1. Introduction
The world’s demand for energy is constantly growing. At the same time, we are facing global warming, which is associated with anthropogenic emissions of greenhouse gasses (GHGs), such as carbon dioxide (38 Gt/year [
1]), to the atmosphere. A thorough analysis for 2012 base year data showed that 474 PJ of energy was originally produced, of which only 28% was used efficiently. As much as 52% of the energy is irretrievably lost as residual heat of flue gases or waste water discarded to the environment as emissions [
2]. The energy losses with effluents of industrial processes may result in excessive CO
2 emissions as high as 19 Gt/year [
2]. In the case of 63% of waste heat, the temperature does not exceed 100 °C. One of the primary and most important solutions to reduce carbon emissions is to increase the energy efficiency of industrial processes [
3]. Heat recovery from waste streams is an extremely challenging issue [
4]. This topic is widely discussed in the chemical engineering industry [
5,
6,
7].
District heating of flats and houses is provided by a heating medium (water) circulating in a closed system. Over the years, there has been a tendency to reduce the circulating water temperature [
8]. Energy-efficient design, including the use of underfloor and wall-heating systems, allows for a further reduction in feed temperature. As mains heat is also used to provide hot domestic water, the mains temperature cannot be reduced below certain levels, usually 65 °C [
8,
9]. The return temperature is dependent on the outside temperature and is usually between 45 °C and 65 °C. These values are consistent with information obtained from the Megatem EC-Lublin CHP plant in Poland [
9]. A simple heat-exchanging system can cool the flue gases to a temperature not lower than the mentioned 45 °C. Further cooling of flue gases is technically feasible yet requires the use of more complex systems.
1.1. Heat Recovery Systems
The residual heat lost with flue gases, that cannot be efficiently used by a heating network, can be recovered by heating the air stream directed to the burner or combustion chamber [
4,
10,
11]. A solution called an air preheater involves the use of a diaphragm heat exchanger, where the residual heat of combustion gases is recovered by heating the combustion air [
4,
12]. A patented solution [
13] with a direct-contact heat exchange to water can also be found. This solution is supposed to increase both the efficiency of the combustion process as well as to improve pollutant reduction by wet scrubbing. The heated water is used to directly preheat combustion air; hence, the combustion temperature is raised and efficiency increased.
There is a number of methods of low-temperature heat management, such as regeneration [
4,
12]. Advanced yet expensive systems such as Organic Rankine Cycle (OCR) or the Kalina cycle can be employed to generate/produce work directly from waste heat [
4,
14,
15]. In addition to the aforementioned methods, there are other less-well-known technologies, such as thermoelectric, piezoelectric and thermophotovoltaic, applied to waste energy harvesting [
4]. The technology readiness level is insufficient for an effective industrial, large-scale application [
16,
17,
18,
19].
Absorption and compressor heat pumps deserve special attention. These are solutions that are being used with increasing frequency [
20,
21,
22]. They significantly increase the efficiency of the system. Their biggest disadvantage is the necessary investment, but the return-on-investment time does not exceed 4 years [
23]. Typically, suppliers provide equipment that delivers heat at temperatures up to 90 °C. Few suppliers offer equipment delivering heat at 120–165 °C, and higher values are in the realm of research or prototypes [
24].
1.2. Condensation
An important phenomenon taking place is the condensation of water in humid flue gases. Depending on the amount of water vapors produced during combustion, this phenomenon is observed once the flue gases have cooled down below a dew point [
25]. The higher the moisture content of the flue gases, the higher the dew point [
11,
25]. Cooling flue gases saturated with water vapor leads to a much higher (by an order of magnitude) heat flux compared to cooling dry flue gases [
4].
The phase change process is often used, for example, in domestic gas boilers, in particular those used for low-temperature heating (e.g., underfloor heating). The condensation of moisture from the flue gases produced by the combustion of natural gas starts at a temperature of around 55 °C. However, combustion of other fuels having a less favorable ratio of hydrogen to other combustible elements in their composition results in lower condensation temperatures [
11].
Condensation can also be employed in a system of direct heating with flue gases. The circulating water is heated directly by flue gases in a scrubber. Heated air is then directed to a second scrubber, where combustion air is preheated directly. The system is patented in France [
26], and in 1981, the French company Seccacier introduced the system under the trade name “Système INNOREX”.
Due to the relatively high moisture content of methane combustion flue gas, diaphragm condensation solutions are widely used, e.g., simple heating condensation boiler fueled with gas, for domestic use [
27]. In district heating systems, the temperature of return water from the network also allows for the condensation of moisture but to a limited extent [
28].
1.3. Proposed Solution
In district heating systems, the boiler generates thermal energy by fuel combustion at a certain excess of air. The heat is transferred to a working medium (water, thermal oil) by the structures inside the boiler (flames, flame tubes, tubes, depending on the design) and later in the economizer [
29]. In typical solid fuel combustion solutions, heat recovery ends here, and the hot flue gases are dumped into the atmosphere. In more sophisticated solutions, particularly those adapted to burn clean fuels (such as natural gas, propane or fuel oil), additional condensing heat exchangers with a large heat transfer area are used [
28].
The change under consideration is that combustion air is not routed directly from the environment [
30]. It is preheated and, to a large extent, humidified or even saturated. The combustion energy is collected by the dedicated piping inside the boiler and the economizer. Hot flue gases are then cooled in a condensation scrubber to a temperature lower than the condensation temperature of the incoming flue gas stream. The recovered heat comes from a reduction in the flue gas temperature as well as from the heat of condensation. The proposed solution is shown in
Figure 1.
