Detection of Impedance Inhomogeneity in Lithium-Ion Battery Packs Based on Local Outlier Factor
Abstract
1. Introduction
2. Methodology
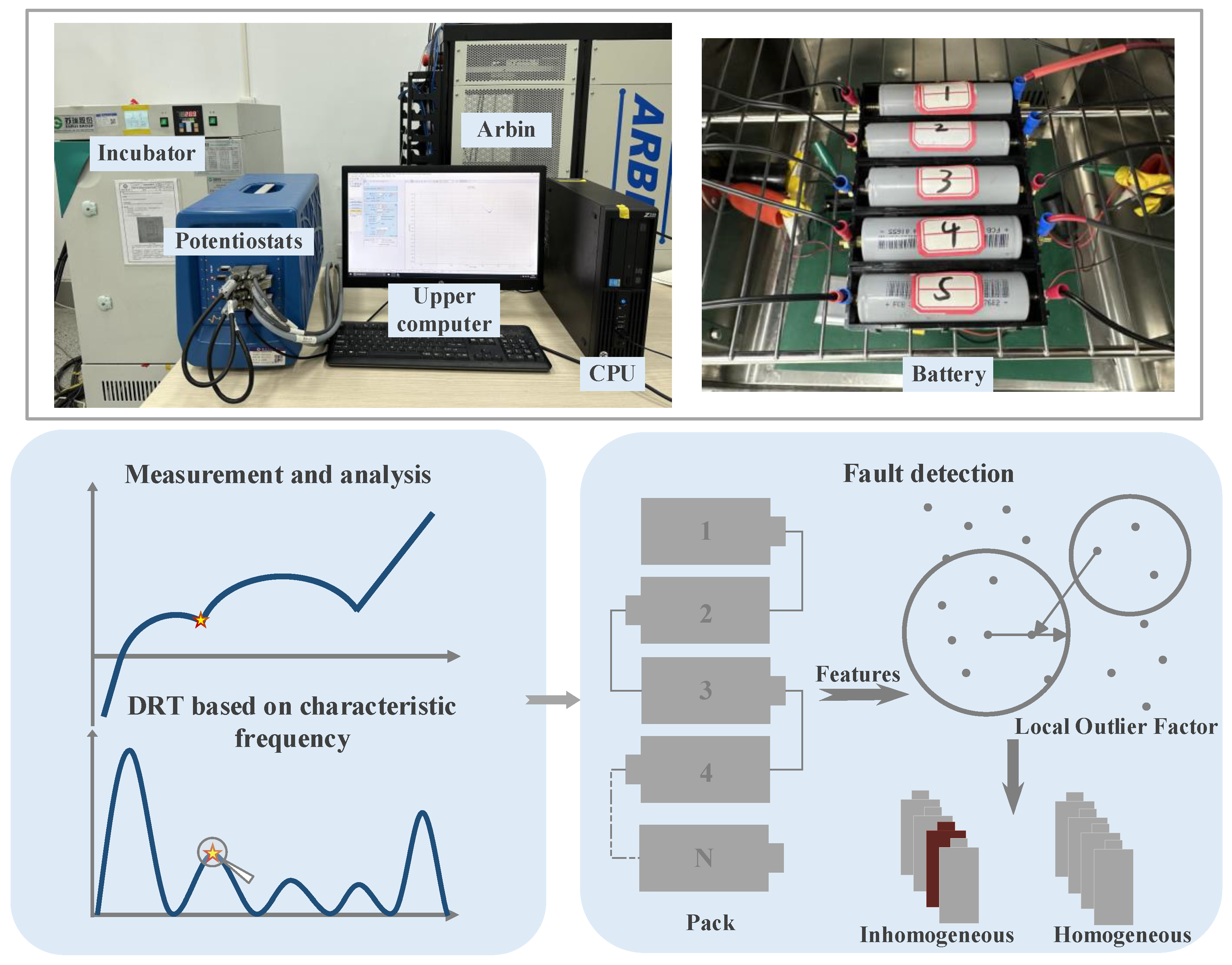
2.1. Battery Specification and Consistency Check
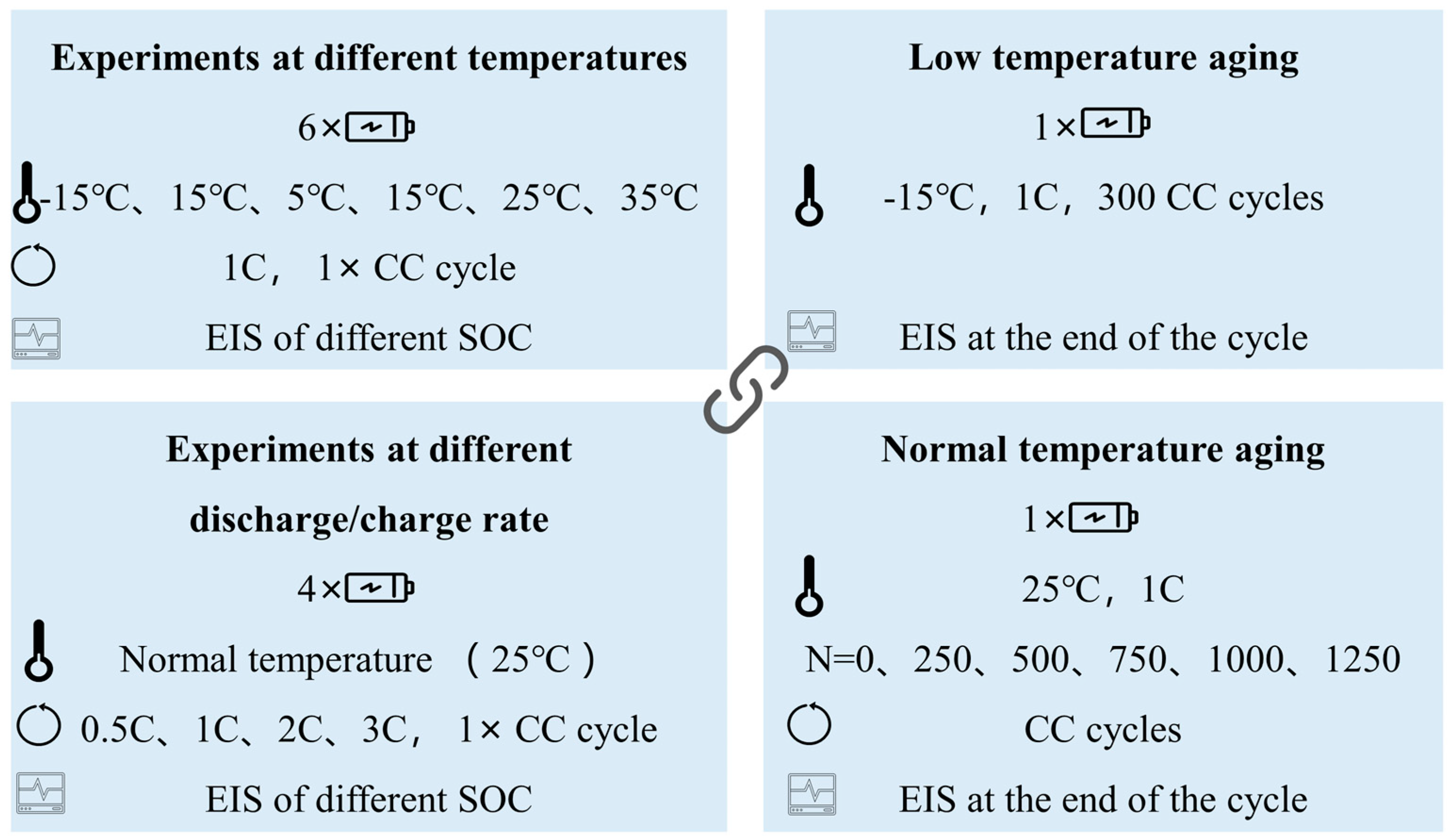
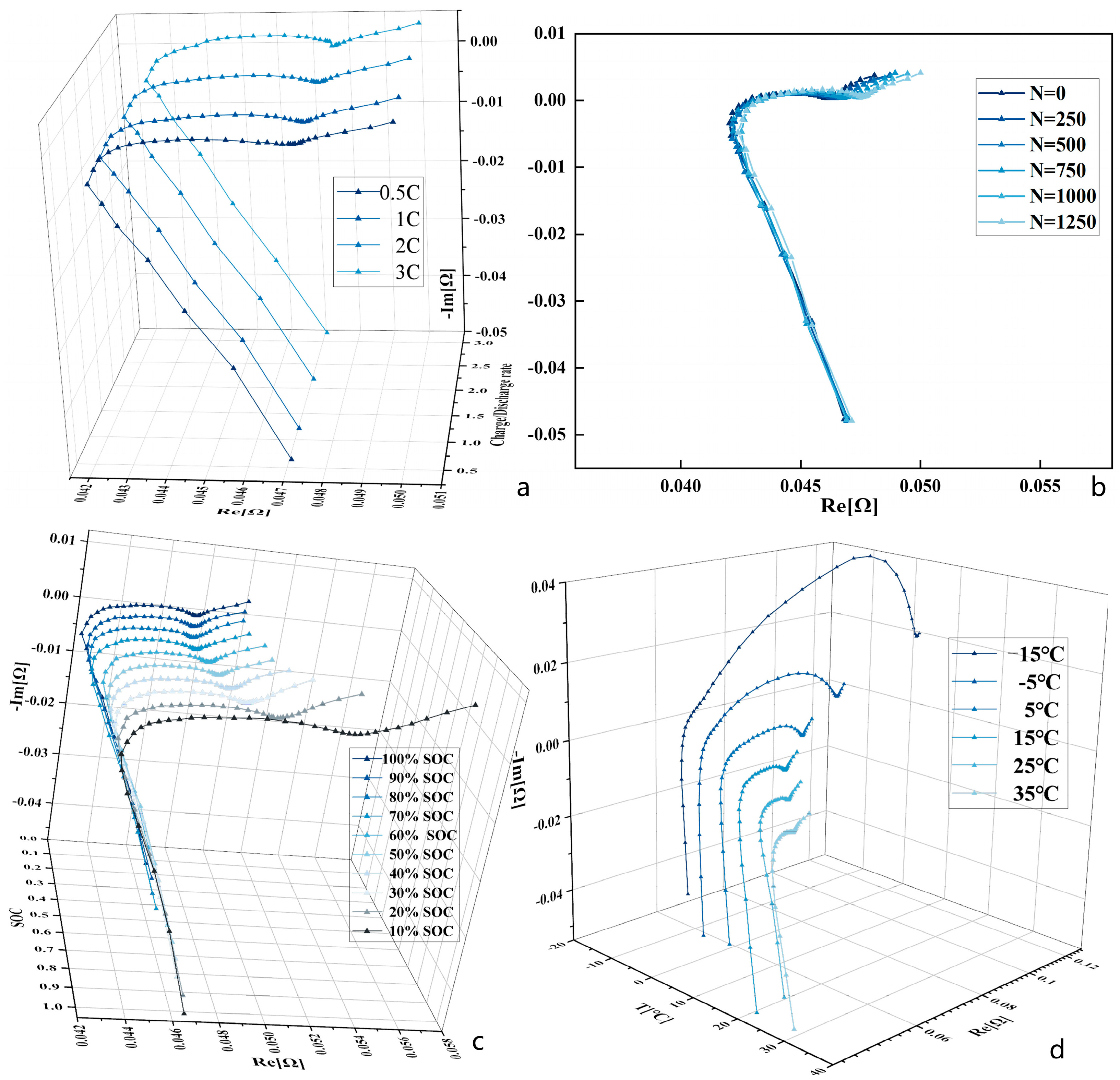
2.2. EIS Testing under Different Conditions
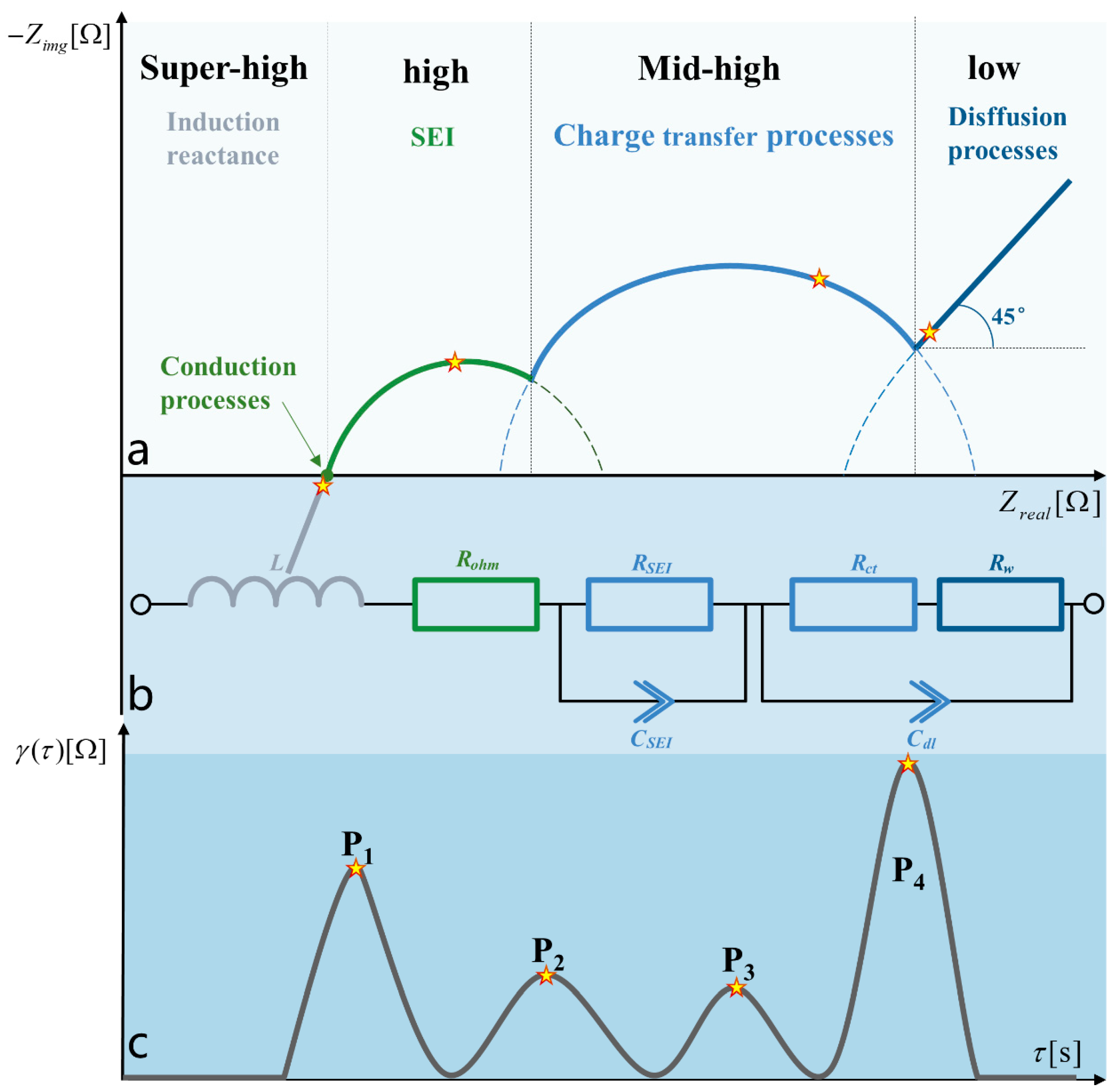
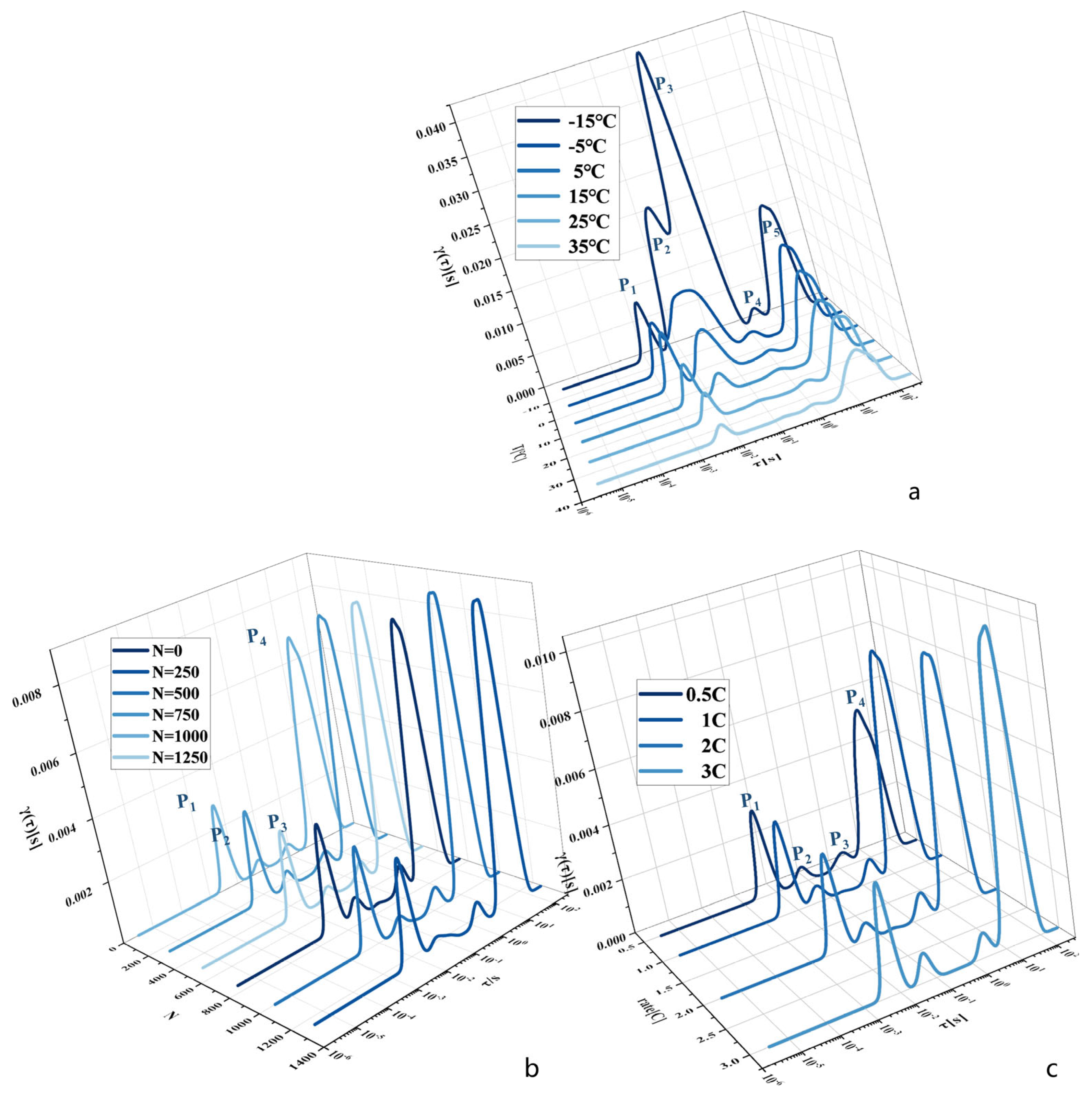
2.3. Principles of the Electrochemical Impedance Spectroscopy Relaxation Time Distribution Method
3. Analysis of Experimental Results and Extraction of Eigenfrequencies
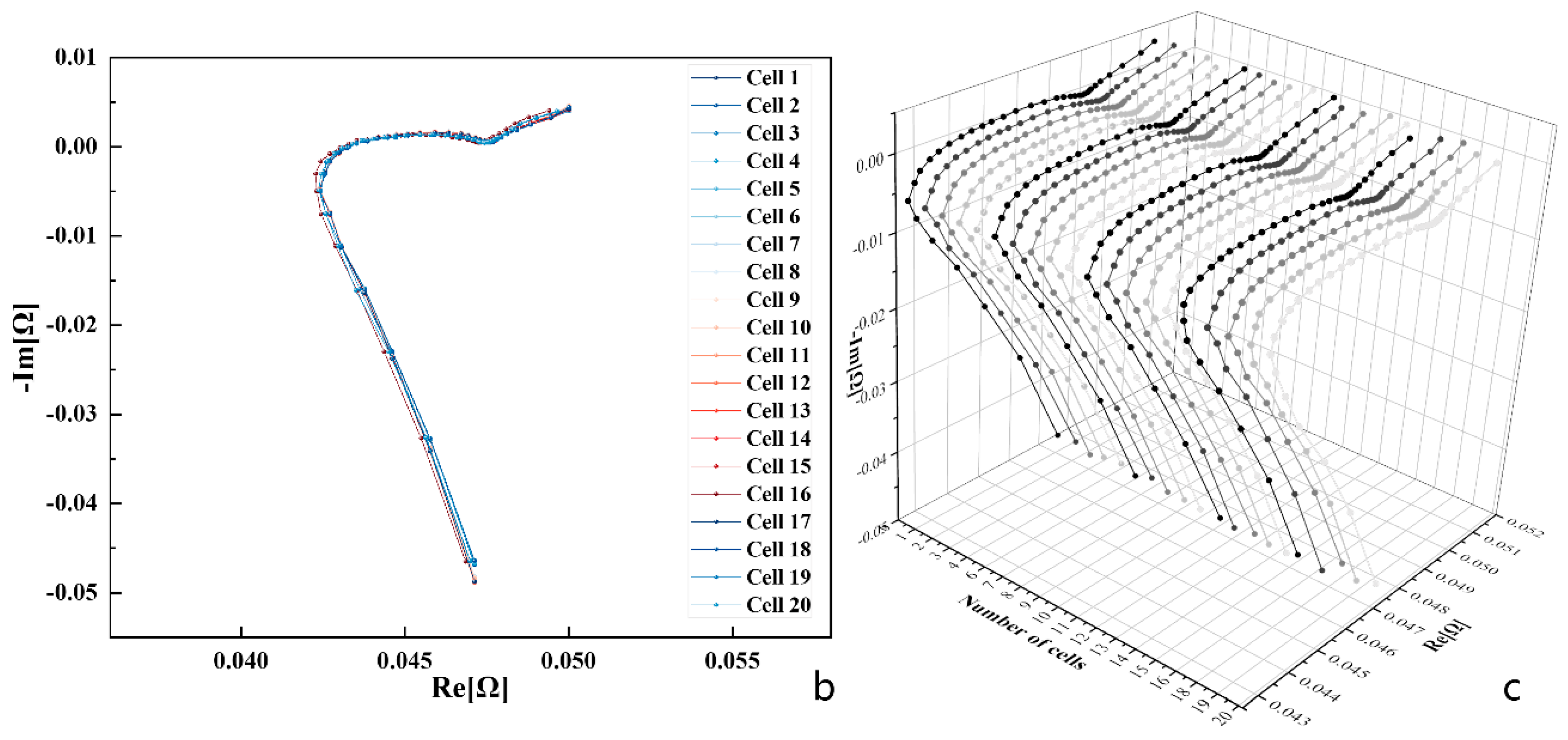
3.1. Results of EIS Tests
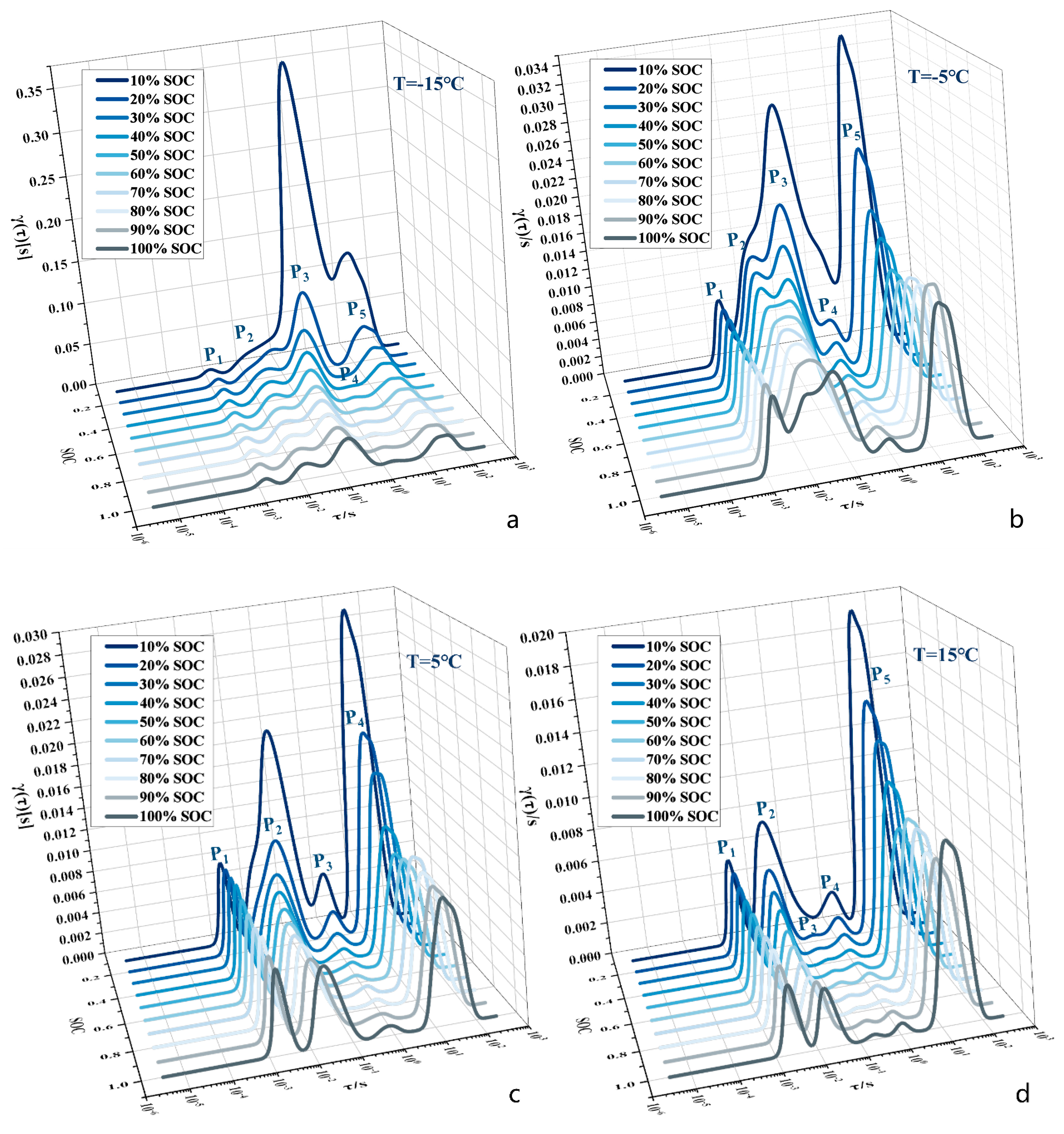
3.2. Eigenfrequency Selection under Multipolarisation Process
4. Inhomogeneity Detection Experiment Based on LOF Outlier Algorithm
4.1. Principle of LOF Outlier Algorithm
- ①
- : Euclidean distance from data point di to dj.
- ②
- Distance: : The distance from data point di to other data points in the dataset and are sorted from smallest to largest, and the distance from di to the k data point.
- ③
- Distance from Neighbourhood: : The dataset includes data points that are within a certain distance, , from a reference point, di.
- ④
- Reachable Distance: : The maximum distance k between the data point di and the Euclidean distance from di to dr. The equation is expressed as follows:
- ⑤
- Local Reachability Density: : The inverse of the k distance of the data point di from the average reachable distance of all data to di in the neighbourhood. is calculated. The equation is expressed as follows:
- ⑥
- Local Outlier Factor: : The average ratio of the local attainable densities of all data points in the neighbourhood to the local attainable density of di at distance k from the data point di should be calculated. The equation is expressed as follows:
