The influence of variables on product yields and their characteristics and process efficiencies were investigated and presented below.
3.2.1. Product Yields
The product yields from the SCWG of the fresh and impregnated coconut shells are presented in
Table 3.
The findings in
Table 3 demonstrate that, under identical biomass loading and residence times, elevating the temperature from 400 to 500 °C resulted in increased gas yields and reductions in solid and liquid content for both the control and impregnated samples. The same trend was observed when the residence time values increased from 20 to 60 min. For the feed concentration, it was noticed that an increase from 20 to 30 wt% led to reductions in liquid and gas yields, while solid production was favoured.
The results of this work are consistent with the findings from the literature [
13,
29,
37,
38]. The authors have explained that higher temperatures and residence times promote hydrothermal reactions associated with gas production, including secondary reactions that break down high molecular weight species into gases [
27]. Researchers also described that higher feed concentrations make the interaction of biomass and supercritical water difficult, leading to the formation of complex components that are not easily gasified. Such conditions inhibit important reactions such as solvolysis and hydrolysis, reducing liquid yield and increasing solid content [
39,
40].
In comparison with the results reported by Marcelino et al. [
13], for control and 1 M impregnated coconut shell samples, it was noticed that increasing the nickel catalyst concentration (2 M impregnated sample) led to decreases in liquid and solid yields and increases in gas content for all the experimental runs. The lowest solid and liquid yields, of 9 and 43 wt%, respectively, and the highest gas content (48 wt%) were obtained for SCWG of 2 M impregnated sample at 450 °C, for 40 min and with 25 wt% of biomass loading. Considering the same experimental conditions, high solid and liquid contents were obtained, of 12.5 and 66.1 wt%, respectively, and a low gas yield of 21.2 wt%, for SCWG of the control sample [
13]. These results confirm the positive influence of increased nickel loading on the SCWG process.
The ability of the nickel catalyst to degrade complex intermediates such as tar components into gas has been reported in the literature by Kumar and Reddy [
23] and Borges et al. [
29]. Other studies have also investigated the effect of nickel metal loading on the SCWG of biomass in the presence of different supports [
20,
21,
22]. Gong et al. [
20] and Lu et al. [
22] demonstrated that increasing the nickel metal loading to the maximum of 16 wt% enhanced the gas yields obtained from the SCWG of sewage sludge and wheat stalk, respectively. According to the authors, this enhancement was due to high metal activity sites, which accelerated the gasification rate, resulting in more gaseous products. Similarly, Kang [
21] conducted experiments by varying nickel loading from 2.5 wt% to 20 wt%, concluding that the best results were achieved at 10 wt% of catalyst concentration. The authors observed that 2.5 wt% nickel loading did not positively influence gas yield because of the absence of active sites. On the other hand, when a higher nickel loading (20 wt%) was employed, a poor nickel dispersion was noticed, which reduced the catalytic activity. It can be inferred that the increase in the unsupported dispersed nickel loading performed in this study, from 1.6 wt% to 5.6 wt%, produced favourable effects on the SCWG process, enhancing gas yield by up to 48 wt%.
Three replications of the central point, represented by 25 wt% biomass loading, 450 °C and 40 min, were conducted to evaluate the experimental error. The results showed that the solid, liquid and gas yields were 10.5 ± 1.25 (wt%), 48.5 ± 7.09 (wt%) and 40.5 ± 8.03 (wt%), respectively. The largest error of 8.03 wt% is considered acceptable, as experimental errors are inevitable and challenging to control, leading to some losses in mass balance.
3.2.2. Gas Composition and Process Efficiency
The influence of nickel impregnation on the SCWG process was evaluated by conducting detailed analyses of gas products from a select number of experiments, and the results obtained for the response variables are shown in
Table 4.
The data presented in
Table 4 show that during SCWG of 2 M coconut shell samples, CO
2 was the main gas component reaching 79.5 mol% at 400 °C, 40 min and 30 wt% of biomass loading. The comparison of CO
2 yields in the same SCWG conditions for control and 1 M impregnated samples revealed that CO
2 yields increased with the higher nickel concentration. A similar trend was observed for H
2 production. For example, at 500 °C, 20 min and 25 wt% biomass loading, a maximum H
2 content of 15.2 mol% was achieved, whereas for the control and 1 M impregnated samples, 9.5 and 13 mol% were obtained [
13]. Kang [
21] reported increases in CO
2 yields with enhanced nickel loading supported on Al
2O
3, from 5 wt% to 20 wt%. Likewise, Lu et al. [
22] observed increments in CO
2 and H
2 concentrations when nickel loading supported on MgO was increased from 4 wt% to 16 wt%. The increase in gas yields with increasing Ni loading corresponded to lower char and liquid yields. These findings suggested that the high nickel content contributed to the enhancement of the water gas reaction rate, thereby enhancing CO
2 and H
2 production. It was likely that Ni redox catalysis was responsible for the reaction of supercritical water with carbon (from char and tar compounds) as represented by Equation (7).
The presence of Ni could be responsible for methane reforming (Equations (8) and (9)) to produce more hydrogen and CO/CO
2 as observed in the gas products for increased NiO loading.
Characterisation of the char products showed the presence of Ni
0 as the main Ni species after the reaction, indicating that the initial redox Ni
2+Ni
0 catalysis occurred. This makes methane reforming plausible, and metallic Ni has been reported to catalyse this reaction [
24,
29].
The results obtained for CH
4 and CO content in most of the runs for the 2 M impregnated sample demonstrated that 5.6 wt% nickel content gave low constituent yields. The maximum CH
4 content (38.9 mol%) was achieved under the conditions shown in run 3 (500 °C, 60 min and 25 wt% biomass loading). However, during SCWG of the control and 1 M impregnated samples under the same conditions, 43.2 and 42.6 mol% of CH
4 were produced [
13]. Similarly, the maximum content of CO decreased from 8.7 mol% (obtained from SCWG of the 1 M impregnated sample) to 2.3 mol% in run 2 due to the increase in nickel loading. However, comparing the results with those obtained for SCWG of the control samples, enhancements in CO production were observed. Nickel acts an an important hydrogenation catalyst, which contributes to the primary decomposition of CO and H
2 [
27]. On the other hand, Borges et al. [
29] reported that the reduction in CO yield with increased nickel loading could be attributed to the competition for CO for methanation and hydrogen production through the water–gas shift reaction.
Azadi et al. [
41] and Kang [
21] reported a decrease in CH
4 formation with an increase in nickel loading supported by Al
2O
3 from 5 wt% to 20 wt%. According to the authors, this results from the high sensitivity of the methanation reaction to the high nickel loading and crystalline size. The decreased nickel dispersion affects the interaction with the support and the catalyst’s activity in methanation reactions.