The flue gases continue into the recovery scrubber where they are washed by water circulating in a second, independent circuit (deep recovery system). Water cools the exhaust gases in the recovery scrubber. Hot water is then directed to the humidifying scrubber, where it contacts the fresh air that is then directed to combustion. Water cools down as a result of this action, simultaneously warming and moistening the air stream directed to the combustion. In this way, energy is returned to the furnace. On the other hand, recycling of the moisture results in a very significant increase in the dew point in the flue gas stream and, thus, significantly increases heat recovery in the condensation column. The presented system allows one to operate with a wide range of fuels, consistently achieving high efficiency each time.
The new proposed solution is called a “recondensation system”. The aim of this study is to compare the proposed system to other existing solutions. This goal is realized by using process simulations in Chemcad 8 software (release 2021).
2. Materials and Methods
2.1. Chemcad 8 Simulation
The proposed technological solution (
Figure 1) and reference systems were simulated by Chemcad 8 software. Simulation was carried out for the assumption that water is fed only into the district heating network. A supply temperature of 120 °C and a return temperature of 50 °C were assumed as the basic operating point of the district heating network. These are typical operating parameters for district heating networks in Poland [
9]. For most of the year, these temperatures are lower, which, in practice, allows for even greater savings to be achieved, as will be discussed later.
In order to study the behavior of the system, a theoretical model was built based on Chemcad 8. The furnace was modelled as a Gibbs Reactor. Henry’s Law Global K-value Model and Latent Heat Enthalpy model [
31,
32] were applied in the software. Henry’s Law describes that solubility of a gas in a liquid is proportional to the pressure of the gas over the solution (Formula (1)).
pg—partial pressure of the species in the gas phase;
xa—equilibrum mole fraction of the species;
H—Henry’s constant for the species.
The temperature dependence is calculated using correlation shown in Formula (2).
T—temperature;
A,
B,
C,
D—constant values depending on the species, as shown in
Table 1.
Simulations were performed with different fuel types—biomass with 0%, 10%, 20%, 30%, 40%, 50%, 60% moisture content, methane and biogas. Biomass with 0% moisture content is not commercially available and should, therefore, be regarded as a purely theoretical consideration with no practical application. In the simulation, the biomass used was bark. The parameters of bark are detailed in
Table 2.
Flue gas outlet temperature was a key recorded parameter in this research. The lower the flue gas temperature, the higher the efficiency of heat recovery. The adopted strategy was to seek the lowest possible flue gas temperature. To find the optimal parameters for the new system, process optimization was performed. The amount of fuel was set so that the power resulting from the LHV value was 1 MW.
2.1.1. Reference System Simulation
Simulations of boiler operation without a heat recovery system and with condensation system were created (
Figure 2a,b, respectively). They are intended to enable comparison of the newly proposed solution with conventional ones.
The simulation shown in
Figure 2a shows the boiler system without heat recovery. Fuel (stream 3) and combustion air (stream 1) are introduced into the boiler (equipment 1). The exhaust gas is discharged to the environment through heat exchangers (equipment 2 and 3). The flue gas temperature is set at 160 °C. A fixed temperature value is used in practice to protect the heat exchanger (equipment 3) from corrosion. After-work water from the district heating system (stream 25) is heated in the heat exchangers (equipment 2 and 3) to 120 °C. The hot water is fed into the district heating system (stream 27).
Figure 2b shows a simulation of a boiler with a condensing heat recovery system. The combustion system remains unchanged. Flue gases are not discharged directly to the atmosphere but are directed to the condensation scrubber (equipment 4). The scrubber’s circulating water receives heat directly from the flue gas stream. The heated circulating water (stream 20, 21) through the pump (equipment 14) goes to the heat exchanger (equipment 11), which preheats the return water from the district heating network. A constant, small stream of water is fed into the scrubber circulation (stream 17; 10 kg/h) to simulate the refreshing of water subject to contamination.
2.1.2. Proposed Recondensation System Simulation
The proposed recondensation system is an extension of the condensation system shown in
Figure 2b. The system proposed in the analysis differs from the previous one in having two additional scrubbers working together (
Figure 3). The flue gases flow through an additional scrubber (equipment 5) receiving heat. The heated water goes to the humidification scrubber (equipment 7), where it preheats and humidifies the air used for combustion. Thus, the system allows for receiving an additional portion of heat and transferring it back to the boiler.
Fuel is introduced into the furnace (
Figure 3, stream 3). Air is introduced into the furnace (stream 2). In furnace (1), the combustion of fuel takes place. The flue gas stream enters heat exchanger (2), which is in fact boiler equipment that directly receives the heat. Flue gases, cooled to 300 °C, reach heat exchanger (3), which simulates the economizer. This is a standard component used in this type of boiler set-up. The economizer cools flue gases to a temperature that is safe in terms of the acid dew point—160 °C [
10] (stream 6). The cooled gas is then transferred to condensation column (4). As a result of the process, flue gases are cooled to low temperatures (stream 7), and heat is transferred to the water circulating in a scrubber. It is drawn from column (4) and pumped to the heat exchanger (11). Heat exchanger (11) is the actual collection point for the energy from condensation. It transfers heat to water (stream 24) returning from the district heating network. Circulating water returns to condensation column (4). On the way from heat exchanger (11) to column (4), two additional elements are used: a mixer (12) and a stream splitter (13). The mixer adds a negligible amount of cold water (17) to enable smooth sensitivity analyses. This flux has no significant effect on the simulation results. The stream splitter (13) removes excess water so that the amount of water circulating in the system is constant. The exhaust gas leaves condensation column (4) and enters recovery column (5). After cooling, exhaust gas leaves the column (stream 8) and enters the atmosphere.
