Recent Progress in Seawater Splitting Hydrogen Production Assisted by Value-Added Electrooxidation Reactions
Abstract
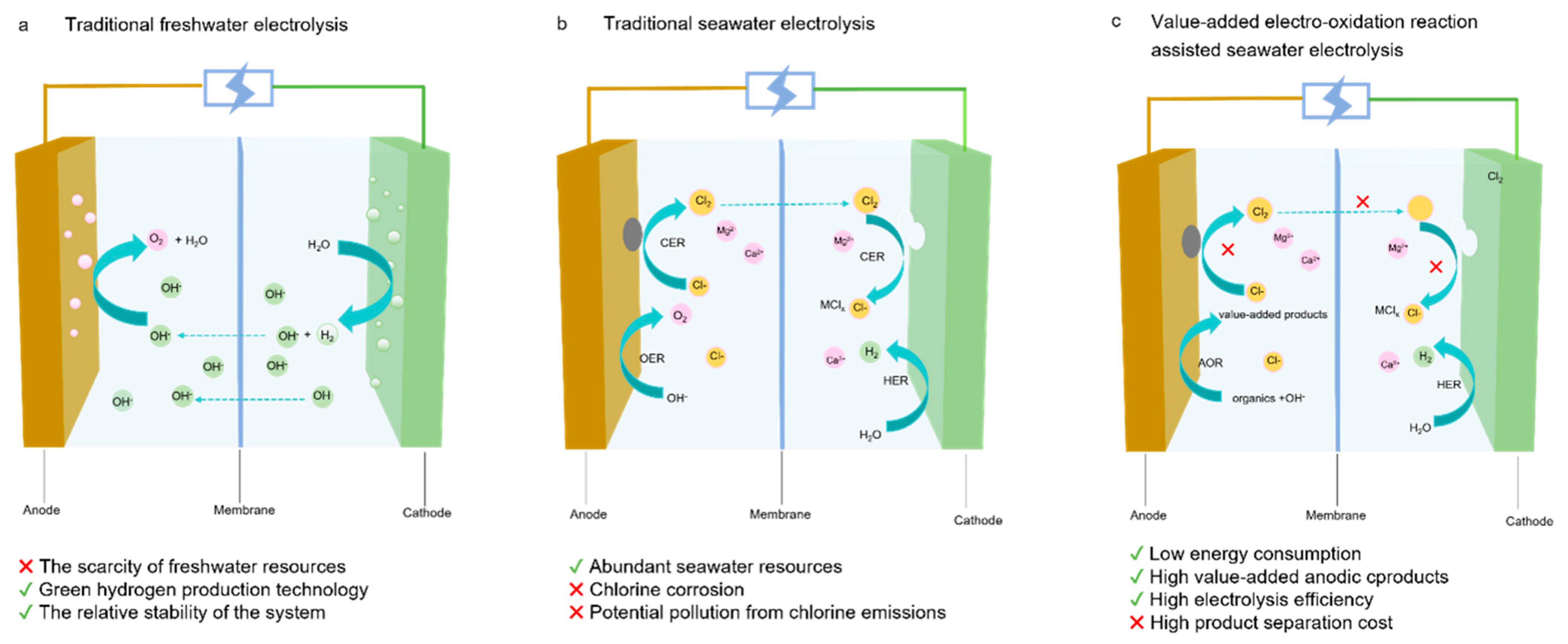
1. Introduction
2. Anodic Oxidation Reaction
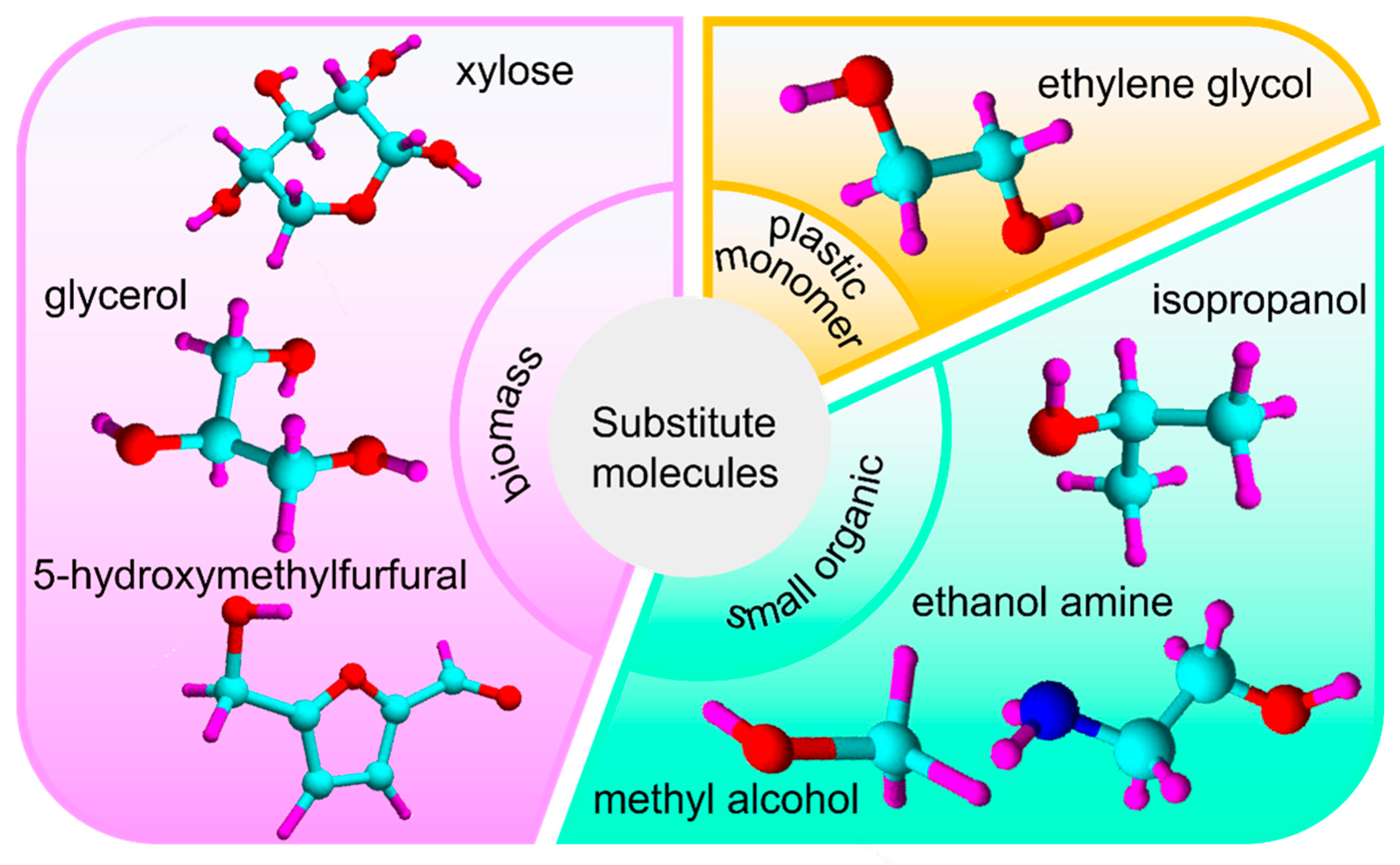
2.1. Oxidation of Organic Small Molecules
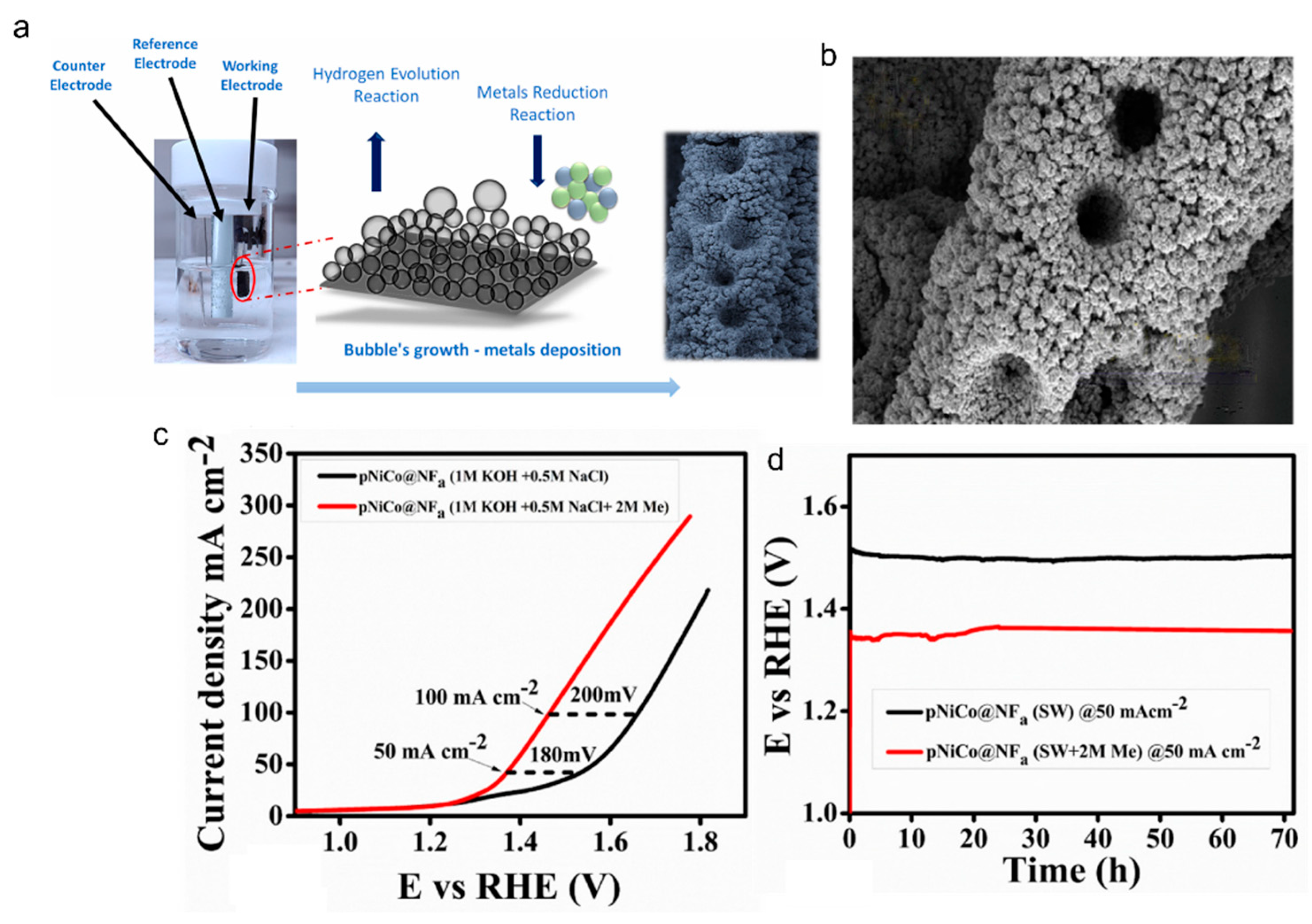
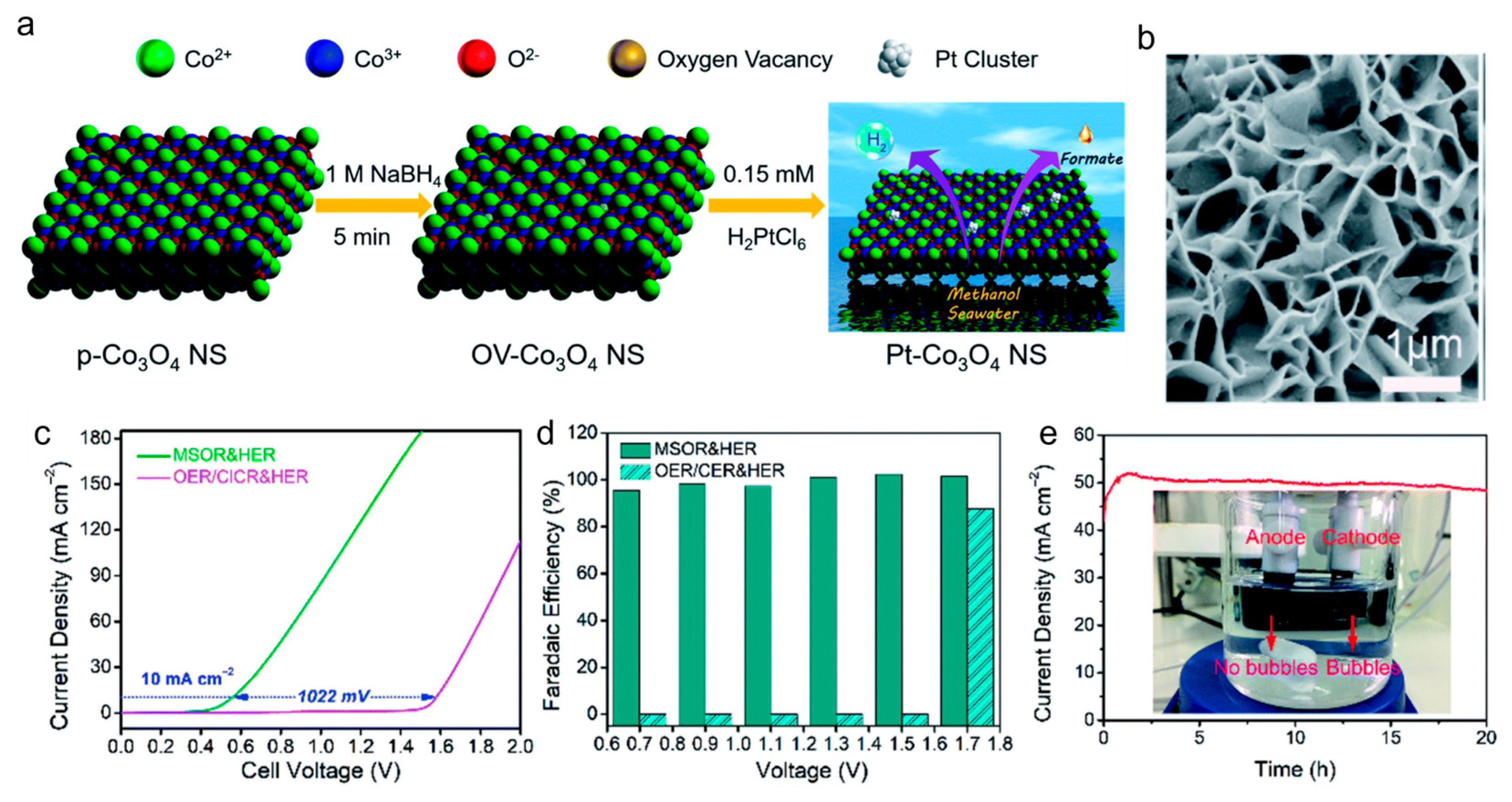
2.1.1. Oxidation of Methanol (MOR)
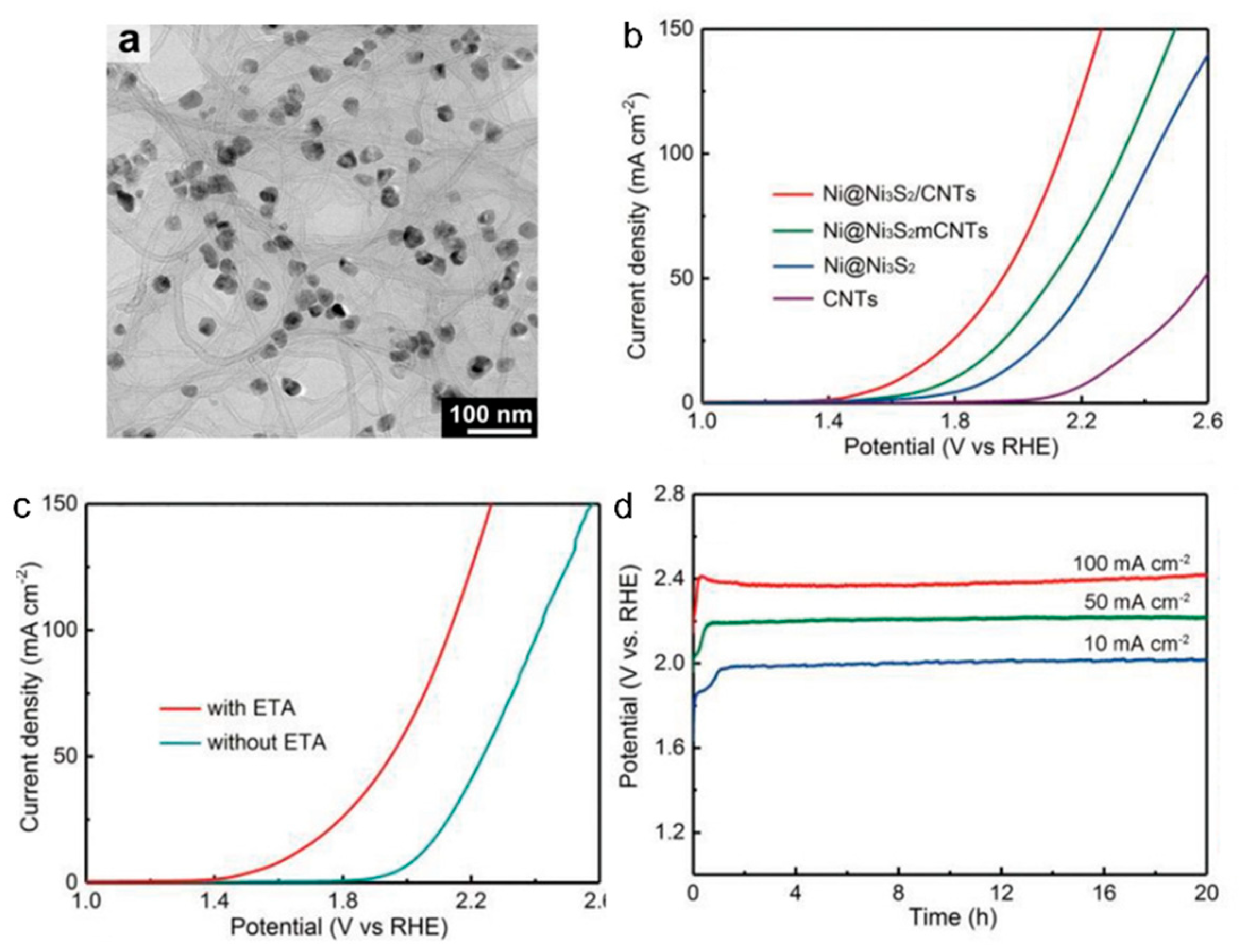
2.1.2. Oxidation Reaction of Ethanolamine
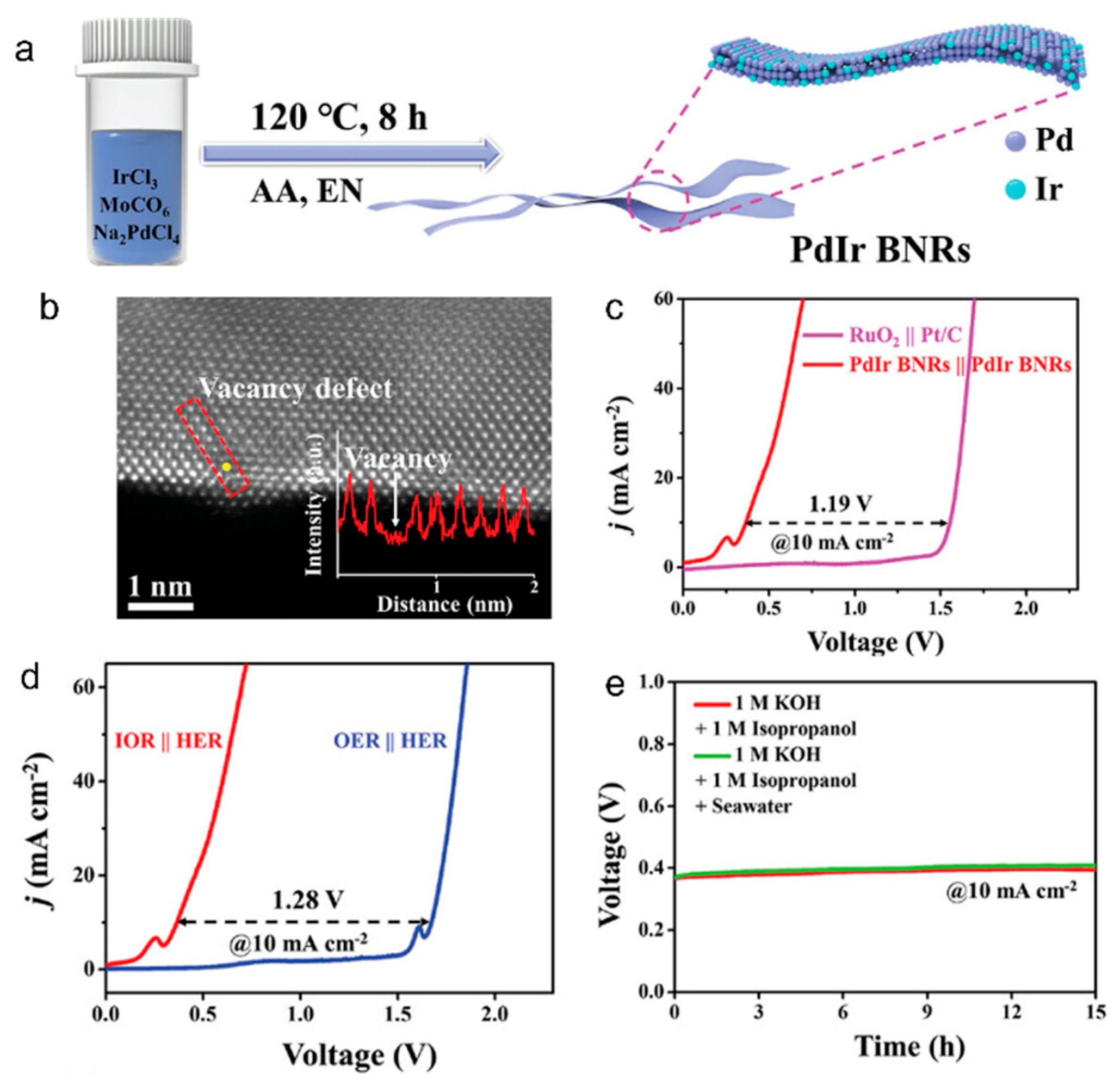
2.1.3. Isopropanol Oxidation Reaction (IPAOR)
2.2. Oxidation of Biomass
