Investigation of the Combustion Properties of Ethylene in Porous Materials Using Numerical Simulations
Abstract
1. Introduction
2. Physical and Mathematical Models
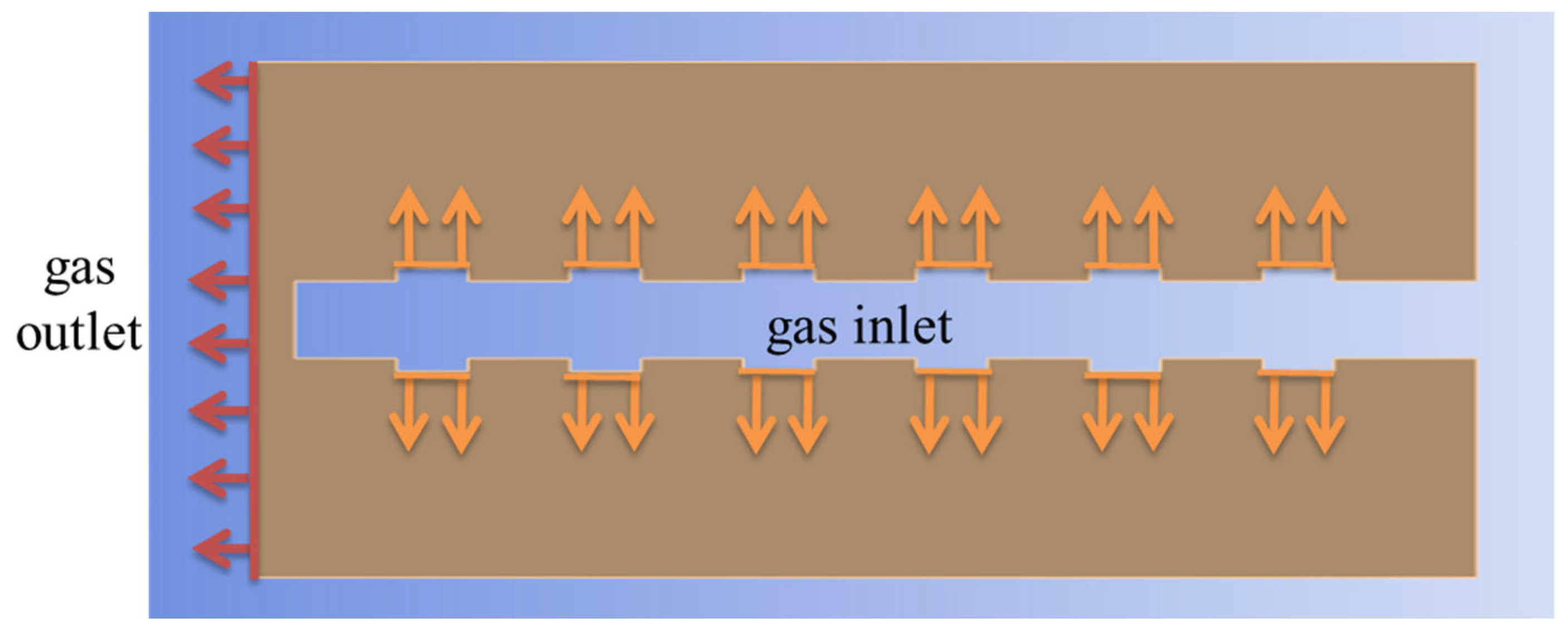
2.1. Physical Models
2.2. Assumptions
- (1)
- Porous dielectric materials in burners will not have a catalytic function in the process of combustion.
- (2)
- Prior to entering the combustion zone, a mixture of ethylene gas has been blended.
- (3)
- Porous dielectric materials are regarded as a diffusion structure that is both isotropic and uniform.
- (4)
- The ideal gases involved in combustion, both reactants and products, remain incompressible before and after the reaction.
- (5)
- The entire furnace disregards the impact of gravity.