Column (5) operates in a coupled system with humidifying scrubber (7) using circulating water. Water from column (5) is pumped into column (7). From column (7), water is transferred back into column (5). Here, analogous to column (4), a system is used that adds a small amount of water to achieve stability during sensitivity analyses. In addition, in the system of columns (5, 7), water must be added to clean the system of impurities that accumulate over time. Column (7) is supplied with air (stream 1), which is then directed for combustion (stream 2).
Water from district heating returns, which is fed (stream 24) into heat exchanger (11) and is then directed to the economizer (3) and the boiler (2), where it draws energy and gradually heats up to the target temperature of 120 °C.
2.2. Optimisation—Sensitivity Study
The aim of this study was to analyze the energy efficiency of the recondensation system for different fuels and different temperatures of water returning from the district heating network. It was noted that flow rates of water in 2 circuits (streams 19 and 11) were critically important parameters. With the use of Chemcad 8 software, we investigated how the flow rates of both the condensation scrubber circuit (
Figure 3—equipment 4) and the deep recovery system (recovery and humidifying scrubbers) (
Figure 3—equipment 5 and 7) affect the temperature of the flue gas discharged into the chimney. The maximum efficiency of the system was examined using a two-dimensional sensitivity analysis. Two-dimensional sensitivity analysis involves running simulations for each combination of two variable values (in one-dimensional analysis, it would be a single variable only). The output data were recorded. Variable values were set for the flow rates of the streams (
Figure 3—stream 19 and 11) directed to the columns (
Figure 3—equipment 4 and 5). The values of each flow rate were varied from 1000 to 20,000 kg/h in steps of 100 kg/h.
The minimum of the exhaust gas temperature was searched using the two-dimensional analysis. A two-step procedure was implemented. First, the analysis was performed for a wide range of values with a low resolution (a large step in sensitivity analysis). Then, the range around the minimum was narrowed down in order to identify the optimal conditions more precisely. Finally, using the selected flow rates, the simulation was repeated, and the parameters of the individual heat exchangers (2, 3, 11) and temperature of the flue gas discharged into the chimney were reported (
Figure 3, Stream 8).
The reference system with a boiler, without any recovery system (
Figure 2a), does not require optimization. It operates under fixed, defined conditions.
The reference system with a condensation scrubber (
Figure 2b) required optimization. A change in water flow results in a change in heat recovery efficiency. One-dimensional sensitivity analysis, a function built into the Chemcad 8 system, was used to determine the optimal operation of the system. Variable values were set for the flow rates of the stream (
Figure 2, stream 19) directed to the condensation column (
Figure 2—equipment 4). The values of flow rates were varied from 1000 to 20,000 kg/h in steps of 100 kg/h. The minimum of the exhaust gas temperature was searched.
It should be pointed out that, due to the methodology for calculating the efficiency of the system, in most of the described cases, we encounter values above 100%. Efficiency is calculated according to Formula (3):
Results of more than 100% do not comply with the definition of efficiency, but the above formula is commonly employed in industrial applications. Values over 100% are observed due to the fact that thermal efficiency is considered [
33]. A similar approach was also used in another study of a similar research arrangement [
30,
34].
2.2.1. Varying Types of Fuel
Firstly, the energy efficiencies gained by combustion of the different types of fuel were studied. The list of examined fuels is as follows: biomass with moisture content 0%, biomass with moisture content 10% (e.g., wood pellets), biomass with moisture content 20% (e.g., grass, bales), biomass with moisture content 30% (e.g., pre-dried woodchips), biomass with moisture content 40% (e.g., poplar wood), biomass with moisture content 50% (e.g., sawdust, bark), biomass with moisture content 60% (e.g., olive residues), methane and biomethane (set as 70% of methane and 30% of carbon dioxide). In this study, we keep the composition of the fuel (bark) constant by changing only the moisture content. This is to make the results comparable. For the analyzed cases, the following constant values were assumed, as shown in
Table 3.
In order to compare the results for systems that are typically used in energy applications with the proposed technology, three sets of simulations were performed. As a result, a comparison of the three variants was conducted:
2.2.2. Varying Temperature of District Heating Water Return Temperature
The temperature of district heating water return is usually about 50 °C [
9]. We tested how changing this value affects the efficiency of heat recovery. For this purpose, biomass with 50% moisture content and methane were used as fuel. The recovery efficiency for biomass as fuel was checked at return temperatures of 30, 40, 50, 60 and 70 °C, while methane was at 20 and 50 °C. An exception was made, and for the test with methane as fuel at a return temperature of 20 °C, the air humidity was set at 0%. This is a theoretical case with no coverage in actual heating systems but resulting in an interesting result. For the analyzed cases, the following constant values were assumed, as shown in
Table 4.
3. Results
3.1. Sensitivity Study Results Interpretaion
In
Section 3.1.1 and
Section 3.1.2, we will focus on a single case to accurately present and explain the result of the sensitivity analysis.
Section 3.2 and
Section 3.3 consider the effect of fuel type and return water temperature from the district heating network on the minimum flue gas temperature.