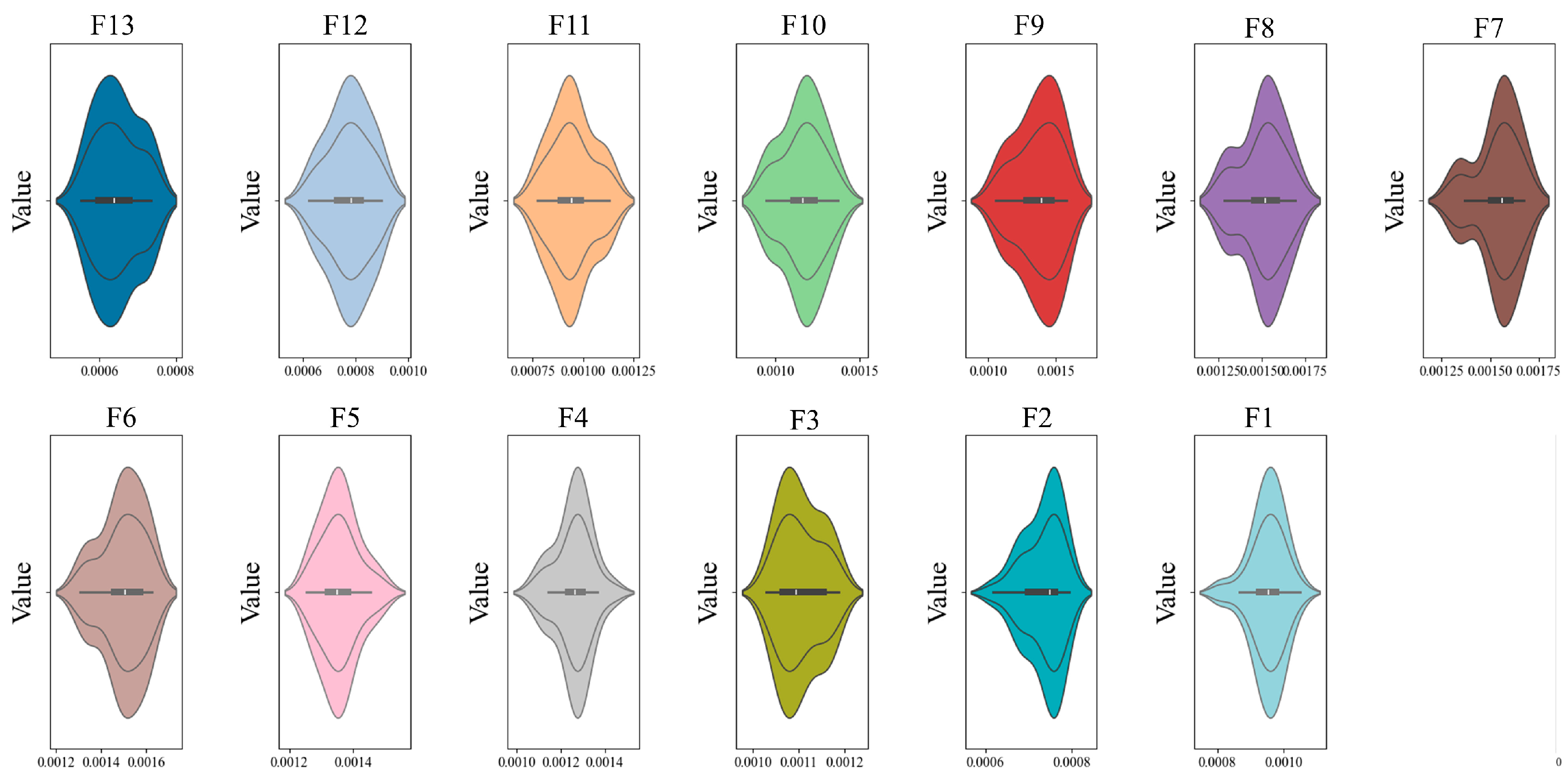
4.2. Experiment on Battery Packs and Extraction of Eigenfrequencies
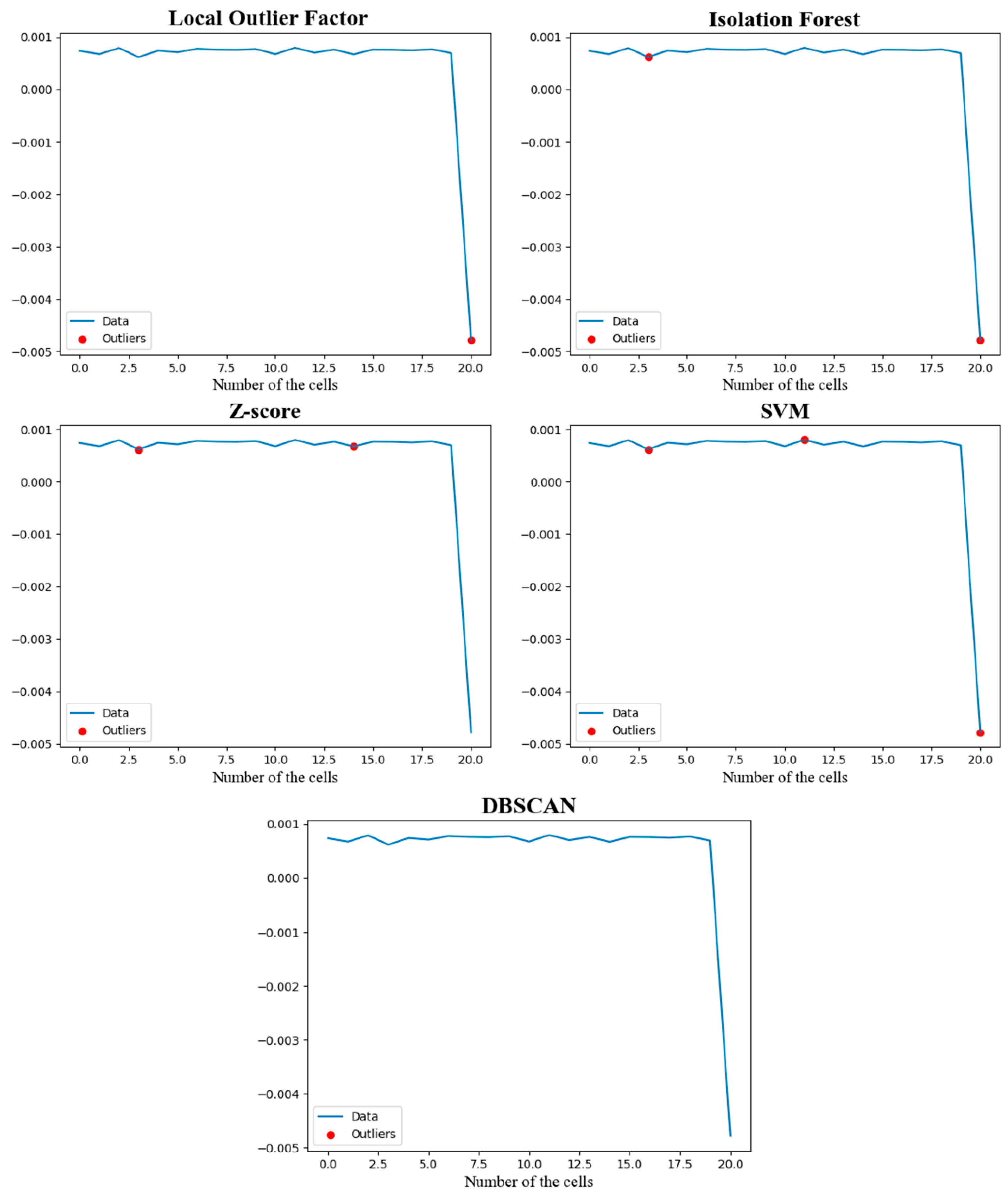
4.3. Experimental Results and Comparison of Battery Pack Algorithm Effectiveness
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, X.; Xu, L.; Lin, X.; Pecht, M. Battery lifetime prognostics. Joule 2020, 4, 310–346. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, Y.; Jiang, J.; Cheng, G.; Diao, W.; Zhang, W. Study on battery pack consistency evolutions and equilibrium diagnosis for serial-connected Lithium-ion battery. J. Appl. Energy 2017, 207, 510–519. [Google Scholar] [CrossRef]
- Ren, D.; Feng, X.; Liu, L.; Hsu, H.; Lu, L.; Wang, L.; Ouyang, M. Investigating the relationship between internal short circuit and thermal runaway of Lithium-ion battery under thermal abuse condition. Energy Storage Mater. 2021, 34, 563–573. [Google Scholar] [CrossRef]
- Jones, P.K.; Stimming, U.; Lee, A.A. Impedance-based forecasting of Lithium-ion battery performance amid uneven usage. Nat. Commun. 2022, 13, 4806. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhao, C.Z.; Huang, J.Q.; Zhang, Q. The timescale identification decoupling complicated kinetic processes in Lithium-ion battery. Joule 2022, 6, 1172–1198. [Google Scholar] [CrossRef]
- Rüther, T.; Plank, C.; Schamel, M.; Danzer, M.A. Detection of inhomogeneities in serially connected Lithium-ion battery. Appl. Energy 2023, 332, 0306–2619. [Google Scholar] [CrossRef]
- Tian, J.; Wang, Y.; Liu, C.; Chen, Z. Consistency evaluation and cluster analysis for Lithium-ion battery pack in electric vehicles. Energy 2018, 194, 116944. [Google Scholar] [CrossRef]
