Biofuel Production in Oleic Acid Hydrodeoxygenation Utilizing a Ni/Tire Rubber Carbon Catalyst and Predicting of n-Alkanes with Box–Behnken and Artificial Neural Networks
Abstract
1. Introduction
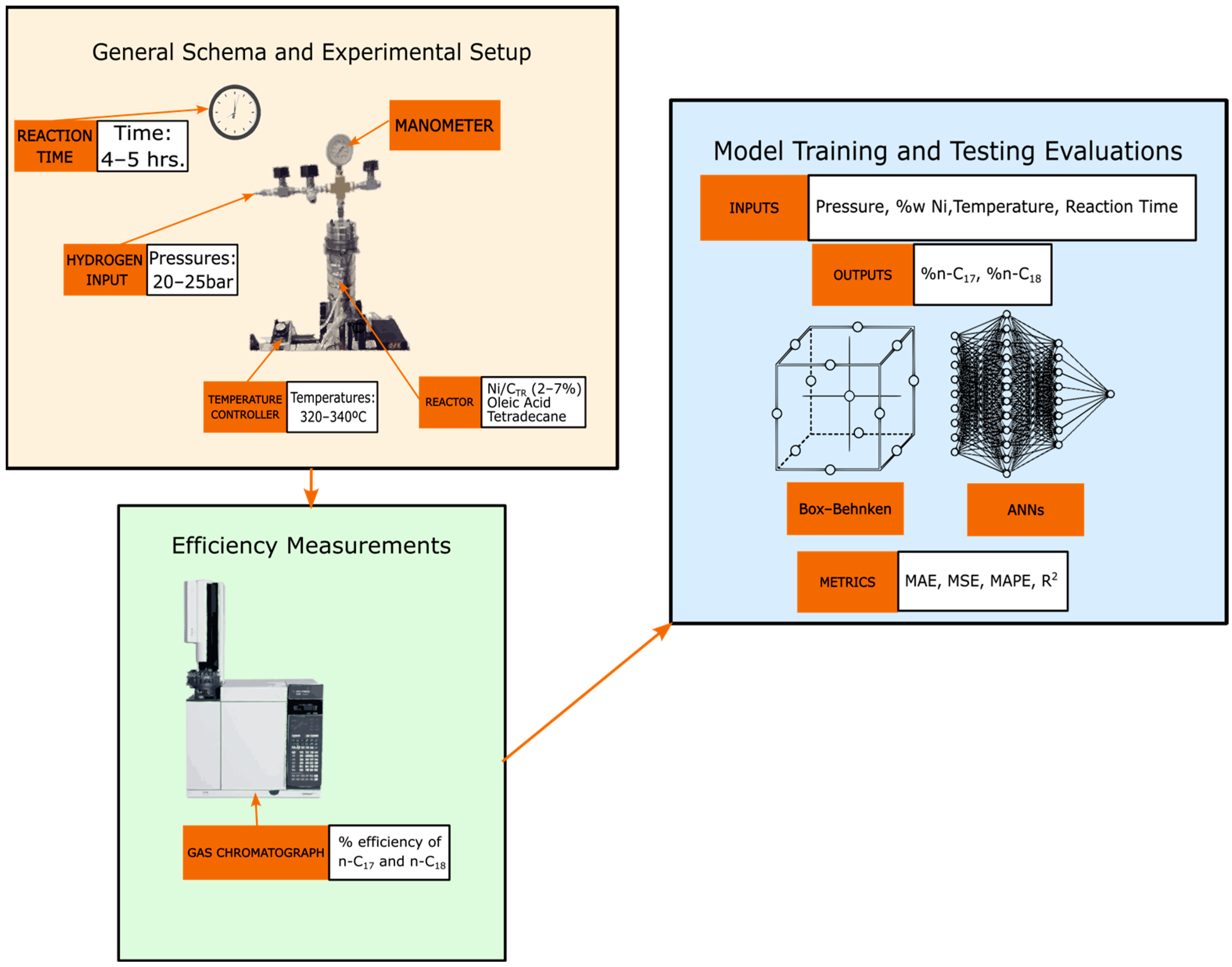
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Characterization of Catalysts
2.3. Hydrodeoxygenation of Oleic Acid
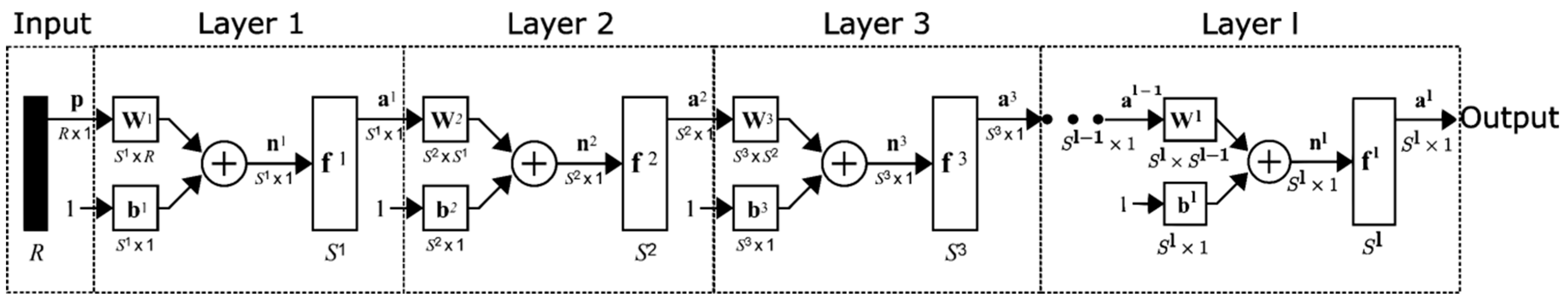
2.4. Development of Box–Behnken and Artificial Neural Networks Optimization Models to Predict n-Alkanes
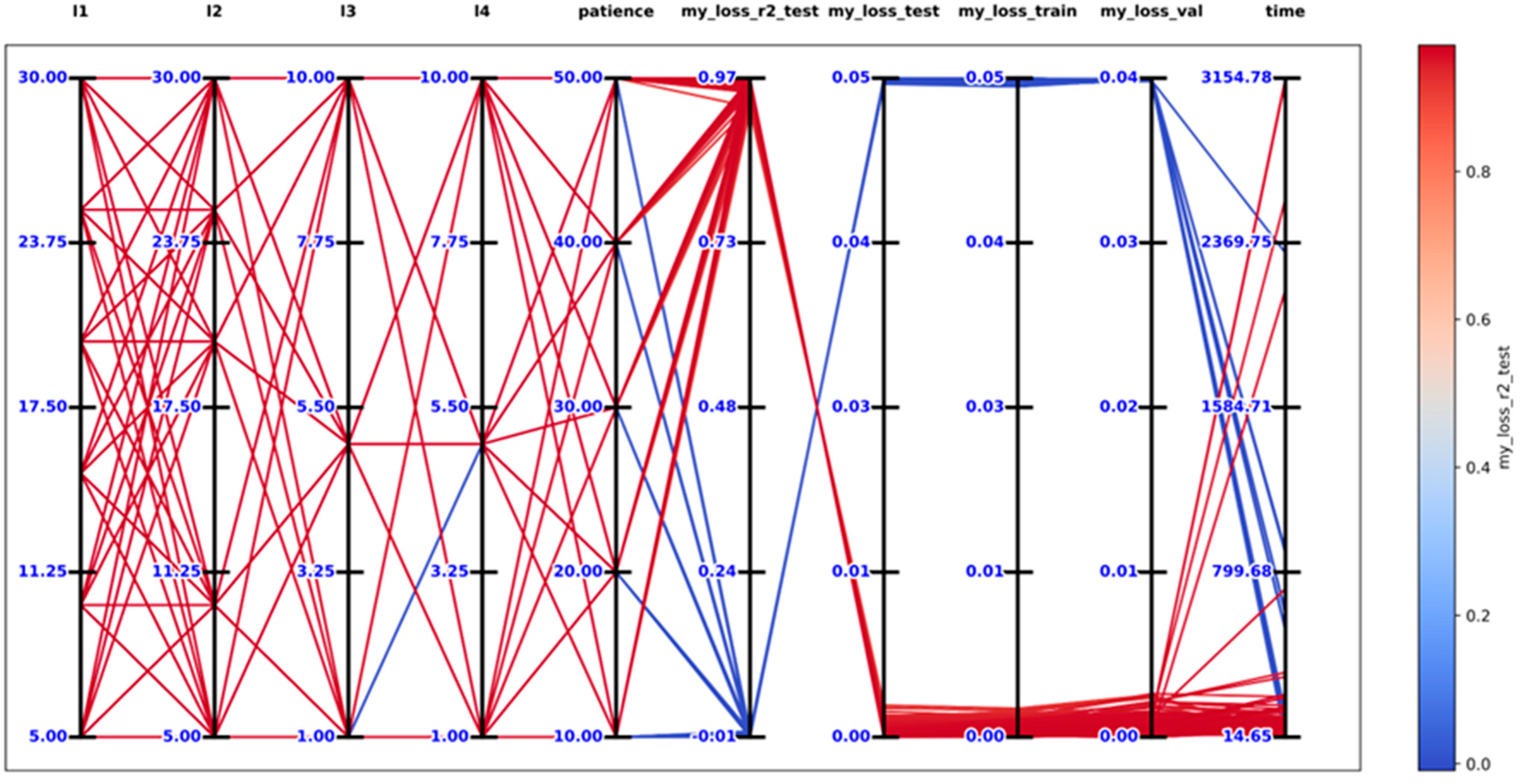
2.5. Development of Artificial Neural Networks Models to Predict n-Alkanes
2.6. Metrics of Comparison
3. Results
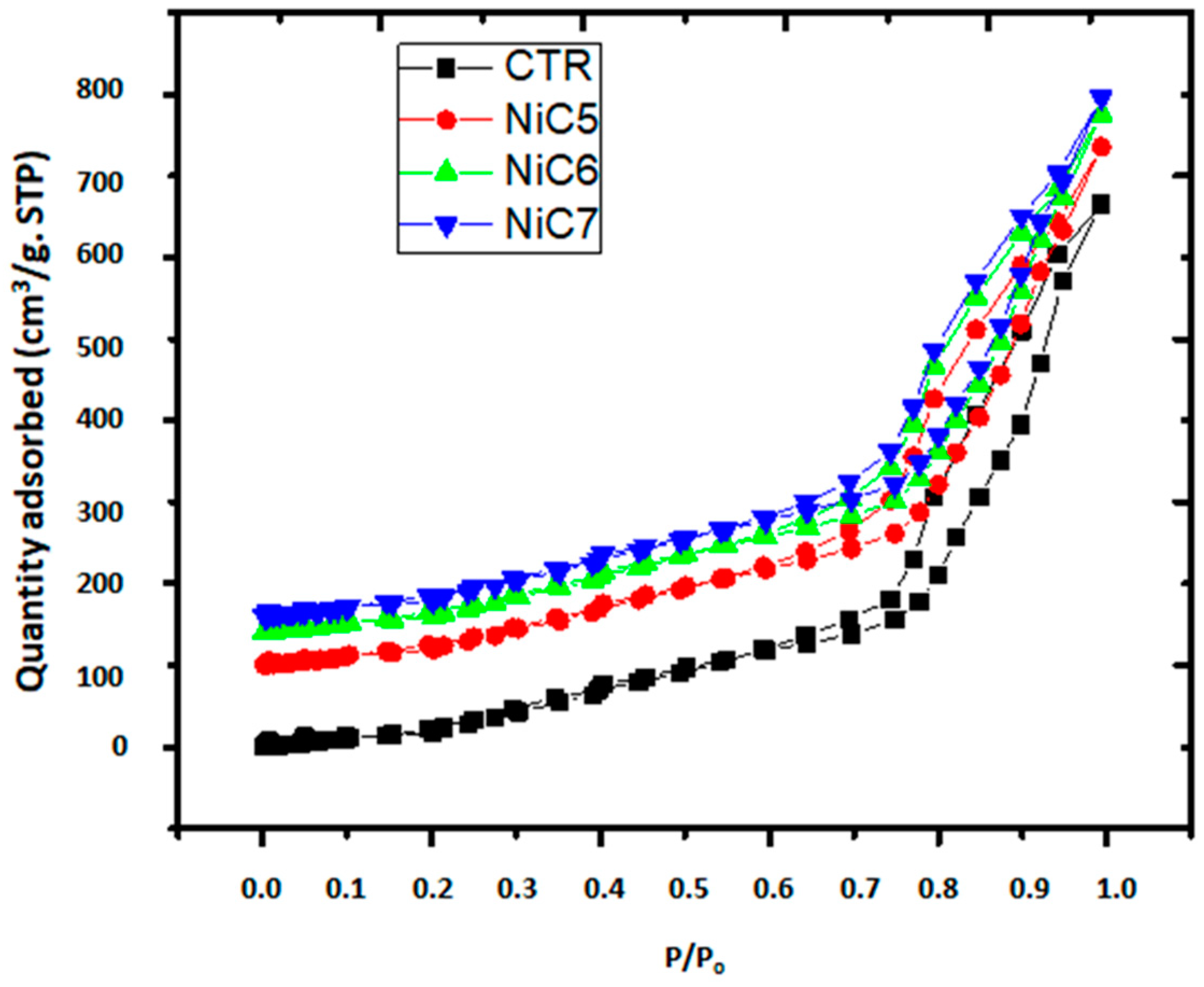
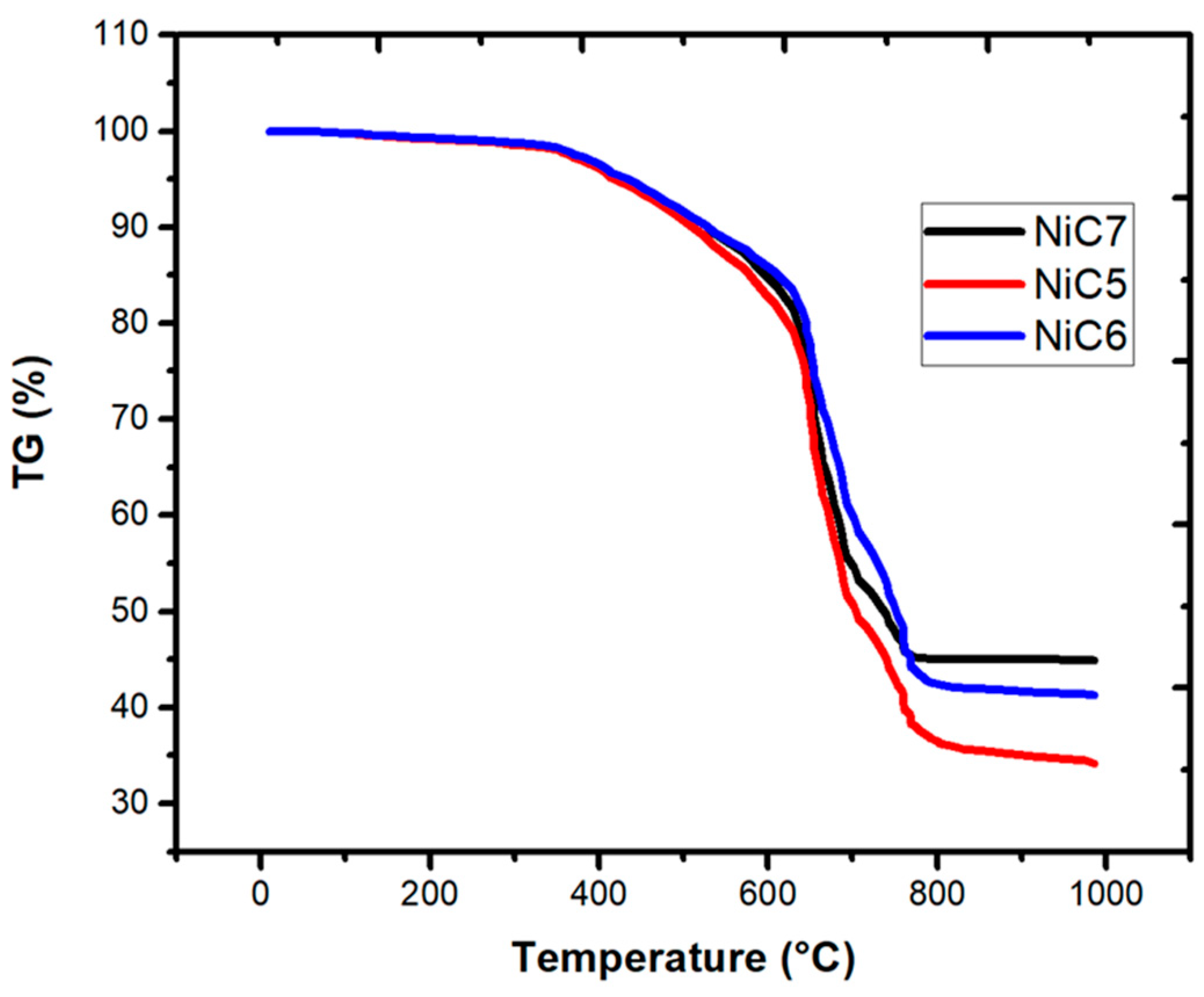
3.1. Ni/C Catalyst Characterization
3.2. Conversion of Oleic Acid to n-C17 and n-C18 by Hydrodeoxygenation
3.3. Box–Behnken Results
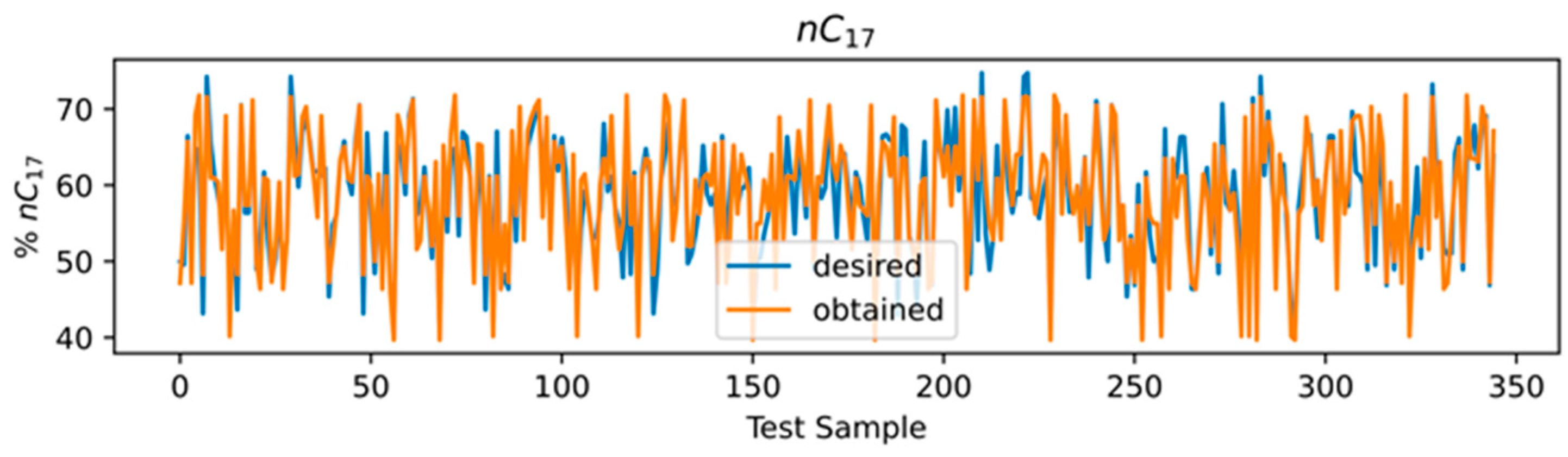
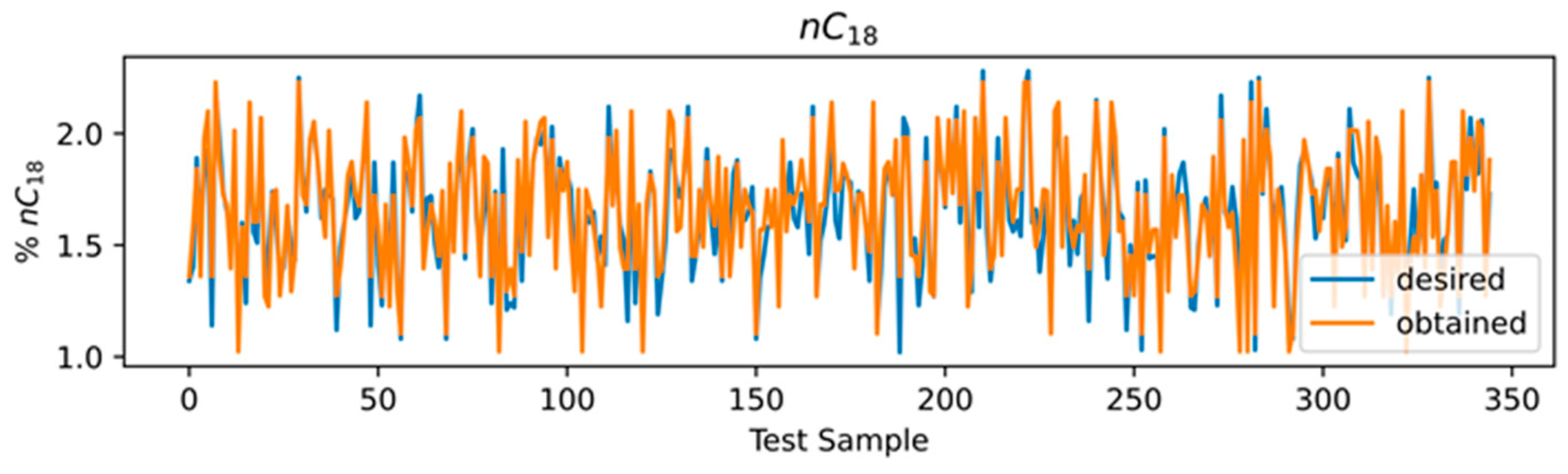
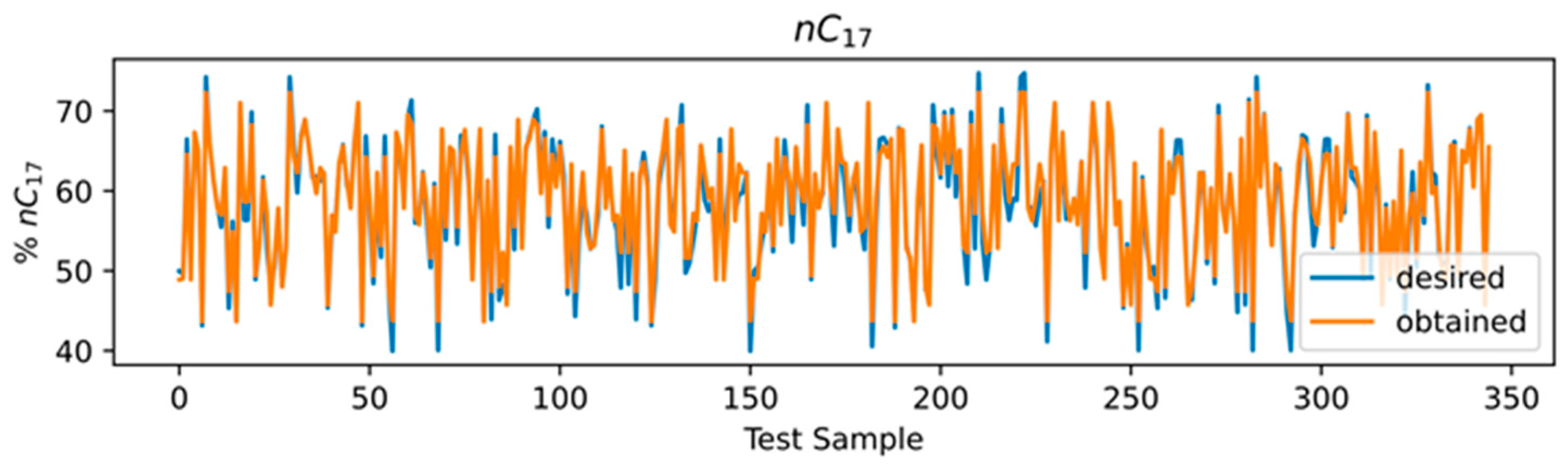
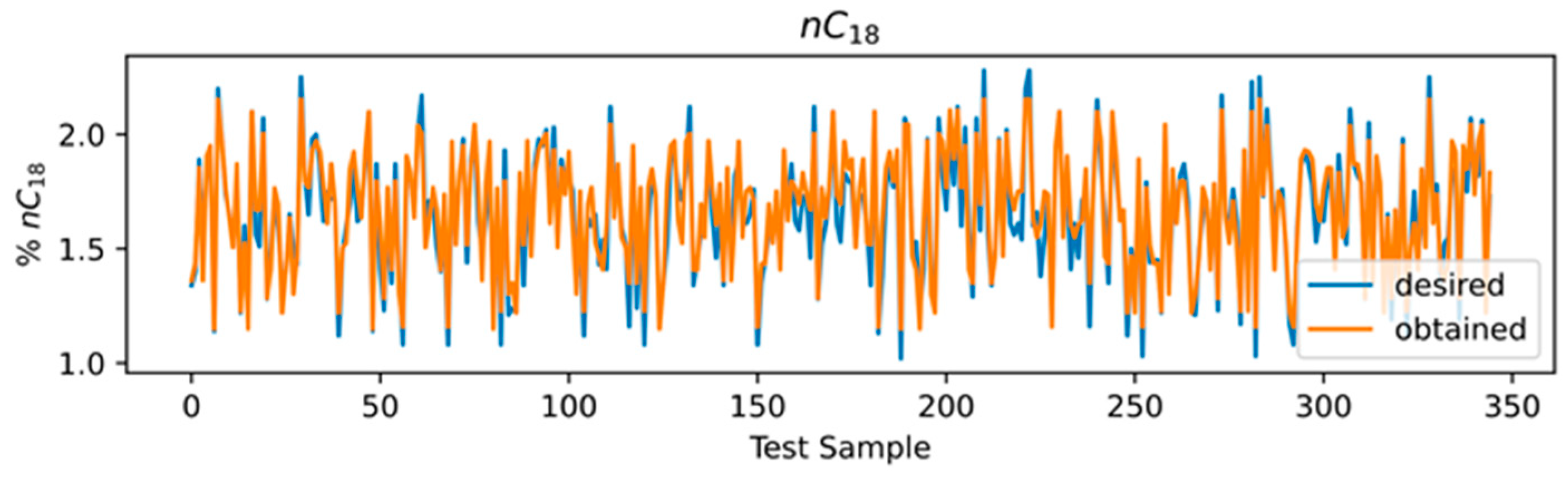
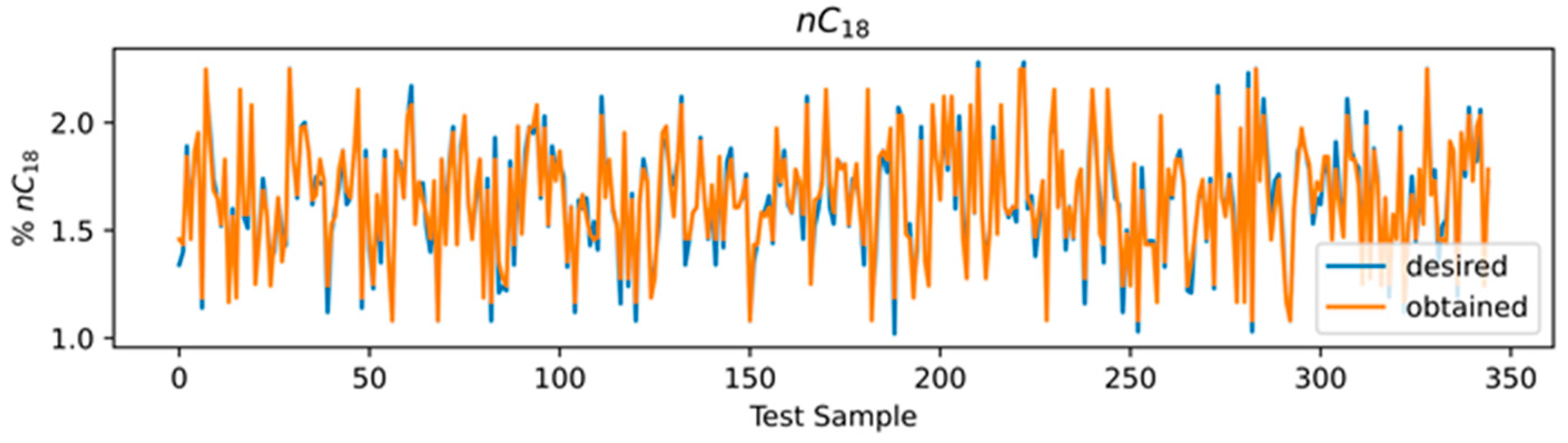
3.4. ANN Results
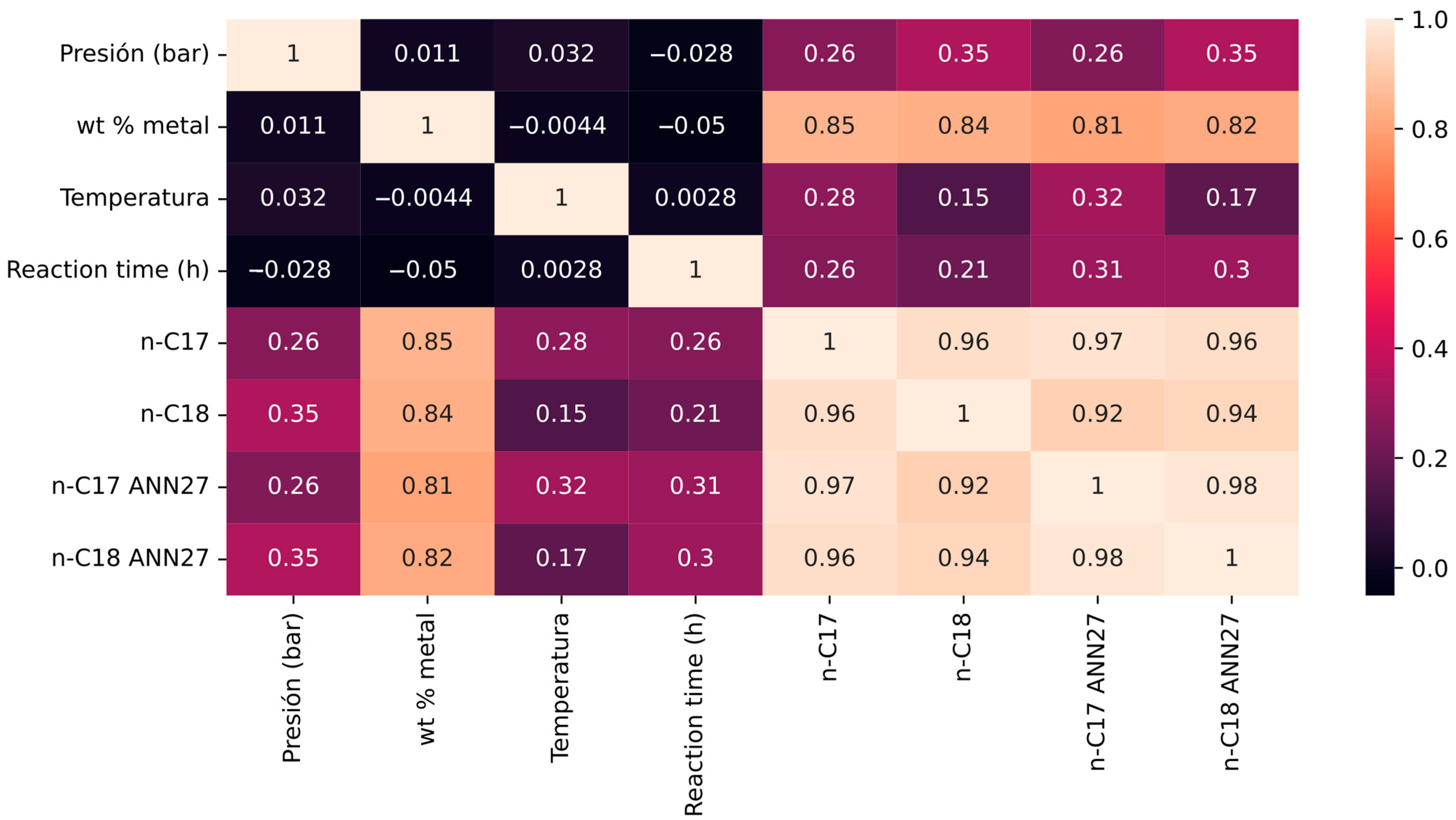
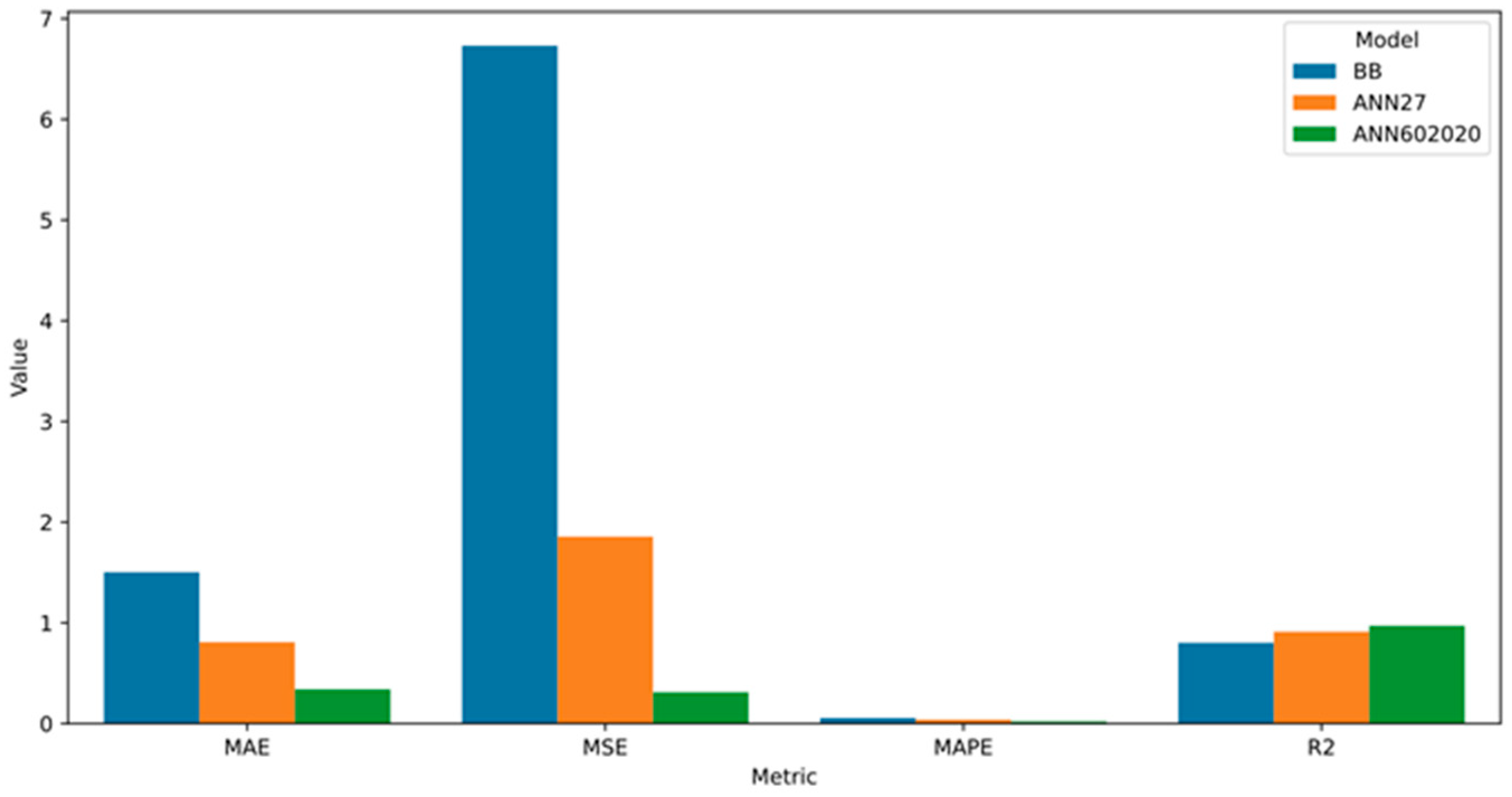
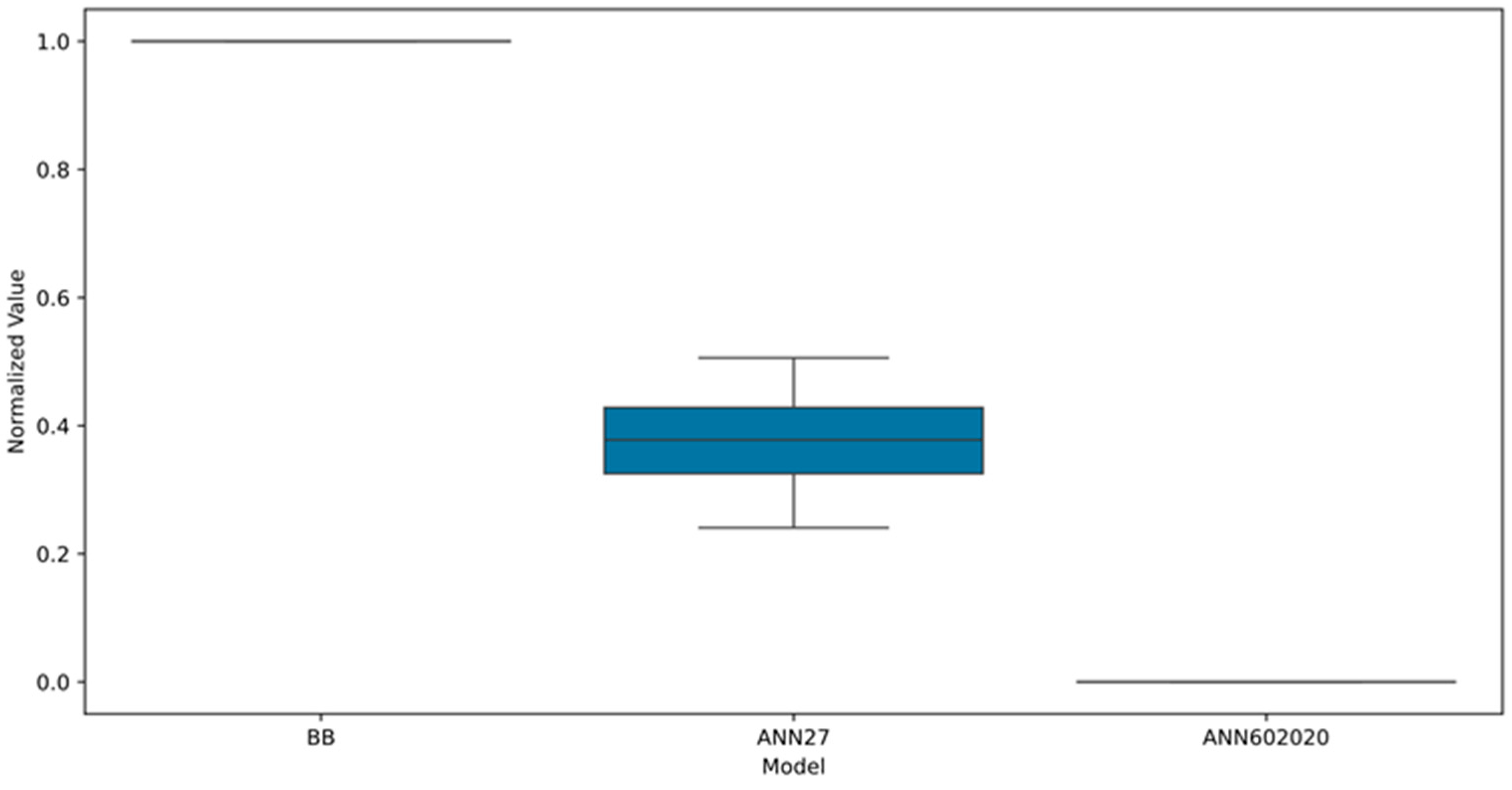
3.5. Comparison of Models
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sánchez-Cárdenas, M.; Medina-Valtierra, J.; Kamaraj, S.K.; Trejo-Zárraga, F.; Antonio Sánchez-Olmos, L. Physicochemical Effect of Pt Nanoparticles/γ-Al2O3 on the Oleic Acid Hydrodeoxygenation to Biofuel. Environ. Prog. Sustain. Energy 2017, 36, 1224–1233. [Google Scholar] [CrossRef]
- Sánchez-Cárdenas, M.; Medina-Valtierra, J.; Kamaraj, S.K.; Ramírez, R.R.M.; Sánchez-Olmos, L.A. Effect of Size and Distribution of Ni Nanoparticles on γ-Al2O3 in Oleic Acid Hydrodeoxygenation to Produce n-Alkanes. Catalysts 2016, 6, 156. [Google Scholar] [CrossRef]
- Wathakit, K.; Sukjit, E.; Kaewbuddee, C.; Maithomklang, S.; Klinkaew, N.; Liplap, P.; Arjharn, W.; Srisertpol, J. Characterization and Impact of Waste Plastic Oil in a Variable Compression Ratio Diesel Engine. Energies 2021, 14, 2230. [Google Scholar] [CrossRef]
- Kittisupakorn, P.; Sae-ueng, S.; Suwatthikul, A. Optimization of Energy Consumption in a Hydrotreating Process for Green Diesel Production from Palm Oil. Comput. Aided Chem. Eng. 2016, 38, 751–756. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, H.; Wang, W.; Zheng, Y.; Hu, P. Hydroprocessing of Waste Cooking Oil over a Dispersed Nano Catalyst: Kinetics Study and Temperature Effect. Appl. Catal. B 2014, 150–151, 238–248. [Google Scholar] [CrossRef]
- Aatola, H.; Larmi, M.; Sarjovaara, T.; Mikkonen, S.; Aatola, H.; Larmi, M.; Sarjovaara, T.; Mikkonen, S. Hydrotreated Vegetable Oil (HVO) as a Renewable Diesel Fuel: Trade-off between NOx, Particulate Emission, and Fuel Consumption of a Heavy Duty Engine. SAE Int. J. Engines 2008, 1, 1251–1262. [Google Scholar] [CrossRef]
- Hulkkonen, T.; Hillamo, H.; Sarjovaara, T.; Larmi, M.; Hulkkonen, T.; Hillamo, H.; Sarjovaara, T.; Larmi, M. Experimental Study of Spray Characteristics Between Hydrotreated Vegetable Oil (HVO) and Crude Oil Based EN 590 Diesel Fuel; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2011. [Google Scholar] [CrossRef]