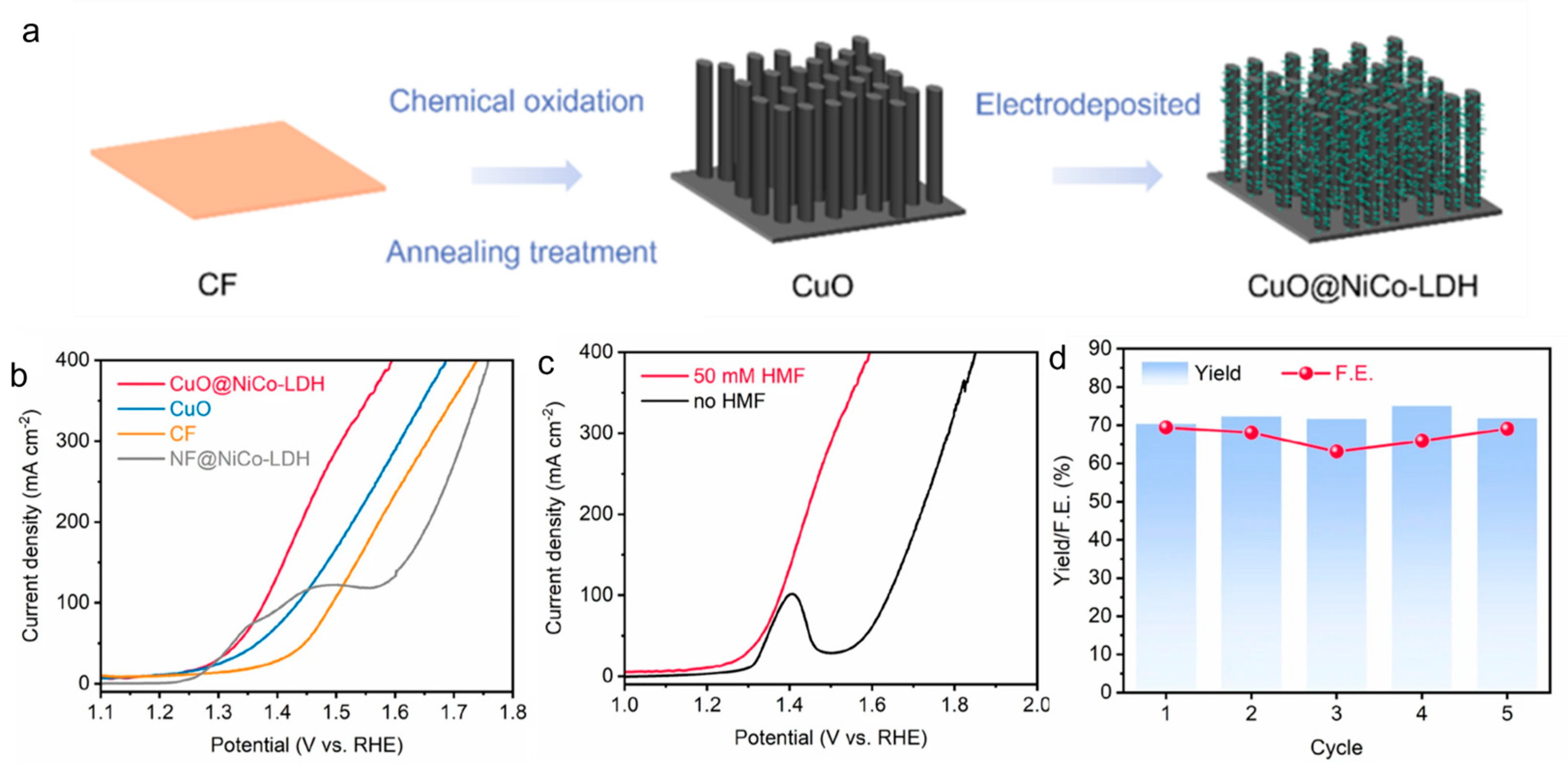
2.2.1. Oxidation of 5-Hydroxymethylfurfural (HMF)
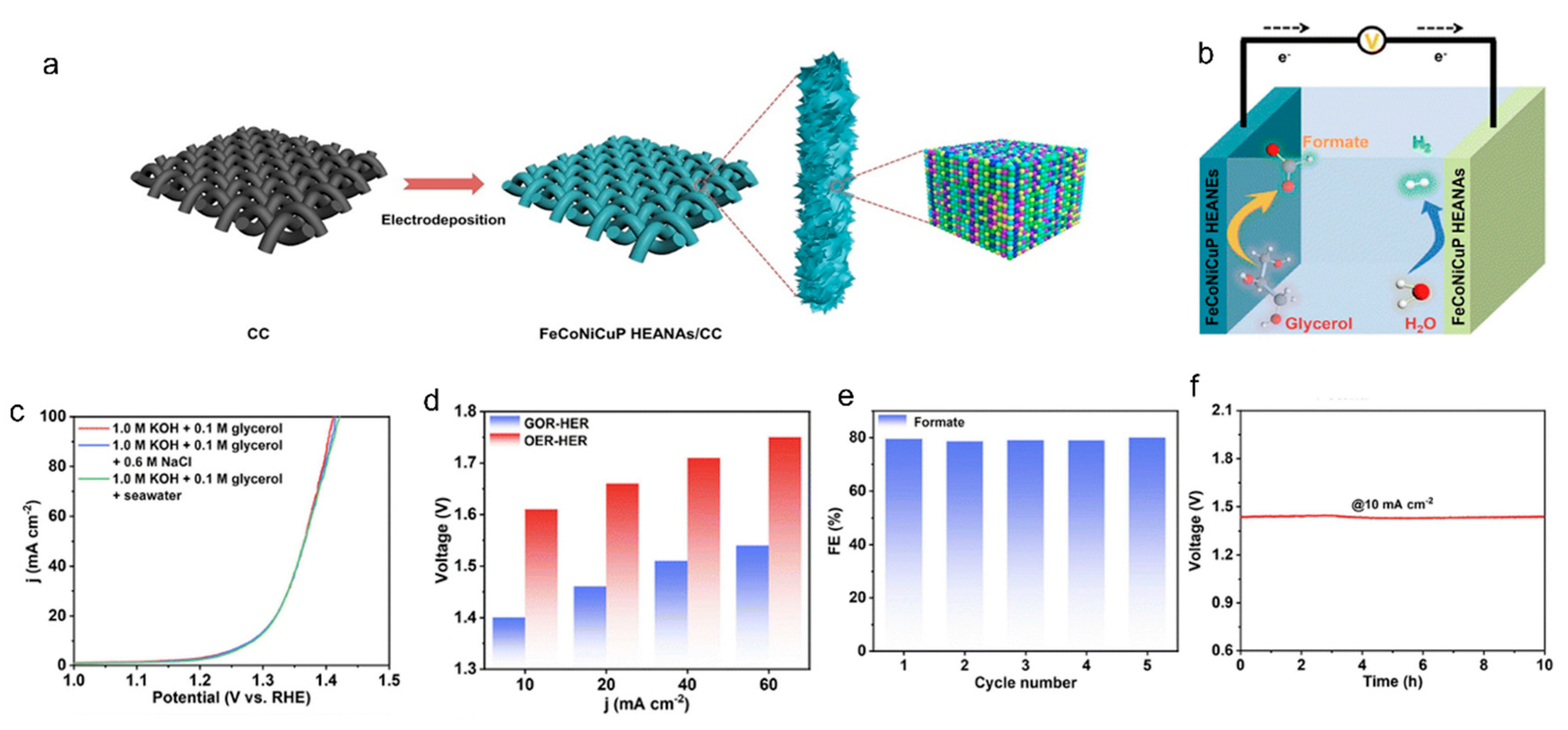
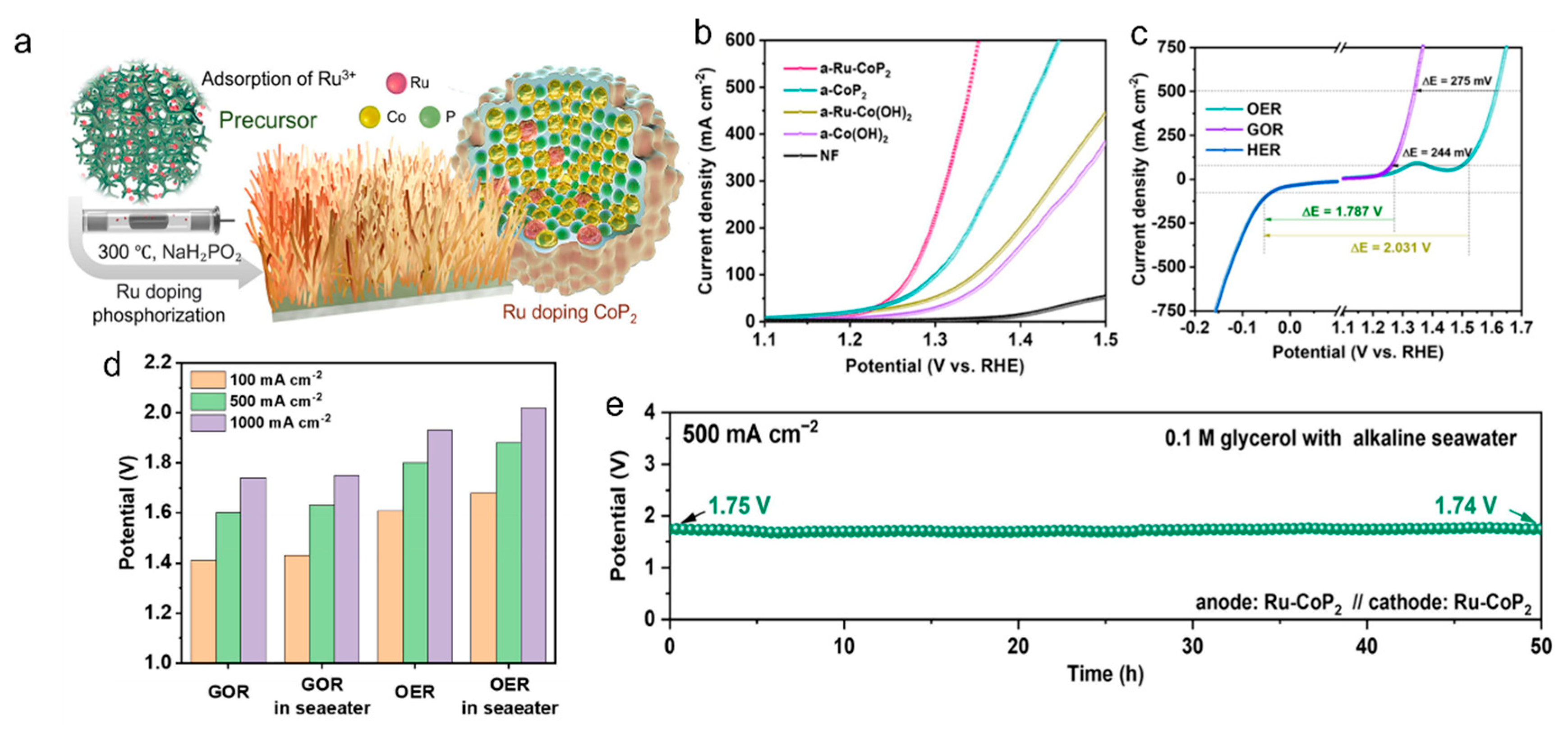
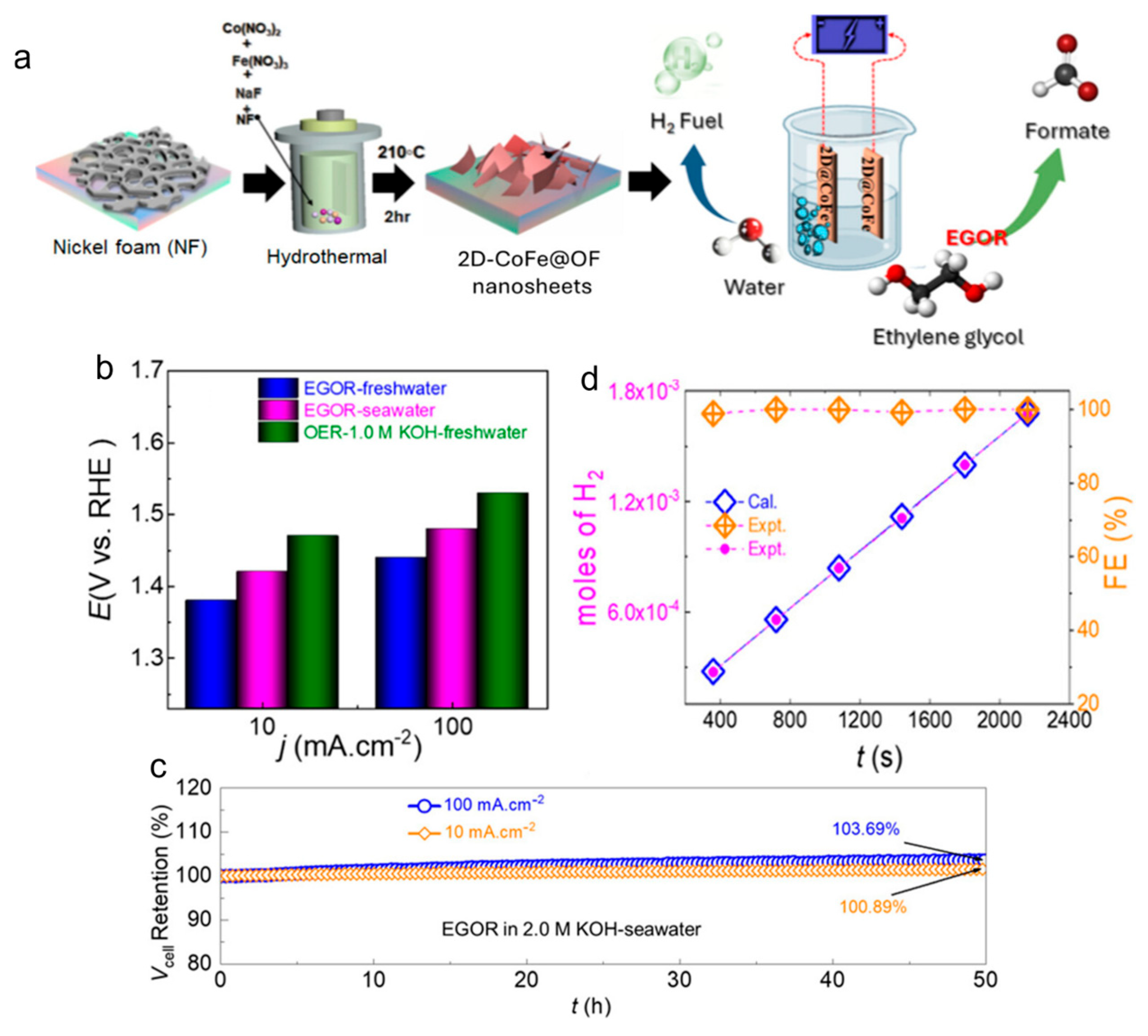
2.2.2. Oxidation Reaction of Glycerol (GOR)
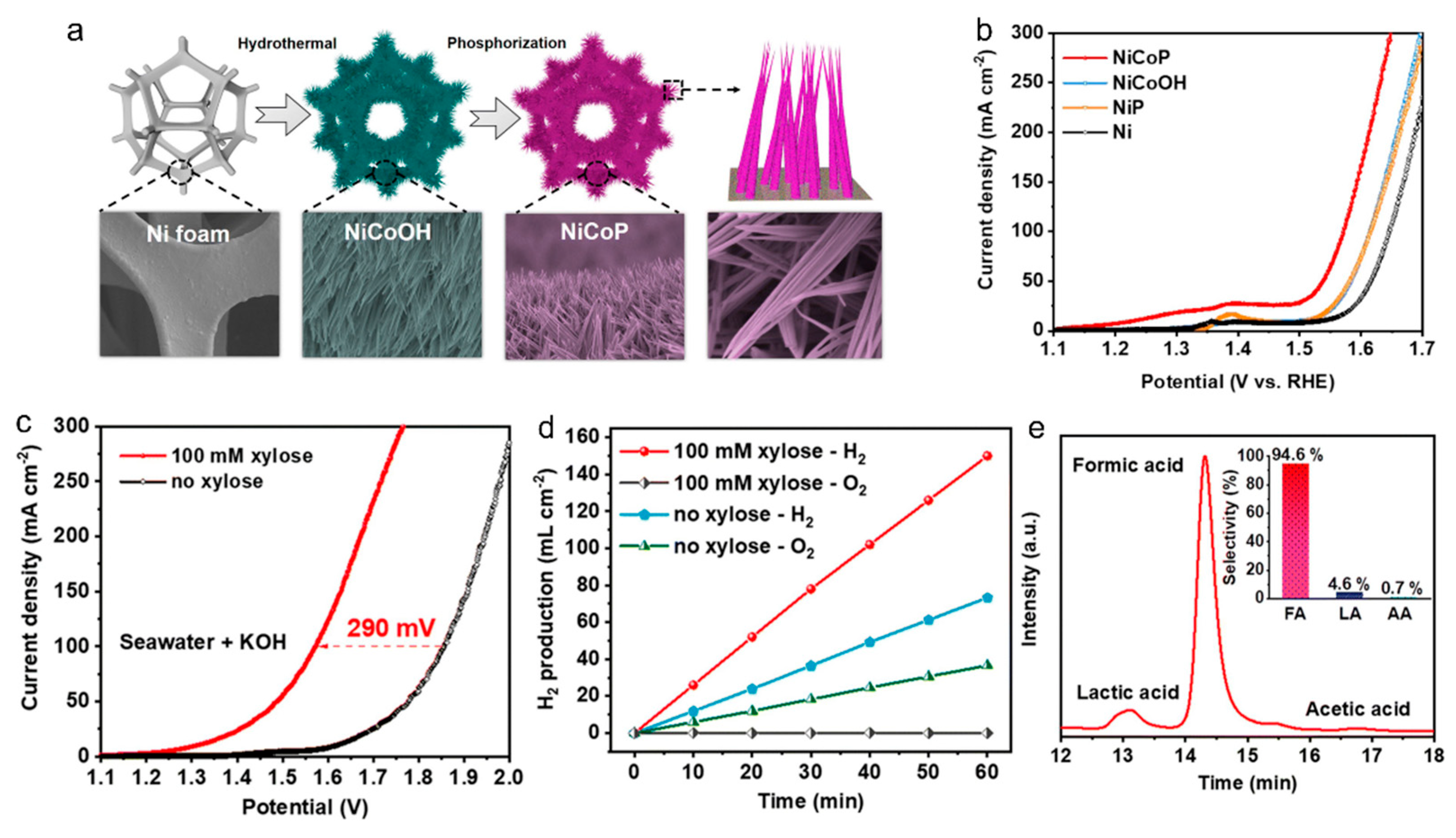
2.2.3. Xylose Oxidation Reaction (XOR)
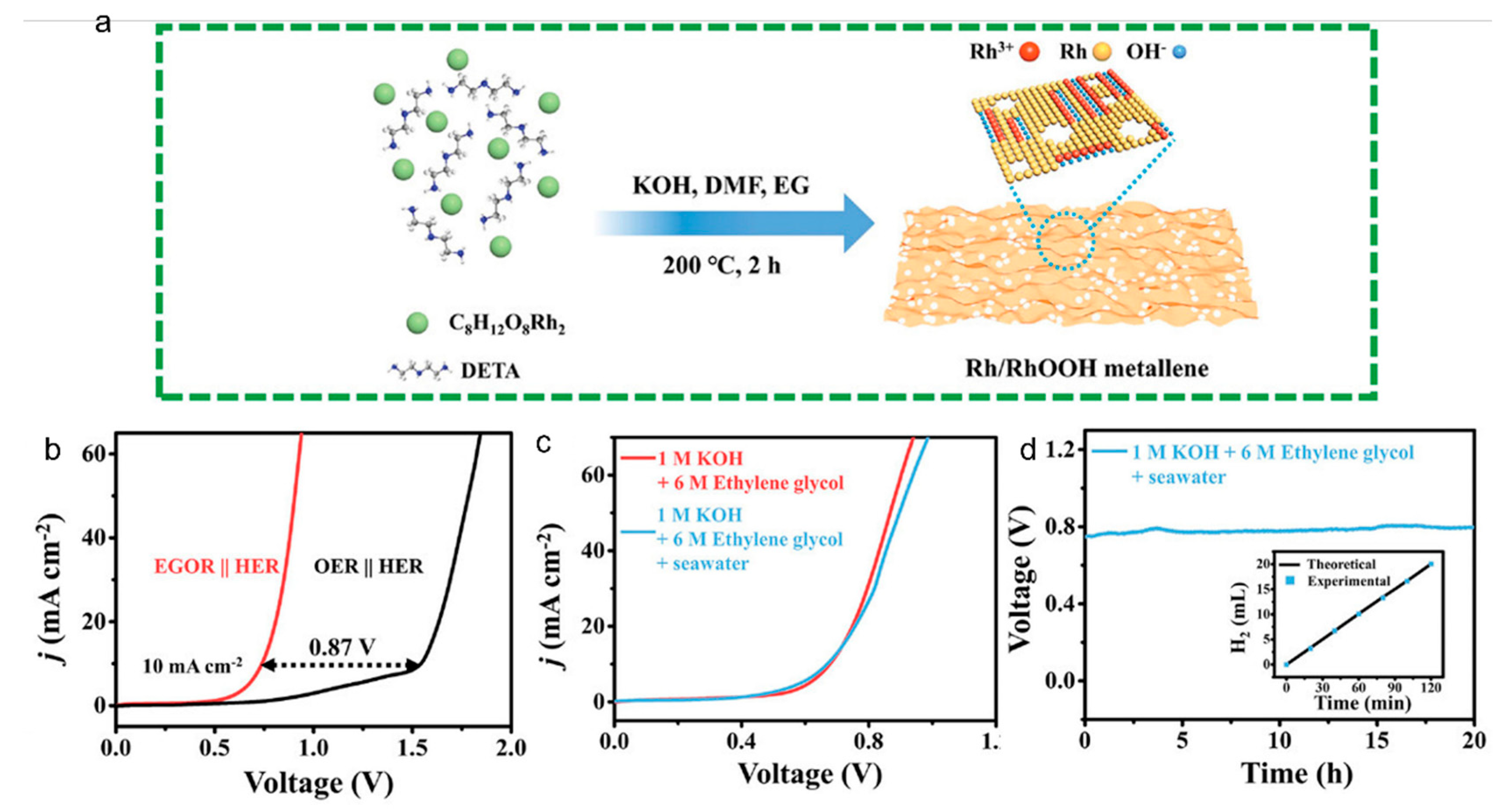
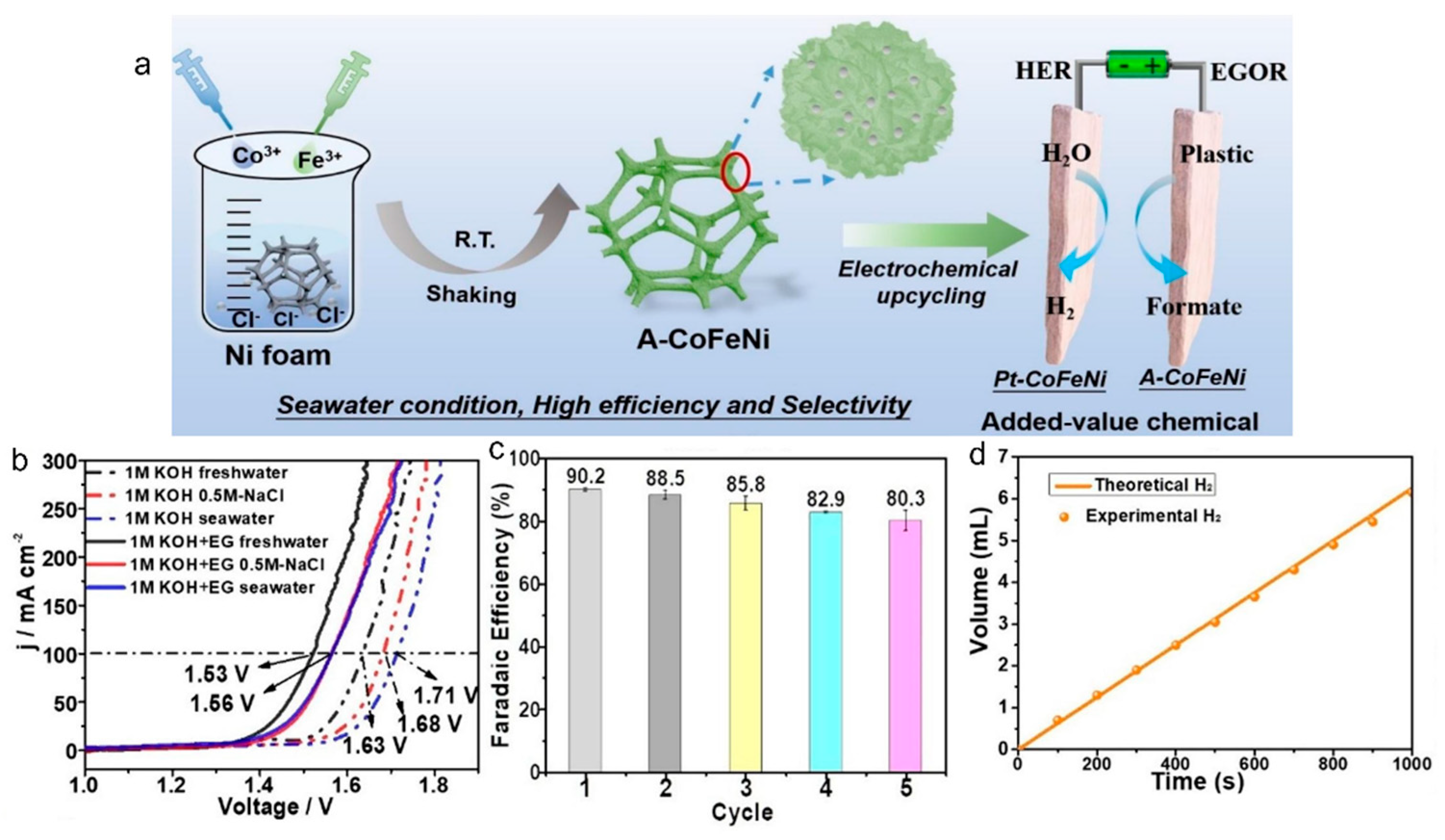
2.3. Oxidation Reactions of Plastics
3. Conclusions and Outlook
Funding
Conflicts of Interest
Appendix A
| Term List Table | ||
