Design of Cobalt-Free Ni-Rich Cathodes for High-Performance Sodium-Ion Batteries Using Electrochemical Li+/Na+ Exchange
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Material Characterization
2.3. Electrochemical Measurements
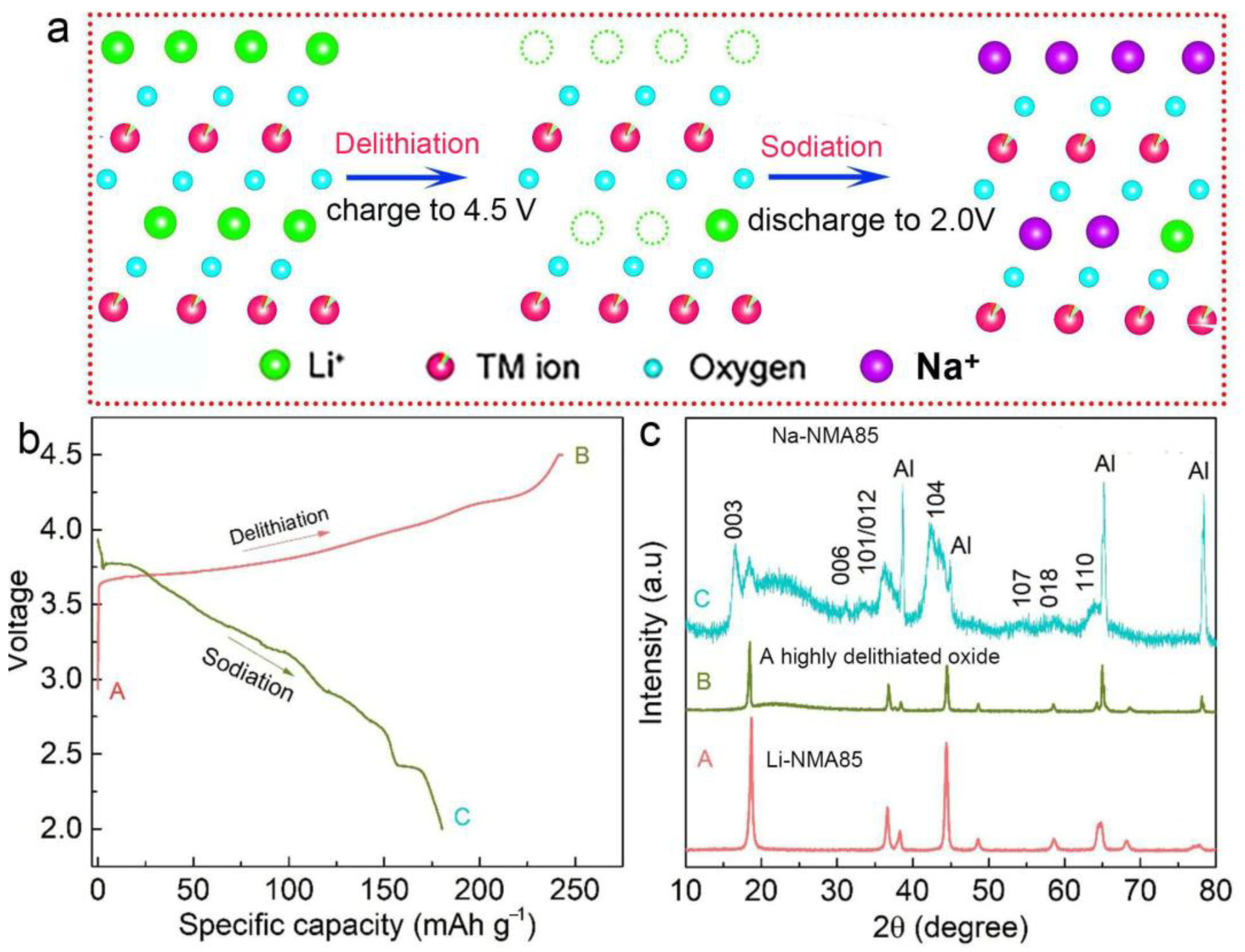
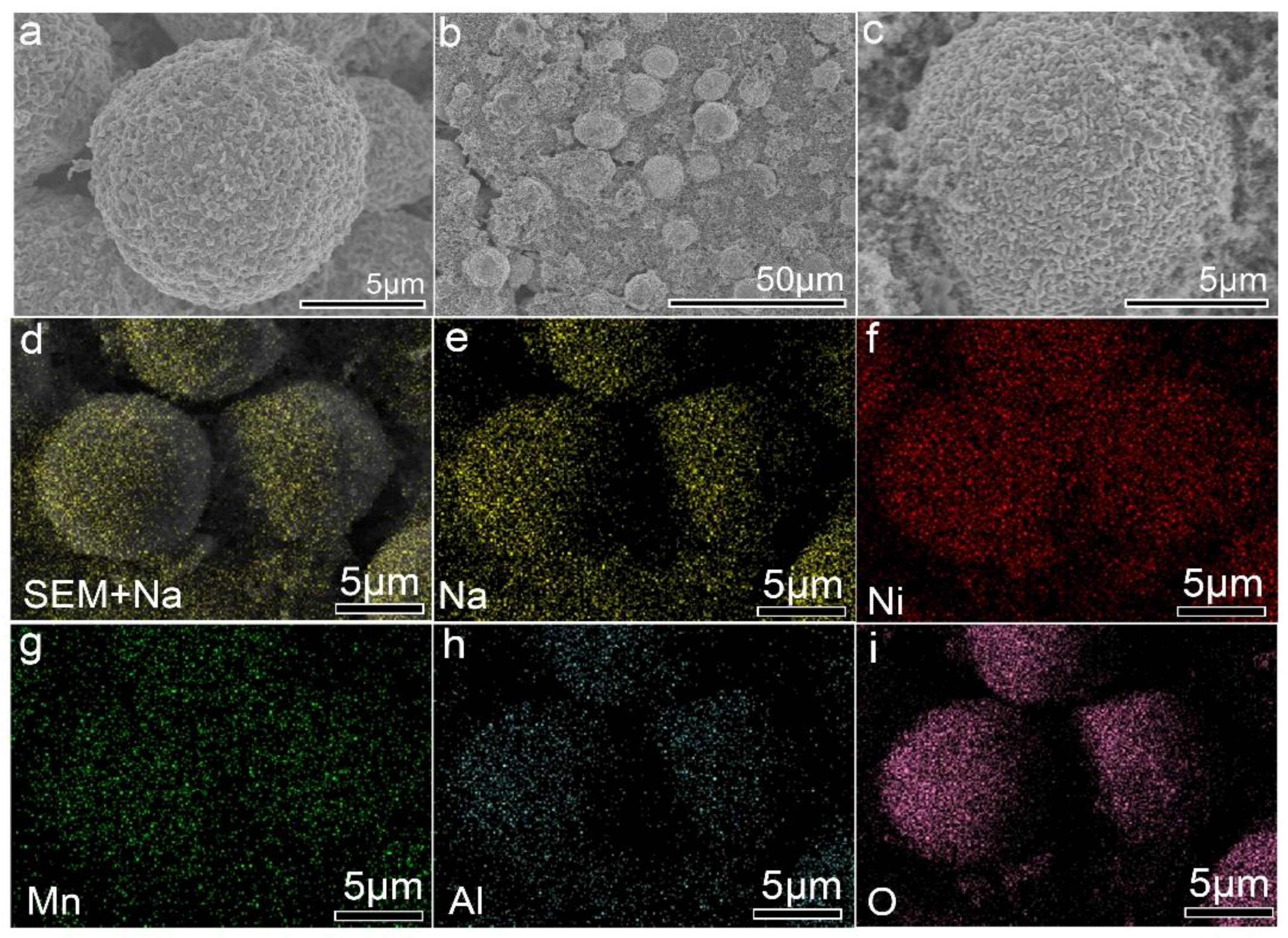
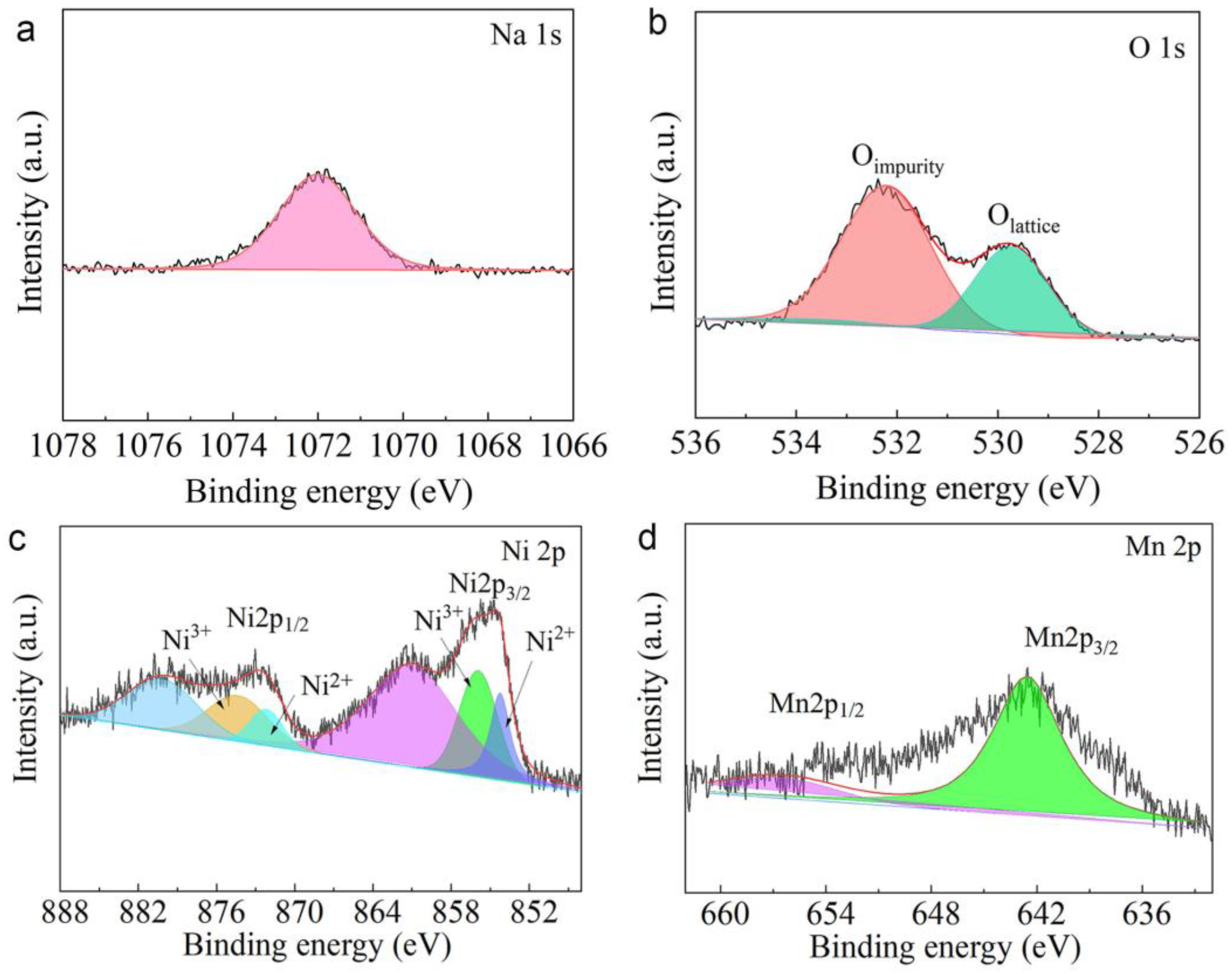
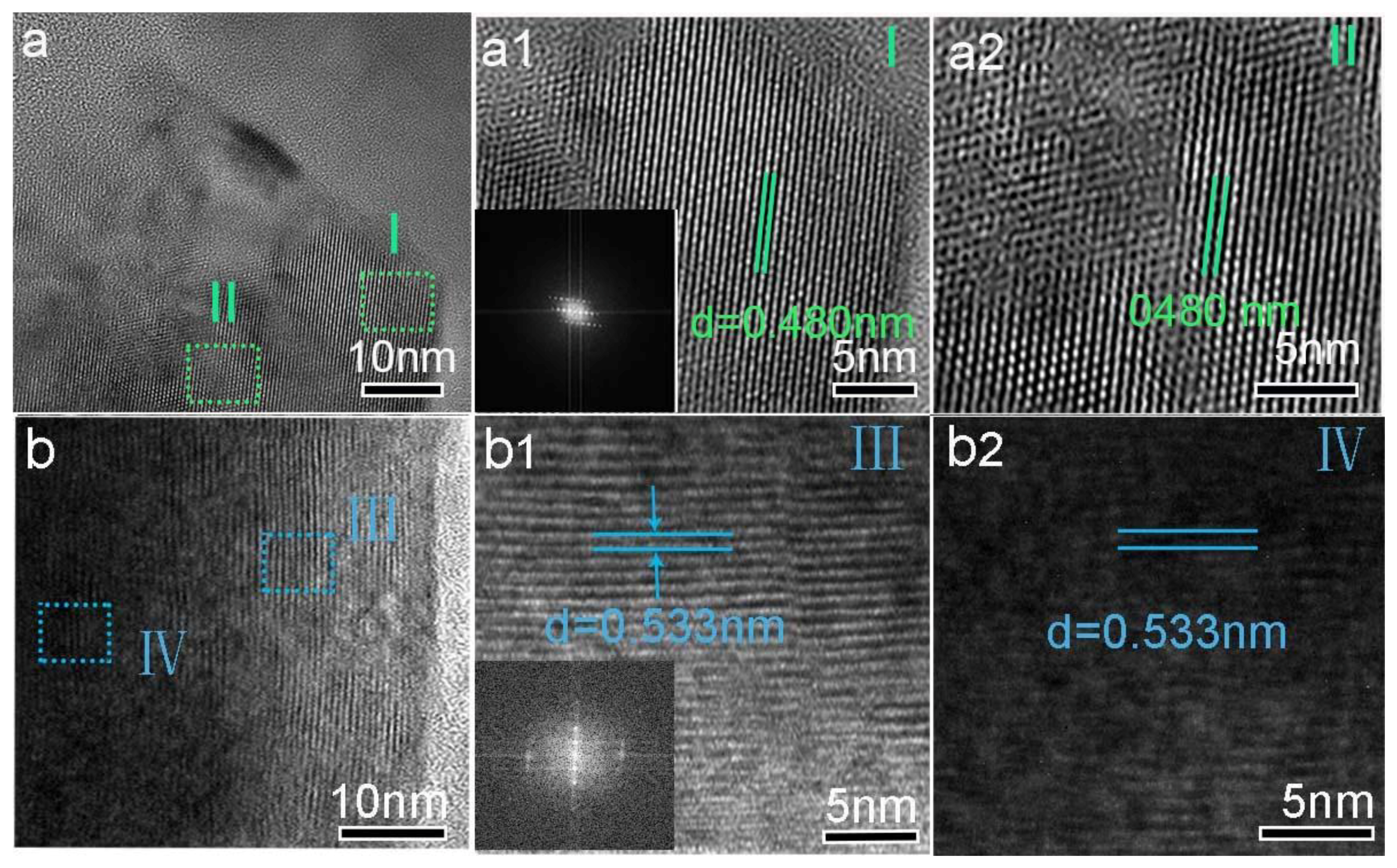
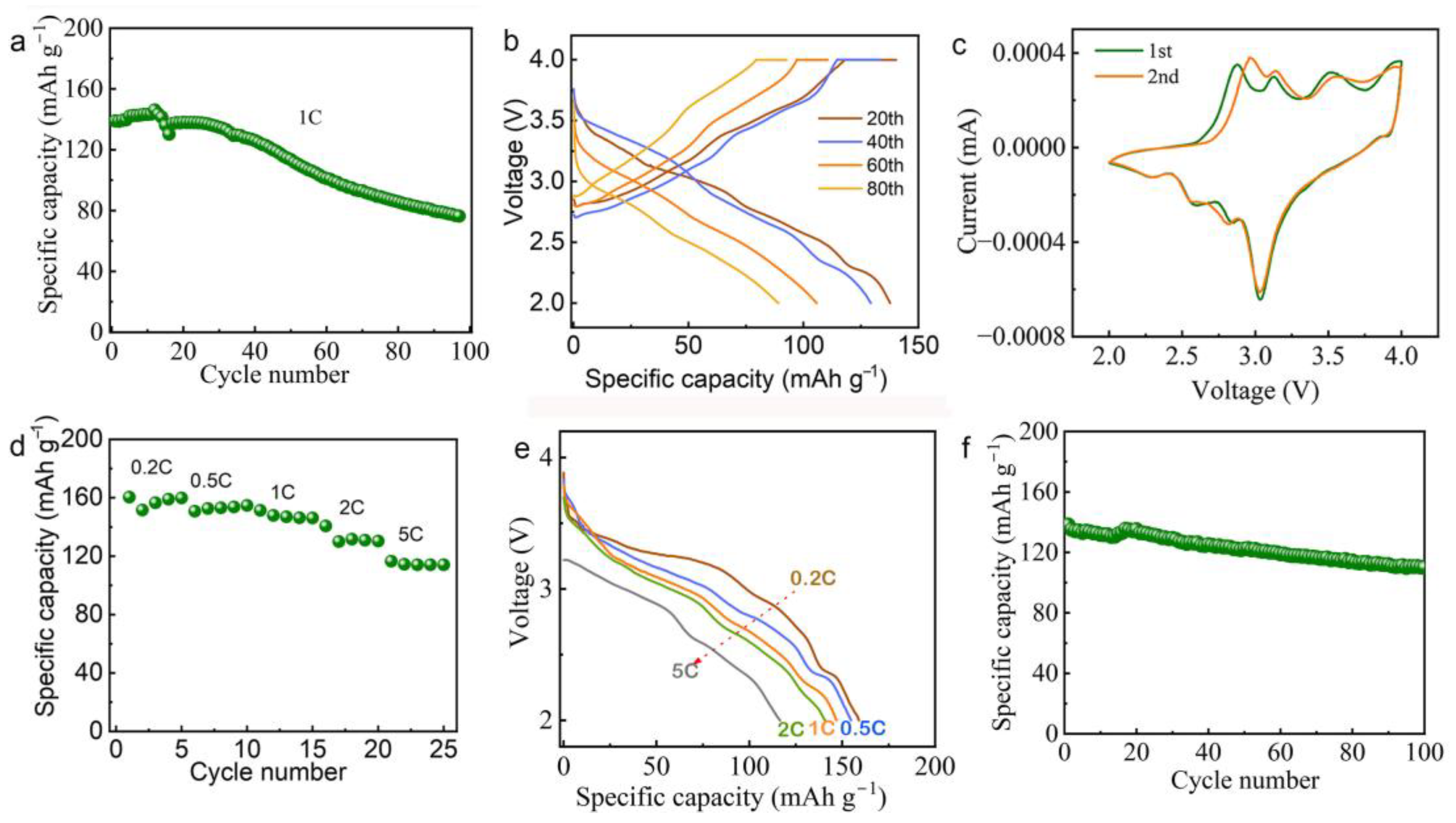
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, F.N.U.; Rasul, M.G.; Sayem, A.S.M.; Mandal, N.K. Design and optimization of lithium-ion battery as an efficient energy storage device for electric vehicles: A comprehensive review. J. Energy Storage 2023, 71, 108033. [Google Scholar] [CrossRef]
- Lv, Y.; Huang, S.; Zhao, Y.; Roy, S.; Lu, X.; Hou, Y.; Zhang, J. A review of nickel-rich layered oxide cathodes: Synthetic strategies, structural characteristics, failure mechanism, improvement approaches and prospects. Appl. Energy 2022, 305, 117849. [Google Scholar] [CrossRef]
- Pu, X.; Wang, H.; Zhao, D.; Yang, H.; Ai, X.; Cao, S.; Chen, Z.; Cao, Y. Recent progress in rechargeable sodium-ion batteries: Toward high-power applications. Small 2019, 15, 1805427. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.S.A.; Nujud Badawi, M.; Liew, J.; Prasankumar, T.; Ramesh, K.; Ramesh, S.; Ramesh, S.; Tiong, S.K. High-performance sodium-ion batteries with graphene: An overview of recent developments and design. ChemSusChem 2025, 18, e202400958. [Google Scholar] [CrossRef]
- Gao, R.M.; Zheng, Z.J.; Wang, P.F.; Wang, C.Y.; Ye, H.; Cao, F.F. Recent advances and prospects of layered transition metal oxide cathodes for sodium-ion batteries. Energy Storage Mater. 2020, 30, 9–26. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.H.; Ma, J.; Zhao, J.; Li, X.; Cui, G. Challenges and strategies toward practical application of layered transition metal oxide cathodes for sodium-ion batteries. Chem. Mater. 2023, 36, 54–73. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, E.; Sumboja, A.; Anggraningrum, I.T.; Syahrial, A.Z.; Yan, Q. Layered 3d transition metal-based oxides for sodium-ion and lithium-ion batteries: Differences, links and beyond. Adv. Funct. Mater. 2025, 35, 2413078. [Google Scholar] [CrossRef]
- Deng, H.; Liu, L.; Shi, Z. Effect of copper substitution on the electrochemical properties of high entropy layered oxides cathode materials for sodium-ion batteries. Mater. Lett. 2023, 340, 134113. [Google Scholar] [CrossRef]
