Combustion Characterization and Kinetic Analysis of Coupled Combustion of Bio-Syngas and Bituminous Coal
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material Properties
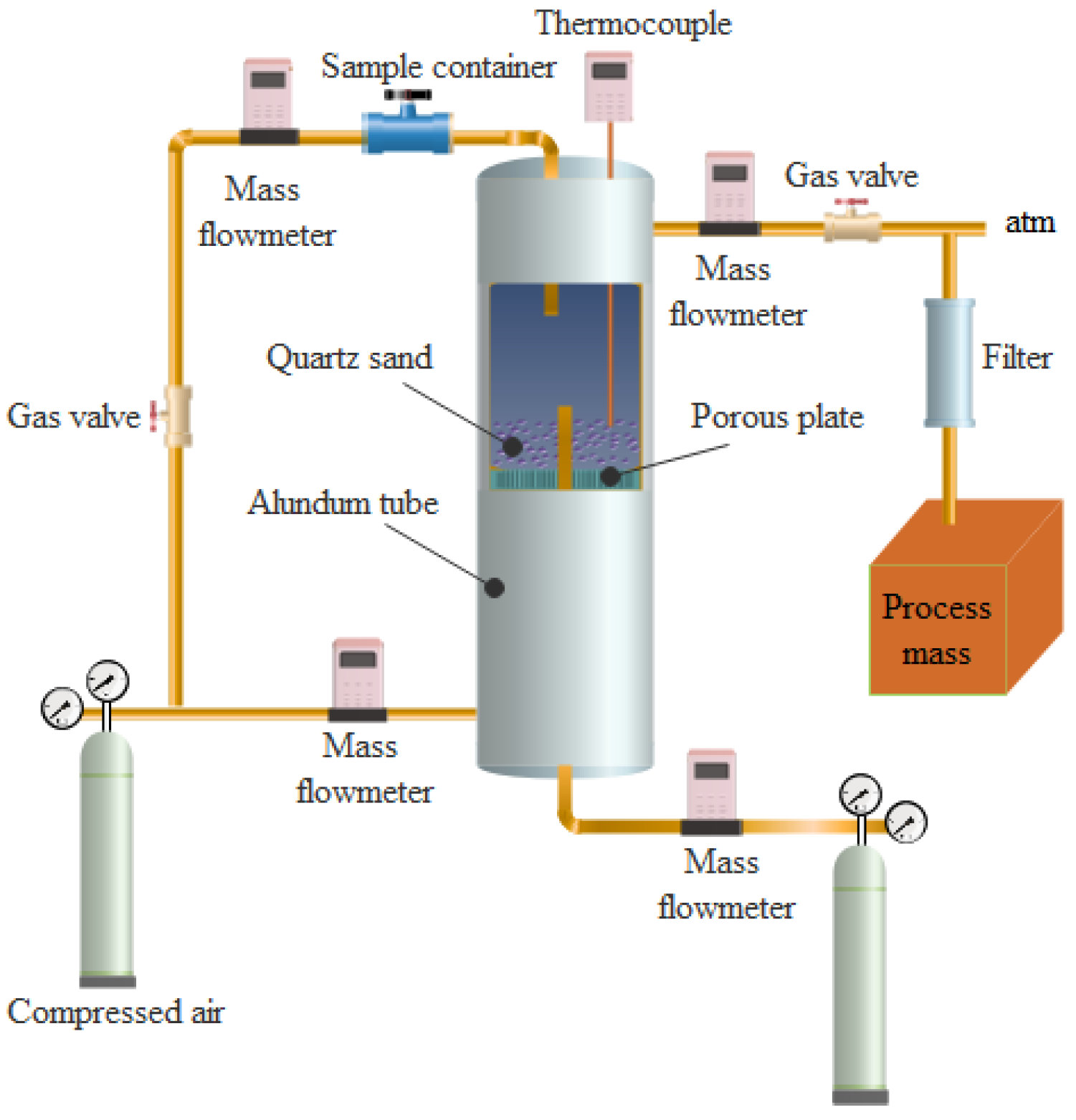
2.2. Experimental Apparatus
2.3. Experimental Setup
2.4. Model Description
2.5. Computational Theory of Kinetic Parameters
- (1)
- Basic kinetic equation
- (2)
- Kinetic mechanism model function
- (3)
- Iso-conversional rate method
3. Results and Discussion
3.1. Combustion Characteristics and Factors
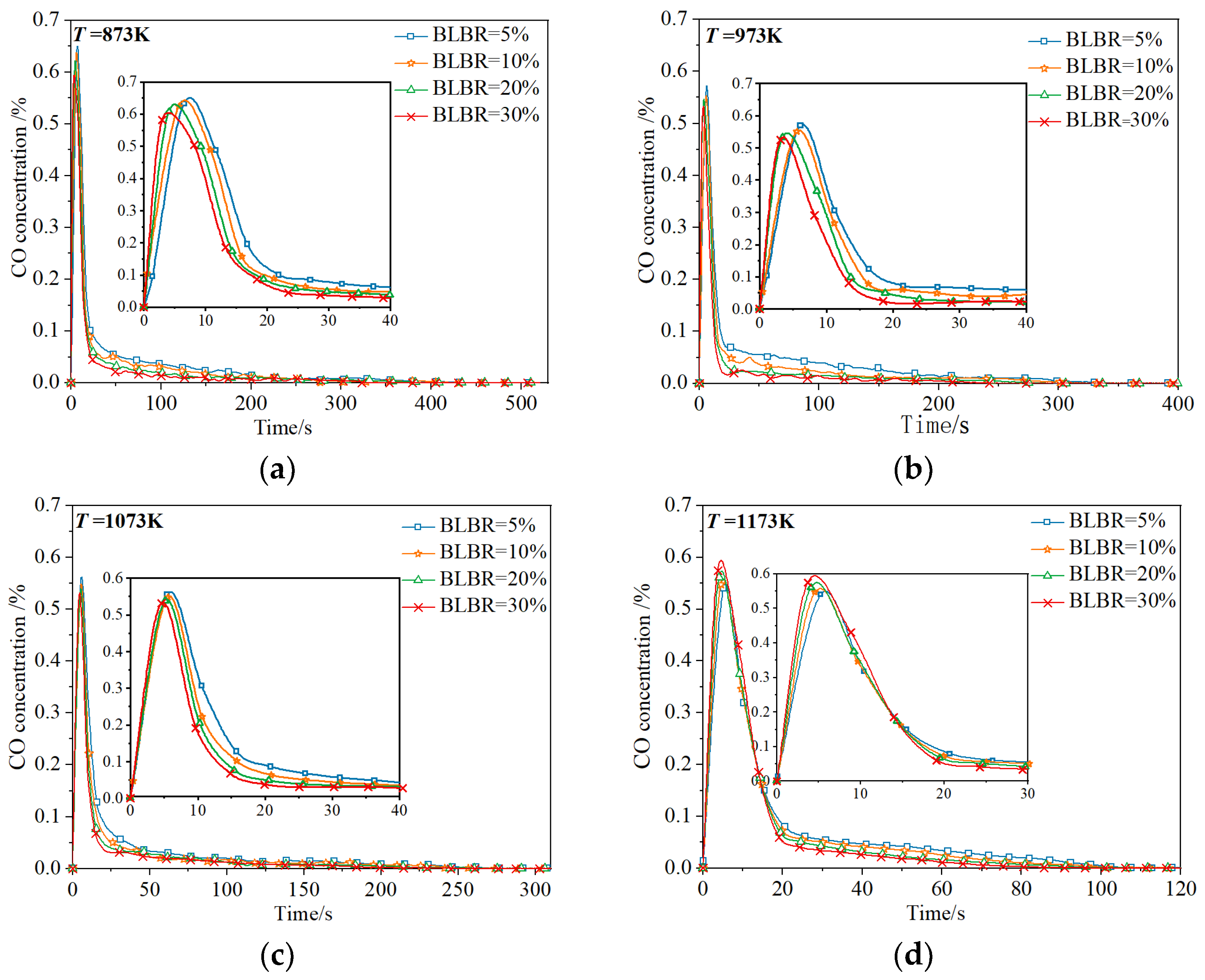
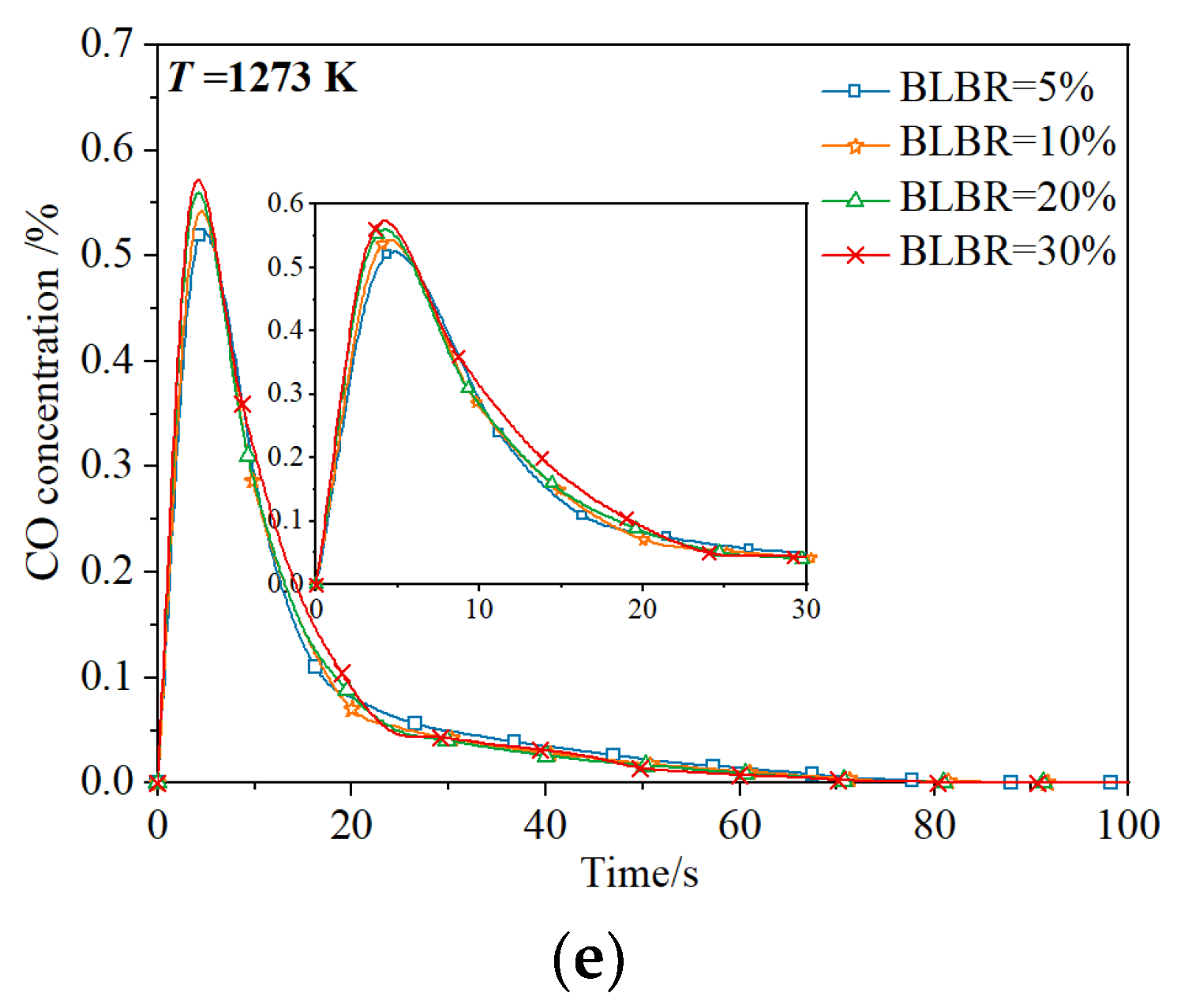
3.1.1. Effect of Temperature on Gas Production
3.1.2. Effect of BLBR on Gas Production
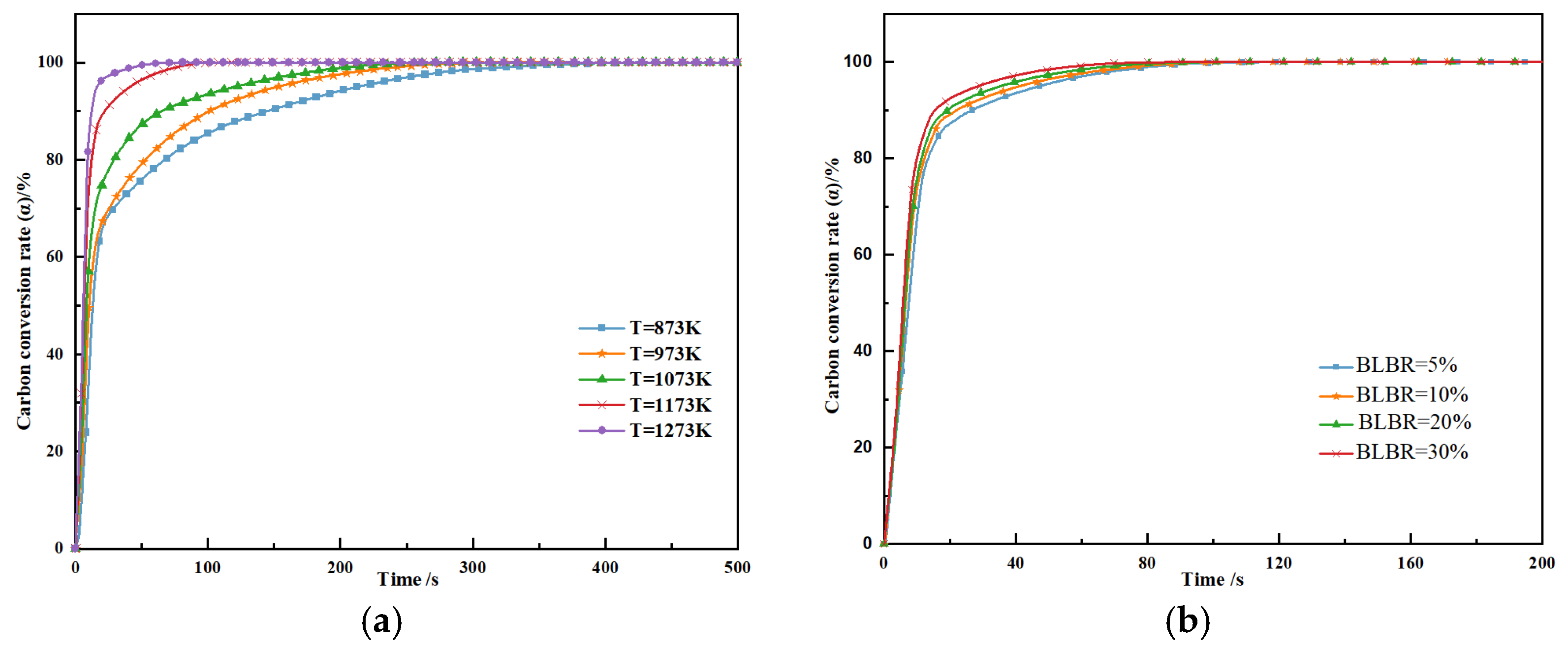
3.1.3. Coupled Combustion Characteristics
3.2. Kinetic Analysis of Bituminous Coal in Coupled Combustion
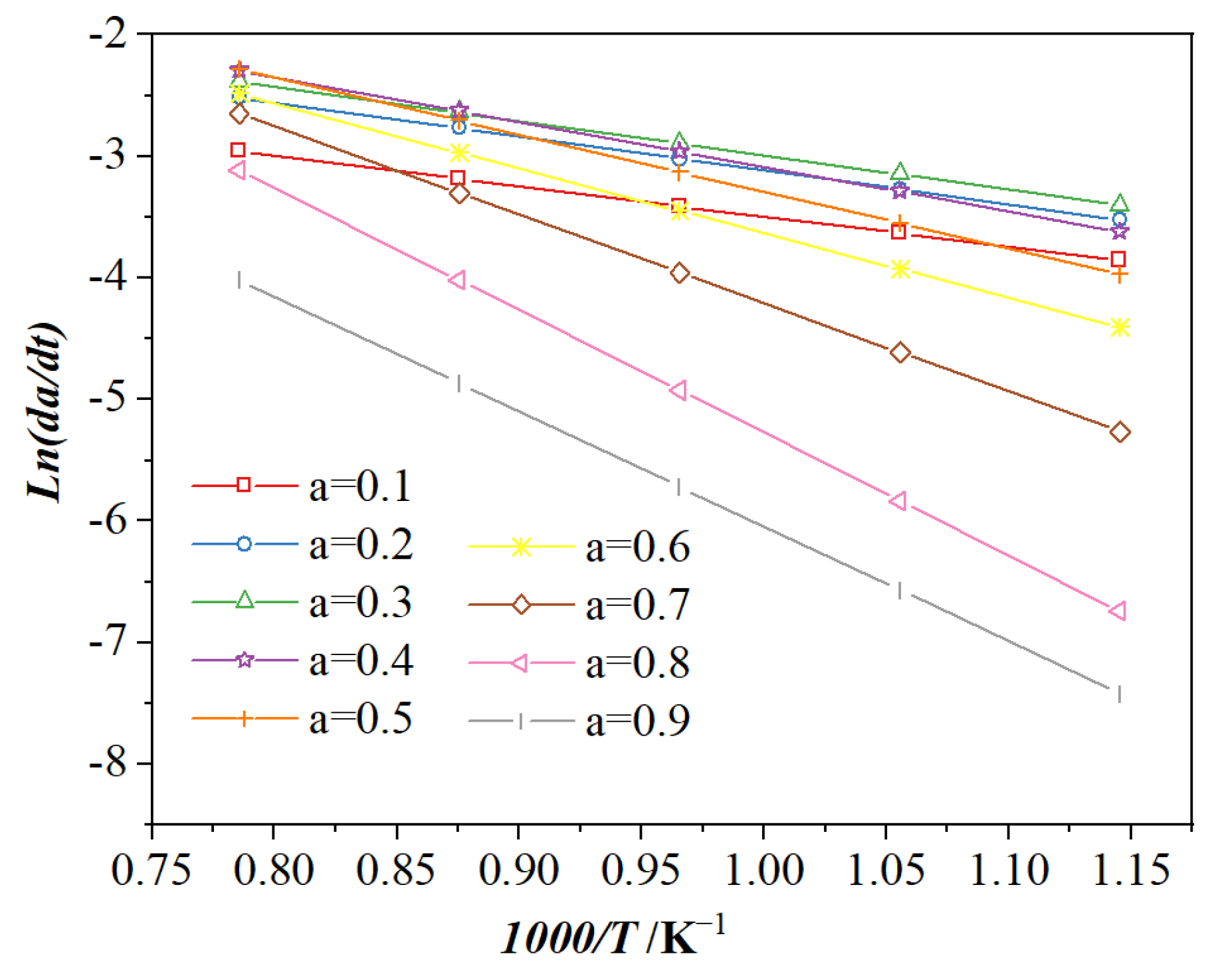
3.2.1. Iso-Conversional Method
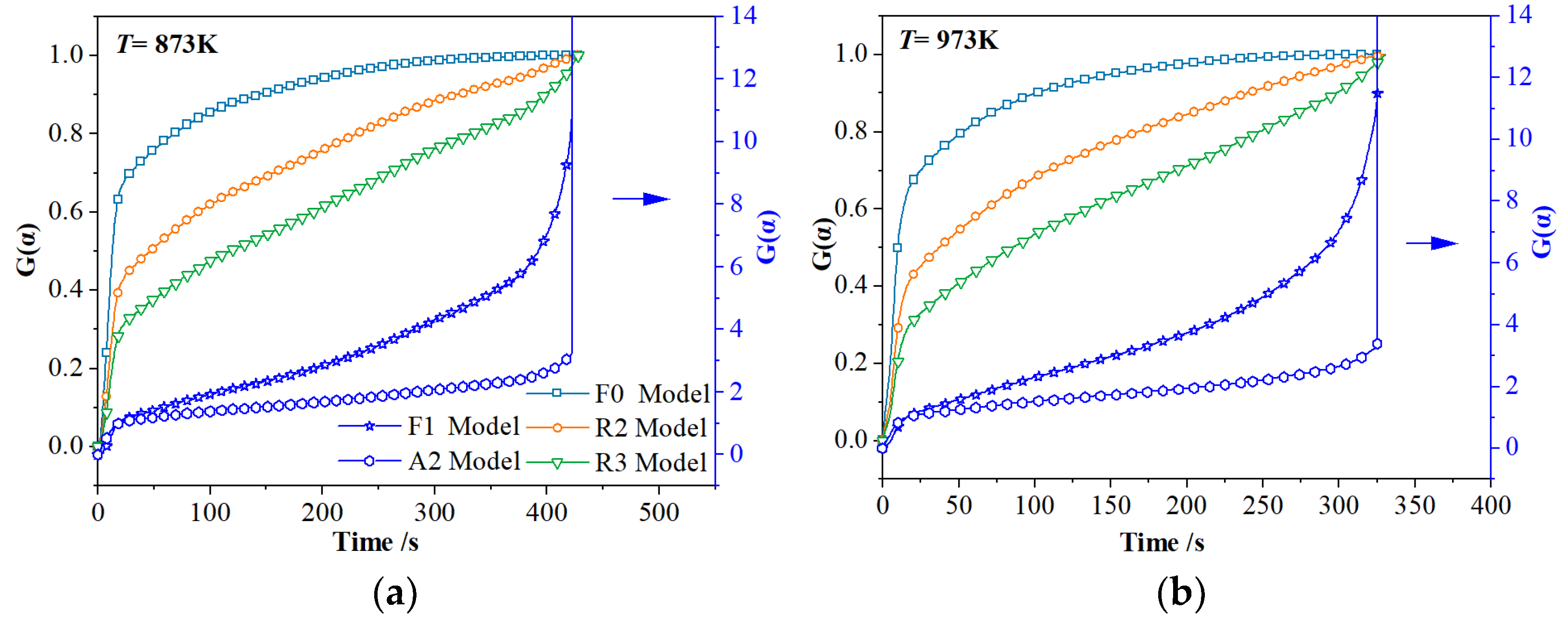
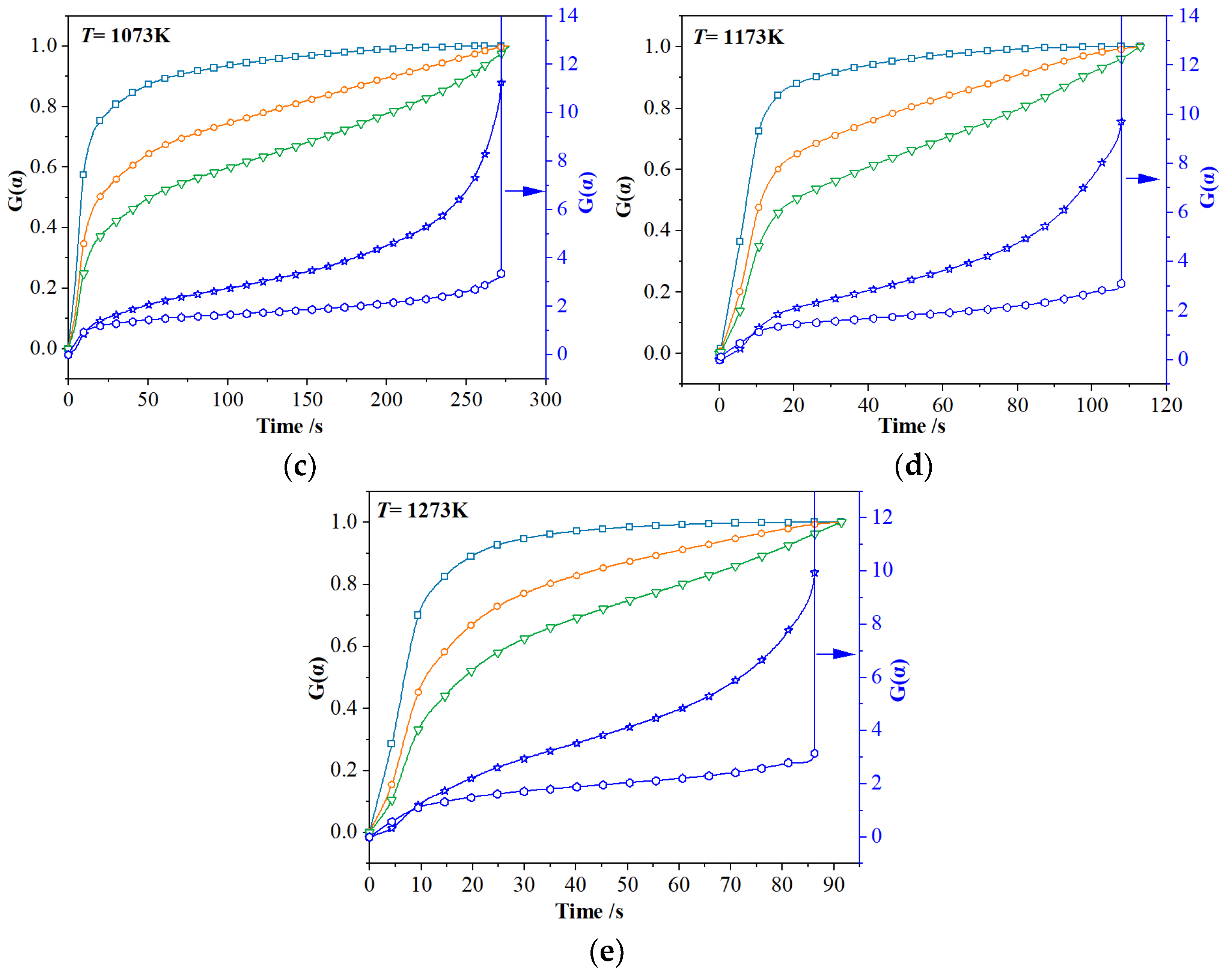
3.2.2. Model-Fitting Method
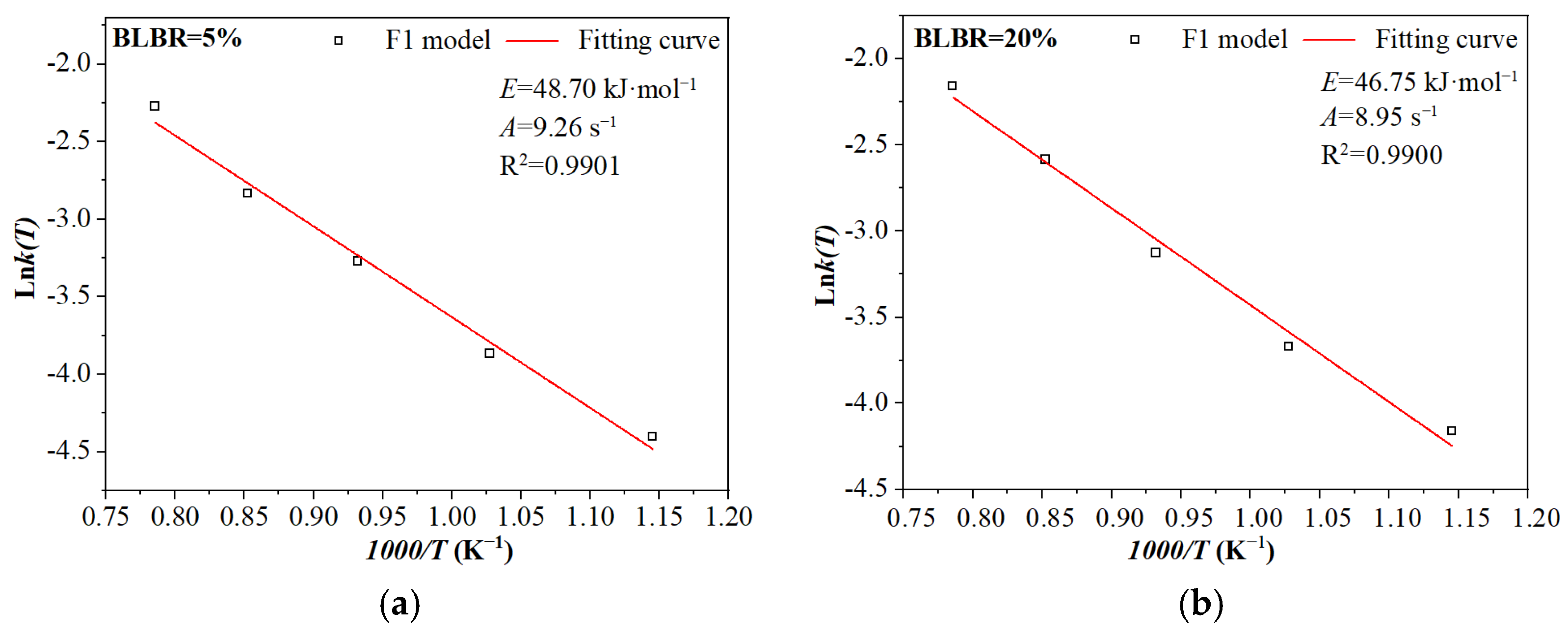
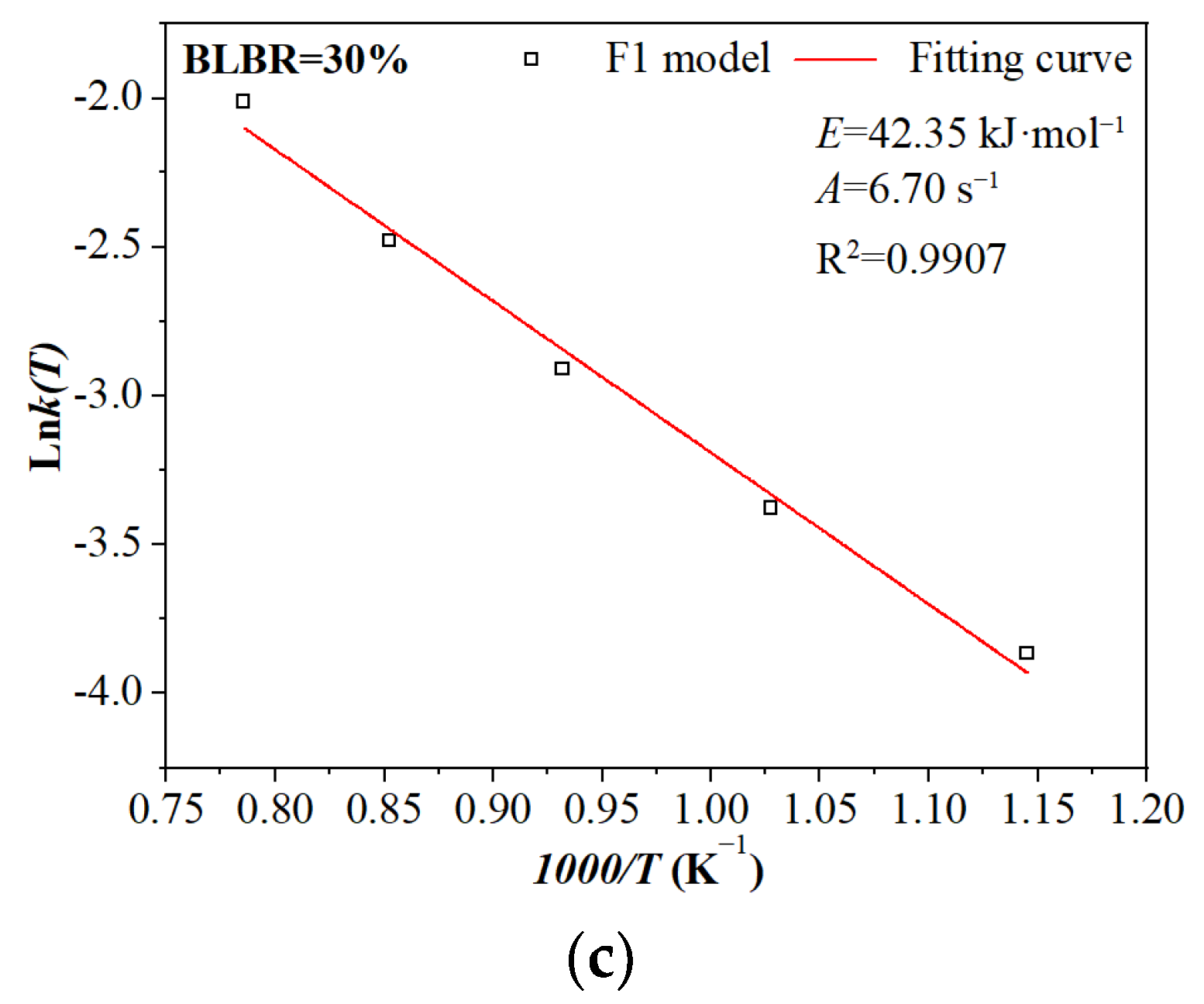
3.2.3. The Effect of BLBR on the Kinetic Parameters
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Y.; Su, Q.; Li, B.; Zhang, Y.; Wang, X.; Zhao, H.; Guo, S. Have Those Countries Declaring “Zero Carbon” or “Carbon Neutral” Climate Goals Achieved Carbon Emissions-Economic Growth Decoupling? J. Clean. Prod. 2022, 363, 132450. [Google Scholar] [CrossRef]
- Mi, Z.; Meng, J.; Guan, D.; Shan, Y.; Song, M.; Wei, Y.-M.; Liu, Z.; Hubacek, K. Chinese CO2 Emission Flows Have Reversed Since the Global Financial Crisis. Nat. Commun. 2017, 8, 1712. [Google Scholar] [CrossRef]
