Catalytic Pyrolysis of Naomaohu Coal Using Combined CaO and Ni/Olivine Catalysts for Simultaneously Improving the Tar and Gas Quality
Abstract
1. Introduction
2. Experimental
2.1. Materials
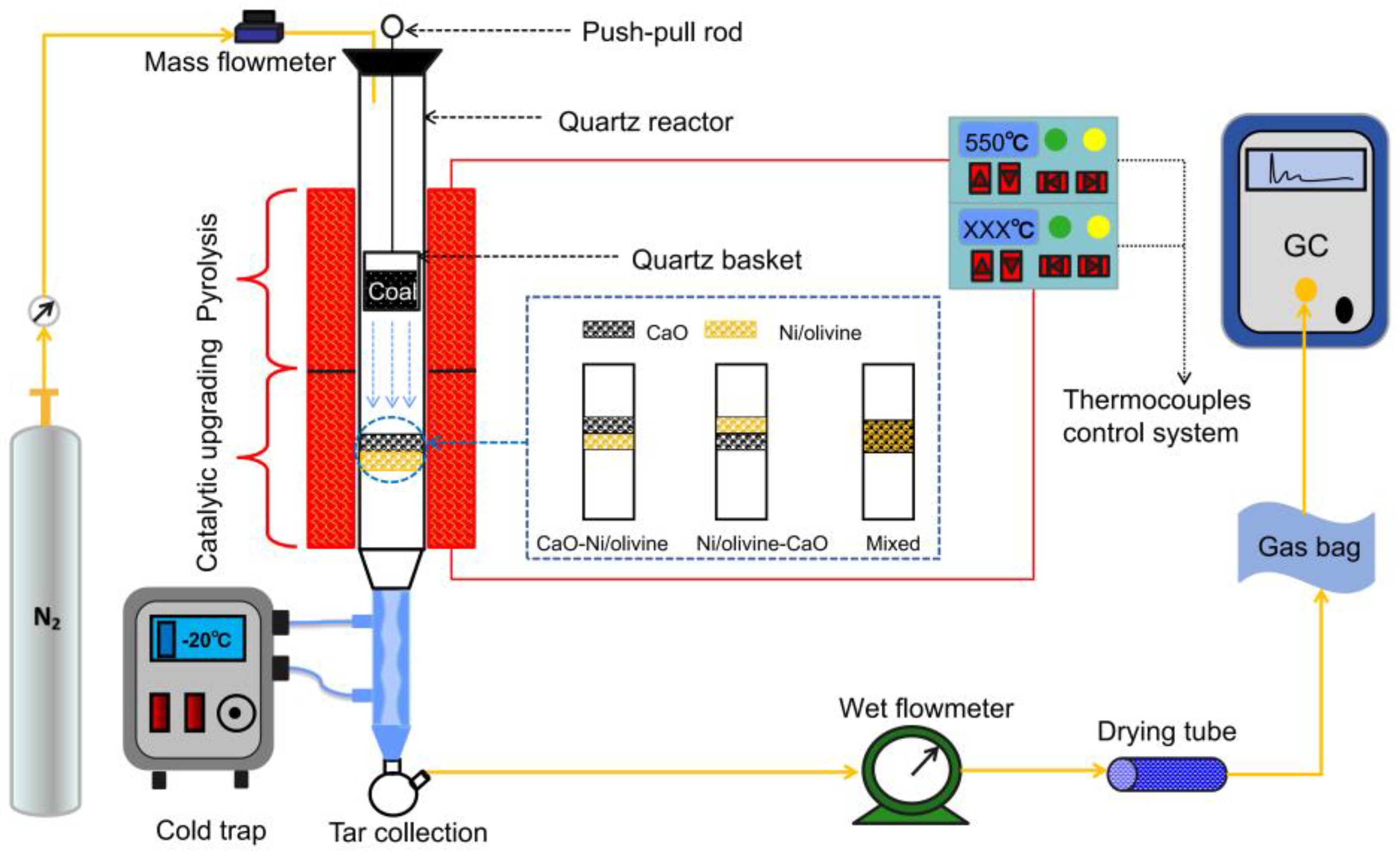
2.2. Experimental Apparatus and Procedure
2.3. Analysis of Pyrolysis Products
3. Results and Discussion
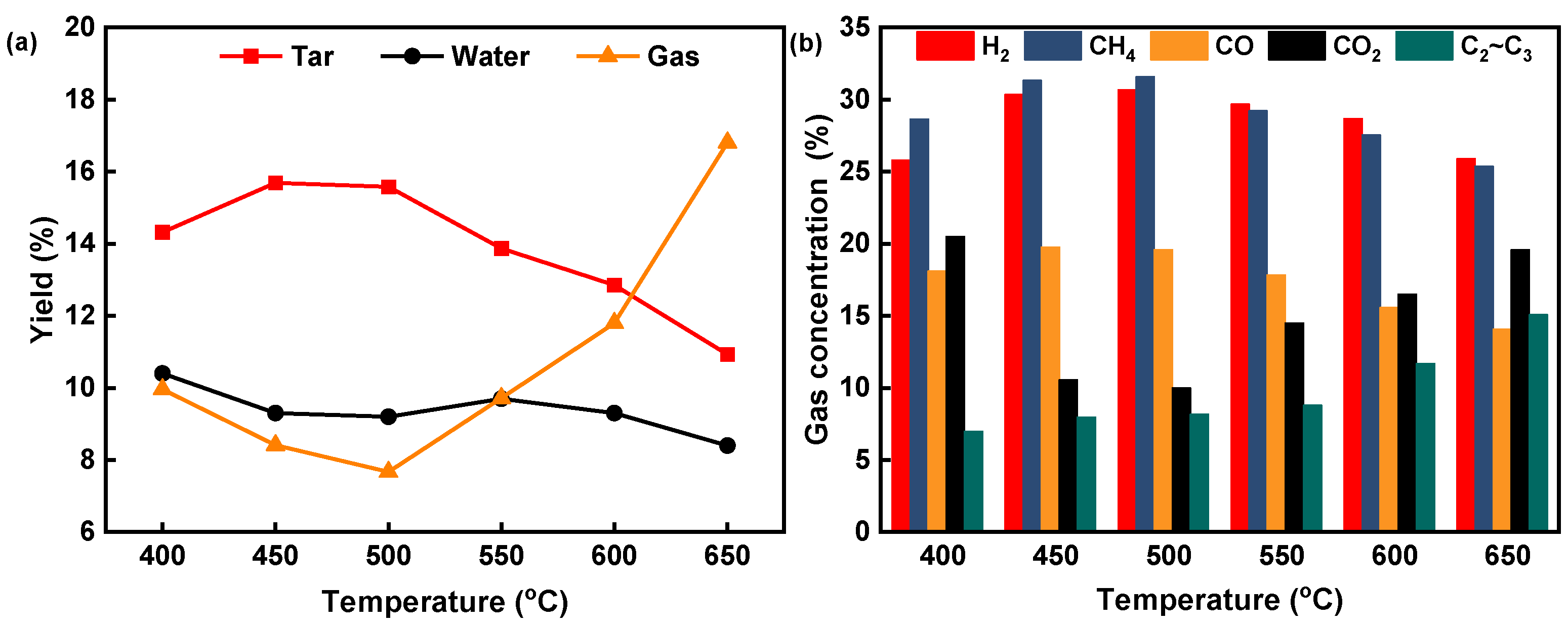
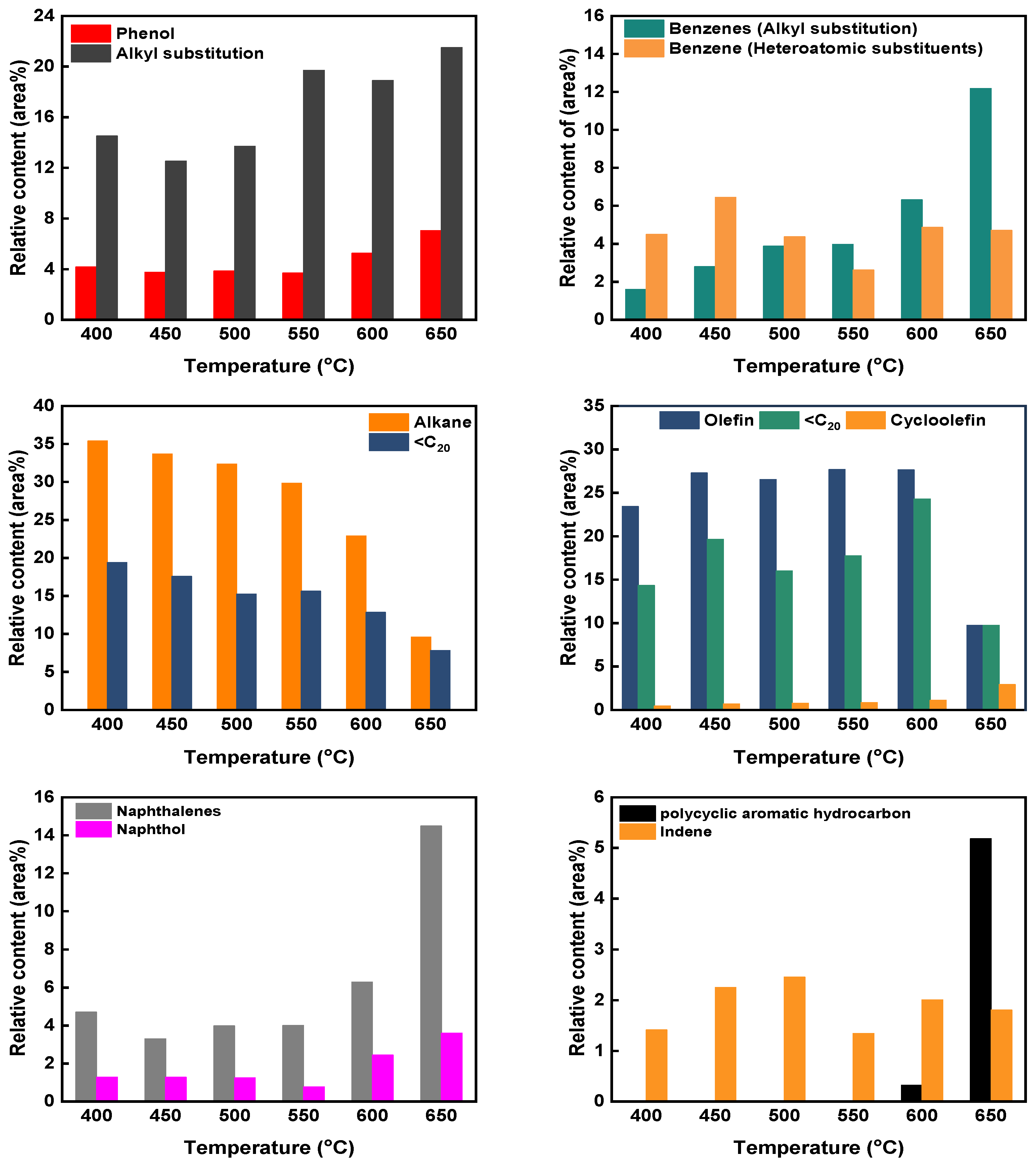
3.1. Pyrolysis Product Distribution at Different Catalytic Modes and Temperatures
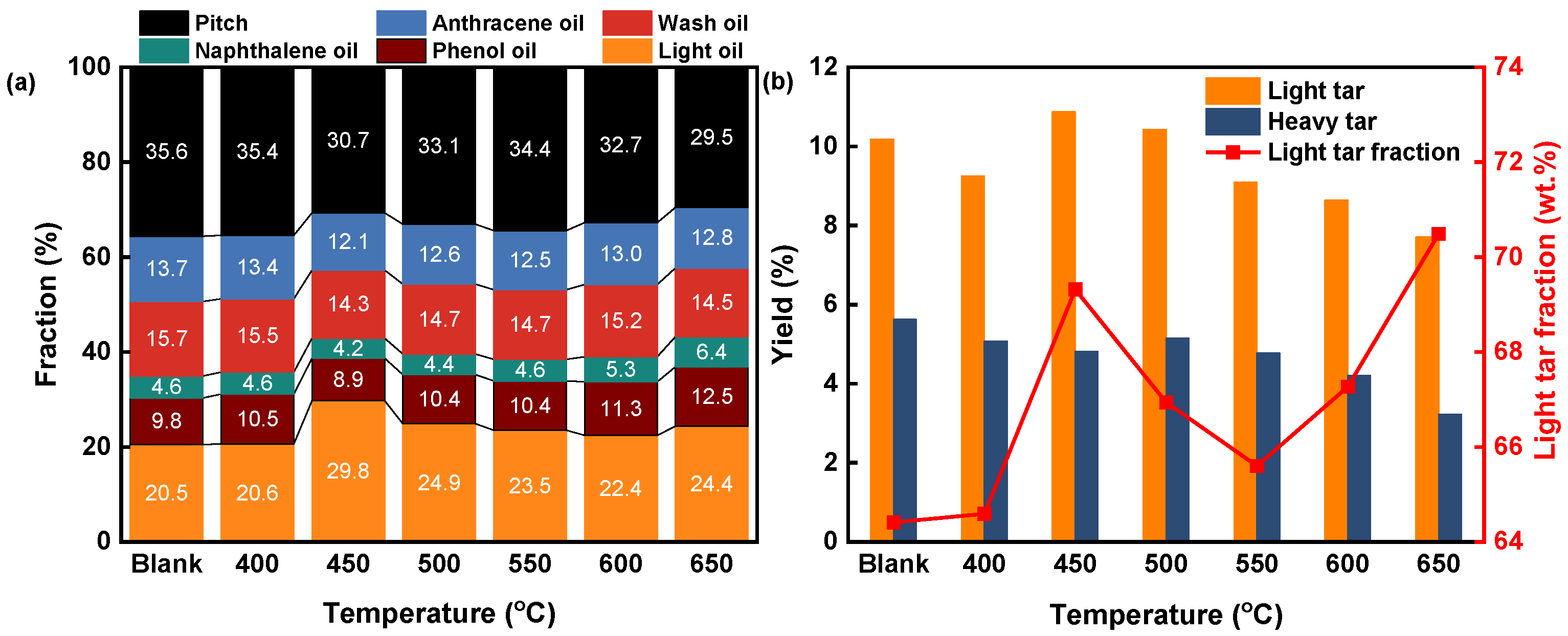
3.2. Simulated Distillation Analysis of the Tar
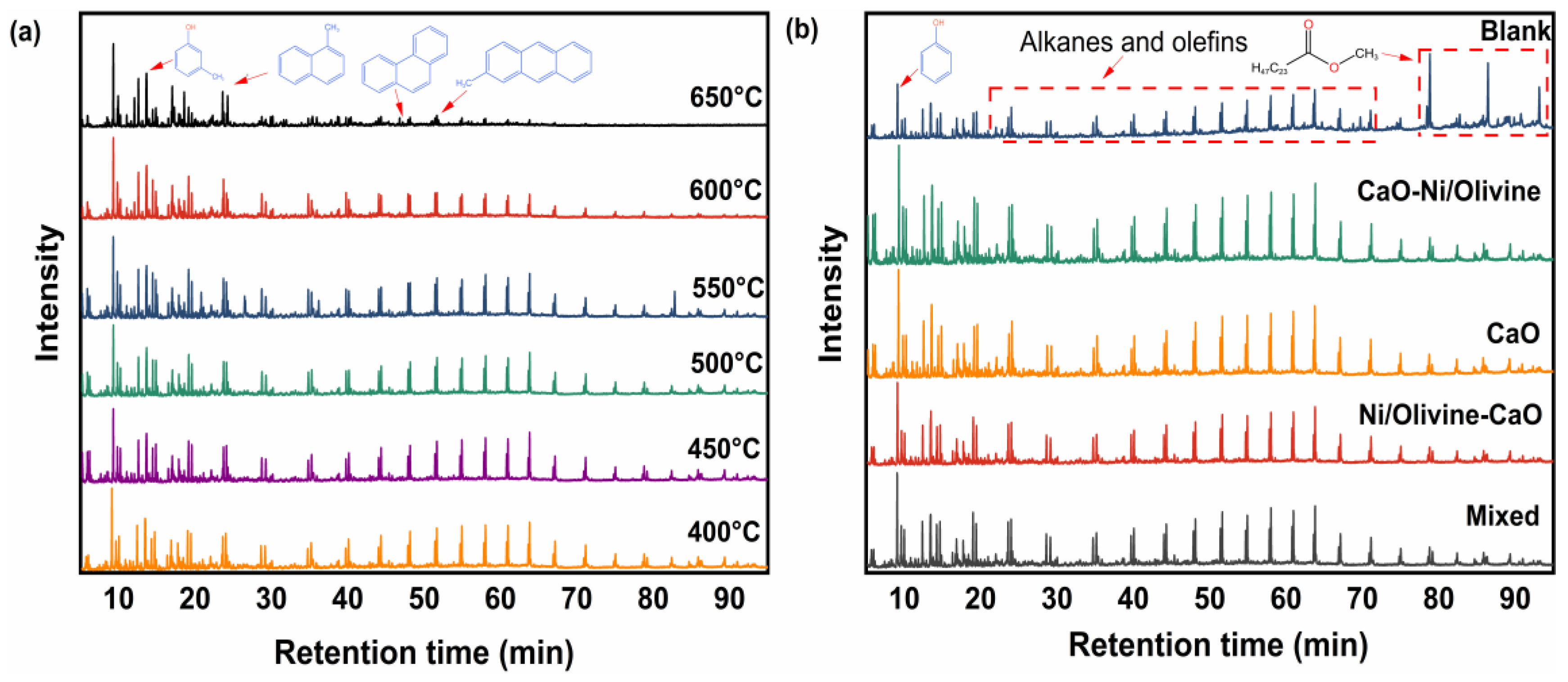
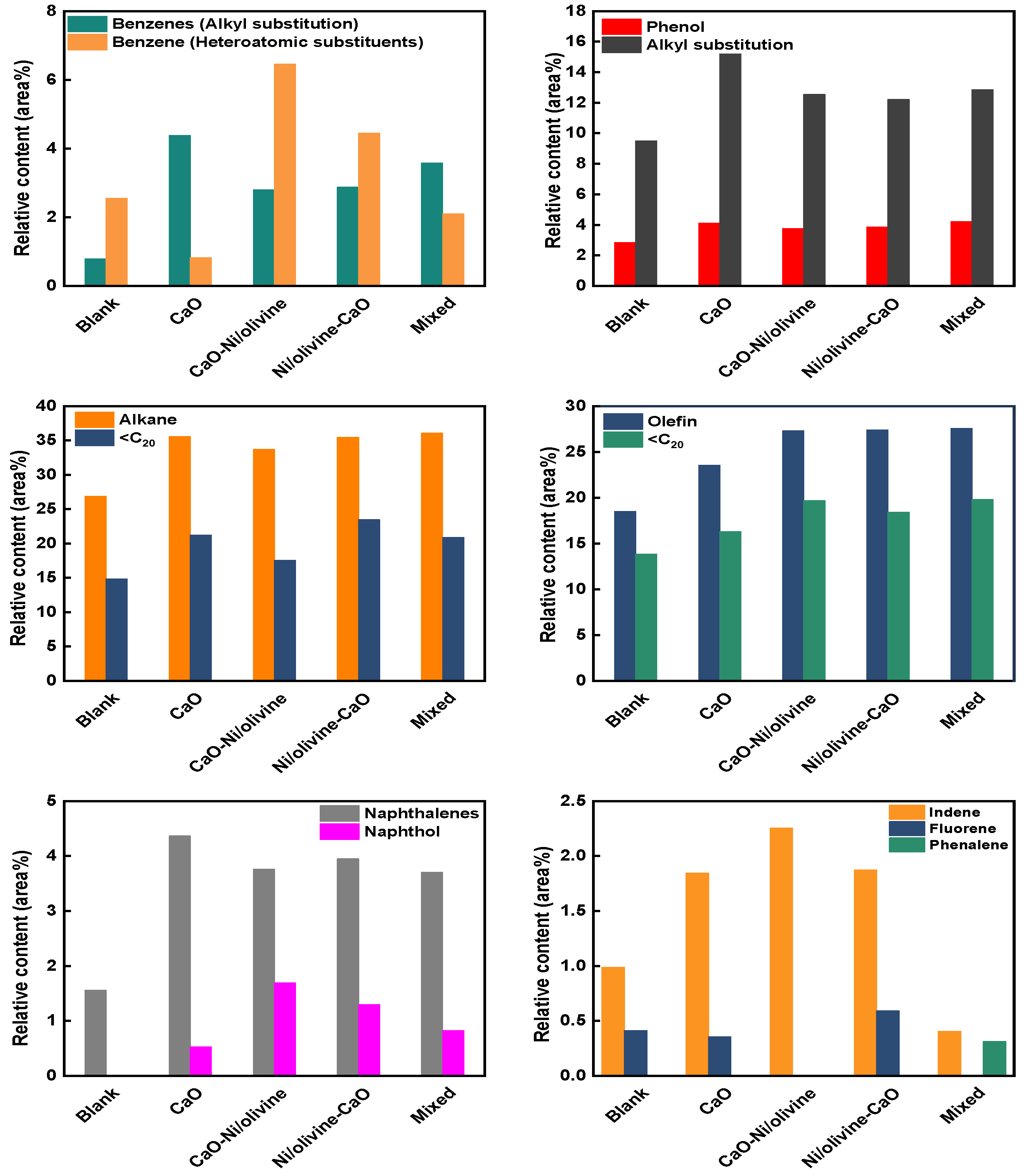
3.3. GC-MS Analysis of the Tar
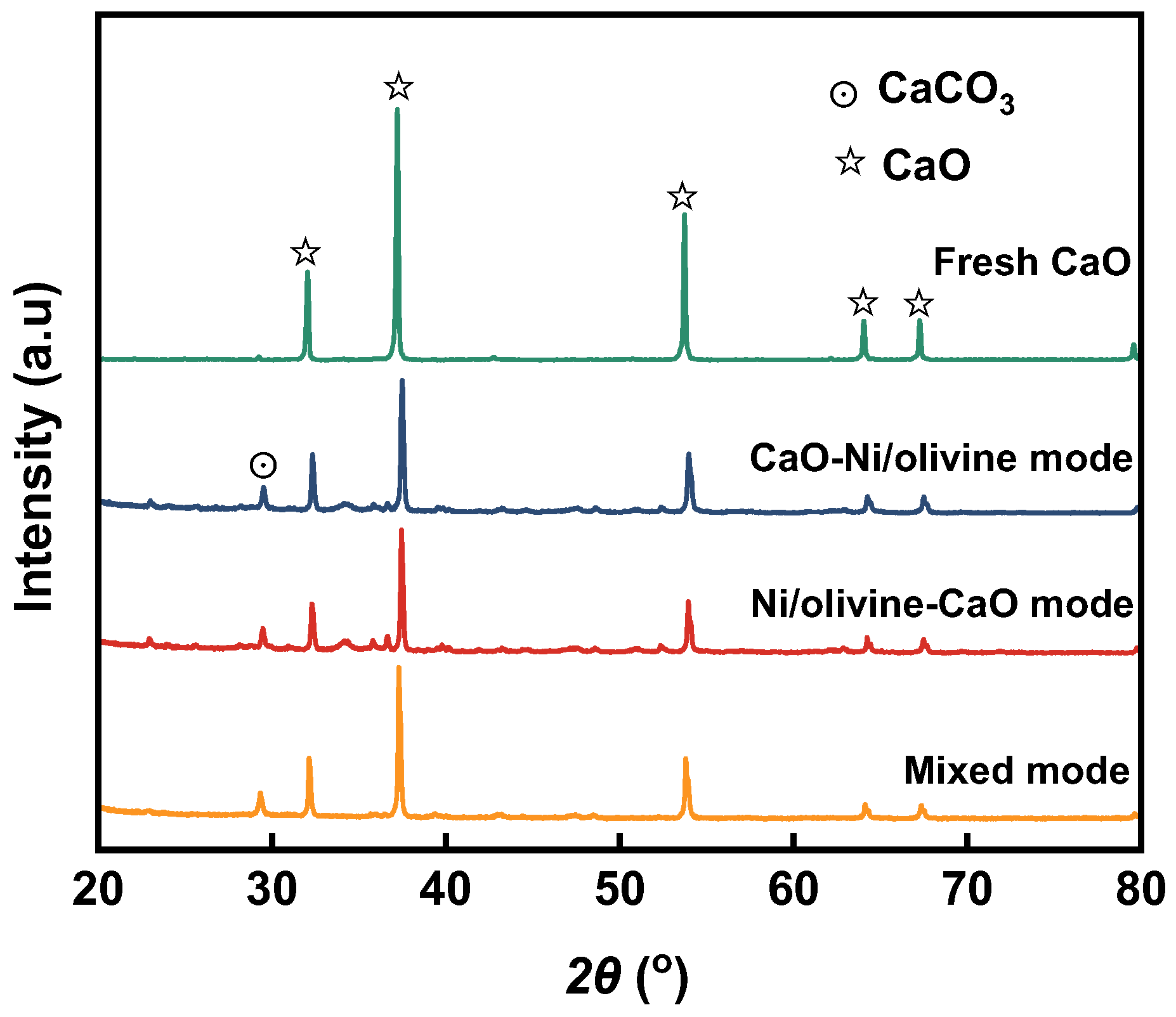
3.4. XRD Analysis
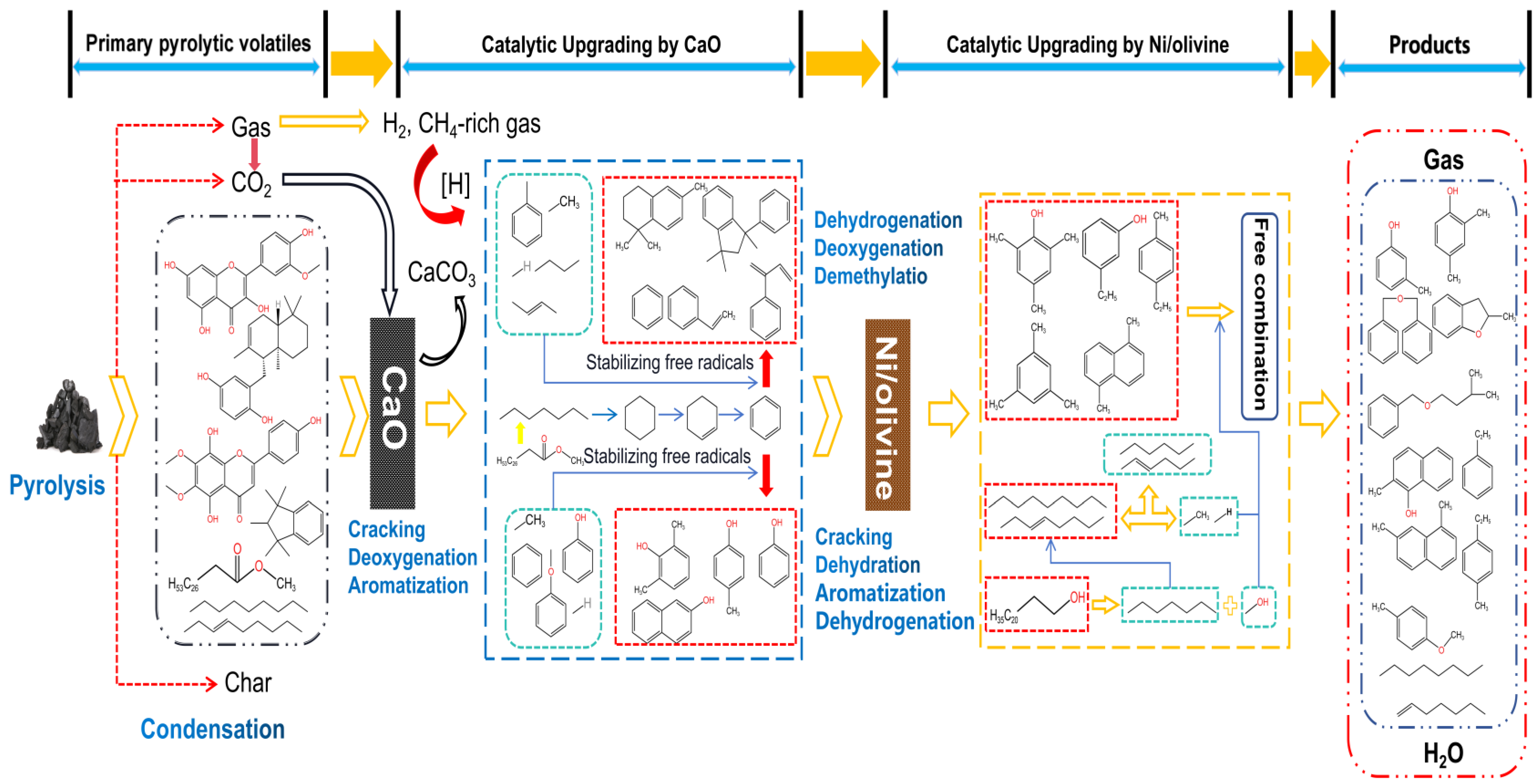
3.5. Proposed Mechanism for Catalytic Upgrading