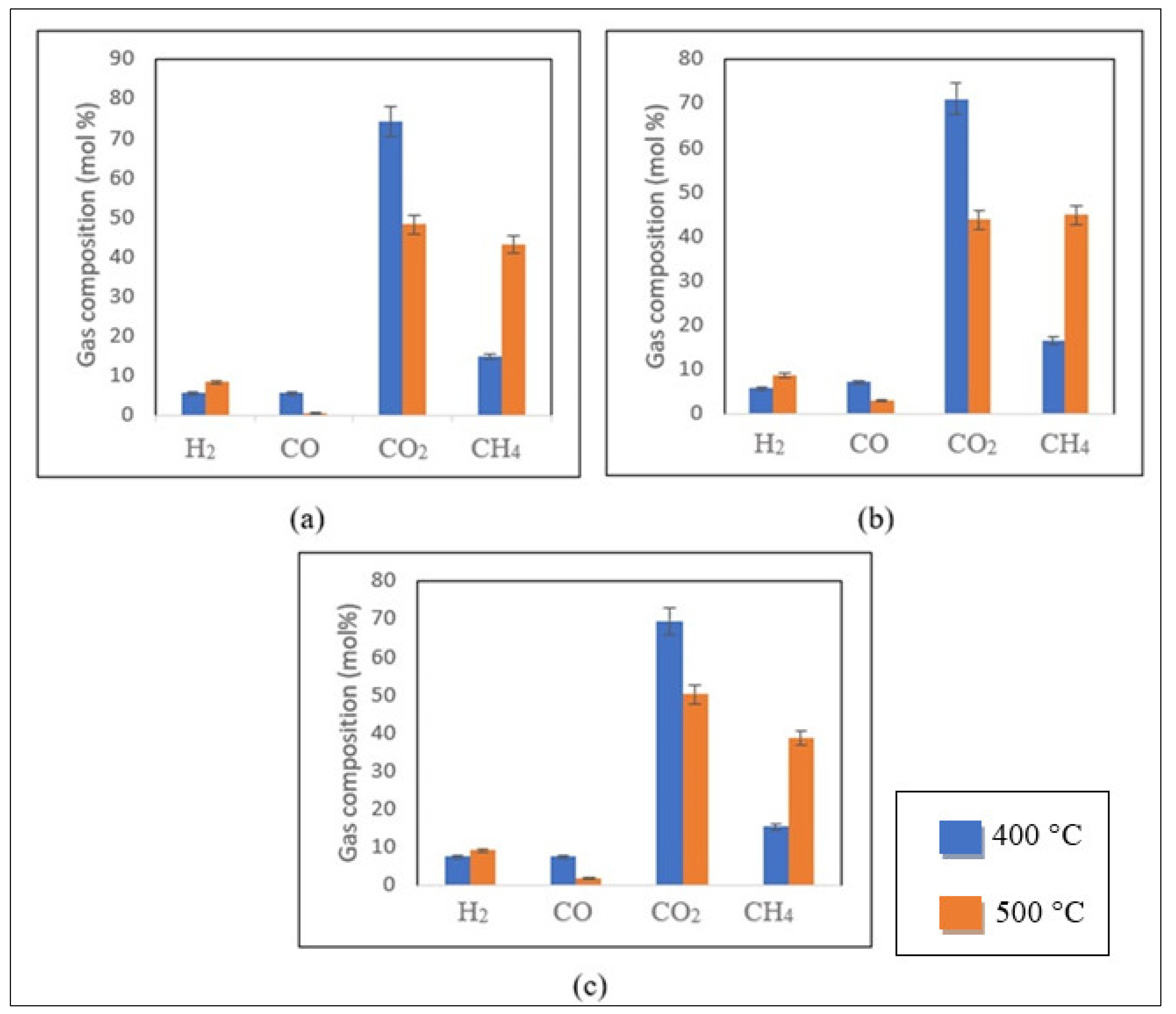
Influence of Temperature
Temperature is recognized as the most crucial variable in determining gas composition and SCWG efficiency [
13]. For a fixed residence time of 40 min and 20% biomass loading, the impact of temperature on the gasification process of control, 1 M and 2 M impregnated samples is presented in
Figure 5a–c.
The increase in process temperature led to higher yields of H
2 and CH
4 in SCWG of the control and 1 M impregnated coconut shell samples, as depicted in
Figure 5a,b [
13]. The same was observed in
Figure 5c, which represents the SCWG of the 2 M impregnated sample. Adversely, the production of CO and CO
2 decreased with the temperature increase for all samples.
The methanation of CO
2 and CO and water–gas shift reactions are enhanced with temperature contributing to the rise in H
2 and CH
4 yields in the product gas [
42]. Steam-reforming endothermic reactions associated with H
2 production are favoured by higher reaction temperatures [
43]. Despite methanation being an exothermic process, the temperature range employed in this study did not significantly affect this reaction [
13].
The gasification reaction rates with increased nickel loading are less dependent on the process temperature. Consequently, reduced increments in H
2 and CH
4 yields were detected. For example, for the 2 M impregnated sample at 40 min and 20% biomass loading, an increase of 150 and 21% in CH
4 and H
2 yields was observed with the temperature elevation from 400 to 500 °C. Under the same conditions, higher percentages of CH
4 and H
2 contents were observed for the control and 1 M impregnated samples [
13].
The impact of temperature on GE, CGE and HGE values obtained from SCWG of control, 1 M and 2 M impregnated samples was also analysed. The results obtained from the SCWG of samples at 20 wt% biomass loading and 40 min residence time are exhibited in
Table 5.
The GE, CGE and HGE values increased with the temperature for all samples due to an increased reaction rate [
44]. The results are in agreement with those described in the literature [
45,
46], where the increase in the HGE value with the increase in temperature is due to the high hydrogen and methane yields in the gas at 500 °C. The highest GE, CGE and HGE values of 46, 53 and 83% were detected for the 2 M impregnated sample at 500 °C.
The most significant increases in GE, CGE and HGE values were found for the SCWG control sample, suggesting a higher sensitivity of the SCWG reactions to temperature in the absence of a catalyst.