- López, L.A.; Arce, G.; Jiang, X. Mapping China’s Flows of Emissions in the World’s Carbon Footprint: A Network Approach of Production Layers. Energy Econ. 2020, 87, 104739. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, Z.; He, G.; Wang, H.; Zhang, X.; Lin, J.; Qi, Y.; Liang, X. Challenges and Opportunities for Carbon Neutrality in China. Nat. Rev. Earth Environ. 2021, 3, 141–155. [Google Scholar] [CrossRef]
- Kao, X.; Liu, Y.; Wang, W.; Wen, Q.; Zhang, P. The Pressure of Coal Consumption on China’s Carbon Dioxide Emissions: A Spatial and Temporal Perspective. Atmos. Pollut. Res. 2024, 15, 102188. [Google Scholar] [CrossRef]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The Role of Renewable Energy in the Global Energy Transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Tian, X.; An, C.; Chen, Z. The Role of Clean Energy in Achieving Decarbonization of Electricity Generation, Transportation, and Heating Sectors by 2050: A Meta-Analysis Review. Renew. Sustain. Energy Rev. 2023, 182, 113404. [Google Scholar] [CrossRef]
- Tong, D.; Zhang, Q.; Liu, F.; Geng, G.; Zheng, Y.; Xue, T.; Hong, C.; Wu, R.; Qin, Y.; Zhao, H.; et al. Current Emissions and Future Mitigation Pathways of Coal-Fired Power Plants in China From 2010 to 2030. Environ. Sci. Technol. 2018, 52, 12905–12914. [Google Scholar] [CrossRef]
- Liu, L.; Memon, M.Z.; Xie, Y.; Gao, S.; Guo, Y.; Dong, J.; Gao, Y.; Li, A.; Ji, G. Recent Advances of Research in Coal and Biomass Co-Firing for Electricity and Heat Generation. Circ. Econ. 2023, 2, 100063. [Google Scholar] [CrossRef]
- Antar, M.; Lyu, D.; Nazari, M.; Shah, A.; Zhou, X.; Smith, D.L. Biomass for A Sustainable Bioeconomy: An Overview of World Biomass Production and Utilization. Renew. Sustain. Energy Rev. 2021, 139, 110691. [Google Scholar] [CrossRef]
- Mishra, S.; Upadhyay, R.K. Review on Biomass Gasification: Gasifiers, Gasifying Mediums, and Operational Parameters. Mater. Sci. Energy Technol. 2021, 4, 329–340. [Google Scholar] [CrossRef]
