High-Performance Carbon Black/Fe3O4/Epoxy Nanodielectrics for Electrostatic Energy Storage and Harvesting Solutions
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Morphology
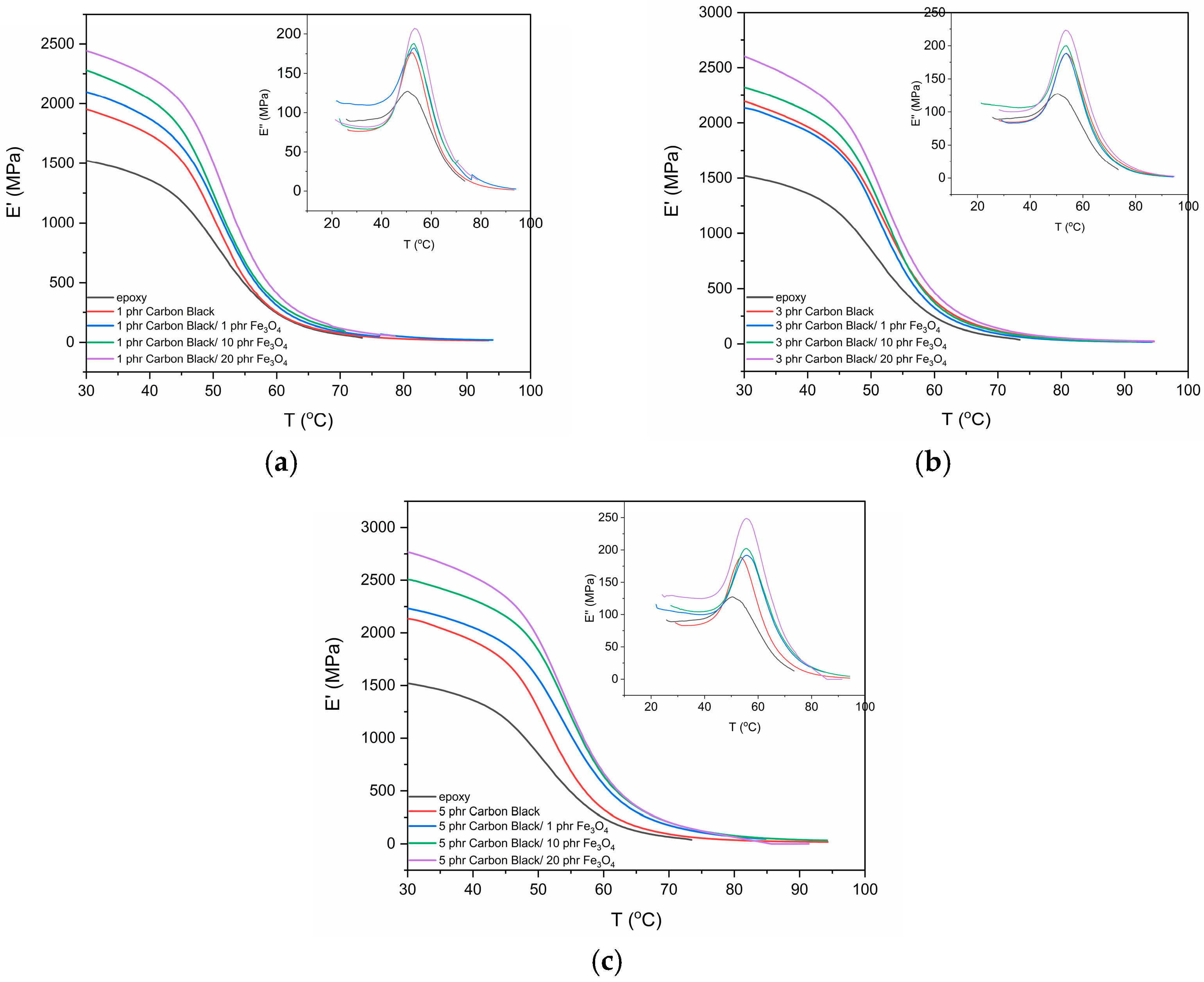
3.2. Thermomechanical Characterization
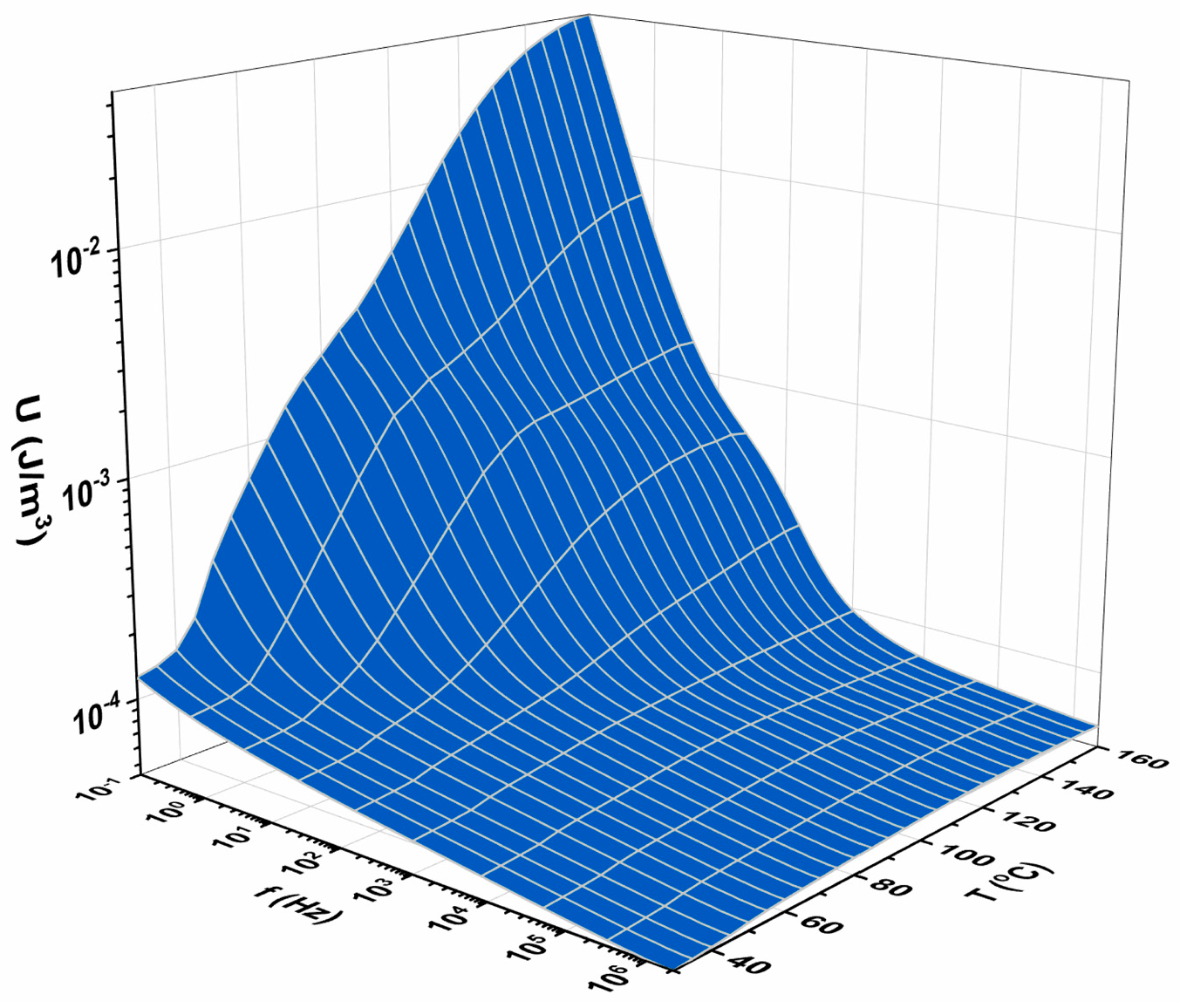
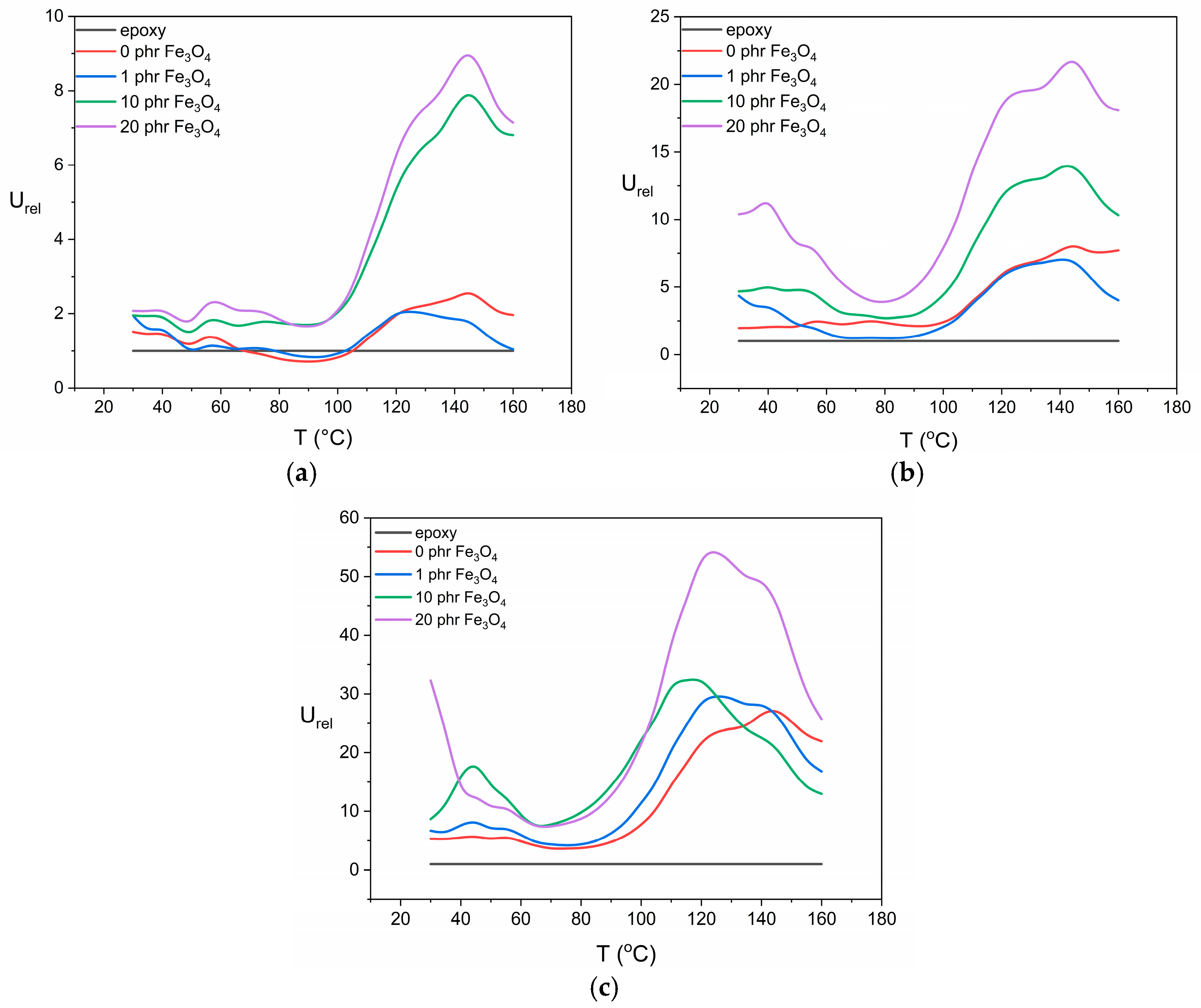
3.3. Energy-Storage Performance Under AC Conditions
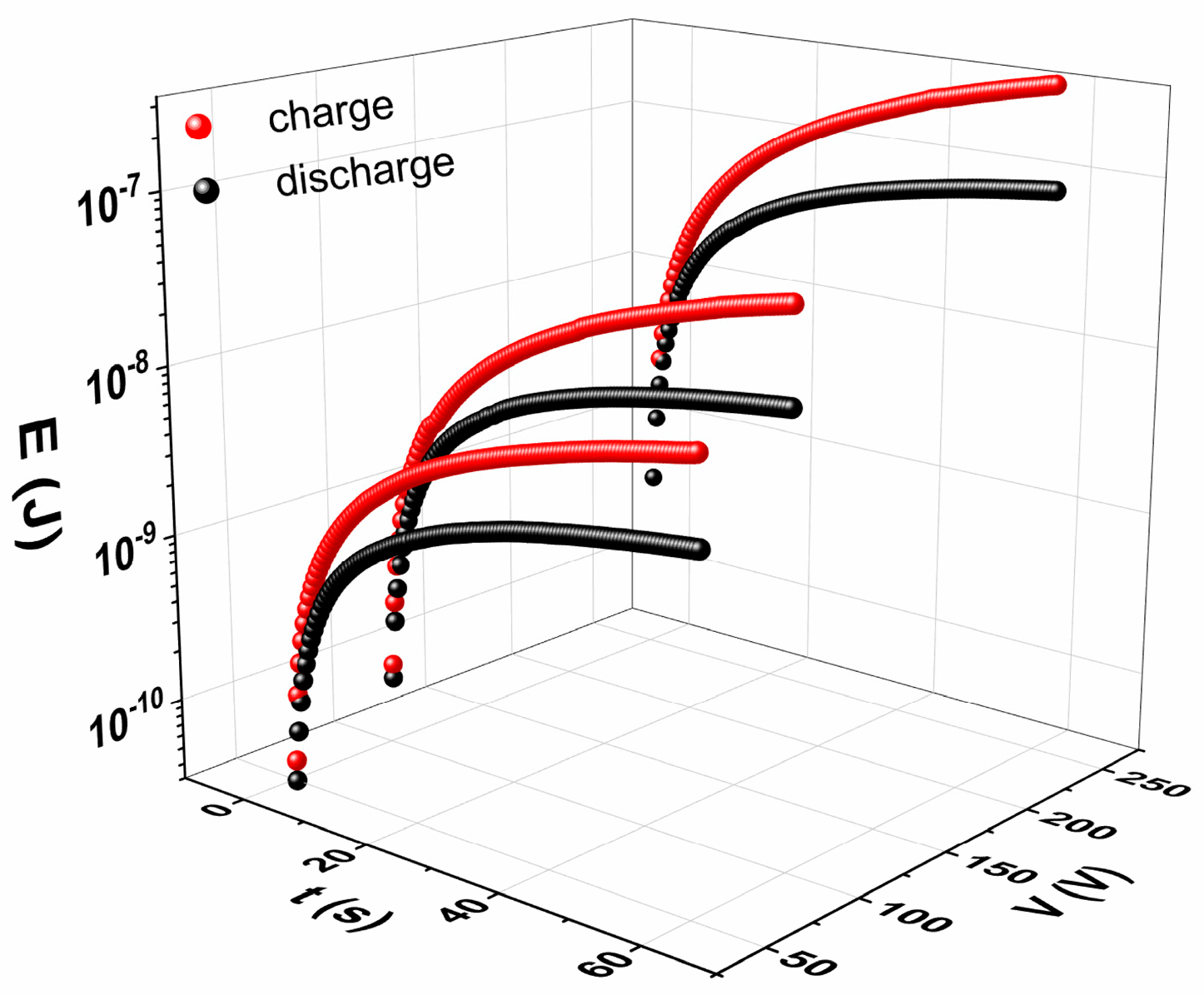
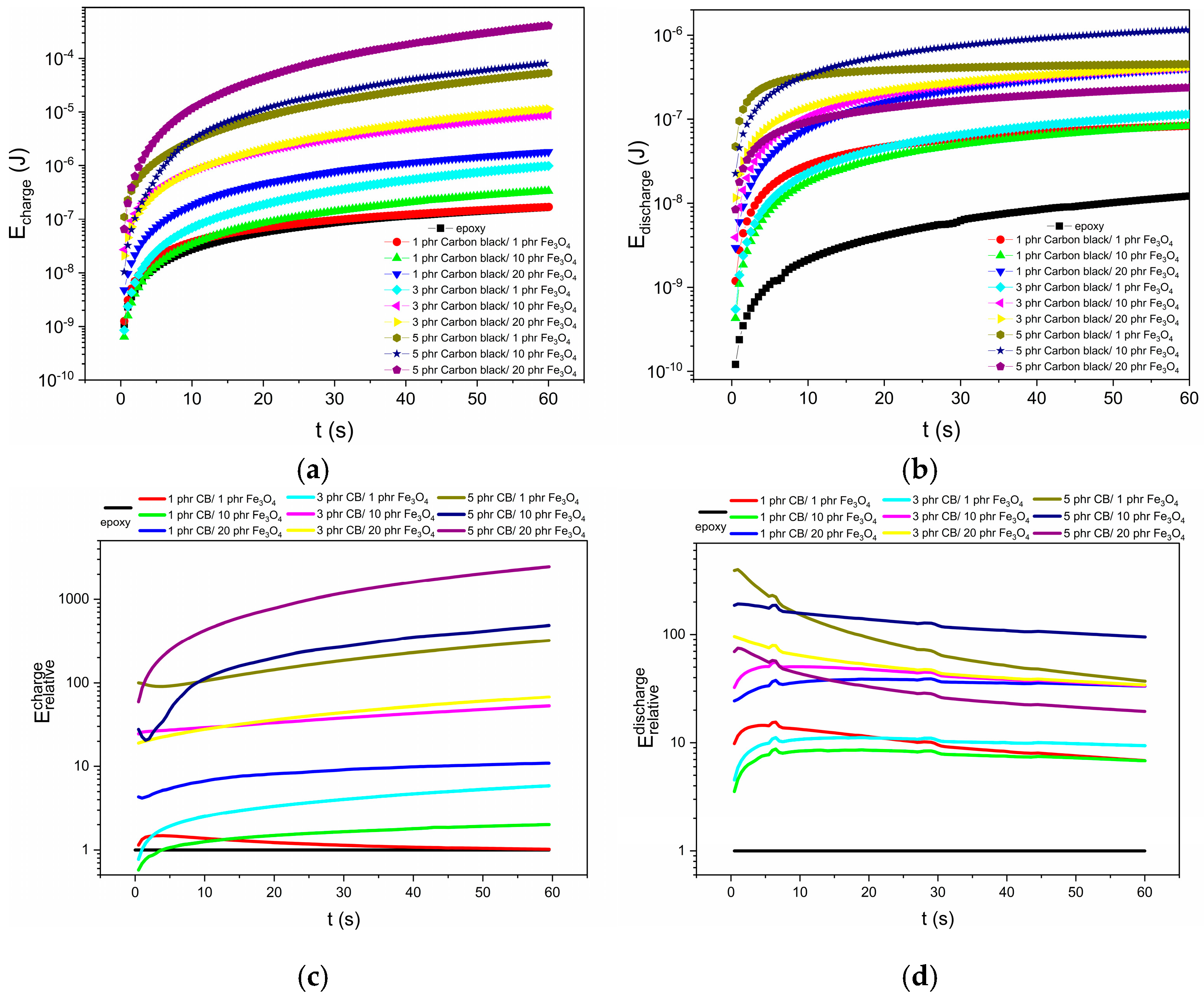
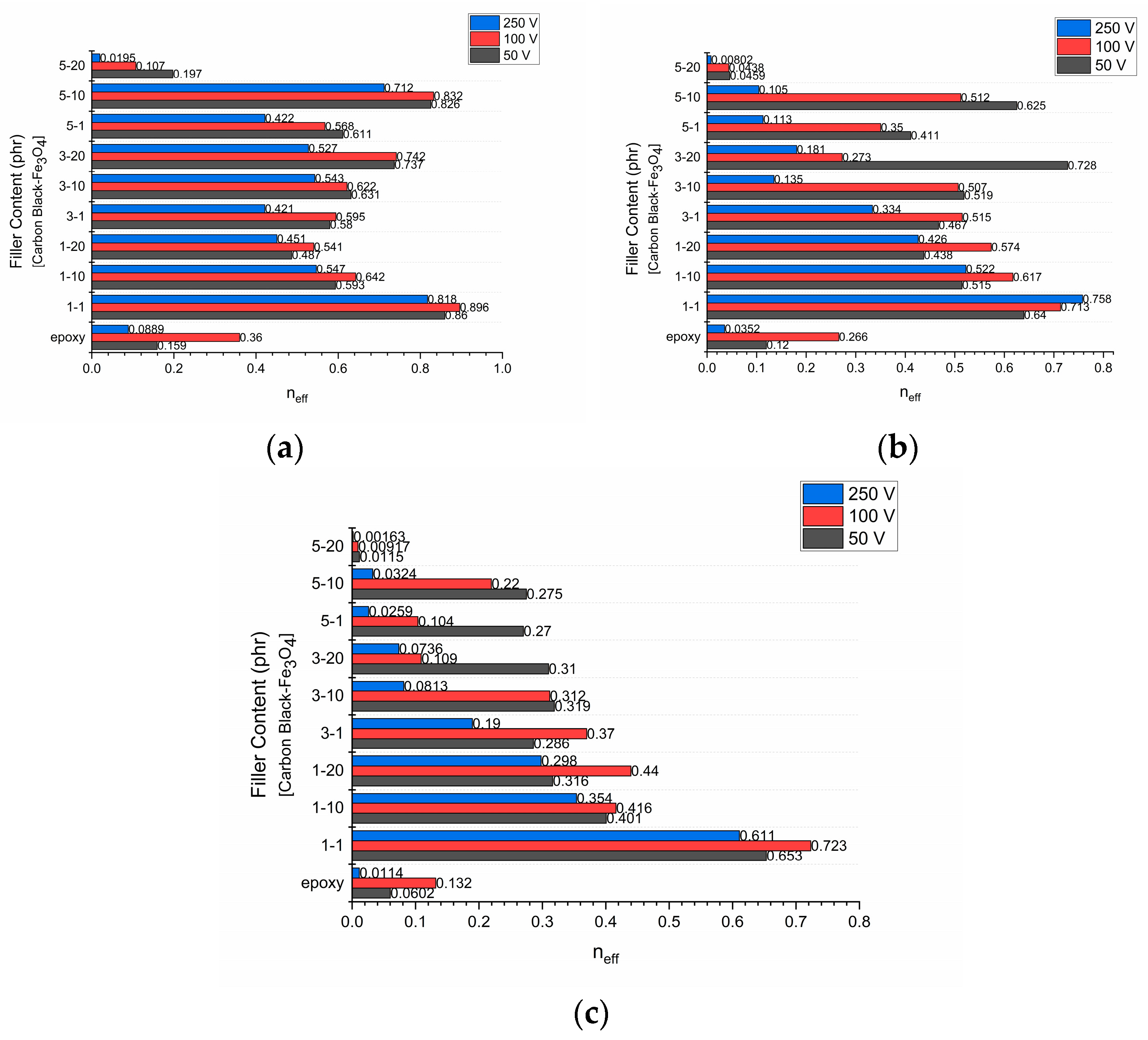
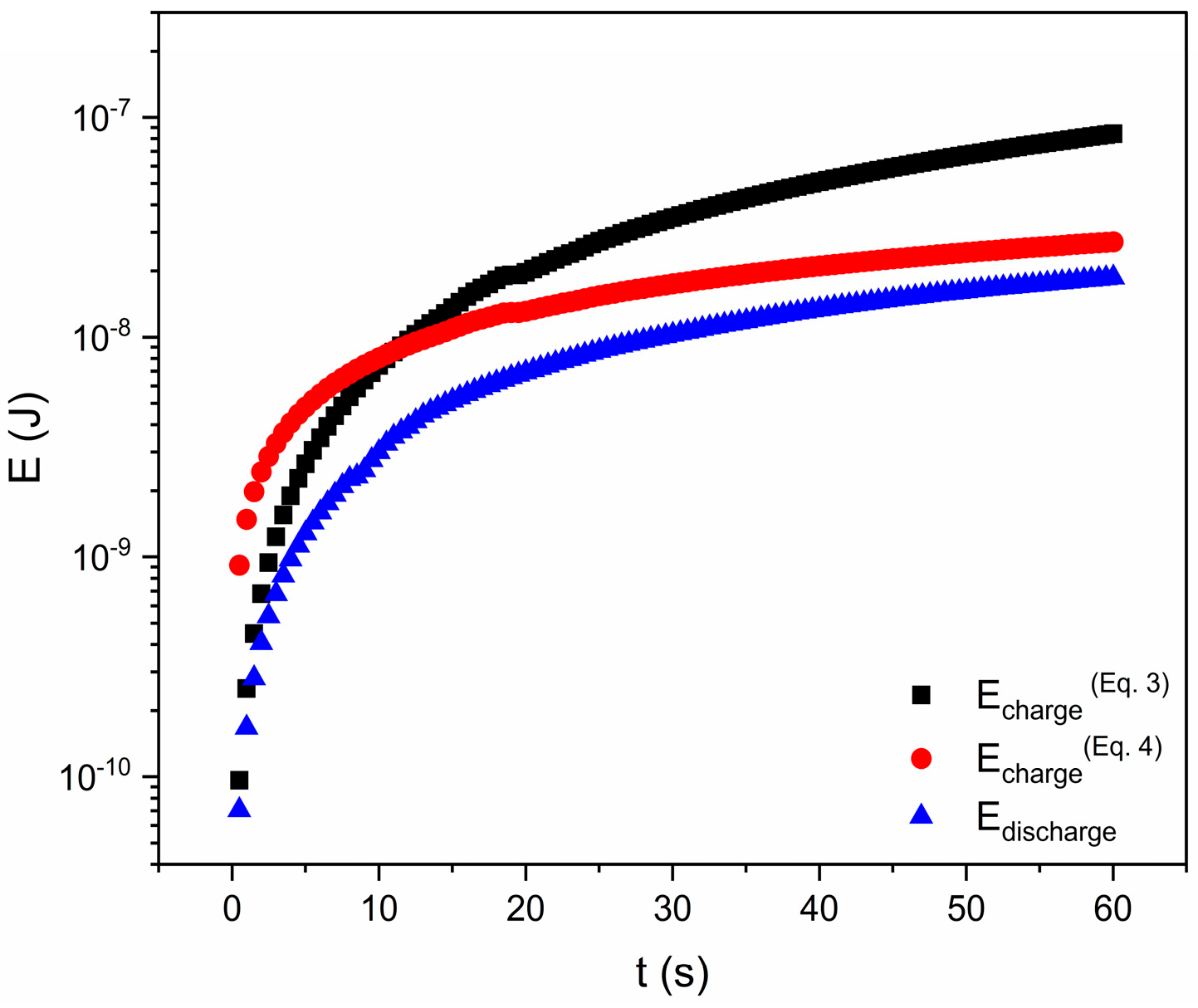
3.4. Energy-Storage and -Harvesting Performance Under DC Conditions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, S.; Skordos, A.; Thakur, V.K. Functional Nanocomposites for Energy Storage: Chemistry and New Horizons. Mater. Today Chem. 2020, 17, 100304. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Wang, J.; Zhen, X.; Lei, C.; Shen, Z.; Zhang, X.; Nan, C.W. Ultrahigh Capacitive Energy Storage in a Heterogeneous Nanolayered Composite. Adv. Funct. Mater. 2024, 34, 2410823. [Google Scholar] [CrossRef]