- Sugiyama, K.; Goto, I.; Kitano, K.; Mogi, K.; Honkanen, M. Effects of Hydrotreated Vegetable Oil (HVO) as Renewable Diesel Fuel on Combustion and Exhaust Emissions in Diesel Engine. SAE Int. J. Fuels Lubr. 2011, 5, 205–217. [Google Scholar] [CrossRef]
- Sánchez-Cárdenas, M.; Sánchez-Olmos, L.A.; Sathish-Kumar, K.; Trejo-Zarraga, F.; Maldonado-Ruelas, V.A.; Ortiz-Medina, R.A. Controlled Evaluation in a Diesel Engine of the Biofuel Obtained with Ni/γ-Al2O3 Nanoparticles in the Hydrodeoxygenation of Oleic Acid. Int. J. Chem. React. Eng. 2020, 18, 20190136. [Google Scholar] [CrossRef]
- Yfanti, V.L.; Lemonidou, A.A. Effect of Hydrogen Donor on Glycerol Hydrodeoxygenation to 1,2-Propanediol. Catal. Today 2020, 355, 727–736. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of Fast Pyrolysis of Biomass and Product Upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Monnier, J.; Sulimma, H.; Dalai, A.; Caravaggio, G. Hydrodeoxygenation of Oleic Acid and Canola Oil over Alumina-Supported Metal Nitrides. Appl. Catal. A Gen. 2010, 382, 176–180. [Google Scholar] [CrossRef]
- Attia, M.; Farag, S.; Chaouki, J. Upgrading of Oils from Biomass and Waste: Catalytic Hydrodeoxygenation. Catalysts 2020, 10, 1381. [Google Scholar] [CrossRef]
- Pinna, F. Supported Metal Catalysts Preparation. Catal. Today 1998, 41, 129–137. [Google Scholar] [CrossRef]
- Marsh, H.; Rodríguez-Reinoso, F. Activated Carbon; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1–536. [Google Scholar] [CrossRef]
- Murillo, R.; Navarro, M.V.; López, J.M.; García, T.; Callén, M.S.; Aylón, E.; Mastral, A.M. Activation of Pyrolytic Tire Char with CO2: Kinetic Study. J. Anal. Appl. Pyrolysis 2004, 71, 945–957. [Google Scholar] [CrossRef]
- El-Sheikh, A.H.; Newman, A.P.; Al-Daffaee, H.; Phull, S.; Cresswell, N.; York, S. Deposition of Anatase on the Surface of Activated Carbon. Surf. Coat. Technol. 2009, 187, 284–292. [Google Scholar] [CrossRef]
- Do, P.T.; Chiappero, M.; Lobban, L.L.; Resasco, D.E. Catalytic Deoxygenation of Methyl-Octanoate and Methyl-Stearate on Pt/Al2O3. Catal. Lett. 2009, 130, 9–18. [Google Scholar] [CrossRef]
- Lestari, S.; Simakova, I.; Tokarev, A.; Mäki-Arvela, P.; Eränen, K.; Murzin, D.Y. Synthesis of Biodiesel via Deoxygenation of Stearic Acid over Supported Pd/C Catalyst. Catal. Lett. 2008, 122, 247–251. [Google Scholar] [CrossRef]
- Zuo, H.; Liu, Q.; Wang, T.; Ma, L.; Zhang, Q.; Zhang, Q. Hydrodeoxygenation of Methyl Palmitate over Supported Ni Catalysts for Diesel-like Fuel Production. Energy Fuels 2012, 26, 3747–3755. [Google Scholar] [CrossRef]
- Şenol, O.I.; Ryymin, E.M.; Viljava, T.R.; Krause, A.O.I. Effect of Hydrogen Sulphide on the Hydrodeoxygenation of Aromatic and Aliphatic Oxygenates on Sulphided Catalysts. J. Mol. Catal. A Chem. 2007, 277, 107–112. [Google Scholar] [CrossRef]
- Phan, D.P.; Lee, E.Y. Phosphoric Acid Enhancement in a Pt-Encapsulated Metal-Organic Framework (MOF) Bifunctional Catalyst for Efficient Hydro-Deoxygenation of Oleic Acid from Biomass. J. Catal. 2020, 386, 19–29. [Google Scholar] [CrossRef]