- Tezer, Ö.; Karabağ, N.; Öngen, A.; Çolpan, C.Ö.; Ayol, A. Biomass Gasification for Sustainable Energy Production: A Review. Int. J. Hydrogen Energy 2022, 47, 15419–15433. [Google Scholar] [CrossRef]
- Mo, W.; Du, K.; Sun, Y.; Guo, M.; Zhou, C.; You, M.; Xu, J.; Jiang, L.; Wang, Y.; Su, S.; et al. Technical-Economic-Environmental Analysis of Biomass Direct and Indirect Co-Firing in Pulverized Coal Boiler in China. J. Clean. Prod. 2023, 426, 139119. [Google Scholar] [CrossRef]
- Agbor, E.; Zhang, X.; Kumar, A. A Review of Biomass Co-Firing in North America. Renew. Sustain. Energy Rev. 2014, 40, 930–943. [Google Scholar] [CrossRef]
- Dong, C.; Yang, Y.; Yang, R.; Zhang, J. Numerical Modeling of the Gasification Based Biomass Co-Firing in A 600MW Pulverized Coal Boiler. Appl. Energy 2010, 87, 2834–2838. [Google Scholar] [CrossRef]
- Si, M.; Cheng, Q.; Yuan, L.; Zhang, Y.; Luo, Z. Study on the Combustion Behavior of Single Coal Particle Using a Thermal-Imaging Technique. Combust. Flame 2022, 242, 112178. [Google Scholar] [CrossRef]
- Eaton, A.M.; Smoot, L.D.; Hill, S.C.; Eatough, C.N. Components, Formulations, Solutions, Evaluation, and Application of Comprehensive Combustion Models. Prog. Energy Combust. Sci. 1999, 25, 387–436. [Google Scholar] [CrossRef]
- Wen, X.; Wang, H.; Luo, Y.; Luo, K.; Fan, J. Numerical Investigation of the Effects of Volatile Matter Composition and Chemical Reaction Mechanism on Pulverized Coal Combustion Characteristics. Fuel 2017, 210, 695–704. [Google Scholar] [CrossRef]
- Guedea, I.; Pallarès, D.; Díez, L.I.; Johnsson, F. Conversion of Large Coal Particles Under O2/N2 and O2/CO2 Atmospheres—Experiments and Modeling. Fuel Process. Technol. 2013, 112, 118–128. [Google Scholar] [CrossRef]
- Hecht, E.S.; Shaddix, C.R.; Geier, M.; Molina, A.; Haynes, B.S. Effect of CO2 and Steam Gasification Reactions on the Oxy-Combustion of Pulverized Coal Char. Combust. Flame 2012, 159, 3437–3447. [Google Scholar] [CrossRef]