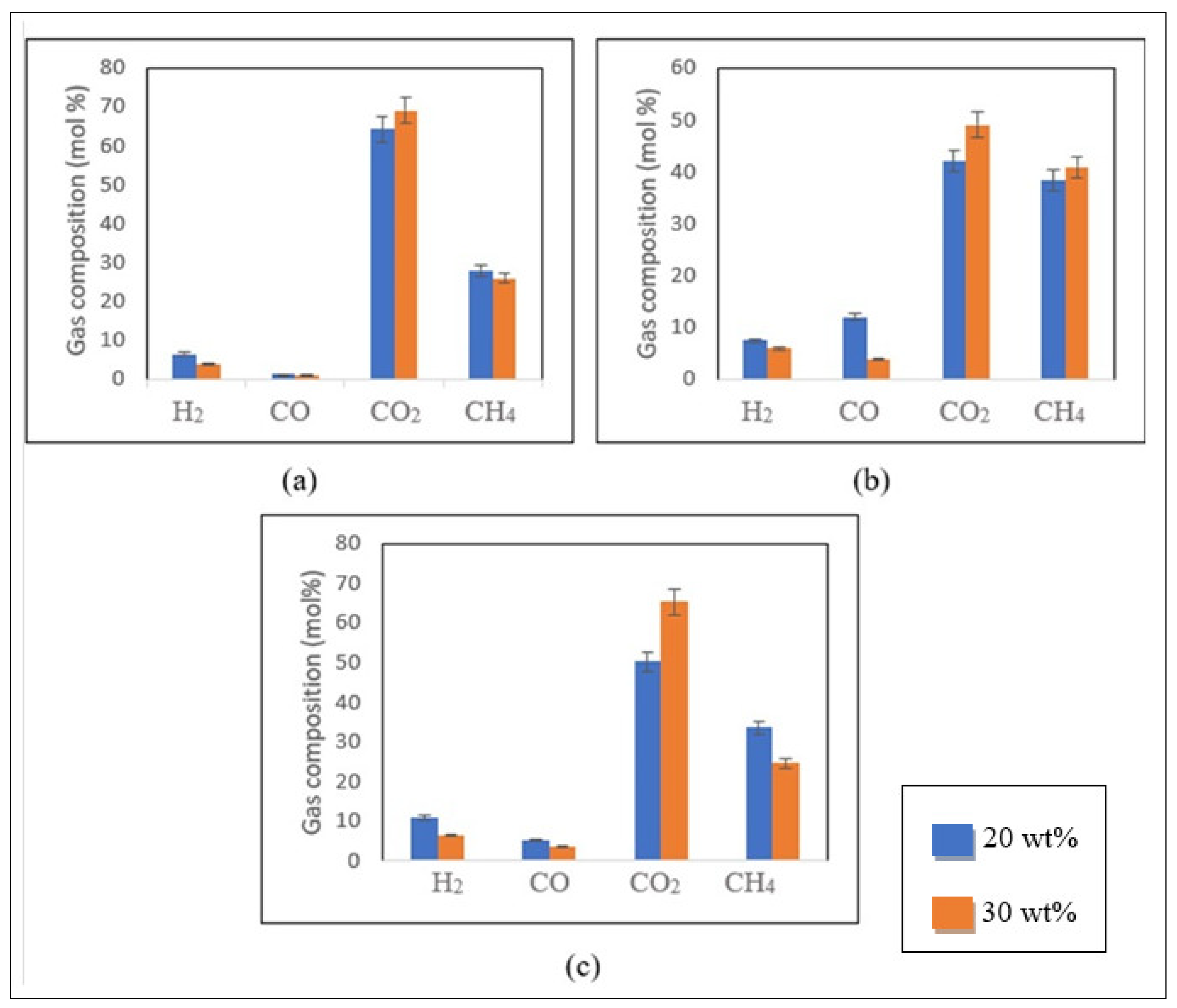
Influence of Biomass Loading
Figure 6a–c present the effect of biomass loading on the gas composition.
According to
Figure 6, the production of CO
2 increased with the feed concentration for control and impregnated samples. However, the molar concentrations of the other gases (H
2, CO and CH
4) were reduced under the same conditions. For instance, for the 2 M impregnated sample, an increase from 50.3 to 65.4 mol% in the CO
2 yield was observed. In contrast, the H
2, CH
4 and CO yields declined from 10.8 to 6.3 mol%, 33.5 to 24.8 mol% and 5.3 to 3.5 mol%, respectively (
Figure 6c).
Compared with the results obtained for the control (
Figure 6a) and 1 M impregnated samples (
Figure 6b), a comparable tendency was noticed for the CO
2, H
2 and CO yields [
13]. Generally, water–gas shift and steam reforming reactions are less favourable at high biomass concentrations, leading to the decreased formation of H
2 and CO
2 [
37]. In the steam-reforming reaction, a significant amount of water is preferable to react with hydrocarbons and produce H
2 and CO as end products [
47,
48]. However, these reactions can be accelerated in the presence of nickel nanocatalysts, leading to higher CO
2 production [
13]. It can be assumed that higher nickel loading produces a greater increase in CO
2 yield. Compared to the 1 M impregnated sample results, the enhancement in H
2 yield with increased nickel loading was insufficient to promote H
2 production at the higher feed concentration. The decrease in CO yield resulted from the combined effect of high biomass loading and nickel content.
When analysing the results obtained for CH
4 content from the 1 M impregnated sample (
Figure 6b), a different profile was noticed [
13]. At 450 °C and 60 min, a modest increase in CH
4 content (from 38.4 to 40.9 mol%) was detected with the increment in biomass concentration. However, in identical temperature conditions and when decreasing the residence time to 20 min, CH
4 increased from 21.9 to 25.8 mol% for the 2 M impregnated sample. This study demonstrated that increasing nickel loading leads to a decline in CH
4 formation, as shown in
Figure 6c, as a result of the increased sensitivity of the methanation reaction to both high nickel loading and nickel crystalline size.
Increasing biomass loading from 20 to 30 wt% for the control and impregnated samples at 450 °C and 60 min decreased GE, CGE and HGE values (
Table 6). It has been reported that increasing biomass loading reduces heat and mass transfer between char and water, so the steam-reforming and gas shift reactions are restricted, reducing gasification efficiencies [
30,
40,
49]. However, the most significant reduction was noticed for the HGE value. This may be due to the decrease in H
2 and CH
4 content with the increased biomass loading. The most considerable reduction of 56% was found for the HGE value obtained for the 2 M impregnated sample. It is possible to assume that the higher influence of the nickel concentration and biomass loading on the HGE value is due to the considerable reduction in H
2 and CH
4 production.
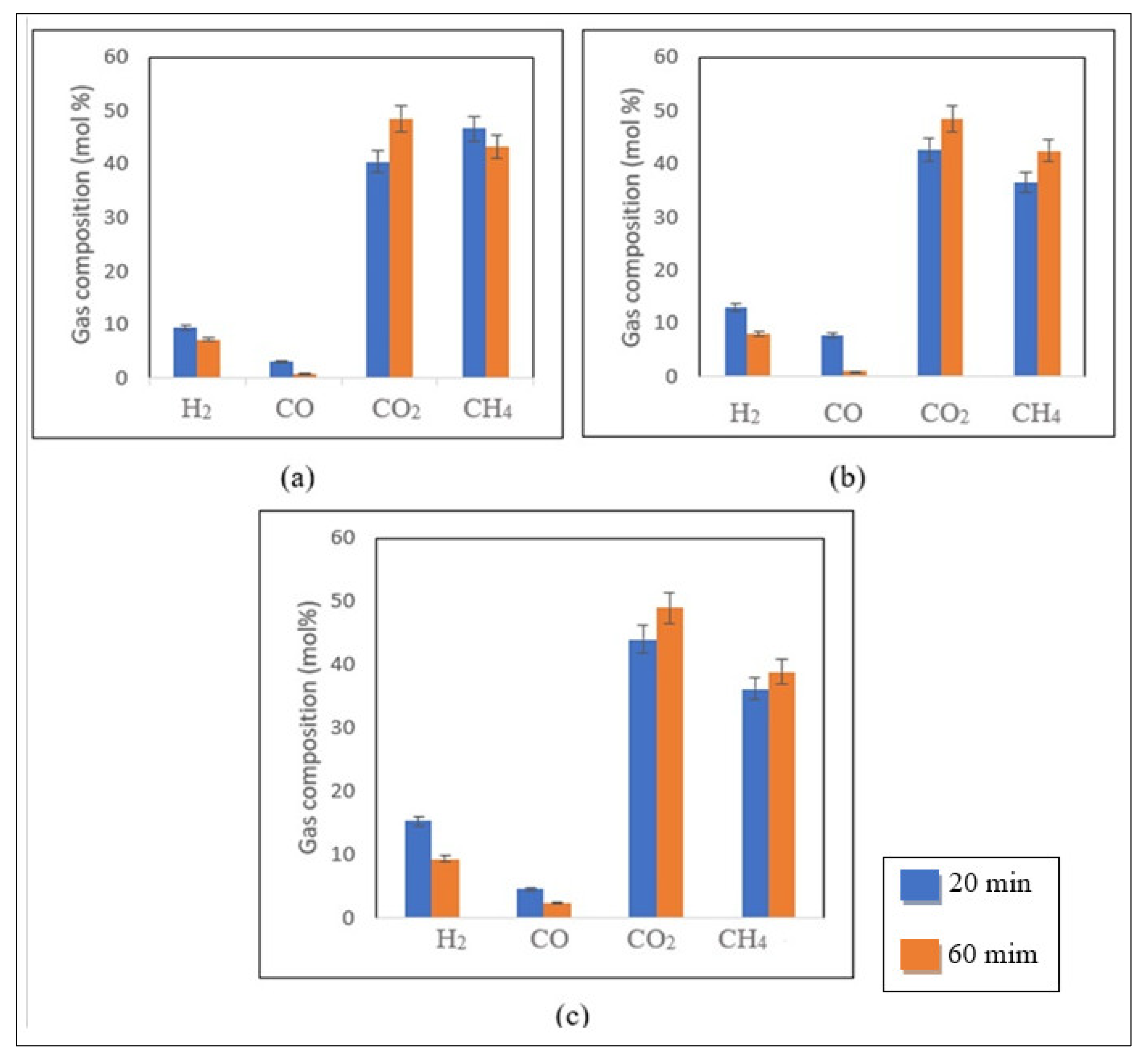
Influence of Residence Time
The impact of residence time on the response variables is seen in
Figure 7a–c for 20 to 60 min residence times for a fixed biomass loading of 25 wt% at 500 °C.
