Abstract
Carbon monoxide (CO) is classified as a simple fuel that contains one carbon and one oxygen atom. The oxidation of CO with an oxidizer is relatively unusual, with the oxidation of CO having a slow reaction time. The addition of a small amount of “hydrogenous” species, such as H2, H2O, and CH4, will substantially increase the reaction time. This study numerically investigated and compared the effects of different hydrogenous species addition on the premixed CO/air flames, which act as the initiation of a CO/air flame, on the adiabatic flame temperature, laminar flame speed, and heat release rates at standard conditions (298 K and 1 atm pressure) using San Diego Mechanism. The results showed that the addition of critical hydrogenous species distinguished the difference between dry and wet CO/air oxidation, in which different hydrogenous species have an identical critical value. Adding different hydrogenous species and different addition ratios has an indistinguishable adiabatic flame temperature, while adding CH4 has a higher laminar flame speed distribution compared with H2 and H2O addition, respectively. Furthermore, the laminar flame speed positively correlates with the net heat release rate, which adding CH4 has a noticeable increase on the net heat release rate. Adding more hydrogenous species makes the reactant more reactive and moves the reaction zone upstream. Finally, the dominant reactions to the heat release rate are identical in different hydrogenous species addition, where R23: CO + O (+M) ⇌ CO2 (+M) becomes the most contributed reaction.
1. Introduction
Carbon monoxide (CO) is a colorless, tasteless, and odorless gas classified as toxic due to its various health problems. When carbon monoxide enters the human lungs, it can cause reactions that dispossess oxygen from the human cells and diminish the oxygen storage in a muscle [1]. Once carbon monoxide is exposed to the air, the CO/air mixture becomes dangerous due to its wide flammability limit range (12.5–74%) [2], especially in a confined space. Carbon monoxide can be produced during the incomplete combustion of carbon fuels or appear naturally from forest fires and volcanic eruptions. Besides its harmful effects, carbon monoxide is also usually used in many industries, such as producing various chemicals, food processing, and agriculture. Also, carbon monoxide is one of the important species in hydrocarbon oxidation, which facilitates the production of CO2 [3].
In the combustion fields, carbon monoxide is classified as a fuel and produces carbon dioxide when it reacts with the oxidizer. Two novel carbon monoxide oxidation mechanisms are classified as dry and wet carbon monoxide oxidation. In the dry carbon monoxide oxidation, CO is oxidized into CO2 following the reaction steps of CO + O2 → CO2 + O and CO + O (+M) → CO2 (+M). Due to the slow reaction time, these prolonged reactions cannot be readily attainable as a pure CO flame. Wet carbon monoxide oxidation can be achieved by adding a small number of impurities as a form of “hydrogenous” species, such as H2, H2O, or hydrocarbon. Introducing some hydrogenous species to the CO is oxidized will change its dominant reaction to CO + OH → CO2 + H, which has a faster chemical reaction time [4].
Few literature studies concerning the fundamental studies and the practical applications focus on carbon monoxide combustion. There are considerable challenges to control and minimize the effect of hydrogen-containing species, such as water vapor in humid air, especially in the experimental study and industrial applications [5,6,7]. In the fundamental and numerical study, Rightley and Williams [8] examine the one-step rate expression, which can be used to find an analytical flame speed of CO/air oxidation with the hydrogen-containing species in the fuel between 1% and 10% in various temperature and pressure. The study found that one-step rate expression can accurately predict the flame speed of a CO/air flame in a wide range of conditions using minimum computational resources. In the same series of studies, Rightley and Williams [9] examine the critical hydrogen-containing species to discern the difference between dry and wet CO/air oxidation. They found that the critical hydrogen-containing species is in a factor of , classified as dry CO oxidation via asymptotic and numerical simulation methods. Kim et al. [10] numerically examine the effect of water vapor addition on CO/O2 flame using counterflow premixed flames configuration. Introducing the water vapor will substantially extend the flammability region of CO/O2 flame. In the experimental study, Shen et al. [2] investigate the visual observation and overpressure characteristics of stoichiometric CO/air flame with and without CO2 addition in a closed duct environment. Their study shows that the addition of CO2 had a remarkable negative effect on flame, which reduced the flame propagation speed and pressure dynamics while increasing the flame instability. Besides the above fundamental studies, the CO-related study mainly blends the CO with H2 to form a syngas (synthetic gas) [11,12,13] in various compositions, pressures, and temperatures. It was then found that hydrogen has a dominant effect on incrementing the laminar flame speed and adiabatic flame temperature, especially in the high hydrogen content.
Such worthy extensive studies have yielded the development of carbon monoxide flames using experimental and numerical methods that are reasonably predictive and comprehensive. On the other hand, most of the studies focus on blending carbon monoxide with hydrogen, which shows the dominance of hydrogen, even though it is a small portion of hydrogen blending. Furthermore, the study of wet CO oxidation with small hydrogenous species impurities (lower than 1%), such as H2, H2O, and CH4, acting as the initiation of CO/air flames has not yet been explored. In the present study, the effects of small hydrogenous species addition as the initiation of the premixed CO/air flame is numerically studied in the one-dimensional freely propagating flame configuration. The critical amount of hydrogenous species addition to distinguish the dry and wet CO oxidation is first examined using laminar flame speed at a stoichiometric mixture. The laminar flame speed, adiabatic flame temperature, and heat release rate for different hydrogenous species additions are analyzed.
2. Methodology
This study used the FreeFlame model from the Cantera library, which uses steady, adiabatic, one-dimensional, unstrained, and freely propagating assumptions of laminar premixed flame. An equilibrate model from the Cantera library was used to calculate the equilibrium species and adiabatic flame temperature using constant enthalpy and pressure settings. Cantera is an open-source tool for solving various thermodynamic and combustion problems, compiling detailed chemical kinetics, thermodynamics, and transport processes using the Python (3.10.16) and a MATLAB (R2024B) interface. The detailed mathematical expression of the Cantera library can be found in the Cantera page documentation [14]. A flame-fixed condition was used for the boundaries of the premixed flame to prevent the slope of species and temperature dependence and make the solution domain-independent. A grid distribution in a 0.08 m width with automatic refinement and a mixture-average transport model was applied with the maximum slope and curve of 0.05 and 0.1, respectively.