- Li, J.Y.; Hu, H.Y.; Zhou, L.F.; Li, H.W.; Lei, Y.J.; Lai, W.H.; Fan, Y.M.; Zhu, Y.F.; Peleckis, G.; Chen, S.Q.; et al. Surface lattice-matched engineering based on in situ spinel interfacial reconstruction for stable heterostructured sodium layered oxide cathodes. Adv. Funct. Mater. 2023, 33, 2213215. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Q.; Zhao, X.; Mu, D.; Tan, G.; Li, L.; Chen, R.; Wu, F. Structure evolution of layered transition metal oxide cathode materials for Na-ion batteries: Issues, mechanism and strategies. Mater. Today 2023, 62, 271–295. [Google Scholar] [CrossRef]
- Lin, C.; Meng, X.; Liang, M.; Li, W.; Liang, J.; Liu, T.; Ke, X.; Liu, J.; Shi, Z.; Liu, L. Facilitating reversible transition metal migration and expediting ion diffusivity via oxygen vacancies for high performance O3-type sodium layered oxide cathodes. J. Mater. Chem. A 2023, 11, 68–76. [Google Scholar] [CrossRef]
- Liu, G.; Xu, W.; Wu, J.; Li, Y.; Chen, L.; Li, S.; Ren, Q.; Wang, J. Unlocking high-rate O3 layered oxide cathode for Na-ion batteries via ion migration path modulation. J. Energy Chem. 2023, 83, 53–61. [Google Scholar] [CrossRef]