- Al-Emran, M.; Griffy-Brown, C. The Role of Technology Adoption in Sustainable Development: Overview, Opportunities, Challenges, and Future Research Agendas. Technol. Soc. 2023, 73, 102240. [Google Scholar] [CrossRef]
- Kong, L.; Tang, C.; Peng, H.; Huang, J.; Zhang, Q. Advanced Energy Materials for Flexible Batteries in Energy Storage: A Review. SmartMat 2020, 1. [Google Scholar] [CrossRef]
- Wang, R.; Zhu, Y.; Fu, J.; Yang, M.; Ran, Z.; Li, J.; Li, M.; Hu, J.; He, J.; Li, Q. Designing Tailored Combinations of Structural Units in Polymer Dielectrics for High-Temperature Capacitive Energy Storage. Nat. Commun. 2023, 14, 2406. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, L.; Chang, Z.; Dastan, D.; Liu, Y.; Fan, R.; Shi, Z. Significantly Enhancing the High-temperature Breakdown and Capacitive Performances of Dielectric Polymers via Incorporating Alumina Nanotubes as Equivalent Crosslinking Points. Adv. Funct. Mater. 2025, 30, 2425001. [Google Scholar] [CrossRef]
- Wang, Y.; Linker, T.; Abu Hurayra Lizu, K.M.; Ortiz-Flores, L.A.; Kamal, D.; Zhou, J.; Nguyen, H.; Li, C.; Gao, W.; Konstantinou, A.; et al. Ultrahigh Energy Density in High-Temperature Polymer Dielectric Reinforced by Bilayer Nanocoating. Chem. Eng. J. 2025, 507, 160613. [Google Scholar] [CrossRef]
- Aklujkar, P.S.; Gurnani, R.; Rout, P.; Khomane, A.R.; Mutegi, I.; Desai, M.; Pollock, A.; Toribio, J.M.; Hao, J.; Cao, Y.; et al. Rationally Designed High-Temperature Polymer Dielectrics for Capacitive Energy Storage: An Experimental and Computational Alliance. Prog. Polym. Sci. 2025, 161, 101931. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.; Konstantinou, A.C.; Baferani, M.A.; Davis-Amendola, K.; Gao, W.; Cao, Y. Sandwiched Polymer Nanocomposites Reinforced by Two-Dimensional Interface Nanocoating for Ultrahigh Energy Storage Performance at Elevated Temperatures. Small 2023, 19, 2208105. [Google Scholar] [CrossRef]
- Chen, C.; Zuo, Y.; Ye, W.; Li, X.; Deng, Z.; Ong, S.P. A Critical Review of Machine Learning of Energy Materials. Adv. Energy Mater. 2020, 10, 1903242. [Google Scholar] [CrossRef]