3.1.1. Reference System
The water flow in the condensation scrubber circuit was optimized. The value of the water flow rate for which the flue gas temperature obtains its minimum was determined. It was assumed that the fuel is biomass with a moisture content of 50%, and the temperature of the return water from the district heating network is 50 °C. From the sensitivity analysis, the dependence of the flue gas temperature on the flow rate of water circulating in the condensation scrubber was derived (
Figure 2b, stream 19).
Figure 4 can be interpreted as follows. Too small a flow rate of circulating water has insufficient heat capacity to accumulate all the heat of the flue gas in the condensation scrubber (Equipment 11). Too much water has excessive heat capacity and has no chance to be heated significantly to a desired parameter. At the same time, the heat exchanger (
Figure 2b, equipment 11) does not cool the water significantly to obtain optimum operating conditions. There is a stabilization of the temperature value of the water entering the scrubber at a higher level than under optimal conditions. Between these extremes, there must be a condition at which the temperature of the exhaust gas is the lowest. The detailed explanation is the same as in the third paragraph of
Section 3.1.2.
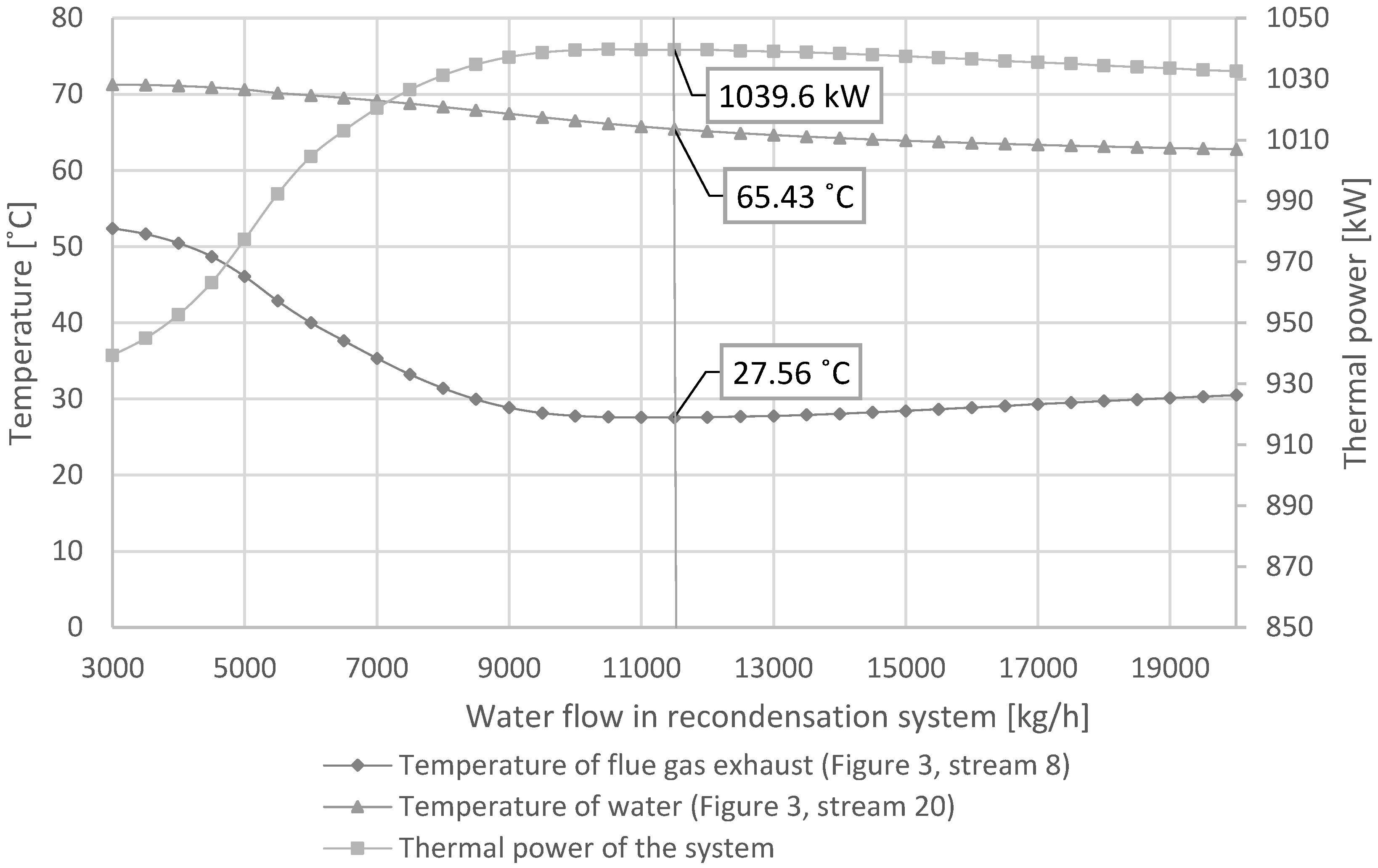
3.1.2. Recondensation System
When performing the two-dimensional sensitivity analysis, it was noted that there is a minimum of flue gas temperature for the flow rate in both the condensation scrubber and the recovery scrubber. The relationship for defined input data is shown in
Figure 5 and
Figure 6 (biomass combustion 50% moisture, district heating water return temperature 50 °C).
From the set of graphs shown (
Figure 5 and
Figure 6), it can be seen that by increasing the recovery scrubber (equipment 5) water flow rate, the flue gas temperature is reduced to a certain value. In the considered example, the lowest value observed was ~28.95 °C. Then, as the flow rate increased, there was a small temperature increase. There was a distinct minimum observed. Depending on the water flow in the condensing system (equipment 4), the minimum is reached for a different flow in the recovery scrubber (equipment 5). The minimum temperature varies for different flow rates in the recondensation system. The scrubbing systems influence each other. As the flow rate in the deep recovery system increases, the minimum temperature decreases down to a certain point (~28.95 °C) and then slowly increases.