As shown in
Figure 7b,c, the production of CO
2 and CH
4 was favoured with the enhancement in residence time from 20 to 60 min for the 1 M and 2 M impregnated samples. At 25 wt% biomass loading, the CO
2 and CH
4 yields increased slightly from 44.1 to 49.5 mol% and 36.2 to 38.9 mol%, respectively, for longer residence times (
Figure 7c). Conversely, the H
2 and CO yields were reduced from 15.2 to 9.2 mol% and 4.5 to 2.5 mol%, respectively, with an increase in residence time.
A similar trend was observed for the 1 M impregnated sample (
Figure 7b). In these conditions, the H
2 and CO yields reduced from 13 to 8 mol% and 7.7 to 1.02 mol% when the reaction time increased from 20 to 60 min, respectively. Meanwhile, the production of CO
2 and CH
4 increased from 42.8 to 48.4 mol% and 36.7 to 42.6 mol%, respectively [
13]. A similar result was observed for the SCWG of the control sample in similar experimental conditions (
Figure 7a). However, a reduction in CH
4 content from 46.8 to 43.2 mol%, was observed [
13]. It can be concluded that the methanation reaction rate related to the CO consumption and CH
4 formation is more favoured in the presence of the catalyst at low loading and high residence time.
The literature reports similar results [
13,
50]. Water–gas shift and methanation reactions are favoured at longer residence times, leading to the improved formation of CO
2 and CH
4, respectively. Studies also show that relatively low temperatures (<500 °C) and long residence times do not favour H
2 production. Under longer reaction times, hydrogenation and methanation reactions involving CO and H
2 consumption are favoured, resulting in reduced H
2 and CO yields [
29,
51].
The impact of changing the residence time from 20 to 60 min during the SCWG of the control, 1 M and 2 M impregnated samples at 500 °C and 25 wt% on GE, CGE and HGE values is presented in
Table 7.
The higher reaction time (60 min) gives higher GE and CGE values for all samples. This observation agrees with Samiee-Zafarghand et al. [
48], who indicated that an enhanced CGE value with increasing residence time indicates increased gas production in supercritical water conditions. Moreover, greater reaction times are essential for cracking reactions that generate gases [
51]. Nevertheless, it can be seen that the HGE values declined with an increase in time to 60 min due to the promotion of hydrogenation and methanation reactions resulting in a decrease in the yields of H
2 and CO and the HGE value [
38]. The highest HGE value of 133% can be related to the high production of H
2 at lower residence times (20 min). Moreover, hydrogen from water is used during the reactions, leading to HGE values larger than 100%.
