reaction of Li with alloy thin films studied by in situ AFM. J. Electrochem. Soc. 2003, 150, A1457. [Google Scholar] [CrossRef]
- Sun, W.; Hu, R.; Liu, H.; Zhang, H.; Liu, J.; Yang, L.; Wang, H.; Zhu, M. Silicon/Wolfram Carbide@Graphene composite: Enhancing conductivity and structure stability in amorphous-silicon for high lithium storage performance. Electrochim. Acta 2016, 191, 462–472. [Google Scholar] [CrossRef]
- Li, J.; Dozier, A.K.; Li, Y.; Yang, F.; Cheng, Y.T. Crack pattern formation in thin film lithium-ion battery electrodes. J. Electrochem. Soc. 2011, 158, A689. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhong, L.; Huang, S.; Mao, S.X.; Zhu, T.; Huang, J.Y. Size-dependent fracture of silicon nanoparticles during lithiation. ACS Nano 2012, 6, 1522–1531. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zheng, J.; Zheng, H.; Mei, Z.; Du, X.; Li, H. Electrochemical performances and volume variation of nano-textured silicon thin films as anodes for lithium-ion batteries. Nanotechnology 2013, 24, 424011. [Google Scholar] [CrossRef]
- Wang, S.; Ren, P.; Takyi-Aninakwa, P.; Jin, S.; Fernandez, C. A critical review of improved deep convolutional neural network for multi-timescale state prediction of lithium-ion batteries. Energies 2022, 15, 5053. [Google Scholar] [CrossRef]
- Li, X.; Wu, M.; Feng, T.; Xu, Z.; Qin, J.; Chen, C.; Wang, D. Graphene enhanced silicon/carbon composite as anode for high performance lithium-ion batteries. RSC Adv. 2017, 7, 48286–48293. [Google Scholar] [CrossRef]
- Liu, R.; Shen, C.; Dong, Y.; Qin, J.; Wang, Q.; Iocozzia, J.; Lin, Z. Sandwich-like CNTs/Si/C nanotubes as high performance anode materials for lithium-ion batteries. J. Mater. Chem. A 2018, 6, 14797–14804. [Google Scholar] [CrossRef]
- Liu, N.; Lu, Z.; Zhao, J.; McDowell, M.T.; Lee, H.W.; Zhao, W.; Cui, Y. A pomegranate-inspired nanoscale design for large-volume-change lithium battery anodes. Nat. Nanotechnol. 2014, 9, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Bang, B.M.; Lee, J.I.; Kim, H.; Cho, J.; Park, S. High-performance macroporous bulk silicon anodes synthesized by template-free chemical etching. Adv. Energy Mater. 2012, 2, 878. [Google Scholar] [CrossRef]
- Jiménez, A.R.; Klöpsch, R.; Wagner, R.; Rodehorst, U.C.; Kolek, M.; Nölle, R.; Placke, T.; Winter, M. A step toward high-energy silicon-based thin film lithium ion batteries. ACS Nano 2017, 11, 4731–4744. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X.; Chen, S.; Zou, M.; Li, Z.; Cao, A.; Yuan, Q. Rational design of hierarchical carbon/mesoporous silicon composite sponges as high-performance flexible energy storage electrodes. ACS Appl. Mater. Interfaces 2017, 9, 22819–22825. [Google Scholar] [CrossRef]
- Heubner, C.; Nikolowski, K.; Reuber, S.; Schneider, M.; Wolter, M.; Michaelis, A. Recent insights into rate performance limitations of Li-ion batteries. Batter. Supercaps 2020, 4, 268–285. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Ren, D.; Wang, L.; He, X. Graphite as anode materials: Fundamental mechanism, recent progress and advances. Energy Storage Mater. 2021, 36, 147–170. [Google Scholar] [CrossRef]
- Gao, B.; Sinha, S.; Fleming, L.; Zhou, O. Alloy formation in nanostructured silicon. Adv. Mater. 2001, 13, 816–819. [Google Scholar] [CrossRef]
- Li, H.; Huang, X.; Chen, L.; Zhou, G.; Zhang, Z.; Yu, D.; Pei, N. The crystal structural evolution of nano-Si anode caused by lithium insertion and extraction at room temperature. Solid State Ion. 2000, 135, 181–191. [Google Scholar] [CrossRef]