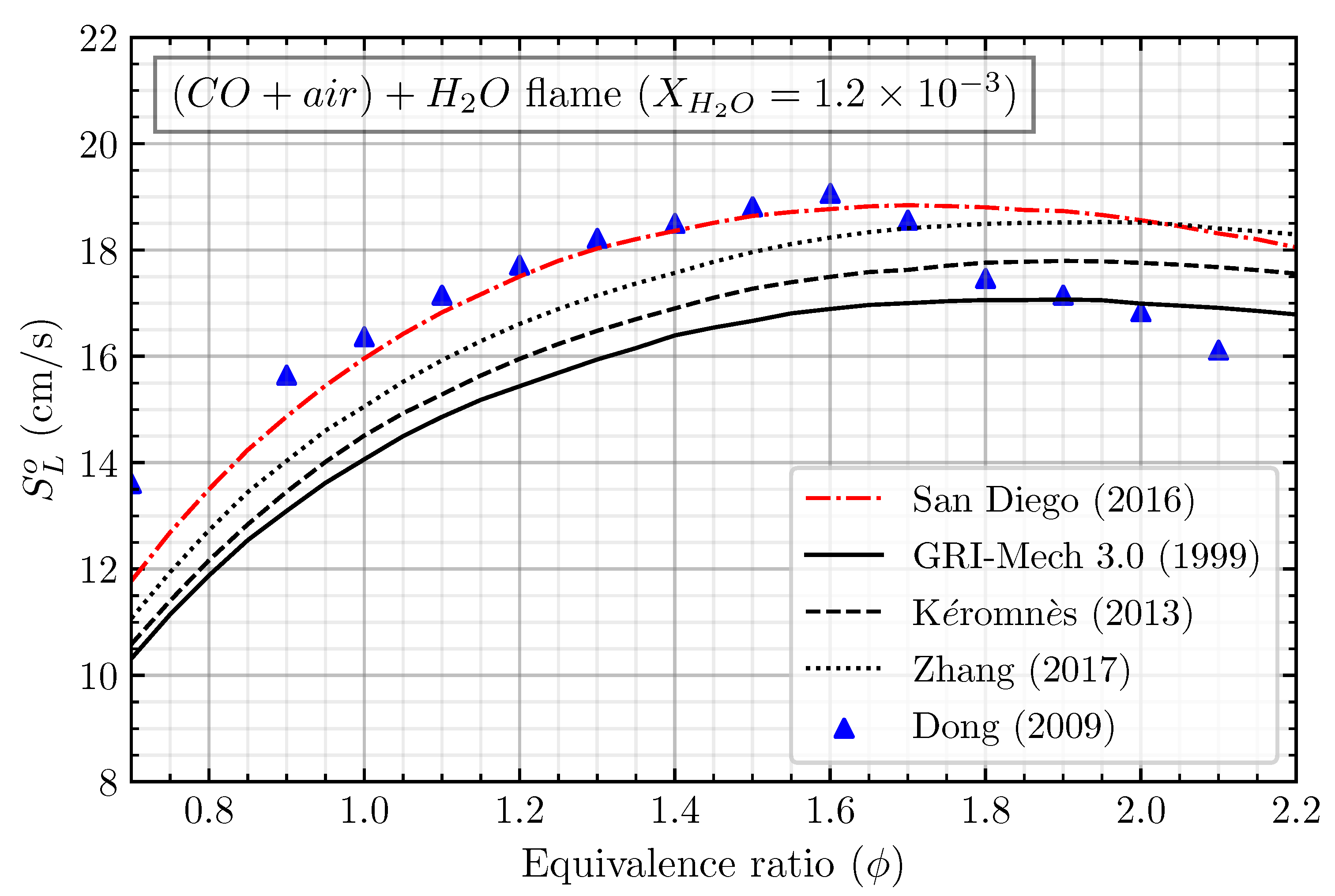
The mechanism validation was first conducted to ensure that the numerical settings and the used mechanism agreed well with the experimental data. Four different kinetic mechanisms, including the San Diego mechanism [15], GRI-Mech 3.0 mechanism [16], Kéromnès mechanism [12], and Zhang mechanism [13], were used to calculate the laminar flame speed of (CO/air) + H2O. The calculated laminar flame speed was then compared with the results of Dong et al. [17], who experimented with the premixed CO/air flame at ambient conditions. It should be noted that, in the numerical simulation of CO/air flame, the solver can only solve the calculation when we treat it as wet carbon monoxide oxidation. Adding any hydrogenous species is necessary to initiate the solver to solve the numerical case of the CO/air flame. When burning carbon monoxide with air, the hydrogenous species may come from the presence of water vapor () in the air to form a humid air. Nevertheless, in the study of Dong et al. [17], they did not specify the amount of , which can be estimated and validated using numerical simulation. The estimation of in the air was firstly conducted by comparing the numerical simulation of stoichiometric (CO/air) + flame with the available measured data by varying the from to . The measured laminar flame speed of CO/air first intersects with the calculated laminar flame speed at = . By these results, we extend the calculation of the laminar flame speed of CO/air flame with = with different equivalence ratios, as shown in Figure 1. The San Diego mechanism result agrees well with the experiment compared to the other mechanisms. The calculated laminar flame speed agrees well with the equivalence ratio between 0.9 and 1.6; meanwhile, it is mostly overestimated in the equivalence ratio lower than 0.9 and higher than 1.6. The discrepancy mainly comes from the uncertainty of the measurement in the lean and rich (CO/air) flame, owing to the lower laminar flame speed of that region. This result indicates that (CO/air) + 1.2 H2O can represent the CO/air flame. Furthermore, the San Diego mechanism will be used for the following analysis.
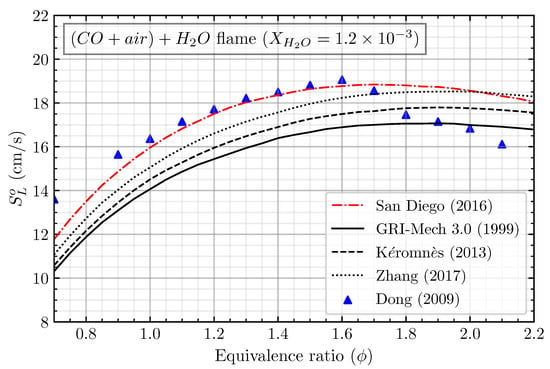
Figure 1.
Mechanism validation of calculated laminar flame speed using San Diego mechanism [15], GRI-Mech 3.0 mechanism [16], Kéromnès mechanism [12], and Zhang mechanism [13] with the experiments of Dong et al. for CO/air flame [17].
The initiation of the premixed CO/air flame study was performed by adding three different hydrogenous species named methane (CH4), hydrogen (H2), and water vapor (H2O). The parameters examined in this study were the species addition ratio () and mixture equivalence ratio (). The species addition ratio () represents the amount of hydrogenous species added to the CO/air mixture. The stoichiometric reaction for the CO/air flame with added species is as follows:
where i represents the CH4, H2, and/or H2O. The mixture equivalence ratio was defined as follows:
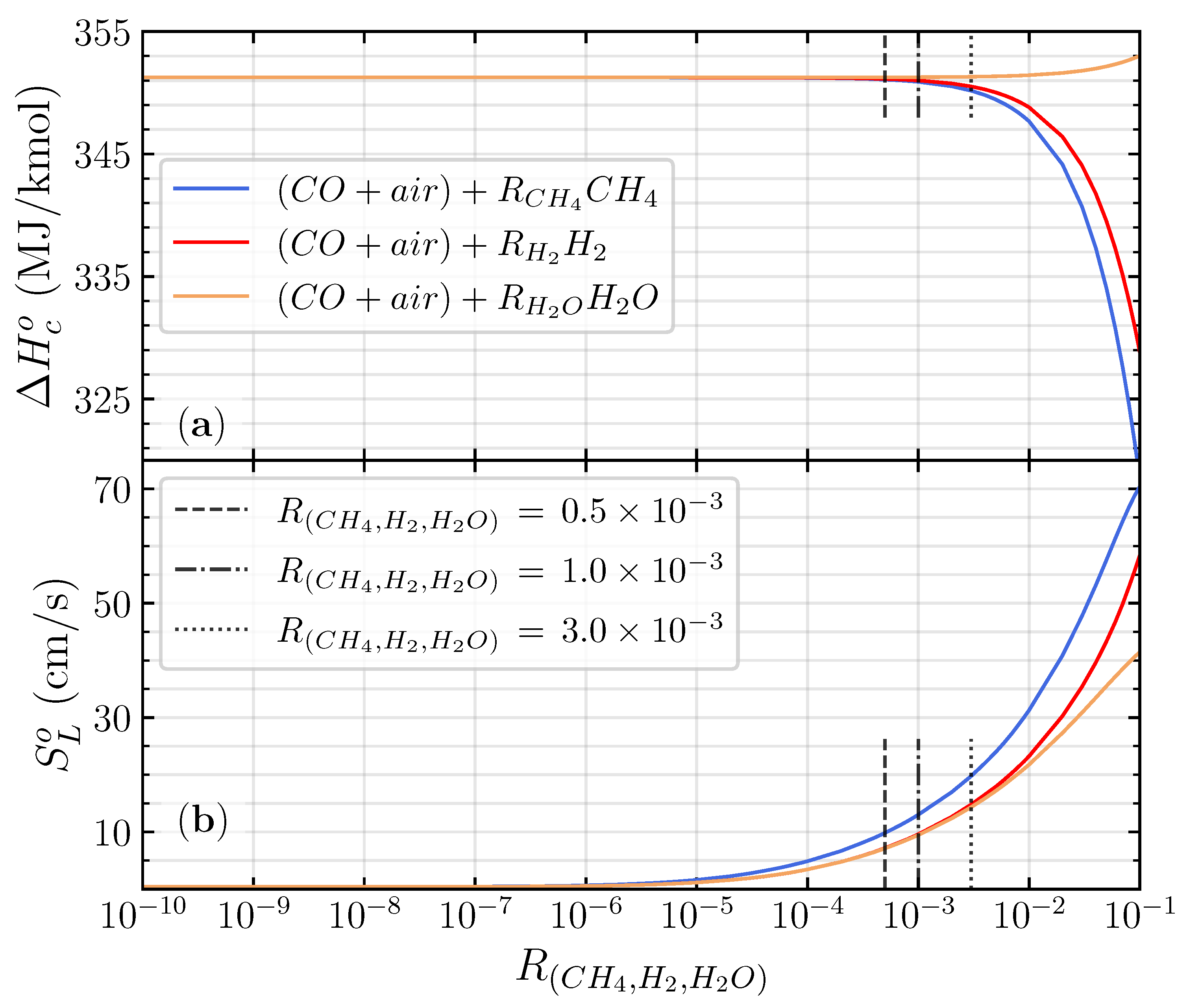
where represents the stoichiometric fuel to oxygen mole fraction of CO/air flame. The investigation involved a value of varied on , , and , which is close to the CO/air flame ((CO/air) + 1.2 H2O). The value of was varied from 0.5 to 4.0 to understand its effect on the wide range of equivalence ratios. It is worth mentioning that varying the from up to in the premixed CO/air flame will not significantly change mole fraction of the mixture. Also, the heat of combustion remains close to the CO/air flames (351 MJ/kmol) in different , as illustrated in Figure 2a. Therefore, the definition of the equivalence ratio in Equation (2) remains the same with the CO/air flame when a small amount of hydrogenous species is introduced to the mixture. The upstream temperature and pressure were set to 300 K, and one atmospheric pressure and all reactants were in the gas phase.