Considering the impact of the nickel loading and residence time on gasification efficiencies, the highest percentage of change was found for the HGE value (reduced to 38.2%) obtained for the 2 M impregnated sample at 60 min residence time. Increasing nickel loading and residence time promotes hydrogenation and methanation reactions, leading to a significant reduction.
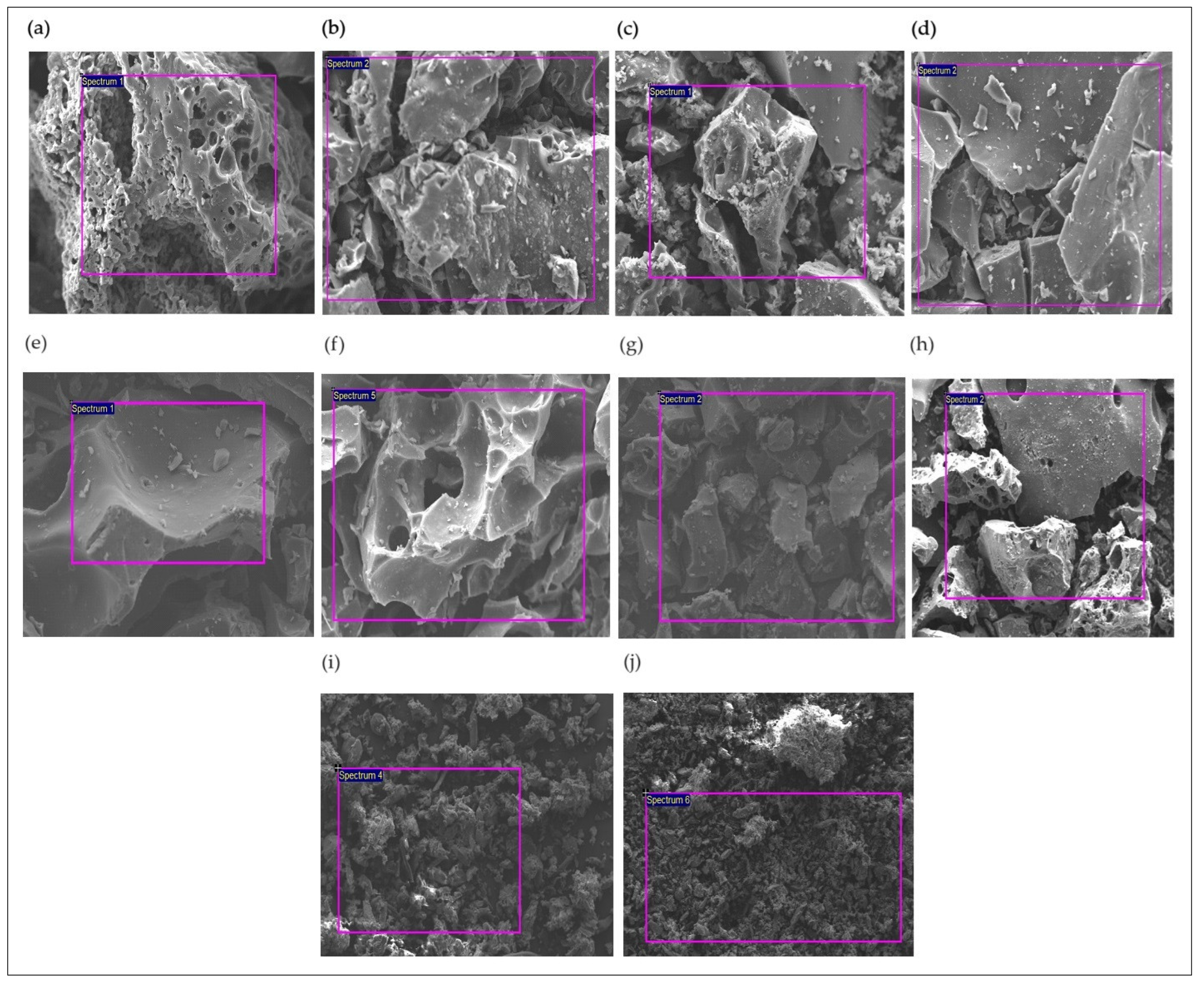
3.2.3. Solid Characterization
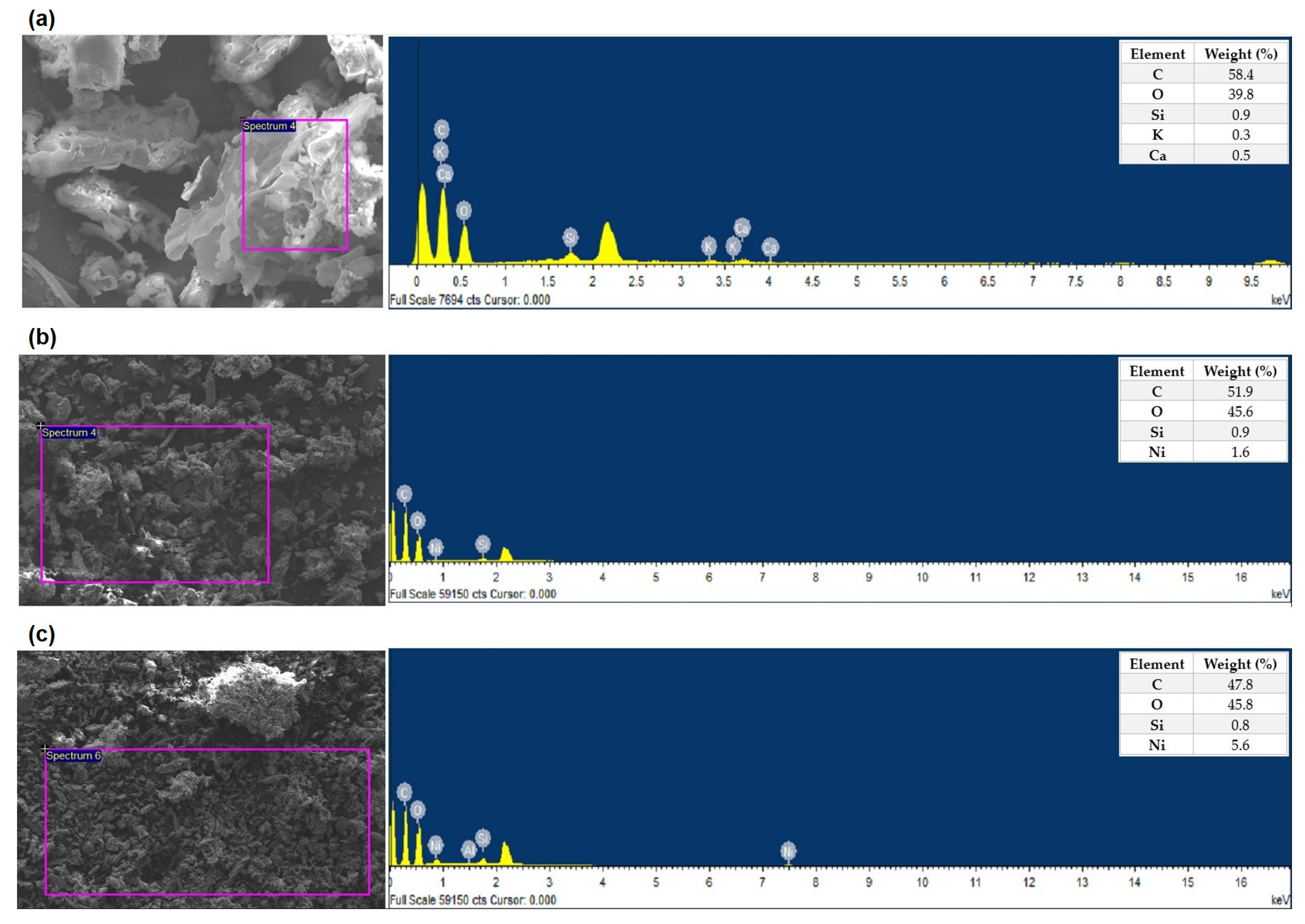
The solid content obtained from SCWG of the impregnated biomass samples at 400 and 500 °C with 20 and 60 min residence times for 25 wt% biomass loading was characterised using SEM-EDX and XRD analysis to assess the impact of nickel concentration and process parameters on the catalyst retention and hydrochar morphology.
The hydrochars (
Figure 8a–h) showed more porous and fragmented surfaces than those observed for the biomass-impregnated samples before SCWG (
Figure 8i,j). Furthermore, the hydrochar produced from the SCWG of the 2 M impregnated samples (
Figure 8a–d) showed a greater appearance of pore structure and fractures.
The hydrochars produced from SCWG at 500 °C for 1 M (
Figure 8g,h) and 2 M (
Figure 8c,d) impregnated samples demonstrated a more fissured structure. The same morphological structure profile appeared with an increment in residence time. These results indicate both conditions promote efficient biomass conversion, compromising the structural conformation to the hydrochar [
40,
47]. Previous studies have also reported the enlargement of pore structure under these experimental conditions, leading to an improvement in the degree of gasification and further gasification reactions, such as free radical reactions. These reactions promote the gradual loss of the oxygenated groups in the hydrochar, causing the disintegration of its surface, as observed in the results obtained from EDX analysis of hydrochars shown in
Table 8 [
15,
40]. The oxygen content in hydrochars decreases with higher temperatures and longer residence times. Conversely, an elevated nickel loading increases the oxygen concentration due to the higher presence of oxygen in 2 M-impregnated biomass.
Ni was found in all hydrochar samples indicating that Ni was retained from the original precursors [
27]. The greatest nickel loading (22.6 wt%) was identified for the hydrochar formed from the SCWG of the 2 M impregnated sample at 500 °C and 60 min. Increased gasification of the solid feedstock would reduce the amount of char product and lead to the enrichment of Ni in the solid residues [
23]. The authors investigated the effect of pyrolysis temperature (from 300 °C to 700 °C) and biomass nickel loading (from 0.1 to 1.0 M) on the nickel concentration in hydrochars with the highest level of nickel (9.9 wt%) found in hydrochars produced from 1.0 M impregnated wood at 700 °C.