| Molecular Formula/Structural Formula | Full Name of the Substance | Acronym |
| — | oxygen evolution reaction | OER |
| — | chlorine evolution reaction | CER |
| — | anodic oxidation reactions | AOR |
| — | hydrogen evolution reaction | HER |
| — | water electrolysis | WE |
| CH3OH | methanol | MeOH |
| — | oxidation of methanol | MOR |
| HCOOH | formic acid | FA |
| — | dynamic hydrogen bubble template | DHBT |
| — | nanosheet | NS |
| HOCH2CH2NH2 | ethanolamine | ETA |
| — | the carbon nanotube | CNT |
| — | chronopotentiometry | CP |
| CH3CH(OH)CH3 | isopropanol | IPA |
| — | isopropanol oxidation reaction | IPAOR |
| — | PdIr bimetallic nanorods | PdIr BNRs |
| C6H6O3 | 5-hydroxymethylfurfural | HMF |
| C6H6O4 | 5-hydroxymethyl-2-furoic acid | HMFCA |
| C6H4O3 | 2,5-furan-dialdehyde | DFF |
| C6H4O4 | 5-formyl-2-furoic acid | FFCA |
| C6H4O5 | 2,5-furan-dicarboxylic acid | FDCA |
| CH2OHCHOHCH2OH | glycerol | Gly |
| — | oxidation reaction of glycerol | GOR |
| — | high-entropy alloy nanoparticles | HEANAs |
| — | glycerol-coupled electrolysis | GOE |
| C5H10O5 | xylose | Xyl |
| — | xylose oxidation reaction | XOR |
| CH3CH(OH)COOH | lactic acid | LA |
| CH3COOH | acetic acid | AA |
| (CH8O4)n | polyethylene terephthalate | PET |
| HOCH2CH2OH | ethylene glycol | EG |
| — | ethylene glycol oxidation reaction | EGOR |
| HOCH2COOH | glycolic acid | GA |
| — | nickel Foam | NF |
| — | integrated seawater electrolyzer | ISE |
| — | faradaic efficiency | FE |
| — | density functional theory | DFT |
References
- IREAN. Hydrogen. Available online: https://www.irena.org/Energy-Transition/Technology/Hydrogen (accessed on 23 February 2025).
- IEA. The Future of Hydrogen. Available online: https://www.iea.org/reports/the-future-of-hydrogen (accessed on 23 February 2025).