To explain the reason for the minimum, it is necessary to consider the operation of the two circuits independently. In the condensation scrubber circuit (
Figure 3—equipment 4), liquid is sprayed into a column, where it gets in direct contact with flue gas in a countercurrent regime. This cools flue gases and causes the condensation of moisture in them. Flue gases cool down to a temperature slightly higher than the water inlet temperature. Water is heated to a temperature that depends on the moisture content in flue gases. Heated water enters heat exchanger (11), where it heats water returning from the district heating network. Cooled water is returned to column (4). A small, insufficient liquid flow rate in the circuit inhibits heat transfer, which results in a lower efficiency of heat recovery. Also, water in the circuit reaches a higher equilibrium temperature, which results in insufficient cooling of the exhaust gas. The heat is not recovered efficiently. By increasing the flow rate of water in the circuit, the situation improves. The temperature of flue gas leaving the scrubber (stream 7) is lower, and heat recovery is more efficient.
For flows higher than the optimum, the situation is different. Water returning from the district heating network has a certain temperature (stream 24). Ideally, its temperature can only be raised to the temperature of water in the scrubber circuit. If the water flow in the circuit is too high, its temperature is only slightly reduced at exchanger (11). Thus, the sub-optimally warm liquid reaches the recovery scrubber without optimal flue gas cooling and heat extraction. The system reaches equilibrium under unfavorable heat recovery conditions. Thus, heat recovery is less efficient. Reducing the flow rate of water in the circuit increases the temperature drop across heat exchanger (11) and increases the heat transfer efficiency.
Increasing the water flow rate in the condensation system from low values decreases the outlet temperature of flue gases (which is the same as taking heat away from flue gases), while decreasing the amount of water on the high side improves heat extraction in the recovery exchanger. Thus, it is to be expected that there must be a minimum between the two extremes where recovery will be most effective. This fact was confirmed by simulations (
Figure 7). Analyzing the data, it can be seen that the maximum efficiency of the system is correlated with the product of the temperature difference in the fluid upstream and downstream of the exchanger and the fluid flow rate. In the same graph, it can be seen that the slope of the change in flue gas temperature as a function of flow to the right of the minimum is small. Significant increases in flow only slightly worsen the performance.
The deep recovery system works in a similar way. In the column (
Figure 3, stream 5), flue gases are cooled with the simultaneous heating of circulating water. Flue gas leaving condensation column (4) is in a 100% moisture-saturated state. Moisture condensation occurs along the entire length of recovery column (5), which ensures the very high efficiency of the unit. Heated water is directed to humidifying column (7). The combustion air (stream 1) enters the same column in a countercurrent. Heat exchange takes place between water and air. Water is cooled by cold air. Due to the evaporation of water, this process is very efficient. The heated air stream (stream 2) carries a large moisture load to the furnace, which results in increased moisture in flue gas.
Performing a sensitivity analysis of the deep recovery system, it can be seen (
Figure 3) that the system works in a similar way to the circuit of condensation column (4); however, the role of heat exchanger (11) is fulfilled here by the humidifying column (7). As indicated by
Figure 8, the highest recovery power achieved in the system coincides with the lowest flue gas temperature and the highest temperature of water (20) leaving condensation scrubber (4). Increasing the flows on the low side results in a significant change in recovery power, similar to that shown according to the condensation scrubber system. In contrast, decreasing the flows on the very high side results in an increase in efficiency that is much more pronounced than was the case with the condensation scrubber.
The occurrence of a local minimum is due to the same reason as for the condensation scrubber system. In contrast to the exchanger (
Figure 3—equipment 11), there is evaporation of the water and humidification of the gas stream in the scrubber (equipment 7). Too much water in the circuit results in a lower water temperature at the inlet of the preheating scrubber. The air stream warms up, but the equilibrium temperature is lower. At the same time, a lower air temperature at the outlet of the preheating scrubber results in fewer water vapors being returned to the boiler.
For each of the examples considered in this publication, the minimum flue gas temperature occurs at a value similar to the one shown in
Figure 8. The minimum for each of the considered examples occurs for different flow rates. It was noted that a change in fuel moisture content or a change in fuel type requires a substantial adjustment to bring the system into an optimum operating state. More on this topic is shown in
Section 3.2 and
Section 3.3.
3.2. System Effeciency Dependence in Relation to Humditiy of Biomass
A relationship can be seen, indicating that the higher the moisture contents of fuel, the lower the efficiency of the system. Nevertheless, the efficiency is very high over the whole range. The results of the investigation are shown in
Table 5.
The performance of the system for a methane combustion with the condensation system was also tested. According to simulations, an efficiency of 97.5% can be achieved in this way (see
Figure 9).
Replacing the fuel with biomethane, simulated as methane (65%) + carbon dioxide (35%), only slightly reduces the efficiency of the system (108.2% vs. 108.1%). Interestingly, the flue gas temperature in Example 8 is lower than the air and fuel inlet temperatures. This state of affairs is possible due to the way humidifying scrubber (7) operates. As combustion air originally has a moisture content of 50%, the evaporation of water can reduce the temperature below the air inlet temperature. A similar situation occurs during cooling tower operation in classical cooling circuits.
