Characterization of Thermal Runaway of Lithium Ternary Power Battery in Semi-Confined Space
Abstract
1. Introduction
2. Experimental Setting
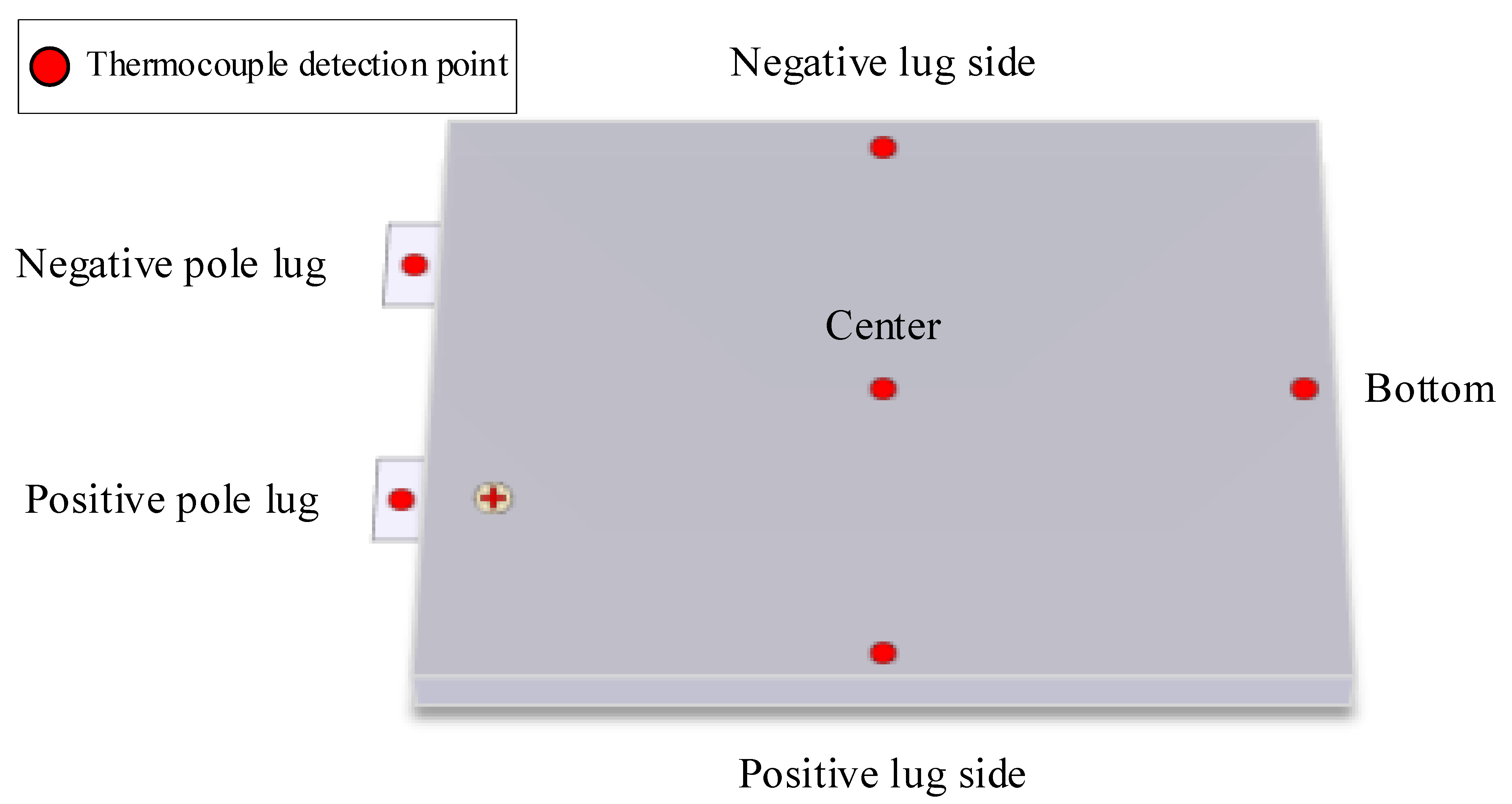
2.1. Battery Specimen
2.2. Experimental Apparatus
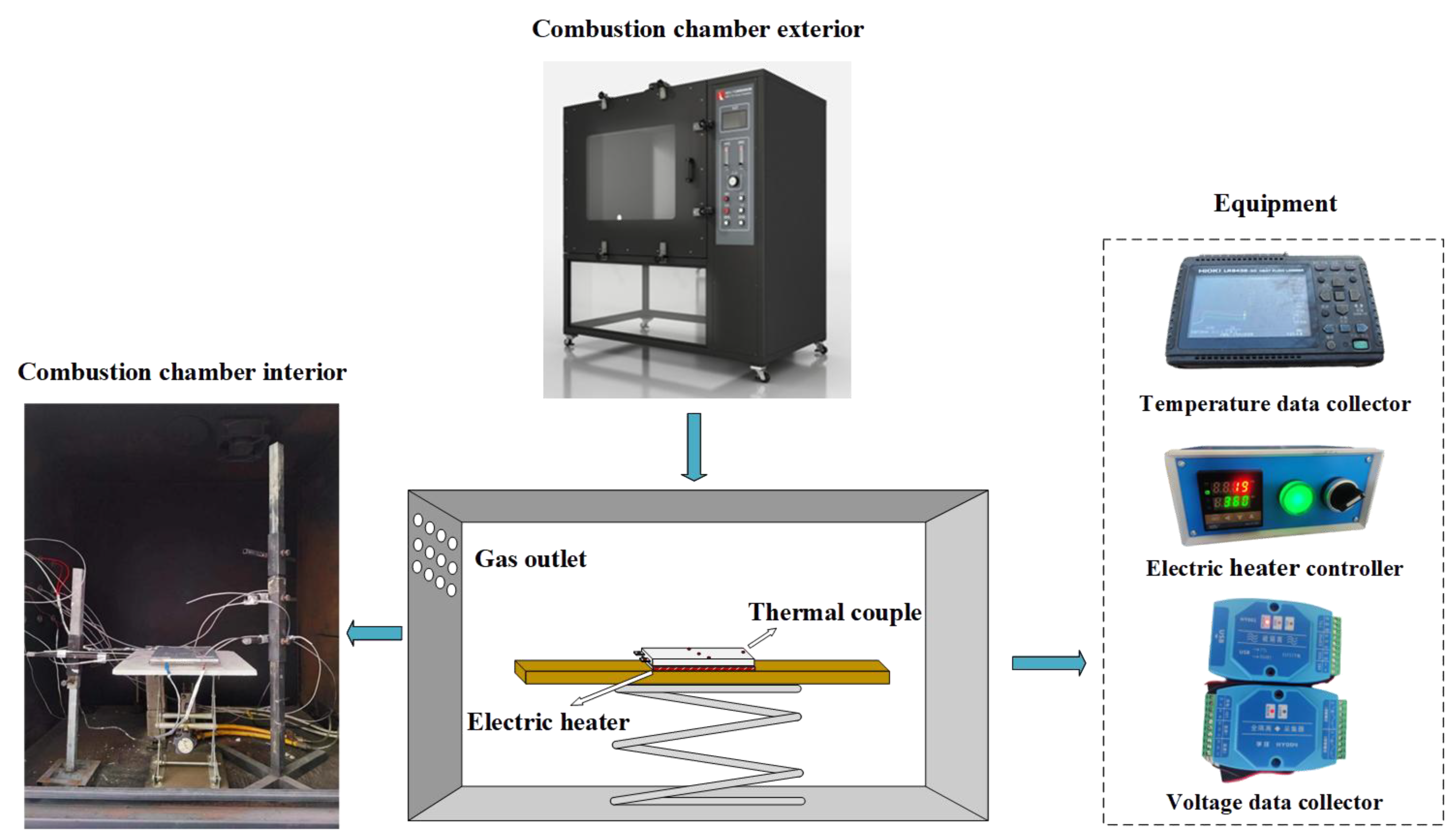
2.2.1. Semi-Confined Space LIB TR Experiment Platform
2.2.2. Open-Space LIB TR Experiment Platform
2.3. Experimental Methods
3. Results and Discussion
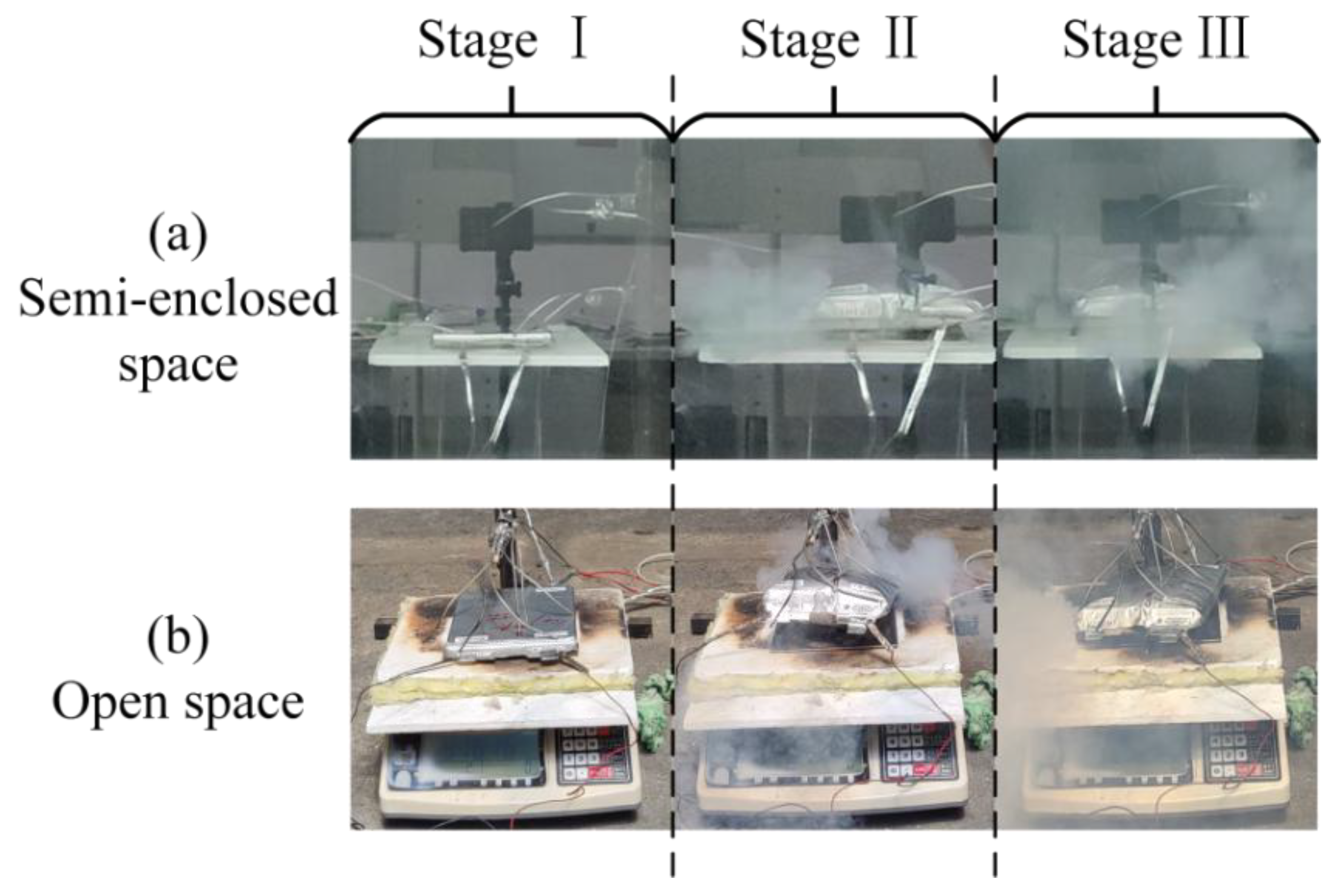
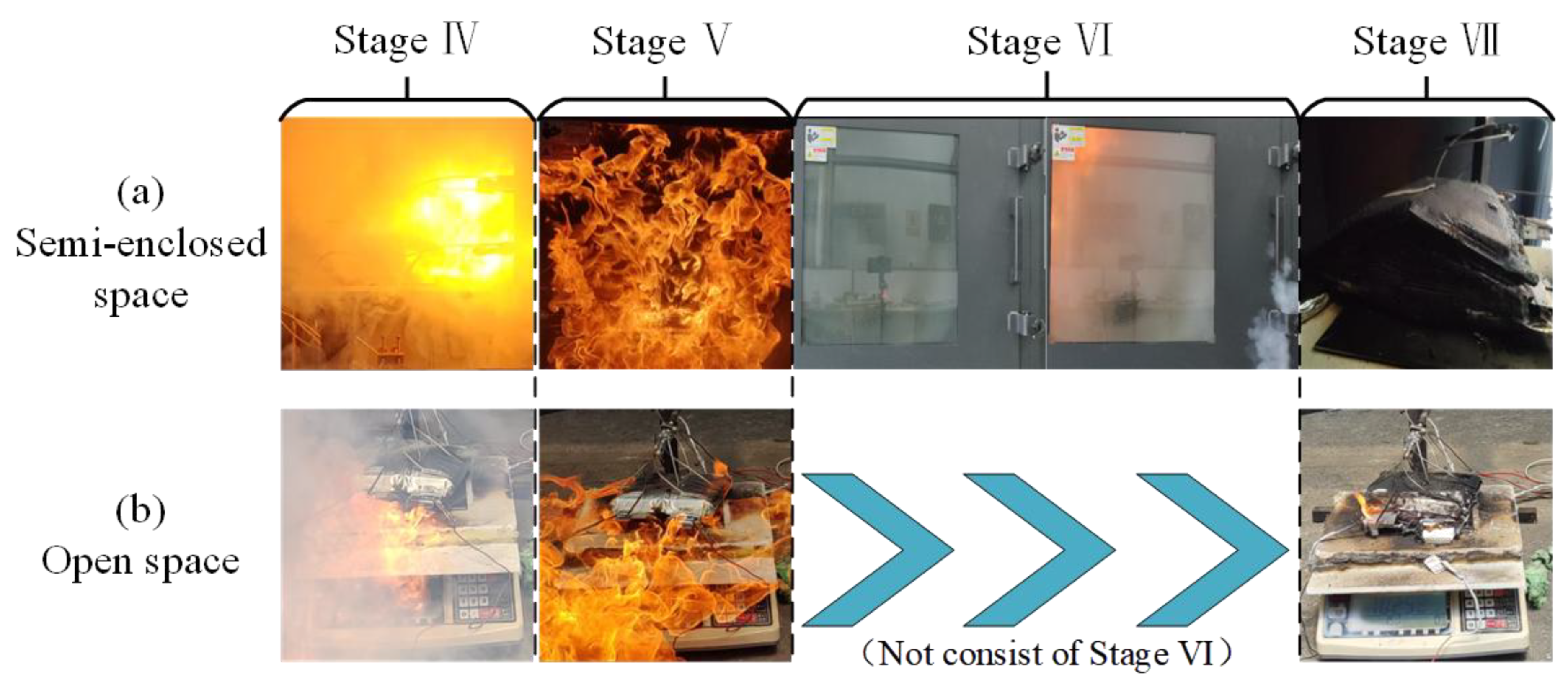
3.1. Analysis of TR Process
- (1)
- Flame-free TR development stage
- (2)
- TR stage with flame
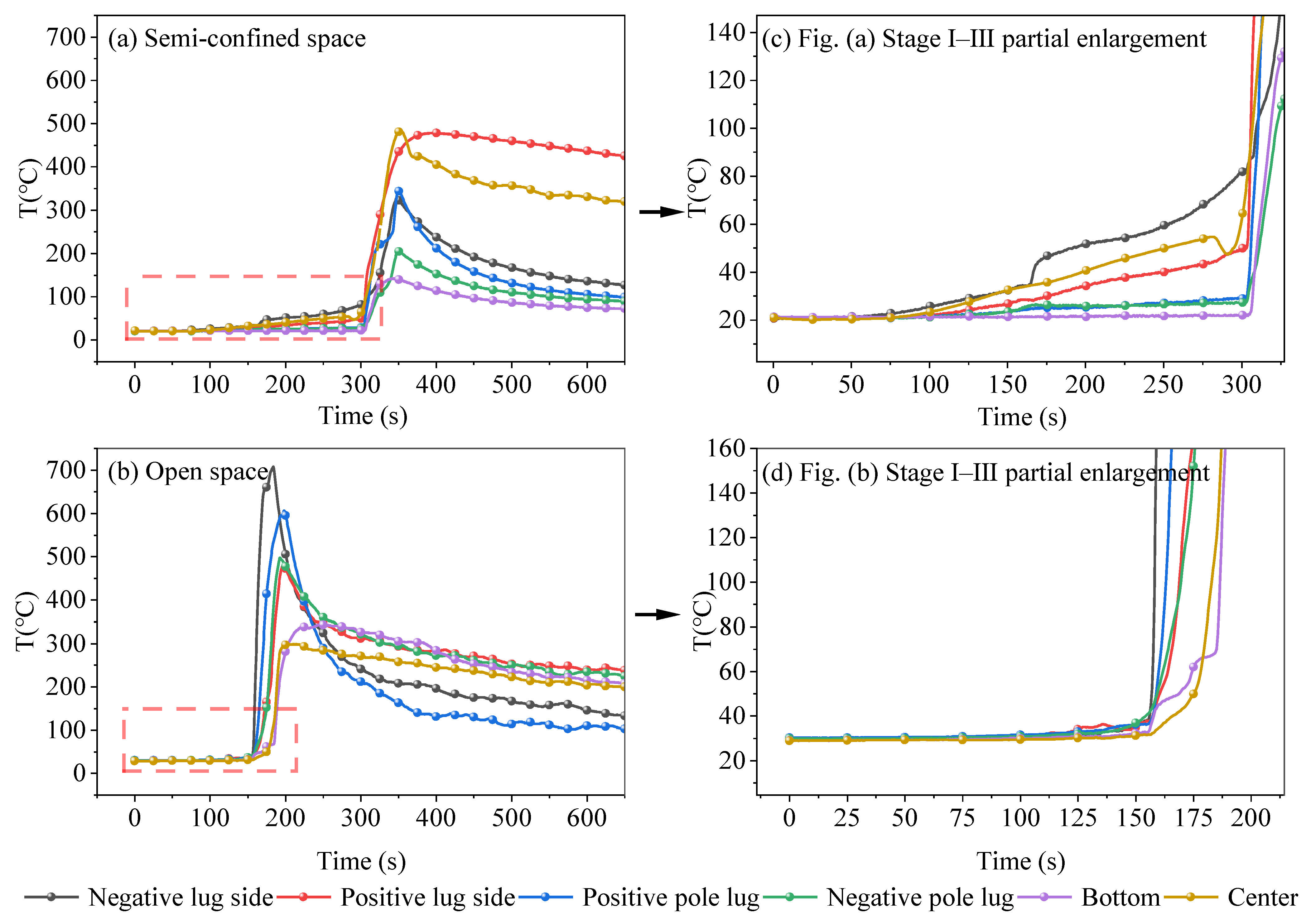
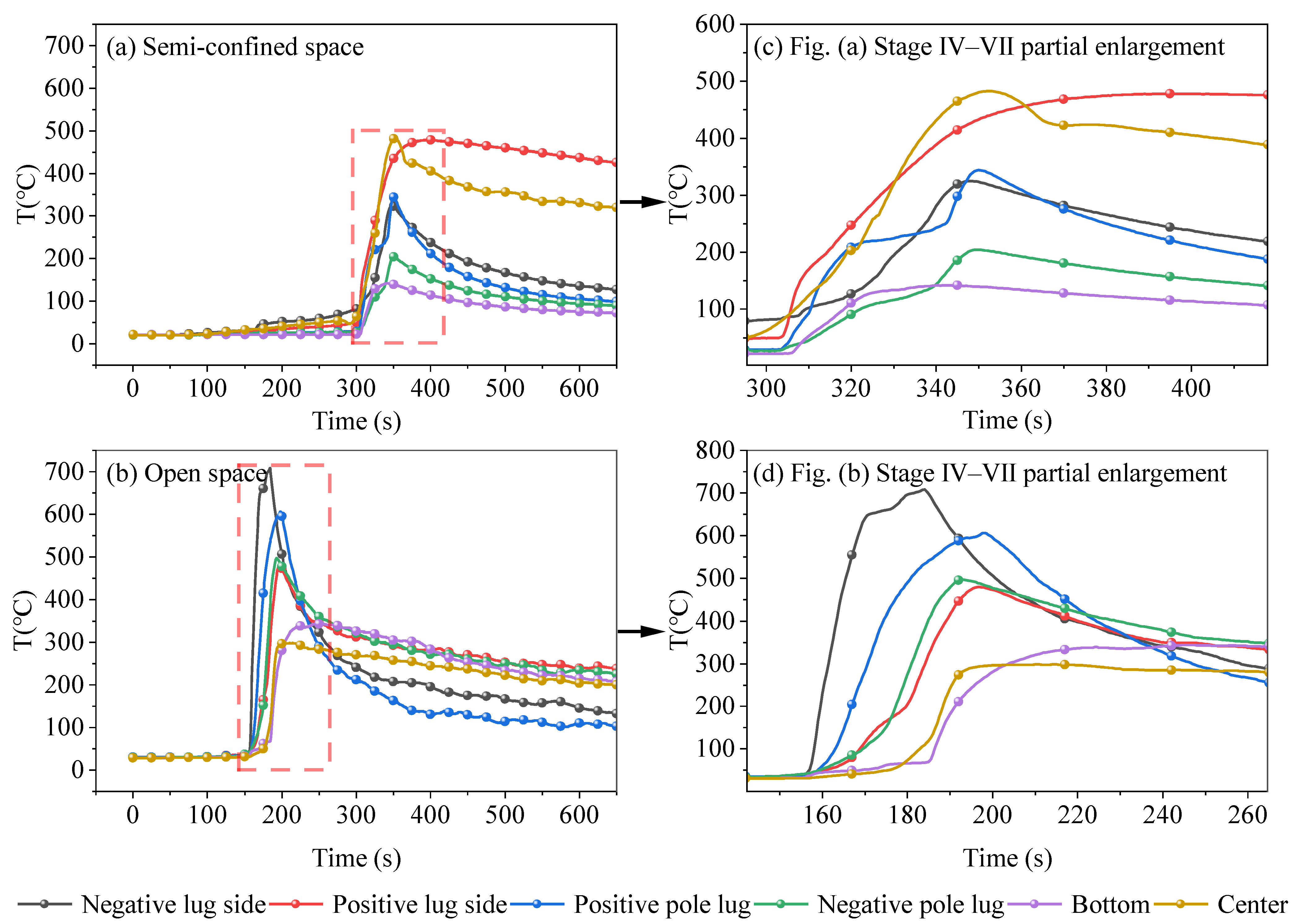
3.2. Characterization of TR Temperature Change
- (1)
- Flame-free TR development stage
- (2)
- TR stage with flame
3.3. Exploration of TR Characteristics of LIB Under Semi-Confined Space
3.4. Future Perspectives
4. Conclusions
- 1.
- The TR behavior of LIBs in semi-confined space and open space is different. The TR process triggered by thermal abuse can be divided into two main stages: the flame-free TR development stage and the flame TR stage. Among them, the flame-free TR development stage includes heating, bulging, and jetting. The flame TR stage includes flame injection, complete combustion, explosion, and the end of the TR reaction. In the TR process of LIBs, no significant difference was observed between the TR performance in semi-confined space and open space during the flame-free TR development stage. However, during TR