High-Energy-Density Lithium–Sulfur Battery Based on a Lithium Polysulfide Catholyte and Carbon Nanofiber Cathode
Abstract
1. Introduction
2. Materials and Methods
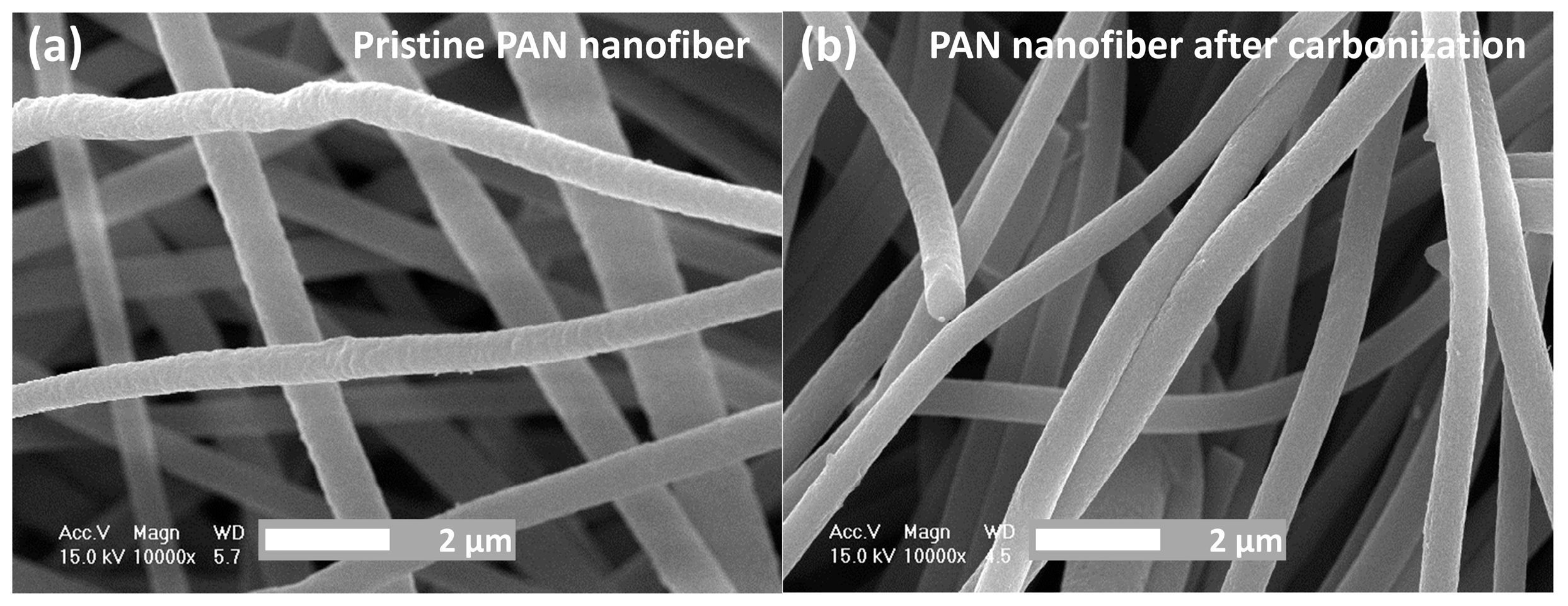
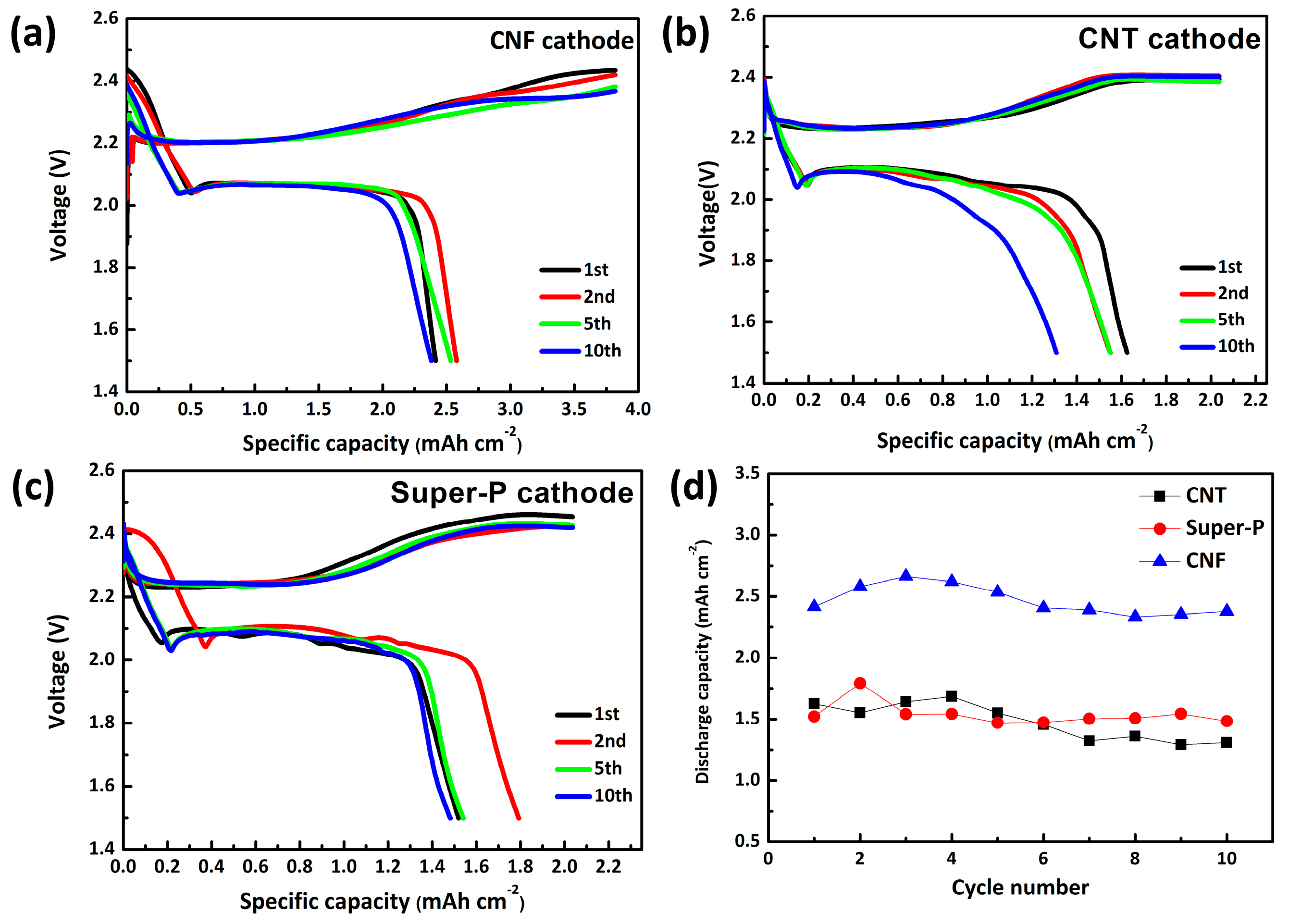
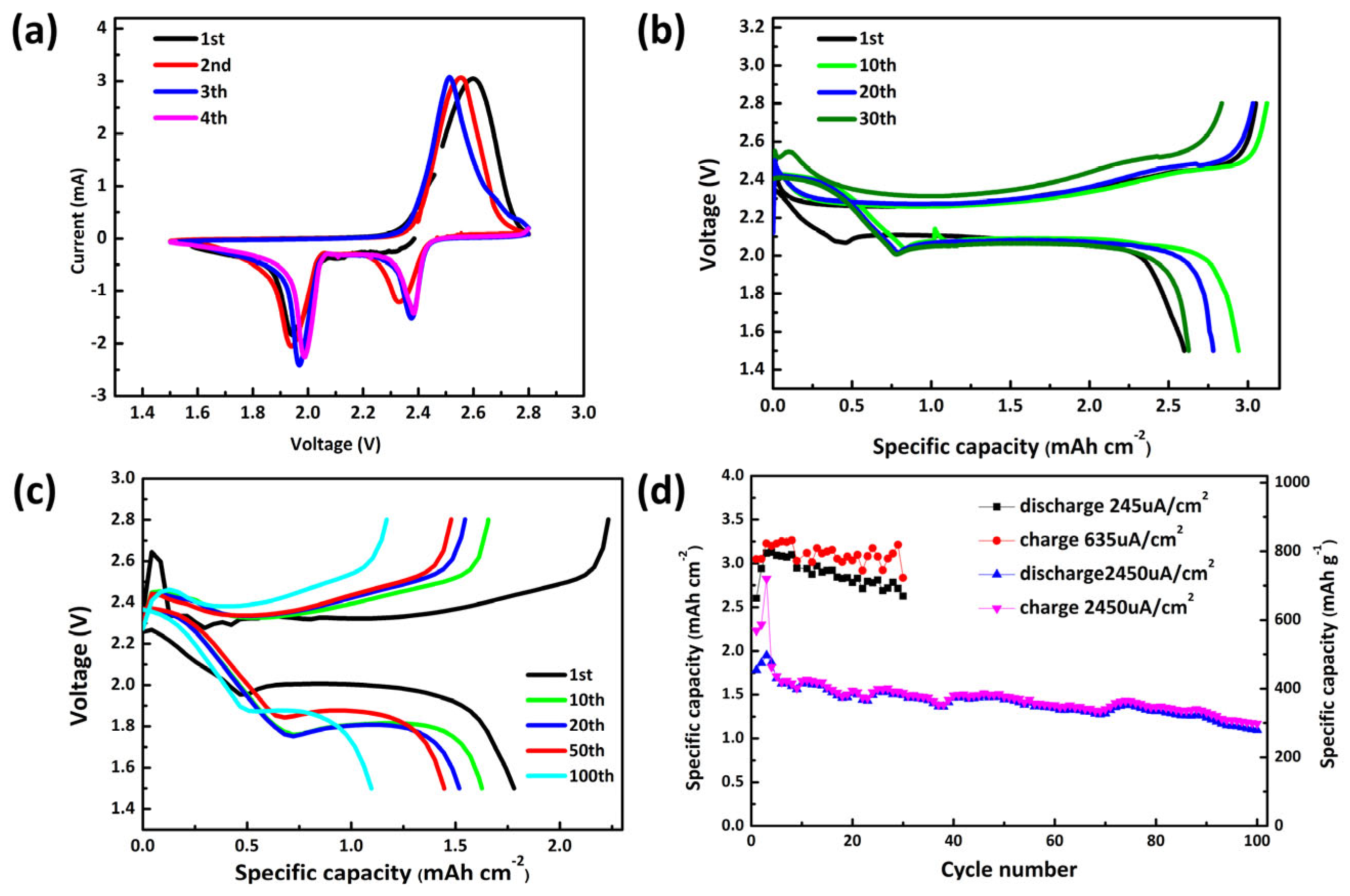
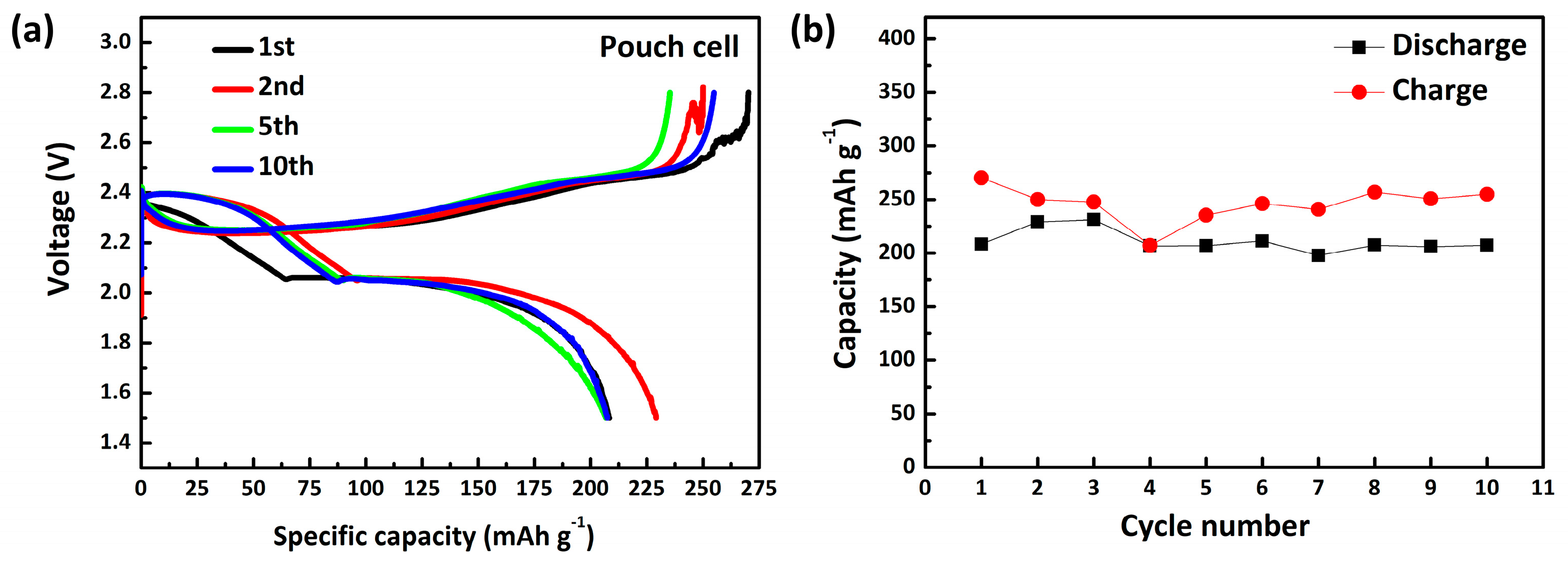
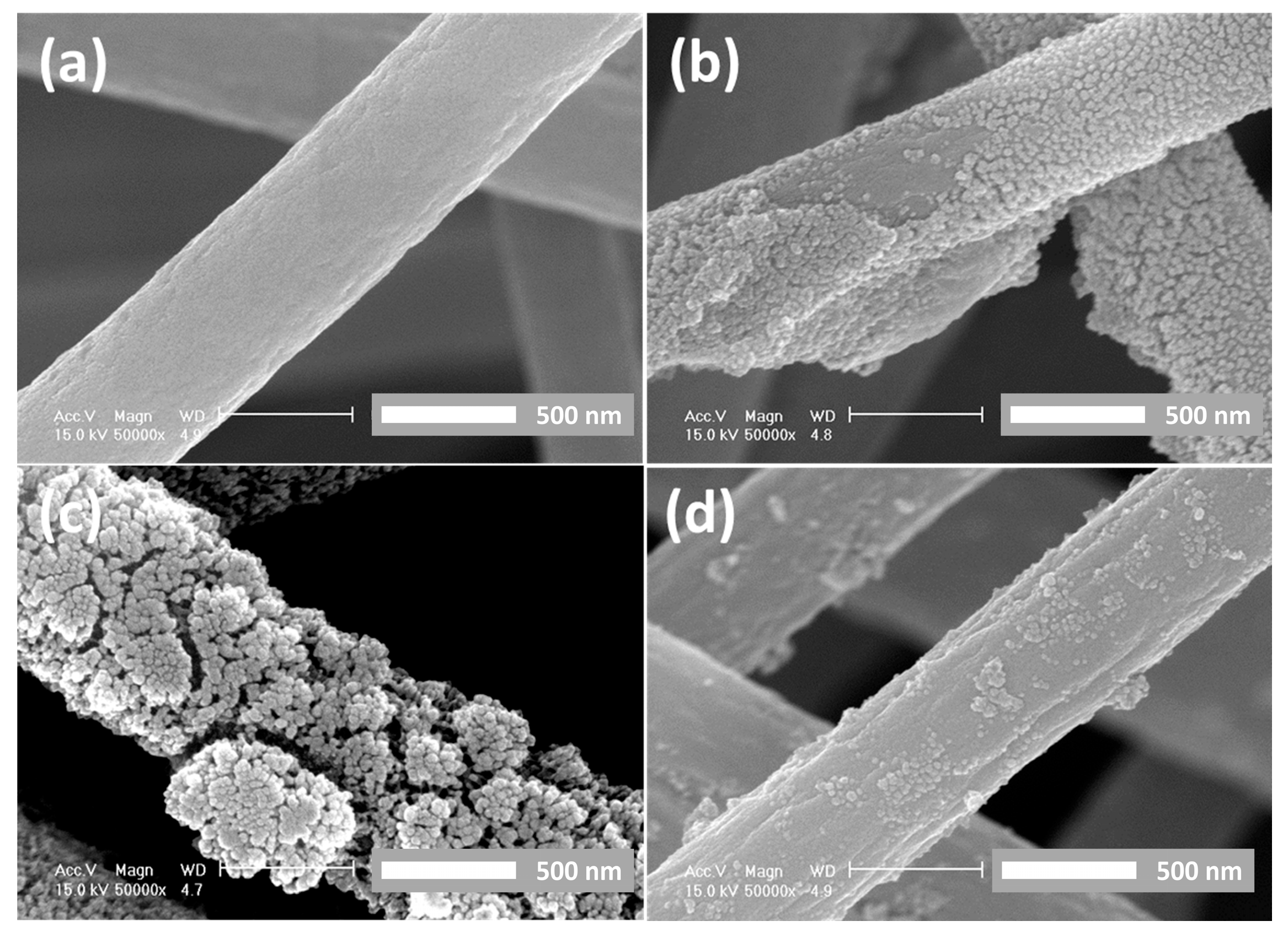
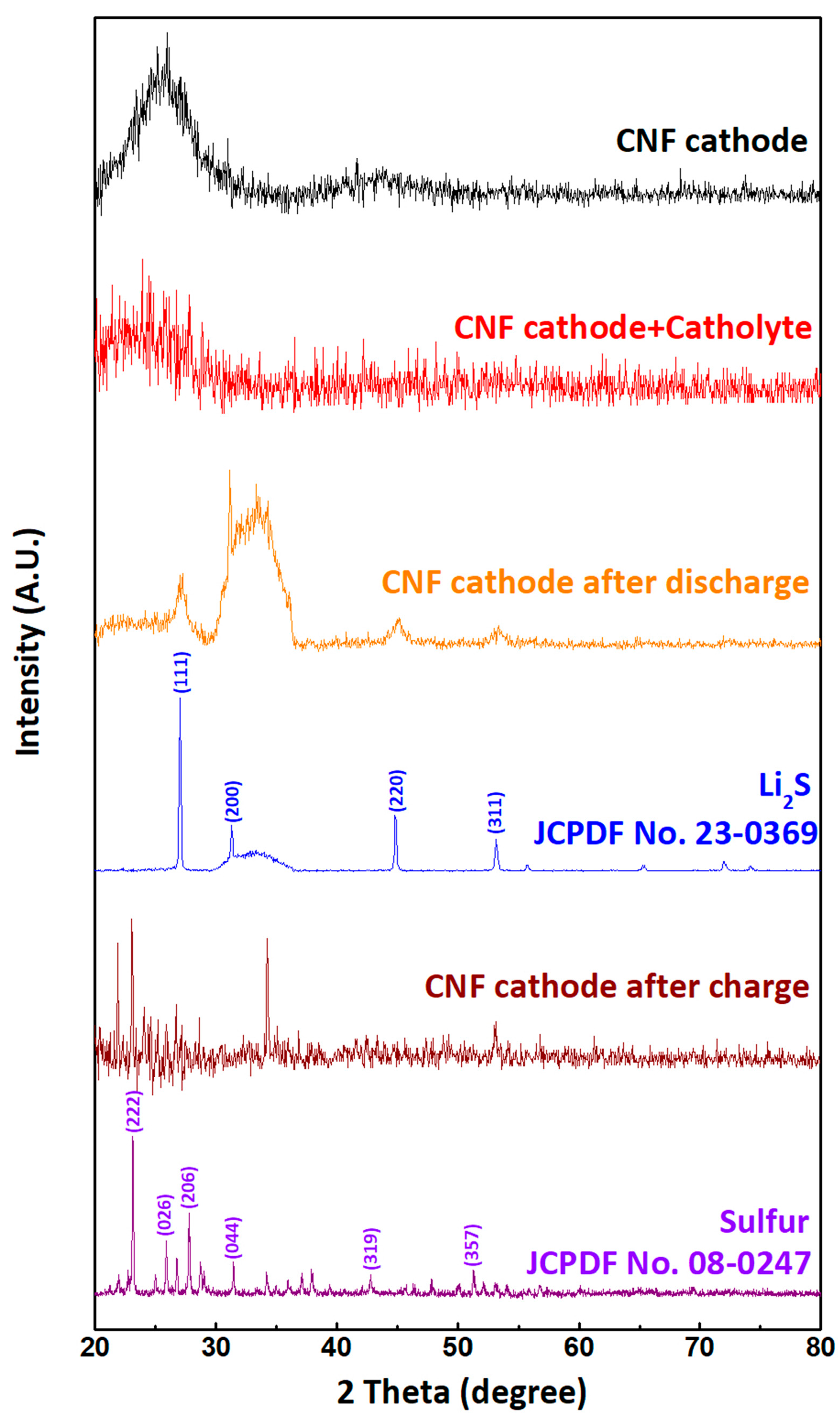
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.-M. Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Wang, H.; Qi, L.; Osaka, T. Electrochemical characteristics of layered LiNi1/3Co1/3Mn1/3O2 and with different synthesis conditions. J. Power Sources 2006, 160, 627–632. [Google Scholar] [CrossRef]
- Gaberscek, M.; Dominko, R.; Jamnik, J. Is small particle size more important than carbon coating? An example study on LiFePO4 cathodes. Electrochem. Commun. 2007, 9, 2778–2783. [Google Scholar] [CrossRef]