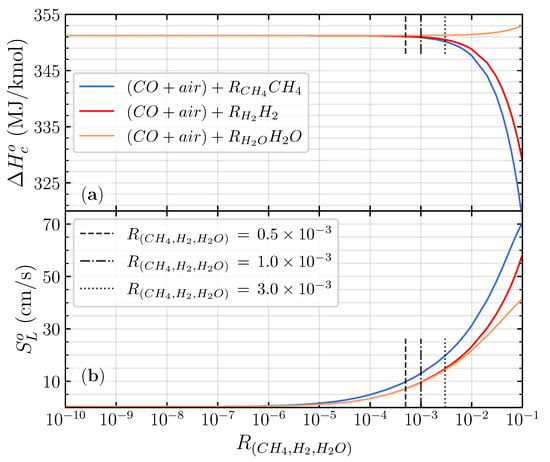
Figure 2.
The heat of combustion (a) and laminar flame speed (b) of the stoichiometric premixed CO/air flames as a function of .
3. Results and Discussion
Figure 2b shows the calculated laminar flame speed of the stoichiometric premixed CO/air flames for different hydrogenous species additions as a function of between and . Adding different hydrogenous species to the CO/air flame has almost an similar laminar flame speed distribution for between and . In the greater than , the laminar flame speed of all different hydrogenous species starts to exponentially increase up to . The exponential increase in laminar flame speed distinguished the difference between the dry and wet oxidation of the premixed CO/air flame [9]. The critical hydrogenous addition ratio () to shift the dry and the wet CO/air oxidation for different hydrogenous species is identical, owing to the promotion of H radicals from hydrogenous species decomposition [4]. The sensitivity analysis shows that the sensitivity coefficient of the top four essential elementary reactions starts to fluctuate when the (detailed analysis in Figure 3 below). Adding CH4 to the premixed CO/air flame has a higher distribution of laminar flame speed compared with H2 and H2O addition, respectively. CH4 will promote more OH radicals to react with CO to produce CO2 compared with H2 and H2O (detailed analysis in Figure 4 below).
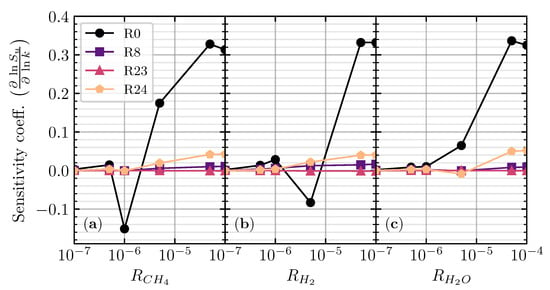
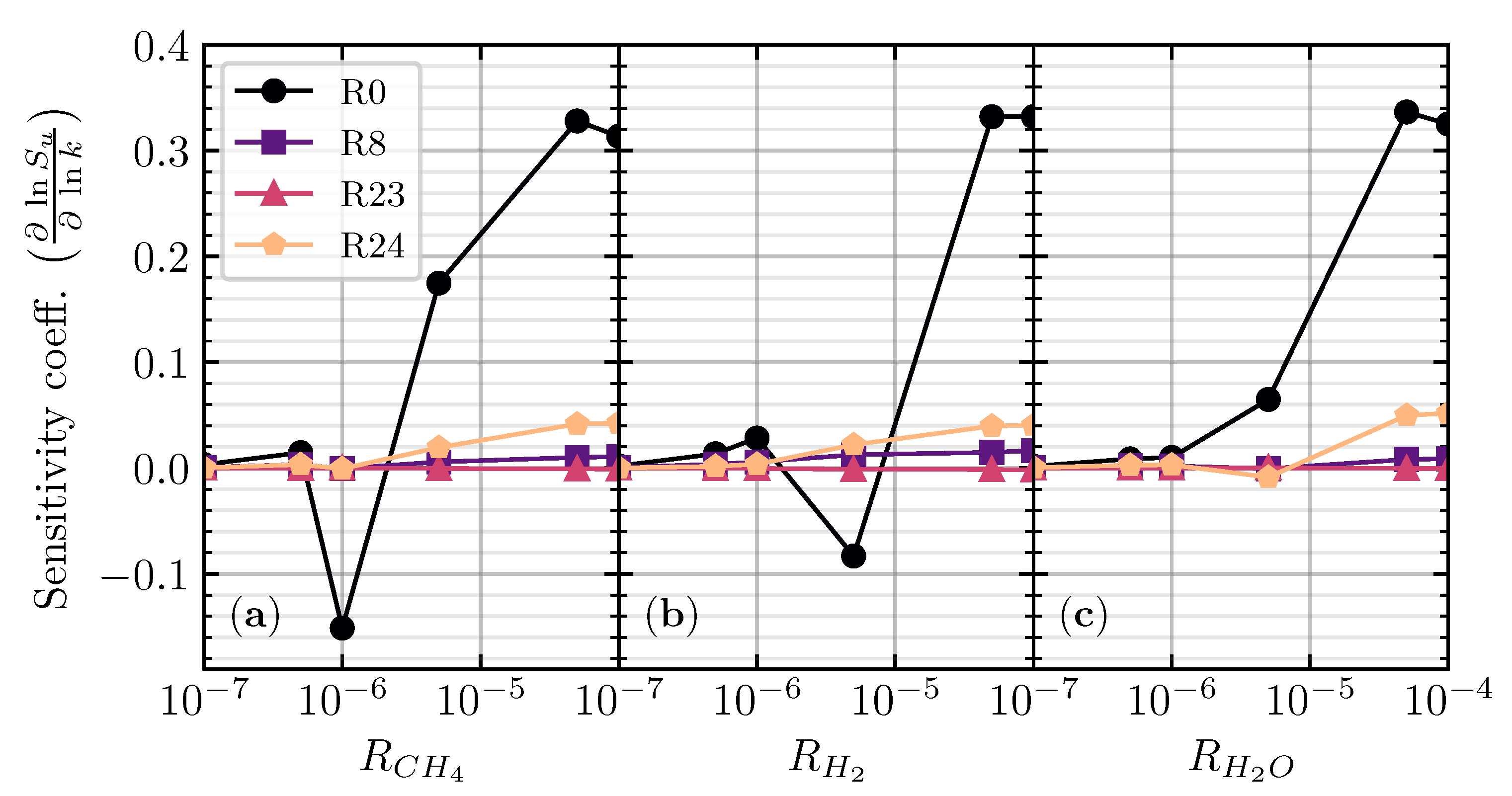
Figure 3.
Sensitivity analysis of the laminar flame speed for stoichiometric CO/air flame with CH4 (a), H2 (b), and H2O (c) addition at different addition ratios ().
