Highly Efficient Process for Producing a Jet-A1 Biofuel Component Through Hydroprocessing Soybean Oil over Ni and Pt Catalysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
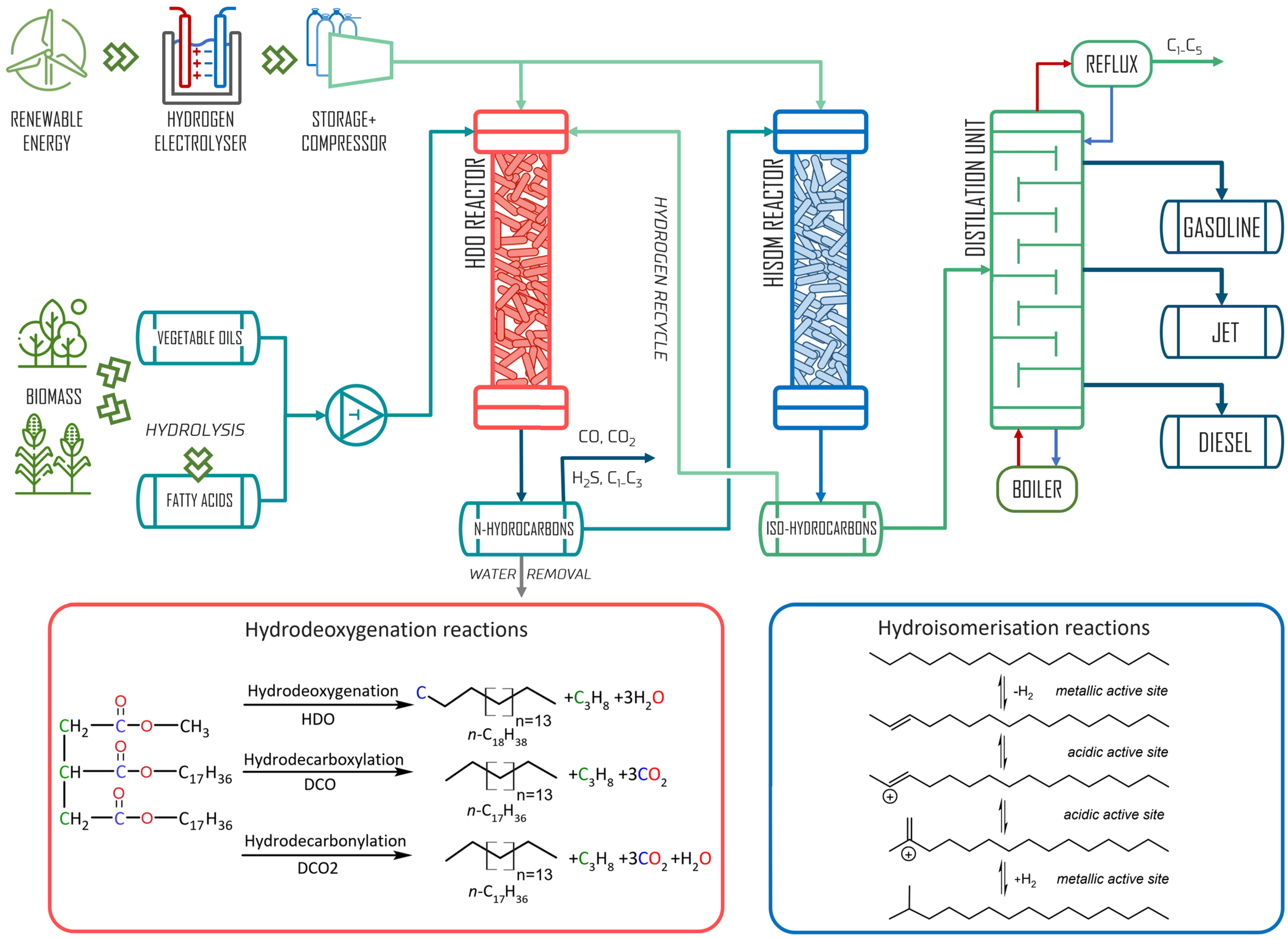