- Villevieille, C.; Novák, P. A metastable β-sulfur phase stabilized at room temperature during cycling of high efficiency carbon fibre–sulfur composites for Li–S batteries. J. Mater. Chem. A 2013, 1, 13089–13092. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Y.; Yan, T.; Gao, X.-P. Perspectives of High-Performance Li–S Battery Electrolytes. Adv. Funct. Mater. 2024, 34, 2309625. [Google Scholar] [CrossRef]
- Kolosnitsyn, V.S.; Karaseva, E. V Lithium-sulfur batteries: Problems and solutions. Russ. J. Electrochem. 2008, 44, 506–509. [Google Scholar] [CrossRef]
- Mikhaylik, Y.V.; Akridge, J.R. Polysulfide Shuttle Study in the Li/S Battery System. J. Electrochem. Soc. 2004, 151, A1969. [Google Scholar] [CrossRef]
- Wang, T.; He, J.; Zhu, Z.; Cheng, X.-B.; Zhu, J.; Lu, B.; Wu, Y. Heterostructures Regulating Lithium Polysulfides for Advanced Lithium-Sulfur Batteries. Adv. Mater. 2023, 35, 2303520. [Google Scholar] [CrossRef]
- Miao, L.-X.; Wang, W.-K.; Wang, A.-B.; Yuan, K.-G.; Yang, Y.-S. A high sulfur content composite with core–shell structure as cathode material for Li–S batteries. J. Mater. Chem. A 2013, 1, 11659–11664. [Google Scholar] [CrossRef]
- Raza, H.; Bai, S.; Cheng, J.; Majumder, S.; Zhu, H.; Liu, Q.; Zheng, G.; Li, X.; Chen, G. Li-S Batteries: Challenges, Achievements and Opportunities. Electrochem. Energy Rev. 2023, 6, 29. [Google Scholar] [CrossRef]
- Guo, J.; Xu, Y.; Wang, C. Sulfur-Impregnated Disordered Carbon Nanotubes Cathode for Lithium–Sulfur Batteries. Nano Lett. 2011, 11, 4288–4294. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Rao, M.; Aloni, S.; Wang, L.; Cairns, E.J.; Zhang, Y. Porous carbon nanofiber–sulfur composite electrodes for lithium/sulfur cells. Energy Environ. Sci. 2011, 4, 5053–5059. [Google Scholar] [CrossRef]
- Chen, H.; Dong, W.; Ge, J.; Wang, C.; Wu, X.; Lu, W.; Chen, L. Ultrafine Sulfur Nanoparticles in Conducting Polymer Shell as Cathode Materials for High Performance Lithium/Sulfur Batteries. Sci. Rep. 2013, 3, 1910. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, G.; Cha, J.J.; Wu, H.; Vosgueritchian, M.; Yao, Y.; Bao, Z.; Cui, Y. Improving the Performance of Lithium–Sulfur Batteries by Conductive Polymer Coating. ACS Nano 2011, 5, 9187–9193. [Google Scholar] [CrossRef]
- Zhao, F.; Xue, J.; Shao, W.; Yu, H.; Huang, W.; Xiao, J. Toward high-sulfur-content, high-performance lithium-sulfur batteries: Review of materials and technologies. J. Energy Chem. 2023, 80, 625–657. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, G.; Cui, Y. A membrane-free lithium/polysulfide semi-liquid battery for large-scale energy storage. Energy Environ. Sci. 2013, 6, 1552–1558. [Google Scholar] [CrossRef]
- Zhang, S.S.; Read, J.A. A new direction for the performance improvement of rechargeable lithium/sulfur batteries. J. Power Sources 2012, 200, 77–82. [Google Scholar] [CrossRef]
- Chen, S.; Dai, F.; Gordin, M.L.; Wang, D. Exceptional electrochemical performance of rechargeable Li–S batteries with a polysulfide-containing electrolyte. RSC Adv. 2013, 3, 3540–3543. [Google Scholar] [CrossRef]
- Agostini, M.; Xiong, S.; Matic, A.; Hassoun, J. Polysulfide-containing Glyme-based Electrolytes for Lithium Sulfur Battery. Chem. Mater. 2015, 27, 4604–4611. [Google Scholar] [CrossRef]
- Jin, P.; Li, L.; Gu, X.; Hu, Y.; Zhang, X.; Lin, X.; Ma, X.; He, X. S-doped porous carbon fibers with superior electrode behaviors in lithium ion batteries and fuel cells. Mater. Rep. Energy 2022, 2, 100160. [Google Scholar] [CrossRef]
- Wei, L.; Ji, D.; Zhao, F.; Tian, X.; Guo, Y.; Yan, J. A Review of Carbon Nanofiber Materials for Dendrite-Free Lithium-Metal Anodes. Molecules 2024, 29, 4096. [Google Scholar] [CrossRef] [PubMed]
- Fitzer, E.; Frohs, W.; Heine, M. Optimization of stabilization and carbonization treatment of PAN fibres and structural characterization of the resulting carbon fibres. Carbon 1986, 24, 387–395. [Google Scholar] [CrossRef]