- Feng, X.; Pan, Y.; He, X.; Wang, L.; Ouyang, M. Detecting the internal short circuit in large-format Lithium-ion battery using model-based fault-diagnosis algorithm. J. Energy Storage 2018, 18, 26–39. [Google Scholar] [CrossRef]
- Cong, X.; Zhang, C.; Jiang, J.; Zhang, W.; Jiang, Y.; Zhang, L. A comprehensive signal-based fault diagnosis method for Lithium-ion battery in electric vehicles. Energies 2021, 14, 1221. [Google Scholar] [CrossRef]
- Yao, L.; Xiao, Y.; Gong, X.; Hou, J.; Chen, X. A novel intelligent method for fault diagnosis of electric vehicle battery system based on wavelet neural network. J. Power Sources 2020, 453, 227870. [Google Scholar] [CrossRef]
- Lin, D.; Li, Z.; Feng, Y.; Wang, X.; Li, S. Research on inconsistency identification of Lithium-ion battery pack based on operational data. In Proceedings of the 2021 4th IEEE International Conference on Industrial Cyber-Physical Systems (ICPS), Victoria, BC, Canada, 10–12 May 2021; pp. 498–502. [Google Scholar]
- Kong, X.; Zheng, Y.; Ouyang, M.; Lu, L.; Li, J.; Zhang, Z. Fault diagnosis and quantitative analysis of micro-short circuits for Lithium-ion battery in battery packs. J. Power Sources 2018, 395, 358–368. [Google Scholar] [CrossRef]
- Lin, T.; Chen, Z.; Zhou, S. Voltage-correlation based multi-fault diagnosis of Lithium-ion battery packs considering inconsistency. J. Clean. Prod. 2022, 336, 130358. [Google Scholar] [CrossRef]
- Hong, J.; Wang, Z.; Chen, W.; Wang, L. Multi-fault synergistic diagnosis of battery systems based on the modified multi-scale entropy. Int. J. Energy Res. 2019, 42, 8350–8369. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Q.; Zhang, Y.; Wang, J.; Stimming, U.; Lee, A.A. Identifying degradation patterns of Lithium-ion battery from impedance spectroscopy using machine learning. Nat. Commun. 2020, 11, 1706. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xie, J. Failure Study of Commercial LiFePO4 Cells in Overcharge Conditions Using Electrochemical Impedance Spectroscopy. J. Electrochem. Soc. 2015, 162, 10. [Google Scholar] [CrossRef]