2.2. Hydrodeoxygenation Process
2.3. Synthesis of Hydroisomerization Catalyst
2.4. Hydroisomerization Process
2.5. Catalyst Characterization
2.5.1. Low-Temperature Nitrogen Adsorption Method
2.5.2. Inductively Coupled Plasma–Optical Emission Spectroscopy (ICP-OES)
2.5.3. Thermogravimetry
3. Results
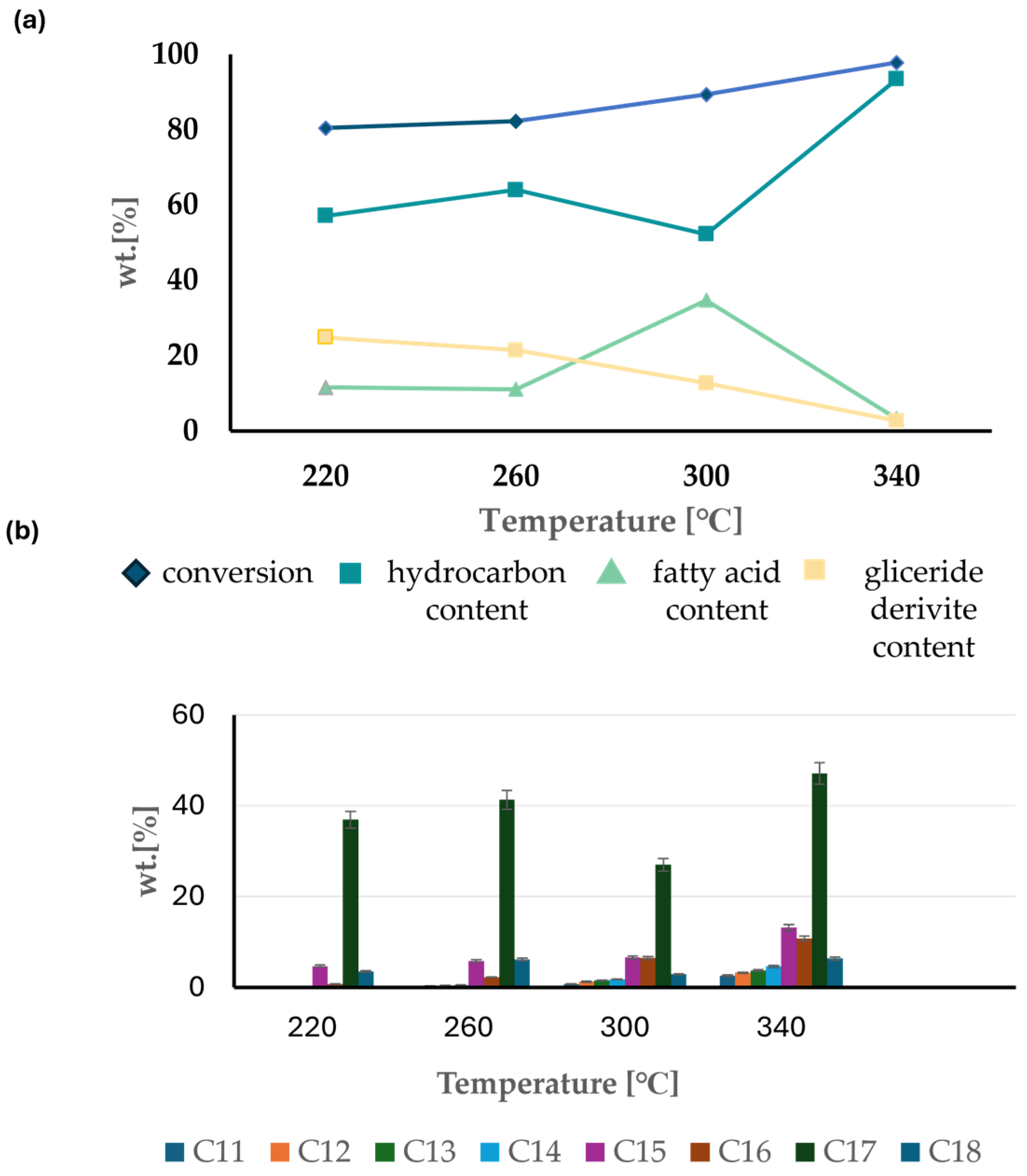
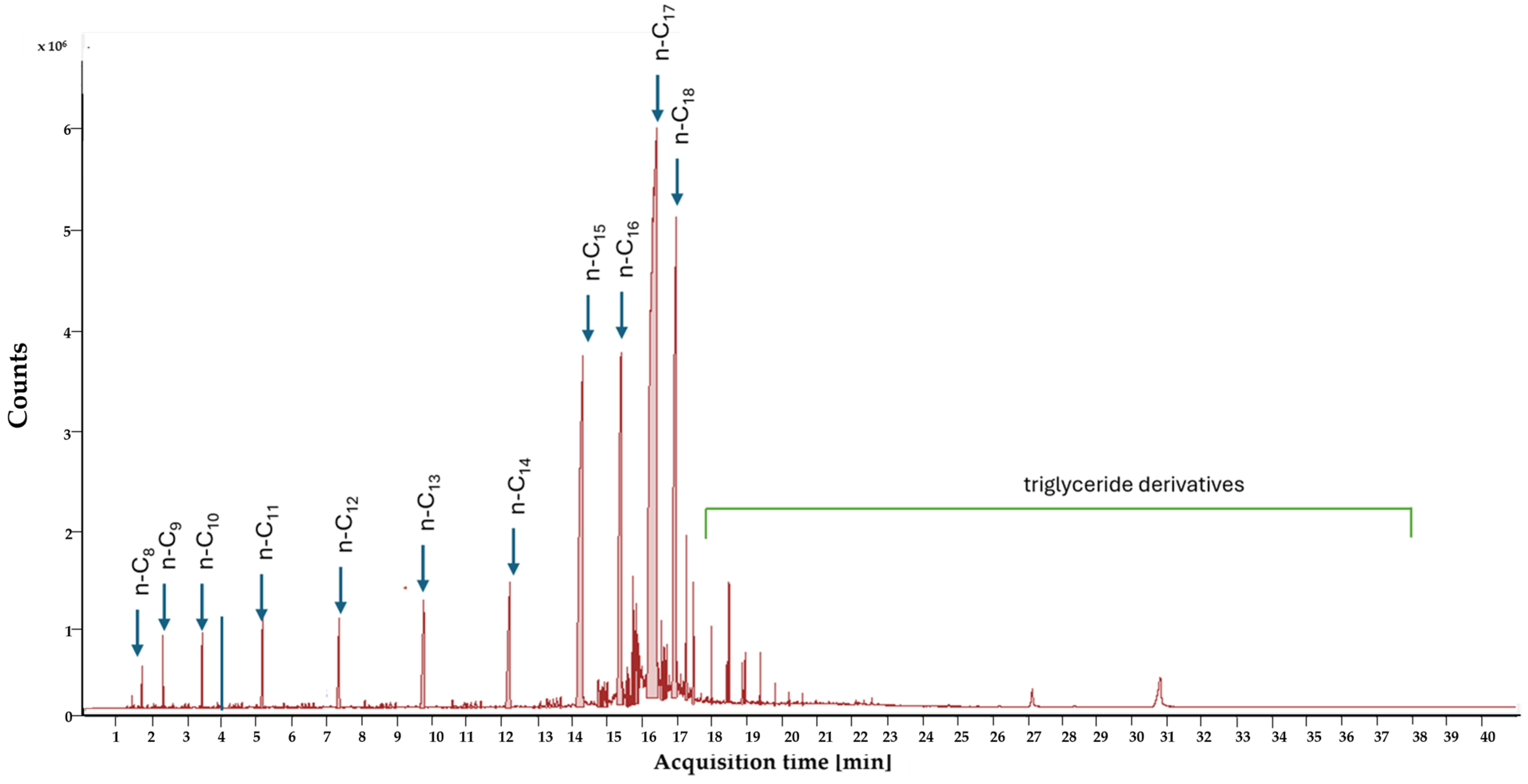
3.1. Hydrodeoxygenation of Degummed Soybean Oil
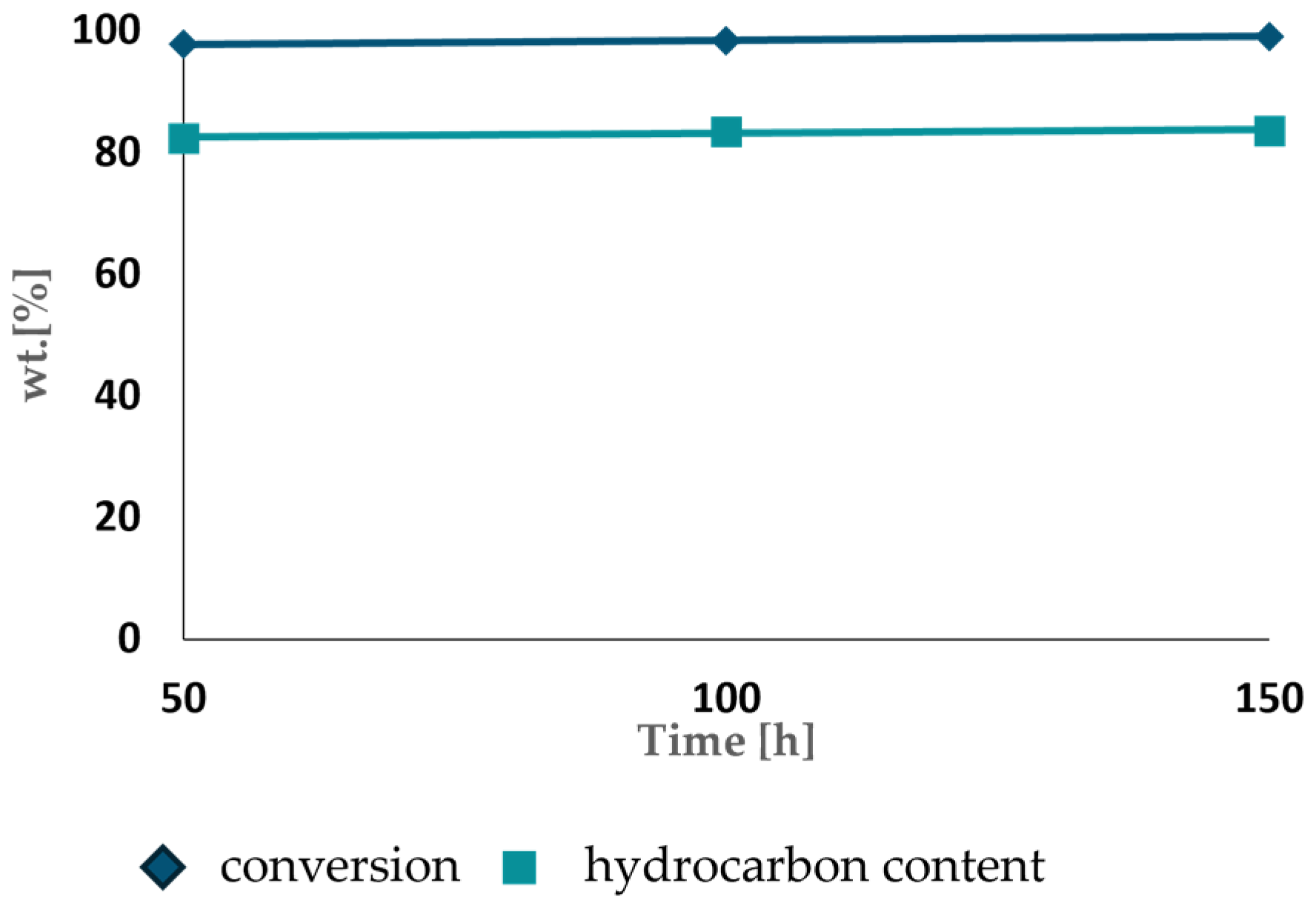
3.2. Hydroisomerization of Hydrocarbons Fraction
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IEA. Aviation. 2022. Available online: https://www.iea.org/energy-system/transport/aviation (accessed on 1 October 2024).