- Li, J.; Duan, H. Recent progress in energy-saving hydrogen production by coupling with value-added anodic reactions. Chem 2024, 10, 3008–3039. [Google Scholar] [CrossRef]
- Pan, U.N.; Kandel, M.R.; Tomar, A.K.; Kim, N.H.; Lee, J.H. Synchronous Surface-Interface and Crystal-Phase Engineered Multifaceted Hybrid Nanostructure of Fe-(1T)-VSe2 Nanosheet and Fe-CoSe2 Nanorods Doped with P for Rapid HER and OER, Kinetics. Small 2023, 20, 2305519. [Google Scholar] [CrossRef]
- Kandel, M.R.; Pan, U.N.; Dhakal, P.P.; Ghising, R.B.; Sidra, S.; Kim, D.H.; Kim, N.H.; Lee, J.H. Manganese-Doped Bimetallic (Co,Ni)2P Integrated CoP in N,S Co−Doped Carbon: Unveiling a Compatible Hybrid Electrocatalyst for Overall Water Splitting. Small 2023, 20, 2307241. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, C.; Qin, M.; Li, B.; Lin, B.; Mao, Q.; Yang, H.; Liu, B.; Wang, Y. Reversible hydrogen spillover in Ru-WO3−x enhances hydrogen evolution activity in neutral pH water splitting. Nat. Commun. 2022, 13, 5382. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Kuang, P.; Wang, L.; Yu, J. Hierarchical porous nickel supported NiFeOxHy nanosheets for efficient and robust oxygen evolution electrocatalyst under industrial condition. Appl. Catal. B Environ. 2021, 299, 120668. [Google Scholar] [CrossRef]
- Tang, J.; Guo, K.; Guan, D.; Hao, Y.; Shao, Z. A semi-vapor electrolysis technology for hydrogen generation from wide water resources. Energy Environ. Sci. 2024, 17, 7394–7402. [Google Scholar] [CrossRef]
- Chen, X.H.; Li, X.L.; Li, T.; Jia, J.H.; Lei, J.L.; Li, N.B.; Luo, H.Q. Enhancing neutral hydrogen production by disrupting the rigid hydrogen bond network on Ru nanoclusters through Nb2O5-mediated water reorientation. Energy Environ. Sci. 2024, 17, 5091–5101. [Google Scholar] [CrossRef]
- Amer, M.S.; Arunachalam, P.; Al-Mayouf, A.M.; Alsaleh, A.A.; Alshalwi, M.; A Almutairi, Z. Effect of Iron and Vanadium Doping on Structural Phase Transition in Cobalt Diselenide Enabling Superior Oxygen/Hydrogen Electrocatalysis. ACS Appl. Energy Mater. 2023, 6, 11718–11731. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, Z.; Liu, T.; Wu, Y.; Lan, C.; Jiang, W.; Zhu, L.; Wang, Y.; Yang, D.; Shao, Z. A membrane-based seawater electrolyser for hydrogen generation. Nature 2022, 612, 673–678. [Google Scholar] [CrossRef]
- Guo, J.; Zheng, Y.; Hu, Z.; Zheng, C.; Mao, J.; Du, K.; Jaroniec, M.; Qiao, S.-Z.; Ling, T. Direct seawater electrolysis by adjusting the local reaction environment of a catalyst. Nat. Energy 2023, 8, 264–272. [Google Scholar] [CrossRef]
- Tong, W.; Forster, M.; Dionigi, F.; Dresp, S.; Sadeghi Erami, R.; Strasser, P.; Cowan, A.J.; Farràs, P. Electrolysis of low-grade and saline surface water. Nat. Energy 2020, 5, 367–377. [Google Scholar] [CrossRef]
- Fan, R.; Liu, C.; Li, Z.; Huang, H.; Feng, J.; Li, Z.; Zou, Z. Ultrastable electrocatalytic seawater splitting at ampere-level current density. Nat. Sustain. 2024, 7, 158–167. [Google Scholar] [CrossRef]
- Hajji, S.; Allouche, N.; Bouri, S.; Aljuaid, A.M.; Hachicha, W. Assessment of Seawater Intrusion in Coastal Aquifers Using Multivariate Statistical Analyses and Hydrochemical Facies Evolution-Based Model. Int. J. Environ. Res. Public Health 2021, 19, 155. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.; Jung, G.Y.; Kim, J.H.; Park, S.O.; Park, J.; Kim, Y.-T.; Kang, S.J.; Jeong, H.Y.; Kwak, S.K.; Joo, S.H. Atomically dispersed Pt–N4 sites as efficient and selective electrocatalysts for the chlorine evolution reaction. Nat. Commun. 2020, 11, 412. [Google Scholar] [CrossRef]
- Liu, J.; Duan, S.; Shi, H.; Wang, T.; Yang, X.; Huang, Y.; Wu, G.; Li, Q. Rationally Designing Efficient Electrocatalysts for Direct Seawater Splitting: Challenges, Achievements, and Promises. Angew. Chem. Int. Ed. 2022, 134, e202210753. [Google Scholar] [CrossRef]
- Hong, W.T.; Risch, M.; Stoerzinger, K.A.; Grimaud, A.; Suntivich, J.; Shao-Horn, Y. Toward the rational design of non-precious transition metal oxides for oxygen electrocatalysis. Energy Environ. Sci. 2015, 8, 1404–1427. [Google Scholar] [CrossRef]
- Dresp, S.; Dionigi, F.; Klingenhof, M.; Strasser, P. Direct Electrolytic Splitting of Seawater: Opportunities and Challenges. ACS Energy Lett. 2019, 4, 933–942. [Google Scholar] [CrossRef]
- Saada, H.; Fabre, B.; Loget, G.; Benoit, G. Is Direct Seawater Splitting Realistic with Conventional Electrolyzer Technologies? ACS Energy Lett. 2024, 9, 3351–3368. [Google Scholar] [CrossRef]
- Liu, D.; Cai, Y.; Wang, X.; Zhuo, Y.; Sui, X.; Pan, H.; Wang, Z. Innovations in electrocatalysts, hybrid anodic oxidation, and electrolyzers for enhanced direct seawater electrolysis. Energy Environ. Sci. 2024, 17, 6897–6942. [Google Scholar] [CrossRef]
- Vos, J.G.; Wezendonk, T.A.; Jeremiasse, A.W.; Koper, M.T.M. MnOx/IrOx as Selective Oxygen Evolution Electrocatalyst in Acidic Chloride Solution. J. Am. Chem. Soc. 2018, 140, 10270–10281. [Google Scholar] [CrossRef]
- Veroneau, S.S.; Hartnett, A.C.; Thorarinsdottir, A.E.; Nocera, D.G. Direct Seawater Splitting by Forward Osmosis Coupled to Water Electrolysis. ACS Appl. Energy Mater. 2022, 5, 1403–1408. [Google Scholar] [CrossRef]
- Ma, T.; Xu, W.; Li, B.; Chen, X.; Zhao, J.; Wan, S.; Jiang, K.; Zhang, S.; Wang, Z.; Tian, Z.; et al. The Critical Role of Additive Sulfate for Stable Alkaline Seawater Oxidation on Nickel-Based Electrodes. Angew. Chem. Int. Ed. 2021, 60, 22740–22744. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Li, J.; Wang, K.; Li, Z.; Zhu, M.; Xu, Z. Structural Buffer Engineering on Metal Oxide for Long-Term Stable Seawater Splitting. Adv. Funct. Mater. 2022, 32, 2201127. [Google Scholar] [CrossRef]
- Li, D.; Tu, J.; Lu, Y.; Zhang, B. Recent advances in hybrid water electrolysis for energy-saving hydrogen production. Green Chem. Eng. 2023, 4, 17–29. [Google Scholar] [CrossRef]
- Doan, T.L.L.; Nguyen, D.C.; Prabhakaran, S.; Kim, D.H.; Tran, D.T.; Kim, N.H.; Lee, J.H. Single-Atom Co-Decorated MoS2 Nanosheets Assembled on Metal Nitride Nanorod Arrays as an Efficient Bifunctional Electrocatalyst for pH-Universal Water Splitting. Adv. Funct. Mater. 2021, 31, 2100233. [Google Scholar] [CrossRef]
- Amer, M.S.; Arunachalam, P.; Al-Mayouf, A.M.; AlSaleh, A.A.; Almutairi, Z.A. Bifunctional vanadium doped mesoporous Co3O4 on nickel foam towards highly efficient overall urea and water splitting in the alkaline electrolyte. Environ. Res. 2023, 236, 116818. [Google Scholar] [CrossRef]
- Deng, J.; Li, R.; Xiang, K.; Zhang, L.; Sun, K.; Kim, Y.D.; Liu, Z.; Ge, J.; Peng, Z. Superior matching between electrochemical and non-electrochemical reactions to boost furfural electro-oxidation. Chem. Eng. J. 2025, 511, 162214. [Google Scholar] [CrossRef]
- Li, S.; Hou, Y.; Feng, G.; Li, Q.; Zhai, H.; Hua, Q.; Hu, R.; Xu, M.; Zhang, C.; Huang, Z.; et al. High-Entropy Alloy Nanoflower Array Electrodes with Optimizable Reaction Pathways for Low-Voltage Hydrogen Production at Industrial-Grade Current Density. Adv. Mater. 2024, 37, 2416200. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, L. Recent Advances in Hybrid Seawater Electrolysis for Hydrogen Production. Adv. Mater. 2023, 36, 2308647. [Google Scholar] [CrossRef]
- Du, X.; Tan, M.; Wei, T.; Kobayashi, H.; Song, J.; Peng, Z.; Zhu, H.; Jin, Z.; Li, R.; Liu, W. Highly efficient and robust nickel-iron bifunctional catalyst coupling selective methanol oxidation and freshwater/seawater hydrogen evolution via CO-free pathway. Chem. Eng. J. 2023, 452, 139404. [Google Scholar] [CrossRef]
- Xiang, K.; Song, Z.; Wu, D.; Deng, X.; Wang, X.; You, W.; Peng, Z.; Wang, L.; Luo, J.-L.; Fu, X.-Z. Bifunctional Pt–Co3O4 electrocatalysts for simultaneous generation of hydrogen and formate via energy-saving alkaline seawater/methanol co-electrolysis. J. Mater. Chem. A 2021, 9, 6316–6324. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Arshad, F.; Haq, T.u.; Sher, F. Highly selective and corrosion resistant interconnected pNiCo@NF electrocatalysts for methanol-assisted seawater electrolysis. Int. J. Hydrog. Energy 2025, 105, 1123–1132. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, J.; Feng, R.; Wang, L.; Zhang, J.; Luo, J.L.; Fu, X.Z. Less-Energy Consumed Hydrogen Evolution Coupled with Electrocatalytic Removal of Ethanolamine Pollutant in Saline Water over Ni@Ni3S2/CNT Nano-Heterostructured Electrocatalysts. Small Methods 2021, 6, 2101195. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Yu, H.; Deng, K.; Wang, Z.; Xu, Y.; Wang, L.; Wang, H. Defect-Rich PdIr Bimetallene Nanoribbons with Interatomic Charge Localization for Isopropanol-Assisted Seawater Splitting. Small 2023, 19, 2300388. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wang, L.; Jia, T.; Wang, X.; Li, C.; Wang, H.; Li, L.; Zhou, Y.; Zhai, C.; Tao, H.; et al. ZIF-67-derived FeCoNi-LDH with a 3D nanoflower hierarchical structure for highly efficient oxidation of 5-Hydroxymethylfurfural and coupling seawater splitting hydrogen production. Chem. Eng. J. 2024, 481, 148429. [Google Scholar] [CrossRef]