- Oladele, I.O.; Adelani, S.O.; Taiwo, A.S.; Akinbamiyorin, I.M.; Olanrewaju, O.F.; Orisawayi, A.O. Polymer-Based Nanocomposites for Supercapacitor Applications: A Review on Principles, Production and Products. RSC Adv. 2025, 15, 7509–7534. [Google Scholar] [CrossRef]
- Fan, B.; Zhou, M.; Zhang, C.; He, D.; Bai, J. Polymer-Based Materials for Achieving High Energy Density Film Capacitors. Prog. Polym. Sci. 2019, 97, 101143. [Google Scholar] [CrossRef]
- Nitinkumar, J.H.; Reghu, N.; Akhilesh, P.K.; Vlad, A.; Balachandran, M.; Raghavan, P. Recent Progress in Nanodielectric Composites and Their Applications; Springer: Berlin/Heidelberg, Germany, 2024; pp. 123–149. [Google Scholar]
- Drakopoulos, S.X.; Loukelis, K.; Triantafyllou-Rundell, M.E.; Stoumpos, C.C.; Chatzinikolaidou, M.; Psarras, G.C. Epoxy/Clay Nanodielectrics: From Relaxation Dynamics to Capacitive Energy Storage. Adv. Compos. Hybrid Mater. 2024, 7, 118. [Google Scholar] [CrossRef]
- Drakopoulos, S.X.; Wu, J.; Maguire, S.M.; Srinivasan, S.; Randazzo, K.; Davidson, E.C.; Priestley, R.D. Polymer Nanocomposites: Interfacial Properties and Capacitive Energy Storage. Prog. Polym. Sci. 2024, 156, 101870. [Google Scholar] [CrossRef]