- Scheelhaase, J.; Grimme, W.; Maertens, S. EU Trilogue Results for the Aviation Sector—Key Issues and Expected Impacts. Transp. Res. Procedia 2024, 78, 206–214. [Google Scholar] [CrossRef]
- Abrantes, I.; Ferreira, A.F.; Silva, A.; Costa, M. Sustainable Aviation Fuels and Imminent Technologies—CO2 Emissions Evolution towards 2050. J. Clean. Prod. 2021, 313, 127937. [Google Scholar] [CrossRef]
- Główka, M.; Wójcik, J.; Boberski, P.; Białecki, T.; Gawron, B.; Skolniak, M.; Suchocki, T. Sustainable Aviation Fuel—Comprehensive Study on Highly Selective Isomerization Route towards HEFA Based Bioadditives. Renew. Energy 2024, 220, 119696. [Google Scholar] [CrossRef]
- Richter, S.; Kukkadapu, G.; Westbrook, C.K.; Braun-Unkhoff, M.; Naumann, C.; Köhler, M.; Riedel, U. A Combined Experimental and Modeling Study of Combustion Properties of an Isoparaffinic Alcohol-to-Jet Fuel. Combust. Flame 2022, 240, 111994. [Google Scholar] [CrossRef]
- Goh, B.H.H.; Chong, C.T.; Ong, H.C.; Seljak, T.; Katrašnik, T.; Józsa, V.; Ng, J.-H.; Tian, B.; Karmarkar, S.; Ashokkumar, V. Recent Advancements in Catalytic Conversion Pathways for Synthetic Jet Fuel Produced from Bioresources. Energy Convers. Manag. 2022, 251, 114974. [Google Scholar] [CrossRef]
- Lim, J.H.K.; Gan, Y.Y.; Ong, H.C.; Lau, B.F.; Chen, W.H.; Chong, C.T.; Ling, T.C.; Klemeš, J.J. Utilization of Microalgae for Bio-Jet Fuel Production in the Aviation Sector: Challenges and Perspective. Renew. Sustain. Energy Rev. 2021, 149, 111396. [Google Scholar] [CrossRef]
- Chevron Renewable Energy Group. Available online: https://www.regi.com/about (accessed on 28 September 2024).
- Weiss, K.R. Sustainable Aviation Fuels (SAF). Available online: https://www.icao.int/Meetings/Stocktaking2020/Documents/ICAO%20Stocktaking%202020%20-%203.3.7%20-%20Kevin%20Weiss.pdf (accessed on 1 October 2024).
- Del Paggio, A. IH2* Technology. 2018. Available online: https://bioenergyinternational.com/wp-content/uploads/2018/09/6.Alan_Del-Paggio.pdf (accessed on 1 October 2024).