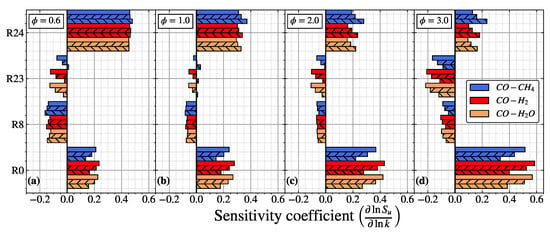
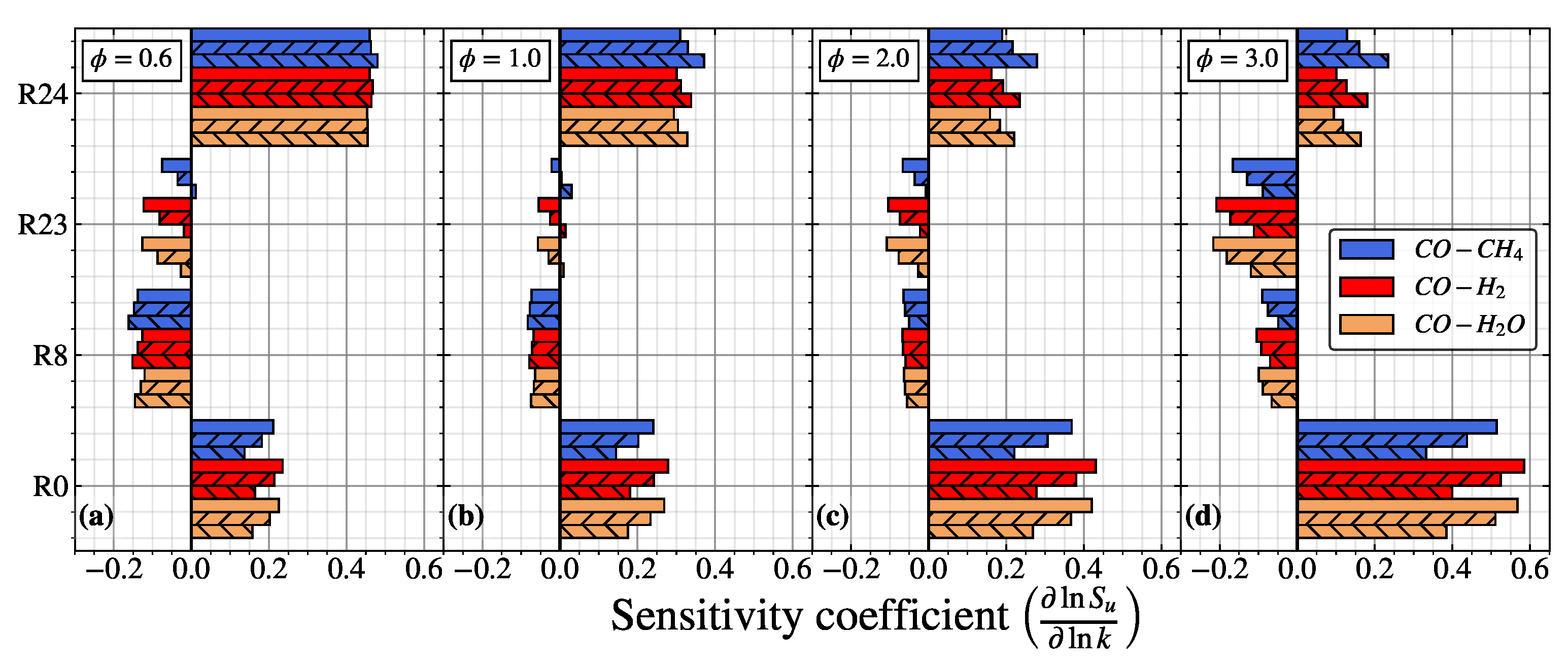
Figure 4.
Sensitivity analysis of the laminar flame speed for CO/air flame at (a), (b), (c), and (d), respectively. The no hatch, forward slash (//), and backward slash () pattern represent , and , respectively.
3.1. Effects of Different Hydrogenous Species Additions on the Adiabatic Flame Temperature and Laminar Flame Speed
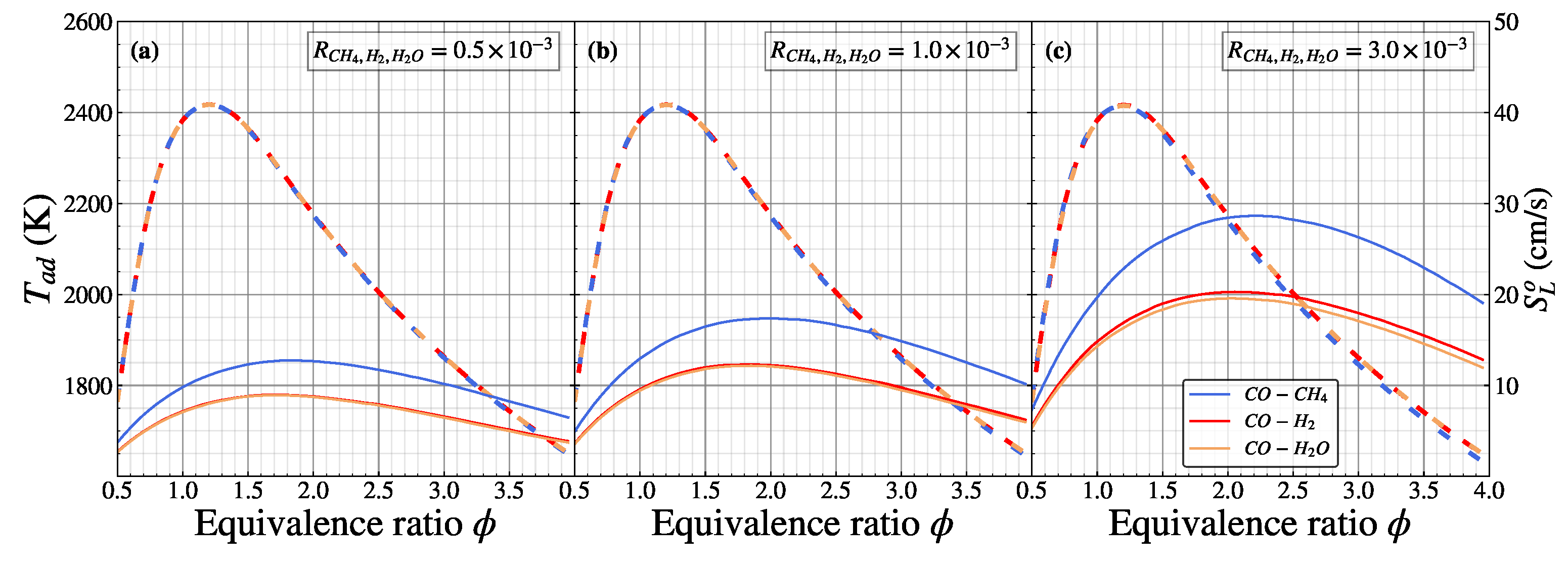
Figure 5 shows the calculated adiabatic flame temperature () and laminar flame speed () of CO/air for different as a function of equivalence ratios. The dashed lines represent the adiabatic flame temperature, while the solid line represents the laminar flame speed. By varying the hydrogenous species addition, the overall distribution of the adiabatic flame temperature of CO/air in the is almost identical by following a parabolic shape. The maximum adiabatic flame temperature is the same for different hydrogenous species additions, located on the equivalence ratio of 1.2 (2417 K), and lower for adiabatic flame temperature on ultra-lean (, 1765 K) and ultra-rich (, 1648 K) conditions. When the increased from to , the adiabatic flame temperature distribution remains identical, owing to the identical value of the heat of combustion, where the heat of combustion remains close to the CO/air flame. Increasing the further to affected the adiabatic flame temperature of the CH4 addition, which was slightly underestimated in the rich conditions () compared with the H2 and H2O addition. Adding CH4 to the rich CO/air combustion slightly reduced the heat of combustion, making the adiabatic flame temperature reduce, as shown in Figure 2a. The laminar flame speed distribution also follows the parabolic graph as adiabatic flame temperature distribution. In the case of , the laminar flame speed for the CH4 addition has a higher distribution compared with the H2 and H2O addition in different equivalence ratios. This indicates that adding CH4 to the CO/air flame will produce a heat release rate more compared with H2 and H2O addition, leading to a higher laminar flame speed distribution (detailed analysis in Figure 6 below). In the case of H2 and H2O addition, the laminar flame speed is indistinguishable, showing a coinciding line. The natural characteristics of H2 and H2O are identical (two hydrogen atoms), which leads to the interchangeable laminar flame speed distribution. The difference effect of the H2 and H2O addition on the laminar flame speed becomes significant starting from , as shown in Figure 2b. For the , H2 has a considerable influence on the laminar flame speed compared to the H2O addition due to its reactivity [18], which is beyond the scope of the current study. For instance, the maximum laminar flame speed appears at 12.75 cm/s () for the CH4 addition and 8.92 cm/s () for the H2 and H2O addition into the CO/air flame, respectively. When the increases to , the overall laminar flame speed has an identical shape with a higher distribution than . The maximum laminar flame speed of the CH4 addition increases to 17.38 cm/s (), while for the H2 and H2O addition, it increases to 12.30 cm/s (). In , the overall laminar flame speed increases more than twice compared with , where the maximum laminar flame speed of the CH4, H2, and H2O addition is 28.60 cm/s (), 20.29 cm/s (), and 19.57 cm/s (), respectively. The laminar flame speed distribution of the H2 addition starts to be slightly higher than the H2O addition due to its reactivity, especially in the rich mixture. Increasing the has the quasi-linear relation with increasing the maximum laminar flame speed of the CO/air flames.
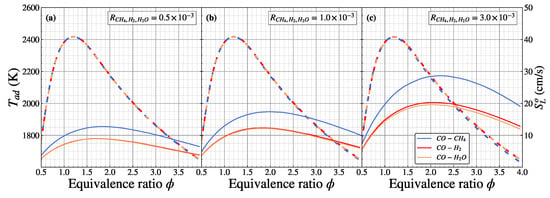
Figure 5.
Adiabatic flame temperature (dashed line) and laminar flame speed (solid line) of premixed CO/air flames for (a), (b), and (c) as a function of equivalence ratio.