- Wu, L.R.; Zhang, Y.H.; Wu, Z.; Tian, J.; Wang, H.; Zhao, H.; Xu, S.; Chen, L.; Duan, X.; Zhang, D.; et al. Stabilized O3-type layered sodium oxides with enhanced rate performance and cycling stability by dual-Site Ti4+/K+ Substitution. Adv. Sci. 2023, 10, 2304067. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, S.; Jie, Y.; Lei, Z.; Cao, R.; Jiao, S. Synthesis and electrochemical investigation of O3 type high-nickel NCM cathodes for sodium-ion batteries. Chem. Res. Chin. Univ. 2021, 37, 280–285. [Google Scholar] [CrossRef]
- Ding, F.; Zhao, C.; Zhou, D.; Meng, Q.; Xiao, D.; Zhang, Q.; Niu, Y.; Li, Y.; Rong, X.; Lu, Y.; et al. A novel Ni-rich O3-Na[Ni0.60Fe0.25Mn0.15]O2 cathode for Na-ion batteries. Energy Storage Mater. 2020, 30, 420–430. [Google Scholar] [CrossRef]
- Yang, J.; Tang, M.; Liu, H.; Chen, X.; Xu, Z.; Huang, J.; Su, Q.; Xia, Y. O3-type layered Ni-rich oxide: A high-capacity and superior-rate cathode for sodium-ion batteries. Small 2019, 15, 1905311. [Google Scholar] [CrossRef]
- Li, J.; Yang, H.; Deng, Q.; Li, W.; Zhang, Q.; Zhang, Z.; Chu, Y.; Yang, C. Stabilizing ni-rich single-crystalline LiNi0.83Co0.07Mn0.10O2 cathodes using Ce/Gd co-doped high-entropy composite surfaces. Angew. Chem. Int. Ed. 2024, 63, e202318042. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, C.; Yuan, F.; Lyu, M.; Yang, P.; Zhang, L.; Zhou, M.; Wang, L.; Zhang, S.; Wang, L. Ni-rich cathode materials for stable high-energy lithium-ion batteries. Nano Energy 2024, 126, 109620. [Google Scholar] [CrossRef]
- Li, W.; Reimers, J.; Dahn, J. In situ x-ray diffraction and electrochemical studies of Li1-xNiO2. Solid State Ion. 1993, 67, 123–130. [Google Scholar] [CrossRef]
- Park, H.; Min, K.; Park, K. A Synergistic effect of Na+ and Al3+ dual doping on electrochemical performance and structural stability of LiNi0.88Co0.08Mn0.04O2 cathodes for Li-ion batteries. ACS Appl. Mater. Interfaces 2022, 14, 5168–5176. [Google Scholar] [CrossRef]
- Qiu, H.; Zhang, R.; Zhang, Y. Na+ lattice doping induces oxygen vacancies to achieve high capacity and mitigate voltage decay of Li-rich cathodes. Int. J. Mol. Sci. 2023, 24, 8035. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Yang, Z.; Tao, L.; Waters, C.K.; Xu, Z.; Li, L.; Sainio, S.; Du, Y.; Xin, H.L.; Nordlund, D.; et al. The sensitive surface chemistry of Co-free, Ni-rich layered oxides: Identifying experimental conditions that influence characterization results. J. Mater. Chem. 2020, 8, 17487–17497. [Google Scholar] [CrossRef]