- Shahabuddin, M.; Alam, M.T.; Krishna, B.B.; Bhaskar, T.; Perkins, G. A Review on the Production of Renewable Aviation Fuels from the Gasification of Biomass and Residual Wastes. Bioresour. Technol. 2020, 312, 123596. [Google Scholar] [CrossRef] [PubMed]
- de Klerk, A.; Chauhan, G.; Halmenschlager, C.; Link, F.; Montoya Sánchez, N.; Gartley, B.; El-Sayed, H.E.M.; Sehdev, R.; Lehoux, R. Sustainable Aviation Fuel: Pathways to Fully Formulated Synthetic Jet Fuel via Fischer–Tropsch Synthesis. Energy Sci. Eng. 2024, 12, 394–409. [Google Scholar] [CrossRef]
- van Dyk, S.; Saddler, J. Progress in Commercialization of Biojet/Sustainable Aviation Fuels (SAF): Technologies and Policies; IEA Bioenergy: Task 39; IEA Bioenergy: Paris, France, 2024; ISBN 9791280907363. [Google Scholar]
- Peters, M.A.; Alves, C.T.; Onwudili, J.A. A Review of Current and Emerging Production Technologies for Biomass-Derived Sustainable Aviation Fuels. Energies 2023, 16, 6100. [Google Scholar] [CrossRef]
- Ameen, M.; Azizan, M.T.; Yusup, S.; Ramli, A.; Yasir, M. Catalytic Hydrodeoxygenation of Triglycerides: An Approach to Clean Diesel Fuel Production. Renew. Sustain. Energy Rev. 2017, 80, 1072–1088. [Google Scholar] [CrossRef]
- Nepomniashchii, A.A.; Buluchevskiy, E.A.; Yurpalov, V.L.; Drozdov, V.A.; Lavrenov, A.V. Hydrodeoxygenation of Vegetable Oil on M/WO3-Al2O3 (M = NiMo, Pt, Pd, Ni) Catalysts for Producing Biofuels. AIP Conf. Proc. 2020, 2301, 020002. [Google Scholar] [CrossRef]
- Yoosuk, B.; Sanggam, P.; Wiengket, S.; Prasassarakich, P. Hydrodeoxygenation of Oleic Acid and Palmitic Acid to Hydrocarbon-like Biofuel over Unsupported Ni-Mo and Co-Mo Sulfide Catalysts. Renew. Energy 2019, 139, 1391–1399. [Google Scholar] [CrossRef]
- Dabbawala, A.A.; Elmutasim, O.; Baker, M.A.; Siakavelas, G.; Anjum, D.H.; Charisiou, N.D.; Hinder, S.J.; Munro, C.J.; Gacesa, M.; Goula, M.A.; et al. Toward Maximizing the Selectivity of Diesel-like Hydrocarbons from Oleic Acid Hydrodeoxygenation Using Ni/Co-Al2O3 Embedded Mesoporous Silica Nanocomposite Catalysts: An Experimental and DFT Approach. Appl. Surf. Sci. 2023, 640, 158294. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Y.; Zhao, J.; Gou, J.; Sun, K.; Liu, C. Zinc-Modified Pt/SAPO-11 for Improving the Isomerization Selectivity to Dibranched Alkanes. Cuihua Xuebao/Chin. J. Catal. 2017, 38, 509–517. [Google Scholar] [CrossRef]
- Veriansyah, B.; Han, J.Y.; Kim, S.K.; Hong, S.-A.; Kim, Y.J.; Lim, J.S.; Shu, Y.-W.; Oh, S.-G.; Kim, J. Production of Renewable Diesel by Hydroprocessing of Soybean Oil: Effect of Catalysts. Fuel 2012, 94, 578–585. [Google Scholar] [CrossRef]
- Hengsawad, T.; Srimingkwanchai, C.; Butnark, S.; Resasco, D.E.; Jongpatiwut, S. Effect of Metal-Acid Balance on Hydroprocessed Renewable Jet Fuel Synthesis from Hydrocracking and Hydroisomerization of Biohydrogenated Diesel over Pt-Supported Catalysts. Ind. Eng. Chem. Res. 2018, 57, 1429–1440. [Google Scholar] [CrossRef]
- Brandão, R.D.; de Freitas Júnior, A.M.; Oliveira, S.C.; Suarez, P.A.Z.; Prauchner, M.J. The Conversion of Coconut Oil into Hydrocarbons within the Chain Length Range of Jet Fuel. Biomass Convers. Biorefin. 2021, 11, 837–847. [Google Scholar] [CrossRef]
- ASTM D7566-22; Standard Specification for Aviation Turbine Fuel Containing Synthesized Hydrocarbons. ASTM International: West Conshohocken, PA, USA, 2022.