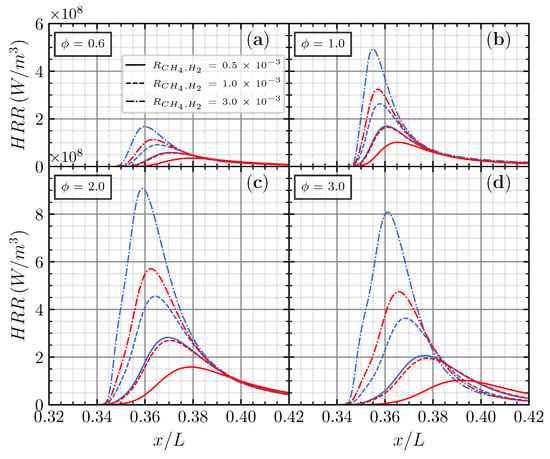
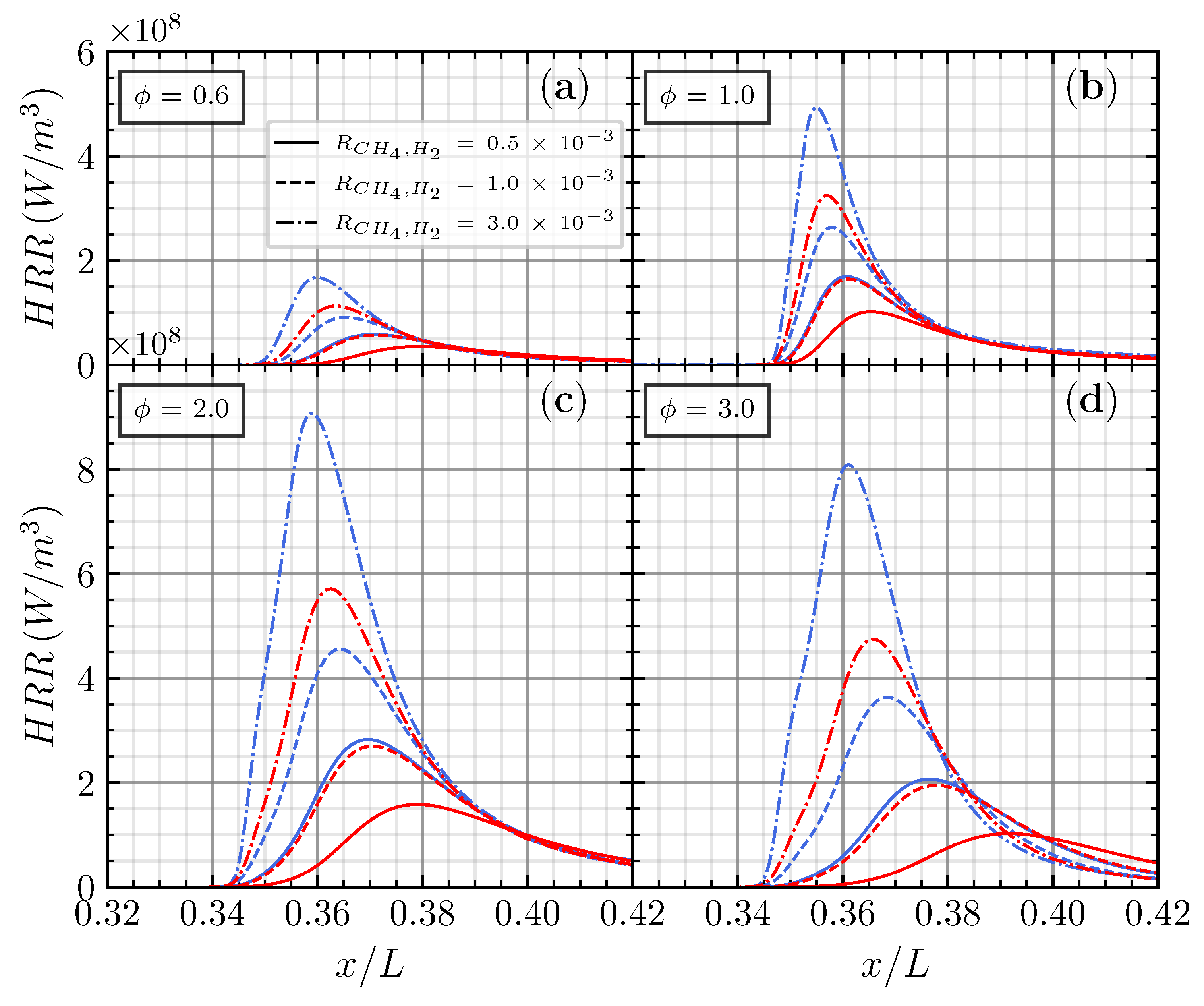
Figure 6.
Net heat release rate for CO/air flame with CH4 (blue) and H2 (red) addition on various additions of (a), (b), (c), and (d), respectively.
The effect of hydrogenous species addition to the laminar flame speed of the CO/air flame can be described as the increment of adiabatic flame temperature (thermal effect), the reduction in activation energy, the increase in active radicals (chemical effect), and the high mobility of hydrogen (transport effect) [19,20,21]. Tang et al. [19] found that the thermal and chemical effects are dominant compared with the transport effect. Nevertheless, adding a small amount of hydrogenous species to the CO/air produces an identical heat of combustion and adiabatic flame temperature, minimizing the thermal effect on the laminar flame speed. Therefore, the change in laminar flame speed mainly comes from the chemical effect.
Sensitivity analysis was performed to understand the major reactions that contributed to the change of the laminar flame speed when CH4, H2, and H2O were added with different additions. Figure 3 and Figure 4 show the sensitivity analysis results of the CO/air flame with different hydrogenous species additions near the and various equivalence ratios, respectively. In Figure 3, the different colors and symbols represent the different reaction numbers. In Figure 4, the no hatch, forward slash (//), and backward slash () pattern represent , and , respectively. The top four essential elementary reactions with their reaction numbers and important properties are shown in Table 1.

Table 1.
Reaction number and reaction equations.
In Figure 3, the sensitivity coefficient of the top four essential elementary reactions is mostly near zero when in different hydrogenous species. When increases to (), the sensitivity coefficient of four reactions becomes fluctuating, indicating the change of the chemical scheme of CO oxidation. R0 becomes the most fluctuated reaction as the hydrogenous species starts to contribute to the production of H radicals from its decomposition, which later reacts with O2 from the air to produce OH radicals (R0). The produced OH radicals then reacted with CO (R24) as the significant reaction contributing to the production of CO2 [4,22]. R24 also starts to have a positive (promotion) sensitivity coefficient as the production mechanism for CO2 is shifted from dry CO oxidation (R23) to wet CO oxidation (R24). It is evidence that R23 stays near zero sensitivity coefficient on as R23 contributed less to the laminar flame speed. Increasing further, the sensitivity coefficients of R0 and R24 become more positive, while R23 is nearly unchanged.
In Figure 4, the dominant reaction contribution in the sensitivity analysis of the laminar flame speed is similar for different in different equivalence ratios. R0 and R24 have a positive sensitivity coefficient, while R8 and R23 have a negative (inhibiting) sensitivity coefficient. In , R24 turns into the most contributed reaction, owing to the presence of O2 becoming less in the lean mixture. Thus, the contribution from the reaction related to the consumption of O2 (R0) becomes inferior. The hydrogenous species produces H radicals, reducing the sensitivity coefficient of R0 slightly (up to ∼6%). In comparison, the sensitivity coefficient of R24 remains the same when increases from to .
In the stoichiometric condition, the sensitivity coefficient of R0 increases, while the sensitivity coefficient of R24 reduces. Increasing the equivalence ratio will generally increase the hydrogenous species and reduce the O2 in the mixture, intensifying the production of OH (R0). It should be noted that the increment of produced OH radicals is not sufficient to react with CO as the amount of hydrogenous species is much less compared with the amount of CO in the premixtures. Consequently, the sensitivity coefficient of R24 reduces in the stoichiometric condition. This implies that the contribution of R0 and R24 is mainly controlled by adding hydrogenous species and primary fuel (CO) species, respectively. When increases from to , the difference of R0 and R24 becomes more distinguishable, with the contribution of R0 becoming weaker, while R24 becomes stronger. The sensitivity coefficient of the third-body reaction (R8 and R23) was also reduced, weakening its inhibiting effect and increasing its laminar flame speed. When increases further to 2.0 and 3.0, the sensitivity coefficients of R0 and R24 repeatedly increase and decrease, respectively. Furthermore, the third body reaction R23 has the minimum inhibiting effects in the = 2.0, which aligns with the pattern illustrated in Figure 5, where the maximum laminar flame speed appears near the = 2.0.
