A Pore Scale Study on Heat Transfer Characteristics of Integrated Thermal Protection Structures with Phase Change Material
Abstract
1. Introduction
2. Models and Materials
2.1. Experimental Models
2.2. Simulation Models
2.3. Material Properties Parameters
3. Methods and Verifications
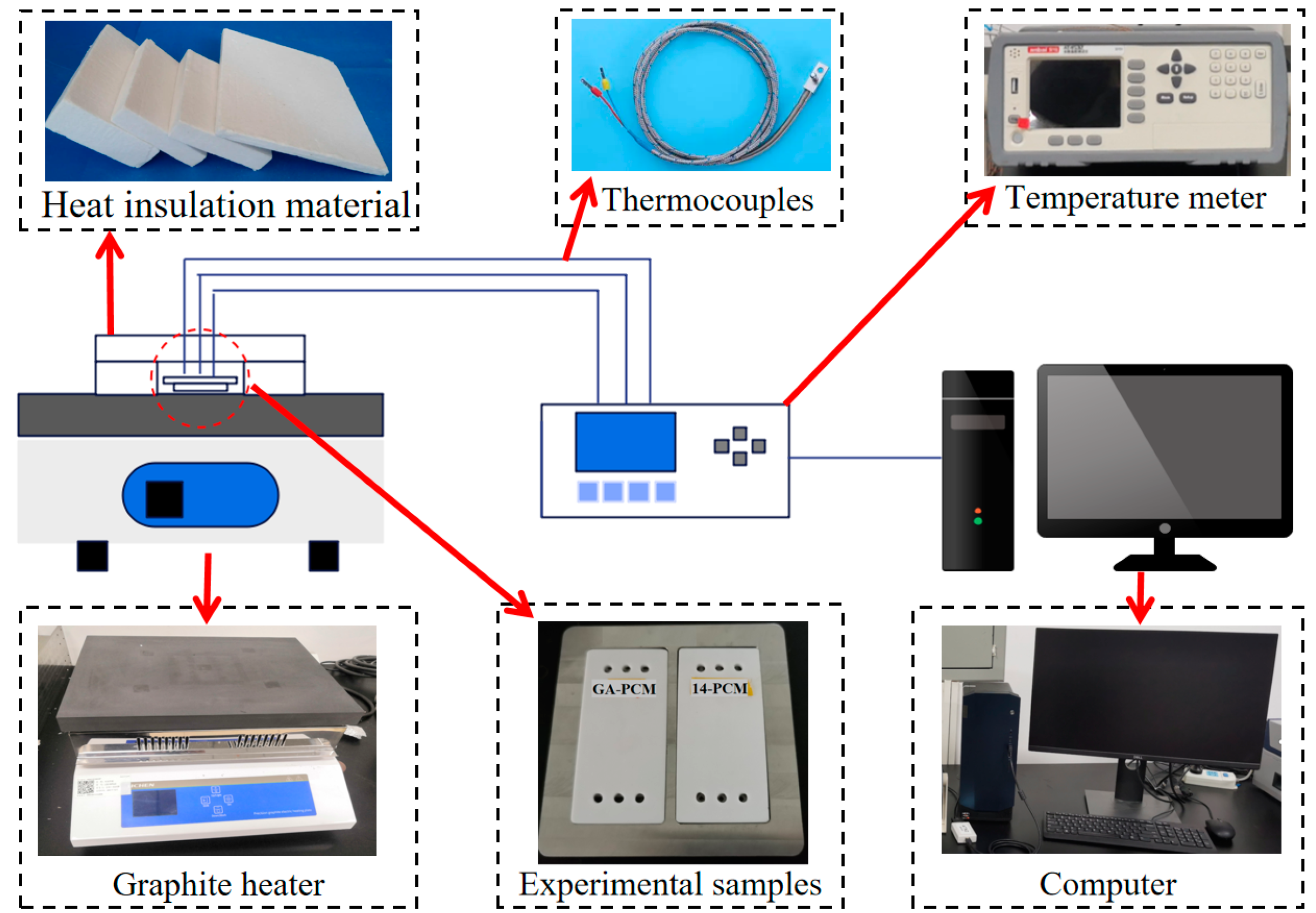
3.1. Experimental Methods
- (1)
- The 3D-printed alumina ceramic components were combined with the metallic base to form the ITPS experimental specimens.