- Limthongkul, P.; Jang, Y.I.; Dudney, N.J.; Chiang, Y.M. Electrochemically-driven solid-state amorphization in lithium-silicon alloys and implications for lithium storage. Acta Mater. 2003, 51, 1103–1113. [Google Scholar] [CrossRef]
- Yu, P.; Popov, B.N.; Ritter, J.A.; White, R.E. Lithium Diffusion in Graphite Carbon and Implications for the Rate Capability of Anodes. J. Electrochem. Soc. 1999, 146, 8–13. [Google Scholar] [CrossRef]
- Ding, N.; Xu, J.; Yao, Y.X.; Wegner, G.; Fang, X.; Chen, C.H.; Lieberwirth, I. Determination of the diffusion coefficient of lithium ions in nano-Si. Solid State Ion. 2009, 180, 222–225. [Google Scholar] [CrossRef]
- Liu, G.; Wang, D.; Zhang, J.; Kim, A.; Lu, W. Preventing dendrite growth by a soft piezoelectric material. ACS Mater. Lett. 2019, 1, 498–505. [Google Scholar] [CrossRef]
- Sagane, F.; Shimokawa, R.; Sano, H.; Sakaebe, H.; Iriyama, Y. In-situ scanning electron microscopy observations of Li plating and stripping reactions at the lithium phosphorus oxynitride glass electrolyte/Cu interface. J. Power Sources 2013, 225, 245–250. [Google Scholar] [CrossRef]
- Gireaud, L.; Grugeon, S.; Laruelle, S.; Yrieix, B.; Tarascon, J.M. Lithium metal stripping/plating mechanisms studies: A metallurgical approach. Electrochem. Commun. 2006, 8, 1639–1645. [Google Scholar] [CrossRef]
- Flügel, M.; Richter, K.; Wohlfahrt-Mehrens, M.; Waldmann, T. Detection of Li deposition on Si/Graphite anodes from commercial Li-ion cells: A post-mortem GD-OES depth profiling study. J. Electrochem. Soc. 2022, 169, 050533. [Google Scholar] [CrossRef]
- Chang, H.J.; Trease, N.M.; Ilott, A.J.; Zeng, D.; Du, L.S.; Jerschow, A.; Grey, C.P. Investigating Li microstructure formation on Li anodes for lithium batteries by in situ 6Li/7Li NMR and SEM. J. Phys. Chem. C 2015, 119, 16443–16451. [Google Scholar] [CrossRef]
- Leskes, M.; Kim, G.; Liu, T.; Michan, A.L.; Aussenac, F.; Dorffer, P.; Paul, S.; Grey, C.P. Surface-sensitive NMR detection of the solid electrolyte interphase layer on reduced graphene oxide. J. Phys. Chem. Lett. 2017, 8, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Flügel, M.; Bolsinger, M.; Marinaro, M.; Knoblauch, V.; Hölzle, M.; Wohlfahrt-Mehrens, M.; Waldmann, T. Onset shift of Li plating on Si/Graphite anodes with increasing Si content. J. Electrochem. Soc. 2023, 170, 060536. [Google Scholar] [CrossRef]
- Koleti, U.R.; Zhang, C.; Dinh, T.Q.; Marco, J.; Amietszajew, T.; Malik, R. A new concept to improve the lithium plating detection sensitivity in lithium-ion batteries. Int. J. Smart Grid Clean Energy 2019, 8, 505–516. [Google Scholar] [CrossRef]
- Schindler, S.; Bauer, M.; Petzl, M.; Danzer, M.A. Voltage relaxation and impedance spectroscopy as in-operando methods for the detection of lithium plating on graphitic anodes in commercial lithium-ion cells. J. Power Sources 2016, 304, 170–180. [Google Scholar] [CrossRef]
- Brown, D.E.; McShane, E.J.; Konz, Z.M.; Knudsen, K.B.; McCloskey, B.D. Detecting onset of lithium plating during fast charging of Li-ion batteries using operando electrochemical impedance spectroscopy. Cell Rep. Phys. Sci. 2021, 2, 100589. [Google Scholar] [CrossRef]
- von Lüders, C.; Zinth, V.; Erhard, S.V.; Osswald, P.J.; Hofmann, M.; Gilles, R.; Jossen, A. Lithium plating in lithium-ion batteries investigated by voltage relaxation and in situ neutron diffraction. J. Power Sources 2017, 342, 17–23. [Google Scholar] [CrossRef]