- Bu, C.; Liu, D.; Chen, X.; Pallarès, D.; Gómez-Barea, A. Ignition Behavior of Single Coal Particle in A Fluidized Bed Under O2/CO2 and O2/N2 Atmospheres: A Combination of Visual Image and Particle Temperature. Appl. Energy 2014, 115, 301–308. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, D.; Cai, L.; Zou, C.; Qiu, J.; Zheng, C. The Chemical and Physical Effects of CO2 on the Homogeneous and Heterogeneous Ignition of the Coal Particle in O2/CO2 Atmospheres. Proc. Combust. Inst. 2017, 36, 2113–2121. [Google Scholar] [CrossRef]
- Lei, M.; Zou, C.; Xu, X.; Wang, C. Effect of CO2 and H2O on the Combustion Characteristics and Ash Formation of Pulverized Coal in Oxy-Fuel Conditions. Appl. Therm. Eng. 2018, 133, 308–315. [Google Scholar] [CrossRef]
- Zhu, T.; Hu, Y.; Tang, C.; Wang, L.; Liu, X.; Deng, L.; Che, D. Experimental Study on NOx Formation and Burnout Characteristics of Pulverized Coal in Oxygen Enriched and Deep-Staging Combustion. Fuel 2020, 272, 117639. [Google Scholar] [CrossRef]
- Fan, W.; Li, Y.; Guo, Q.; Chen, C.; Wang, Y. Coal-Nitrogen Release and NOx Evolution in the Oxidant-Staged Combustion of Coal. Energy 2017, 125, 417–426. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, C.; Chen, X.; Peng, Z.; Gao, H.; Gong, X. Performance Analysis of a Novel Biomass Gasification System Coupled to a Coal-Fired Power Plant Based on Heat and Water Recovery. Energy Convers. Manag. 2024, 299, 117822. [Google Scholar] [CrossRef]
- Abid, S.; Zhang, X.; Zhang, W.; Mu, H.; Zhang, C.; Wang, A. System Performance and Pollution Emission of Biomass Gas Co-Firing in A Coal-Fired Boiler. J. Power Energy Eng. 2020, 8, 18–25. [Google Scholar] [CrossRef]
- Ye, Y.L.; Wang, H.M.; An, X.; Jiang, W.H. Thermal Efficiency Calculating Model of Pulverized Coal and Blast Furnace Gas Co-Fired Boilers Based on Separate Combustion Calculation. IOP Conf. Ser. Earth Environ. Sci. 2018, 186, 012018. [Google Scholar] [CrossRef]