- Nur Azreena, I.; Lau, H.L.N.; Asikin-Mijan, N.; Hassan, M.A.; Izham, S.M.; Safa Gamal, M.; Nor Adira Wan Khalit, W.; Arumugam, M.; Kennedy, E.; Stockenhuber, M.; et al. Hydrodeoxygenation of Fatty Acid over La-Modified HZSM5 for Premium Quality Renewable Diesel Production. J. Anal. Appl. Pyrolysis 2022, 161, 105406. [Google Scholar] [CrossRef]
- Chang, H.; Abdulkareem-Alsultan, G.; Taufiq-Yap, Y.H.; Mohd Izham, S.; Sivasangar, S. Development of Porous MIL-101 Derived Catalyst Application for Green Diesel Production. Fuel 2024, 355, 129459. [Google Scholar] [CrossRef]
- Aziz, I.; Sugita, P.; Darmawan, N.; Dwiatmoko, A.A.; Rustyawan, W. Green Diesel Synthesis from Palm Fatty Acid Distillate Using a Nickel Phosphide Catalyst: Optimization by Box Behnken Design. Bioresour. Technol. Rep. 2024, 27, 101897. [Google Scholar] [CrossRef]
- Abdullah, N.H.B.; Mijan, N.A.; Taufiq-Yap, Y.H.; Ong, H.C.; Lee, H.V. Environment-Friendly Deoxygenation of Non-Edible Ceiba Oil to Liquid Hydrocarbon Biofuel: Process Parameters and Optimization Study. Environ. Sci. Pollut. Res. 2022, 29, 51143–51152. [Google Scholar] [CrossRef] [PubMed]
- Yusuff, A.S.; Ishola, N.B.; Gbadamosi, A.O.; Azeez, T.M.; Onibonoje, M.O. An Artificial Intelligence Approach to Model and Optimize Biodiesel Production from Used Cooking Oil Using CaO Incorporated Zeolite Catalyst. Energy Convers. Manag. X 2023, 20, 100452. [Google Scholar] [CrossRef]
- Sumayli, A.; Alshahrani, S.M. Modeling and Prediction of Biodiesel Production by Using Different Artificial Intelligence Methods: Multi-Layer Perceptron (MLP), Gradient Boosting (GB), and Gaussian Process Regression (GPR). Arab. J. Chem. 2023, 16, 104801. [Google Scholar] [CrossRef]
- Pflaum, H.; Hofmann, P.; Geringer, B.; Weissel, W.; Pflaum, H.; Hofmann, P.; Geringer, B.; Weissel, W. Potential of Hydrogenated Vegetable Oil (HVO) in a Modern Diesel Engine; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2010. [Google Scholar] [CrossRef]
- Singh, D.; Subramanian, K.A.; Singal, S.K. Emissions and Fuel Consumption Characteristics of a Heavy Duty Diesel Engine Fueled with Hydroprocessed Renewable Diesel and Biodiesel. Appl. Energy 2015, 155, 440–446. [Google Scholar] [CrossRef]
- Gong, S.; Shinozaki, A.; Shi, M.; Qian, E.W. Hydrotreating of Jatropha Oil over Alumina Based Catalysts. Energy Fuels 2012, 26, 2394–2399. [Google Scholar] [CrossRef]
- Calzada, L.A.; Pérez-Estrada, D.; Sánchez-Ramírez, M.; Gómora-Herrera, D.; Gómez-Cortés, A.; Díaz, G.; Klimova, T.E. Boosting the Hydrodeoxygenation Activity and Selectivity of Ni/(M)-SBA-15 Catalysts by Chemical Alteration of the Support. ACS Omega 2023, 8, 42849–42866. [Google Scholar] [CrossRef]
- Madsen, A.T.; Ahmed, E.H.; Christensen, C.H.; Fehrmann, R.; Riisager, A. Hydrodeoxygenation of Waste Fat for Diesel Production: Study on Model Feed with Pt/Alumina Catalyst. Fuel 2011, 90, 3433–3438. [Google Scholar] [CrossRef]
- Kumar, V.; Muthuraj, M.; Palabhanvi, B.; Ghoshal, A.K.; Das, D. Evaluation and Optimization of Two Stage Sequential in Situ Transesterification Process for Fatty Acid Methyl Ester Quantification from Microalgae. Renew. Energy 2014, 68, 560–569. [Google Scholar] [CrossRef]
- Das, A.; Kumawat, P.K.; Chaturvedi, N.D. A Study to Target Energy Consumption in Wastewater Treatment Plant Using Machine Learning Algorithms. Comput. Aided Chem. Eng. 2021, 50, 1511–1516. [Google Scholar] [CrossRef]