- Fan, X.; Burton, R.; Austic, G. Conversion of Degummed Soybean Oil to Biodiesel: Optimization of Degumming Methods and Evaluation of Fuel Properties. Int. J. Green Energy 2010, 7, 593–599. [Google Scholar] [CrossRef]
- More, N.S.; Gogate, P.R. Intensified Degumming of Crude Soybean Oil Using Cavitational Reactors. J. Food Eng. 2018, 218, 33–43. [Google Scholar] [CrossRef]
- Talpur, M.Y.; Sherazi, S.T.H.; Mahesar, S.A.; Kandhro, A.A. Effects of Chicken Frying on Soybean, Sunflower and Canola Oils. Pak. J. Anal. Environ. Chem. 2009, 10, 59–66. [Google Scholar]
- Arun, N.; Sharma, R.V.; Dalai, A.K. Green Diesel Synthesis by Hydrodeoxygenation of Bio-Based Feedstocks: Strategies for Catalyst Design and Development. Renew. Sustain. Energy Rev. 2015, 48, 240–255. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, G.; Miao, C. Green and Renewable Bio-Diesel Produce from Oil Hydrodeoxygenation: Strategies for Catalyst Development and Mechanism. Renew. Sustain. Energy Rev. 2019, 101, 568–589. [Google Scholar] [CrossRef]
- Li, T.; Cheng, J.; Huang, R.; Zhou, J.; Cen, K. Conversion of Waste Cooking Oil to Jet Biofuel with Nickel-Based Mesoporous Zeolite Y Catalyst. Bioresour. Technol. 2015, 197, 289–294. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Rozmysłowicz, B.; Lestari, S.; Simakova, O.; Eränen, K.; Salmi, T.; Murzin, D.Y. Catalytic Deoxygenation of Tall Oil Fatty Acid over Palladium Supported on Mesoporous Carbon. Energy Fuels 2011, 25, 2815–2825. [Google Scholar] [CrossRef]
- Verma, D.; Rana, B.S.; Kumar, R.; Sibi, M.G.; Sinha, A.K. Diesel and Aviation Kerosene with Desired Aromatics from Hydroprocessing of Jatropha Oil over Hydrogenation Catalysts Supported on Hierarchical Mesoporous SAPO-11. Appl. Catal. A Gen. 2015, 490, 108–116. [Google Scholar] [CrossRef]
- Di Vito Nolfi, G.; Gallucci, K.; Rossi, L. Green Diesel Production by Catalytic Hydrodeoxygenation of Vegetables Oils. Int. J. Environ. Res. Public Health 2021, 18, 13041. [Google Scholar] [CrossRef] [PubMed]
- Šimáček, P.; Kubička, D.; Šebor, G.; Pospíšil, M. Hydroprocessed Rapeseed Oil as a Source of Hydrocarbon-Based Biodiesel. Fuel 2009, 88, 456–460. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, J.; Ding, J.; Liu, T.; Shi, G.; Li, X.; Dang, W.; Cheng, Y.; Guo, R. Pore Structure and Fractal Characteristics of Different Shale Lithofacies in the Dalong Formation in the Western Area of the Lower Yangtze Platform. Minerals 2020, 10, 72. [Google Scholar] [CrossRef]
- Jaroszewska, K.; Masalska, A.; Grzechowiak, J.R. Hydroisomerization of Long-Chain Bio-Derived n-Alkanes into Monobranched High Cetane Isomers via a Dual-Component Catalyst Bed. Fuel 2020, 268, 117239. [Google Scholar] [CrossRef]
- Chen, Y.K.; Hsieh, C.H.; Wang, W.C. The Production of Renewable Aviation Fuel from Waste Cooking Oil. Part II: Catalytic Hydro-Cracking/Isomerization of Hydro-Processed Alkanes into Jet Fuel Range Products. Renew. Energy 2020, 157, 731–740. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chen, Y.-K.; Wang, W.-C. The Production of Bio-Jet Fuel from Palm Oil Derived Alkanes. Fuel 2020, 260, 116345. [Google Scholar] [CrossRef]
- ASTM D1655-23; Standard Specification for Aviation Turbine Fuels. ASTM International: West Conshohocken, PA, USA, 2023.