with flame, the two cases exhibit significantly different characteristics. It is particularly noteworthy that the explosion stage only occurs in the TR process of LIBs in semi-confined space, which is a unique stage differentiating it from open space.
- 2.
- The TR temperatures of LIBs in semi-confined space and open space are different. In the flame-free TR development stage, the temperature of both semi-confined space and open-space LIBs can be stabilized at about 40 °C for a certain length of time. In the stage of TR with flame, the TR flame under the open space environment is violent and persistent, with a maximum temperature of 709 °C and a maximum warming rate of 72.3 °C/s. For the semi-confined space, due to the low level of gas circulation with the outside world, combustion ends early, the heat is not fully released, the maximum temperature is only 482.6 °C, and the maximum temperature rises at a rate of 22.7 °C/s.
- 3.
- The key factor influencing differences in TR outcomes between semi-confined and open spaces lies in air circulation. In open spaces with unrestricted airflow, TR reactions of LIBs are markedly intensified. This manifests through a sharp temperature increase in flames and an accelerated heating rate. Conversely, semi-confined spaces with restricted ventilation suppress TR reactions, resulting in premature flame extinguishment and diminished heating rates. However, such restricted airflow may concurrently allow combustible gas accumulation in localized zones, substantially elevating explosion risks and overall hazard potential. For future electric aircraft designs implementing LIBs in semi-confined compartments, meticulous evaluation of spatial impacts on TR propagation is imperative to optimize safety standards.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| TR | Thermal runaway |
| LIB | Lithium-ion battery |
| SOC | State of charge |
References
- Olabi, A.G.; Abbas, Q.; Shinde, P.A.; Abdelkareem, M.A. Rechargeable batteries: Technological advancement, challenges, current and emerging applications. Energy 2023, 266, 126408. [Google Scholar] [CrossRef]
- Ghiji, M.; Edmonds, S.; Moinuddin, K. A review of experimental and numerical studies of lithium ion battery fires. Appl. Sci. 2021, 11, 1247. [Google Scholar] [CrossRef]
- Hu, X.; Gao, F.; Xiao, Y.; Wang, D.; Gao, Z.; Huang, Z.; Ren, S.; Jiang, N.; Wu, S. Advancements in the safety of Lithium-Ion Battery: The trigger, consequence and mitigation method of thermal runaway. Chem. Eng. J. 2024, 481, 148450. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, X.; Huang, W.; He, X.; Wang, L.; Ouyang, M. Challenges and opportunities to mitigate the catastrophic thermal runaway of high-energy batteries. Adv. Energy Mater. 2023, 13, 2203841. [Google Scholar] [CrossRef]
- Mallick, S.; Gayen, D. Thermal behaviour and thermal runaway propagation in lithium-ion battery systems—A critical review. J. Energy Storage 2023, 62, 106894. [Google Scholar] [CrossRef]