- Gantenbein, S.; Weiss, M.; Ivers-Tiffée, E. Impedance based time-domain modeling of lithium-ion batteries: Part I. J. Power Sources 2018, 379, 317–327. [Google Scholar] [CrossRef]
- Zhu, D.; Ma, T.; Yang, Y. Optimization and application of the distribution of relaxation times based on characteristic frequency resolution and hyperparameters. J. Power Sources 2022, 545, 231955. [Google Scholar] [CrossRef]
- Zhang, L. Study on State Estimation of Ternary Lithium-Ion Battery Based on Electrochemical Impedance Spectroscopy. Master’s Thesis, Jilin University, Changchun, China, 2018. [Google Scholar]
- Wang, J.; Huang, Q.; Li, W.; Wang, J.; Zhuang, Q.-C.; Zhang, J.-J. Fundamentals of distribution of relaxation times for electrochemical impedance spectroscopy. J. Electrochem. 2020, 26, 607–627. [Google Scholar]
- Zhou, X.; Pan, Z.; Han, X.; Lu, L.; Ouyang, M. An easy-to-implement multi-point impedance technique for monitoring aging of Lithium-ion battery. J. Power Sources 2019, 417, 188–192. [Google Scholar] [CrossRef]
- Iurilli, P.; Brivio, C.; Wood, V. On the use of electrochemical impedance spectroscopy to characterize and model the aging phenomena of Lithium-ion battery: A critical review. J. Power Sources 2021, 505, 229860. [Google Scholar] [CrossRef]
- Li, X.; Ahmadi, M.; Collins, L.; Kalinin, S.V. Deconvolving distribution of relaxation times, resistances and inductance from electrochemical impedance spectroscopy via statistical model selection: Exploiting structural-sparsity regularization and data-driven parameter tuning. Electrochim. Acta 2019, 313, 570–583. [Google Scholar] [CrossRef]