3.2. Effects of Different Hydrogenous Species Additions on Heat Release Rate
The effects of different hydrogenous species additions on the net heat release rate (HRR) were investigated. Since the effect of the H2 and H2O addition are identical, the results will focus on the comparison between the addition of CH4 and H2 in different and . Figure 6 shows the net heat release rate of the CH4 and H2 addition with respect to non-dimensional axial distance () ranging from 0 to 1. Four different equivalence ratios were chosen in order to observe different conditions, including lean (), stoichiometric (), maximum laminar flame speed (), and rich ) conditions.
Increasing from from to linearly increases the net heat release rate for both the CH4 and H2 addition in different equivalence ratios. The maximum net heat release rate () occurred in the , close to the maximum laminar flame speed for CH4 and H2 additions. These findings have linear results presented by Chen et al. [23], in which the laminar flame speed positively correlates with the net heat release rate. Adding CH4 results in a noticeable increase in the net heat release rate, with increasing more than 300% when increases to .
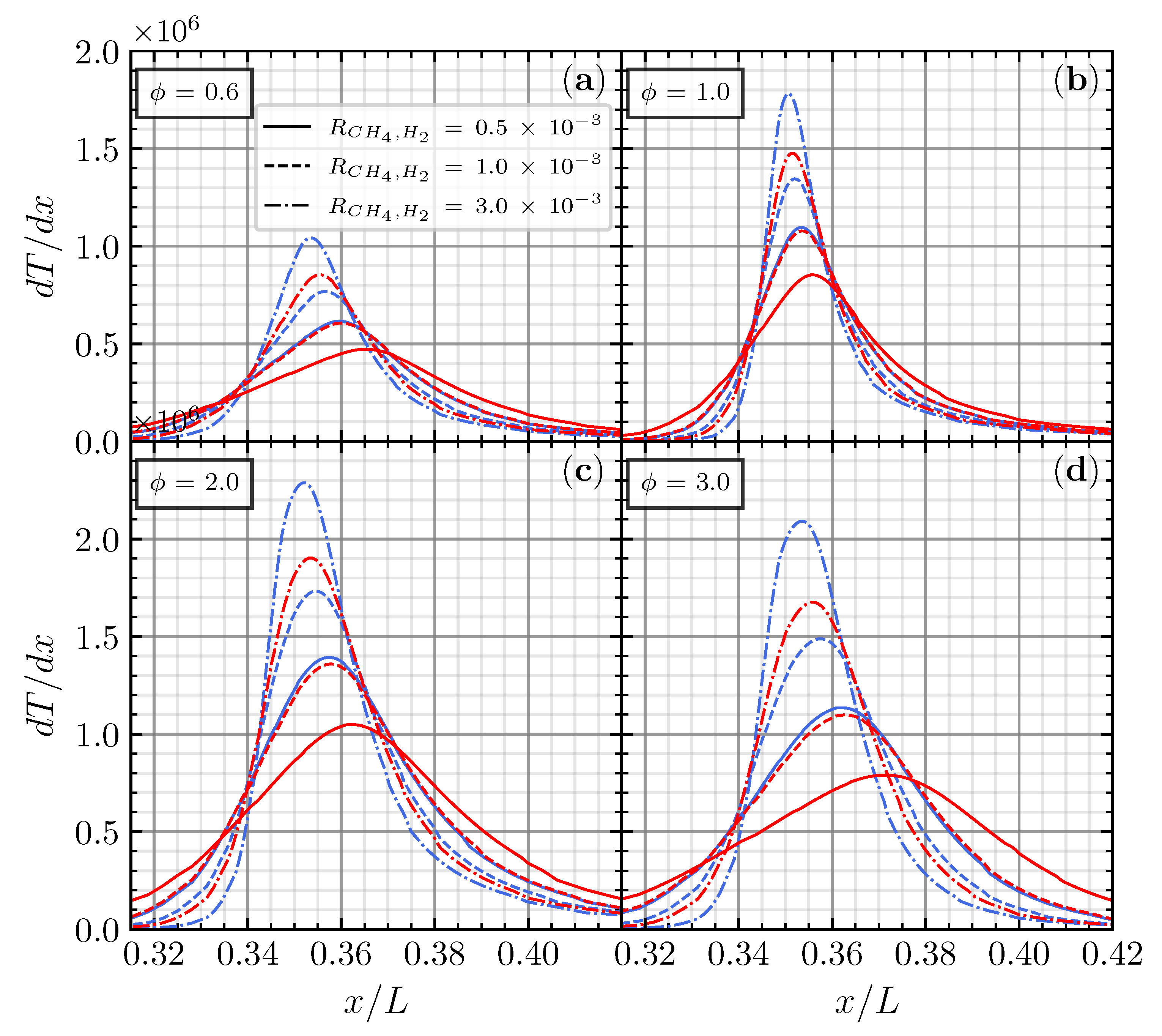
The increment of also affected the location of , which moves upstream in every equivalence ratio. These results are also confirmed with the identical distribution and location of the maximum temperature gradient , presented in Figure 7. usually defines the location of the flame front, representing the fast reaction zones [24]. Also, has a relation with the flame thickness () [25] through the following equation:
where is the unburned mixture temperature, and is the burned combustion product temperature. Since and are identical for different i and (see the distribution of in Figure 5), is a significant factor to affect the . Based on the above equation, the increase in will reduce , indicating the stronger reaction rate in the reaction zone. Smaller would be more prone to having a higher heat release rate. This may infer that the increment and upstream shift of and indicate that increasing on the CO/air flame enhances the production of OH radicals through R0 and makes the reactant more reactive [20].
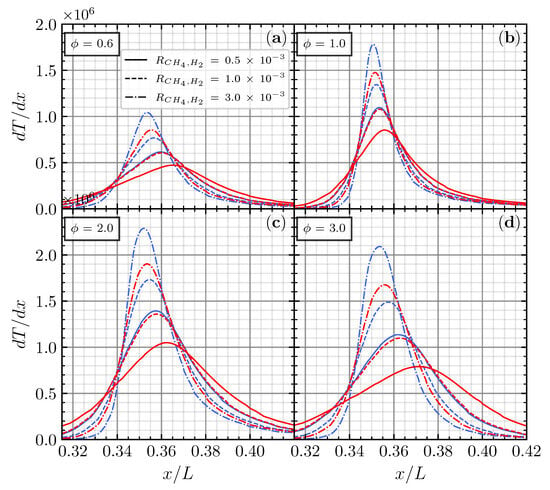
Figure 7.
Temperature gradient for CO/air flame with CH4 (blue) and H2 (red) addition on (a), (b), (c), and (d), respectively.