2.3. Governing Equation
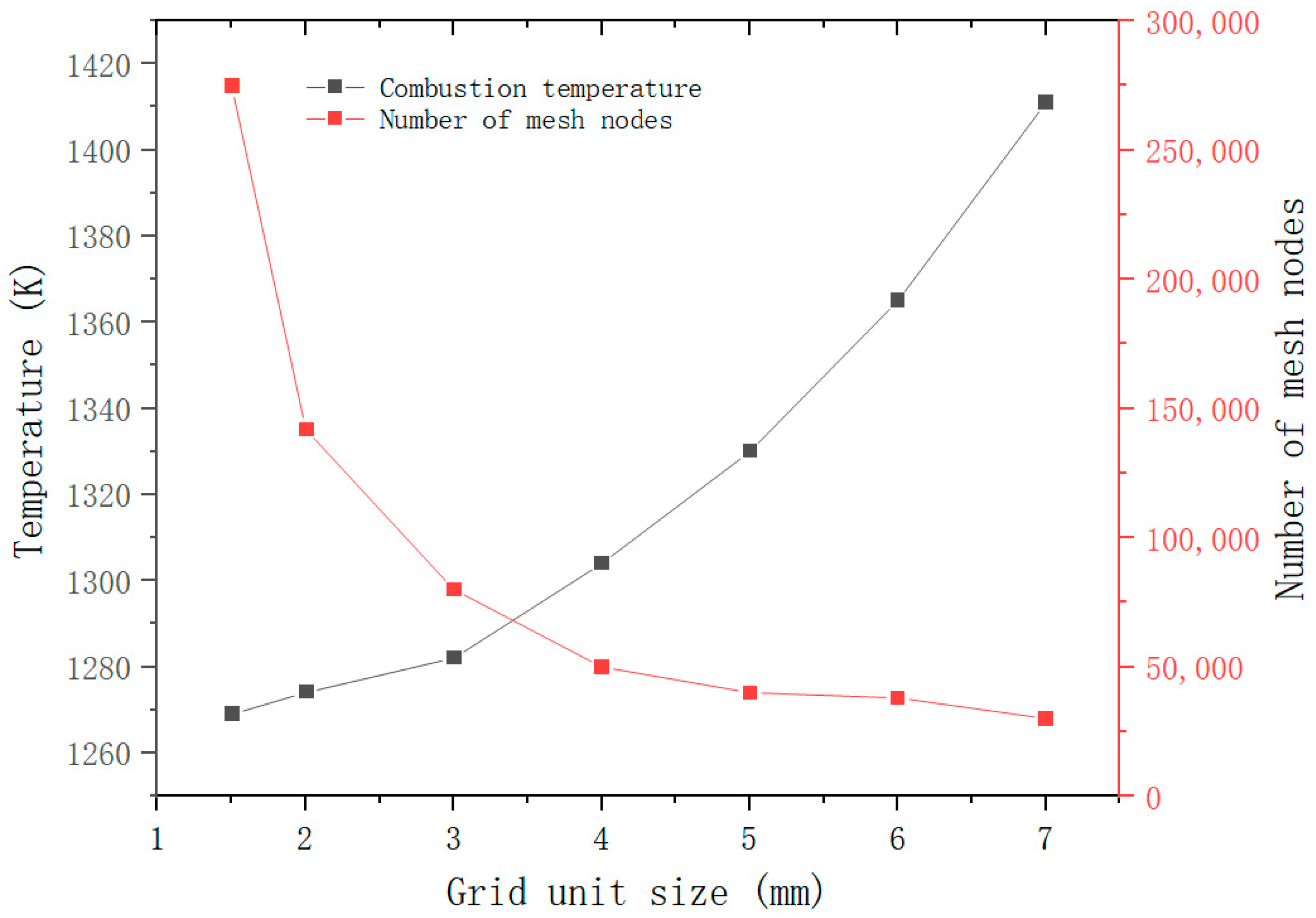
3. Numerical Method and Grid-Independent Verification
3.1. Thermal Property Parameters
3.2. Boundary Conditions
4. Results and Discussion
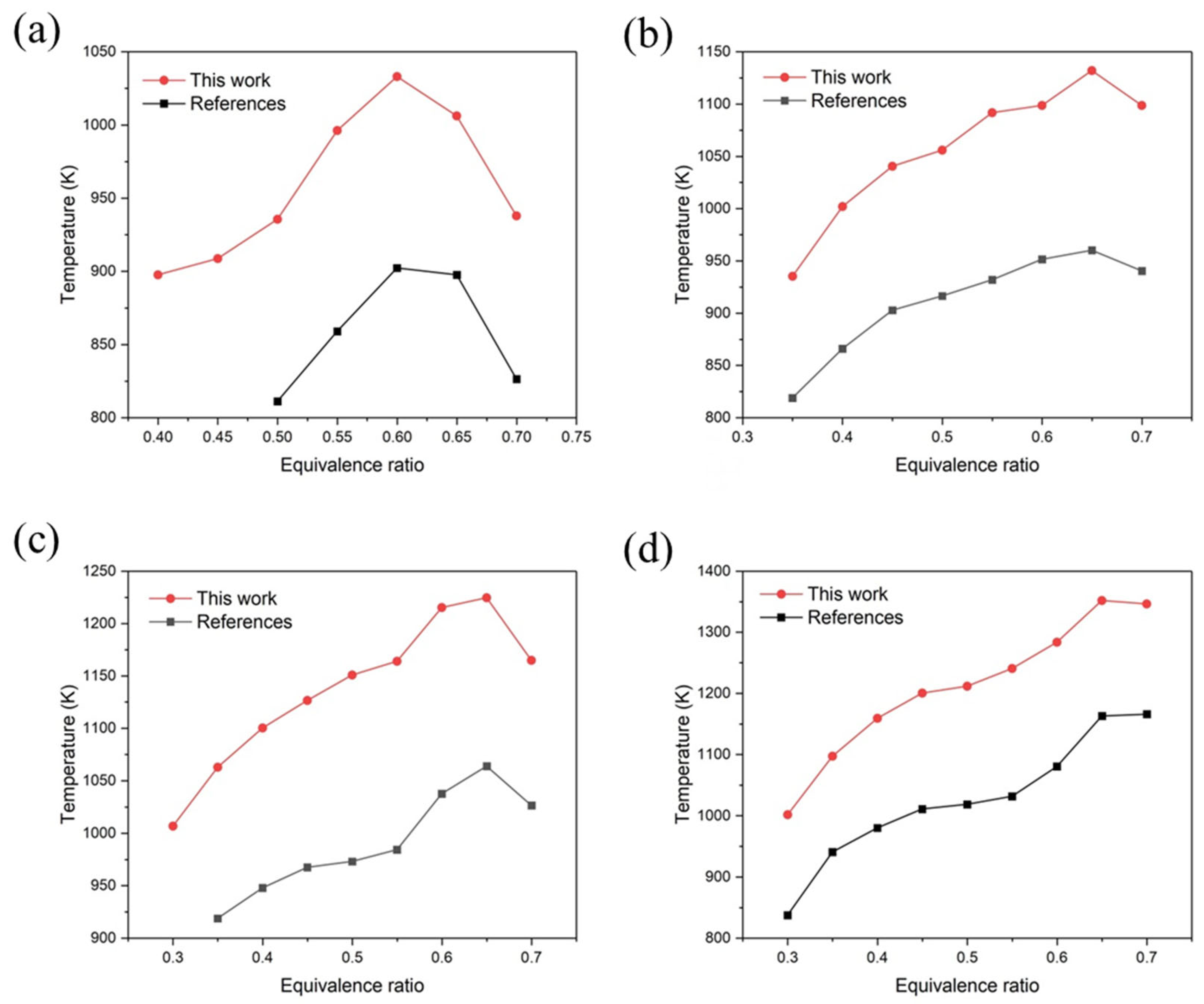
4.1. Computational Validations
4.2. Influence of Different Working Conditions on the Combustion Characteristics
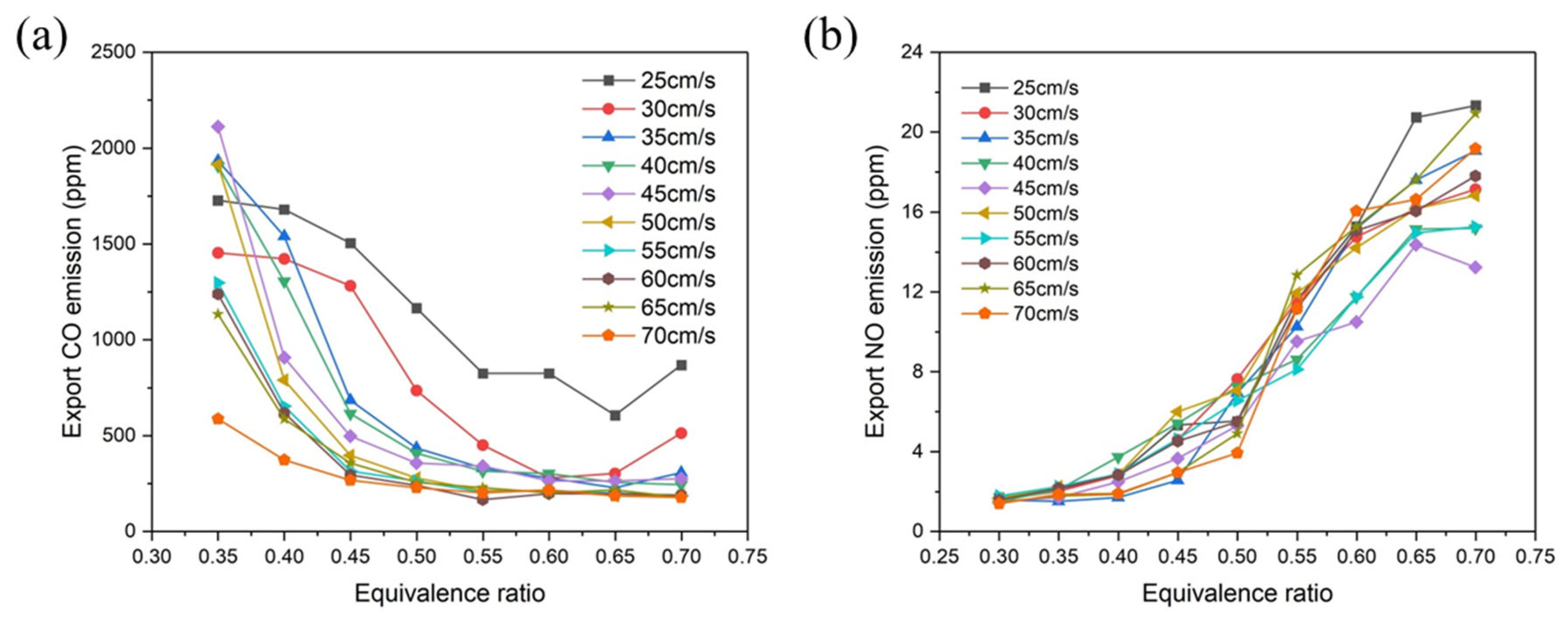