- Wang, G.; Ping, P.; Peng, R.; Lv, H.; Zhao, H.; Gao, W.; Kong, D. A semi reduced-order model for multi-scale simulation of fire propagation of lithium-ion batteries in energy storage system. Renew. Sustain. Energy Rev. 2023, 186, 113672. [Google Scholar] [CrossRef]
- Shan, T.; Zhang, P.; Wang, Z.; Zhu, X. Insights into extreme thermal runaway scenarios of lithium-ion batteries fire and explosion: A critical review. J. Energy Storage 2024, 88, 111532. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhao, J.-R.; Yin, L.; Li, B.; Tian, Y. Staged thermal runaway behaviours of three typical lithium-ion batteries for hazard prevention. J. Therm. Anal. Calorim. 2024, 149, 10321–10333. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, R.; Shen, Z.; Yu, Q.; Xiong, R.; Shen, W. Towards a safer lithium-ion batteries: A critical review on cause, characteristics, warning and disposal strategy for thermal runaway. Adv. Appl. Energy 2023, 11, 100146. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Liu, F.; Fang, Z.; Gu, N.; Chen, B.; Yang, N.; Jia, Y. A comparative study of overcharge thermal runaway force-electrical-thermal characteristics and safety assessment of lithium batteries with different cathode materials. Appl. Therm. Eng. 2024, 256, 124092. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, L.; Wu, Z.; Dong, J.; Yuan, Z.; Han, J.; Li, J. Thermal runaway and combustion of lithium-ion batteries in engine room fires on oil/electric-powered ships. Appl. Therm. Eng. 2024, 254, 123838. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, J.; Zhu, L.; Wu, J.; Wei, D.; Wang, Q.; Chen, H.; Li, K.; Gao, Z.; Xu, C. Experimental and simulation investigation of thermal runaway propagation in lithium-ion battery pack systems. J. Energy Storage 2024, 77, 109868. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Bai, J. Influences of multi factors on thermal runaway induced by overcharging of lithium-ion battery. J. Energy Chem. 2022, 70, 531–541. [Google Scholar] [CrossRef]
- Lee, S.-K.; Kim, H.-K.; Lee, K.-J. Investigation of the risk of thermal runaway in a pouch-type lithium-ion battery with an internal short based on a multiphysics simulation. Appl. Therm. Eng. 2024, 236, 121582. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, K.; Niu, J.; Liu, T.; Hu, J. Research on the lower explosion limit of thermal runaway gas in lithium batteries under high-temperature and slight overcharge conditions. J. Energy Storage 2024, 79, 109976. [Google Scholar] [CrossRef]
- Jin, C.; Sun, Y.; Wang, H.; Zheng, Y.; Wang, S.; Rui, X.; Xu, C.; Feng, X.; Wang, H.; Ouyang, M. Heating power and heating energy effect on the thermal runaway propagation characteristics of lithium-ion battery module: Experiments and modeling. Appl. Energy 2022, 312, 118760. [Google Scholar] [CrossRef]
- Jie, D.; Baohui, C.; Jiazheng, L.; Tiannian, Z.; Chuanping, W. Thermal runaway and combustion characteristics, risk and hazard evaluation of lithium-iron phosphate battery under different thermal runaway triggering modes. Appl. Energy 2024, 368, 123451. [Google Scholar] [CrossRef]