- Hagan, M.T.; Demuth, H.B.; Beale, M.H.; De Jesús, O. Neural Network Design, 2nd ed.; Campus Pub. Service, University of Colorado Bookstore: Boulder, CO, USA, 1996; ISBN 9780971732117. [Google Scholar]
- Lederer, J. Activation Functions in Artificial Neural Networks: A Systematic Overview. arXiv 2021, arXiv:2101.09957. [Google Scholar]
- Olvera-Gonzalez, E.; Rivera, M.M.; Escalante-Garcia, N.; Flores-Gallegos, E. Modeling Energy LED Light Consumption Based on an Artificial Intelligent Method Applied to Closed Plant Production System. App. Sci. 2021, 11, 2735. [Google Scholar] [CrossRef]
- Ayodele, O.B.; Togunwa, O.S.; Abbas, H.F.; Daud, W.M.A.W. Preparation and Characterization of Alumina Supported Nickel-Oxalate Catalyst for the Hydrodeoxygenation of Oleic Acid into Normal and Iso-Octadecane Biofuel. Energy Convers. Manag. 2014, 88, 1104–1110. [Google Scholar] [CrossRef]
- Matam, S.K.; Kondratenko, E.V.; Aguirre, M.H.; Hug, P.; Rentsch, D.; Winkler, A.; Weidenkaff, A.; Ferri, D. The Impact of Aging Environment on the Evolution of Al2O3 Supported Pt Nanoparticles and Their NO Oxidation Activity. Appl. Catal. B 2013, 129, 214–224. [Google Scholar] [CrossRef]
- Lu, A.H.; Spliethoff, B.; Schüth, F. Aqueous Synthesis of Ordered Mesoporous Carbon via Self-Assembly Catalyzed by Amino Acid. Chem. Mater. 2008, 20, 5314–5319. [Google Scholar] [CrossRef]
- Chen, X.; Ma, X.; Peng, X. Role of Filamentous Coke in Deactivation of Ni/Bio-Char Catalyst During Dry Reforming of Non-Oxygenates Tar. J. Anal. Appl. Pyrolysis 2021, 159, 105314. [Google Scholar] [CrossRef]
- Miguel, G.S.; Fowler, G.D.; Sollars, C.J. Pyrolysis of Tire Rubber: Porosity and Adsorption Characteristics of the Pyrolytic Chars. Ind. Eng. Chem. Res. 1998, 37, 2430–2435. [Google Scholar] [CrossRef]
- Broglia, F.; Rimoldi, L.; Meroni, D.; De Vecchi, S.; Morbidelli, M.; Ardizzone, S. Guaiacol Hydrodeoxygenation as a Model for Lignin Upgrading. Role of the Support Surface Features on Ni-Based Alumina-Silica Catalysts. Fuel 2019, 243, 501–508. [Google Scholar] [CrossRef]
- Li, G.; Hu, L.; Hill, J.M. Comparison of Reducibility and Stability of Alumina-Supported Ni Catalysts Prepared by Impregnation and Co-Precipitation. Appl. Catal. A Gen. 2006, 301, 16–24. [Google Scholar] [CrossRef]
- Chen, S.; Pan, X.; Miao, C.; Xie, H.; Zhou, G.; Jiao, Z.; Zhang, X. Study of Catalytic Hydrodeoxygenation Performance for the Ni/KIT-6 Catalysts. J. Saudi Chem. Soc. 2018, 22, 614–627. [Google Scholar] [CrossRef]
- Immer, J.G.; Kelly, M.J.; Lamb, H.H. Catalytic Reaction Pathways in Liquid-Phase Deoxygenation of C18 Free Fatty Acids. Appl. Catal. A Gen. 2010, 375, 134–139. [Google Scholar] [CrossRef]
- Ahmadi, M.; Macias, E.E.; Jasinski, J.B.; Ratnasamy, P.; Carreon, M.A. Decarboxylation and Further Transformation of Oleic Acid over Bifunctional, Pt/SAPO-11 Catalyst and Pt/Chloride Al2O3 Catalysts. J. Mol. Catal. A Chem. 2014, 386, 14–19. [Google Scholar] [CrossRef]
- Srifa, A.; Faungnawakij, K.; Itthibenchapong, V.; Assabumrungrat, S. Roles of Monometallic Catalysts in Hydrodeoxygenation of Palm Oil to Green Diesel. Chem. Eng. J. 2015, 278, 249–258. [Google Scholar] [CrossRef]