- Han, Z.; Yue, J.; Zeng, X.; Yu, J.; Wang, F.; Sun, S.; Yao, H.; Luo, G.; Liu, X.; Sun, Y.; et al. Characteristics of Gas-Solid Micro Fluidized Beds for Thermochemical Reaction Analysis. Carbon Resour. Convers. 2020, 3, 203–218. [Google Scholar] [CrossRef]
- Yu, J.; Zeng, X.; Zhang, J.; Zhong, M.; Zhang, G.; Wang, Y.; Xu, G. Isothermal Differential Characteristics of Gas–Solid Reaction in Micro-Fluidized Bed Reactor. Fuel 2013, 103, 29–36. [Google Scholar] [CrossRef]
- Silva, J.; Teixeira, S.; Teixeira, J. A Review of Biomass Thermal Analysis, Kinetics and Product Distribution for Combustion Modeling: From the Micro to Macro Perspective. Energies 2023, 16, 6705. [Google Scholar] [CrossRef]
- Wang, F.; Zeng, X.; Wang, Y.; Su, H.; Yu, J.; Xu, G. Non-Isothermal Coal Char Gasification with CO2 in A Micro Fluidized Bed Reaction Analyzer and A Thermogravimetric Analyzer. Fuel 2016, 164, 403–409. [Google Scholar] [CrossRef]
- Zhang, H.; Dou, B.; Li, J.; Zhao, L.; Wu, K. Thermogravimetric Kinetics on Catalytic Combustion of Bituminous Coal. J. Energy Inst. 2020, 93, 2526–2535. [Google Scholar] [CrossRef]
- Aidabulov, M.; Zhakupov, D.; Zhunussova, K.; Temireyeva, A.; Shah, D.; Sarbassov, Y. Thermal Characterization, Kinetic Analysis and Co-Combustion of Sewage Sludge Coupled with High Ash Ekibastuz Coal. Energies 2023, 16, 6634. [Google Scholar] [CrossRef]
- Tan, J.; He, Y.; Zhu, R.; Zhu, Y.; Wang, Z. Experimental Study on Co-Firing Characteristics of Ammonia with Pulverized Coal in A Staged Combustion Drop Tube Furnace. Proc. Combust. Inst. 2023, 39, 3217–3225. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Z.; Fang, W.; He, Y.; Hsu, E.; Li, Q.; Gul-e-Rana, J.; Cen, K. High-Temperature Pyrolysis Behavior of A Bituminous Coal in A Drop Tube Furnace and Further Characterization of The Resultant Char. J. Anal. Appl. Pyrol. 2019, 137, 163–170. [Google Scholar] [CrossRef]