- Liu, T.; Huang, J.; Hu, X.; Cui, S.; Zhu, G. Study on the variation of normalized heat and gas release of overcharge-induced thermal runaway in confined space. Appl. Therm. Eng. 2024, 243, 122636. [Google Scholar] [CrossRef]
- Liu, Y.; Niu, H.; Li, Z.; Liu, J.; Xu, C.; Huang, X. Thermal runaway characteristics and failure criticality of massive ternary Li-ion battery piles in low-pressure storage and transport. Process Saf. Environ. Prot. 2021, 155, 486–497. [Google Scholar] [CrossRef]
- Wang, Z.; Mao, N.; Jiang, F. Study on the effect of spacing on thermal runaway propagation for lithium-ion batteries. J. Therm. Anal. Calorim. 2020, 140, 2849–2863. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.; Wang, H.; Jia, J.; Xie, S.; Zhi, M.; Fu, J.; Sun, Q. Influence of Ambient Pressure and Heating Power on the Thermal Runaway Features of Lithium-Ion Battery. J. Electrochem. Energy Convers. Storage 2021, 18, 1–24. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Zhou, L.; Han, C.; He, T.; Wang, Z. Influencing factors of lithium-ion battery thermal runaway in confined space. J. Energy Storage 2023, 73, 109125. [Google Scholar] [CrossRef]
- Mao, B.; Yu, S.; Zhang, X.; Shi, J.; Zhang, Y. Characterization of the deflagration behavior of the lithium-ion battery module within a confined space under different ventilation conditions. Process Saf. Environ. Prot. 2024, 184, 1034–1040. [Google Scholar] [CrossRef]
- Kong, L.; Li, Y.; Feng, W. Strategies to solve lithium battery thermal runaway: From mechanism to modification. Electrochem. Energy Rev. 2021, 4, 633–679. [Google Scholar] [CrossRef]