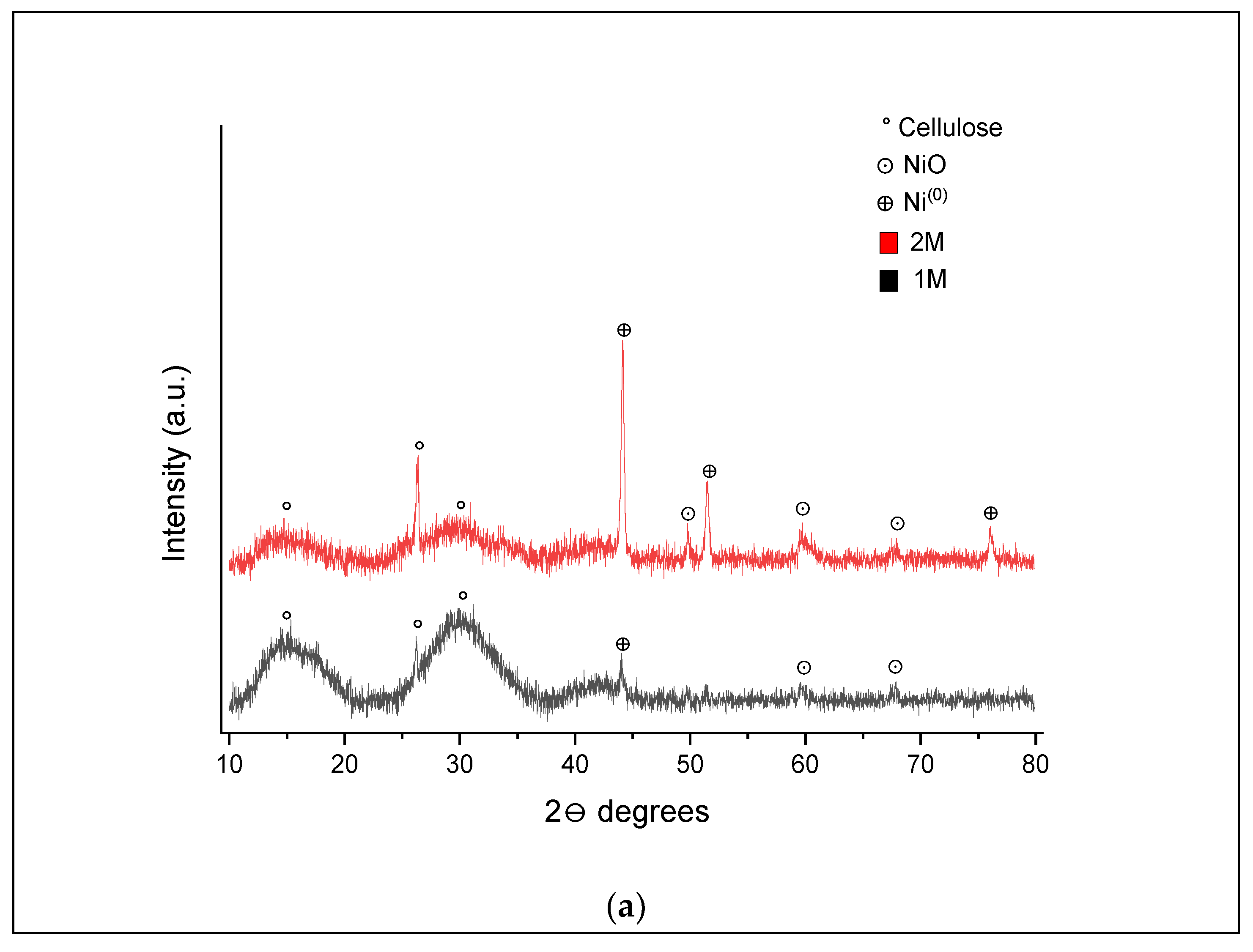
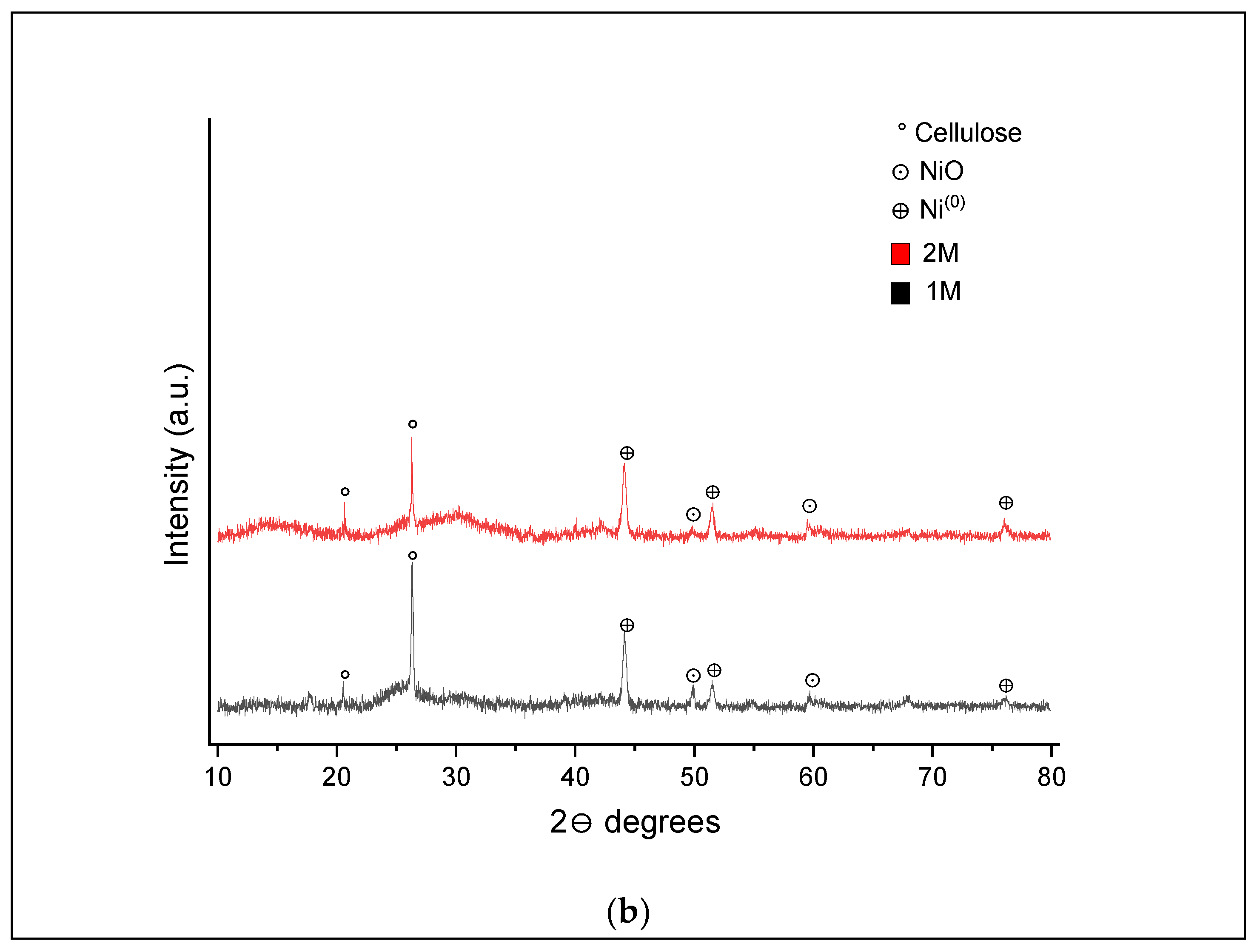
XRD analysis of the hydrochar samples obtained from SCWG of 1 and 2 M impregnated samples at 400 and 500 °C was also conducted, and the patterns are shown in
Figure 9 and
Figure 10, respectively.