- Babaeiyazdi, I.; Rezaei-Zare, A.; Shokrzadeh, S. State of charge prediction of EV Lithium-ion battery using EIS: A machine learning approach. J. Energy 2021, 223, 120116. [Google Scholar] [CrossRef]
- Ivers-Tiff’ee, E.; Weber, A. Evaluation of electrochemical impedance spectra by the distribution of relaxation times. J. Ceram. Soc. Jpn. 2017, 125, 193–201. [Google Scholar] [CrossRef]
- Wan, T.H.; Saccoccio, M.; Chen, C.; Ciucci, F. Influence of the Discretization Methods on the Distribution of Relaxation Times Deconvolution: Implementing Radial Basis Functions with DRTtools. J. Electrochim. Acta 2015, 184, 483–499. [Google Scholar] [CrossRef]
- Gavrilyuk, A.L.; Osinkin, D.A.; Bronin, D.I. On a variation of the Tikhonov regularization method for calculating the distribution function of relaxation times in impedance spectroscopy. J. Electrochim. Acta 2020, 354, 136683. [Google Scholar] [CrossRef]
- Tagayi, R.K.; Ezahedi, S.E.; Kim, J.; Kim, J. Employment of relaxation times distribution with improved elastic net regularization for advanced impedance data analysis of a Lithium-ion battery. J. Energy Storage 2023, 70, 107970. [Google Scholar] [CrossRef]
- Effat, M.B.; Ciucci, F. Bayesian and hierarchical Bayesian based regularization for deconvolving the distribution of relaxation times from electrochemical impedance spectroscopy data. J. Electrochim. Acta 2017, 247, 1117–1129. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.; Liu, M.; Huang, T.; Yu, A. Detection of Lithium-ion plating in Lithium-ion battery by distribution of relaxation times. J. Power Sources 2021, 496, 229867. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, J.; Pan, Z.; Ouyang, M. Impedance characterization of Lithium-ion battery aging under high-temperature cycling: Importance of electrolyte-phase diffusion. J. Power Sources 2019, 426, 216–222. [Google Scholar] [CrossRef]