4.3. Pollutant Emission Characteristics during Ethylene Combustion
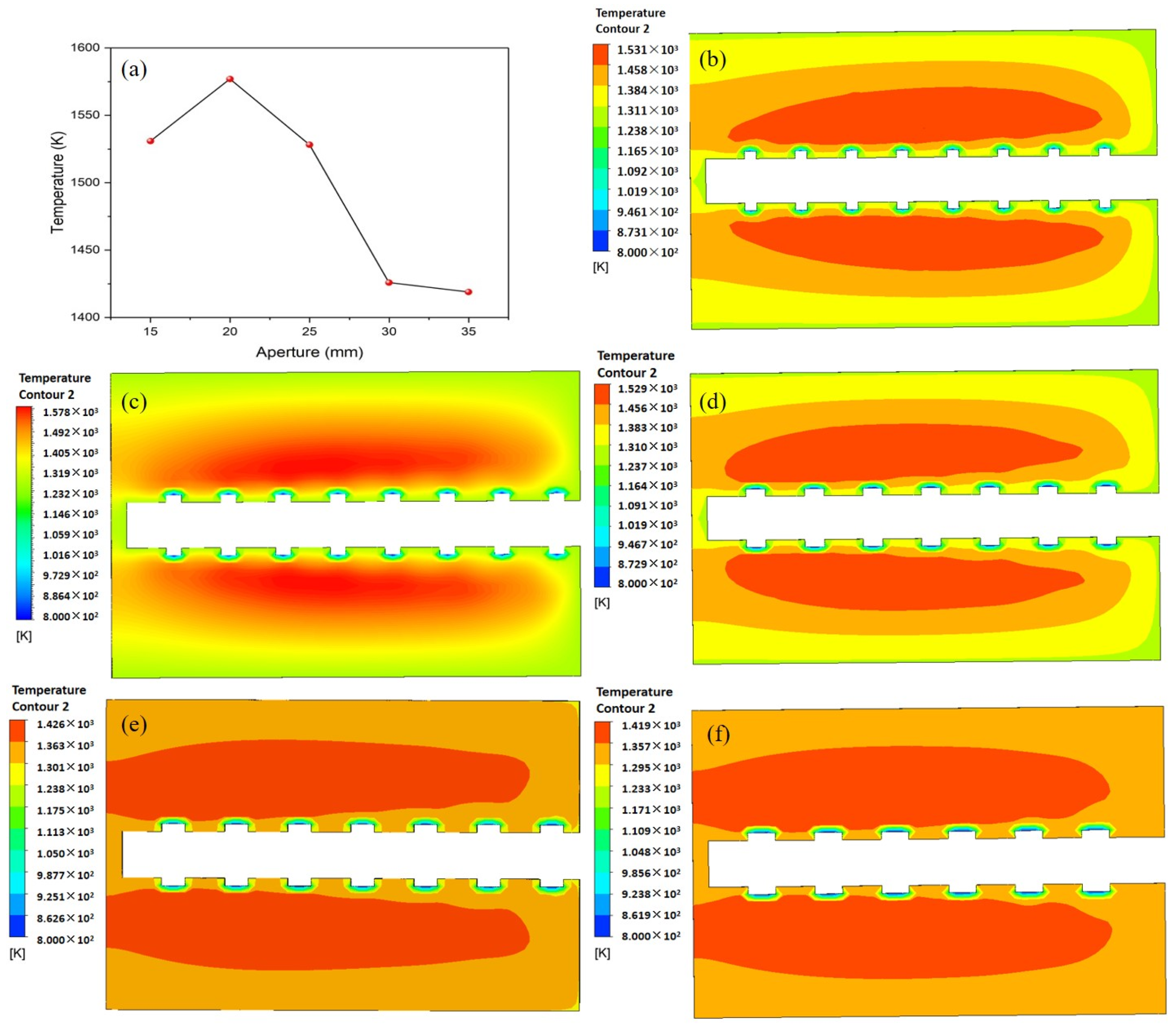
4.4. Effect of Combustion Aperture on Combustion Characteristics
4.5. Influence of Porosity of Porous Media on Combustion Characteristics
5. Conclusions
- (1)
- Following the enhancement of the porous medium burner’s structure, there was a noticeable increase in the overall temperature; the highest temperature reached 1426 K. The central area is where the high-temperature distribution is primarily focused, ensuring that the combustion chamber effectively expels the fully combusted ethylene gas.
- (2)
- Typically, the emission of CO initially decreases and subsequently gradually rises as the equivalence ratio increases. The CO emission reaches the lowest value when the equivalence ratio increases to 0.55. The generated NO is mainly instantaneous NO. As the equivalence ratio increases, the burner experiences a greater temperature rise, leading to the production of instantaneous NO. However, the emission of NO remains consistently low.
- (3)
- The pore diameter affects the temperature change in the combustion chamber by changing the inflow diameter of the ethylene gas. As the aperture decreases and the number of combustion holes increases, the temperature rises.