- Huang, B.; Liu, D.; Qian, K.; Zhang, L.; Zhou, K.; Liu, Y.; Kang, F.; Li, B. A simple method for the complete performance recovery of degraded Ni-rich LiNi0.70Co0.15Mn0.15O2 cathode via surface reconstruction. ACS Appl. Mater. Interfaces 2019, 11, 14076–14084. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Nie, Y.; Miao, C.; Tan, Y.; Wen, M.; Xiao, W. Enhanced electrochemical properties of Ni-rich layered cathode materials via Mg2+and Ti4+ co-doping for lithium-ion batteries. J. Colloid Interface Sci. 2021, 601, 853–862. [Google Scholar] [CrossRef]
- Lv, Y.; Huang, S.; Lu, S.; Ding, W.; Yu, X.; Liang, G.; Zou, J.; Kang, F.; Zhang, J.; Cao, Y. B2O3/LiBO2 dual-modification layer stabilized ni-rich cathode for lithium-ion battery. J. Power Sources 2022, 536, 231510. [Google Scholar] [CrossRef]
- Lv, Y.; Huang, S.; Zhang, J.; Kang, G.; Liu, Y.; Li, N.; Liang, Y.; Zhong, X.; Jia, T.; Ouyang, Y.; et al. Antimony doping enabled radially aligned microstructure in LiNi0.91Co0.06Al0.03O2 cathode for high-voltage and low-temperature lithium battery. Adv. Funct. Mater. 2024, 34, 2312284. [Google Scholar] [CrossRef]
- Xu, M.; Gammaitoni, G.; Häfner, M.; Villalobos-Portillo, E.; Marini, C.; Bianchini, M. Understanding and optimizing Li substitution in P2-type Sodium Layered Oxides for Sodium-Ion Batteries. Adv. Funct. Mater. 2024, 2425499. [Google Scholar] [CrossRef]