- Zou, K.; Lu, S.; Chen, X.; Gao, E.; Cao, Y.; Bi, Y. Thermal and gas characteristics of large-format LiNi0. 8Co0. 1Mn0. 1O2 pouch power cell during thermal runaway. J. Energy Storage 2021, 39, 102609. [Google Scholar] [CrossRef]
- Yang, X.; Wang, H.; Li, M.; Li, Y.; Li, C.; Zhang, Y.; Chen, S.; Shen, H.; Qian, F.; Feng, X. Experimental study on thermal runaway behavior of lithium-ion battery and analysis of combustible limit of gas production. Batteries 2022, 8, 250. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, T.; Wang, Q. Experimental study on the influence of different heating methods on thermal runaway of lithium-ion battery. J. Energy Storage 2021, 42, 103063. [Google Scholar] [CrossRef]
- Zhang, H. Study on Thermal Runaway Behavior and Jet Characteristics of a 156 Ah Prismatic Ternary Lithium Battery. Batteries 2024, 10, 282. [Google Scholar] [CrossRef]
- Chen, M.; Ouyang, D.; Weng, J.; Liu, J.; Wang, J. Environmental pressure effects on thermal runaway and fire behaviors of lithium-ion battery with different cathodes and state of charge. Process Saf. Environ. Prot. 2019, 130, 250–256. [Google Scholar] [CrossRef]
- Xie, S.; Yang, X.; Sun, Q.; Wang, Z.; He, Y. Research progress and prospects on thermal safety of lithium-ion batteries in aviation low-temperature and low-pressure environments. J. Energy Storage 2024, 83, 110734. [Google Scholar] [CrossRef]