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, K.-Y.; Wu, Y.-F.; Wang, D.-C.; Jin, L.-J.; Hu, H.-Q. Synergistic effect of co-pyrolysis of pre-dechlorination treated PVC residue and Pingshuo coal. J. Fuel Chem. Technol. 2021, 49, 1086–1094. [Google Scholar] [CrossRef]
- Li, T.; Du, T.-Z.; Shen, Y.-F.; Yan, L.-J.; Kong, J.; Wang, M.-J.; Wang, J.-C.; Chang, L.-P.; Bao, W.-R. Effect of char powder on gaseous tar reaction during low-rank coal pyrolysis. J. Fuel Chem. Technol. 2021, 49, 626–633. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, S.; Li, X.; Xu, J.; Wu, Z.; Yang, B. Process intensification on suspension pyrolysis of ultra-fine low-rank pulverized coal via conveyor bed on pilot scale: Distribution and characteristics of products. Fuel 2021, 286, 119341. [Google Scholar] [CrossRef]
- Zhao, J.-P.; Cao, J.-P.; Wei, F.; Zhao, X.-Y.; Feng, X.-B.; Huang, X.; Zhao, M.; Wei, X.-Y. Sulfation-acidified HZSM-5 catalyst for in-situ catalytic conversion of lignite pyrolysis volatiles to light aromatics. Fuel 2019, 255, 115784. [Google Scholar] [CrossRef]
- Cao, S.; Wang, D.; Wang, M.; Zhu, J.; Jin, L.; Li, Y.; Hu, H. In-Situ Upgrading of Coal Pyrolysis Tar with Steam Catalytic Cracking over Ni/Al2O3 Catalysts. ChemistrySelect 2020, 5, 4905–4912. [Google Scholar] [CrossRef]
- Xue, B.-W.; Han, Z.-N.; Wang, C.; Xu, G.-W. Progress and Breakthrough in Solid Heat Carrier Coal Pyrolysis Technology. Liaoning Chem. Ind. 2020, 149, 199–203. [Google Scholar]
- Fu, D.-Q.; Li, X.-H.; Li, W.-Y.; Feng, J. Catalytic upgrading of coal pyrolysis products over bio-char. Fuel Process. Technol. 2018, 176, 240–248. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, Q.; Shi, L.; Yan, Y.; Wu, J.; Xiang, C.; Wang, T.; Liu, Z. Effect of volatiles’ reaction on composition of tars derived from pyrolysis of a lignite and a bituminous coal. Fuel 2019, 242, 140–148. [Google Scholar] [CrossRef]