- Guo, S.; Ma, M.; Ge, R.; Algadi, H.; Shao, Q. Hierarchical copper oxide@nickel-cobalt layered double hydroxide for efficient 5-hydroxymethylfurfural electro-oxidation in alkaline seawater. Adv. Compos. Hybrid Mater. 2023, 6, 158. [Google Scholar] [CrossRef]
- Song, L.; Ma, C.; Shi, P.; Zhu, X.; Qu, K.; Zhu, L.; Lu, Q.; Wang, A.-L. Self-supported FeCoNiCuP high-entropy alloy nanosheet arrays for efficient glycerol oxidation and hydrogen evolution in seawater electrolytes. Green Chem. 2024, 26, 10921–10928. [Google Scholar] [CrossRef]
- Deng, B.; Shen, J.; Lu, J.; Huang, C.; Chen, Z.; Peng, F.; Liu, Y. Ru doping triggering reconstruction of cobalt phosphide for coupling glycerol electrooxidation with seawater electrolysis. J. Energy Chem. 2025, 100, 317–326. [Google Scholar] [CrossRef]
- Mao, Q.; Deng, K.; Yu, H.; Xu, Y.; Wang, Z.; Li, X.; Wang, L.; Wang, H. In Situ Reconstruction of Partially Hydroxylated Porous Rh Metallene for Ethylene Glycol-Assisted Seawater Splitting. Adv. Funct. Mater. 2022, 32, 2201081. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Yu, Z.-R.; Zhang, Y.; Barras, A.; Addad, A.; Roussel, P.; Tang, L.-C.; Szunerits, S.; Boukherroub, R. Seawater corrosive engineering assisted in-situ room temperature synthesis of Ni/Co/Fe trimetallic composition to achieve polyester plastics upgrading and green hydrogen production. Chem. Eng. J. 2024, 498, 155472. [Google Scholar] [CrossRef]
- Patil, S.A.; Patil, D.V.; Katkar, P.; Hussain, S.; Nazir, G.; Cho, S.; Inamdar, A.I.; Im, H.; Shrestha, N.K. Sustainable Hydrogen Generation Facilitated through Ethylene Glycol Oxidation in Fresh/Seawater with Cobalt- and Iron-Based Fluorinated Nanosheets. Energy Fuels 2024, 38, 22393–22401. [Google Scholar] [CrossRef]
- Liu, K.; Gao, X.; Liu, C.X.; Shi, R.; Tse, E.C.M.; Liu, F.; Chen, Y. Energy-Saving Hydrogen Production by Seawater Splitting Coupled with PET Plastic Upcycling. Adv. Energy Mater. 2024, 14, 2304065. [Google Scholar] [CrossRef]
- Freitas, D.S.; Teixeira, P.; Pinheiro, I.B.; Castanheira, E.M.S.; Coutinho, P.J.G.; Alves, M.J. Chitosan Nano/Microformulations for Antimicrobial Protection of Leather with a Potential Impact in Tanning Industry. Materials 2022, 15, 1750. [Google Scholar] [CrossRef]
- Sujisha, S.S.; Rema, P.; Sarathkumar, A. Systemic Effects and Medicolegal Aspects of Formic Acid Poisoning—An Autopsy Study. J. Evol. Med. Dent. Sci. 2020, 9, 2166–2170. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, W.; Yu, L.; García-Melchor, M.; Wang, D.; Zhi, L.; Zhang, H. Enabling heterogeneous catalysis to achieve carbon neutrality: Directional catalytic conversion of CO2 into carboxylic acids. Carbon Energy 2023, 6, e362. [Google Scholar] [CrossRef]
- Schwarz, F.; Larenz, E.; Mechler, A.K. Sustainable electrochemical synthesis of dry formaldehyde from anhydrous methanol. Green Chem. 2024, 26, 4645–4652. [Google Scholar] [CrossRef]
- Singh, B.; Goyal, A.; Verma, S.; Singh, L.; Draksharapu, A. Exploring hybrid seawater electrolysis with anodic oxidation reactions (AORs): Recent progress and prospects. Sustain. Energy Fuels 2024, 8, 5131–5146. [Google Scholar] [CrossRef]
- Ullah, A.; Hashim, N.A.; Rabuni, M.F.; Mohd Junaidi, M.U. A Review on Methanol as a Clean Energy Carrier: Roles of Zeolite in Improving Production Efficiency. Energies 2023, 16, 1482. [Google Scholar] [CrossRef]
- Liu, X.; Mao, H.; Liu, G.; Yu, Q.; Wu, S.; Li, B.; Zhou, G.; Li, Z.; Wang, L. Metal doping and Hetero-engineering of Cu-doped CoFe/Co embedded in N-doped carbon for improving trifunctional electrocatalytic activity in alkaline seawater. Chem. Eng. J. 2023, 451, 138699. [Google Scholar] [CrossRef]
- Hedlund, F.H.; Aldrich, P.T. Inherently ill-defined nature of waste: Fatal outdoor poisoning of hazmat waste collection driver—Lessons learned. Process Saf. Prog. 2021, 40, 165–172. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, J.; Xu, C.; Feng, R.; Sui, P.; Wang, L.; Zhang, J.; Luo, J.L.; Fu, X.Z. Hollow NiSe Nanocrystals Heterogenized with Carbon Nanotubes for Efficient Electrocatalytic Methanol Upgrading to Boost Hydrogen Co-Production. Adv. Funct. Mater. 2020, 31, 2008812. [Google Scholar] [CrossRef]
- Li, T.; Li, S.; Liu, Q.; Yin, J.; Sun, D.; Zhang, M.; Xu, L.; Tang, Y.; Zhang, Y. Immobilization of Ni3Co Nanoparticles into N-Doped Carbon Nanotube/Nanofiber Integrated Hierarchically Branched Architectures toward Efficient Overall Water Splitting. Adv. Sci. 2019, 7, 1902371. [Google Scholar] [CrossRef] [PubMed]
- Burlage, H.M.; Hawkins, D.B. Pharmaceutical Applications of Isopropyl Alcohol. I. As a Solvent in Pharmaceutical Manufacturing. J. Am. Pharm. Assoc. 1946, 35, 379–384. [Google Scholar] [CrossRef]
- Mangoufis-Giasin, I.; Fusek, L.; Yang, T.; Khanipour, P.; Brummel, O.; Libuda, J.; Mayrhofer, K.J.J.; Calle-Vallejo, F.; Katsounaros, I. Activity Trends for the Selective Oxidation of 2-Propanol to Acetone on Noble Metal Electrodes in Alkaline Electrolyte. ACS Catal. 2023, 13, 14562–14569. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, W.; Cao, Y.; Sun, J.; Zhong, Y.; Shi, S.; Duan, X.; Zhou, X. Mechanistic insights into the formation pathway of acetone in selective oxidation of propane with H2 and O2 over Au/uncalcined TS-1 catalyst. J. Catal. 2025, 447, 116138. [Google Scholar] [CrossRef]
- Zhao, K.; Yang, W.; Li, L.; Wang, S.; Wang, L.; Qi, Z.; Yang, Y.; Chen, Z.; Zhuang, J.; Hao, J.; et al. Discharge Induced-Activation of Phosphorus-Doped Nickel Oxyhydroxide for Oxygen Evolution Reaction. Chem. Eng. J. 2022, 435, 135049. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Yang, J.; Pang, Y.; Zhu, X.; Lu, Y.; Wu, Y.; Wang, J.; Chen, H.; Kou, Z.; et al. Quench-Induced Surface Engineering Boosts Alkaline Freshwater and Seawater Oxygen Evolution Reaction of Porous NiCo2O4 Nanowires. Small 2021, 18, 2106187. [Google Scholar] [CrossRef]
- Deng, K.; Mao, Q.; Wang, W.; Wang, P.; Wang, Z.; Xu, Y.; Li, X.; Wang, H.; Wang, L. Defect-rich low-crystalline Rh metallene for efficient chlorine-free H2 production by hydrazine-assisted seawater splitting. Appl. Catal. B Environ. 2022, 310, 121338. [Google Scholar] [CrossRef]
- He, J.; Peng, J.; Ling, R.; Wang, J. Recent Progress on the Production of Liquid Fuel 2,5-Dimethylfuran via Chemoselective Hydrogenolysis Biomass-Derived 5-Hydroxymethylfurfural. Catalysts 2024, 15, 31. [Google Scholar] [CrossRef]
- Kwon, Y.; Schouten, K.J.P.; van der Waal, J.C.; de Jong, E.; Koper, M.T.M. Electrocatalytic Conversion of Furanic Compounds. ACS Catal. 2016, 6, 6704–6717. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Liu, S.; Cai, W.; Jin, M.; Zhang, T.; Zhang, Z.; Liu, Q.; Liu, X.; Zhang, X.; Wang, F. CoSe2@NiSe-CoSe2 Heterojunction for Enhanced Electrocatalytic 5-Hydroxymethylfurfural Oxidation Coupled with Hydrogen Evolution. Adv. Funct. Mater. 2025, 35, 2421447. [Google Scholar] [CrossRef]
- Pei, A.; Wang, P.; Zhang, S.; Zhang, Q.; Jiang, X.; Chen, Z.; Zhou, W.; Qin, Q.; Liu, R.; Du, R.; et al. Enhanced electrocatalytic biomass oxidation at low voltage by Ni2+-O-Pd interfaces. Nat. Commun. 2024, 15, 5899. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, Z.; Fan, K.; Ren, Z.; Xie, W.; Yang, Y.; Shao, M.; Wei, M. Ultrathin layered double hydroxides nanosheets array towards efficient electrooxidation of 5-hydroxymethylfurfural coupled with hydrogen generation. Appl. Catal. B Environ. 2021, 299, 120669. [Google Scholar] [CrossRef]
- Yang, C.; Wang, C.; Zhou, L.; Duan, W.; Song, Y.; Zhang, F.; Zhen, Y.; Zhang, J.; Bao, W.; Lu, Y.; et al. Refining d-band center in Ni0.85Se by Mo doping: A strategy for boosting hydrogen generation via coupling electrocatalytic oxidation 5-hydroxymethylfurfural. Chem. Eng. J. 2021, 422, 130125. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, S.; Wang, F.; Liu, W.; He, X.; Liu, Q.; Zhang, X.; Liu, X. Modulation strategies of electrocatalysts for 5-hydroxymethylfurfural oxidation-assisted water splitting. Microstructures 2024, 4, 2024043. [Google Scholar] [CrossRef]
- Xia, T.; Yang, J.; Ren, Q.; Fu, Y.; Zhang, Z.; Li, Z.; Shao, M.; Duan, X. Promoting Alcohols Electrooxidation Coupled with Hydrogen Production via Asymmetric Pulse Potential Strategy. Angew. Chem. Int. Ed. 2024, 64, e202420992. [Google Scholar] [CrossRef]