- Tanaka, T. Progress in Nanodielectrics: Future from the Past Decade. In IEEE Transactions on Dielectrics and Electrical Insulation; IEEE: Piscataway Township, NJ, USA, 2024; p. 1. [Google Scholar] [CrossRef]
- Chen, K.; Liu, Z.; Zheng, W.; Liu, S.; Chi, Z.; Xu, J.; Zhang, Y. Research Progress of Intrinsic Polymer Dielectrics with High Permittivity. IET Nanodielectr. 2023, 6, 182–211. [Google Scholar] [CrossRef]
- Yang, M.; Guo, M.; Xu, E.; Ren, W.; Wang, D.; Li, S.; Zhang, S.; Nan, C.-W.; Shen, Y. Polymer Nanocomposite Dielectrics for Capacitive Energy Storage. Nat. Nanotechnol. 2024, 19, 588–603. [Google Scholar] [CrossRef]
- Stavropoulos, S.G.; Sanida, A.; Psarras, G.C. Carbon Allotropes/Epoxy Nanocomposites as Capacitive Energy Storage/Harvesting Systems. Appl. Sci. 2021, 11, 7059. [Google Scholar] [CrossRef]
- Stavropoulos, S.G.; Sanida, A.; Psarras, G.C. A Comparative Study on the Electrical Properties of Different Forms of Carbon Allotropes–Epoxy Nanocomposites. Express Polym. Lett. 2020, 14, 477–490. [Google Scholar] [CrossRef]