- (2)
- The paraffin and the ITPS specimens were heated to 353.15 K to completely liquefy paraffin.
- (3)
- The liquefied paraffin was poured into the porous lattice units through pre-designed holes, and the system was allowed to thermally equilibrate for a certain period to ensure complete filling of the porous lattice.
- (4)
- The paraffin was cooled and solidified at room temperature (298.15 K), resulting in the formation of ITPS specimens containing PCMs.
- (1)
- First, heat the temperature of the graphite heating plate to 353.15 K.
- (2)
- Then, wrap the experimental sample with thermal insulation cotton around its surroundings. And place a thermocouple at the center point on the surface of the inner plate.
- (3)
- Next, place the experimental sample on a graphite heating platform at a temperature of 353.15 K. The temperature data are transmitted to the temperature tester through the thermocouple.
- (4)
- Finally, the collected temperature data was processed and analyzed using a computer.
3.2. Numerical Methods
- (1)
- The molten liquid paraffin is considered as an incompressible Newtonian fluid, and its flow within the enclosed space is assumed to be laminar.
- (2)
- Except for density differences caused by the Boussinesq assumption, all other thermophysical properties of the material are assumed to be constant.
- (3)
- Thermal radiation within the PCMs is neglected.
- (4)
- The distribution of paraffin and aluminum oxide is assumed to be uniform and isotropic.
3.3. Method Verification
3.3.1. Numerical Method Verification
3.3.2. Mesh Independence Analysis
4. Results and Discussion
4.1. Heat Transfer Characteristics of ITPS
4.2. Evolution of Solid–Liquid Interface
4.3. Effects of Structure
4.4. Effects of Pore Size
5. Conclusions
- (1)
- PCMs can effectively enhance the thermal insulation capability of ITPS. The addition of paraffin can decrease the average temperature of the inner plate of ITPS by approximately 15%.
- (2)
- Porous lattices can significantly improve the heat storage efficiency of PCMs. The incorporation of porous lattices can increase the melting rate of paraffin wax by around 50%.
- (3)
- The geometrical characteristics of the porous lattices have a significant effect on the melting rate of PCMs, and the heat storage efficiency of PCMs can be accurately adjusted by modifying these geometrical characteristics. Among different lattice structures, a larger specific surface area results in a faster melting rate of PCMs. Regarding different pore sizes, a smaller pore size leads to a faster melting rate when thermal conduction dominates heat transfer. However, a larger pore size results in a faster melting rate when natural convection dominates the heat transfer.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Specific surface area (mm−1). | |
| Pore size (mm). | |
| Specific heat (J/(kg·K)). | |
| Source term used in the energy equation (W/m3). | |
| Acceleration due to gravity (m/s2). | |
| Specific enthalpy (kJ/kg). | |
| Released latent heat (kJ/kg). | |
| Sensible enthalpy (J/(kg·K)). | |
| Reference sensible enthalpy (J/(kg·K)). | |
| Latent heat (kJ/kg). | |
| Characteristic length (mm). | |
| Pressure (Pa). | |
| Rayleigh number. | |
| Source term used in the momentum equation (N/m3). | |
| Temperature (K). | |
| Constant temperature (K). | |
| Liquidus temperature (K). | |
| Solidus temperature (K). | |