The comparison result of systems, without heat recovery, with condensation system and with recondensation system, is shown in
Figure 9. Using the proposed recondensation technology for absolutely dry fuel, the efficiency is 104.5%, and for 60% moisture fuel, it is 101.9%. When using single-stage condensing recovery (see
Figure 2b), efficiencies of 95.5% and 90.9%, respectively, are achieved. In solutions where the energy recovery path ends at the economizer (see
Figure 2a), efficiencies of 90.8% and 70.8% are reported. Without heat recovery, the efficiency drops sharply with increasing fuel moisture content.
On the left-hand side, three points are marked, corresponding to the methane combustion efficiencies. The use of a deep recovery system raises the efficiency to 108.2%. This is close to the maximum theoretically achievable efficiency (111% [
34]).
3.3. System Effeciency Dependence in District Heating Return Water Temperature
To determine how the recondensation system behaves while the district heating water return temperature is higher or lower than 50 °C, a few additional tests were carried out. The efficiencies of different cases are shown in
Table 6.
In trial number 6, where the return temperature was 20 °C, absolutely dry air was used in the amount needed for the stoichiometric combustion reaction, without excess. Although an efficiency of 111% was achieved, less energy was generated in the chemical reaction due to the suboptimal fuel/air ratio. The result is only illustrative and is not reflected in practical application.
The comparison result of systems, without heat recovery, with condensation system and with recondensation system, is shown in
Figure 10. The efficiency of the system without recovery is constant. The flue gas temperature is much higher than the temperature of heated water. The condensation system has high efficiency, while the temperature of return water is low. That is the reason why such systems are used in domestic underfloor heating systems.
The recondensation system works only slightly more efficiently in that case. As the return water temperature increases, the recondensation system achieves a greater advantage over the condensation system. It is important to say that the condensation system has slightly lower efficiency than the system without heat recovery, while the return temperature is 70 °C. The reason is that the return water is heating water circulating through the condensation scrubber. Heat flux is oriented in a different direction. The recondensation system seems to work fine, but the amount of humidity in combustion air (
Figure 3, stream 2) is very high (about 32%
mass). It may result in combustion problems due to low-oxygen concentrations. A temperature of 70 °C does not happen in district heating systems in Poland [
9], so it is only an emergency case consideration.
4. Discussion
A comparison of three systems is presented: 1. an ordinary heating boiler, without heat recovery; 2. a boiler with condensing heat recovery; 3. a boiler with condensing heat recovery and an additional deep heat recovery node. The most significant difference between these systems is the thermal efficiency, especially for wet biomass but also for methane.
Figure 9 shows that, as the moisture content of the fuel increases, the solution with an ordinary boiler loses efficiency. A system with condensing heat recovery performs slightly better. The proposed system with deep heat recovery is the most favorable. For a district heating return temperature of 50 °C and biomass with 50% moisture content, deep heat recovery scored an increase in efficiency of ~32% relative to the system without recovery and ~11% relative to the system with condensing recovery. For a return temperature from the district heating network equal to 60 °C, the increase in efficiency is ~28% and ~18%, respectively.
Another important aspect within the operation of the recondensation system is that the efficiency of the system strongly depends on the liquid flow rate in the scrubber circuits. This means that with a change in fuel quality, the system must be tuned to the new operating conditions. Similarly, with a change in the temperature of the return water from the district heating network, the system must be brought to an optimal state. Based on this, an obvious conclusion can be drawn that an automation system will be necessary, which, based on data from the process, will be able to tune the system.
The presented solution may offer significant savings in fuel consumption when heating water or other media in low- and moderate-temperature ranges. The solution is particularly beneficial using domestic water heating and residential heating.
In specific conditions (absolutely dry air for combustion, stoichiometric air/methane mixture at the temperature of 20 °C and district return water heating temperature 20 °C), the maximum physically possible heat recovery can be achieved (efficiency 111% [
34]).
Wang [
30] obtained a thermal efficiency of 105.9% for natural gas combustion. The system using an enthalpy boiler, on the other hand, declares an efficiency of 106.5% [
34]. Kuck [
11], on the other hand, declared the efficiency of recovery analogous to that presented in this publication at 106.6%.
However, the systems described were modeled for slightly different conditions. The results cannot be compared directly, but a higher flue gas temperature means lower thermal efficiency in the system, which, in our calculations, was over 108%. Since the results are quite close to each other, one can presume that the process model in Chemcad 8 is correct. Thus, the results obtained for the combustion of damp biomass are promising and allow us to develop the technology to reduce energy losses.
5. Conclusions
The aim of the herein presented study was to determine the optimum process parameters of the system meant for a deep recovery of the latent heat of condensation lost with flue gases from the combustion of different fuels. The assumed technical conditions included return temperatures varying from 30 to 70 °C, and the fuels were biomass (with humidity varying 0–60%), methane and biomethane. Different cases of heat recovery were analyzed, including the proposed recondensation system; a classical condensation heat recovery system with a single scrubber system; the simplest combustion system without heat recovery.
The main subjects of analysis were the flow rates of water in the scrubbing recovery system and the deep recovery system, since maintaining the optimum operating conditions for this circuit is crucial to the economics of the solution. Efficiencies of 102–106% for biomass and over 108% for natural gas are very high.
Therefore, the proposed solution may offer significant savings in fuel consumption when heating water or other media in low- and moderate-temperature ranges. The solution is particularly beneficial using domestic water heating and residential heating.