- Sanida, A.; Stavropoulos, S.G.; Speliotis, T.; Psarras, G.C. Probing the Magnetoelectric Response and Energy Efficiency in Fe3O4/Epoxy Nanocomposites. Polym. Test 2020, 88, 106560. [Google Scholar] [CrossRef]
- Sanida, A.; Stavropoulos, S.G.; Speliotis, T.; Psarras, G.C. Evaluating the Multifunctional Performance of Polymer Matrix Nanodielectrics Incorporating Magnetic Nanoparticles: A Comparative Study. Polymer 2021, 236, 124311. [Google Scholar] [CrossRef]
- Song, K.; Guo, J.Z.; Liu, C. Polymer-Based Multifunctional Nanocomposites and Their Applications; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128150672. [Google Scholar]
- Manika, G.C.; Psarras, G.C. Barium Titanate/Epoxy Resin Composite Nanodielectrics as Compact Capacitive Energy Storing Systems. Express Polym. Lett. 2019, 13, 749–758. [Google Scholar] [CrossRef]
- Sanida, A.; Stavropoulos, S.G.; Speliotis, T.; Psarras, G.C. Development, Characterization, Energy Storage and Interface Dielectric Properties in SrFe12O19/Epoxy Nanocomposites. Polymer 2017, 120, 73–81. [Google Scholar] [CrossRef]