- Zinth, V.; von Lüders, C.; Hofmann, M.; Hattendorff, J.; Buchberger, I.; Erhard, S.; Gilles, R. Lithium plating in lithium-ion batteries at sub-ambient temperatures investigated by in situ neutron diffraction. J. Power Sources 2014, 271, 152–159. [Google Scholar] [CrossRef]
- Senyshyn, A.; Mühlbauer, M.J.; Dolotko, O.; Ehrenberg, H. Low-temperature performance of Li-ion batteries: The behavior of lithiated graphite. J. Power Sources 2015, 282, 235–240. [Google Scholar] [CrossRef]
- II, R.E.G.; Klingler, R.J.; Sandí, G.; Johnson, C.S.; Scanlon, L.G.; Rathke, J.W. 7Li NMR study of intercalated lithium in curved carbon lattices. J. Power Sources 2000, 89, 237–243. [Google Scholar]
- Hsieh, Y.-C.; Leißing, M.; Nowak, S.; Hwang, B.-J.; Winter, M.; Brunklaus, G. Quantification of dead lithium via in situ nuclear magnetic resonance spectroscopy. Cell Rep. Phys. Sci. 2020, 1, 100139. [Google Scholar] [CrossRef]
- Wandt, J.; Marino, C.; Gasteiger, H.A.; Jakes, P.; Eichel, R.A.; Granwehr, J. Operando electron paramagnetic resonance spectroscopy–formation of mossy lithium on lithium anodes during charge–discharge cycling. Energy Environ. Sci. 2015, 8, 1358–1368. [Google Scholar] [CrossRef]
- Petzl, M.; Danzer, M.A. Nondestructive detection, characterization, and quantification of lithium plating in commercial lithium-ion batteries. J. Power Sources 2014, 254, 80–87. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Liu, S.; Sun, Y. Revealing the overlithiation effect on cycling and calendar aging of a silicon/graphite electrode for high-energy lithium-ion batteries. Chem. Sci. 2024, 15, 17979–17987. [Google Scholar] [CrossRef]
- Waldmann, T.; Wohlfahrt-Mehrens, M. Effects of rest time after Li plating on safety behavior—ARC tests with commercial high-energy 18650 Li-ion cells. Electrochim. Acta 2017, 230, 454–460. [Google Scholar] [CrossRef]
- Tao, J.; Liu, L.; Han, J.; Peng, J.; Chen, Y.; Yang, Y.; Lin, Y. New perspectives on spatial dynamics of lithiation and lithium plating in graphite/silicon composite anodes. Energy Storage Mater. 2023, 60, 102809. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, Y.; Sun, Q.; Yang, R.; Zhang, Q.; Xu, Y. Fundamental understanding and facing challenges in structural design of porous Si-based anodes for lithium-ion batteries. Adv. Funct. Mater. 2023, 33, 2301109. [Google Scholar] [CrossRef]
- Chen, Y.; Torres-Castro, L.; Chen, K.H.; Penley, D.; Lamb, J.; Karulkar, M.; Dasgupta, N.P. Operando detection of Li plating during fast charging of Li-ion batteries using incremental capacity analysis. J. Power Sources 2022, 539, 231601. [Google Scholar] [CrossRef]
- Arora, P.; Doyle, M.; White, R.E. Mathematical modeling of the lithium deposition overcharge reaction in lithium-ion batteries using carbon-based negative electrodes. J. Electrochem. Soc. 1999, 146, 3543–3553. [Google Scholar] [CrossRef]
- Chang, H.J.; Ilott, A.J.; Trease, N.M.; Mohammadi, M.; Jerschow, A.; Grey, C.P. Correlating microstructural lithium metal growth with electrolyte salt depletion in lithium batteries using 7Li MRI. J. Am. Chem. Soc. 2015, 137, 15209–15216. [Google Scholar] [CrossRef]