- Kaewmeesri, R.; Srifa, A.; Itthibenchapong, V.; Faungnawakij, K. Deoxygenation of Waste Chicken Fats to Green Diesel over Ni/Al2O3: Effect of Water and Free Fatty Acid Content. Energy Fuels 2015, 29, 833–840. [Google Scholar] [CrossRef]
- Serrano, D.P.; Escola, J.M.; Briones, L.; Arroyo, M. Selective Hydrodecarboxylation of Fatty Acids into Long-Chain Hydrocarbons Catalyzed by Pd/Al-SBA-15. Microporous Mesoporous Mater. 2019, 280, 88–96. [Google Scholar] [CrossRef]






| Independent Variable | Symbol | Coded Levels | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Pressure (bar) | P | 20 | 22.5 | 25 |
| Weight % Ni | W | 2 | 4.5 | 7 |
| Temperature (°C) | T | 320 | 330 | 340 |
| Reaction time (h) | t | 4 | 4.5 | 5 |
| Textural Properties | ||||
|---|---|---|---|---|
| Catalyst | Weight % Ni | SBET (m2/g) | Vp (cm3/g) | Dp (nm) |
| CTR | 0 | 114.21 | 0.39 | 12.27 |
| NiC2 | 2 | 108.34 | 0.36 | 12.02 |
| NiC3 | 3 | 104.32 | 0.341 | 11.77 |
| NiC4 | 4 | 100.18 | 0.318 | 11.54 |
| NiC5 | 5 | 95.01 | 0.31 | 11.28 |
| NiC6 | 6 | 90.24 | 0.302 | 11.25 |
| NiC7 | 7 | 87.75 | 0.287 | 10.99 |
| Catalyst | Pressure (bar) | wt% Metal | Temperature | Reaction Time (h) | Yield of n-C17 (% Mole) | Yield of n-C18 (% Mole) |
|---|---|---|---|---|---|---|
| NiC2 | 20 | 2 | 340 | 5 | 48.38 | 1.29 |
| NiC2 | 20 | 2 | 340 | 4 | 44.82 | 1.17 |
| NiC3 | 20 | 3 | 340 | 5 | 56.95 | 1.41 |
| NiC3 | 20 | 3 | 340 | 4 | 52.92 | 1.49 |
| NiC4 | 20 | 4 | 340 | 5 | 59.54 | 1.61 |
| NiC4 | 20 | 4 | 340 | 4 | 55.48 | 1.52 |
| NiC5 | 20 | 5 | 340 | 5 | 64.55 | 1.82 |
| NiC5 | 20 | 5 | 340 | 4 | 61.86 | 1.75 |
| NiC6 | 20 | 6 | 340 | 5 | 65.44 | 1.83 |
| NiC6 | 20 | 6 | 340 | 4 | 62.28 | 1.76 |
| NiC7 | 20 | 7 | 340 | 5 | 68.94 | 1.95 |
| NiC7 | 20 | 7 | 340 | 4 | 66.34 | 1.87 |
| NiC2 | 20 | 2 | 320 | 5 | 46.87 | 1.27 |
| NiC2 | 20 | 2 | 320 | 4 | 40.05 | 1.08 |
| NiC3 | 20 | 3 | 320 | 5 | 53.33 | 1.5 |
| NiC3 | 20 | 3 | 320 | 4 | 47.11 | 1.33 |
| NiC4 | 20 | 4 | 320 | 5 | 56.37 | 1.56 |
| NiC4 | 20 | 4 | 320 | 4 | 50.43 | 1.45 |
| NiC5 | 20 | 5 | 320 | 5 | 61.7 | 1.74 |
| NiC5 | 20 | 5 | 320 | 4 | 56.66 | 1.6 |
| NiC6 | 20 | 6 | 320 | 5 | 63.67 | 1.75 |
| NiC6 | 20 | 6 | 320 | 4 | 58.3 | 1.65 |
| NiC7 | 20 | 7 | 320 | 5 | 66.17 | 1.87 |
| NiC7 | 20 | 7 | 320 | 4 | 61.3 | 1.73 |
| NiC4.5 | 22.5 | 4.5 | 330 | 4.5 | 60.31 | 1.72 |
| NiC2 | 22.5 | 2 | 330 | 4 | 44.23 | 1.18 |
| NiC4.5 | 22.5 | 4.5 | 340 | 5 | 64.42 | 1.83 |
| NiC2 | 22.5 | 2 | 340 | 4.5 | 52.15 | 1.3 |
| NiC4.5 | 25 | 4.5 | 330 | 5 | 64.71 | 1.91 |
| NiC4.5 | 25 | 4.5 | 320 | 4.5 | 59.64 | 1.77 |
| NiC4.5 | 20 | 4.5 | 320 | 4.5 | 56.29 | 1.58 |
| NiC4.5 | 25 | 4.5 | 330 | 4 | 59.88 | 1.71 |
| NiC2 | 20 | 2 | 330 | 4.5 | 45.03 | 1.2 |
| NiC2 | 22.5 | 2 | 330 | 5 | 49.87 | 1.38 |
| NiC7 | 20 | 7 | 330 | 4.5 | 65.68 | 1.85 |
| NiC7 | 22.5 | 7 | 340 | 4.5 | 70.06 | 2.03 |
| NiC4.5 | 25 | 4.5 | 340 | 4.5 | 64.95 | 1.85 |
| NiC7 | 22.5 | 7 | 330 | 5 | 65.73 | 2.06 |
| NiC4.5 | 20 | 4.5 | 330 | 4 | 48.82 | 1.36 |
| NiC2 | 25 | 2 | 330 | 4.5 | 49.08 | 1.35 |
| NiC4.5 | 22.5 | 4.5 | 320 | 5 | 60.83 | 1.76 |
| NiC7 | 25 | 7 | 330 | 4.5 | 69.88 | 2.1 |