In the XRD pattern shown in
Figure 9a, three peaks are highlighted, which relate to some cellulose residue (15.3°, 26.2° and 30.0°) in both samples after SCWG [
47]. In the 1 M impregnated sample, peaks were detected at 59.7°and 67.5°, indicating the occurrence of nickel nanoparticles in oxide form (NiO) and one peak at 44°, characterizing the presence of nickel nanoparticles in the Ni
0 form. In the 2 M impregnated samples, three additional peaks were found at 49.7° (NiO), 51.5° and 76° (Ni
0). From these results, it can be inferred that the transition of nickel from Ni
n+ to Ni
0 and from Ni(OH)
2 to NiO was intensified with increased nickel loading at the process conditions. Kumar and Reddy [
24] described that hydroxides of nickel are initially dehydrated to NiO, which then interacts with char or the produced gases and is reduced to nanoactive forms (Ni
0).
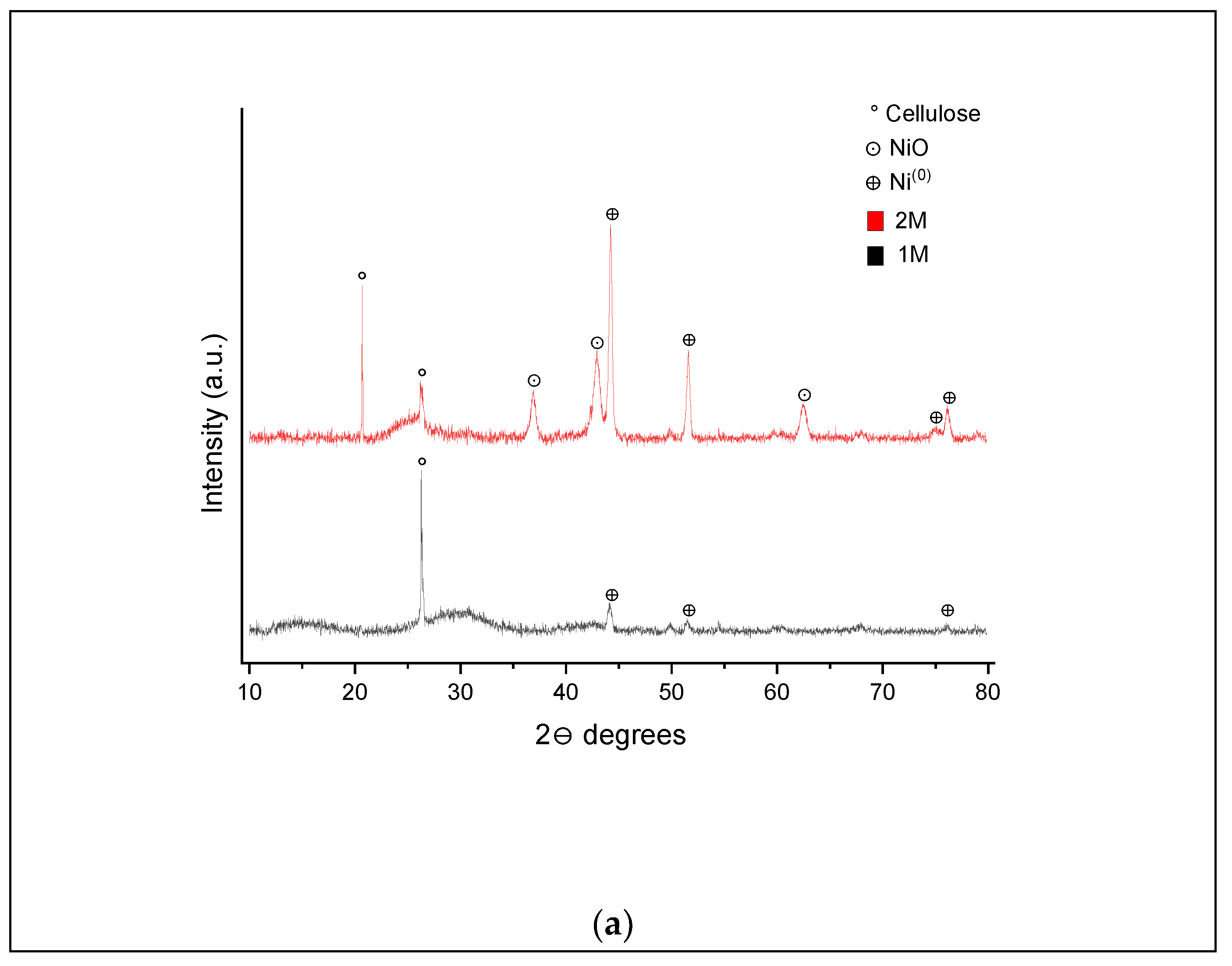
For the 400 °C and 60 min (
Figure 9b) conditions, the absence of peaks at 15.3° and 30° is attributed to the loss of cellulose, and 67.5° to the presence of NiO for the 1 M and 2 M samples. Additional peaks at 49.8° (NiO), 51.5° and 76° (Ni
0) in the XRD pattern were produced for the 1 M impregnated sample. It is possible to assume that increasing the residence time causes the degradation of cellulose and a further reduction from the NiO phase to the pure Ni
0 form [
23].
Many peaks can be seen for the XRD pattern presented in
Figure 10a for hydrochars produced at 500 °C and 20 min, especially for the 2 M impregnated biomass sample. The additional peaks include the presence of nanoparticles for NiO (36.8°, 43° and 62.5°) and Ni
0 (74.8°).