- Liu, C.; Hirohara, M.; Maekawa, T.; Chang, R.; Hayashi, T.; Chiang, C.-Y. Selective electro-oxidation of glycerol to dihydroxyacetone by a non-precious electrocatalyst—CuO. Appl. Catal. B Environ. 2020, 265, 118543. [Google Scholar] [CrossRef]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, S.; Meng, A.C.; Zheng, B.; Chen, Z.; Tour, J.M.; Lin, J. Laser-induced high-entropy alloys as long-duration bifunctional electrocatalysts for seawater splitting. Energy Environ. Sci. 2024, 17, 8670–8682. [Google Scholar] [CrossRef]
- Wang, X.; Peng, Q.; Zhang, X.; Lv, X.; Wang, X.; Fu, Y. Carbonaceous-assisted confinement synthesis of refractory high-entropy alloy nanocomposites and their application for seawater electrolysis. J. Colloid Interface Sci. 2022, 607, 1580–1588. [Google Scholar] [CrossRef]
- Shi, M.; Tang, T.; Xiao, L.; Han, J.; Bai, X.; Sun, Y.; Chen, S.; Sun, J.; Ma, Y.; Guan, J. Nanoflower-like high-entropy Ni–Fe–Cr–Mn–Co (oxy)hydroxides for oxygen evolution. Chem. Commun. 2023, 59, 11971–11974. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yao, Y.; Ouyang, W.; Liu, Z.; Yin, H.; Wang, D. A stable oxygen evolution splitting electrocatalysts high entropy alloy FeCoNiMnMo in simulated seawater. J. Mater. Sci. Technol. 2023, 138, 29–35. [Google Scholar] [CrossRef]
- Liu, X.; Chi, J.; Mao, H.; Wang, L. Principles of Designing Electrocatalyst to Boost Reactivity for Seawater Splitting. Adv. Energy Mater. 2023, 13, 2301438. [Google Scholar] [CrossRef]
- Zhu, J.; Chi, J.; Cui, T.; Guo, L.; Wu, S.; Li, B.; Lai, J.; Wang, L. F doping and P vacancy engineered FeCoP nanosheets for efficient and stable seawater electrolysis at large current density. Appl. Catal. B Environ. 2023, 328, 122487. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, C.; Liu, Y.; Ye, Q.; Lu, J.; Zhao, Y.; Ma, J.; Cheng, Y. Room-Temperature Synthesis of Amorphous Fe-Doped Nickel Phosphate for High-Efficiency Alkaline Seawater Oxidation. Small 2025, 21, 2410739. [Google Scholar] [CrossRef]
- Song, Y.; Sun, M.; Zhang, S.; Zhang, X.; Yi, P.; Liu, J.; Huang, B.; Huang, M.; Zhang, L. Alleviating the Work Function of Vein-Like CoXP by Cr Doping for Enhanced Seawater Electrolysis. Adv. Funct. Mater. 2023, 33, 2214081. [Google Scholar] [CrossRef]
- Liu, X.; Yu, Q.; Qu, X.; Wang, X.; Chi, J.; Wang, L. Manipulating Electron Redistribution in Ni2P for Enhanced Alkaline Seawater Electrolysis. Adv. Mater. 2023, 36, 2307395. [Google Scholar] [CrossRef]
- Yang, Y.; Zou, R.; Gan, J.; Wei, Y.; Chen, Z.; Li, X.; Admassie, S.; Liu, Y.; Peng, X. Integrating electrocatalytic seawater splitting and biomass upgrading via bifunctional nickel cobalt phosphide nanorods. Green Chem. 2023, 25, 4104–4112. [Google Scholar] [CrossRef]
- Teke, S.; Saud, S.; Bhattarai, R.M.; Ali, A.; Nguyen, L.; Denra, A.; Nguyen, D.B.; Mok, Y.S. Optimization of PET depolymerization for enhanced terephthalic acid recovery from commercial PET and post consumer PET-bottles via low-temperature alkaline hydrolysis. Chemosphere 2024, 365, 143391. [Google Scholar] [CrossRef]
- Sanko, V.; Sahin, I.; Aydemir Sezer, U.; Sezer, S. A versatile method for the synthesis of poly(glycolic acid): High solubility and tunable molecular weights. Polym. J. 2019, 51, 637–647. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Umer, M.; Son, Y.; Govindaraju, S.; Chellasamy, G.; Panda, A.; Park, J.; Umer, S.; Kim, J.; Choi, S.I.; et al. An Amiable Design of Cobalt Single Atoms as the Active Sites for Oxygen Evolution Reaction in Desalinated Seawater. Small 2023, 20, 2305289. [Google Scholar] [CrossRef]
- Wang, S.; Wang, M.; Liu, Z.; Liu, S.; Chen, Y.; Li, M.; Zhang, H.; Wu, Q.; Guo, J.; Feng, X.; et al. Synergetic Function of the Single-Atom Ru–N4 Site and Ru Nanoparticles for Hydrogen Production in a Wide pH Range and Seawater Electrolysis. ACS Appl. Mater. Interfaces 2022, 14, 15250–15258. [Google Scholar] [CrossRef]
- Kim, M.S.; Tran, D.T.; Nguyen, T.H.; Dinh, V.A.; Kim, N.H.; Lee, J.H. Ni Single Atoms and Ni Phosphate Clusters Synergistically Triggered Surface-Functionalized MoS2 Nanosheets for High-performance Freshwater and Seawater Electrolysis. Energy Environ. Mater. 2022, 5, 1340–1349. [Google Scholar] [CrossRef]
- Guo, L.; Chi, J.; Zhu, J.; Cui, T.; Lai, J.; Wang, L. Dual-doping NiMoO4 with multi-channel structure enable urea-assisted energy-saving H2 production at large current density in alkaline seawater. Appl. Catal. B Environ. 2023, 320, 121977. [Google Scholar] [CrossRef]
- Lin, Y.; Sun, K.; Chen, X.; Chen, C.; Pan, Y.; Li, X.; Zhang, J. High-precision regulation synthesis of Fe-doped Co2P nanorod bundles as efficient electrocatalysts for hydrogen evolution in all-pH range and seawater. J. Energy Chem. 2021, 55, 92–101. [Google Scholar] [CrossRef]
- Wang, H.; Chen, L.; Tan, L.; Liu, X.; Wen, Y.; Hou, W.; Zhan, T. Electrodeposition of NiFe-layered double hydroxide layer on sulfur-modified nickel molybdate nanorods for highly efficient seawater splitting. J. Colloid Interface Sci. 2022, 613, 349–358. [Google Scholar] [CrossRef]
- Jin, H.; Liu, X.; Vasileff, A.; Jiao, Y.; Zhao, Y.; Zheng, Y.; Qiao, S.-Z. Single-Crystal Nitrogen-Rich Two-Dimensional Mo5N6 Nanosheets for Efficient and Stable Seawater Splitting. ACS Nano 2018, 12, 12761–12769. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, C.; Sun, S.; Zhang, H.; Geng, M.; He, X.; Dong, K.; Luo, Y.; Zheng, D.; Zhuang, W.; et al. Boosting Alkaline Seawater Oxidation of CoFe-layered Double Hydroxide Nanosheet Array by Cr Doping. Small 2023, 20, 2307294. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, C.; Weng, S.; Lv, H.; Li, P.; Deng, S.; Hao, W. Construction of an “environment-friendly” CuBx@PU self-supporting electrode toward efficient seawater electrolysis. Green Chem. 2022, 24, 5918–5929. [Google Scholar] [CrossRef]




| Substance Class | Reactant | Catalyst | Electrolyte | Potential (vs. RHE)/Cell Voltage (V) | Current Density (mA cm−2) | Main Product | Refs. |
|---|---|---|---|---|---|---|---|
| small organic molecule | methanol | NiFe2O4/NF | Seawater + methanol | 1.74 | 100 | formate | [32] |
| methanol | Pt–Co3O4 | 1.0 M NaOH + 3.5%NaCl + 2.0 Mmethanol. | 0.533 | 10 | formate | [33] | |
| methanol | Pt–Co3O4 | 1.0 M NaOH + 3.5% NaCl + 2.0 M methanol. | 0.647 | 50 | formate | [33] | |
| methanol | pNiCo@NF | 1.0 M KOH + 0.5NaCl + 2.0 M methanol | 1.38 | 50 | formate | [34] | |
| ethanolamine | Ni@Ni3S2/CNT | 1.0 M NaCl + 0.5M ethanolamine | 1.63 | 10 | glycinate | [35] | |
| ethanolamine | Ni@Ni3S2/CNT | 1.0 M NaCl + 0.5M ethanolamine | 2.13 | 100 | glycinate | [35] | |
| Isopropanol | PdIr BNRs | Seawater + isopropanol | 0.38 | 10 | acetone | [36] | |
| biomass | HMF | FeCoNi-S@NF | 1 M KOH HMF + 0.5 M NaCl | 1.7 | 10 | FDCA | [37] |
| HMF | CuO@NiCo-LDH | 1.0 M KOH + 0.5 M NaCl + 50 Mm HMF | 1.4 | 133.4 | FDCA | [38] | |
| glycerol | FeCoNiCuP HEANAs | 1.0 M KOH + 0.1 M | 1.4 | 10 | formate | [39] | |
| Glycerol + 0.6NaCl | |||||||
| glycerol | Ru-CoP2 | seawater + 1 M glycerol | 1.43 | 100 | formate | [40] | |
| xylose. | NiCoP | 1.0 M KOH seawater+ | 1.57 | 100 | Formic acid | [35] | |
| 100 mM xylose. | |||||||
| plastic monomer | ethylene glycol | Rh/RhOOH metallene | 1 M KOH seawater + 6 M EG | 0.678 | 10 | glycolate | [41] |
| ethylene glycol | A-CoFeNi | 1 M KOH + 0.3 M EG + seawater | 1.38 | 10 | formate | [42] | |
| ethylene glycol | A-CoFeNi | 1 M KOH + 0.3 M EG + seawater | 1.56 | 100 | formate | [42] | |
| ethylene glycol | 2DCoFe@OF/NF | alkaline seawater + EG | 1.42 | 10 | formate | [43] | |
| ethylene glycol | 2DCoFe@OF/NF | alkaline seawater + EG | 1.48 | 100 | formate | [43] | |
| ethylene glycol | Pd-CuCo2O4 | alkaline seawater + EG | 0.68 | 100 | glycolate | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Yang, C.; Yang, J.; Xiao, X.; Ran, M.; Li, J. Recent Progress in Seawater Splitting Hydrogen Production Assisted by Value-Added Electrooxidation Reactions. Energies 2025, 18, 3016. https://doi.org/10.3390/en18123016
Guo Y, Yang C, Yang J, Xiao X, Ran M, Li J. Recent Progress in Seawater Splitting Hydrogen Production Assisted by Value-Added Electrooxidation Reactions. Energies. 2025; 18(12):3016. https://doi.org/10.3390/en18123016
Chicago/Turabian StyleGuo, Yuanping, Chenghao Yang, Jianli Yang, Xin Xiao, Maofei Ran, and Jing Li. 2025. "Recent Progress in Seawater Splitting Hydrogen Production Assisted by Value-Added Electrooxidation Reactions" Energies 18, no. 12: 3016. https://doi.org/10.3390/en18123016
APA StyleGuo, Y., Yang, C., Yang, J., Xiao, X., Ran, M., & Li, J. (2025). Recent Progress in Seawater Splitting Hydrogen Production Assisted by Value-Added Electrooxidation Reactions. Energies, 18(12), 3016. https://doi.org/10.3390/en18123016