| Fatty Acid | [%] |
|---|---|
| C14:0 | 0.10 |
| C16:0 | 11.33 |
| C18:0 | 4.55 |
| C18:1 cis | 22.24 |
| C18;1 n-9 trans | 1.02 |
| C18:2 n9,n-12 cis | 54.67 |
| C18:2 n9,n-12 trans | 0.01 |
| C18:3 n9,n-12,n-15 cis | 6.07 |
| C18:3 n9,n-12,n-15 trans | 0.01 |
| Property | Unit | Results |
|---|---|---|
| Acid Number | mgKOH/g | 0.78 (±0.01) |
| Iodine Number | gI2/100 g | 127.4 (±0.4) |
| Density at 20 °C | g/cm3 | 0.919 (±0.001) |
| Average Kinematic Viscosity | mm2/s | 32.18 (±0.02) |
| Catalyst | SBET | VT | VMIKRO | La | Lm |
|---|---|---|---|---|---|
| [m2/g] | [cm3/g] | [cm3/g] | [nm] | [nm] | |
| Ni/γ-Al2O3 + SiO2 (fresh) | 179 | 0.316 | 0.00589 | 6.13 | 9.6 |
| Ni/γ-Al2O3 + SiO2 (used) | 113 | 0.257 | 0.00105 | 6.13 | 9.6 |
| Catalyst | SBET | VT | VMikro | La | Lm |
|---|---|---|---|---|---|
| [m2/g] | [cm3/g] | [cm3/g] | [nm] | [nm] | |
| 0.5% Pt/Al2O3 + SAPO-11 + 3% PEG-4 (fresh) | 208 | 0.397 | 0.0245 | 8.08 | 12.1 |
| 0.5% Pt/Al2O3 + SAPO-11 + 3% PEG-4 (after used) | 142 | 0.297 | 0.0050 | 8.20 | 12.4 |
| 0.5% Pt/Al2O3 + SAPO-11 + 3% PEG-4 (after used calcinated) | 191 | 0.375 | 0.0213 | 8.11 | 12.3 |
| Property | Unit | Requirements (ASTM D1655 (1)/D7566 (2)) | Bio-Component Jet-A1 |
|---|---|---|---|
| Flash point | °C | min 38 | 100 |
| Freezing point | °C | max −47 | −58.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Główka, M.; Wójcik, J.K.; Boberski, P.; Woszczyński, P.J.; Sabura, E. Highly Efficient Process for Producing a Jet-A1 Biofuel Component Through Hydroprocessing Soybean Oil over Ni and Pt Catalysts. Energies 2024, 17, 6195. https://doi.org/10.3390/en17236195
Główka M, Wójcik JK, Boberski P, Woszczyński PJ, Sabura E. Highly Efficient Process for Producing a Jet-A1 Biofuel Component Through Hydroprocessing Soybean Oil over Ni and Pt Catalysts. Energies. 2024; 17(23):6195. https://doi.org/10.3390/en17236195
Chicago/Turabian StyleGłówka, Marek, Jan Krzysztof Wójcik, Przemysław Boberski, Piotr Józef Woszczyński, and Ewa Sabura. 2024. "Highly Efficient Process for Producing a Jet-A1 Biofuel Component Through Hydroprocessing Soybean Oil over Ni and Pt Catalysts" Energies 17, no. 23: 6195. https://doi.org/10.3390/en17236195
APA StyleGłówka, M., Wójcik, J. K., Boberski, P., Woszczyński, P. J., & Sabura, E. (2024). Highly Efficient Process for Producing a Jet-A1 Biofuel Component Through Hydroprocessing Soybean Oil over Ni and Pt Catalysts. Energies, 17(23), 6195. https://doi.org/10.3390/en17236195