- Khan, I.; Khan, I.; Saeed, K.; Ali, N.; Zada, N.; Khan, A.; Ali, F.; Bilal, M.; Akhter, M.S. Polymer Nanocomposites: An Overview. In Smart Polymer Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2023; pp. 167–184. [Google Scholar]
- Zaghloul, M.M.Y.; Zaghloul, M.M.Y.; Fuseini, M. Recent Progress in Epoxy Nanocomposites: Corrosion, Structural, Flame Retardancy and Applications—A Comprehensive Review. Polym. Adv. Technol. 2023, 34, 3438–3472. [Google Scholar] [CrossRef]
- Siwal, S.S.; Zhang, Q.; Devi, N.; Thakur, V.K. Carbon-Based Polymer Nanocomposite for High-Performance Energy Storage Applications. Polymers 2020, 12, 505. [Google Scholar] [CrossRef]
- Khodabakhshi, S.; Fulvio, P.F.; Andreoli, E. Carbon Black Reborn: Structure and Chemistry for Renewable Energy Harnessing. Carbon 2020, 162, 604–649. [Google Scholar] [CrossRef]
- ASTM D150-22; Standard Test Methods for AC Loss Characteristics and Permittivity (Dielectric Constant) of Solid Electrical Insulation. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM D257-14(2021)e1; Standard Test Methods for DC Resistance or Conductance of Insulating Materials. ASTM International: West Conshohocken, PA, USA, 2022.