- Balusamy, S.; Kamal, M.M.; Lowe, S.M.; Tian, B.; Gao, Y.; Hochgreb, S. Laser Diagnostics of Pulverized Coal Combustion in O2/N2 and O2/CO2 Conditions: Velocity And Scalar Field Measurements. Exp. Fluids 2015, 56, 108. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, Y.; Gao, D.; Liu, P.; Meng, S.; Sun, S. Kinetics of Steam Gasification of In-Situ Chars in A Micro Fluidized Bed. Int. J. Hydrogen Energy 2016, 41, 15187–15198. [Google Scholar] [CrossRef]
- Yu, J.; Yao, C.; Zeng, X.; Geng, S.; Dong, L.; Wang, Y.; Gao, S.; Xu, G. Biomass Pyrolysis in A Micro-Fluidized Bed Reactor: Characterization and Kinetics. Chem. Eng. J. 2011, 168, 839–847. [Google Scholar] [CrossRef]
- Zaghib, K.; Song, X.; Kinoshita, K. Thermal Analysis of the Oxidation of Natural Graphite: Isothermal Kinetic Studies. Thermochim. Acta 2001, 371, 57–64. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Wight, C.A. Isothermal and Nonisothermal Reaction Kinetics in Solids: In Search of Ways Toward Consensus. J. Phys. Chem. A 1997, 101, 8279–8284. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Goryachko, V. Potentialities of Software for Kinetic Processing of Thermoanalytical Data by the Isoconversion Method. Thermochim. Acta 1992, 194, 221–230. [Google Scholar] [CrossRef]
- He, A.; Li, Z.; Li, J.; Wang, X.; Zhang, L.; Yang, S.; Xin, X.; Sun, T.; Lei, T. Effects of Bio-Syngas Blends on Combustion Characteristics of Coal: Kinetic Analysis. J. Biobased Mater. Bioenergy 2021, 15, 269–277. [Google Scholar] [CrossRef]
- Bai, W.; Li, H.; Deng, L.; Liu, H.; Che, D. Air-Staged Combustion Characteristics of Pulverized Coal under High Temperature and Strong Rreducing Atmosphere Conditions. Energy Fuel 2014, 28, 1820–1828. [Google Scholar] [CrossRef]