- Yang, J.; Liu, W.; Zhao, H.; Zhang, Q. Experimental investigation of lithium-ion batteries thermal runaway propagation consequences under different triggering modes. Aerospace 2024, 11, 438. [Google Scholar] [CrossRef]
- Zhi, M.; Liu, Q.; Xu, Q.; Pan, Z.; Sun, Q.; Su, B.; Zhao, H.; Cui, H.; He, Y. Review of prevention and mitigation technologies for thermal runaway in lithium-ion batteries. Aerosp. Traffic Saf. 2024, 1, 55–72. [Google Scholar] [CrossRef]


| Name | Parameters |
|---|---|
| Size | 220 × 160 × 10 mm |
| Capacity | 50 Ah |
| Rated Voltage | 3.6 V |
| Operating Voltage Range | 2.5 V–4.3 V |
| Type | Ternary lithium-ion battery |
| Cathode | Ternary cathode material |
| Anode | Graphite |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Hou, C.; Hu, P.; Chen, Y. Characterization of Thermal Runaway of Lithium Ternary Power Battery in Semi-Confined Space. Energies 2025, 18, 2444. https://doi.org/10.3390/en18102444
Xu H, Hou C, Hu P, Chen Y. Characterization of Thermal Runaway of Lithium Ternary Power Battery in Semi-Confined Space. Energies. 2025; 18(10):2444. https://doi.org/10.3390/en18102444
Chicago/Turabian StyleXu, Hai, Chenghao Hou, Po Hu, and Yanhe Chen. 2025. "Characterization of Thermal Runaway of Lithium Ternary Power Battery in Semi-Confined Space" Energies 18, no. 10: 2444. https://doi.org/10.3390/en18102444
APA StyleXu, H., Hou, C., Hu, P., & Chen, Y. (2025). Characterization of Thermal Runaway of Lithium Ternary Power Battery in Semi-Confined Space. Energies, 18(10), 2444. https://doi.org/10.3390/en18102444