- Virginie, M.; Courson, C.; Niznansky, D.; Chaoui, N.; Kiennemann, A. Characterization and reactivity in toluene reforming of a Fe/olivine catalyst designed for gas cleanup in biomass gasification. Appl. Catal. B Environ. 2010, 101, 90–100. [Google Scholar] [CrossRef]
- Shen, Y. Chars as carbonaceous adsorbents/catalysts for tar elimination during biomass pyrolysis or gasification. Renew. Sustain. Energy Rev. 2015, 43, 281–295. [Google Scholar] [CrossRef]
- Ren, X.-Y.; Feng, X.-B.; Cao, J.-P.; Tang, W.; Wang, Z.-H.; Yang, Z.; Zhao, J.-P.; Zhang, L.-Y.; Wang, Y.-J.; Zhao, X.-Y. Catalytic Conversion of Coal and Biomass Volatiles: A Review. Energy Fuels 2020, 34, 10307–10363. [Google Scholar] [CrossRef]
- Niu, J.; Du, X.; Ran, J.; Wang, R. Dry (CO2) reforming of methane over Pt catalysts studied by DFT and kinetic modeling. Appl. Surf. Sci. 2016, 376, 79–90. [Google Scholar] [CrossRef]
- Jin, L.; Xie, T.; Ma, B.; Li, Y.; Hu, H. Preparation of carbon-Ni/MgO-Al2O3 composite catalysts for CO2 reforming of methane. Int. J. Hydrog. Energy 2017, 42, 5047–5055. [Google Scholar] [CrossRef]
- Jeong, M.-G.; Kim, S.Y.; Kim, D.H.; Han, S.W.; Kim, I.H.; Lee, M.; Hwang, Y.K.; Kim, Y.D. High-performing and durable MgO/Ni catalysts via atomic layer deposition for CO2 reforming of methane (CRM). Appl. Catal. A Gen. 2016, 515, 45–50. [Google Scholar] [CrossRef]
- Han, J.; Wang, X.; Yue, J.; Gao, S.; Xu, G. Catalytic upgrading of coal pyrolysis tar over char-based catalysts. Fuel Process. Technol. 2014, 122, 98–106. [Google Scholar] [CrossRef]
- Han, J.; Liu, X.; Yue, J.; Xi, B.; Gao, S.; Xu, G. Catalytic Upgrading of in Situ Coal Pyrolysis Tar over Ni-Char Catalyst with Different Additives. Energy Fuels 2014, 28, 4934–4941. [Google Scholar] [CrossRef]