By using deep heat recovery with a recondensation circuit, a high degree of operational stability can be achieved, regardless of the degree of moisture in the fuel. The proposed solution is insensitive to moisture in the fuel. It has high efficiency with low and high amounts of water in the fuel.
The systems for heat production when equipped with a proposed solution can be, therefore, fueled with low-quality fuels, such as wet wood chips from tree debarking. These are not only a source of cheap renewable energy but also as a difficult byproduct of the wood industry, a waste-acidifying soil, which inhibits plant growth [
35].
Hence, the conclusion is that maintaining optimum operating conditions for this circuit is crucial to the economics of the solution. It has not been considered how to control the system to automatically find its optimum. Automation requires further research.
Another advantage of the solution is that it can provide simultaneous wet gas cleaning carried out at the first condensation stage, which is a water scrubber that may remove particulates, dust and acid oxides from flue gas when solid fuels are used. The removal of pollutants in this way is now widely used in the power and heating industries. It should be added that if emissions of acidic oxides such as HCl or SO2 need to be reduced, it is necessary to maintain an alkaline pH. An alkaline pH enables the efficient absorption of acidic oxides.
Author Contributions
Conceptualization, J.K. and R.K.; methodology, J.K.; formal analysis, R.K.; investigation, J.K.; resources, J.K.; data curation, R.K.; writing—original draft preparation, J.K.; writing—review and editing, J.K and R.K.; visualization, J.K.; supervision, R.K. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support was provided for this research by the Ministry of Education and Science of the Republic of Poland. Funding under the programme named “Doktorat wdrożeniowy” under tripartite agreement No. RJO15/SDW/003_22.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Crippa, M.; Guizzardi, D.; Banja, M.; Solazzo, E.; Muntean, M.; Schaaf, E.; Pagani, F.; Monforti-Ferrario, F.; Olivier, J.G.J.; Quadrelli, R.; et al. CO2 Emissions of All World Countries; European Commission: Brussels, Belgium, 2022. [Google Scholar]
- Forman, C.; Muritala, I.K.; Pardemann, R.; Meyer, B. Estimating the Global Waste Heat Potential. Renew. Sustain. Energy Rev. 2016, 57, 1568–1579. [Google Scholar] [CrossRef]
- Cullen, J.M.; Allwood, J.M. Theoretical Efficiency Limits for Energy Conversion Devices. Energy 2010, 35, 2059–2069. [Google Scholar] [CrossRef]
- Jouhara, H.; Khordehgah, N.; Almahmoud, S.; Delpech, B.; Chauhan, A.; Tassou, S.A. Waste Heat Recovery Technologies and Applications. Therm. Sci. Eng. Prog. 2018, 6, 268–289. [Google Scholar] [CrossRef]
- Yang, B.; Yuan, W.; Fu, L.; Zhang, S.; Wei, M.; Guo, D. Techno-Economic Study of Full-Open Absorption Heat Pump Applied to Flue Gas Total Heat Recovery. Energy 2020, 190, 116429. [Google Scholar] [CrossRef]
- Chantasiriwan, S. Optimum Installation of Flue Gas Dryer and Additional Air Heater to Increase the Efficiency of Coal-Fired Utility Boiler. Energy 2021, 221, 119769. [Google Scholar] [CrossRef]
- Wang, H.; Wu, X.; Liu, Z.; Granlund, K.; Lahdelma, R.; Li, J.; Teppo, E.; Yu, L.; Duamu, L.; Li, X.; et al. Waste Heat Recovery Mechanism for Coal-Fired Flue Gas in a Counter-Flow Direct Contact Scrubber. Energy 2021, 237, 121531. [Google Scholar] [CrossRef]
- Smyk, A. Wykorzystanie Wody Sieciowej Powrotnej Do Zasilania w Ciepło Budynków Energooszczędnych. Instal 2018, 393, 5–11. (In Polish) [Google Scholar]
- District Heating Informations. Available online: https://megatem-ec.pl/files/Informacje_o_sieci.pdf (accessed on 21 March 2024). (In Polish).
