Biohydrogen Production and Quantitative Determination of Monosaccharide Production Using Hyperthermophilic Anaerobic Fermentation of Corn Stover
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Strain, Media, and Growth Conditions
2.3. Ezymes Extraction from T. maritima Bacterium
2.4. Experimental Setup-Batch Fermentation Process
2.5. Enzyme Activity Essay
2.6. HPLC Analysis of Inhibitory Products
Derivatization Process
3. Results and Discussion
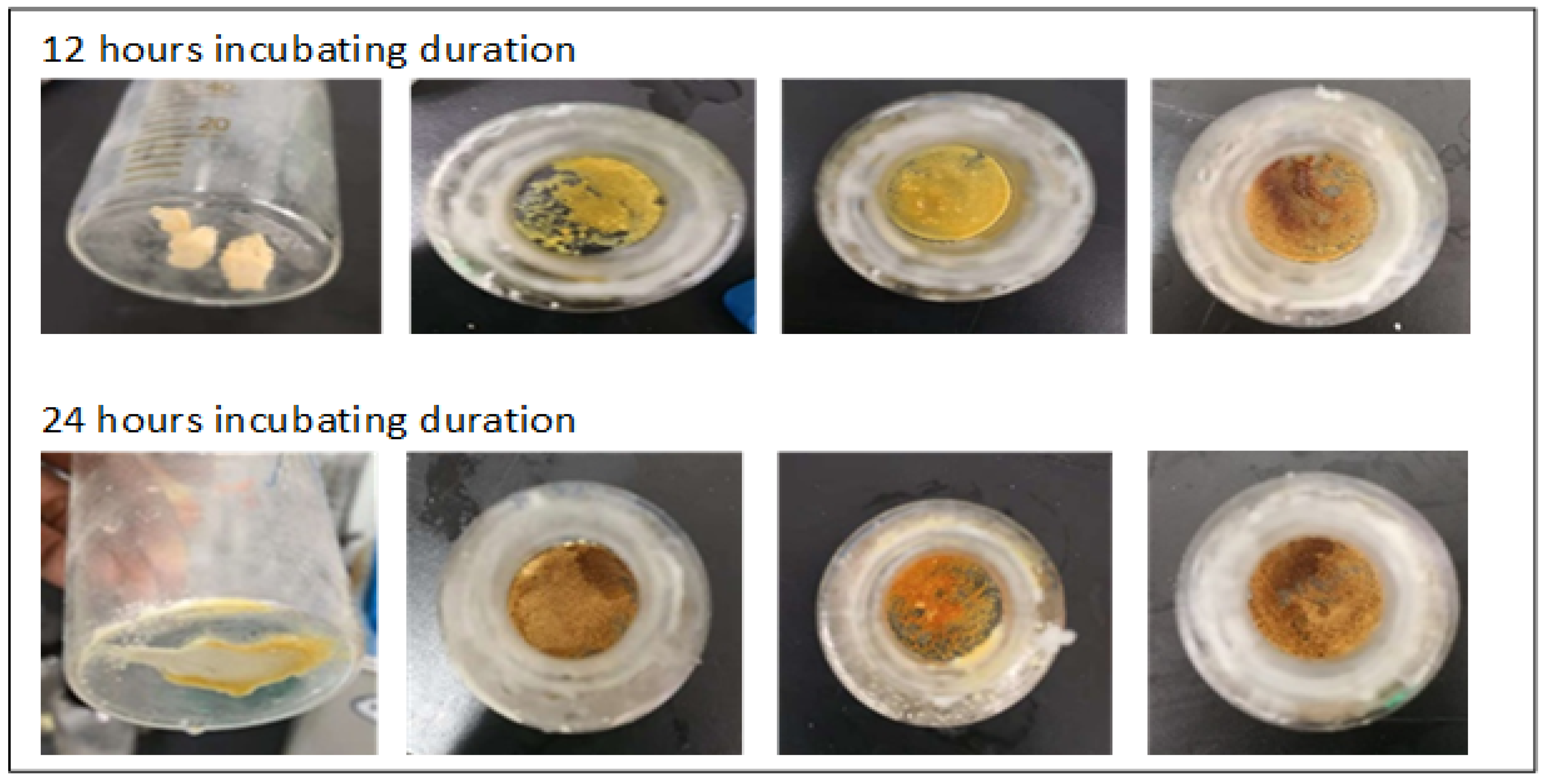
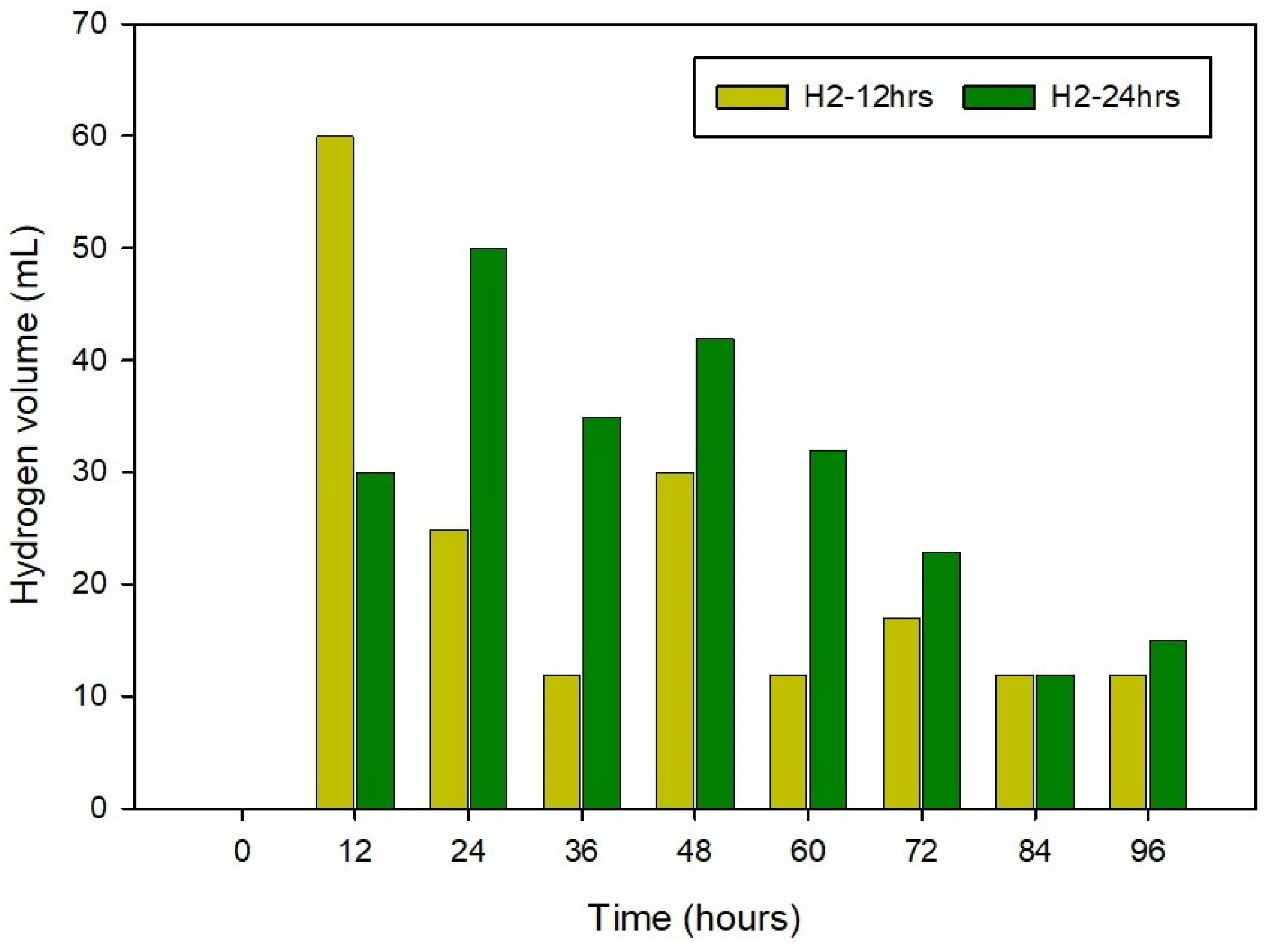
3.1. Physical Monitoring of the Substrate during the 80-Degree Incubation
3.2. Enzymatic Degradation during the Dark Fermentation of Corn Stover Using T. maritima
3.3. Determination of Sugar Monomers of Delignified Corn Stover
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kutlu, L. Greenhouse gas emission efficiencies of world countries. Int. J. Environ. Res. Public Health 2020, 17, 8771. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Lv, Y.; Wu, R.; Sui, Y.; Chen, C.; Xin, F.; Zhou, J.; Dong, W.; Jiang, M. Current status and perspectives on biobutanol production using lignocellulosic feedstocks. Bioresour. Technol. Rep. 2019, 7, 100245. [Google Scholar] [CrossRef]
- Singh, A.; Sevda, S.; Abu Reesh, I.M.; Vanbroekhoven, K.; Rathore, D.; Pant, D. Biohydrogen production from lignocellulosic biomass: Technology and sustainability. Energies 2015, 8, 13062–13080. [Google Scholar] [CrossRef]
- Tun, M.M.; Juchelkova, D.; Win, M.M.; Thu, A.M.; Puchor, T. Biomass energy: An overview of biomass sources, energy potential, and management in Southeast Asian countries. Resources 2019, 8, 81. [Google Scholar] [CrossRef]
- Binod, P.; Gnansounou, E.; Sindhu, R.; Pandey, A. Enzymes for second generation biofuels: Recent developments and future perspectives. Bioresour. Technol. Rep. 2019, 5, 317–325. [Google Scholar] [CrossRef]
- Gargaud, M.; Amils, R.; Cleaves, H.J. Encyclopedia of Astrobiology, Hyperthermophiles; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011; Volume 1. [Google Scholar]
- Shao, W.; Wang, Q.; Rupani, P.F.; Krishnan, S.; Ahmad, F.; Rezania, S.; Rashid, M.A.; Sha, C.; Din, M.F.M. Biohydrogen production via thermophilic fermentation: A prospective application of Thermotoga species. Energy 2020, 197, 117199. [Google Scholar] [CrossRef]
- Maru, B.T.; Constanti, M.; Stchigel, A.M.; Medina, F.; Sueiras, J.E. Biohydrogen production by dark fermentation of glycerol using Enterobacter and Citrobacter sp. Biotechnol. Prog. 2013, 29, 31–38. [Google Scholar] [CrossRef]