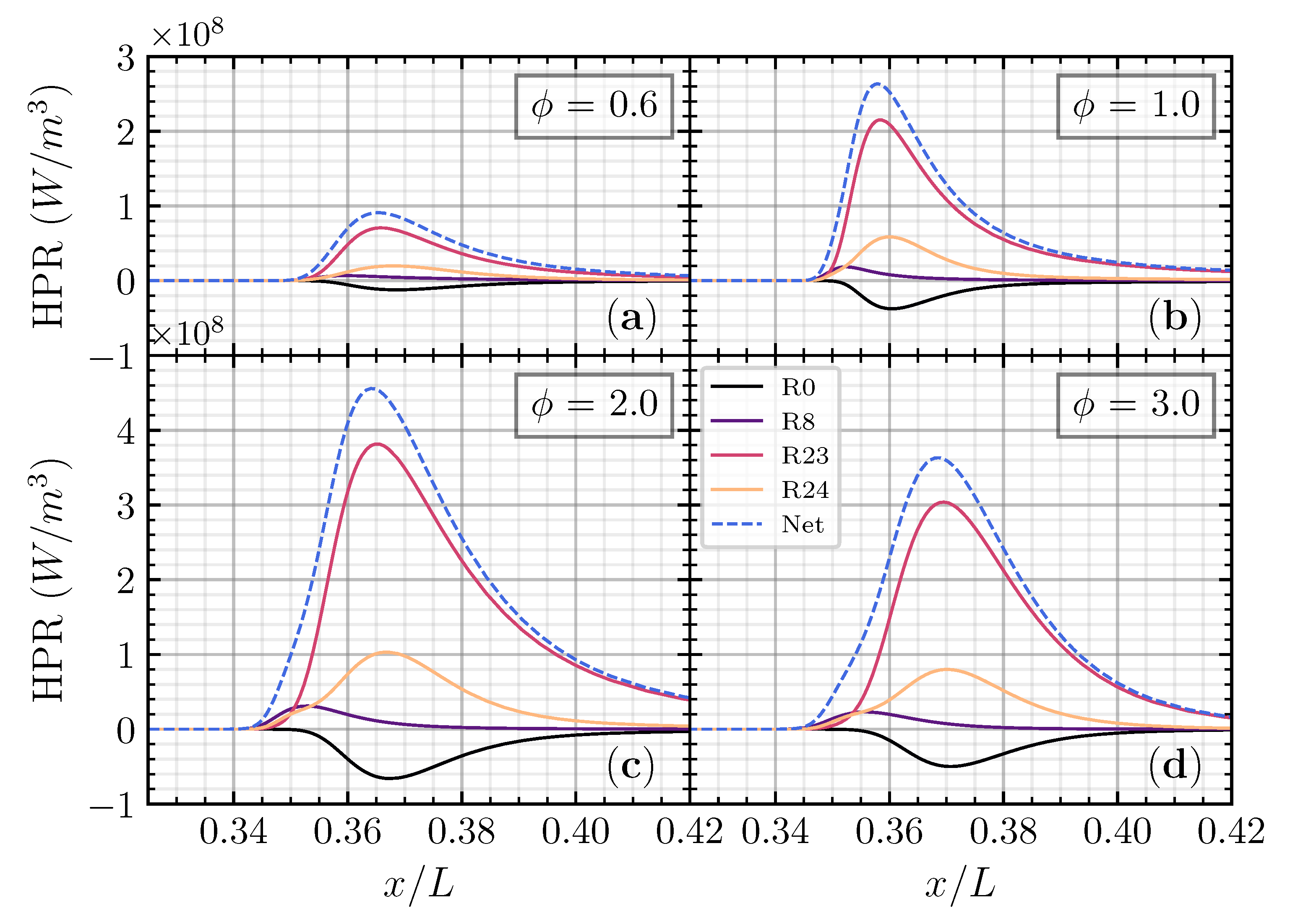
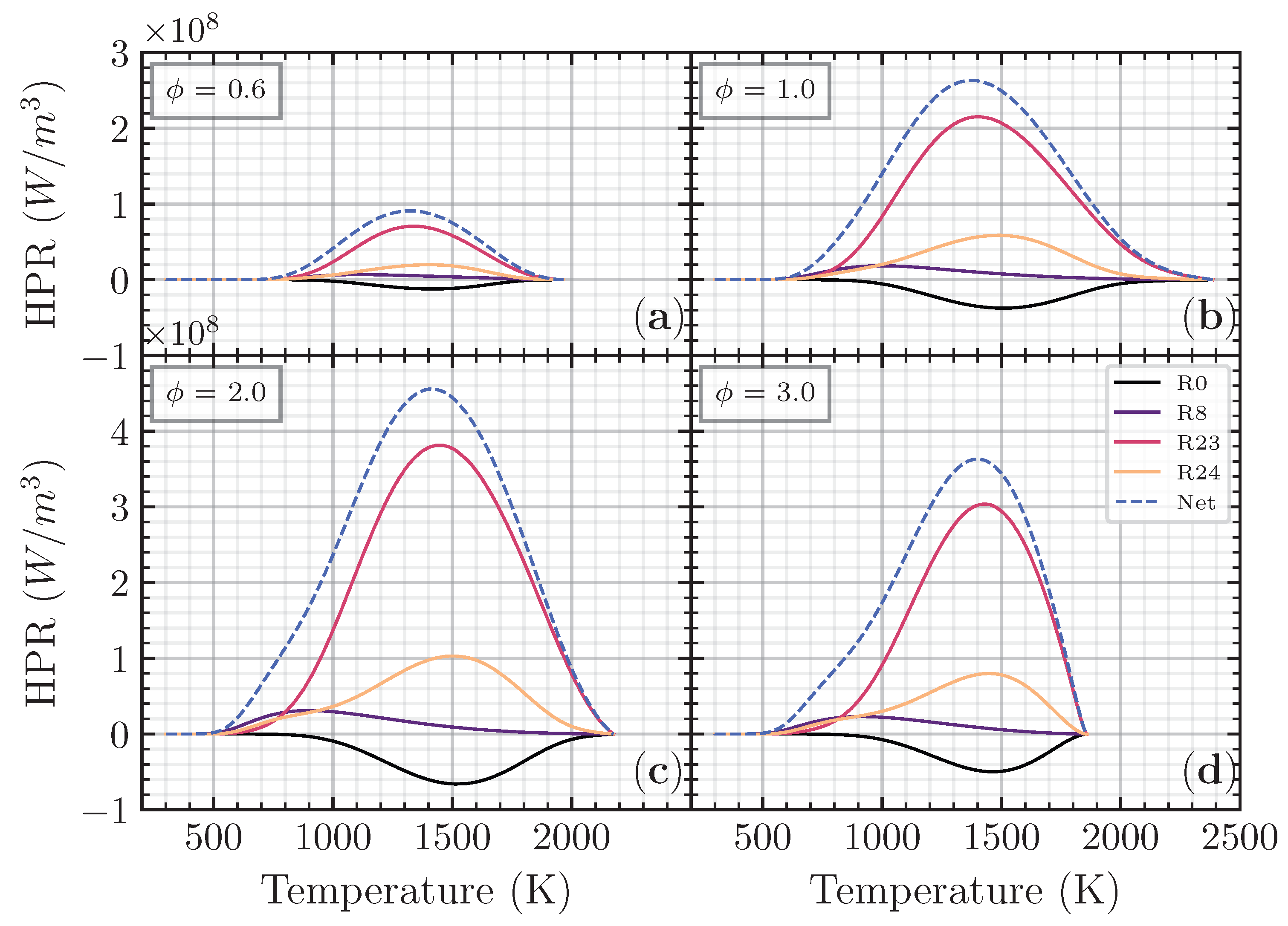
The heat production rate was analyzed to understand the reaction that contributed to the heat release rate. Figure 8 shows that the major reaction contributed to the heat production rate of the CH4 addition on the CO/air flame as a function of non-dimensional axial distance () in different equivalence ratios. was chosen as this addition ratio is close to the CO/air flame case. As we observed previously, adding CH4 to the CO/air flame will increase both the laminar flame speed and net heat release rate compared to H2 and H2O in different and . Thus, the heat production rate analysis will be performed on CH4 in addition to the CO/air flame in various . R23 becomes the major contributing reaction to the heat production rate, followed by R24 and R8. R0 has a negative distribution, meaning that R0 has a heat-absorbing effect on the net heat release rate.
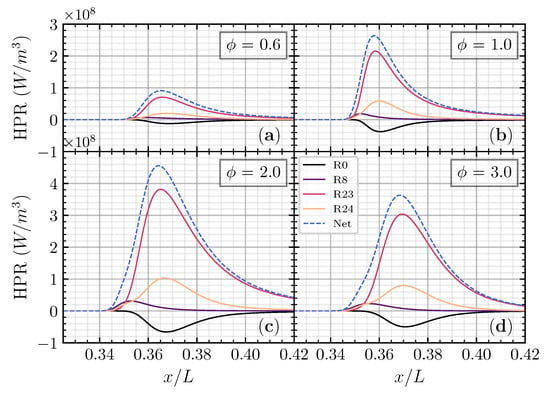
Figure 8.
Heat production rate for CO/air flame with CH4 addition () as a function of non-dimensional axial distance () on (a), (b), (c), and (d), respectively.
The third-body reaction R23 becomes the most contributed reaction with more than 60% contribution to the net heat release rate. The oxidation of the fuel (CO) to become CO2 is an essential reaction contributing to the net heat release rate through R23 and R24. The heat production from R24 has an almost proportional heat absorption of R0, making the net contribution to the total heat release of these two reactions limited. The third-body reaction R8 appears first compared to another reaction in every equivalence ratio, meaning that the heat produced from R8 initiates total heat release. Although the contribution from R8 is relatively small, it is essential to initiate the reaction. Figure 9 shows the major reaction contributed to the heat production rate of the CH4 addition as a function of temperature in different equivalence ratios. R8 has a peak in a relatively low temperature (<1000 K), while R0, R23, and R24 have a peak in a relatively higher temperature (between 1300 K and 1500 K).
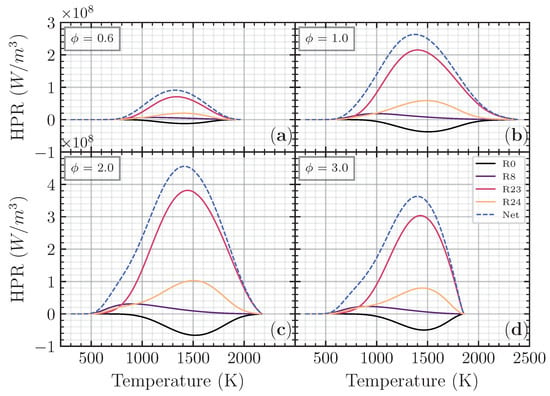
Figure 9.
Heat production rate for CO/air flame with CH4 addition () as a function of temperature (K) on (a), (b), (c), and (d), respectively.