| NiC7 | 22.5 | 7 | 330 | 4 | 65.69 | 1.9 |
| NiC4.5 | 20 | 4.5 | 340 | 4.5 | 60.35 | 1.67 |
| NiC4.5 | 22.5 | 4.5 | 340 | 4 | 60.89 | 1.7 |
| NiC7 | 22.5 | 7 | 320 | 4.5 | 65.51 | 1.92 |
| NiC4.5 | 20 | 4.5 | 330 | 5 | 62.36 | 1.73 |
| NiC2 | 22.5 | 2 | 320 | 4.5 | 45.23 | 1.25 |
| NiC4.5 | 22.5 | 4.5 | 340 | 5 | 64.42 | 1.83 |
| NiC2 | 25 | 2 | 340 | 5 | 53.38 | 1.49 |
| NiC2 | 25 | 2 | 340 | 4 | 48.92 | 1.28 |
| NiC3 | 25 | 3 | 340 | 5 | 61.65 | 1.62 |
| NiC3 | 25 | 3 | 340 | 4 | 57.12 | 1.61 |
| NiC4 | 25 | 4 | 340 | 5 | 64.44 | 1.83 |
| NiC4 | 25 | 4 | 340 | 4 | 59.78 | 1.65 |
| NiC5 | 25 | 5 | 340 | 5 | 69.15 | 2.06 |
| NiC5 | 25 | 5 | 340 | 4 | 66.46 | 1.89 |
| NiC6 | 25 | 6 | 340 | 5 | 70.54 | 2.1 |
| NiC6 | 25 | 6 | 340 | 4 | 66.98 | 1.93 |
| NiC7 | 25 | 7 | 340 | 5 | 74.24 | 2.25 |
| NiC7 | 25 | 7 | 340 | 4 | 70.74 | 2.07 |
| NiC2 | 25 | 2 | 320 | 5 | 50.87 | 1.47 |
| NiC2 | 25 | 2 | 320 | 4 | 43.15 | 1.19 |
| NiC3 | 25 | 3 | 320 | 5 | 57.23 | 1.71 |
| NiC3 | 25 | 3 | 320 | 4 | 50.01 | 1.45 |
| NiC4 | 25 | 4 | 320 | 5 | 60.07 | 1.78 |
| NiC4 | 25 | 4 | 320 | 4 | 53.63 | 1.58 |
| NiC5 | 25 | 5 | 320 | 5 | 65.2 | 1.98 |
| NiC5 | 25 | 5 | 320 | 4 | 59.66 | 1.74 |
| NiC6 | 25 | 6 | 320 | 5 | 67.37 | 2.02 |
| NiC6 | 25 | 6 | 320 | 4 | 61.2 | 1.82 |
| NiC7 | 25 | 7 | 320 | 5 | 70.17 | 2.17 |
| NiC7 | 25 | 7 | 320 | 4 | 64.4 | 1.93 |
| Yield (% Mole) | ||||||
|---|---|---|---|---|---|---|
| Catalyst | n-C18 | n-C17 | n-C16 | n-C15 | n-C14–n-C8 | Others |
| NiC7 | 2.25 | 74.24 | 4.5 | 1.2 | 0.9 | 16.91 |
| Model 1 Regression Coefficients for the Yield of n-C17 and Its F-Ratio and p-Value Values. | |||
| Factor | Coefficient | F-Ratio | p-Value |
| constant | 60.31 | ||
| A:P | 2.4675 | 10.53 | 0.0070 |
| B:W | 9.74667 | 164.33 | 0.0001 |
| C:T | 1.74167 | 5.25 | 0.0409 |
| D:t | 1.99917 | 6.91 | 0.0220 |
| PP | −0.80875 | 0.50 | 0.4918 |
| PW | 0.0375 | 0.00 | 0.9778 |
| PT | 0.3125 | 0.06 | 0.8164 |
| Pt | −2.1775 | 2.73 | 0.1241 |
| WW | −3.125 | 7.51 | 0.0179 |
| WT | −0.5925 | 0.20 | 0.6608 |
| Wt | −1.4 | 1.13 | 0.3087 |
| TT | 1.45 | 1.62 | 0.2277 |
| Tt | 1.78 | 1.83 | 0.2014 |
| tt | −0.16125 | 0.02 | 0.8899 |
| Model 1 Yield of n-C17 (% mole) = 60.31 + 2.4675 × P + 9.74667 × W + 1.74167 × T + 1.99917 × t − 0.80875 × P2 + 0.0375 × P × W+ 0.3125 × P × T − 2.1775 × P × t − 3.125 × W2 − 0.5925 × W × T − 1.4 × W × t + 1.45 × T2 + 1.78 × T × t − 0.16125 × t2 | |||
| Model 2 Regression Coefficients for the Yield of n-C18 and Its F-Ratio and p-Value Values. | |||
| Factor | Coefficient | F-Ratio | p-Value |
| constant | 1.72 | ||
| A:P | 0.108333 | 30.46 | 0.0001 |
| B:W | 0.35 | 317.98 | 0.0000 |
| C:T | 0.0225 | 1.31 | 0.2740 |
| D:t | 0.0825 | 17.67 | 0.0012 |
| PP | −0.0258333 | 0.77 | 0.3975 |
| PW | 0.025 | 0.54 | 0.4762 |
| PT | −0.0025 | 0.01 | 0.9426 |
| Pt | −0.0425 | 1.56 | 0.2351 |
| WW | −0.0958333 | 10.60 | 0.0069 |
| WT | 0.015 | 0.19 | 0.6669 |
| Wt | −0.01 | 0.09 | 0.7737 |
| TT | 0.0254167 | 0.75 | 0.4049 |
| Tt | 0.05 | 2.16 | 0.1671 |
| tt | 0.00791667 | 0.07 | 0.7926 |
| Model 2 Yield of n-C18 = 1.72 + 0.108333 × P + 0.35 × W + 0.0225 × T + 0.0825 × t − 0.0258333 × P2 + 0.025 × P × W − 0.0025 × P × T − 0.0425 × P × t − 0.0958333 × W2 + 0.015 × W × T − 0.01 × W × t + 0.0254167 × T2 + 0.05 × T × t + 0.00791667 × t2 | |||