| Materials | Proximate Analysis | Ultimate Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| V | FC c | A | M | C | H | N | S | O c | kJ/kg | |
| Bituminous coal | 29.91 | 57.51 | 5.41 | 7.17 | 68.31 | 5.62 | 0.72 | 0.83 | 11.94 | 25228 |
| CO/% | CO2/% | H2/% | N2 c/% | CH4/% | c/(kg/m3) | c/(kJ/m3) | |
|---|---|---|---|---|---|---|---|
| Bio-syngas | 21.99 | 13.01 | 14.01 | 48.99 | 2.00 | 1.17 | 5003 |
| Model | Mechanism | ||
|---|---|---|---|
| 1 | One-dimensional diffusion, 1D | ||
| 2 | Zero order, F0/R1 | 1 | |
| 3 | First order, F1 | ||
| 4 | Second order, F2 | ||
| 5 | Third order, F3 | ||
| 6 | Four order, F4 | ||
| 7 | Contracting area, R2 | ||
| 8 | Contracting volume, R3 | ||
| 9 | Nucleation and growth | ||
| 10 | Nucleation and growth, A2 | ||
| 11 | Nucleation and growth, A3 | ||
| 12 | Nucleation and growth, A4 | ||
| 13 | One-dimensional diffusion, D1 | ||
| 14 | Two-dimensional diffusion, D2 | ||
| 15 | Shrinkage geometrical, P2 | ||
| 16 | Shrinkage geometrical, P3 | ||
| 17 | Shrinkage geometrical, P4 |
| 0.10 | 0.20 | 0.30 | 0.40 | 0.50 | 0.60 | 0.70 | 0.80 | 0.90 | Average Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| 20.54 | 23.32 | 23.45 | 30.71 | 38.83 | 44.27 | 60.47 | 83.80 | 78.54 | 44.88 | |
| 0.9709 | 0.9904 | 0.9565 | 0.9405 | 0.9448 | 0.9176 | 0.9014 | 0.9470 | 0.9000 | ||
| 0.2069 | 0.2933 | 0.0851 | 0.1312 | 0.1595 | 0.2247 | 0.3381 | 0.3372 | 0.4432 |
| F1 | 873 K | 0.0147 s−1 | 0.9530 | 0.15~0.92 | E = 45.45 kJ·mol−1 A = 7.10 s−1 R2 = 0.99 |
| 973 K | 0.0236 s−1 | 0.9619 | 0.10~0.90 | ||
| 1073 K | 0.0421 s−1 | 0.9560 | 0.20~0.89 | ||
| 1173 K | 0.0644 s−1 | 0.9826 | 0.15~0.90 | ||
| 1273 K | 0.1054 s−1 | 0.9922 | 0.10~0.95 | ||
| R2 | 873 K | 0.0049 s−1 | 0.9088 | 0.10~0.89 | E = 46.07 kJ·mol−1 A = 3.19 s−1 R2 = 0.91 |
| 973 K | 0.0108 s−1 | 0.9196 | 0.20~0.85 | ||
| 1073 K | 0.0224 s−1 | 0.9483 | 0.25~0.83 | ||