- Ko, T.-H. The influence of pyrolysis on physical properties and microstructure of modified PAN fibers during carbonization. J. Appl. Polym. Sci. 1991, 43, 589–600. [Google Scholar] [CrossRef]
- Zhang, S.S.; Tran, D.T. A proof-of-concept lithium/sulfur liquid battery with exceptionally high capacity density. J. Power Sources 2012, 211, 169–172. [Google Scholar] [CrossRef]
- Zhang, S.S. New insight into liquid electrolyte of rechargeable lithium/sulfur battery. Electrochim. Acta 2013, 97, 226–230. [Google Scholar] [CrossRef]
- Miles, M.H.; McManis, G.E.; Fletcher, A.N. Reactions Involving Silver Ions and Silver Metal in Molten Nitrates. J. Electrochem. Soc. 1984, 131, 2075. [Google Scholar] [CrossRef]
- Zhang, L.; Ling, M.; Feng, J.; Mai, L.; Liu, G.; Guo, J. The synergetic interaction between LiNO3 and lithium polysulfides for suppressing shuttle effect of lithium-sulfur batteries. Energy Storage Mater. 2018, 11, 24–29. [Google Scholar] [CrossRef]
- Mao, Y.; Li, T.; Abuelgasim, S.; Hao, X.; Xiao, Y.; Li, C.; Wang, W.; Li, Y.; Bao, E. Systematic insight of the behavior of LiNO3 additive in LiS batteries with gradient S loading. J. Energy Storage 2024, 79, 110215. [Google Scholar] [CrossRef]
- Ji, X.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon–sulphur cathode for lithium–sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Yermukhambetova, A.; Bakenov, Z.; Chen, P. Ternary sulfur/polyacrylonitrile/Mg0.6Ni0.4O composite cathodes for high performance lithium/sulfur batteries. J. Mater. Chem. A 2013, 1, 295–301. [Google Scholar] [CrossRef]
- Fu, Y.; Su, Y.-S.; Manthiram, A. Highly Reversible Lithium/Dissolved Polysulfide Batteries with Carbon Nanotube Electrodes. Angew. Chemie Int. Ed. 2013, 52, 6930–6935. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, B.; Yoon, B.; Ahn, S.; Jang, J.; Lim, D.; Seo, I. High-Energy-Density Lithium–Sulfur Battery Based on a Lithium Polysulfide Catholyte and Carbon Nanofiber Cathode. Energies 2024, 17, 5258. https://doi.org/10.3390/en17215258
Oh B, Yoon B, Ahn S, Jang J, Lim D, Seo I. High-Energy-Density Lithium–Sulfur Battery Based on a Lithium Polysulfide Catholyte and Carbon Nanofiber Cathode. Energies. 2024; 17(21):5258. https://doi.org/10.3390/en17215258
Chicago/Turabian StyleOh, Byeonghun, Baeksang Yoon, Suhyeon Ahn, Jumsuk Jang, Duhyun Lim, and Inseok Seo. 2024. "High-Energy-Density Lithium–Sulfur Battery Based on a Lithium Polysulfide Catholyte and Carbon Nanofiber Cathode" Energies 17, no. 21: 5258. https://doi.org/10.3390/en17215258
APA StyleOh, B., Yoon, B., Ahn, S., Jang, J., Lim, D., & Seo, I. (2024). High-Energy-Density Lithium–Sulfur Battery Based on a Lithium Polysulfide Catholyte and Carbon Nanofiber Cathode. Energies, 17(21), 5258. https://doi.org/10.3390/en17215258