| Metric | Value |
|---|---|
| MAE | 2.9109908579710146 |
| MSE | 13.448980818453808 |
| MAPE | 5.1193371481436586% |
| R2 | 0.7880365513288001 |
| Metric | Value |
|---|---|
| MAE | 0.09526569939130435 |
| MSE | 0.014199636479194434 |
| MAPE | 6.0985543534315845% |
| R2 | 0.8153592131544917 |
| Metric | Value |
|---|---|
| MAE | 1.503128278681158 |
| MSE | 6.731590227466498 |
| MAPE | 5.6089457507876216% |
| R2 | 0.8016978822416458 |
| Tested Correlation | Pearson Coefficient | p-Value |
|---|---|---|
| n-C17 to n-C18 BB | 0.91473 | 4.8 × 10−137 |
| n-C18 to n-C18 BB | 0.912676 | 2.4 × 10−135 |
| n-C17 to n-C17 BB | 0.901432 | 9.3 × 10−127 |
| n-C18 to n-C17 BB | 0.860052 | 2.9 × 10−102 |
| wt% metal to n-C18 BB | 0.829173 | 1.19 × 10−88 |
| wt% metal to n-C17 BB | 0.80735 | 1.4 × 10−80 |
| Pressure (bar) to n-C18 BB | 0.388761 | 6.81 × 10−14 |
| Pressure (bar) to n-C17 BB | 0.315907 | 1.96 × 10−9 |
| Reaction time (h) to n-C18 BB | 0.247907 | 3.15 × 10−6 |
| Reaction time (h) to n-C17 BB | 0.214262 | 6.02 × 10−5 |
| Temperature to n-C17 BB | 0.198049 | 0.000214 |
| Temperature to n-C18 BB | 0.077803 | 0.149284 |
| Maximum Yield of n-C17 (% Mole) = 71.5468 | |
| Factor | Optimized Values |
| Pressure (bar) | 24.98 |
| wt% metal | 6.94 |
| Temperature (°C) | 340 |
| Reaction time (h) | 4.74 |
| Maximum Yield of n-C18 (% Mole) = 2.22304 | |
| Factor | Optimized Values |
| Pressure | 24.82 |
| wt% metal | 6.95 |
| Temperature | 340 |
| Reaction time | 5 |
| Metric | Value |
|---|---|
| MAE | 1.5393119812011713 |
| MSE | 3.7038989347370617 |
| MAPE | 2.7534556120058794% |
| R2 | 0.9416244842390435 |
| Metric | Value |
|---|---|
| MAE | 0.0775767906575963 |
| MSE | 0.009133386009309954 |
| MAPE | 4.936592052944989% |
| R2 | 0.8812367075879945 |
| Metric | Value |
|---|---|
| MAE | 0.8084443859293845 |
| MSE | 1.8565161603731877 |
| MAPE | 3.8450238324754346% |
| R2 | 0.911430595913519 |
| Tested Correlation | Pearson Coefficient | p-Value |
|---|---|---|
| n-C17 to n-C17 ANN27 | 0.973276 | 3 × 10−221 |
| n-C17 to n-C18 ANN27 | 0.962993 | 2.2 × 10−197 |
| n-C18 to n-C18 ANN27 | 0.941994 | 1.1 × 10−164 |
| n-C18 to n-C17 ANN27 | 0.921942 | 2.4 × 10−143 |
| wt% metal to n-C18 ANN27 | 0.816651 | 6.9 × 10−84 |
| wt% metal to n-C17 ANN27 | 0.807074 | 1.75 × 10−80 |
| Pressure (bar) to n-C18 ANN27 | 0.351109 | 1.91 × 10−11 |
| Temperature to n-C17 ANN27 | 0.321232 | 1.01 × 10−9 |
| Reaction time (h) to n-C17 ANN27 | 0.306582 | 6.08 × 10−9 |
| Reaction time (h) to n-C18 ANN27 | 0.302872 | 9.44 × 10−9 |
| Pressure (bar) to n-C17 ANN27 | 0.256717 | 1.35 × 10−6 |
| Temperature to n-C18 ANN27 | 0.172889 | 0.001264 |
| Maximum Yield of n-C17 (% Mole) = 72.2953 | |
| Factor | Optimized Values |
| Pressure (bar) | 25 |
| wt% metal | 7 |
| Temperature (°C) | 340 |
| Reaction time (h) | 5 |
| Maximum Yield of n-C18 (% Mole) = 2.1534 | |
| Factor | Optimized Values |
| Pressure | 25 |
| wt% metal | 7 |
| Temperature | 340 |
| Reaction time | 5 |
| Metric | Value |
|---|---|
| MAE | 0.6345773381772246 |
| MSE | 0.6185777595032578 |