| Reference temperature (K). | |
| Temperature difference (K). | |
| Time (s). | |
| Velocity (m/s). | |
| Thermal expansion coefficient (K−1). | |
| Liquid fraction. | |
| Porosity. | |
| Thermal diffusivity (m2/s). | |
| Thermal conductivity (W/(m·K)). | |
| Kinematic viscosity (m2/s). | |
| Density (kg/m3). |
References
- Yuan, Y.P.; Xiang, B.; Cao, X.L.; Zhang, N.; Sun, L.L. Research status and development on latent energy storage technology of building. J. Southwest Jiaotong Univ. 2016, 51, 585–598. [Google Scholar]
- Jiang, Z.; Zou, B.Y.; Cong, L.; Xie, C.; Li, C.; Qiao, G.; Zhao, Y.; Nie, B.; Zhang, T.; Ge, Z.; et al. Research progress and outlook of thermal energy storage technologies. Energy Storage Sci. Technol. 2022, 11, 2746–2771. [Google Scholar]
- Li, Z.; Li, B.R.; Chen, H.Z.; Wen, B.; Du, X.Z. State of the art review on phase change thermal energy storage technology. Chem. Ind. Eng. Prog. 2020, 39, 5066–5085. [Google Scholar]
- Sun, W.C.; Feng, J.X.; Zhang, Z.G.; Fang, X.M. Research progress of phase change heat storage technology for passive energy conservation in buildings. Chem. Ind. Eng. Prog. 2020, 39, 1824–1834. [Google Scholar]
- Shang, B.; Hu, J.; Hu, R.; Cheng, J.; Luo, X. Modularized thermal storage unit of metal foam/paraffin composite. Int. J. Heat Mass Transf. 2018, 125, 596–603. [Google Scholar] [CrossRef]
- Verdier, D.; Falcoz, Q.; Ferriere, A. Design of a protection thermal energy storage using phase change material coupled to a solar receiver. High Temp. Mater. Process. 2014, 33, 509–523. [Google Scholar] [CrossRef]
- Cao, C.Y.; Wang, R.X.; Xing, X.D.; Song, H.W.; Huang, C.G. Design and experimental investigation of integrated thermal protection structure containing phase change material layer. J. Astronaut. 2019, 40, 352–361. [Google Scholar]
- Du, Y.X.; Xiao, G.M.; Gui, Y.W.; He, L.X.; Liu, L. Heat transfer characteristics inside phase change thermal control device. J. Chem. Ind. Eng. 2012, 63, 107–113. [Google Scholar]
- Zhao, C.Y.; Zhang, G.H. Review on microencapsulated phase change materials (MEPCMs): Fabrication, characterization and applications. Renew. Sustain. Energy Rev. 2011, 15, 3813–3832. [Google Scholar] [CrossRef]
- Zhang, N.; Yuan, Y. Synthesis and thermal properties of nanoencapsulation of paraffin as phase change material for latent heat thermal energy storage. Energy Built Environ. 2020, 1, 410–416. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, J.; Wang, X.; Yang, R.; Lin, K. Influence of additives on thermal conductivity of shape-stabilized phase change material. Sol. Energy Mater. Sol. Cells 2006, 90, 1692–1702. [Google Scholar] [CrossRef]
- Motahar, S.; Alemrajabi, A.A.; Khodabandeh, R. Experimental study on solidification process of a phase change material containing TiO2 nanoparticles for thermal energy storage. Energy Convers. Manag. 2017, 138, 162–170. [Google Scholar] [CrossRef]
- Agyenim, F.; Eames, P.; Smyth, M. A comparison of heat transfer enhancement in a medium temperature thermal energy storage heat exchanger using fins. Sol. Energy 2009, 83, 1509–1520. [Google Scholar] [CrossRef]
- Yang, X.; Lu, Z.; Bai, Q.; Zhang, Q.; Jin, L.; Yan, J. Thermal performance of a shell-and-tube latent heat thermal energy storage unit: Role of annular fins. Appl. Energy 2017, 202, 558–570. [Google Scholar] [CrossRef]