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Psarras, G.C. Fundamentals of Dielectric Theories. In Dielectric Polymer Materials for High-Density Energy Storage; Elsevier: Amsterdam, The Netherlands, 2018; Volume 268, pp. 11–57. ISBN 9780128132159. [Google Scholar]
- Manika, G.C.; Psarras, G.C. Energy Storage and Harvesting in BaTiO3/Epoxy Nanodielectrics. High Volt. 2016, 1, 151–157. [Google Scholar] [CrossRef]
- Sun, J.; Luo, B.; Li, H. A Review on the Conventional Capacitors, Supercapacitors, and Emerging Hybrid Ion Capacitors: Past, Present, and Future. Adv. Energy Sustain. Res. 2022, 3, 2100191. [Google Scholar] [CrossRef]
- Xie, Z.; Li, H.; Peng, Z.; Liu, Y. High-Temperature Energy Storage Polymer Dielectrics for Capacitors. In High Temperature Polymer Dielectrics: Fundamentals and Applications in Power Equipment; Wiley-VCH: Weinheim, Germany, 2023. [Google Scholar]
- Li, Z.; Min, D.; Niu, H.; Li, S.; Zhang, Y.; Huang, Y.; Li, S. Enhanced DC Breakdown Strength of Epoxy Nanocomposites at Elevated Temperature and Its Mechanisms. J. Appl. Phys. 2021, 130, 065101. [Google Scholar] [CrossRef]
- Zhou, Y.; Yuan, C.; Wang, S.; Zhu, Y.; Cheng, S.; Yang, X.; Yang, Y.; Hu, J.; He, J.; Li, Q. Interface-Modulated Nanocomposites Based on Polypropylene for High-Temperature Energy Storage. Energy Storage Mater. 2020, 28, 255–263. [Google Scholar] [CrossRef]
- Yi, Z.; Lyu, B.; Gao, D.; Hao, J.; Liu, Z.; Ning, Z.; Jiang, B. Preparation Strategy and Composition Design of Polymer-Based Layered Composites for Improving Energy Storage Performances. J. Energy Storage 2024, 92, 111995. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, H.; Yin, C.; Jung, Y.H.; Min, S.; Zhang, Y.; Zhang, C.; Chen, Q.; Lee, K.J.; Chi, Q. Recent Progress in Polymer Dielectric Energy Storage: From Film Fabrication and Modification to Capacitor Performance and Application. Prog. Mater. Sci. 2023, 140, 101207. [Google Scholar] [CrossRef]
- He, Q.; Sun, K.; Shi, Z.; Liu, Y.; Fan, R. Polymer Dielectrics for Capacitive Energy Storage: From Theories, Materials to Industrial Capacitors. Mater. Today 2023, 68, 298–333. [Google Scholar] [CrossRef]
- Stavropoulos, S.G.; Sanida, A.; Psarras, G.C. Assessing the Effect of Fe3O4 Nanoparticles on the Thermomechanical Performance of Different Forms of Carbon Allotropes/Epoxy Hybrid Nanocomposites. Appl. Mech. 2022, 3, 560–572. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, S.; Xie, P.; Miao, X.; Liu, S.; Sun, N.; Guo, M.; Xu, Z.; Zhong, T.; Shen, Y. Research Progress and Prospect of Polymer Dielectrics. Appl. Phys. Rev. 2023, 10, 031310. [Google Scholar] [CrossRef]
- Behera, R.; Elanseralathan, K. A Review on Polyvinylidene Fluoride Polymer Based Nanocomposites for Energy Storage Applications. J. Energy Storage 2022, 48, 103788. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Q.; Allahyarov, E.; Li, Y.; Zhu, L. Challenges and Opportunities of Polymer Nanodielectrics for Capacitive Energy Storage. ACS Appl. Mater. Interfaces 2021, 13, 37939–37960. [Google Scholar] [CrossRef]
- Bera, S.; Singh, M.; Thantirige, R.; Tiwary, S.K.; Shook, B.T.; Nieves, E.; Raghavan, D.; Karim, A.; Pradhan, N.R. 2D-Nanofiller-Based Polymer Nanocomposites for Capacitive Energy Storage Applications. Small Sci. 2023, 3, 2300016. [Google Scholar] [CrossRef]
- Li, H.; Ren, L.; Zhou, Y.; Yao, B.; Wang, Q. Recent Progress in Polymer Dielectrics Containing Boron Nitride Nanosheets for High Energy Density Capacitors. High Volt. 2020, 5, 365–376. [Google Scholar] [CrossRef]
- Li, X.; Hu, P.; Jiang, J.; Pan, J.; Nan, C.W.; Shen, Y. High-Temperature Polymer Composite Dielectrics: Energy Storage Performance, Large-Scale Preparation, and Device Design. Adv. Mater. 2025, 37, 2411507. [Google Scholar] [CrossRef]
- Xie, Y.; Fan, X.; Li, X.; Zhang, Y.; Zhang, Z.; Huang, X. Perspective on Interface Engineering for Capacitive Energy Storage Polymer Nanodielectrics. Phys. Chem. Chem. Phys. 2022, 24, 19624–19633. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, Y.; Liu, Y.; Li, L.; Liu, Y.; Wang, Q. Dielectric Polymers for High-Temperature Capacitive Energy Storage. Chem. Soc. Rev. 2021, 50, 6369–6400. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stavropoulos, S.; Sanida, A.; Psarras, G. High-Performance Carbon Black/Fe3O4/Epoxy Nanodielectrics for Electrostatic Energy Storage and Harvesting Solutions. Energies 2025, 18, 3147. https://doi.org/10.3390/en18123147
Stavropoulos S, Sanida A, Psarras G. High-Performance Carbon Black/Fe3O4/Epoxy Nanodielectrics for Electrostatic Energy Storage and Harvesting Solutions. Energies. 2025; 18(12):3147. https://doi.org/10.3390/en18123147
Chicago/Turabian StyleStavropoulos, Sotirios, Aikaterini Sanida, and Georgios Psarras. 2025. "High-Performance Carbon Black/Fe3O4/Epoxy Nanodielectrics for Electrostatic Energy Storage and Harvesting Solutions" Energies 18, no. 12: 3147. https://doi.org/10.3390/en18123147
APA StyleStavropoulos, S., Sanida, A., & Psarras, G. (2025). High-Performance Carbon Black/Fe3O4/Epoxy Nanodielectrics for Electrostatic Energy Storage and Harvesting Solutions. Energies, 18(12), 3147. https://doi.org/10.3390/en18123147