- Mertens, A.; Granwehr, J. Two-dimensional impedance data analysis by the distribution of relaxation times. J. Energy Storage 2017, 13, 401–408. [Google Scholar] [CrossRef]
- Leng, F.; Wei, Z.; Ming, C.; Yazami, R. Hierarchical degradation processes in Lithium-ion battery during ageing. J. Electrochim. Acta 2017, 256, 52–62. [Google Scholar] [CrossRef]
- Ko, C.-J.; Chen, K.-C. Constructing battery impedance spectroscopy using partial current in constant-voltage charging or partial relaxation voltage. Appl. Energy 2024, 356, 122454. [Google Scholar] [CrossRef]
- Fan, W.; Xu, G.; Yu, B. On-line estimation method for internal temperature of Lithium-ion battery based on electrochemical impedance spectroscopy. J. Proc. CSEE 2021, 41, 3283–3293. [Google Scholar]
- Carthy, K.M.; Gullapalli, H.; Ryan, K.M.; Kennedy, T. Electrochemical impedance correlation analysis for the estimation of Lithium-ion battery state of charge, state of health and internal temperature. J. Energy Storage 2022, 50, 104608. [Google Scholar] [CrossRef]
- Risse, S.; Cañas, N.A.; Wagner, N.; Härk, E.; Ballauff, M.; Friedrich, K.A. Correlation of capacity fading processes and electrochemical impedance spectra in Lithium-ion/sulfur cells. J. Power Sources 2016, 323, 107–114. [Google Scholar] [CrossRef]
- Beelen, H.P.G.J.; Raijmakers, L.H.J.; Donkers, M.C.F.; Notten, P.H.L.; Bergveld, H.J. A comparison and accuracy analysis of impedance-based temperature estimation methods for Lithium-ion battery. J. Appl. Energy 2016, 175, 128–140. [Google Scholar] [CrossRef]
- Li, X.; Xu, J.; Hu, Z. The health parameter estimation method for LiFePO4 battery echelon use. J. Trans. China Electrotech. Soc. 2018, 33, 9–16. [Google Scholar]