- Le, J.; Liu, P.; Liu, D.; Lu, X.; Pan, T.; Zhang, D. Effect of Catalysts on the Yields of Light Components and Phenols Derived from Shenmu Coal Low Temperature Pyrolysis. Energy Fuels 2017, 31, 7033–7041. [Google Scholar] [CrossRef]
- Fredriksson, H.O.A.; Lancee, R.J.; Thüne, P.C.; Veringa, H.J.; Niemantsverdriet, J. Olivine as tar removal catalyst in biomass gasification: Catalyst dynamics under model conditions. Appl. Catal. B Environ. 2013, 130–131, 168–177. [Google Scholar] [CrossRef]
- Świerczyński, D.; Courson, L. Oxidation Reduction Behavior of Iron-Bearing Olivines (FexMg1-x)2SiO4 Used as Catalysts for Biomass Gasification. Chem. Mater. 2006, 18, 897–905. [Google Scholar] [CrossRef]
- Courson, C.; Udron, L. Hydrogen production from biomass gasification on nickel catalysts Tests for dry reforming of methane. Catal. Today 2002, 76, 75–86. [Google Scholar] [CrossRef]
- Yi, R.-X.; Tursun, Y.; Hashan, A.; Wang, K. Study on in-situ catalytic pyrolysis of Naomaohu coal with Ni/olivine catalyst for upgrading its pyrolysis oil and gas. Pet. Process. Petrochem. 2023, 54, 57–63. [Google Scholar]
- Jia, Y.; Huang, J.; Wang, Y. Effects of Calcium Oxide on the Cracking of Coal Tar in the Freeboard of a Fluidized Bed. Energy Fuels 2004, 18, 1625–1632. [Google Scholar]
- Lu, Q.; Zhang, N.; Yang, Q.; Li, Y.; Cao, Q.; Liu, S.; Ling, J.; Xie, X.; Xu, Y.; Wang, L.; et al. Influence of calcium promoter on catalytic pyrolysis characteristics of iron-loaded brown coal in a fixed bed reactor. J. Energy Inst. 2020, 93, 695–710. [Google Scholar] [CrossRef]
- ASTM D95-05e1; Standard Test Method for Water in Petroleum Products and Bituminous Materials by Distillation. ASTM International: West Conshohocken, PA, USA, 2005.