- (4)
- The reduction in porosity will cause an increase in gas flow resistance, leading to the accumulation of heat in the combustion chamber. The temperature increase clearly indicates a significant surge, with the maximum temperature rise reaching 266 K.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xi, S.; Li, H.; Ma, K.; Lu, Y.; Xi, W. Study on the Transformation of Combustion Mechanism and Ejection Phenomenon of Aluminum Particles in Methane Flame. Energies 2023, 16, 4057. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.; Wang, Y.; Guo, Z.; Liu, X. Fundamental flame characteristics of premixed H2–air combustion in a planar porous micro-combustor. Chem. Eng. J. 2016, 283, 1187–1196. [Google Scholar] [CrossRef]
- Ning, D.; Liu, Y.; Xiang, Y.; Fan, A. Experimental investigation on non-premixed methane/air combustion in Y-shaped meso-scale combustors with/without fibrous porous media. Energy Convers. Manag. 2017, 138, 22–29. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Tseng, H.-H.; Wey, M.-Y.; Lin, M.-D. Characteristics of two types of stabilized nano zero-valent iron and transport in porous media. Sci. Total Environ. 2010, 408, 2260–2267. [Google Scholar] [CrossRef] [PubMed]
- Sathe, S.B.; Tong, T.W.; Faruque, M.A. Experimental study of natural convection in a partially porous enclosure. J. Thermophys. Heat Transf. 1987, 1, 260–267. [Google Scholar] [CrossRef]
- Farzaneh, M.; Reza, E.; Shams, M.; Shafiey, M. Two-dimensional Numerical Simulation of Combustion and Heat Transfer in Porous Burners. Eng. Lett. 2007, 15, 370–375. [Google Scholar]
- Deng, Y.; Xie, M.; Liu, H.; Ma, S. Experimental Study on Superadiabatic Combustion in Porous Media with Reciprocating Flow. J. Combust. Sci. Technol. 2004, 10, 82–87. [Google Scholar]
- Ma, S.H.; Xie, M.Z.; Deng, Y.B. One-dimensional Numerical Simulation of Reciprocating-flow Combustion in Porous Media. J. Eng. Therm. Energy Power 2004, 384–388, 438–439. [Google Scholar]
- Quaye, E.K.; Pan, J.; Lu, Q.; Zhang, Y.; Wang, Y.; Alubokin, A.A. Study on Combustion Characteristics of Premixed Methane-Oxygen in a Cylindrical Porous Media Combustor. Chem. Eng. Process.-Process Intensif. 2021, 159, 108207. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, J.R.; Li, B.W.; Wu, Z.P.; Xue, Z.J. Progress in the Research on Diffusion Filtration Combustion in Porous Medium. Adv. Mater. Res. 2013, 781–784, 2758–2761. [Google Scholar] [CrossRef]
- Vandadi, V.; Park, C. Analytical solutions of superadiabatic filtration combustion. Int. J. Heat Mass Transf. 2018, 117, 740–747. [Google Scholar] [CrossRef]
- Ling, Z.; Zhou, C.; Zeng, X.; Ling, B.; Qian, J. Experimental study on pollutant emission characteristics of lower-heat-value ethylene combustion in porous media. CIESC J. 2019, 70, 4346–4355. [Google Scholar]
- Talukdar, P.; Mishra, S.C.; Trimis, D.; Durst, F. Combined radiation and convection heat transfer in a porous channel bounded by isothermal parallel plates. Int. J. Heat Mass Transf. 2004, 47, 1001–1013. [Google Scholar] [CrossRef]
- Talukdar, P.; Mishra, S.C.; Trimis, D.; Durst, F. Heat transfer characteristics of a porous radiant burner under the influence of a 2-D radiation field. J. Quant. Spectrosc. Radiat. Transf. 2004, 84, 527–537. [Google Scholar] [CrossRef]