- Eriksen, N.T.; Riis, M.L.; Holm, N.K.; Iversen, N. H2 synthesis from pentoses and biomass in Thermotoga spp. Biotechnol. Lett. 2011, 33, 293–300. [Google Scholar] [CrossRef]
- Abreu, A.A.; Tavares, F.; Alves, M.M.; Pereira, M.A. Boosting dark fermentation with co-cultures of extreme thermophiles for biohythane production from garden waste. Bioresour. Technol. 2016, 219, 132–138. [Google Scholar] [CrossRef]
- Saidi, R.; Liebgott, P.P.; Gannoun, H.; Ben Gaida, L.; Miladi, B.; Hamdi, M.; Bouallagui, H.; Auria, R. Biohydrogen production from hyperthermophilic anaerobic digestion of fruit and vegetable wastes in seawater: Simplification of the culture medium of Thermotoga maritima. Waste Manag. 2018, 71, 474–484. [Google Scholar] [CrossRef]
- Pradhan, N.; Dipasquale, L.; d’Ippolito, G.; Panico, A.; Lens, P.; Esposito, G.; Fontana, A. Hydrogen production by the thermophilic bacterium Thermotoga neapolitana. Int. J. Mol. Sci. 2015, 16, 12578–12600. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, E.W.; Claassen, P.A.; Stams, A.J. Substrate and product inhibition of hydrogen production by the extreme thermophile, Caldicellulosiruptor saccharolyticus. Biotechnol. Bioeng. 2003, 81, 255–262. [Google Scholar] [CrossRef]
- Mohan, S.V. Waste to renewable energy: A sustainable and green approach towards production of biohydrogen by acidogenic fermentation. In Sustainable Biotechnology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 129–164. [Google Scholar]
- Herrmann, A.; Sieling, K.; Wienforth, B.; Taube, F.; Kage, H. Short-term effects of biogas residue application on yield performance and N balance parameters of maize in different cropping systems. J. Agric. Sci. 2013, 151, 449–462. [Google Scholar] [CrossRef]
- Wu, H.; Pei, J.; Wu, G.; Shao, W. Overexpression of GH10 endoxylanase XynB from Thermotoga maritima in Escherichia coli by a novel vector with potential for industrial application. Enzym. Microb. Technol. 2008, 42, 230–234. [Google Scholar] [CrossRef]
- Leonard, S.R.; Mammel, M.K.; Lacher, D.W.; Elkins, C.A. Application of metagenomic sequencing to food safety: Detection of Shiga toxin-producing Escherichia coli on fresh bagged spinach. Appl. Environ. Microbiol. 2015, 81, 8183–8191. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhou, Q.; Wu, K.; Li, X.-Q.; Shao, W.-L. A highly efficient method for liquid and solid cultivation of the anaerobic hyperthermophilic eubacterium Thermotoga maritima. FEMS Microbiol. Lett. 2006, 259, 254–259. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, H.; Pei, J.; Jiang, Y.; Song, X.; Shao, W. pHsh vectors, a novel expression system of Escherichia coli for the large-scale production of recombinant enzymes. Biotechnol. Lett. 2010, 32, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Yin, E.; Le, Y.; Pei, J.; Shao, W.; Yang, Q. High-level expression of the xylanase from Thermomyces lanuginosus in Escherichia coli. World J. Microbiol. Biotechnol. 2008, 24, 275–280. [Google Scholar] [CrossRef]
- Lever, M. A new reaction for colorimetric determination of carbohydrates. Anal. Biochem. 1972, 47, 273–279. [Google Scholar] [CrossRef]
- Doelle, H.W.; Rokem, J.S.; Berovic, M. Biotechnology-Volume VIII: Fundamentals in Biotechnology; EOLSS Publications: Abu Dhabi, United Arab Emirates, 2009; Volume 8. [Google Scholar]
- Moreira, M.; Guido, L.; Cruz, J.; Barros, A. Determination of galacturonic acid content in pectin from fruit juices by liquid chromatographydiode array detection-electrospray ionization tandem mass spectrometry. Open Chem. 2010, 8, 1236–1243. [Google Scholar] [CrossRef]
- Horn, S.J.; Vaaje-Kolstad, G.; Westereng, B.; Eijsink, V. Novel enzymes for the degradation of cellulose. Biotechnol. Biofuels 2012, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, A.; Bischoff, K.M.; Sani, R.K. Highly thermostable xylanase production from a thermophilic Geobacillus sp. strain WSUCF1 utilizing lignocellulosic biomass. Front. Bioeng. Biotechnol. 2015, 3, 84. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; van der Heide, E.; Wang, H.; Li, B.; Yu, G.; Mu, X. Alkaline twin-screw extrusion pretreatment for fermentable sugar production. Biotechnol. Biofuels 2013, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Dixon, R.A. Lignin modification improves fermentable sugar yields for biofuel production. Nat. Biotechnol. 2007, 25, 759. [Google Scholar] [CrossRef] [PubMed]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Wacht, M.; Meyer, A. Simultaneous and Sensitive HPLC Determination of Mono-and Disaccharides, Uronic Acids, and Amino Sugars after Derivatization by Reductive Amination. Acta Hydrochim. Et Hydrobiol. 2003, 31, 134–144. [Google Scholar] [CrossRef]
- Kumar, A.; Chandra, R. Ligninolytic enzymes and its mechanisms for degradation of lignocellulosic waste in environment. Heliyon 2020, 6, e03170. [Google Scholar] [CrossRef] [PubMed]
- Akinbomi, J.; Taherzadeh, M.J. Evaluation of fermentative hydrogen production from single and mixed fruit wastes. Energies 2015, 8, 4253–4272. [Google Scholar] [CrossRef]