| 1173 K | 0.0353 s−1 | 0.9770 | 0.15~0.88 | ||
| 1273 K | 0.0305 s−1 | 0.9599 | 0.10~0.85 | ||
| R3 | 873 K | 0.0047 s−1 | 0.9253 | 0.10~0.89 | E = 44.01 kJ·mol−1 A = 1.97 s−1 R2 = 0.87 |
| 973 K | 0.0067 s−1 | 0.9300 | 0.10~0.85 | ||
| 1073 K | 0.0184 s−1 | 0.9655 | 0.25~0.80 | ||
| 1173 K | 0.0269 s−1 | 0.9824 | 0.15~0.88 | ||
| 1273 K | 0.0240 s−1 | 0.9723 | 0.10~0.85 | ||
| F0 | 873 K | 0.0066 s−1 | 0.9662 | 0.05~0.99 | E = 43.17 kJ·mol−1 A = 3.43 s−1 R2 = 0.80 |
| 973 K | 0.0218 s−1 | 0.9096 | 0.20~0.78 | ||
| 1073 K | 0.0333 s−1 | 0.9267 | 0.25~0.83 | ||
| 1173 K | 0.0499 s−1 | 0.9561 | 0.15~0.90 | ||
| 1273 K | 0.0399 s−1 | 0.9209 | 0.10~0.85 | ||
| A2 | 873 K | 0.0111 s−1 | 0.9837 | 0.10~0.94 | E = 42.34 kJ·mol−1 A = 4.97 s−1 R2 = 0.85 |
| 973 K | 0.0344 s−1 | 0.9051 | 0.20~0.78 | ||
| 1073 K | 0.0526 s−1 | 0.9266 | 0.25~0.83 | ||
| 1173 K | 0.0712 s−1 | 0.9405 | 0.15~0.90 | ||
| 1273 K | 0.0687 s−1 | 0.9435 | 0.10~0.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, A.; Li, Z.; Gu, B.; Lei, T. Combustion Characterization and Kinetic Analysis of Coupled Combustion of Bio-Syngas and Bituminous Coal. Energies 2024, 17, 6205. https://doi.org/10.3390/en17236205
He A, Li Z, Gu B, Lei T. Combustion Characterization and Kinetic Analysis of Coupled Combustion of Bio-Syngas and Bituminous Coal. Energies. 2024; 17(23):6205. https://doi.org/10.3390/en17236205
Chicago/Turabian StyleHe, Ailing, Zaifeng Li, Bingdong Gu, and Tingzhou Lei. 2024. "Combustion Characterization and Kinetic Analysis of Coupled Combustion of Bio-Syngas and Bituminous Coal" Energies 17, no. 23: 6205. https://doi.org/10.3390/en17236205
APA StyleHe, A., Li, Z., Gu, B., & Lei, T. (2024). Combustion Characterization and Kinetic Analysis of Coupled Combustion of Bio-Syngas and Bituminous Coal. Energies, 17(23), 6205. https://doi.org/10.3390/en17236205