- Ren, D.; Feng, X.; Lu, L.; He, X.; Ouyang, M. Overcharge behaviors and failure mechanism of Lithium-ion battery under different test conditions. J. Appl. Energy 2019, 250, 323–332. [Google Scholar] [CrossRef]
- Galeotti, M.; Cinà, L.; Giammanco, C.; Di Carlo, A.; Santoni, F.; De Angelis, A.; Moschitta, A.; Carbone, P. LiPo battery dataset: Capacity, electrochemical impedance spectra, and fit of equivalent circuit model at various states-of-charge and states-of-health. J. Data Brief 2023, 50, 215–223. [Google Scholar] [CrossRef]
- Schmidt, J.P.; Berg, P.; Schönleber, M.; Weber, A.; Ivers-Tiffée, E. The distribution of relaxation times as basis for generalizedtime-domain models for Lithium-ion battery. J. Power Sources 2013, 221, 70–77. [Google Scholar] [CrossRef]
- Manikandan, B.; Ramar, V.; Yap, C.; Balaya, P. Investigation of physico-chemical processes in Lithium-ion battery by deconvolution of electrochemical impedance spectra. J. Power Sources 2017, 361, 300–309. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, K.; Wei, J.; Dong, G. Voltage fault detection for Lithium-ion battery pack using local outlier factor. J. Meas. 2019, 146, 544–556. [Google Scholar] [CrossRef]
- Zhao, S.; Luo, L.; Wang, B. An improved outlier delection algorithm K-LOF based on density. Comput. Perform. Commun. Syst. 2017, 2, 1–7. [Google Scholar]
- Hu, G. Research on Lithium-Ion Evolution Characteristics and Safety of Lithium-Ion Battery Based on AC Impedance Characteristics; Hunan University: Changsha, China, 2023. [Google Scholar]
- Mei, L.; Zhang, F.; Gao, Q. Overview of outlier detection technology. J. Appl. Res. Comput. 2020, 37, 3521–3527. [Google Scholar]


| Item | General Parameter |
|---|---|
| Minimum Capacity | 2400 mAh |
| Rated Capacity | 2500 mAh |
| Nominal voltage | 3.6 V |
| Lower cut-off voltage | 2.5 V |
| Upper cut-off voltage | 4.2 ± 0.03 V |
| Charging current | 2.5 A |