- Gomis, D.B.; Tamayo, D.M.; Alonso, J.M. Determination of monosaccharides in cider by reversed-phase liquid chromatography. Anal. Chim. Acta 2001, 436, 173–180. [Google Scholar] [CrossRef]
- Guttman, A. Analysis of monosaccharide composition by capillary electrophoresis. J. Chromatogr. A 1997, 763, 271–277. [Google Scholar] [CrossRef]
- Zhang, R.-E.; Cao, Y.-L.; Hearn, M.W. Synthesis and application of Fmoc-hydrazine for the quantitative determination of saccharides by reversed-phase high-performance liquid chromatography in the low and subpicomole range. Anal. Biochem. 1991, 195, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Anumula, K. Quantitative determination of monosaccharides in glycoproteins by high-performance liquid chromatography with highly sensitive fluorescence detection. Anal. Biochem. 1994, 220, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Raba, C.; Fischer, K. Ion-pair RP-HPLC determination of sugars, amino sugars, and uronic acids after derivatization with p-aminobenzoic acid. Anal. Chem. 2001, 73, 2377–2382. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, S.R.; Shockley, K.R.; Conners, S.B.; Scott, K.L.; Wolfinger, R.D.; Kelly, R.M. Carbohydrate-induced differential gene expression patterns in the hyperthermophilic bacterium Thermotoga maritima. J. Biol. Chem. 2003, 278, 7540–7552. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rupani, P.F.; Sakrabani, R.; Sadaqat, B.; Shao, W. Biohydrogen Production and Quantitative Determination of Monosaccharide Production Using Hyperthermophilic Anaerobic Fermentation of Corn Stover. Energies 2024, 17, 1734. https://doi.org/10.3390/en17071734
Rupani PF, Sakrabani R, Sadaqat B, Shao W. Biohydrogen Production and Quantitative Determination of Monosaccharide Production Using Hyperthermophilic Anaerobic Fermentation of Corn Stover. Energies. 2024; 17(7):1734. https://doi.org/10.3390/en17071734
Chicago/Turabian StyleRupani, Parveen Fatemeh, Ruben Sakrabani, Beenish Sadaqat, and Weilan Shao. 2024. "Biohydrogen Production and Quantitative Determination of Monosaccharide Production Using Hyperthermophilic Anaerobic Fermentation of Corn Stover" Energies 17, no. 7: 1734. https://doi.org/10.3390/en17071734
APA StyleRupani, P. F., Sakrabani, R., Sadaqat, B., & Shao, W. (2024). Biohydrogen Production and Quantitative Determination of Monosaccharide Production Using Hyperthermophilic Anaerobic Fermentation of Corn Stover. Energies, 17(7), 1734. https://doi.org/10.3390/en17071734