- Meng, Z.N.; Zhang, P. Experimental and numerical investigation of a tube-in-tank latent thermal energy storage unit using composite PCM. Appl. Energy 2017, 190, 524–539. [Google Scholar] [CrossRef]
- Zhu, F.; Zhang, C.; Gong, X. Numerical analysis and comparison of the thermal performance enhancement methods for metal foam/phase change material composite. Appl. Therm. Eng. 2016, 109, 373–383. [Google Scholar] [CrossRef]
- Xiao, G.M.; Du, Y.X.; Liu, L.; Wei, D.; Yang, X.F.; Gui, Y.W. Numerical simulation and experimental study on the thermal characteristics of composite phase change materials for heat storage. J. Exp. Fluid Mech. 2018, 32, 6. [Google Scholar]
- Zhang, N.; Liu, H.; Xia, Q.; Zhang, Z.; Tong, Z.; Du, Y.; Yuan, Y. Experimental investigation of the effect of copper foam pore structure on the thermal performance of phase change material in different centrifugal force fields. Int. J. Heat Mass Transf. 2023, 206, 123945. [Google Scholar] [CrossRef]
- Chen, H.; Liu, X.L.; Yang, Y.X.; Zhong, L.Q.; Wang, L.; Gao, N. Numerical simulation of foam metal copper/paraffin phase change thermal storage process. CIESC J. 2019, 70, 86–92. [Google Scholar]
- Chintakrinda, K.; Weinstein, R.D.; Fleischer, A.S. A direct comparison of three different material enhancement methods on the transient thermal response of paraffin phase change material exposed to high heat fluxes. Int. J. Therm. Sci. 2011, 50, 1639–1647. [Google Scholar] [CrossRef]
- Yang, X.; Yu, J.; Guo, Z.; Jin, L.; He, Y.-L. Role of porous metal foam on the heat transfer enhancement for a thermal energy storage tube. Appl. Energy 2019, 239, 142–156. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, P.; Li, M. Preparation and thermal characterization of paraffin/metal foam composite phase change material. Appl. Energy 2013, 112, 1357–1366. [Google Scholar] [CrossRef]
- Lafdi, K.; Mesalhy, O.; Shaikh, S. Experimental study on the influence of foam porosity and pore size on the melting of phase change materials. J. Appl. Phys. 2007, 102, 759. [Google Scholar] [CrossRef]
- Al-Ketan, O.; Rowshan, R.; Abu Al-Rub, R.K. Topology-mechanical property relationship of 3D printed strut, skeletal, and sheet based periodic metallic cellular materials. Addit. Manuf. 2018, 19, 167–183. [Google Scholar] [CrossRef]
- Hu, X.; Gong, X. Pore-scale numerical simulation of the thermal performance for phase change material embedded in metal foam with cubic periodic cell structure. Appl. Therm. Eng. 2019, 151, 231–239. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, F.; Deng, X.; Zhao, M.; Zhou, H.; Zhang, D.Z. Experimental study on the thermal storage performance of phase change materials embedded with additively manufactured triply periodic minimal surface architected lattices. Int. J. Heat Mass Transf. 2022, 199, 123452. [Google Scholar] [CrossRef]
- Qureshi, Z.A.; Omari, S.A.; Elnajjar, E.; Mahmoud, F.; Al-Ketan, O.; Abu Al-Rub, R. Thermal characterization of 3D-Printed lattices based on triply periodic minimal surfaces embedded with organic phase change material. Case Stud. Therm. Eng. 2021, 27, 101315. [Google Scholar] [CrossRef]
- Sundarram, S.S.; Li, W. The effect of pore size and porosity on thermal management performance of phase change material infiltrated microcellular metal foams. Appl. Therm. Eng. 2014, 64, 147–154. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, F.; Zhang, T.; Deng, X.; Zhang, Z.W. Numerical simulation and experiment of heat storage process of TPMS porous aluminum-paraffin composite phase change material. Chem. Ind. Eng. Prog. 2022, 41, 4918–4927. [Google Scholar]