- Zuo, W.; Zhang, X.; Li, Y. Review of Flue Gas Acid Dew-Point and Related Low Temperature Corrosion. J. Energy Inst. 2020, 93, 1666–1677. [Google Scholar] [CrossRef]
- Kuck, J. Efficiency of Vapour-Pump-Equipped Condensing Boilers. Appl. Therm. Eng. 1996, 16, 233–244. [Google Scholar] [CrossRef]
- Nicholson, R. Recuperative and Regenerative Techniques at High Temperature. J. Heat Recovery Syst. 1983, 3, 385–404. [Google Scholar] [CrossRef]
- Ostrowski, P.; Pronobis, M.; Gramatyka, F.; Olewiński, H.; Habram, T. Sposób oraz Instalacja Odzysku Ciepła i Mokrego Oczyszczania Niskotemperaturowych Spalin Odprowadzanych do Otoczenia, Zwłaszcza z Komór. Spalania. Patent PL 217784, 15 March 2010. [Google Scholar]
- Haddad, C.; Périlhon, C.; Danlos, A.; François, M.X.; Descombes, G. Some Efficient Solutions to Recover Low and Medium Waste Heat: Competitiveness of the Thermoacoustic Technology. Energy Procedia 2014, 50, 1056–1069. [Google Scholar] [CrossRef]
- Lhermet, G.; Tauveron, N.; Caney, N.; Blondel, Q.; Morin, F. A Recent Advance on Partial Evaporating Organic Rankine Cycle: Experimental Results on an Axial Turbine. Energies 2022, 15, 7559. [Google Scholar] [CrossRef]
- Casi, Á.; Araiz, M.; Catalán, L.; Astrain, D. Thermoelectric Heat Recovery in a Real Industry: From Laboratory Optimization to Reality. Appl. Therm. Eng. 2021, 184, 116275. [Google Scholar] [CrossRef]
- Aridi, R.; Faraj, J.; Ali, S.; Lemenand, T.; Khaled, M. Thermoelectric Power Generators: State-of-the-Art, Heat Recovery Method, and Challenges. Electricity 2021, 2, 359–386. [Google Scholar] [CrossRef]
- Hur, S.; Kim, S.; Kim, H.S.; Kumar, A.; Kwon, C.; Shin, J.; Kang, H.; Sung, T.H.; Ryu, J.; Baik, J.M.; et al. Low-Grade Waste Heat Recovery Scenarios: Pyroelectric, Thermomagnetic, and Thermogalvanic Thermal Energy Harvesting. Nano Energy 2023, 114, 108596. [Google Scholar] [CrossRef]
- Chen, S.; Guo, Y.; Pan, Q.; Shuai, Y. A Review on Current Development of Thermophotovoltaic Technology in Heat Recovery. Int. J. Extrem. Manuf. 2024, 6, 022009. [Google Scholar] [CrossRef]
- Waite, M.; Modi, V. Potential for Increased Wind-Generated Electricity Utilization Using Heat Pumps in Urban Areas. Appl. Energy 2014, 135, 634–642. [Google Scholar] [CrossRef]
- Carmona-Martínez, A.A.; Fresneda-Cruz, A.; Rueda, A.; Birgi, O.; Khawaja, C.; Janssen, R.; Davidis, B.; Reumerman, P.; Vis, M.; Karampinis, E.; et al. Renewable Power and Heat for the Decarbonisation of Energy-Intensive Industries. Processes 2023, 11, 18. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y.; Wu, K.; Ge, Z.; Yang, Y. Analysis of a New Super High Temperature Hybrid Absorption-Compression Heat Pump Cycle. Energies 2022, 15, 7515. [Google Scholar] [CrossRef]
- Wang, X.; Zhuo, J.; Liu, J.; Li, S. Synergetic Process of Condensing Heat Exchanger and Absorption Heat Pump for Waste Heat and Water Recovery from Flue Gas. Appl. Energy 2020, 261, 114401. [Google Scholar] [CrossRef]
- Arpagaus, C.; Bless, F.; Uhlmann, M.; Schiffmann, J.; Bertsch, S.S. High Temperature Heat Pumps: Market Overview, State of the Art, Research Status, Refrigerants, and Application Potentials. Energy 2018, 152, 985–1010. [Google Scholar] [CrossRef]
- Maalouf, S.; Boulawz Ksayer, E.; Clodic, D. Investigation of Direct Contact Condensation for Wet Flue-Gas Waste Heat Recovery Using Organic Rankine Cycle. Energy Convers. Manag. 2016, 107, 96–102. [Google Scholar] [CrossRef]
- Guillet, R. Dispositif de Traitement Des Fumees et Des Gaz Comburants d’un Foyer. Patent FR 2508616 A1, 29 June 1981. (In French). [Google Scholar]
- Bălănescu, D.T.; Homutescu, V.M.; Ianuş, G.; Popescu, A. In Situ Study on the Condensate Latent Heat Recovery and Its Economic Impact in the Case of a 60 KW Condensing Boilers System. IOP Conf. Ser. Mater. Sci. Eng. 2020, 997, 012139. [Google Scholar] [CrossRef]
- Sinha, R.; Kumari Thakur, K. Heat Recovery from Condensing Heat Exchanger. Mater. Today Proc. 2023, 72, 1965–1969. [Google Scholar] [CrossRef]
- Filkoski, R.; Lazarevska, A.; Mladenovska, D.; Kitanovski, D. Steam System Optimization of an Industrial Heat and Power Plant. Therm. Sci. 2020, 24, 3649–3662. [Google Scholar] [CrossRef]
- Wang, J.; Hua, J.; Fu, L.; Zhou, D. Effect of Gas Nonlinearity on Boilers Equipped with Vapor-Pump (BEVP) System for Flue-Gas Heat and Moisture Recovery. Energy 2020, 198, 117375. [Google Scholar] [CrossRef]
- Ye, B.; Liu, J.; Xu, X.; Chen, G.; Zheng, J. A New Open Absorption Heat Pump for Latent Heat Recovery from Moist Gas. Energy Convers. Manag. 2015, 94, 438–446. [Google Scholar] [CrossRef]
- Schmitz, K.S. Thermodynamics of the Liquid State. In Physical Chemistry; Elsevier: Boston, MA, USA, 2017; ISBN 9780128005149. [Google Scholar]
- Demirel, Y. Entropy and Exergy. In Nonequilibrium Thermodynamics; Elsevier: Amsterdam, The Netherlands, 2002; pp. 102–123. [Google Scholar]
- Men, Y.; Liu, X.; Zhang, T.; Xu, X.; Jiang, Y. Novel Flue Gas Waste Heat Recovery System Equipped with Enthalpy Wheel. Energy Convers. Manag. 2019, 196, 649–663. [Google Scholar] [CrossRef]
- Kulikova, Y.; Sukhikh, S.; Babich, O.; Yuliya, M.; Krasnovskikh, M.; Noskova, S. Feasibility of Old Bark and Wood Waste Recycling. Plants 2022, 11, 1549. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).