| Number of the Cell | LOF | Number of the Cell | LOF |
|---|---|---|---|
| 1 | 0.9999999603104642 | 11 | 1.0005747349187861 |
| 2 | 1.000577847674403 | 12 | 0.9996478433621423 |
| 3 | 0.9994948657361418 | 13 | 1.0003190130016608 |
| 4 | 1.001113965094464 | 14 | 0.9997786215631451 |
| 5 | 0.9999541066596755 | 15 | 1.0006076330331992 |
| 6 | 1.0002361489721572 | 16 | 0.9997693879356649 |
| 7 | 0.9996234787060005 | 17 | 0.9998019338422555 |
| 8 | 0.9997658478546321 | 18 | 0.9999107279827717 |
| 9 | 0.9998175547351235 | 19 | 0.9997069900363661 |
| 10 | 0.9996647441359714 | 20 | 0.9994457946193019 |
| Type of Algorithm | FAR/% | MAR/% | Detection Time/ms |
|---|---|---|---|
| Isolation Forest | 1/20 | 0/1 | 59.15600000298582 |
| Z-score | 2/20 | 1 | 0.19610000890679657 |
| SVM | 2/20 | 0/1 | 0.5737000028602779 |
| LOF | 0/20 | 0/1 | 0.1517999917268753 |
| DBSCAN | 1 | 1 | 0.7514000026276335 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Wang, J.; Wang, Y.; Pan, B.; Wang, L. Detection of Impedance Inhomogeneity in Lithium-Ion Battery Packs Based on Local Outlier Factor. Energies 2024, 17, 5123. https://doi.org/10.3390/en17205123
Zhu L, Wang J, Wang Y, Pan B, Wang L. Detection of Impedance Inhomogeneity in Lithium-Ion Battery Packs Based on Local Outlier Factor. Energies. 2024; 17(20):5123. https://doi.org/10.3390/en17205123
Chicago/Turabian StyleZhu, Lijun, Jian Wang, Yutao Wang, Bin Pan, and Lujun Wang. 2024. "Detection of Impedance Inhomogeneity in Lithium-Ion Battery Packs Based on Local Outlier Factor" Energies 17, no. 20: 5123. https://doi.org/10.3390/en17205123
APA StyleZhu, L., Wang, J., Wang, Y., Pan, B., & Wang, L. (2024). Detection of Impedance Inhomogeneity in Lithium-Ion Battery Packs Based on Local Outlier Factor. Energies, 17(20), 5123. https://doi.org/10.3390/en17205123