- Yang, X.G.; Ge, S.; Liu, T.; Leng, Y.; Wang, C.Y. A look into the voltage plateau signal for detection and quantification of lithium plating in lithium-ion cells. J. Power Sources 2018, 395, 251–261. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Christensen, L. Structural changes in silicon anodes during lithium insertion/extraction. Electrochem. Solid-State Lett. 2004, 7, A93–A96. [Google Scholar] [CrossRef]
- Song, W.; Chae, O.B. Surface-Coating Strategies of Si-Negative Electrode Materials in Lithium-Ion Batteries. Batteries 2024, 10, 327. [Google Scholar] [CrossRef]
- McDowell, M.T.; Lee, S.W.; Nix, W.D.; Cui, Y. Understanding the lithiation of silicon and other alloying anodes for lithium-ion batteries. Adv. Mater. 2013, 25, 4966–4985. [Google Scholar] [CrossRef]
- Sitinamaluwa, H.S.; Lee, M.V.; Lee, H.W.; Chen, Y.; Dasgupta, N.P. Lithium concentration dependent structure and mechanics of amorphous silicon. J. Appl. Phys. 2016, 119, 245102. [Google Scholar] [CrossRef]
- Wang, X.; Lee, H.; Chen, Y.; Dasgupta, N.P. High damage tolerance of electrochemically lithiated silicon. Nat. Commun. 2015, 6, 8417. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Liu, Z.; Lv, X.; Zhou, G.; Yin, J. Electrochemical studies of LiB compound as anode material for lithium-ion battery. Electrochim. Acta 2006, 51, 5731–5737. [Google Scholar] [CrossRef]
- Morita, T.; Takami, N. Characterization of oxidized boron-doped carbon fiber anodes for Li-ion batteries by analysis of heat of immersion. Electrochim. Acta 2004, 49, 2591–2599. [Google Scholar] [CrossRef]
- Funabiki, A.; Inaba, M.; Ogumi, Z. A.c. impedance analysis of electrochemical lithium intercalation into highly oriented pyrolytic graphite. J. Power Sources 1997, 68, 227–231. [Google Scholar] [CrossRef]
- Li, J.; Murphy, E.; Winnick, J.; Kohl, P.A. Studies on the cycle life of commercial lithium ion batteries during rapid charge–discharge cycling. J. Power Sources 2001, 102, 294–301. [Google Scholar] [CrossRef]
- Tanim, T.R.; Dufek, E.J.; Dickerson, C.C.; Wood, S.M. Electrochemical quantification of lithium plating: Challenges and considerations. J. Electrochem. Soc. 2019, 166, A2689–A2696. [Google Scholar] [CrossRef]
- Zhu, Y.; Yan, L.; Han, S.; Gu, M.; Ma, S.; Luo, Y.; Meng, A.; Zhao, C.; Xie, J.; Yang, C.; et al. In situ lithium plating monitor for lithium-ion batteries enabled by operando pressure detection. J. Power Sources 2025, 631, 236281. [Google Scholar] [CrossRef]


| (Ω) | (Ω) | (Ω) | = + (Ω) | Chi-Squared (χ2) | |
|---|---|---|---|---|---|
| Gr | 1.7 | 2.17 | 3.96 | 6.13 | 5.87 10−5 |
| Si | 2.6 | 3.2 | 21.6 | 24.8 | 2.27 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, M.; Ha, S.-H.; Jeong, J.; Shin, H.-C. Selective Lithium Plating on Graphite–Silicon Composite Anodes During Fast Charging in Rechargeable Lithium Batteries. Energies 2025, 18, 3423. https://doi.org/10.3390/en18133423
Park M, Ha S-H, Jeong J, Shin H-C. Selective Lithium Plating on Graphite–Silicon Composite Anodes During Fast Charging in Rechargeable Lithium Batteries. Energies. 2025; 18(13):3423. https://doi.org/10.3390/en18133423
Chicago/Turabian StylePark, Minkyu, Seong-Hyeok Ha, Jiung Jeong, and Heon-Cheol Shin. 2025. "Selective Lithium Plating on Graphite–Silicon Composite Anodes During Fast Charging in Rechargeable Lithium Batteries" Energies 18, no. 13: 3423. https://doi.org/10.3390/en18133423
APA StylePark, M., Ha, S.-H., Jeong, J., & Shin, H.-C. (2025). Selective Lithium Plating on Graphite–Silicon Composite Anodes During Fast Charging in Rechargeable Lithium Batteries. Energies, 18(13), 3423. https://doi.org/10.3390/en18133423