- Leonardi, S.A.; Viskanta, R.; Gore, J.P. Radiation and thermal performance measurements of a metal fiber burner. J. Quant. Spectrosc. Radiat. Transf. 2002, 73, 491–501. [Google Scholar] [CrossRef]
- Nikitin, V.F.; Skryleva, E.I.; Manakhova, A.N. Accounting for the instability of a liquid flow front through a porous medium under conditions of low gravity and in the presence of chemical interactions between the phases. Acta Astronaut. 2023, 213, 197–203. [Google Scholar] [CrossRef]
- Smirnova, M.N.; Nikitin, V.F.; Skryleva, E.I.; Weisman, Y.G. Capillary driven fluid flows in microgravity. Acta Astronaut. 2023, 204, 892–899. [Google Scholar] [CrossRef]
- Skryleva, E.I. Numerical simulation of multiphase flow in a porous medium in the presence of heat and mass transfer between phases. Heat Transf. Res. 2023, 54, 1–10. [Google Scholar] [CrossRef]
- Liu, H.; Dong, S.; Li, B.-W.; Chen, H.-G. Parametric investigations of premixed methane–air combustion in two-section porous media by numerical simulation. Fuel 2010, 89, 1736–1742. [Google Scholar] [CrossRef]
- Donoso-García, P.; Henríquez-Vargas, L. Numerical study of turbulent porous media combustion coupled with thermoelectric generation in a recuperative reactor. Energy 2015, 93, 1189–1198. [Google Scholar] [CrossRef]
- Zheng, C.H.; Cheng, L.M.; Li, T.; Luo, Z.Y.; Ni, M.J.; Cen, K.F. Numerical Simulation of Combustion Fronts in Porous Media. Proc. CSEE 2009, 29, 48–53. [Google Scholar]
- Zhu, R.; Pan, D.; Gao, H.; Gao, N.; Zhu, T. Study on Surface Combustion Simulation of Metal Fiber Burner. Gas Heat 2020, 40, 36–43. [Google Scholar]
- Gao, Y.; Wang, Y.; Yan, L.; Fan, X.-Y.; Li, J.; Pu, F. Influence of Fuel Flow Rate on Combustion Characteristics of Coke Oven Gas. Contemp. Chem. Ind. 2018, 47, 938–941. [Google Scholar]
- Liu, Y. Numerical Simulation Study of Flameless Combustion in Ammonia Gas Porous Medium. Master’s Thesis, University of Science and Technology Liaoning, Anshan, China, 2021. [Google Scholar]


| No. | Equivalence Ratio | Flow Velocity (cm/s) |
|---|---|---|
| 1 | 0.30 | 45, 50, 55, 60, 65, 70 |
| 2 | 0.35 | 30, 35, 40, 45, 50, 55, 60, 65, 70 |
| 3 | 0.40 | 25, 30, 35, 40, 45, 50, 55, 60, 65, 70 |
| 4 | 0.45 | 25, 30, 35, 40, 45, 50, 55, 60, 65, 70 |
| 5 | 0.50 | 25, 30, 35, 40, 45, 50, 55, 60, 65, 70 |
| 6 | 0.55 | 25, 30, 35, 40, 45, 50, 55, 60, 65, 70 |
| 7 | 0.6 | 25, 30, 35, 40, 45, 50, 55, 60, 65, 70 |
| 8 | 0.65 | 25, 30, 35, 40, 45, 50, 55, 60, 65, 70 |
| 9 | 0.70 | 25, 30, 35, 40, 45, 50, 55, 60, 65, 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, L.; Ding, S.; Li, S.; Zhang, H.; Feng, W. Investigation of the Combustion Properties of Ethylene in Porous Materials Using Numerical Simulations. Energies 2024, 17, 2153. https://doi.org/10.3390/en17092153
Tu L, Ding S, Li S, Zhang H, Feng W. Investigation of the Combustion Properties of Ethylene in Porous Materials Using Numerical Simulations. Energies. 2024; 17(9):2153. https://doi.org/10.3390/en17092153
Chicago/Turabian StyleTu, Linyu, Siyu Ding, Shefeng Li, Haitao Zhang, and Wei Feng. 2024. "Investigation of the Combustion Properties of Ethylene in Porous Materials Using Numerical Simulations" Energies 17, no. 9: 2153. https://doi.org/10.3390/en17092153
APA StyleTu, L., Ding, S., Li, S., Zhang, H., & Feng, W. (2024). Investigation of the Combustion Properties of Ethylene in Porous Materials Using Numerical Simulations. Energies, 17(9), 2153. https://doi.org/10.3390/en17092153