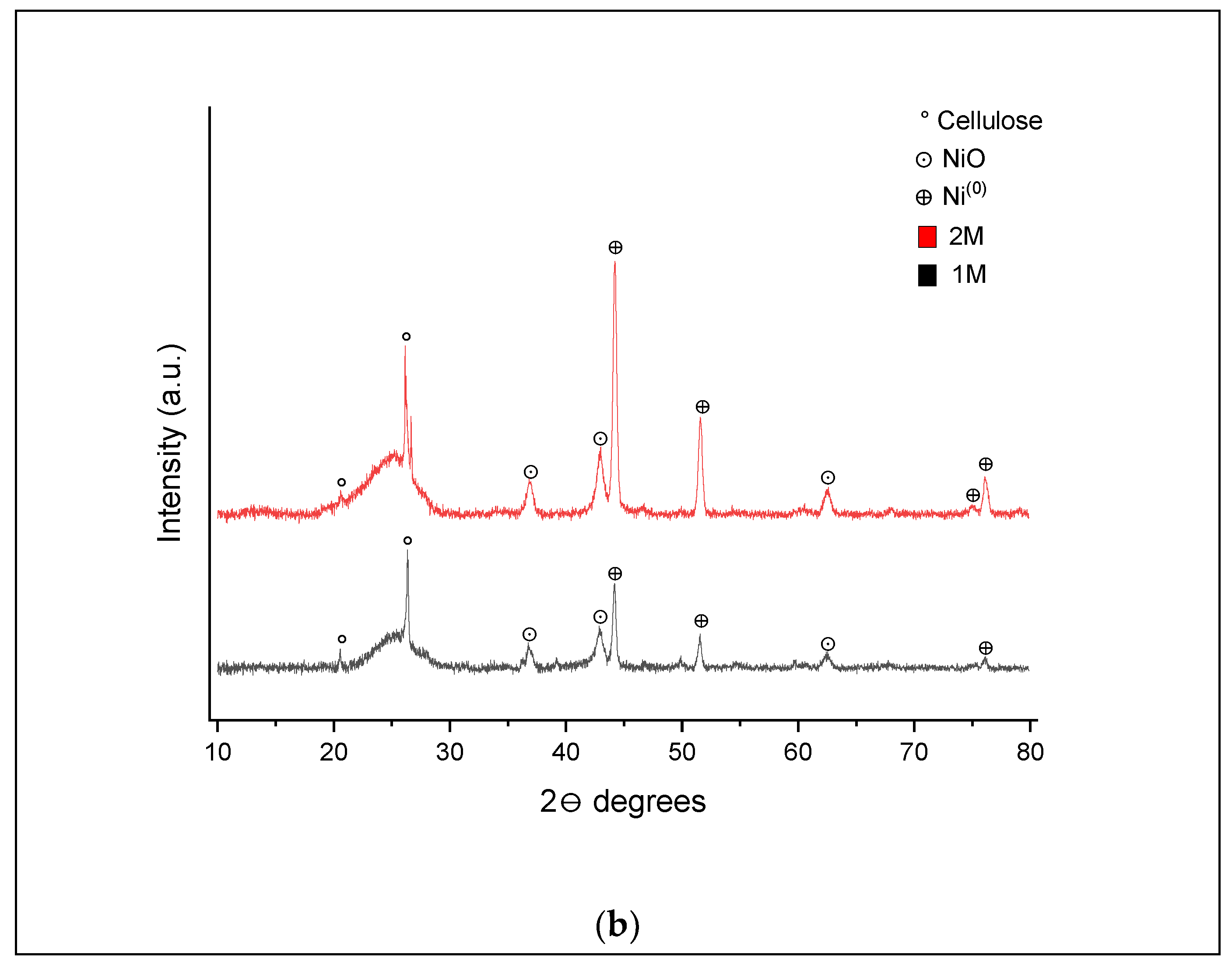
In comparison to the results for the 1 M impregnated sample in
Figure 9a, the absence of peaks associated with cellulose (15.3°, 30.0°) and the presence of peaks for NiO (59.7°and 67.5°) was evident; however, additional peaks were seen at 51.5° and 76°, indicating the presence of Ni
0. For the 2 M impregnated sample, additional peaks at 36.8°, 43°and 62.5° associated with NiO forms can be seen.
As shown in
Figure 10b, the XRD pattern for both hydrochar samples at 500 °C and 60 min presented a similar profile to those for 20 min. Peaks at 20.5° and 26.3° were attributed to residual cellulose, while peaks at 36.8°, 43° and 62.5° were due to the presence of NiO and peaks at 44.2°, 51.6°, 75° and 76° characterized the Ni
0 form. When compared to the XRD patterns in
Figure 9b for hydrochar formed at 400 °C and 60 min, further peaks at 36.8, 43° and 62.5° were observed, indicating the presence of NiO, and a peak at 75° for Ni
0.
These findings confirm that the higher the temperature, the higher the reduction from NiO to Ni
0 forms. It can also be inferred that increasing nickel loading enhanced the transition of nickel stages. It has been reported that the conversion of nickel forms to Ni
0 nanocrystallites is desirable in the SCWG process since it is a catalytic active phase for in situ H
2 production, promoting the additional degradation of biomass, leading to the high tar and char conversion into gas [
23,
26].
3.2.4. Liquid Analysis
The water-insoluble phase of the liquid effluent produced from SCWG of control and impregnated samples (1 M and 2 M) at 25 wt% feed loading, 400 and 500 °C and 20 and 60 min residence times was collected and analysed to determine the main components using GC-MS analysis. The results are presented in
Table 9,
Table 10 and
Table 11. The percentages associated with each constituent were determined by dividing its specific chromatographic peak area by the total peak area of all identified components [
52]. The compounds were identified using computer matching and an NIST (National Institute of Standards and Technology) spectral library.
Onwudili and Williams [
53] categorized oil components into aliphatic, alkylbenzenes, phenols and polycyclic aromatic hydrocarbons (PAHs). According to the results presented, the primary products identified in the liquid output from the SCWG of the samples can be classified into ketones, phenol, alcohols, organic acids, furans, aldehydes, aromatics and other compounds (a relative area of <1% of the total integrated area [
38]).
As shown in
Table 9, phenolic compounds were largely seen at high temperatures and residence times. According to the literature, the dealkylation and hydrolysis of lignin molecules promote the cleavage of C–C bonds, facilitating the production of phenol and its derivatives in the hydrothermal process. Additionally, the existence of inorganic species in the biomass, such as alkali and alkaline earth elements, enhances the formation of phenolic compounds to a greater extent [
54].
The presence of furan derivative compounds (benzofuran 2.3-dihydro-2-methyl) at 500 °C is likely a consequence of the degradation of C–C bonds in the cellulose and hemicellulose molecules [
54]. It can also be seen that the total content of ketones decreased as the temperature and residence time increased. Yu et al. [
55] reported a similar result and found that the decomposition of ketones in the liquid into gas contributes to increased CGE.
The presence of the nickel catalyst significantly altered the chemical composition of the liquid products. In comparison to the liquid composition presented in
Table 9, the absence of furan derivative compounds (benzofuran,2,3,dihydro-2-methyl) is evident in
Table 10, which is likely due to their conversion to compounds with lower molecular weight, such as 2-cyclopenten-1-one, 2.3-dimethyl in the presence of a nickel catalyst. Borges et al. [
29] observed a similar finding.
It was also observed that N-heterocyclic compounds, specifically 3-aminoisoxazole and N-(2-furymethyl)methanesulfonamide, were present in a small amount (<3.0%) in the liquid product from the SCWG of 1 M impregnated sample at 400 °C. However, with increasing temperature, the formation of these compounds was not observed. According to Babaei et al. [
54], a decrease in N-heterocyclic compound formation in the presence of a nickel catalyst is expected. The active catalyst induces the interaction of radicals with each other, formatting gaseous products and subsequently inhibiting the generation of N compounds.
Analysing the composition of the liquid product obtained from the SCWG of the 2 M impregnated sample (
Table 11) shows the presence of 2-cyclopenten-1-one,2-methyl. According to Yu et al. [
55], the presence of this compound can be derived from the partial hydrogenation of phenol. Zhang et al. [
56] also explained that the hydrogenation of phenol is a crucial step in the SCWG pathways as it results in saturated compounds that can be more readily decomposed into gaseous compounds.
Similar to Zhang et al. [
56], cyclohexanol,2,3-dimethyl, which is produced as a hydrogenated intermediate product from the catalytic SCWG of phenol, was also identified. The absence of these compounds at 500 °C indicates that catalyst action promoted their conversion.
Furfural, a principal product of carbohydrate decomposition, was found in the liquid product from the catalytic SCWG (
Table 10 and
Table 11). However, its content decreased significantly with the increase in nickel loading. Increased nickel loading favoured the conversion of carbohydrates through reactions like decarboxylation, hydroxylation, tautomerism and cyclization [
57]. Moreover, the liquid produced by the 2 M impregnated sample at 500 °C and 60 min exhibited a discrete spectrum of substances, indicating that the majority of previously formed components were converted into gases due to the effective action of the nickel catalyst under these conditions.