| MAPE | 1.0887789453225924% |
| R2 | 0.9902508690475856 |
| Metric | Value |
|---|---|
| MAE | 0.046886737533237625 |
| MSE | 0.0036509875484390993 |
| MAPE | 2.9908108061085723% |
| R2 | 0.952525459740136 |
| Metric | Value |
|---|---|
| MAE | 0.34073203785523093 |
| MSE | 0.31111437352584853 |
| MAPE | 2.0397948757155816% |
| R2 | 0.9713881643938609 |
| Tested Correlation | Pearson Coefficient | p-Value |
|---|---|---|
| n-C17 to n-C17 ANN602020 | 0.995199804 | 0 |
| n-C18 to n-C18 ANN602020 | 0.976071019 | 2.2912 × 10−229 |
| n-C17 to n-C18 ANN602020 | 0.970654942 | 2.2636 × 10−214 |
| n-C18 to n-C17 ANN602020 | 0.952805659 | 1.1885 × 10−179 |
| wt% metal to n-C18 ANN602020 | 0.851587787 | 3.12006 × 10−98 |
| wt% metal to n-C17 ANN602020 | 0.84645346 | 6.65384 × 10−96 |
| Pressure (bar) to n-C18 ANN602020 | 0.364380744 | 2.84642 × 10−12 |
| Temperature to n-C17 ANN602020 | 0.27855838 | 1.44305 × 10−7 |
| Pressure (bar) to n-C17 ANN602020 | 0.276223795 | 1.85016 × 10−7 |
| Reaction time (h) to n-C17 ANN602020 | 0.269802864 | 3.62168 × 10−7 |
| Reaction time (h) to n-C18 ANN602020 | 0.22970418 | 1.64118 × 10−5 |
| Temperature to n-C18 ANN602020 | 0.152945617 | 0.004409192 |
| Maximum Yield of n-C17 (% Mole) = 73.8834 | |
| Factor | Optimized Values |
| Pressure (bar) | 25 |
| wt% metal | 7 |
| Temperature (°C) | 340 |
| Reaction time (h) | 5 |
| Maximum Yield of n-C18 (% Mole) = 2.2503 | |
| Factor | Optimized Values |
| Pressure | 25 |
| wt% metal | 7 |
| Temperature | 340 |
| Reaction time | 5 |
| Source of Variation | sum_sq | df | F | PR (>F) |
|---|---|---|---|---|
| C (Model) | 2.041302 | 2 | 252.6423 | 1.24 × 10−8 |
| Residual | 0.036359 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Olmos, L.A.; Sánchez-Cárdenas, M.; Trejo, F.; Montes Rivera, M.; Olvera-Gonzalez, E.; Hernández Guerrero, B.A. Biofuel Production in Oleic Acid Hydrodeoxygenation Utilizing a Ni/Tire Rubber Carbon Catalyst and Predicting of n-Alkanes with Box–Behnken and Artificial Neural Networks. Energies 2024, 17, 5717. https://doi.org/10.3390/en17225717
Sánchez-Olmos LA, Sánchez-Cárdenas M, Trejo F, Montes Rivera M, Olvera-Gonzalez E, Hernández Guerrero BA. Biofuel Production in Oleic Acid Hydrodeoxygenation Utilizing a Ni/Tire Rubber Carbon Catalyst and Predicting of n-Alkanes with Box–Behnken and Artificial Neural Networks. Energies. 2024; 17(22):5717. https://doi.org/10.3390/en17225717
Chicago/Turabian StyleSánchez-Olmos, Luis A., Manuel Sánchez-Cárdenas, Fernando Trejo, Martín Montes Rivera, Ernesto Olvera-Gonzalez, and Benito Alexis Hernández Guerrero. 2024. "Biofuel Production in Oleic Acid Hydrodeoxygenation Utilizing a Ni/Tire Rubber Carbon Catalyst and Predicting of n-Alkanes with Box–Behnken and Artificial Neural Networks" Energies 17, no. 22: 5717. https://doi.org/10.3390/en17225717
APA StyleSánchez-Olmos, L. A., Sánchez-Cárdenas, M., Trejo, F., Montes Rivera, M., Olvera-Gonzalez, E., & Hernández Guerrero, B. A. (2024). Biofuel Production in Oleic Acid Hydrodeoxygenation Utilizing a Ni/Tire Rubber Carbon Catalyst and Predicting of n-Alkanes with Box–Behnken and Artificial Neural Networks. Energies, 17(22), 5717. https://doi.org/10.3390/en17225717