- Caket, A.G.; Wang, C.; Nugroho, M.A.; Celik, H.; Mobedi, M. Recent studies on 3D lattice metal frame technique for enhancement of heat transfer: Discovering trends and reasons. Renew. Sustain. Energy Rev. 2022, 167, 112697. [Google Scholar] [CrossRef]
- Molteni, M.; Candidori, S.; Graziosi, S.; Gariboldi, E. Improving the thermal response flexibility of 2- and 3-phase composite phase change materials by metallic triply periodic minimal surface structures. J. Energy Storage 2023, 72, 108185. [Google Scholar] [CrossRef]
- Cai, W.; Yang, W.; Zhang, K.; Fan, J.H.; Wu, J.Y.; Xing, T.; He, R. Experimental and numerical simulation of the melting process of paraffin phase change material. J. Southwest Univ. Sci. Technol. 2018, 33, 6–9+81. [Google Scholar]
- Qureshi, Z.A.; Elnajjar, E.; Al-Ketan, O.; Al-Rub, R.A.; Al-Omari, S.B. Heat transfer performance of a finned metal foam-phase change material (FMF-PCM) system incorporating triply periodic minimal surfaces (TPMS)—ScienceDirect. Int. J. Heat Mass Transf. 2021, 170, 121001. [Google Scholar] [CrossRef]
- Voller, V.R.; Prakash, C. A fixed grid numerical modelling methodology for convection-diffusion mushy region phase-change problems. Int. J. Heat Mass Transf. 1987, 30, 1709–1719. [Google Scholar] [CrossRef]
- Kamkari, B.; Shokouhmand, H.; Bruno, F. Experimental investigation of the effect of inclination angle on convection-driven melting of phase change material in a rectangular enclosure—ScienceDirect. Int. J. Heat Mass Transf. 2014, 72, 186–200. [Google Scholar] [CrossRef]













| Material | ρ (kg/m3) | cp (J/(kg·K)) | λ (W/(m·K)) | L (kJ/kg) | Ts (K) | μ (kg/(m·s)) | α (K−1) |
|---|---|---|---|---|---|---|---|
| Paraffin | 824 | 2200 | 0.2977 | 213.05 | 324.45 | 0.00689 | 0.00583 |
| Steel (S-06) | 8030 | 502.48 | 16.27 | - | - | - | - |
| Alumina ceramic | 3500 | 880 | 27.5 | - | - | - | - |
| Lattice Structure | (mm) | A (mm−1) | l (mm) | |
|---|---|---|---|---|
| Tetradecahedron | 8 | 0.75 | 3.29 | 8 |
| Gibson–Ashby | 8 | 0.75 | 2.61 | 8 |
| Gibson–Ashby | 6 | 0.75 | 3.48 | 6 |
| Gibson–Ashby | 4 | 0.75 | 5.12 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Zhang, H.; Zhang, C.; Tang, W.; Xiao, G.; Du, Y. A Pore Scale Study on Heat Transfer Characteristics of Integrated Thermal Protection Structures with Phase Change Material. Energies 2024, 17, 465. https://doi.org/10.3390/en17020465
Huang Z, Zhang H, Zhang C, Tang W, Xiao G, Du Y. A Pore Scale Study on Heat Transfer Characteristics of Integrated Thermal Protection Structures with Phase Change Material. Energies. 2024; 17(2):465. https://doi.org/10.3390/en17020465
Chicago/Turabian StyleHuang, Ziyuan, Hongming Zhang, Chao Zhang, Wei Tang, Guangming Xiao, and Yanxia Du. 2024. "A Pore Scale Study on Heat Transfer Characteristics of Integrated Thermal Protection Structures with Phase Change Material" Energies 17, no. 2: 465. https://doi.org/10.3390/en17020465
APA StyleHuang, Z., Zhang, H., Zhang, C., Tang, W., Xiao, G., & Du, Y. (2024). A Pore Scale Study on Heat Transfer Characteristics of Integrated Thermal Protection Structures with Phase Change Material. Energies, 17(2), 465. https://doi.org/10.3390/en17020465