- ASTM D2887; Standard Test Method for Boiling Range Distribution of Petroleum Fractions by Gas Chromatography. ASTM International: West Conshohocken, PA, USA, 2023.
- Yang, H.; Zhang, J.; Chen, Z.; Wan, L.; Li, C.; Zhang, X.; Li, J.; Tian, R.; Yu, J.; Gao, S. Base-acid relay catalytic upgrading of coal pyrolysis volatiles over CaO and HZSM-5 catalysts. J. Anal. Appl. Pyrolysis 2023, 170, 105926. [Google Scholar] [CrossRef]
- Li, Y.; Amin, M.N.; Lu, X.; Li, C.; Ren, F.; Zhang, S. Pyrolysis and catalytic upgrading of low-rank coal using a NiO/MgO–Al2O3 catalyst. Chem. Eng. Sci. 2016, 155, 194–200. [Google Scholar] [CrossRef]
- Yi, L.; Liu, H.; Xiao, K.; Wang, G.; Zhang, Q.; Hu, H.; Yao, H. In situ upgrading of bio-oil via CaO catalyst derived from organic precursors. Proc. Combust. Inst. 2019, 37, 3119–3126. [Google Scholar] [CrossRef]
- Kumagai, S.; Yamasaki, R.; Kameda, T.; Saito, Y.; Watanabe, A.; Watanabe, C.; Teramae, N.; Yoshioka, T. Aromatic hydrocarbon selectivity as a function of CaO basicity and aging during CaO-catalyzed PET pyrolysis using tandem µ-reactor-GC/MS. Chem. Eng. J. 2018, 332, 169–173. [Google Scholar] [CrossRef]
- Che, Q.; Yang, M.; Wang, X.; Chen, X.; Chen, W.; Yang, Q.; Yang, H.; Chen, H. Aromatics production with metal oxides and ZSM-5 as catalysts in catalytic pyrolysis of wood sawdust. Fuel Process. Technol. 2019, 188, 146–152. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, Z.; Ding, K.; Zhang, B.; Deng, A.; Min, M.; Chen, P.; Ruan, R. Co-pyrolysis of bamboo residual with waste tire over dual catalytic stage of CaO and co-modified HZSM-5. Energy 2017, 133, 90–98. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.; Liang, L.; Guan, G.; Huang, W. Catalytic depolymerization of coal char over iron-based catalyst: Potential method for producing high value-added chemicals. Fuel 2017, 210, 329–333. [Google Scholar] [CrossRef]
- Zhong, M.; Zhao, Y.; Zhai, J.-R.; Jin, L.-J.; Hu, H.-Q.; Bai, Z.-Q.; Li, W. Effects of nickel additives with different anions on the structure and pyrolysis behavior of Hefeng coal. Fuel Process. Technol. 2019, 193, 273–281. [Google Scholar] [CrossRef]


| Proximate Analysis (wt.%, ad) | Ultimate Analysis (wt.%, daf) | |||||||
|---|---|---|---|---|---|---|---|---|
| Moisture | Ash | Volatile | Fixed Carbon * | C | H | O * | N | S |
| 4.88 | 12.77 | 48.52 | 33.83 | 68.40 | 3.55 | 24.86 | 2.77 | 0.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tursun, Y.; Wang, K.; Yi, R.; Abduhani, H.; Dai, Z.; Zhong, M.; Jin, L.; Li, J.; Liu, Y. Catalytic Pyrolysis of Naomaohu Coal Using Combined CaO and Ni/Olivine Catalysts for Simultaneously Improving the Tar and Gas Quality. Energies 2024, 17, 1613. https://doi.org/10.3390/en17071613
Tursun Y, Wang K, Yi R, Abduhani H, Dai Z, Zhong M, Jin L, Li J, Liu Y. Catalytic Pyrolysis of Naomaohu Coal Using Combined CaO and Ni/Olivine Catalysts for Simultaneously Improving the Tar and Gas Quality. Energies. 2024; 17(7):1613. https://doi.org/10.3390/en17071613
Chicago/Turabian StyleTursun, Yalkunjan, Ke Wang, Runxiao Yi, Hairat Abduhani, Zhenghua Dai, Mei Zhong, Lijun Jin, Jian Li, and Yang Liu. 2024. "Catalytic Pyrolysis of Naomaohu Coal Using Combined CaO and Ni/Olivine Catalysts for Simultaneously Improving the Tar and Gas Quality" Energies 17, no. 7: 1613. https://doi.org/10.3390/en17071613
APA StyleTursun, Y., Wang, K., Yi, R., Abduhani, H., Dai, Z., Zhong, M., Jin, L., Li, J., & Liu, Y. (2024). Catalytic Pyrolysis of Naomaohu Coal Using Combined CaO and Ni/Olivine Catalysts for Simultaneously Improving the Tar and Gas Quality. Energies, 17(7), 1613. https://doi.org/10.3390/en17071613