4. Conclusions
In this study, the effects of small hydrogenous species (CH4, H2, and H2O) additions as the initiation of the premixed CO/air flame were numerically investigated using a one-dimensional freely propagating flame configuration. The addition ratios of various hydrogenous species ranging from up to vary with a wide range of equivalence ratios in STP conditions. The combustion characteristics such as adiabatic flame temperature, laminar flame speed, and heat release rate were studied. The major conclusions of this study are as follows:
Adding different hydrogenous species to the premixed CO/air flame has an identical critical value, distinguishing the difference between dry and wet CO/air oxidation, which is . In the greater than , the laminar flame speed of all different hydrogenous species starts to exponentially increase up to .
Adding different hydrogenous species and different addition ratios has an identical adiabatic flame temperature, while adding CH4 has a higher laminar flame speed distribution compared with H2 and H2O addition, respectively. The chemical effects have a noticeable contribution to the laminar flame speed, where R0: H +O2 ⇌ O + OH and R24: CO + OH ⇌ CO2 + H become the most contributed reactions to the flame speed.
The laminar flame speed positively correlates with the net heat release rate, where adding CH4 results in a noticeable increase in the net heat release rate. The hydrogenous species makes the reactant more reactive, and the reaction zone moves upstream. The dominant reactions to the heat release rate are identical in different hydrogenous species additions, where R23: CO + O (+M) ⇌ CO2 (+M) becomes the most contributed reaction.
Author Contributions
Conceptualization, T.-H.L. and G.-B.C.; methodology, A.F. and T.-H.L.; software, A.F.; validation, G.-B.C. and T.-H.L.; formal analysis, A.F. and T.-H.L.; investigation, A.F.; resources, T.-H.L. and G.-B.C.; data curation, A.F.; writing—original draft preparation, A.F.; writing—review and editing, A.F. and T.-H.L.; visualization, A.F.; supervision, G.-B.C. and T.-H.L.; project administration, T.-H.L.; funding acquisition, T.-H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially supported by the National Science and Technology Council of the Republic of China under grant number NSTC 112-2622-E-006-030 and NSTC 113-2622-E-006-004.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CO | Carbon monoxide |
| CH4 | Methane |
| H2 | Hydrogen |
| H2O | Water vapor |
| Mole fraction of species i | |
| Laminar flame speed | |
| Mixture equivalence ratio | |
| Species addition ratio | |
| Adiabatic flame temperature | |
| HRR | Heat release rate |
| HPR | Heat production rate |
| Temperature gradient | |
| Flame thickness |
References
- Majstorović, A.; Babić, V.; Todić, M. Carbon monoxide in the process of uncontrolled combustion-occurrence, hazards and first aid. J. Phys. Conf. Ser. 2020, 1426, 012008. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, Z.; Dou, Z.; Zhang, C. Premixed CO/air combustion in a closed duct with inhibition. Energy 2021, 230, 120782. [Google Scholar] [CrossRef]
- Chen, G.B.; Li, Y.H.; Cheng, T.S.; Chao, Y.C. Chemical effect of hydrogen peroxide addition on characteristics of methane-air combustion. Energy 2013, 55, 564–570. [Google Scholar] [CrossRef]
- Von Elbe, G.; Lewis, B. Free-radical reactions in glow and explosion of carbon monoxide-oxygen mixtures. Combust. Flame 1986, 63, 135–150. [Google Scholar] [CrossRef]
- Hadman, G.; Thompson, H.W.; Hinshelwood, C.N. The explosive oxidation of carbon monoxide at lower pressures. Proc. R. Soc. Lond. Ser. A-Math. Phys. Eng. Sci. 1932, 138, 297–311. [Google Scholar] [CrossRef]
- Leah, A.; Watson, H. Radiation from explosion flames of carbon monoxide. Combust. Flame 1959, 3, 169–186. [Google Scholar] [CrossRef]
- Wang, W.; Rogg, B. Reduced kinetic mechanisms and their numerical treatment I: Wet CO flames. Combust. Flame 1993, 94, 271–292. [Google Scholar] [CrossRef]
- Rightley, M.; Williams, F. Analytical approximations for structures of wet CO flames with one-step reduced chemistry. Combust. Flame 1995, 101, 287–301. [Google Scholar] [CrossRef]
- Rightley, M.L.; Williams, F.A. Burning velocities of CO flames. Combust. Flame 1997, 110, 285–297. [Google Scholar] [CrossRef]
- Taek Kim, G.; Park, J.; Ho Chung, S.; Sang Yoo, C. Effects of water vapor addition on downstream interaction in CO/O2 counterflow premixed flames. Fuel 2023, 342, 127888. [Google Scholar] [CrossRef]
- Davis, S.G.; Joshi, A.V.; Wang, H.; Egolfopoulos, F. An optimized kinetic model of H2/CO combustion. Proc. Combust. Inst. 2005, 30, 1283–1292. [Google Scholar] [CrossRef]
- Kéromnès, A.; Metcalfe, W.K.; Heufer, K.A.; Donohoe, N.; Das, A.K.; Sung, C.J.; Herzler, J.; Naumann, C.; Griebel, P.; Mathieu, O.; et al. An experimental and detailed chemical kinetic modeling study of hydrogen and syngas mixture oxidation at elevated pressures. Combust. Flame 2013, 160, 995–1011. [Google Scholar] [CrossRef]
- Zhang, Y.; Mathieu, O.; Petersen, E.L.; Bourque, G.; Curran, H.J. Assessing the predictions of a NOx kinetic mechanism on recent hydrogen and syngas experimental data. Combust. Flame 2017, 182, 122–141. [Google Scholar] [CrossRef]
- Goodwin, D.G.; Moffat, H.K.; Schoegl, I.; Speth, R.L.; Weber, B.W. Cantera: An Object-Oriented Software Toolkit for Chemical Kinetics, Thermodynamics, and Transport Processes. Version 3.1.0. 2024. Available online: https://zenodo.org/records/14455267 (accessed on 7 May 2025).
- Williams, F. The San Diego Mechanism, Chemical-Kinetic Mechanisms for Combustion Applications; Combustion Research Group: San Diego, CA, USA, 2018; Available online: http://web.eng.ucsd.edu/mae/groups/combustion/mechanism.html (accessed on 30 September 2024).
- Smith, G.P.; Golden, D.M.; Frenklach, M.; Moriarty, N.W.; Eiteneer, B.; Goldenberg, M.; Bowman, C.T.; Hanson, R.K.; Song, S.; Gardiner, W.C., Jr.; et al. GRI-Mech 3.0. 1999. Available online: http://combustion.berkeley.edu/gri-mech/ (accessed on 30 September 2024).
- Dong, C.; Zhou, Q.; Zhao, Q.; Zhang, Y.; Xu, T.; Hui, S. Experimental study on the laminar flame speed of hydrogen/carbon monoxide/air mixtures. Fuel 2009, 88, 1858–1863. [Google Scholar] [CrossRef]
- Sun, H.; Yang, S.; Jomaas, G.; Law, C. High-pressure laminar flame speeds and kinetic modeling of carbon monoxide/hydrogen combustion. Proc. Combust. Inst. 2007, 31, 439–446. [Google Scholar] [CrossRef]
- Tang, C.; Huang, Z.; Law, C. Determination, correlation, and mechanistic interpretation of effects of hydrogen addition on laminar flame speeds of hydrocarbon–air mixtures. Proc. Combust. Inst. 2011, 33, 921–928. [Google Scholar] [CrossRef]
- Fauzy, A.; Chen, G.B.; Lin, T.H. Numerical Analysis of Hydrogen Peroxide Addition and Oxygen-Enriched Methane Combustion. ACS Omega 2023, 8, 16094–16105. [Google Scholar] [CrossRef]
- Chen, G.B.; Wu, F.H. Numerical Simulation of Ammonia Combustion at Different Hydrogen Peroxide Concentrations. Int. J. Energy Res. 2023, 2023, 1–15. [Google Scholar] [CrossRef]
- Glassman, I.; Yetter, R.A. Combustion, 4th ed.; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar] [CrossRef]
- Chen, K.; Tan, K.; Wei, Z.; Kahangamage, U. Numerical investigation of laminar flame speed and NO emission of hydrogen-enriched ammonia at elevated pressures or temperatures. Fuel 2025, 386, 134337. [Google Scholar] [CrossRef]
- Lafay, Y.; Renou, B.; Cabot, G.; Boukhalfa, M. Experimental and numerical investigation of the effect of H2 enrichment on laminar methane–air flame thickness. Combust. Flame 2008, 153, 540–561. [Google Scholar] [CrossRef]
- Portarapillo, M.; Sanchirico, R.; Luciani, G.; Di Benedetto, A. Flame propagation of combustible dusts: A Mallard-Le Chatelier inspired model. Combust. Flame 2023, 251, 112737. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).