- Hu, W.; Peng, Y.; Wei, Y.; Yang, Y. Application of electrochemical impedance spectroscopy to degradation and aging research of lithium-ion batteries. J. Phys. Chem. C 2023, 127, 4465–4495. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Y.; Shi, L.; Yu, J.; Huang, S. Design of Cobalt-Free Ni-Rich Cathodes for High-Performance Sodium-Ion Batteries Using Electrochemical Li+/Na+ Exchange. Energies 2025, 18, 3205. https://doi.org/10.3390/en18123205
Lv Y, Shi L, Yu J, Huang S. Design of Cobalt-Free Ni-Rich Cathodes for High-Performance Sodium-Ion Batteries Using Electrochemical Li+/Na+ Exchange. Energies. 2025; 18(12):3205. https://doi.org/10.3390/en18123205
Chicago/Turabian StyleLv, Yao, Liqiu Shi, Jianfeng Yu, and Shifei Huang. 2025. "Design of Cobalt-Free Ni-Rich Cathodes for High-Performance Sodium-Ion Batteries Using Electrochemical Li+/Na+ Exchange" Energies 18, no. 12: 3205. https://doi.org/10.3390/en18123205
APA StyleLv, Y., Shi, L., Yu, J., & Huang, S. (2025). Design of Cobalt-Free Ni-Rich Cathodes for High-Performance Sodium-Ion Batteries Using Electrochemical Li+/Na+ Exchange. Energies, 18(12), 3205. https://doi.org/10.3390/en18123205






